Abstract
Satisfactory results in terms of functional and oncological outcomes can be obtained in sacral and pelvic malignant bone tumors.
Preoperative planning, adequate imaging, and a multidisciplinary approach are needed.
3D-printed prostheses have to fulfill several requirements: (i) mechanical stability, (ii) biocompatibility, (iii) implantability, and (iv) diagnostic compatibility.
In this review, we highlight current standards in the use of 3D-printed technology for sacropelvic reconstruction.
Keywords: malignant pelvic tumors, 3DP, pelvic reconstruction, custom implants, pelvic resection, sacrectomy, spinal reconstruction
Introduction
In recent years, various titanium 3D printing applications are available in a broad spectrum of medical device types (1). In the field of musculoskeletal oncology, the improvement of 3D printing technology allows the creation of customized implants to handle complex reconstructions. This topic is closely related to computer-assisted surgery (CAS) and the optimization of data derived from preoperative imaging studies for the improvement of clinical and surgical outcomes such as the accuracy of bone cuts (2, 3, 4, 5). The first objective of surgery in the oncological setting is local control with complete excision of the tumor, while obtaining wide resection margins (6). However, it is obvious that the orthopedic surgeon must first take into consideration the local and systemic adjuvant treatments that the patient can receive based on the correct histopathological diagnosis. Limb salvage with endoprosthetic replacement surgery is today used for 90–95% of all patients with primary malignant bone tumors without compromising the oncological outcome (7, 8, 9). Prosthetic reconstructions after primary bone tumor removal can be divided into two groups based on (i) availability of modular implants or (ii) custom implants for unusual sites where massive allografts are the main alternative. Modular megaprostheses can be used for reconstruction of the entire humerus and lower limb long bones (femur, tibia) and have shown acceptable performance scores (range 65–82% at the Musculoskeletal Tumor Society score) with a 5-year estimated revision-free survival of 65–86% (10, 11, 12, 13, 14, 15). Sites where reconstruction after large tumor resection is extremely difficult are the forearm, foot and ankle, and spinopelvic areas. Advances in anesthesiologic and surgical techniques also allow the removal of some extensive tumors of the sacropelvic bone with adequate margins despite the difficulty to perform multiplanar osteotomies (16, 17, 18), whereas the development of surgical techniques allows demanding reconstructions (19, 20, 21, 22). With the use of CAS and custom 3D-printed reconstructions, satisfactory results in terms of functional and oncological outcomes have been also reported in recent series of patients affected by malignant bone tumors of unusual sites including sacrum and pelvis (20, 23, 24, 25, 26). These patient-specific special implants and related surgical tools have been initially studied for revision hip arthroplasties in selected cases (27, 28, 29, 30, 31) and then applied for pelvic tumor surgery (20, 25, 30). The planning of the surgical intervention in oncology depends on several decisive factors: the technique of the previous biopsy and the histopathological diagnosis (complemented by molecular, cytogenetic, and immunological studies), the tumor volume, the soft tissue involvement, and the patient’s general health status (32, 33). Without taking these aspects into account, therapeutic decisions would not be adequate and effective and long-term survival of the patient cannot be expected. This review is focused on the principles, concepts, and use of 3D-printed prosthetic reconstructions after sacral and pelvic resections, reporting the state of the art and the authors experience on this specific topic.
Planning the surgical approach
Once the resection has been planned and confirmed at the multidisciplinary consultation meeting, the surgeon can begin the second stage of surgical treatment planning (34). In addition to the type of tumor and extent of bony invasion, indications for pelvic reconstruction are based on the type of pelvic resection. The Enneking and Dunham surgical classification of pelvic resections into four zones (35) is the most frequently used. Angelini et al. reported an algorithm based on the above classification to guide the reconstructive strategies (30), and custom 3D-printed prosthesis should be used in type I or type I–IV pelvic resections and in pelvic acetabular resections when a cup with modular stem cannot be used due to the small size of the residual ilium. For tumors of the sacrum, the resection procedure must be prepared in terms of oncological adequacy, but also considering the possible complications deriving from secondary dysfunctions of the axial and supportive functions of the spine and pelvis. Loss or dysfunction of any component of this complex anatomical system causes failure of other adjacent organs of movement, such as the hip joints. Spinopelvic reconstruction should be considered, in relation to expected neurologic loss and functional instability (Fig. 1), following a total or high sacrectomy or sacroiliac joint removal.
Figure 1.
Types of sacral bone resection: type 1 – low sacral amputation (partial sacrectomy below S2), type 2 – high sacral amputation (partial sacrectomy through S1 or S1–S2), type 3 – total sacrectomy (sacrectomy at L5-S1 level), and type 4 – extended sacrectomy (resection of tumor extending beyond the sacroiliac joint or the L5 vertebra).
Once the decision to perform a reconstruction with a custom 3D-printed prosthesis has been made, it is important to have adequate imaging studies for the virtual model and a multidisciplinary discussion with the engineers (36, 37).
Radiological imaging
Comprehensive oncological staging is based on imaging studies: computed tomography (CT) of the pelvis and chest, magnetic resonance imaging (MRI), and bone scan or PET/CT (33, 38). Each of the tests mentioned earlier has its own sensitivity and specificity which complete the patient's clinical evaluation. CT perfectly shows bone morphology, the extension of the tumor in the soft tissues, and the presence of calcifications in the tumor mass. The MRI exam evaluates the tumor infiltration in the medullary cavity and in the soft tissues and the presence of micrometastases (the so-called 'skip metastases') within the same bone or in the adjacent one. Both exams are used to evaluate the response of the tumor to preoperative chemoradiotherapy. Bone scintigraphy and PET/CT are useful for evaluating systemic spread, the metabolic activity of the tumor, and detecting viable tumor tissue throughout the bone.
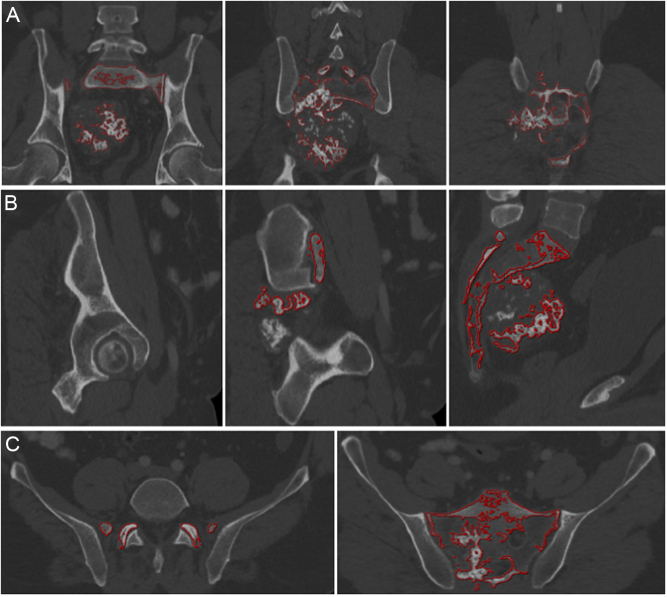
Patient-specific implants (PSI) are implants custom-made to the individual patient's anatomical bone structures that can be used intraoperatively to allow safe resection and reconstruction of the bone defect (39). The modeling is based on a high-resolution CT scan, converting the imaging data (Digital Imaging and Communications in Medicine) into a digital virtual 3D model (40). Preoperative determination of bone resection is possible manually, by placing cutting planes and drawing the tumor volume, but is a technically demanding and time-consuming task. This process called ‘segmentation’ is the delineation of tumor and anatomic structures by defining their contours. Nowadays, software exists that allows automatic segmentation of healthy bone, identification of tumor volume (Fig. 2), and generation of bone tumor resection plans, with the possibility of manual changes (slice-by-slice), in an acceptable time-frame of 20–30 min.
Figure 2.
A 46-year-old patient with extensive chondrosarcoma of the sacrum: preoperative planning of the extent of resection according to the ICAPS computer system (Germany): (A) coronal (B) sagittal, and (C) axial view. Tumor and sacrum are automatically mapped with red lines
Patient positioning and surgical approach
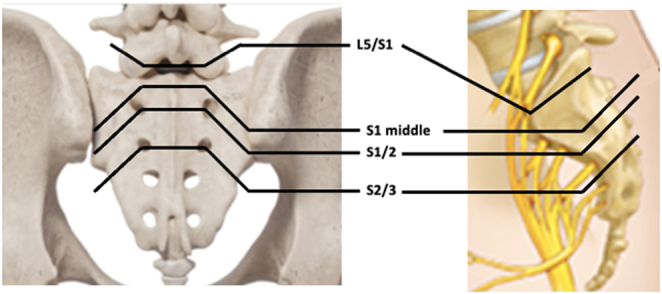
The close multidisciplinary collaboration between surgeons and engineers is essential for implant design: the surgeon contributes by transferring the surgical approach and anatomical landmarks that can be reached within the surgical field to the team. Furthermore, it is well known that the choice of surgical approach is one of the most important factors determining wound healing in the postoperative period (41). Incisions in the midline of the sacrum that reach into the gluteal fissure have a potential risk of infective complications (42). On the other hand, the surgical approach passing through both buttocks, transverse or straight cuts at the level of L5 to S3 extending bilaterally to the buttocks in the shape of the well-known star symbol is recommended for partial or total transverse sacrectomies in selected cases (Fig. 3), accepting the risk of flap necrosis (42, 43, 44). In type 4 extended sacrectomies, the best surgical approach appears to be the ‘Marcy–Fletcher posterior pelvic access’ which allows dissection of the L5 vertebra, the sacroiliac joint, the ilium, and even the hip joint (Fig. 4). In pelvic surgery, the patient positioning and surgical incision depend on the portion of bone to be resected, surgeon preference, and experience: supine position or lateral decubitus, combined approaches, and simultaneous or staged procedures are only some technical aspects that need to be considered in preoperative planning. Different surgical approaches have been described with pros and cons, such as the Kocher–Langenbach approach, Ollier's lateral U, ‘reverse question mark’, the utilitarian pelvic incision, and an ‘S’ shaped incision (45).
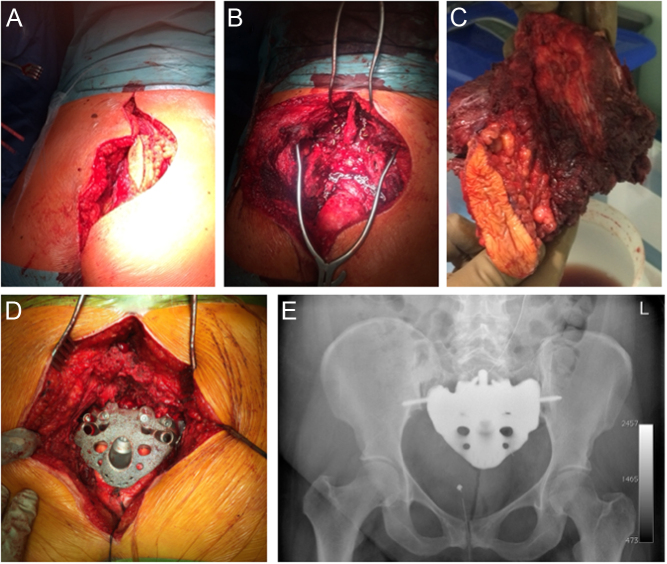
Figure 3.
A 36-year-old patient with G3 mesenchymal chondrosarcoma. (A) intraoperative picture of modified epaulette intraoperative access for total sacrectomy including open biopsy tract. (B) Intraoperative picture after tumor removal and (C) surgical specimen. (D) Intraoperative custom-made endoprosthesis realized with 3D-printing technology (MUTARS® Implantcast Gmbh, Buxtehüde, Germany). (E) Postoperative radiograph.
Figure 4.
(A) Modified posterior Marcy–Fletcher approach for type 4 sacrectomies: intraoperative picture shows the wide exposure of the sacrum and pelvic area. (B) Preoperative axial and (C) 3D CT reconstruction of the pelvis in a patient with osteosarcoma of the sacroiliac joint and L5 vertebra. (D) Clinical results after wound healing.
Implant details
Due to the value and complexity of the anatomical pelvic area, custom-made 3D-printed prostheses have to fulfill several requirements: (i) mechanical stability, (ii) biocompatibility, (iii) implantability, and (iv) diagnostic compatibility.
Mechanical stability
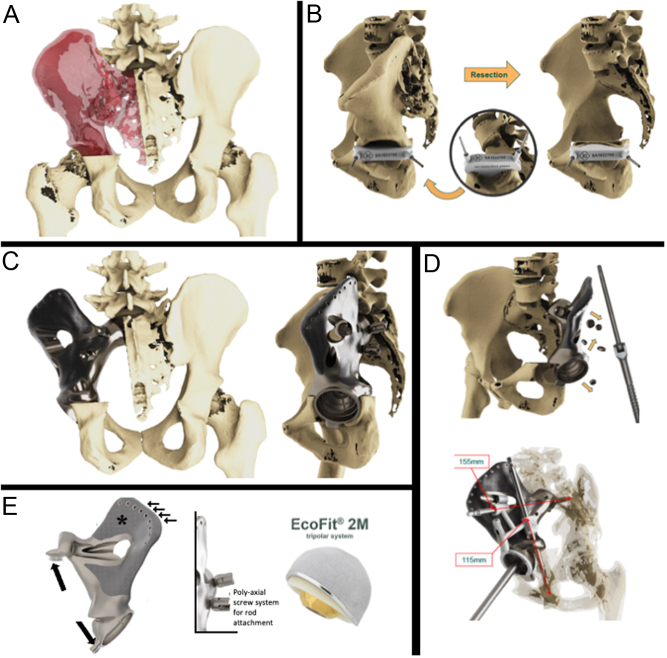
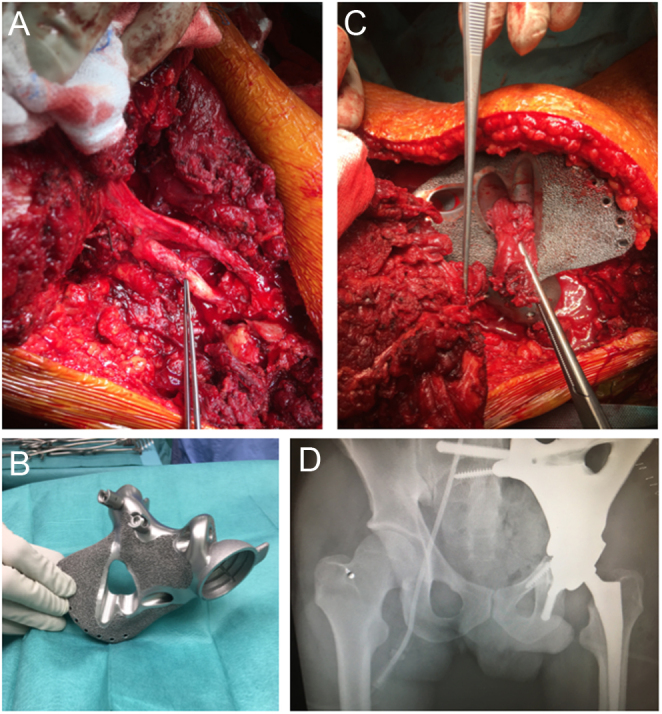
Starting from the virtual model, it is possible to create any complex shape with solid and porous sections to be combined to provide optimal strength and performance (46, 47). Ideally, the resistance and stiffness of the implant, and forces distribution should be identical to the removed bone it replaces. In real life, there are different opinions and prosthetic design based on surgeons’ experience. In sacral reconstructions, some authors preferred a prosthetic implant closely matching the anatomical structure of the sacrum (48), while others opt for an implant reduced in size (20, 23, 49) (Fig. 5). Huang et al. performed a biomechanical comparison of a 3D-printed sacrum prosthesis vs rod-screw systems for reconstruction after total sacrectomy, concluding that the prosthesis has the biomechanical advantages of a more uniform stress distribution (50). However, a combined custom pelvic prosthesis with posterior pedicle screw-rod fixation directly connected to tulip-head screws should be considered to increase stability in the proximal part of the ilium (Fig. 5) (51). Mechanical stability can be improved by adding porous surfaces in the contact areas with host bone facilitating bone ingrowth and long-term mechanical strength (Fig. 6) (52, 53). Specific porous or textured surfaces can be realized to favor strict adherence of vascularized soft tissues (p.e. muscles) obtaining stability, coverage of the implant, reduction of dead space, and the consequent risk of infection (20, 24, 25, 30). Other relevant aspects are the need for a primary stable fixation using long cancellous screws, cortical screws, press-fit porous stems, and small hooks for stabilization, often used in combination (Fig. 6) (20, 24, 25, 30, 54).
Figure 5.
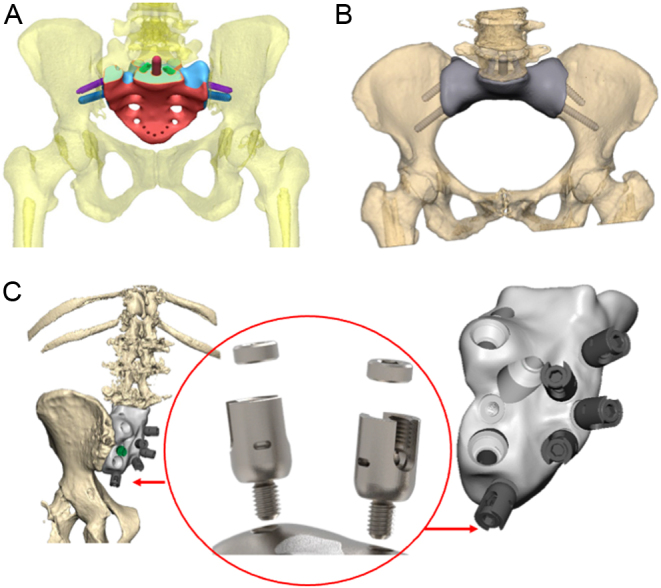
3D-printed sacral endoprostheses (additive manufacturing, EBM - electron beam melting, Implantcast Gmbh, Buxtehüde, Germany) in (A) anatomical version and (B) reduced in size. (C) Different design of sacral 3D-printed custom prosthesis with the possibility of connection to posterior spine stabilization using polyaxial screws.
Figure 6.
Wide margin hemisacrectomy plus hemipelvectomy (type I–II) for huge osteosarcoma after a good response to induction chemotherapy in a 25-year-old patient. (A) Scope of resection; (B) surgical technique with a custom cutting guide (in the circle) fixed with two pins in a virtual model; (C) 3D-printed implant with acetabular component and sacral proximal fixation. (D) Specific technical tricks: safety screws and 3D-printed plastic guide for adequate drilling. (E) Further details of the implant that increase long-term fixation: hooks for additional stability (black arrows), holes for soft tissue attachment (small arrows), EPORE® structure (asterisks), polyaxial screw system for spino-implant attachment, and tripolar acetabular system (Ecofit® 2M), (MUTARS ® Implantcast Gmbh, Buxtehüde, Germany).
Biocompatibility
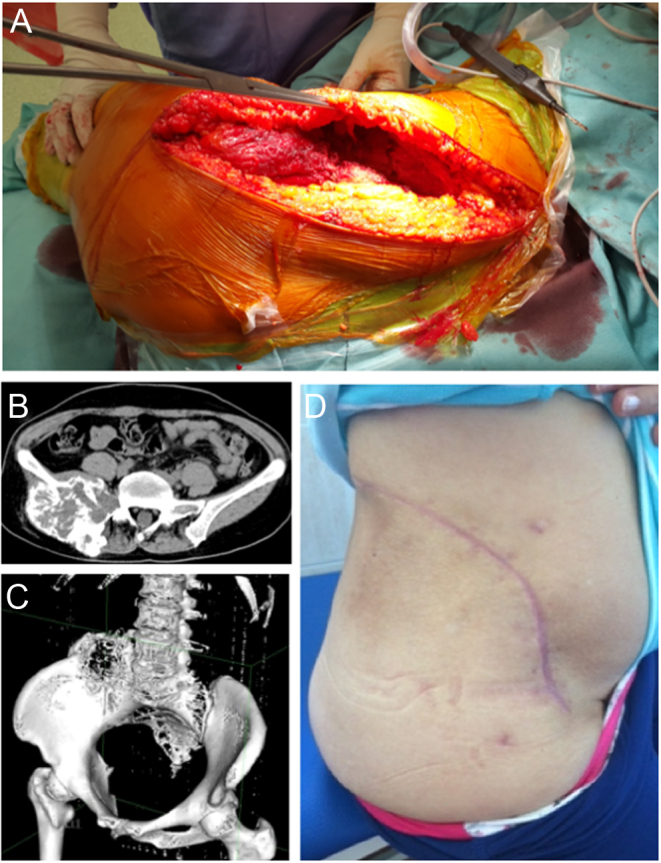
The main complications of massive allograft in pelvic reconstruction are infection and mechanical failures (55, 56). Even if cadaveric bone should have the maximum biocompatibility, the host considers that bone as a non-vascularized foreign body. Studies on biomaterials tried to minimize the adverse interactions of the prosthesis with the surrounding bone and soft tissue ((57). 3D-printed technology is able to create titanium alloy implants through two strategies (powder bed method and power deposit method), exploiting the known characteristics of the metal such as high strength, low density, high corrosion resistance, and excellent biocompatibility (58, 59). Some studies demonstrated that titanium alloy materials have a very good effect on promoting the proliferation and differentiation of osteoblasts (60, 61). Others confirmed that 3D printing process technology can integrate dense parts and porous structures to promote osteoblast adhesion and autologous bone ingrowth (62, 63). In the pelvis and sacrum, there are vascular and neurologic structures that should be protected from possible friction with the prosthetic implant (64). With 3D-printed technology is possible to realize smooth areas in close proximity to the vascular structures (Fig. 7).
Figure 7.
A 30-year-old patient with osteosarcoma of the left hemipelvis. (A) Intraoperative image of nerve root preservation; (B) 3D-printed implant with designed holes for muscle reconstructions and EPORE® structure; (C) hip muscle transfer through the implant; (D) postoperative radiograph shows definitive implant.
Implantability
One significant advantage of PSI is the specific cutting guides (jigs) that are designed by the multidisciplinary team considering the surgical approach (65). These guides can be sterilized and fixed to the host bone to exactly define resection planes, which could prevent misfitting of the definitive custom prosthesis (Fig. 6).
Diagnostic compatibility
The properties of the custom implant used for pelvic reconstruction should cause no or minimal artifacts with imaging studies, considering the need of strict follow-up evaluation of the malignant underlying pathology. The risk of local recurrence in primary tumors such as chondrosarcoma is high (Fig. 8). The event-free survival to local recurrence in a series of 409 chondrosarcomas was 85% at 5 years and 78% at 10 and 15 years (66). Analyzing a series of 215 chondrosarcomas of the pelvis, the overall 5- and 10-year survival to local recurrence drops to 75 and 66%, respectively (67).
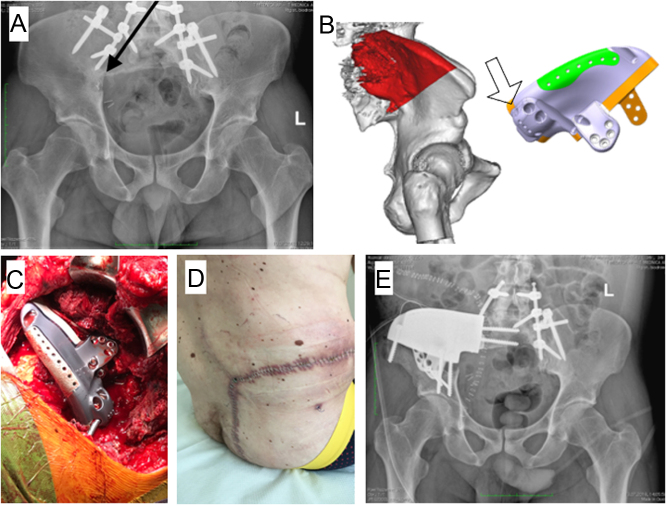
Figure 8.
Total sacrectomy and spinopelvic stabilization in a patient with G3 chondrosarcoma. (A) Radiograph at 3 years of follow-up shows local recurrence (black arrow) in the posterior pelvic column; (B) virtual model with new resection-planes highlighted in red color and the custom 3D prosthetic reconstruction with a hole (empty arrow) to connect the previously implanted posterior stabilization rods; (C) intraoperative picture of the reconstruction; (D) surgical approach wide good healing of the wound; (E) postoperative radiograph.
Pure titanium implants are unfortunately associated with several drawbacks regarding subsequent RT (68). In fact, these implants make notable artifacts on CT scans (used for both oncologic follow ups and to generate RT plans). Ongoing studies are evaluating the further improvement in 3D-printed technology to overcome those problems with metal hardware, such it happens in carbon fiber reinforced polyetheretherketone and titanium (CFP-T) that are now widely used in spine surgery (69).
Conclusion
An increasing role of surgery in the treatment of musculoskeletal sarcomas can be recently observed, particularly in axial localization. The 3D-printed technology associated with CAS dramatically changed the approach to malignant tumors of the pelvis and sacrum, allowing wide resection and stable reconstructions with good oncologic and functional outcomes. Improving surgeon and engineer expertise is changing the use of 3D-printed technology in musculoskeletal oncology, as implant design is moving from customized prostheses to a ‘standardized’ implant that can be tailored to a specific patient based on tumor site.
ICMJE conflict of interest statement
Ruggieri reports he is a consultant for Stryker and Exactech. The other authors declare that there are no relationships/conditions/circumstances that present a potential conflict of interest with the present manuscript.
Funding statement
There was no external funding source in support of this study.
References
- 1.Popov VV Muller-Kamskii G Kovalevsky A Dzhenzhera G Strokin E Kolomiets A & Ramon J. Design and 3D-printing of titanium bone implants: brief review of approach and clinical cases. Biomedical Engineering Letters 20188337–344. ( 10.1007/s13534-018-0080-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosma SE Cleven AHG & Dijkstra PDS. Can navigation improve the ability to achieve tumor-free margins in pelvic and sacral primary bone sarcoma re- sections? A historically controlled study. Clinical Orthopaedics and Related Research 20194771548–1559. ( 10.1097/corr.0000000000000766) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheong D & Letson GD. Computer-assisted navigation and musculoskeletal sarcoma surgery. Cancer Control 201118171–176. ( 10.1177/107327481101800304) [DOI] [PubMed] [Google Scholar]
- 4.Ieguchi M Hoshi M Takada J Hidaka N & Nakamura H. Navigation-assisted surgery for bone and soft tissue tumors with bony extension. Clinical Orthopaedics and Related Research 2012470275–283. ( 10.1007/s11999-011-2094-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abraham JA Kenneally B Amer K & Geller DS. Can navigation-assisted surgery help achieve negative margins in resection of pelvic and sacral tumors? Clinical Orthopaedics and Related Research 2018476499–508. ( 10.1007/s11999.0000000000000064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young PS Bell SW & Mahendra A. The evolving role of computer-assisted navigation in musculoskeletal oncology. Bone and Joint Journal 201597–B258–264. ( 10.1302/0301-620X.97B2.34461) [DOI] [PubMed] [Google Scholar]
- 7.Jeys LM Grimer RJ Carter SR & Tillman RM. Risk of amputation following limb salvage surgery with endoprosthetic replacement, in a consecutive series of 1261 patients. International Orthopaedics 200327160–163. ( 10.1007/s00264-003-0429-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aksnes LH Bauer HCF Jebsen NL Follerås G Allert C Haugen GS & Hall KS. Limb-sparing surgery preserves more function than amputation: a Scandinavian sarcoma group study of 118 patients. Journal of Bone and Joint Surgery. British Volume 200890786–794. ( 10.1302/0301-620X.90B6.19805) [DOI] [PubMed] [Google Scholar]
- 9.Tsantes AG Altsitzioglou P Papadopoulos DV Lorenzo D Romanò CL Benzakour T Tsukamoto S Errani C Angelini A & Mavrogenis AF. Infections of tumor prostheses: an updated review on risk factors, microbiology, diagnosis, and treatment strategies. Biology (Basel) 202312 314. ( 10.3390/biology12020314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pala E Henderson ER Calabrò T Angelini A Abati CN Trovarelli G & Ruggieri P. Survival of current production tumor endoprostheses: complications, functional results, and a comparative statistical analysis. Journal of Surgical Oncology 2013108403–408. ( 10.1002/jso.23414) [DOI] [PubMed] [Google Scholar]
- 11.Qadir I Umer M & Baloch N. Functional outcome of limb salvage surgery with mega-endoprosthetic reconstruction for bone tumors. Archives of Orthopaedic and Trauma Surgery 20121321227–1232. ( 10.1007/s00402-012-1542-3) [DOI] [PubMed] [Google Scholar]
- 12.Ahlmann ER Menendez LR Kermani C & Gotha H. Survivorship and clinical outcome of modular endoprosthetic reconstruction for neoplastic disease of the lower limb. Journal of Bone and Joint Surgery. British Volume 200688790–795. ( 10.1302/0301-620X.88B6.17519) [DOI] [PubMed] [Google Scholar]
- 13.Pala E Trovarelli G Calabrò T Angelini A Abati CN & Ruggieri P. Survival of modern knee tumor megaprostheses: failures, functional results, and a comparative statistical analysis. Clinical Orthopaedics and Related Research 2015473891–899. ( 10.1007/s11999-014-3699-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henderson ER, Groundland JS, Pala E, Dennis JA, Wooten R, Cheong D, Windhager R, Kotz RI, Mercuri M, Funovics PT, et al. Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. Journal of Bone and Joint Surgery 201193418–429. ( 10.2106/JBJS.J.00834) [DOI] [PubMed] [Google Scholar]
- 15.Trovarelli G Cappellari A Angelini A Pala E & Ruggieri P. What is the survival and function of modular reverse total shoulder prostheses in patients undergoing tumor resections in whom an innervated deltoid muscle can be preserved? Clinical Orthopaedics and Related Research 20194772495–2507. ( 10.1097/CORR.0000000000000899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Docquier PL Paul L Cartiaux O Delloye C & Banse X. Computer-assisted resection and reconstruction of pelvic tumor sarcoma. Sarcoma 20102010 125162. ( 10.1155/2010/125162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hufner T Kfuri M Galanski M Bastian L Loss M Pohlemann T & Krettek C. New indications for computer-assisted surgery: tumor resection in the pelvis. Clinical Orthopaedics and Related Research 2004426219–225. ( 10.1097/01.blo.0000138958.11939.94) [DOI] [PubMed] [Google Scholar]
- 18.Puchner SE Funovics PT Böhler C Kaider A Stihsen C Hobusch GM Panotopoulos J & Windhager R. Oncological and surgical outcome after treatment of pelvic sarcomas. PLoS One 201712 e0172203. ( 10.1371/journal.pone.0172203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bus MP Boerhout EJ Bramer JA & Dijkstra PD. Clinical outcome of pedestal cup endoprosthetic reconstruction after resection of a peri-acetabular tumour. Bone and Joint Journal 201496–B1706–1712. ( 10.1302/0301-620X.96B12.34622) [DOI] [PubMed] [Google Scholar]
- 20.Angelini A Kotrych D Trovarelli G Szafrański A Bohatyrewicz A & Ruggieri P. Analysis of principles inspiring design of three-dimensional-printed custom-made prostheses in two referral centres. International Orthopaedics 202044829–837. ( 10.1007/s00264-020-04523-y) [DOI] [PubMed] [Google Scholar]
- 21.Abudu A Grimer RJ Cannon SR Carter SR & Sneath RS. Reconstruction of the hemipelvis after the excision of malignant tumours. Complications and functional outcome of prostheses. Journal of Bone and Joint Surgery. British Volume 199779773–779. ( 10.1302/0301-620x.79b5.6749) [DOI] [PubMed] [Google Scholar]
- 22.Bus MP, Szafranski A, Sellevold S, Goryn T, Jutte PC, Bramer JA, Fiocco M, Streitbürger A, Kotrych D, van de Sande MA, et al. LUMiC® endoprosthetic reconstruction after periacetabular tumor resection: short-term results. Clinical Orthopaedics and Related Research 2017475686–695. ( 10.1007/s11999-016-4805-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei R Guo W Ji T Zhang Y & Liang H. One-step reconstruction with a 3D-printed, custom-made prosthesis after total en bloc sacrectomy: a technical note. European Spine Journal 2017261902–1909. ( 10.1007/s00586-016-4871-z) [DOI] [PubMed] [Google Scholar]
- 24.Liang H Ji T Zhang Y Wang Y & Guo W. Reconstruction with 3D-printed pelvic endoprostheses after resection of a pelvic tumour. Bone and Joint Journal 201799–B267–275. ( 10.1302/0301-620X.99B2.BJJ-2016-0654.R1) [DOI] [PubMed] [Google Scholar]
- 25.Wang B Hao Y Pu F Jiang W & Shao Z. Computer-aided designed, three dimensional-printed hemipelvic prosthesis for peri-acetabular malignant bone tumour. International Orthopaedics 201842687–694. ( 10.1007/s00264-017-3645-5) [DOI] [PubMed] [Google Scholar]
- 26.Albergo JI Farfalli GL Ayerza MA Ritacco LE & Aponte-Tinao LA. Computer-assisted surgery (CAS) in orthopedic oncology. Which were the indications, problems and results in our first consecutive 203 patients? European Journal of Surgical Oncology 202147424–428. ( 10.1016/j.ejso.2020.06.008) [DOI] [PubMed] [Google Scholar]
- 27.Citak M Kochsiek L Gehrke T Haasper C Suero EM & Mau H. Preliminary results of a 3D-printed acetabular component in the management of extensive defects. Hip International 201828266–271. ( 10.5301/hipint.5000561) [DOI] [PubMed] [Google Scholar]
- 28.Hao Y, Luo D, Wu J, Wang L, Xie K, Yan M, Dai K & Hao Y. A novel revision system for complex pelvic defects utilizing 3D-printed custom prosthesis. Journal of Orthopaedic Translation 202131102–109. ( 10.1016/j.jot.2021.09.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wyatt MC. Custom 3D-printed acetabular implants in hip surgery--innovative breakthrough or expensive bespoke upgrade? Hip International 201525375–379. ( 10.5301/hipint.5000294) [DOI] [PubMed] [Google Scholar]
- 30.Angelini A Trovarelli G Berizzi A Pala E Breda A & Ruggieri P. Three-dimension-printed custom-made prosthetic reconstructions: from revision surgery to oncologic reconstructions. International Orthopaedics 201943123–132. ( 10.1007/s00264-018-4232-0) [DOI] [PubMed] [Google Scholar]
- 31.Berasi CC Berend KR Adams JB Ruh EL & Lombardi AV. Are custom triflange acetabular components effective for reconstruction of catastrophic bone loss? Clinical Orthopaedics and Related Research 2015473528–535. ( 10.1007/s11999-014-3969-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mavrogenis AF Angelini A Errani C & Rimondi E. How should musculoskeletal biopsies be performed? Orthopedics 201437585–588. ( 10.3928/01477447-20140825-03) [DOI] [PubMed] [Google Scholar]
- 33.Mavrogenis AF Angelini A Vottis C Palmerini E Rimondi E Rossi G Papagelopoulos PJ & Ruggieri P. State-of-the-art approach for bone sarcomas. European Journal of Orthopaedic Surgery and Traumatology: Orthopedie Traumatologie 2015255–15. ( 10.1007/s00590-014-1468-2) [DOI] [PubMed] [Google Scholar]
- 34.Angelini A Piazza M Pagliarini E Trovarelli G Spertino A & Ruggieri P. The orthopedic-vascular multidisciplinary approach improves patient safety in surgery for musculoskeletal tumors: a large-volume center experience. Journal of Personalized Medicine 202111 462. ( 10.3390/jpm11060462) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enneking WF & Dunham WK. Resection and reconstruction for primary neoplasms involving the innominate bone. Journal of Bone and Joint Surgery. American Volume 197860731–746. ( 10.2106/00004623-197860060-00002) [DOI] [PubMed] [Google Scholar]
- 36.Song B Zhao X Li S Han CJ Wei QS Wen SF Liu J & Shi Y. Differences in microstructure and properties between selective laser melting and traditional manufacturing for fabrication of metal parts: a review. Frontiers of Mechanical Engineering 201510111–125. ( 10.1007/s11465-015-0341-2) [DOI] [Google Scholar]
- 37.Ruggieri P Cerchiaro M & Angelini A. Multidisciplinary approach in patients with metastatic fractures and oligometastases. Injury 202354268–270. ( 10.1016/j.injury.2022.11.027) [DOI] [PubMed] [Google Scholar]
- 38.Caracciolo JT & Letson GD. Radiologic approach to bone and soft tissue sarcomas. Surgical Clinics of North America 201696963–976. ( 10.1016/j.suc.2016.05.007) [DOI] [PubMed] [Google Scholar]
- 39.Döring K Staats K Puchner S & Windhager R. Patient-specific implants for pelvic tumor resections. Journal of Personalized Medicine 202111 683. ( 10.3390/jpm11080683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ritacco LE Milano FE Farfalli GL Ayerza MA Muscolo DL & Aponte-Tinao LA. Accuracy of 3-D planning and navigation in bone tumor resection. Orthopedics 201336e942–e950. ( 10.3928/01477447-20130624-27) [DOI] [PubMed] [Google Scholar]
- 41.Angelini A Pala E Calabrò T Maraldi M & Ruggieri P. Prognostic factors in surgical resection of sacral chordoma. Journal of Surgical Oncology 2015112344–351. ( 10.1002/jso.23987) [DOI] [PubMed] [Google Scholar]
- 42.Ruggieri P Angelini A Pala E & Mercuri M. Infections in surgery of primary tumors of the sacrum. Spine (Phila Pa 1976) 201237420–428. ( 10.1097/BRS.0b013e3182213a44) [DOI] [PubMed] [Google Scholar]
- 43.Verlaan JJ Kuperus JS Slooff WB Hennipman A & Oner FC. Complications, secondary interventions and long term morbidity after en bloc sacrectomy. European Spine Journal 2015242209–2219. ( 10.1007/s00586-014-3729-5) [DOI] [PubMed] [Google Scholar]
- 44.Angelini A Mavrogenis AF & Ruggieri P. Chondrosarcoma of the sacrum. In Tumors of the Sacrum. Ruggieri P Angelini A Vanel D & Picci P Eds. Cham: Springer; 2017. [Google Scholar]
- 45.Angelini A Crimì A Pala E & Ruggieri P. Surgical approaches in pelvic bone tumors. In Surgery of Pelvic Bone Tumors. Ruggieri P & Angelini A. Eds. Cham: Springer; 2021. ( 10.1007/978-3-030-77007-5_1). [DOI] [Google Scholar]
- 46.Sing SL Wang S Agarwala S Wiria FE Ha TMH & Yeong WY. Fabrication of titanium based biphasic scaffold using selective laser melting and collagen immersion. International Journal of Bioprinting 20173007. ( 10.18063/IIB.2017.01.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiu P, Jia Z, Lv J, Yin C, Cheng Y, Zhang K, Song C, Leng H, Zheng Y, Cai H, et al. Tailored surface treatment of 3D printed porous Ti6Al4V by microarc oxidation for enhanced osseointegration via optimized bone in-growth patterns and interlocked bone/implant interface. ACS Applied Materials and Interfaces 2016817964–17975. ( 10.1021/acsami.6b05893) [DOI] [PubMed] [Google Scholar]
- 48.Kim D Lim JY Shim KW Han JW Yi S Yoon DH Kim KN Ha Y Ji GY & Shin DA. Sacral reconstruction with a 3D-printed implant after hemisacrectomy in a patient with sacral osteosarcoma: 1-year follow-up result. Yonsei Medical Journal 201758453–457. ( 10.3349/ymj.2017.58.2.453) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei R Guo W Yang R Tang X Yang Y Ji T & Liang H. Reconstruction of the pelvic ring after total en bloc sacrectomy using a 3D-printed sacral endoprosthesis with re-establishment of spinopelvic stability: a retrospective comparative study. Bone and Joint Journal 2019101–B880–888. ( 10.1302/0301-620X.101B7.BJJ-2018-1010.R2) [DOI] [PubMed] [Google Scholar]
- 50.Huang S Ji T & Guo W. Biomechanical comparison of a 3D-printed sacrum prosthesis versus rod-screw systems for reconstruction after total sacrectomy: a finite element analysis. Clinical Biomechanics (Bristol, Avon) 201970203–208. ( 10.1016/j.clinbiomech.2019.10.019) [DOI] [PubMed] [Google Scholar]
- 51.Mindea SA Salehi SA Ganju A Rosner MK O’Shaughnessy BA Jorge A & Ondra SL. Lumbosacropelvic junction reconstruction resulting in early ambulation for patients with lumbosacral neoplasms or osteomyelitis. Neurosurgical Focus 2003151–6. ( 10.3171/foc.2003.15.2.6) [DOI] [PubMed] [Google Scholar]
- 52.Dai KR Yan MN Zhu ZA & Sun YH. Computer-aided custom-made hemipelvic prosthesis used in extensive pelvic lesions. Journal of Arthroplasty 200722981–986. ( 10.1016/j.arth.2007.05.002) [DOI] [PubMed] [Google Scholar]
- 53.Fang C Cai H Kuong E Chui E Siu YC Ji T & Drstvenšek I. ISurgical applications of three-dimensional printing in the pelvis and acetabulum: from models and tools to implants. Der Unfallchirurg 2019122278–285. ( 10.1007/s00113-019-0626-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jackson RJ & Gokaslan ZL. Spinal-pelvic fixation in patients with lumbosacral neoplasms. Journal of Neurosurgery 20009261–70. ( 10.3171/spi.2000.92.1.0061) [DOI] [PubMed] [Google Scholar]
- 55.Angelini A Drago G Trovarelli G Calabrò T & Ruggieri P. Infection after surgical resection for pelvic bone tumors: an analysis of 270 patients from one institution. Clinical Orthopaedics and Related Research 2014472349–359. ( 10.1007/s11999-013-3250-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ayvaz M Bekmez S Mermerkaya MU Caglar O Acaroglu E & Tokgozoglu AM. Long-term results of reconstruction with pelvic allografts after wide resection of pelvic sarcomas. TheScientificWorldJournal 20142014 605019. ( 10.1155/2014/605019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sheng X Wang A Wang Z Liu H Wang J & Li C. Advanced surface modification for 3D-printed titanium alloy implant interface functionalization. Frontiers in Bioengineering and Biotechnology 202210 850110. ( 10.3389/fbioe.2022.850110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Z Wang C Li C Qin Y Zhong L Chen B Li Z Liu H Chang F & Wang J. Analysis of factors influencing bone ingrowth into three-dimensional printed porous metal scaffolds: a review. Journal of Alloys and Compounds 2017717271–285. ( 10.1016/j.jallcom.2017.05.079) [DOI] [Google Scholar]
- 59.Kermavnar T Shannon A O'Sullivan KJ McCarthy C Dunne CP & O'Sullivan LW. Three-dimensional printing of medical devices used directly to treat patients: a systematic review. 3D Printing and Additive Manufacturing 20218366–408. ( 10.1089/3dp.2020.0324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Q, Zhou J, Zhi P, Liu L, Liu C, Fang A & Zhang Q. 3D printing method for bone tissue engineering scaffold. Medicine in Novel Technology and Devices 202317. ( 10.1016/j.medntd.2022.100205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palmquist A Shah FA Emanuelsson L Omar O & Suska F. A technique for evaluating bone ingrowth into 3D printed, porous Ti6Al4V implants accurately using X-ray micro-computed tomog- raphy and histomorphometry. Micron 2017941–8. ( 10.1016/j.micron.2016.11.009) [DOI] [PubMed] [Google Scholar]
- 62.Dhawan A Kennedy PM Rizk EB & Ozbolat IT. Three-dimensional bioprinting for bone and cartilage restoration in orthopaedic surgery. Journal of the American Academy of Orthopaedic Surgeons 201927e215–e226. ( 10.5435/JAAOS-D-17-00632) [DOI] [PubMed] [Google Scholar]
- 63.Wixted CM Peterson JR Kadakia RJ & Adams SB. Three-dimensional printing in orthopaedic surgery: current applications and future developments. Respiratory Research 20215e20.00230–e20.00211. ( 10.5435/JAAOSGlobal-D-20-00230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Angelini A Calabrò T Pala E Trovarelli G Maraldi M & Ruggieri P. Resection and reconstruction of pelvic bone tumors. Orthopedics 20153887–93. ( 10.3928/01477447-20150204-51) [DOI] [PubMed] [Google Scholar]
- 65.Gouin F Paul L Odri GA & Cartiaux O. Computer-assisted planning and patient-specific instruments for bone tumor resection within the pelvis: a series of 11 patients. Sarcoma 20142014 842709. ( 10.1155/2014/842709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Angelini A Guerra G Mavrogenis AF Pala E Picci P & Ruggieri P. Clinical outcome of central conventional chondrosarcoma. Journal of Surgical Oncology 2012106929–937. ( 10.1002/jso.23173) [DOI] [PubMed] [Google Scholar]
- 67.Mavrogenis AF Angelini A Drago G Merlino B & Ruggieri P. Survival analysis of patients with chondrosarcomas of the pelvis. Journal of Surgical Oncology 201310819–27. ( 10.1002/jso.23351) [DOI] [PubMed] [Google Scholar]
- 68.Henzen D, Schmidhalter D, Guyer G, Stenger-Weisser A, Ermiş E, Poel R, Deml MC, Fix MK, Manser P, Aebersold DM, et al. Feasibility of postoperative spine stereotactic body radiation therapy in proximity of carbon and titanium hybrid implants using a robotic radiotherapy device. Radiation Oncology 202217 94. ( 10.1186/s13014-022-02058-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li CS Vannabouathong C Sprague S & Bhandari M. The use of carbon-fiber-reinforced (CFR) peek material in orthopedic implants: a systematic review. Clinical Medicine Insights. Arthritis and Musculoskeletal Disorders 2015833–45. ( 10.4137/CMAMD.S20354) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a