Abstract
In the long term, limited fasciectomy is currently the most reliable treatment for Dupuytren’s contracture.
The risk for complications is significant, certainly in recurrent disease and in the presence of abundant scar tissue.
Meticulous surgical technique is mandatory.
Microsurgery increases magnification from four times (with surgical loupes) up to 40 times.
Using the microscope in Dupuytren’s surgery, a technique named microfasciectomy is likely to increase both safety and efficiency by preventing instead of treating surgical complications.
Increased experience with microsurgery will benefit Dupuytren’s treatment and hand surgery in general.
Keywords: Dupuytren, microfasciectomy, fasciectomy, microsurgery, hand
Introduction
Dupuytren’s disease (DD) is a common hand disorder with mild to devastating consequences for function of the hand. A scar tissue-like process (fibrosis) forms nodules and strands in the palms, which may cause disabling finger contractures that impact the performance of activities and quality of life (1). The disease is highly prevalent: nodules or strands can be found in up to 30% of the population over 50 years, although a minority may seek medical attention or even require treatment due to functional disability (2, 3). Treatment modalities to correct the finger contractures are variable in invasiveness, which impacts recovery time, complication risks and chances of recurrence (4). Minimal invasive techniques such as needle fasciotomy and collagenase injections have a faster recovery, but incomplete correction and recurrence are markedly more frequent than open surgery (5). Fasciectomy can be augmented with skin grafting in cases of incomplete skin closure or to minimize recurrence (6).
Outcome in Dupuytren’s treatment has been studied intensively, mostly focused on range of motion and recurrence, but the definition and clinical significance of reported parameters are not well standardized (7). For instance, recurrence in DD is not uniformly defined. There is a difference between residual deformity, extension (or progression) of the disease and true recurrence of nodules and strands in the operated field, and at best, definitions are consensus based (8, 9).
However, recurrence is a challenge this can lead to an unsatisfied patient and a disappointed surgeon and may raise the need for surgical re-intervention. Surgical treatment for recurrent contractures is challenging. Not uncommonly, more extensive procedures are required, such as skin grafting, and in selected cases even joint arthrodesis and amputation may be preferred (10). However, even in repeated surgery, clinical improvement can be worthwhile (11, 12). However, there is significant risk for injury to digital nerves and arteries in primary cases and possibly even more in recurrent disease due to excessive scar tissue formation (13, 14).
Rationale for microsurgery in Dupuytren’s fasciectomy
Risk for complications
Possible complications in the treatment for DD are numerous: nerve injury with pain and sensory loss, chronic regional pain syndrome with functional impairment, ischemia due to digital arterial damage, stiffness or contractures due to scar tissue, arthrofibrosis or tendon lesions, delayed wound healing, skin necrosis, infections and hematoma. Even recurrence itself is often counted as a complication, even though this could also be considered disease progression (15).
In a 20-year review, Denkler mentioned that injury to digital nerves and arteries was 10 times more likely in recurrent (20%) than in primary (2%) surgery (14). To avoid neurovascular damage, it seems obvious that meticulous surgery is required. Good surgical planning, hand surgery experience and optimal visualization are prerequisites to success. That way, tissue manipulation can be minimized, neurovascular damage avoided and skin reconstruction successful.
Visual enhancement
To optimize the visualization of the operating field, loupe surgery is mandatory in hand surgery in general and in fasciectomy more particularly. Surgical loupes can enlarge the field of view by 2.5–6 times; usually 4 times is preferred in hand surgery. However, operating microscopes can provide up to 40 times magnification (16, 17). The operating microscope was introduced into small vessel surgery by Jacobson in 1960 (18). Ever since, the focus of microsurgery in hand surgery has been on microvascular indications. The microscope is mostly used in acute trauma settings (replantation and revascularization) and elective surgery with a need for vascular anastomosis such as free flaps or compound tissue transfers such as free toe to finger transplantation. In DD, the microscope is sometimes applied as well. However, this is mostly reserved for complications: the need for vascular repair in unintended digital arterial injury if ischemia is encountered after tourniquet release (19, 20, 21). For some reason, the use of the microscope to avoid such complications is highly resisted by many hand surgeons and is believed to be unnecessary and cumbersome (22).
Added value of microsurgery
Loupes are obviously less expensive than operating microscopes. Often, loupes are purchased on a personal basis by the surgeon, and operating microscopes are provided by the hospital, to be used by different surgeons and specialties. Also, loupes are easier to use and portable. However, once operative anatomical structures are less than 1.5 mm in diameter, a four times loupe magnification may be insufficient for adequate visualization, instrumental positioning, quality and duration of surgery, as demonstrated in a microvascular anastomosis permeability study (16, 23, 24). This may render surgical procedures less safe and feasible. Higher intraoperative magnification reduces complications and reduces surgeon’s fatigue due to superior vision, confidence and ergonomics. It is, however, a challenge to demonstrate the added value and exact position or indications of microsurgery in general. Numerous surgical specialties use operating microscopes for different reasons and indications. In hand surgery, loupes are more frequently used than microscopes. In orthopedic surgery, even the use of loupes is infrequent in contrast to plastic surgery, where both loupes and microscopes are commonly used (16).
Few studies have reported on the added value of microsurgery vs loupe surgery using different methodologies. In retrospective studies without randomization in other domains than hand surgery, any difference in outcome is rarely significant, but obviously there is an indication bias (25). Microsutures of vessels and nerves under 1.5 mm are significantly better if performed with an operating microscope (26).
But these few studies mostly focus on vascular anastomosis and nerve repair. Hardly any research is found on the possible advantage of microdissection to increase efficiency and decrease complications such as neurovascular damage in DD. Here, the digital arteries and nerves are less than 1 mm in size, certainly in the fifth digit and at the level of the proximal interphalangeal (PIP) joint, which is most commonly involved in challenging finger contractures in DD (27, 28).
Microfasciectomy in DD
Microsurgery is used mostly in complications during or after fasciectomy in Dupuytren’s contractures (19). If ischemia is discovered after tourniquet release, urgent repair can be needed but challenging. Thus, preventing such complications may be preferable. Microscope-assisted surgery may have a significant advantage over loupe surgery in DD due to the enhanced visual magnification of small neurovascular structures, embedded in fibrous tissue, certainly in recurrent contractures after earlier treatment (29). A successful microsurgical technique in DD was first described in a Russian paper in 1992 (30). Since neurovascular injury in Dupuytren’s surgery is reported in up to 8% of cases and these structures are even more at risk in surgery for recurrent disease. This technique may decrease such complications and improve clinical outcome significantly (31). Small series of fasciectomy where only parts of the procedure are microscope-assisted, have been reported with successful outcome (29). Here the authors suggested to consider using the microscope in recurrent disease, a history of digital vascular injury and neurovascular encasement in primary DD.
Additionally, the microscope will aid in skin dissection (Fig. 1). Overlying skin is often involved in Dupuytren’s contractures, and the presence of myofibroblasts within the skin and subcutaneous tissue had been demonstrated histologically to be correlated with recurrence of disease (32). However, these myofibroblasts are only present in the dermis and do not cross the basal lamina at the dermoepidermal junction to the epidermis but tempt to retract the skin if attached to this portion of the basement membrane that can be visualized with the operating microscope (33, 34). Thorough surgical dissection upon the basement membrane of the skin may be preferable to prevent recurrence. Also, if limited fasciectomy is preferred over dermatofasciectomy, meticulous skin dissection to preserve skin survival and prevent postoperative necrosis may be improved with microsurgical techniques.
Figure 1.
(A, B) Clinical pictures of the hands before surgery in a primary case with palmar nodules and strands; (C) microdissection of the digital nerves and arteries; and (D) microdissection of the skin with minacious removal of the Dupuytren’s tissue from the basement membrane of the skin.
Not only may microscopically assisted fasciectomy, named with the neologism ‘microfasciectomy’, decrease complications and improve outcome of Dupuytren’s surgery, it will also improve the surgeon’s microsurgical skills. This will benefit the Dupuytren’s patient in general elective surgery, but also in acute settings, since secondary referral for urgent revascularization to a replantation center has been reported (20). Most likely, since practical training and exposure is the most important factor to improve performance, the skills of the surgeon will increase as a consequence of increased exposure to microsurgery, which probably benefits education and other hand surgical cases such as trauma and revascularization (35). Even more, as proficiency-based progression research and training has clearly demonstrated, it is the skill of the surgeon that has the highest impact on improving surgical outcome (36).
Surgical technique of microfasciectomy
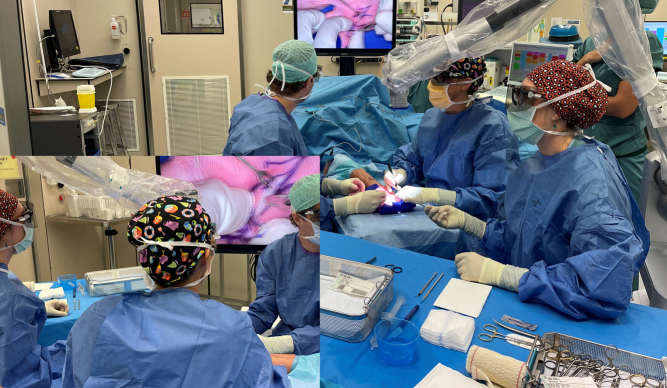
The surgical microscope is installed before the preparation of the patient and adapted to the surgeons’ eyes and kept ready, in order to avoid loss of tourniquet time. Although initially the microscope was only introduced once the procedure became more challenging (e.g. recurrent disease, distal and deep finger contractures and revascularization), we now have the habit to start every Dupuytren’s procedure from the very beginning under the microscope. This includes the skin incisions. We have a projecting screen, so that not only the surgeon but also the nurses and the patient under regional anesthesia (plexus) can follow the procedure. A useful mounting is shown in Fig. 2.
Figure 2.
Schematical presentation of a useful microfasciectomy mounting in theater. The surgeon (1) is seated in the axillary region, with the microscope pedal at the side of the patient and joystick at the head end. The assisting surgeon (2) is seated across him and the instrument nurse (3) sidewise to the surgeon, the microscope stand is positioned at the side of the assisting surgeon. Screen is visible to the nurse and the patient.
After a strategic approach with skin marks, the skin is incised under the microscope on low magnification, which gives the surgeon the best overview with a 4× enlargement, comparable to surgical loupe magnification. The skin edges are undermined, separating DD tissue from the basal lamina of the skin under the needed magnification, usually 10– 15×. Stay sutures can be placed on the skin strategically to allow a surgical field overview of the Dupuytren’s tissue.
Then, from proximal to distal, the DD tissue is isolated. Proximally, a beaver knife is used to cut the strands. The neurovascular bundles are isolated, and the three-dimensional affected palmar fascia is separated from their attachments to the unaffected neighboring and deep edges of its network. Adjacent palmar ray nodules can be removed subcutaneously from within the approached ray, lifting and tilting the hand to allow for microscopic exposure.
Distal to the natatory ligaments, dissection usually becomes more challenging, and, if needed, surgical micro-instruments (mostly scissors) are used to perform a safe neurolysis and smooth arterial dissection. Especially in recurrent disease and scar tissue formation, the latter can be challenging and take time, mostly at the level of the PIP joints. Straight and hooked beaver knives are mounted and ready for alternating use. A fine coated bipolar forceps is required for safe micro-cauterization.
Depending on the extension of the disease and affected fingers, all or strategically chosen digits are treated. We prefer to avoid exceeding a 2-h operating time, even if this would mean that one session is insufficient, and the patient may require another surgical procedure after 10 weeks. This mostly happens in extensive disease, certainly in recurrences. For surgical, tourniquet time, and rehabilitation reasons, we believe it is an advantage to avoid overdoing and aiming to correct all fingers within a bleeding operating field in exceptionally challenging cases.
A bipolar fine-point isolated cauterization is needed in microsurgery for thorough hemostasis. Unnecessary fascial innervation damage is avoided as much as possible. Skin closer Z-plasty and even full-thickness skin grafting (and harvesting) are all performed under the microscope. Often, both surgeons are operating at the same time, certainly in skin closure (zoom out) (Fig. 3).
Figure 3.
(A) Challenging recurrent Dupuytren’s case in the fifth finger with extensile scar tissue and severe contracture of the PIP joint; (B) the microscope is installed before incision; (C) the patient can observe the procedure on screen; (D) microfasciectomy dissection of a neuroma within the recurrent Dupuytren’s tissue (separate from the digital nerve), which is often encountered in recurrent disease; (E) microsurgical dissection of the digital arteries; and (F, G, H) en bloc Dupuytren and skin resection with full-thickness grafting under the microscope.
Outcome
As mentioned earlier, different parameters for outcome of microsurgery vs other surgical techniques, such as loupe surgery, can be investigated, depending on the surgical goal and applied techniques. In Dupuytren’s surgery, dissection of fibrous tissue and the prevention of complications such as ischemia are the primary goals. Up to now, only one limited outcome series was published on 17 Dupuytren patients (29).
The most evident and possible major challenge in the implementation of microsurgery in DD (next to the purchase cost) is the increase in operating time. Indeed, a preliminary study at our department did confirm this increase, mostly at the beginning of a learning curve. Next to intense training and routine of the surgeon and assisting staff, future technical developments of the operating microscope are likely to improve that issue (37).
In a recent preliminary series at our own hospital, a 1-year historical loop cohort was compared to a current microfasciectomy cohort. Both groups were comparable in age and gender distribution, recurrent vs primary disease and number of treated digits per patient (Table 1). In 33 patients, operated for primary DD in 2017, the mean operating time was 46 min. An extra 14 patients were operated for recurrent disease, and this took a mean of 82 min. Sixteen patients received full-thickness skin grafts, and these procedures needed a mean of 87 min. Compared to microfasciectomy, the standard of care at our department since 2020, primary surgery in 30 patients required a mean of 69 min, recurrence was treated in 22 patients for 96 min on average and skin grafting in 7 patients required a mean of 120 min. In this latter group, three digital arteries were repaired. In the microsurgery recurrent group, two arteries were repaired and one was sacrificed, but there was no ischemia. In the primary group, there were two vascular lesions who were repaired. In loop surgery, one patient needed amputation due to ischemia in the full-thickness graft group and two had numbness. Two patients had numbness in the recurrent group, four arteries needed repair whereas two were ligated. In the primary group, one artery needed coagulation and two fingers had numbness. Since microfasciectomy, it seems obvious that although the number of challenging cases has increased (more in recurrent), the number of complications decreased. Even more, the arterial anastomosis was perhaps not strictly necessary for digital survival but was performed due to feasibility of having a double-artery flow during microsurgery, and no ischemia was encountered in this series. This did, however, impact the operating time with a mean absolute increase by 14–20 min (Table 1).
Table 1.
Overview of historical loop cohort in 2017 vs microfasciectomy cohort in 2020. Both groups were comparable in age and gender distribution, recurrent vs primary disease and number of treated digits per patient.
| Loop fasciectomy | Microfasciectomy | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Primary |
Recurrent |
FTG |
Primary |
Recurrent |
FTG | |||||
| Combined | Recurrent | Primary | Combined | Recurrent | Primary | |||||
| n | 33 | 14 | 10 | 6 | 30 | 22 | 8 | 0 | ||
| Age (years) | 63.27 | 57.64 | 59.44 | 64.67 | 63.09 | 60.75 | ||||
| Females, n (%) | 8 (24) | 2 (14) | 0 (0) | 6 (20) | 5 (23) | 1 (12) | ||||
| Rays, mean n | 1.33 | 1.64 | 1.44 | 1.27 | 1.45 | 1.5 | ||||
| Mean operation time (min) | 46 (16–119) | 82 (25–179) | 100 (48–247) | 66 (44–104) | 69 (21–133) | 96 (39–139) | 120 (40–217) | 0 | ||
| Vascular lesion | 1 (3%) | 6 (43%) | 1 (10%) | 0 (0%) | 2 (7%) | 3 (14%) | 3 (38%) | 0 (0%) | ||
| Sensibility disturbances postoperative | 2 (6%) | 2 (14%) | 2 (20%) | 0 (0%) | 0 (0%) | 1 (5%) | 1 (13%) | 0 (0%) | ||
| % NV complications | 9 | 57 | 30 | 0 | 7 | 19 | 51 | 0 | ||
| % NV total | ||||||||||
| Loop fasciectomy = 22 | ||||||||||
| Microfasciectomy = 17 | ||||||||||
FTG, full-thickness skin graft; recurrent, recurrent disease; NV, neurovascular; primary, primary Dupuytren’s contracture.
Back to the future
Since its introduction in the 1960s, the operating microscope has evolved to a standard surgical instrument for the microsurgeon, somehow adapted to the discipline-specific requirements. However, a rigid angle of the lenses and the bifocal optics for three-dimensional vision limited to the surgeons looking through them, are reasons for ergonomic stress (37). Any digital projection and registration includes a two-dimensional vision, limiting stereotaxis and depth perception. To address these challenges, a stereo video camera was developed and added to the surgical microscope, renamed ‘exoscope’. With polarized glasses, depth perception is added to a high-resolution monitor and oculars are no longer required. With digital enhancement, image quality can be improved, and an even higher focus depth can be achieved. Not only can this contribute to better surgical quality, but increased micro-anatomical visualization is likely to contribute to the development of new observations and the acquirement of basic scientific knowledge, insights and translational research of DD (Fig. 4).
Figure 4.
With the exoscope development, microfasciectomy may be performed in the near future with high-resolution visual enhancement. This includes three-dimensional visualization without the need of ocular lenses, improving ergonomics to the surgeon and maneuverability in surgery for Dupuytren’s contractures.
Conclusion
Microfasciectomy may be considered in the surgical treatment of DD. With this technique, microanatomical visualization is enhanced. That way, the dissection of small nerves, vessels and skin away from the fibrous tissue improves. Superior vision is therefore likely to decrease complications and increase clinical outcome in Dupuytren’s surgery. Due to intense and regular training, the superior microsurgical skills of the surgeon are likely to reflect in better hand surgery outcome in general.
ICMJE conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding Statement
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- 1.Wilburn J McKenna SP Perry-Hinsley D & Bayat A. The impact of Dupuytren disease on patient activity and quality of life. Journal of Hand Surgery 2013381209–1214. ( 10.1016/j.jhsa.2013.03.036) [DOI] [PubMed] [Google Scholar]
- 2.Degreef I & de Smet L. A high prevalence of Dupuytren’s disease in Flanders. Acta Orthopaedica Belgica 201076316–320. Available at: https://pubmed.ncbi.nlm.nih.gov/20698450/ [PubMed] [Google Scholar]
- 3.Broekstra DC Kuo RYL Burn E Prieto-Alhambra D Furniss D & Frcs DM. Plastic and Reconstructive Surgery Advance Online Article Dupuytren’s disease: Prevalence, incidence, and lifetime risk of surgical intervention A population-based cohort analysis; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cichocki MN Chung WT Kane RL & Chung KC. Dupuytren contracture: using qualitative data to inform a conceptual framework for shared decision-making. Journal of Hand Surgery (European Volume) 2022. Available at: https://pubmed.ncbi.nlm.nih.gov/36329565/ [DOI] [PubMed] [Google Scholar]
- 5.Soreide E, Murad MH, Denbeigh JM, Dudakovic A, Kakar S, Lewallen EA, et al. Treatment of Dupuytren’s contracture: a systematic review. Bone and Joint Journal 2018100-B1138–1145. ( 10.1302/0301-620x.100b9.bjj-2017-1194.r2) [DOI] [PubMed] [Google Scholar]
- 6.Torrekens M van Nuffel M Couck I de Smet L & Degreef I. Skin grafting prevents recurrence in Dupuytren’s disease and extension correlates with fibrosis diathesis score. Hand Surgery and Rehabilitation 202140495–499. ( 10.1016/j.hansur.2021.03.008) [DOI] [PubMed] [Google Scholar]
- 7.Karpinski M Moltaji S Baxter C Murphy J Petropoulos JA & Thoma A. A systematic review identifying outcomes and outcome measures in Dupuytren’s disease research. Journal of Hand Surgery, European Volume 202045513–520. ( 10.1177/1753193420903624) [DOI] [PubMed] [Google Scholar]
- 8.Radhamony NG Nair RR Sreenivasan S Walkay S Soni A & Kakkar R. Residual deformity versus recurrence following Dupuytren’s palmar fasciectomy-a long term follow-up of 142 cases. Annals of Medicine and Surgery 202273. 103224. ( 10.1016/j.amsu.2021.103224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kan HJ Verrijp FW Hovius SER van Nieuwenhoven CA Dupuytren Delphi Group & Selles RW. Recurrence of Dupuytren’s contracture: a consensus-based definition. PLoS One 201712. e0164849. ( 10.1371/journal.pone.0164849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hozack BA & Rayan GM. Surgical treatment for recurrent Dupuytren disease. Hand 202115589447211060447. ( 10.1177/15589447211060447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendelaar NHA Poelstra R van Nieuwenhoven CA Slijper HP Feitz R Hovius SER & Selles RW. Outcome of recurrent surgery in Dupuytren’s disease: comparison with initial treatment. Plastic and Reconstructive Surgery 2019144828e–835e. ( 10.1097/PRS.0000000000006150) [DOI] [PubMed] [Google Scholar]
- 12.Könneker S Broelsch GF Krezdorn N Dastagir K Kuhbier JW Paprottka FJ & Vogt PM. Multiple recurrences in aggressive forms of Dupuytren’s disease-can patients benefit from repeated selective fasciectomy? Plastic and Reconstructive Surgery. Global Open 20175. e1247. ( 10.1097/GOX.0000000000001247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pillukat T Walle L Stüber R Windolf J & van Schoonhoven J. Treatment of recurrent Dupuytren’s disease. Der Orthopade 201746342–352. ( 10.1007/s00132-017-3385-7) [DOI] [PubMed] [Google Scholar]
- 14.Denkler K. Surgical complications associated with fasciectomy for Dupuytren’s disease: a 20-year review of the English literature. EPlasty 201010 e15. Available at: https://pubmed.ncbi.nlm.nih.gov/20204055/ [PMC free article] [PubMed] [Google Scholar]
- 15.Eberlin KR & Mudgal CS. Complications of treatment for Dupuytren disease. Hand Clinics 201834387–394. ( 10.1016/j.hcl.2018.03.007) [DOI] [PubMed] [Google Scholar]
- 16.Jarrett PM. Intraoperative magnification: who uses it? Microsurgery 200424420–422. ( 10.1002/micr.20066) [DOI] [PubMed] [Google Scholar]
- 17.Stanbury SJ & Elfar J. The use of surgical loupes in microsurgery. Journal of Hand Surgery 201136154–156. ( 10.1016/j.jhsa.2010.09.016) [DOI] [PubMed] [Google Scholar]
- 18.Rickard RF & Hudson DA. A history of vascular and microvascular surgery. Annals of Plastic Surgery 201473465–472. ( 10.1097/SAP.0b013e3182710027) [DOI] [PubMed] [Google Scholar]
- 19.Horta R Burnay T & Silva A. Microsurgical finger revascularization after long warm ischemia time following Dupuytren’s contracture release. Microsurgery 201434415–416. ( 10.1002/micr.22224) [DOI] [PubMed] [Google Scholar]
- 20.Jones NF & Huang JI. Emergency microsurgical revascularization for critical ischemia during surgery for Dupuytren’s contracture: a case report. Journal of Hand Surgery 2001261125–1128. ( 10.1053/jhsu.2001.28428) [DOI] [PubMed] [Google Scholar]
- 21.Chung KC & Segalman KA. Microvascular solution for vascular complication in surgery for Dupuytren’s contracture: a case report. Journal of Hand Surgery 199621711–713. ( 10.1016/S0363-5023(9680035-9) [DOI] [PubMed] [Google Scholar]
- 22.Nancarrow JD. Avoidance of critical ischemia in the surgery of Dupuytren’s disease. Journal of Hand Surgery 2002271109–1110. ( 10.1053/jhsu.2002.36521) [DOI] [PubMed] [Google Scholar]
- 23.Andrades P Benítez S Danilla S Erazo C Hasbun A & Fix J. Vascular diameter determining the magnification for a microvascular anastomosis. Journal of Reconstructive Microsurgery 200824177–181. ( 10.1055/s-2008-1076084) [DOI] [PubMed] [Google Scholar]
- 24.Pieptu D & Luchian S. Loupes-only microsurgery. Microsurgery 200323181–188. ( 10.1002/micr.10126) [DOI] [PubMed] [Google Scholar]
- 25.Ehanire T Singhal D Mast B & Leyngold M. Safety of microsurgery under loupes versus microscope: a head-to-head comparison of 2 surgeons with similar experiences. Annals of Plastic Surgery 201880(6S Supplement 6) S340–S342. ( 10.1097/SAP.0000000000001324) [DOI] [PubMed] [Google Scholar]
- 26.Bernstein DT Hamilton KL Foy C Petersen NJ & Netscher DT. Comparison of magnification in primary digital nerve repair: literature review, survey of practice trends, and assessment of 90 cadaveric repairs. Journal of Hand Surgery 2013382144–2150. ( 10.1016/j.jhsa.2013.04.010) [DOI] [PubMed] [Google Scholar]
- 27.Leslie BM Ruby LK Madell SJ & Wittenstein F. Digital artery diameters: an anatomic and clinical study. Journal of Hand Surgery 198712740–743. ( 10.1016/s0363-5023(8780060-6) [DOI] [PubMed] [Google Scholar]
- 28.Ortiz R Westenberg RF Langhammer CG Knaus WJ Chen NC & Eberlin KR. Nerve diameter in the hand: a cadaveric study. Plastic and Reconstructive Surgery. Global Open 20197e2155. ( 10.1097/GOX.0000000000002155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikkhah D & Blair J. Microscope assisted surgery for dupuytrens disease. Journal of Plastic, Reconstructive and Aesthetic Surgery 201972335–354. ( 10.1016/j.bjps.2018.10.033) [DOI] [PubMed] [Google Scholar]
- 30.Tsarev T & Savchenko VI. [The surgical treatment of Dupuytren's contracture].Vestnik Khirurgii Imeni I I Grekova 1992148169-174. [PubMed] [Google Scholar]
- 31.Hever P Smith OJ & Nikkhah D. Dupuytren’s fasciectomy: surgical pearls in planning and dissection. Plastic and Reconstructive Surgery. Global Open 20208e2832. ( 10.1097/GOX.0000000000002832) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen W Zhou H Pan ZJ Chen JS & Wang L. LThe role of skin and subcutaneous tissues in Dupuytren’s contracture: an electron microscopic observation. Orthopaedic Surgery 20091216–221. ( 10.1111/j.1757-7861.2009.00028.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iqbal SA Manning C Syed F Kolluru V Hayton M Watson S & Bayat A. Identification of mesenchymal stem cells in perinodular fat and skin in Dupuytren’s disease: a potential source of myofibroblasts with implications for pathogenesis and therapy. Stem Cells and Development 201221609–622. ( 10.1089/scd.2011.0140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCann BG Logan A Belcher H Warn A & Warn RM. The presence of myofibroblasts in the dermis of patients with Dupuytren’s contracture. A possible source for recurrence. Journal of Hand Surgery 199318656–661. ( 10.1016/0266-7681(9390029-f) [DOI] [PubMed] [Google Scholar]
- 35.Lefevre E, Ganau M, Zaed I, de Macedo Machado-Filho G, Scibilia A, Mallereau CH, Bresson D, Todeschi J, Cebula H, Proust F, et al. Learning curve and influencing factors of performing microsurgical anastomosis: a laboratory prospective study. Neurosurgical Review 2022453271–3280. ( 10.1007/s10143-022-01856-7) [DOI] [PubMed] [Google Scholar]
- 36.Mazzone E Puliatti S Amato M Bunting B Rocco B Montorsi F Mottrie A & Gallagher AG. A systematic review and meta-analysis on the impact of proficiency-based progression simulation training on performance outcomes. Annals of Surgery 2021274281–289. ( 10.1097/SLA.0000000000004650) [DOI] [PubMed] [Google Scholar]
- 37.Samaha Y & Ray E. Three-dimensional video microscopy: potential for improved ergonomics without increased operative time? Archives of Plastic Surgery 202350125–129. ( 10.1055/s-0042-1758768) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a