Abstract
Management of severely injured patients remains a challenge, characterised by a number of advances in clinical practice over the last decades. This evolution refers to all different phases of patient treatment from prehospital to the long-term rehabilitation of the survivors.
The spectrum of injuries and their severity is quite extensive, which dictates a clear understanding of the existing nomenclature.
What is defined nowadays as polytrauma or major trauma, together with other essential terms used in the orthopaedic trauma literature, is described in this instructional review.
Furthermore, an analysis of contemporary management strategies (early total care (ETG), damage control orthopaedics (DCO), early appropriate care (EAC), safe definitive surgery (SDS), prompt individualised safe management (PRISM) and musculoskeletal temporary surgery (MuST)) advocated over the last two decades is presented.
A focused description of new methods and techniques that have been introduced in clinical practice recently in all different phases of trauma management will also be presented.
As the understanding of trauma pathophysiology and subsequently the clinical practice continuously evolves, as the means of scientific interaction and exchange of knowledge improves dramatically, observing different standards between different healthcare systems and geographic regions remains problematic.
Positive impact on the survivorship rates and decrease in disability can only be achieved with teamwork training on technical and non-technical skills, as well as with efficient use of the available resources.
Keywords: major trauma, polytrauma, management, damage control, early appropriate care, review
Introduction
Trauma, either accidental or violence related, has always been one of the major health problems in human history. The evolution of medicine and surgical procedures was traditionally based on its effective management. At a global scale, the resources and means of trauma-related health services vary significantly, as documented consistently in the annual reports of the World Health Organization (WHO) (1).
Types of severe trauma are the ones mostly responsible for the 4.4 million injury-related deaths recorded across the world in 2021 (8% of all deaths). In its different forms (e.g. road traffic accidents, homicide, suicide) trauma remains the primary cause of death for those below the age of 45 years (1, 2). Furthermore, it is also responsible for more than 20% of all causes of severe disability, adversely affecting the quality of life of those that survive and of their immediate social circle. Beyond death and physical disability, an often-underestimated major problem is the risk of chronic mental illness, substance abuse and secondary chronic problems that stem from a previous traumatic event. Subsequently, the socioeconomic burden is huge, in terms of loss in productivity and consumption of resources towards trauma prevention, acute health services, rehabilitation and lifelong support of the severely disabled survivors.
The effective management of these patients is one of the most difficult clinical quests influenced by a large spectrum of complex and dynamic factors. In between all trauma-related surgical interventions, fracture fixation remains the most frequently required. Defining its optimal timing and choosing between the different fixation methods remains an area of debate (3).
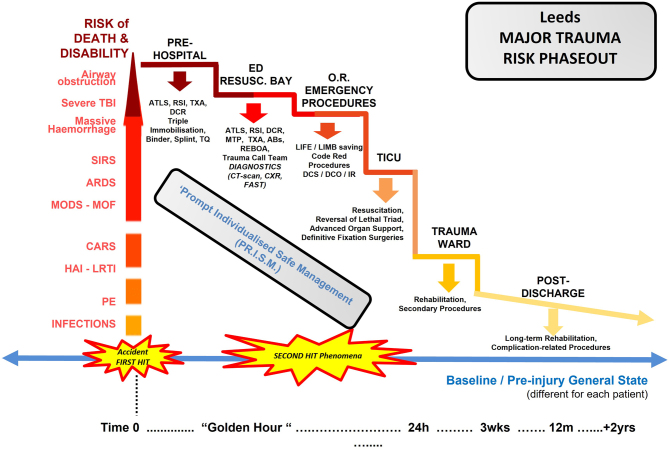
The objectives of this instructional review are (i) to clarify contemporary terms and methods commonly described in the relevant literature and (ii) to offer a comprehensive evaluation of the contemporary principles of management of severely injured patients at all phases of their care (Fig. 1).
Figure 1.
The ‘Leeds Major Trauma Risk Phaseout’ represents a graphic representation of the gradual decrease in the risk of death/disability following major trauma. These risks, at the time of the accident/’first hit’, increase significantly. All patients rely on the subsequent healthcare response to gradually restore their general state ideally to the preinjury levels (blue line). The causes of death/disability at the first stages are mostly associated with airway obstruction, severe brain injury and/or massive haemorrhage. Subsequently, complications relevant to the innate immune response to the ‘first hit’ and the magnitude and timing of surgical interventions (’second hit’) may be fatal due to multiorgan failure, infections and later pulmonary embolism. In all different phases of modern trauma care, a number of different protocols, interventions, drug therapies and surgical procedures aim to reduce the risk of death/disability (pointing-down arrows). A well-coordinated individualised strategy of management following the ‘Leeds PR.I.S.M.’ appears as a sensible model to follow. (30) ABs, antibiotics; ARDS, acute respiratory distress syndrome; ATLS, advanced trauma life support; CARS, compensatory anti-inflammatory response syndrome; CXR, chest x-ray; DCO, damage control orthopaedics; DCR, damage control resuscitation; DCS, damage control surgery; ED, emergency department; FAST, focused assessment with sonography for trauma; HAI, hospital-acquired infection; IR, interventional radiology; LRTI, lower respiratory tract infection; MOF, multiple organ failure; MTP, massive transfusion protocol; O.R., operation room/theatres; PE, pulmonary embolism; PR.I.S.M., prompt individualised safe management strategy; REBOA, resuscitative endovascular balloon occlusion of the aorta; RSI, rapid sequence induction of anaesthesia; SIRS, systemic inflammatory response syndrome; TBI, traumatic brain injury; TICU, trauma intensive care unit; TQ, tourniquet; TXA, tranexamic acid.
Definitions and classification systems
Trauma in general is the result of a physical (even psychological according to the more recent nomenclature) insult to a living organism. It has been subclassified according to its causative mechanism (i.e. blunt, penetrating, blast and deceleration), intention (i.e. accidental, self-inflicted and assault) or severity (i.e. minor, moderate, serious, severe, critical and maximal/untreatable) (4).
The abbreviated injury scale (AIS), since its introduction in 1971 and following its updates (latest in 2015), is unanimously used to cipher the anatomic description of all potential injuries in all anatomic body regions. It is based on the analysis of hundreds of thousands of patients and includes a severity mark for each distinct injury (AIS severity = last digit of the code ranging from 1 to 6). This AIS severity is strongly associated with the risk of each particular injury to cause death (4).
As a surrogate indicator of severity, clinicians still use the injury severity score (ISS) which derives from the AIS coding. It considers the most severe injuries from three different body regions. The body regions can be one of the following: 1) head and neck, including the cervical spine; 2) the face; 3) the chest including the thoracic spine; 4) the abdomen including the lumbar spine and the pelvic organs; 5) the pelvic skeleton and the extremities; and 6) the external referring to extensive skin lesions/burns (5). The ISS (range from 1 to 75) has been proven to be associated with trauma mortality, morbidity and the length of hospital stay (6). A degree of confusion exists between overlapping terminology relevant to ‘severe trauma’. It is defined as any single injury with AIS last digit of 4 or a combination of injuries with ISS score ≥ 16.
‘Polytrauma’ is a Greek term that is also widely used in the trauma literature since 1975 (7). It derives from the merge of two words (’poly’ which means many or too much and ‘trauma’). It should be used when we need to describe a combination of serious injuries in different body regions (in contrast to the term of ‘monotrauma’). In 2014, a clear definition of polytrauma was published. The term should be used when certain anatomic and physiology conditions are met (Table 1) (8).
Table 1.
Parameters considered to guide clinicians and researchers for the appropriate use of the term ‘polytrauma’ following the important publication of 2014 (8) – ‘Berlin Consensus’.
| Anatomic characteristics (both present) | Physiological characteristics (one of five present) |
|---|---|
| AIS last digit – severity ≥ 3 | Hypotension: SBP ≤90 mmHg |
| AIS body regions > 1 | Acidosis: lactate < –6mmol/L |
| Age: ≥70 years | |
| Cognitive state: GCS ≤ 8 | |
| Coagulopathy: INR ≥ 1.4, or aPTT ≥ 40sec |
AIS, Abbreviated Injury Scale; aPTT, arterial partial thromboplastin time; GCS, Glasgow coma scale; INR, international normalised ratio; SBP, systolic blood pressure..
In the United Kingdom, a different term is widely used over the last decade. It refers to the subgroup of injured patients who would benefit from a transfer to a specialised hospital with 24/7 readiness of its services (9). ‘Major trauma’ patients are those with any injury or combination of injuries that are likely to be fatal or cause long-term disability. The term includes poly- and monotrauma, in any body region, from any cause and in any age group.
There are a number of other subgroups that require utilisation of supplementary resources and methods in their management. More specifically, ‘paediatric trauma’ refers to trauma below the age of 16 years. It is the leading cause of deaths in this sensitive population and is associated with higher incidence of severe head injuries as well as trauma-induced coagulopathy (TIC) in comparison with the adults. Furthermore, particular attention is given to the radiation exposure during diagnostic imaging, whilst fracture fixation is adapted to the open epiphyses of their growing skeleton (10).
‘Silver trauma’ refers to the increasing number of elderly patients who often sustain a wide range of serious injuries (AIS ≥ 3) following even a low-energy mechanism. Emphasis is rising towards their limited tolerance to the initial traumatic insult, to early frailty assessment and to special diagnostic and therapeutic protocols that allow the early re-enablement of this vulnerable population. Contemporary multidisciplinary trauma teams include specialist geriatricians from the early stages of a ‘silver trauma’ admission (11).
Similarly, trauma victims whilst in pregnancy also represent patients who require modifications to the standard emergency management protocols and more complex monitoring of their course of treatment (12). Higher risks have been recorded for both fetal and maternal mortality. Additionally, there is often the concern of potential teratogenic effect to the fetus following ionising radiation, especially in the first 15 weeks of gestation. However, the risk of delayed or missed diagnosis of a serious injury is significantly higher for both the fetus and the mother; therefore, at least the acute imaging protocols usually include the standard CT trauma scan. Interdisciplinary input in these cases dictates the involvement of obstetricians from the early stages of management of the injured mother (13).
Lastly, another special situation that has a significant effect in the management of severely injured patients and may alter the routine protocols of management is when they are part of a mass casualty incident (MCI). These are defined as incidents where usually the number of injured patients and/or the nature of injuries are beyond the available capacity and resources (14). Under these circumstances, the initial triage and transfer protocols from the scene of the accident, as well as the type of primary interventions, are modified to allow the effective treatment of the larger possible number of victims and the minimisation of the fatalities. Damage-controlled, staged management protocols are mostly applied to save time and allow efficient use of the stretched resources (15).
Established management strategies
Since its introduction in 1978, the Advanced Trauma Life Support (ATLS) system has provided an irreplaceable framework to the acute management of trauma (1). Its simplicity and clarity has allowed generations of clinicians to train uniformly and practice reliably. All the more importantly, it has created the invaluable culture of coordinated multidisciplinary teamwork in the crucial early period of trauma management. The value of ATLS has been validated in numerous publications and, together with the updates of its training course, represents one of the main pillars of contemporary trauma management (2, 3, 4).
After the ATLS stage, a number of strategies have been advocated over the last 30 years, particularly relevant to the specifics of orthopaedic trauma. In the past, these different strategies have generated heated debates and have been the focus of a number of studies. In reality, they were all proven useful and justified, yet in different patient cohorts relevant to the specific features of the injuries and the innate and adaptive immune response of each individual patient (Table 2) (16).
Table 2.
Different periods in the evolution of management strategies for the severely injured in the orthopaedic trauma literature.
| Period | Rationale | Management | Evidence |
|---|---|---|---|
| 1940–1970 | ‘The fracture patient is too unwell to be operated’ | Patients left in traction for weeks | Case series |
| ETC 1980–1990 | ‘The fracture patient is too unwell not to be operated’ | Definitive fixation in a single stage | RCT, case–control studies |
| DCO 1990–2000 | ‘Second hit phenomenon’ Classification of patients’ status into stable/borderline/unstable/in extremis | Patients with unstable physiology require the least invasive initial fixation, with secondary definitive fixation days later | RCT, registry analysis, matched paired analysis |
| EAC 2013 | Resuscitate to reverse metabolic acidosis – lactate ≤ 4 mmol/L | Early definitive fixation within the first 36 h following initial resuscitation | Case–control study |
| SDS 2015 | Emphasis on continuous evaluation of patients at risk. Risk factors: coagulation/fluid balance/lung function/vasopressor needs/ICP | Certain patients require a staged approach with initial external fixation and definitive fixation as soon as risk factors are optimised | Expert opinion |
| PR.I.S.M. 2016 | ‘Do no further harm’. Additional factors such as genetic predisposition, resources and special groups (silver trauma, pregnancy, children) | Individualised approach – dynamic assessment and as early as possible definitive fixation in a single stage or more stages | Expert opinion |
| MuST 2021 | Differentiation between the staged strategy for distinctly different reasons | Staged fixation in stable patients due to specific local conditions (severe soft tissue trauma, segmental bone loss and complex periarticular fractures) | Expert opinion |
DCO, damage control orthopaedics; EAC, early appropriate care; ETC, early total care; ICP, intracranial pressure; MuST, musculoskeletal temporary surgery; PR.I.S.M., prompt individualised safe management; RCT, randomised controlled trial; SDS, safe definitive surgery.
The early total care (ETC) strategy of the 1980s followed the advances of fracture fixation techniques and anaesthesia of that time. The ETC rationale that ‘the injured patient is too sick not to operate early’ replaced the previous historical teaching of delaying the management of long bone fractures for the fear of severe perioperative complications. The most recognised ETC study is that of Bone and Johnson in 1989 (17). They recommended definitive fixation within 24-h of high-energy femoral fractures, identifying a reduction of secondary pulmonary complications and acute respiratory distress syndrome. This strategy gained global traction and remains applicable still for the majority of our patients under certain conditions.
However, a few years later, it became apparent that the more severely injured patients cannot tolerate prolonged definitive procedures (‘second hit’) (17). This led in the late 90s to the inception of an alternative strategy named damage control orthopaedics (DCO). The term was adopted from the US Navy, originally describing the use of simple but critical interventions in stages allowing a damaged ship to stay afloat and gain time until it reaches a repair dock (18, 19). This strategy employs a staged protocol for the most severely injured based on the following principles:
Immediate control of haemorrhage, decompression of critical cavities (skull, chest, abdomen and fascial compartments), early temporary stabilisation of long bone fractures (usually with bridging external fixators), and decontamination of soiled wounds.
Vital organ monitoring and resuscitation in the intensive care.
Secondary definitive fracture fixation at a later stage.
This type of staged strategy optimally should be applied to a smaller subgroup of patients that cannot tolerate aggressive all-inclusive definitive management due to the multiplicity and severity of either their trauma (’first hit’) and/or their frailty – poor physiologic reserve and mostly the instability of their physiology (20, 21). A number of studies have attempted to define clear criteria that could help the clinicians to decide which of their injured patients would benefit most from DCO (22, 23). Each patient following the initial ATLS manoeuvres is usually classified as being stable/borderline/unstable/in extremis. Each different state was defined according to specific criteria that reflect the presence or not of metabolic shock, coagulopathy, hypothermia (lethal triad) and severe lung, abdominal or soft tissue trauma. More recently, these criteria have been revisited in order to adapt to the evolution of resuscitation protocols, as well as of our better understanding of trauma pathophysiology (24, 25).
The early appropriate care (EAC) strategy was introduced in 2013 (26). It advocated in favour of early definitive management of all major orthopaedic injuries within 36 h, as long as the resuscitation has improved the state of metabolic acidosis (lactate ≤ 4 mmol/L or base excess ≥ 5.5 mmol/L). The evidence supporting a generalised adoption of the above mentioned thresholds was criticised as the metabolic state is crucial but not the only factor. Lactate levels are less reliable in certain scenarios which are common in trauma (e.g. following excessive alcohol consumption, or with uncontrolled diabetes, or in the elderly or in the presence of pre-existing impaired renal function). Furthermore, patients with critical head trauma with high intracranial pressures and/or severe chest trauma with poor respiratory function (p/f ratio <200 mmHg) often are not in the state to allow transfer to the operating theatres for prolonged procedures irrespective of their metabolic state (27, 28).
The safe definitive surgery (SDS) (29) and the prompt individualised safe management (PR.I.S.M.) strategies (30) were introduced more recently attempting to rationalise and balance between those previously described. Both refer to a more holistic approach where decisions are individualised and balanced on additional parameters besides the established ones (i.e. injury severity, age, presence of specific high-risk conditions as the lethal triad, severe head or chest injuries, bilateral femoral shaft fractures, poor response to resuscitation manoeuvres, frailty and pregnancy). The additional parameters include the type of local resources in terms of both manpower/expertise, and hospital capacity. The authors describe it as representing a philosophy of ‘doing no further harm’ aiming to achieve the best possible outcome in any given patient, hospital and health system (25, 30).
In 2021, a new term (MuST surgery – musculoskeletal temporary surgery) was introduced to differentiate between staged management offered for complex monotrauma and to conserve the term DCO for the unstable or in extremis polytrauma patients with the features described previously (31). MuST surgery is mostly applied and should be used as a term when there is severe soft tissue trauma, gross wound contamination, segmental bone loss or complex periarticular fractures in a physiologically stable patient without other serious associated injuries. According to the authors, If this terminology would be uniformly applied, it would allow a better comparison between DCO and ETC patient groups.
Pillars of modern trauma management
Over the last decades, there are certainly numerous aspects that have evolved in all phases of trauma management. There is certainly a wide variation between health systems, influenced not only by resources but also by demographic and geographic variations. Nevertheless, a number of widely accepted contemporary trends and key elements, verified by scientific evidence, exist and will be described.
Key elements at the prehospital phase
During the prehospital phase, a number of crucial measures and interventions contribute significantly to the overall outcome of severely injured patients. According to the WHO, these include the safe extraction from the accident area, correct triage and fast transfer to the appropriate hospital, effective communication, employment of the ATLS, early resuscitation and protection from secondary injuries at the time of extrication and transfer (32, 33).
A number of modern triage tools have been developed reflecting mostly differences between rural/urban regions, levels of readiness and resources between different health systems. They all share common characteristics and principles, aiming to avoid undertriage which can put injured patients at risk and at the same time minimise the over-triage which wastes the valuable resources in the dedicated trauma centres (34, 35).
Over the last decade, certain time-dependent interventions have been introduced at the prehospital trauma care. These aim to reverse the lethal triad (i.e. the vicious cycle of hypothermia, coagulopathy and acidosis) at the earliest possible time. They focus on improving the oxygenation of the injured tissues, reducing/replacing blood loss and decreasing the risk of infections.
Prehospital airway management with rapid sequence induction (RSI) of anaesthesia is considered an established life-saving intervention for a large number of major trauma patients (36). However, it is a challenging procedure with recorded higher risks than inhospital RSIs. The continuous training of prehospital emergency physicians and paramedics via standards, as the interchangeable operator model, is considered essential nowadays (37).
Two major randomised trials (crash-2 and -3) have proven the great value (reduction of all-cause mortality, bleeding as well as head injury-related deaths) of tranexamic acid (TXA). This simple and inexpensive antifibrinolytic agent administered early improves the outcome of bleeding injured patients, as well as of those with severe head trauma (38, 39). In modern clinical practice, the initial administration of the TXA (1 g bolus intravenously and later another 1g infused over a period of 6–8 hours) has clearly moved into the prehospital phase to maximise its effect (40).
Since 2010, the wide acceptance of the damage control resuscitation (DCR) principles in blunt trauma (41, 42) has also had an effect on prehospital interventions. The volume and infusion speed of fluids administered prior to diagnostics is reduced, aiming for a period of permissive hypotension (43). Furthermore and following the rationale of prompt haemostatic resuscitation (administration of blood products), transfusion protocols during the transfer of bleeding trauma patients to the hospital have been introduced (44, 45).
The first use of REBOA (resuscitative endovascular balloon occlusion of the aorta) as a roadside intervention occurred in June 2014. Studies demonstrate that REBOA can have a positive impact in trauma cases where patients have suffered traumatic cardiac arrests (59% return of spontaneous circulation) (46). In reality, the literature still does not have a clear consensus on its effect on mortality with a variety of studies published, suggesting both positive and negative effects of this procedure. REBOA is currently used more as an inhospital technique (47).
The application of pelvic binders in haemodynamically unstable blunt trauma patients or in those with suspicion of a pelvic ring injury has been adopted almost universally. They represent a non-invasive effective method to control the pelvic volume, as well as a method of providing mechanical stability that allows the formation of the ‘first clot’ within the injured pelvis (48, 49). There are multiple devices with some slight nuances between themselves (50). The overarching principle is that they should be placed over the greater trochanters because at that level they provide circumferential compression to the true pelvis. However, there are reports that successful prehospital placement occurs in just 40–70% (51). The pelvic binder can remain in situ till definitive pelvic ring imaging is taken. However, it should be removed or loosened up after the restoration of haemodynamic stability and/or completion of diagnostic imaging. Retainment over 24-h leads to increased risk of skin necrosis and pressure sores and can obstruct secondary surveys especially of the perineal area (52).
Splinting extremities with suspected or apparent injuries are also established good practice at the prehospital stage. Especially for the femur they have been shown to reduce morbidity and mortality and decrease blood transfusions and secondary pulmonary complications (53). Keeping the two legs rapped together has also been shown to add to the volume reduction and stability of a disrupted pelvic ring and is nowadays used as an adjunct to the pelvic binders (54).
The earliest possible administration of broad-spectrum antibiotics in open injuries reduces, in conjunction with other important measures (48), the incidence of fracture-related infections and non-union (55), which are heavily contributing to the morbidity of orthopaedic trauma (56, 57). This has recently generated protocols that include their use at the prehospital phase (58).
Key elements at the Emergency Department
At this phase, the most vital factor in trauma management is the coordinated teamwork of a ‘trauma team’ that is assembled following the launch of a ‘trauma call’ or trauma team activation (TTA). The roles of each team member are specific and should be undertaken after successful completion of the ATLS training (59). A typical ‘trauma team’ consists of the following members: trauma call leader (TCL – most experienced clinician), a primary survey doctor, an anaesthetist with an operating department practitioner (to secure the airway/RSI), an orthopaedic trauma surgeon, a resuscitative surgeon (general/vascular surgeon), emergency care nurses, a script (to record events), a healthcare assistant (runner/porter) and a radiographer. Contemporary practice encourages detailed simulation training of all team members as a group besides the gold standard ATLS course (60).
The value of a pre-alert call (prompt notification to the receiving hospital) from the prehospital crew is highlighted in a number of recent studies, allowing time to the team to assemble, sign in and prepare the trauma bay at the emergency department (ED) to receive the injured patient (61, 62).
An important, but often underestimated, element of a mature trauma system is the quality of handover between the prehospital and hospital clinicians. The use of structured tools like the SBAR (Situation, Background, Assessment, Recommendation) or the ATMIST mnemonics (Age, Time, Mechanism, Injuries found/suspected, Symptoms and signs, Treatment/interventions initiated) and/or use of checklists allow the TCL to get informed efficiently by the ambulance crew whilst the ‘trauma call team’ initiates the ATLS primary ABCDE survey (referring to an hierarchic sequel of assessments of the Airway/Breathing/Circulation/Disability/Environmental factors and exposure) (63).
Establishing different tiers of TTAs has been an area of debate. Different tiers lead to mobilisation of different/additional team members (64, 65, 66). Therefore, there are EDs that have adopted two- (full/limited TTA) or three-tier systems. The main concern introducing tiers is that it can lead to significant undertriage which can mostly affect patients following a low energy mechanism, elderly, or those with only head/chest injuries (65). The main advantage is that it can lead to a better correlation between the patient needs and the use of resources (67).
Tier 1: ED TTA – High-risk injury mechanism with normal physiology.
Tier 2: Hospital TTA – High-risk injury mechanism with abnormal physiology.
Tier 3: Code Red TTA –- These are activated at the discretion of the TCL when the pre-alert communication indicates the arrival of a haemodynamically unstable/in extremis patient. It leads to the mobilisation of additional staff and resources (interventional radiologist, senior resuscitation surgeon and massive haemorrhage packs).
As part of the European ‘Stop the Bleeding Campaign’, guidelines have been created to guide the management of bleeding patients. The recommendations are detailed and emphasise the importance of having established protocols fulfilling a ‘goal-directed treatment strategy’ (68). DCR, optimally initiated at the prehospital stage, continues. For the severely injured patients with haemodynamic instability, until completion of emergency diagnostic investigations and control of bleeding sites, volume restoration is restricted (restrictive fluid resuscitation). Rapid infusion of blood products in balanced ratios (between red cells, plasma and platelets) rather than other fluids (haemostatic resuscitation) is the second pillar of modern DCR (69).
Massive haemorrhage protocols (MHPs) are widely used in hospitals that consistently receive severe trauma. MHPs facilitate an expedited access to blood products to achieve prompt haemostatic resuscitation (70). They are activated under specific conditions and senior clinical authorisation. Four to eight units of ‘O-negative’ blood products, as well as other haemostatic agents (factor V, cryoprecipitate), are readily available. Group-specific blood products and secondarily fully cross-matched units should be given at the earliest possible time. All different blood products should be counted and reach a balanced ratio of 1:1:1 (red cells:plasma:platelets) within the first 6-h (68).
The endpoint of DCR is a systolic blood pressure 70–90 mmHg (permissive hypotension), which allows adequate perfusion of end organs and does not lead to dilutional coagulopathy or risking hydrostatic dislodgement of clots at the arterial bleeding sides. Exceptions in the permissive hypotension practice should be patients with severe brain trauma, as cerebral perfusion is very vulnerable. Similarly, frail elderly patients with severe atherosclerosis need higher systolic pressures to avoid secondary ischaemic problems of sensitive viscera (brain, heart, kidneys and bowel) (42, 71).
Major trauma patients are at risk of TIC (72). TIC carries significant mortality. Its early diagnosis and reversal are of paramount importance. To date, there is no validated rapid coagulation assay to diagnose coagulopathy in the actively bleeding patient (73). In light of this, viscoelastic monitoring (VEM) was developed to provide rapid, accurate, visual representations of patients’ coagulation profiles. The two primary platforms for such tests are the thromboelastography (TEG®, Haemonetics®, Boston, MA, USA) and the thromboelastometry (ROTEM, Tem International GmbH, Munich, Germany) (74). The evidence supporting trauma resuscitation under VEM guidance as compared to standard coagulation assays remains relatively limited (75). Of note, VEM studies can be influenced from certain medications (antiplatelet/anticoagulation), age, gender and alcohol intoxication (76, 77).
Key elements of trauma imaging
The role of early cross-sectional diagnostic imaging of the severely injured remains critical. Before the validated report of a trauma CT scan (contrast-enhanced multiphasic thin sliced from the head to the hips), diagnosis is considered incomplete at least for the adult patients. Expediting the access to a modern scanner, minimising the time to the radiologist report as well as extending the initial imaging to a whole-body trauma CT scan when the patient is unstable and/or when we suspect associated complex extremity trauma represent the current focus of attention. In rare occasions, patients ‘in extremis’ get a trauma CT scan with a delay. They are transferred before completion of diagnostics to theatres for emergency life-saving procedures (emergency thoracotomy, laparotomy and pelvic packing) (78, 79).
Despite the positive impact of CT imaging, there are concerns, as 20–40% of patients end up having negative scans, which is a waste of both resources and time and is considered as unnecessary radiation exposure (80). This is advocated especially in patients who are fully conscious and alert and in a stable haemodynamic state (78). Imaging during paediatric TTAs is also governed by more restrictive protocols. For children, the published risk of developing radiation-associated cancer is significantly higher (81). Therefore, the use of standard trauma CT scans is not recommended, and the onus is on targeted imaging. The only clear indication for a CT for them is in suspected neurotrauma where imaging of the brain should be obtained fast (within 30 min from arrival) (48).
In the modern trauma setting, interventional radiology (IR) has proven its efficiency as a selective definitive method to control sources of arterial and solid organ bleeding (82, 83). The presence of 24/7 access to IR services is considered as a prerequisite in many developed trauma systems (48). The literature indicates that delays in IR interventions are linked to poor patient outcomes (24, 84). Nowadays, a contrast-enhanced multiphasic CT scan is an absolute prerequisite for IR, whilst non-selective proximal embolisations are extremely rare.
In most specialist trauma centres, IR is mostly used in patients with relative haemodynamic stability, whilst patients ‘in extremis’ are treated with open surgery and open control of the bleeding sites/packing. The rationale is that an exsanguinating/peri-arrest patient needs an immediate intervention potentially in many areas, which can happen in theatres within minutes. In these occasions, there is always need for further procedures (as in all DCS/DCO protocols). In contrast, the IR technique is a definitive method that takes hours to be completed and rarely (<10%) requires secondary procedures. In 20–40% of the ‘in extremis’ group, a CT angiography +/- subsequent embolisation follows the surgery to complete adequate bleeding control (85). Endovascular techniques employed in the trauma setting, besides the embolisation, also include the use of the REBOA, the insertion of stents/grafts for large peripheral vessel reconstruction and hybrid procedures. It is critical to highlight the importance of a functioning coagulation cascade which allows the embolisation to work. Therefore, established lethal triad, hypothermia and TIC should be reversed or even better prevented for optimal effect of the endovascular techniques (24, 86).
Key elements at the operating theatres
The bulk of severely injured patients requires multiple major surgical procedures; over than 10% of these patients need even more than five. About 60% of these procedures occur during the first 24 h from admission. The ad hoc nature of trauma incidents mandates direct access to fully staffed specialist operating theatres 24/7 (27, 87, 88).
Contemporary understanding supports the immediate transfer of the major trauma patient to a single specialist centre that can offer all required surgical interventions (24, 88). Nevertheless, the selection of the most appropriate intervention and its timing remain a challenge. Classifying trauma patients into different risk groups, follows criteria based on injury-related anatomic factors, patient-related physiology markers, available resources and expertise, as previously described (27) (Table 3).
Table 3.
Description of parameters defining a number of risk factors in two different time periods. Frontline clinicians use them to define different patient groups that can be safely treated with early definitive fixation or need a different approach with fixation in a number of stages.
| Stable | Borderline | Unstable | In extremis | High-risk factors | |
|---|---|---|---|---|---|
| Publications between 2000 and 2015 (18, 20, 21, 22) | |||||
| Physiology | |||||
| Metabolic state – shock | |||||
| Blood pressure (mmHg) | >100 | 80–100 | <90 | ≤70 | |
| Transfused units (last 2-h period) | 0–2 | 2–8 | 5–15 | >15 | |
| Lactate (mg/dL) | Normal | –2.5 | >2.5 | >4 | |
| ATLS classification of shock | 1 | 2–3 | 3–4 | 4 | |
| Coagulation | |||||
| PLT count (/mm3) | ? | 90–110 k | < 70–90 k | <70 k | |
| Factor II and V (%) | 90–100 | 70–89 | 50–70 | <50 | |
| Fibrinogen (g/L) | >1 | 1 | <1 | DIC | |
| d-Dimer (μg/mL) | Normal | Abnormal | Abnormal | DIC | |
| Temperature, o C | >35 | 33–35 | 30–32 | <30 | |
| Anatomy | |||||
| Soft tissue – visceral injuries | |||||
| Chest AIS score | 1 or 2 | ≥2 | ≥3 | ≥3 | |
| Lung function (PaO2/FiO2, mmHg) | 350–400 | 300–350 | 200–300 | <200 | |
| Abdominal trauma (Moore classification) | 1 or 2 | ≤3 | 3 | ≥3 | |
| Pelvic trauma (AO types) | A or none | B | C | C | |
| External (AIS score) | 1 or 2 | 2 or 3 | 3–4 | ≥4 | |
| ISS > 20 and chest AIS score >2 | Yes | ||||
| Multiple long bone fractures and chest/abdominal AIS score >2 | Yes | ||||
| Other | |||||
| Massive transfusion 10 units over 24 h | Yes | ||||
| Exaggerated inflammatory response. IL6 > 500 pg/mL | Yes | ||||
| Presumed operation time >6 h | Yes | ||||
| Publications 2015–2022 (3, 24, 27, 28, 29, 30, 41) | If absent, usually DCR + ETC (definitive surgery within 24–48 h). If present, staged protocol of management.
|
||||
| Physiology | |||||
| Lactate clearance (of high initial lactate): <60% from baseline; <2/5mmol/L (last 24 h) | |||||
| Intracranial pressure unstable – low CPP <60 mmHg | |||||
| PaO2/FiO2 <250 mmHg | |||||
| Anatomy | |||||
| ISS > 20 and chest AIS score >2 | |||||
| Multiple long bone fractures and chest/abdominal AIS score >2 | |||||
| Polytrauma with abdo/pelvic trauma and haemorrhagic shock III | |||||
| Lung injuries – bilateral or unilateral bi-segmental contusions/flail chest | |||||
| Other | |||||
| Massive transfusion 10 units over 6 h | |||||
| Long extrication times | |||||
| Elder patients >65 years of age and/or frailty score >6 | |||||
AIS, abbreviated injury scale; AO, Arbeitsgemeinschaft für Osteosynthesefragen; ATLS, advanced trauma life support; CPP, cerebral perfusion pressure; DCO, damage control orthopaedics; DCR, damage control resuscitation; DCS, damage control surgery; DIC, disseminated intravascular coagulation; ETC, early total care; ISS, injury severity score; PaO2/FiO2, ratio of arterial oxygen partial pressure to fractional inspired oxygen (expressing respiratory distress); PLT, platelet.
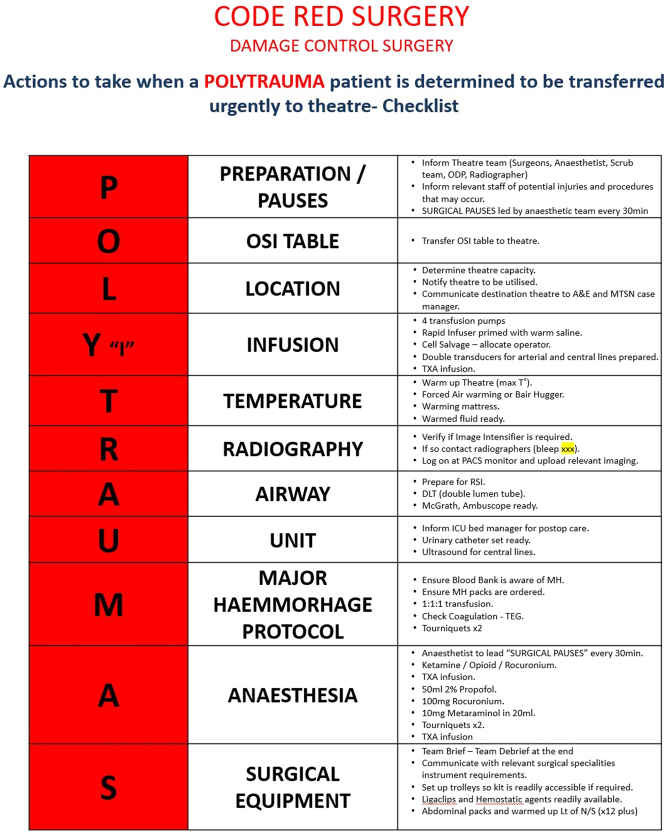
Besides the importance of special equipment, expertise and continuous readiness of the major trauma theatres, recent publications highlight the value of having specific activation protocols (24, 89). As a result, most level 1 trauma hospitals have introduced ‘Code Red’ theatre protocols (Fig. 2). In our major trauma centre (MTC), we manage over 900 patients with severe injuries (ISS ≥ 16) with activation of the ‘Code Red theatre protocol’ in just 50 times per year. When this protocol is activated, theatre staff prepare the operating theatres conditions to receive an exsanguinating patient (room temperature, fluoroscopy, operating table allowing full access to fluoroscopic control, damage laparotomy and external fixation kits, rapid infuser and cell salvage systems). Optimally, these patients are transferred to a dedicated hybrid operating suite where endovascular IR techniques together with standard DCS/DCO can be performed parallel to the continuation of the DCR.
Figure 2.
Example of the ‘Code Red theatre’ checklist used at one of the UK’s major trauma centres.
The value of having a ‘Code Red’ theatre protocol is not solely to provide a checklist of preparative actions or predetermined resources that need mobilisation. Its consistent use strengthens the culture of close teamwork and the focus on having in theatres a structured, well-orchestrated response for these relatively rare and unforgiving clinical situations – similar to the effect of the ATLS protocol during the ‘trauma calls’ (41, 89). In these scenarios, DCS/DCO/MuST surgeries are performed in parallel to haemostatic resuscitation support. The patient is managed in stages, and definitive fracture fixation follows in a later phase when patients’ physiology is recovered and/or soft tissue coverage is achieved (23, 27).
Key elements at the trauma intensive care
The intensive care units (ICUs) that receive severely injured patients have specialised over the last few decades, and adhere to trauma-specific protocols and routines. They mostly receive intubated patients with severe head trauma and/or critical chest – intrabdominal – extremity injuries.
At this phase, respiratory and haemodynamic resuscitation targets individualised rather than the general endpoints of the earlier phases of care. Trauma ICUs are not supporting only the survivorship of the injured patients but proactively decrease the adverse effects of ischaemia and sepsis on the vital organs, balance the immune response to the injury (first hit) and to the subsequent interventions (second hit) and also optimise the patient to endure and recover from complex surgical procedures (90). Furthermore, the focus is also directed to the facilitation of an early extubation, prompt nutritional support, pain relief and safe thromboprophylaxis (91).
The ICU expertise is also essential to determine the opposite when all efforts are futile, and death is inevitable. Appropriate end-of-life care and consideration of suitability for organ donation represent additional important elements in contemporary trauma care (92). Regular coordination with the surgical specialties is essential, as the state of the severely injured patients is very dynamic, timing of the interventions is important, a number of competing priorities exist and the overall outcome is multifactorial.
Key elements of the trauma ward care
The value of cohorting patients to a specific ward has been advocated for years on many different clinical scenarios (93, 94). Severely injured patients, after the ICU phase (if required), still need a well-supervised and coordinated care by experienced clinicians and adequate resources (95). These can be easier obtained if these patients are cohorted in one ward.
The concept of a ‘major trauma ward’ was introduced in the United Kingdom in 2010 following the inception of centralised service for the severely injured patients (87, 88). It currently represents one of the key features in all MTCs. In addition, this has led to the development of groups of specialist nurses that coordinate the care of these patients from the time of initial trauma call till discharge. These cohort wards have enhanced staff resources and developed protocols and standards of care that allow the close monitoring of these patients. Prompt completion of accurate secondary/tertiary surveys, early initiation of rehabilitation and provision of comprehensive nutrition, pain relief and clinical psychology support are key indicators of their performance (96). In 2022, a US study reported that each severely injured patient requires on average input from approximately 80 healthcare clinicians upon their arrival to a hospital (97). This is indicative of the complexity of their problems and the magnitude of resources that need to be readily available in an MTC.
The ageing of the general population in many countries has created a distinct challenge in modern trauma care. Studies have predicted that by 2050 approximately 40% of trauma admissions will be of patients over the age of 65 years. The majority is caused by low-energy mechanisms and are often undertriaged (9, 98). The recorded outcome of elderly trauma remains comparatively worse, despite the significant advances of modern systems (9, 99, 100). The reasons can be attributed to their undertriage, decreased physiologic reserves, frailty due to pre-existing comorbidities, worse tolerance to hypotension, higher complication and delirium risks, and challenges with rehabilitation and re-enablement (101). The importance of the inclusion of dedicated geriatric medicine specialists, for early assessment and perioperative support at the trauma ward, is becoming apparent and incorporated in many modern trauma systems (102), mimicking the shared care model of the hip fracture population (103, 104).
Key elements of the aftercare/rehabilitation phase
Severe trauma produces a major long-term impact to the anatomy, physiology and psychology of the survivors. The importance of rehabilitation cannot be overstated, as well as the deficiency of specialised resources in many healthcare systems. Modern improvement is also the early involvement of rehabilitation services in the course of treatment of severely injured patients. The targets and means of rehabilitation should be defined from the first day and dynamically evolve parallel to the progress of each patient.
Exposure to serious injuries, particularly in children or vulnerable patient groups, is strongly associated to disability, chronic diseases, substance abuse, mental illness, suicides, deprivation, even crime and violence problems. Contemporary trauma rehabilitation refers to a wide spectrum of fields that aim to reverse these. It maybe that predominantly neurorehabilitation and musculoskeletal expertise are employed, but other important elements have recently attracted the attention in developed health systems such as advanced respiratory physiotherapy, speech and language therapy, occupational therapy, mental and psychological assessment and support of the communicating patients but often also of their close social circle. The aim is the earliest possible safe discharge of the patient from the hospital and the best possible re-integration of the injured into the society.
Contemporary trauma management includes the development of quality control systems auditing the provided care that should extend to the post-discharge from the hospital period. The largest trauma registry and auditing system in Europe is the Trauma Audit and Research Network (2). It has a pivotal role in the planning and development of the modern trauma system in the UK. However, the follow-up period it currently captures is limited to the first 3 months post discharge. Besides mortality and complication rates, it has recently focused on the capture of patient-recorded outcomes (PROMs) and experience measures (PREMs). Mostly due to resource limitations, at present, these are recorded over just the first 6months following major trauma (105).
PROMs and PREMs provide a surrogate marker of effectiveness of treatment and burden of a disease and have emerged as optimal assessment tools over the last decade (106, 107, 108). In the literature, there are scarce reports of the late outcome of trauma patients which document the severe impact of certain injuries long term and the need for longitudinal monitoring of this population (109, 110).
Conclusion
Managing severely injured patients represents a challenging clinical need since the beginning of medicine. The variety of different causes, circumstances and conditions, as well as the need for individualised methodologies and solutions, offered universally in an organised sustainable manner, has not changed over the recent decades. New techniques and protocols have developed and refer to all different stages of trauma care from the scene till the final recovery phases. Our understanding and practice are continuously evolving, whilst trauma clinicians are nowadays able to interact and exchange knowledge faster than ever. The observed positive impact on the survivorship rates and decrease of disability can only be sustained with teamwork and efficient use of the available resources even in the most privileged health systems.
Declaration of interest
All authors declare no conflict of interest.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author contribution statement
VG contributed to the planning and contributed to preparation of the draft and the final manuscript. PR contributed to the preparation of the final manuscript. PVG contributed to the preparation of the final manuscript. NK contributed to the conception, planning and preparation of the final manuscript.
References
- 1.Wolrd Health Organisation. Injuries and violence. WHO; 2021. Available at: https://www.who.int/news-room/fact-sheets/detail/injuries-and-violence) (date last accessed 26 February 2023). [Google Scholar]
- 2.Trauma Audit & Research Network, (TARN). Available at: https://www.tarn.ac.uk/ (date last accessed 26 February 2023). [Google Scholar]
- 3.Nauth A Hildebrand F Vallier H Moore T Leenen L McKinley T & Pape HC. Polytrauma: update on basic science and clinical evidence. OTA International 20214e116. ( 10.1097/OI9.0000000000000116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Association for the Advancement of Automotive Medicine 2019. Abbreviated Injury Scale (AIS) - overview. Available at: https://www.aaam.org/abbreviated-injury-scale-ais/ (date last accessed 26 February 2023). [Google Scholar]
- 5.Baker SP O'Neill B Haddon W & Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. Journal of Trauma 197414187–196. ( 10.1097/00005373-197403000-00001) [DOI] [PubMed] [Google Scholar]
- 6.Copes WS Champion HR Sacco WJ Lawnick MM Keast SL & Bain LW. The Injury Severity Score revisited. Journal of Trauma 19882869–77. ( 10.1097/00005373-198801000-00010) [DOI] [PubMed] [Google Scholar]
- 7.Border JR LaDuca J & Seibel R. Priorities in the management of the patient with polytrauma. Progress in Surgery 19751484–120. ( 10.1159/000398211) [DOI] [PubMed] [Google Scholar]
- 8.Pape HC, Lefering R, Butcher N, Peitzman A, Leenen L, Marzi I, Lichte P, Josten C, Bouillon B, Schmucker U, et al. The definition of polytrauma revisited: an international consensus process and proposal of the new 'Berlin definition'. Journal of Trauma and Acute Care Surgery 201477780–786. ( 10.1097/TA.0000000000000453) [DOI] [PubMed] [Google Scholar]
- 9.Moran CG Lecky F Bouamra O Lawrence T Edwards A Woodford M Willett K & Coats TJ. Changing the system - major trauma patients and their outcomes in the NHS (England) 2008–17. EClinicalmedicine 20182–313–21. ( 10.1016/j.eclinm.2018.07.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paneitz DC & Ahmad S. Pediatric trauma update. Missouri Medicine 2018115438–442. [PMC free article] [PubMed] [Google Scholar]
- 11.Carter B Short R Bouamra O Parry F Shipway D Thompson J Baxter M Lecky F & Braude P. A national study of 23 major trauma centres to investigate the effect of frailty on clinical outcomes in older people admitted with serious injury in England (FiTR 1): a multicentre observational study. Lancet. Healthy Longevity 20223e540–e548. ( 10.1016/S2666-7568(2200122-2) [DOI] [PubMed] [Google Scholar]
- 12.Huls CK & Detlefs C. Trauma in pregnancy. Seminars in Perinatology 20184213–20. ( 10.1053/j.semperi.2017.11.004) [DOI] [PubMed] [Google Scholar]
- 13.Metcalfe D Perry DC Bouamra O Salim A Woodford M Edwards A Lecky FE & Costa ML. Regionalisation of trauma care in England. Bone and Joint Journal 201698–B1253–1261. ( 10.1302/0301-620X.98B9.37525) [DOI] [PubMed] [Google Scholar]
- 14.Craigie RJ Farrelly PJ Santos R Smith SR Pollard JS & Jones DJ. Manchester Arena bombing: lessons learnt from a mass casualty incident. BMJ Military Health 202016672–75. ( 10.1136/jramc-2018-000930) [DOI] [PubMed] [Google Scholar]
- 15.McIsaac J & Gentz BA. Preparing for mass casualty events. Anesthesiology Clinics 202038821–837. ( 10.1016/j.anclin.2020.08.008) [DOI] [PubMed] [Google Scholar]
- 16.Lasanianos NG Kanakaris NK Dimitriou R Pape HC & Giannoudis PV. Second hit phenomenon: existing evidence of clinical implications. Injury 201142617–629. ( 10.1016/j.injury.2011.02.011) [DOI] [PubMed] [Google Scholar]
- 17.Bone LB Johnson KD Weigelt J & Scheinberg R. Early versus delayed stabilization of femoral fractures. A prospective randomized study. Journal of Bone and Joint Surgery. American Volume 198971336–340. ( 10.2106/00004623-198971030-00004) [DOI] [PubMed] [Google Scholar]
- 18.Pape HC Giannoudis P & Krettek C. The timing of fracture treatment in polytrauma patients: relevance of damage control orthopedic surgery. American Journal of Surgery 2002183622–629. ( 10.1016/s0002-9610(0200865-6) [DOI] [PubMed] [Google Scholar]
- 19.Scalea TM Boswell SA Scott JD Mitchell KA Kramer ME & Pollak AN. External fixation as a bridge to intramedullary nailing for patients with multiple injuries and with femur fractures: damage control orthopedics. Journal of Trauma 200048613–62. ( 10.1097/00005373-200004000-00006) [DOI] [PubMed] [Google Scholar]
- 20.Giannoudis PV Tan HB Perry S Tzioupis C & Kanakaris NK. The systemic inflammatory response following femoral canal reaming using the reamer-irrigator-aspirator (RIA) device. Injury 201041(Supplement 2) S57–S61. ( 10.1016/S0020-1383(1070011-5) [DOI] [PubMed] [Google Scholar]
- 21.Roberts CS Pape HC Jones AL Malkani AL Rodriguez JL & Giannoudis PV. Damage control orthopaedics: evolving concepts in the treatment of patients who have sustained orthopaedic trauma. Instructional Course Lectures 200554447–462. ( 10.2106/00004623-200502000-00030) [DOI] [PubMed] [Google Scholar]
- 22.Lasanianos NG Kanakaris NK & Giannoudis PV. Intramedullary nailing as a 'second hit' phenomenon in experimental research: lessons learned and future directions. Clinical Orthopaedics and Related Research 20104682514–2529. ( 10.1007/s11999-009-1191-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pape HC Tornetta P Tarkin I Tzioupis C Sabeson V & Olson SA. Timing of fracture fixation in multitrauma patients: the role of early total care and damage control surgery. Journal of the American Academy of Orthopaedic Surgeons 200917541–549. ( 10.5435/00124635-200909000-00001) [DOI] [PubMed] [Google Scholar]
- 24.Cimbanassi S, O'Toole R, Maegele M, Henry S, Scalea TM, Bove F, Mezzadri U, Capitani D, Sala F, Kanakaris N, et al. Orthopedic injuries in patients with multiple injuries: results of the 11th trauma update international consensus conference Milan, December 11, 2017. Journal of Trauma and Acute Care Surgery 202088e53–e76. ( 10.1097/TA.0000000000002407) [DOI] [PubMed] [Google Scholar]
- 25.Pfeifer R Klingebiel FK Halvachizadeh S Kalbas Y & Pape HC. How to Clear polytrauma Patients for Fracture Fixation: results of a systematic review of the literature. Injury 202354292–317. ( 10.1016/j.injury.2022.11.008) [DOI] [PubMed] [Google Scholar]
- 26.Vallier HA Wang X Moore TA Wilber JH & Como JJ. Timing of orthopaedic surgery in multiple trauma patients: development of a protocol for early appropriate care. Journal of Orthopaedic Trauma 201327543–551. ( 10.1097/BOT.0b013e31829efda1) [DOI] [PubMed] [Google Scholar]
- 27.Pape HC Halvachizadeh S Leenen L Velmahos GD Buckley R & Giannoudis PV. Timing of major fracture care in polytrauma patients - an update on principles, parameters and strategies for 2020. Injury 2019501656–1670. ( 10.1016/j.injury.2019.09.021) [DOI] [PubMed] [Google Scholar]
- 28.Pape HC Andruszkow H Pfeifer R Hildebrand F & Barkatali BM. Options and hazards of the early appropriate care protocol for trauma patients with major fractures: towards safe definitive surgery. Injury 201647787–791. ( 10.1016/j.injury.2016.03.020) [DOI] [PubMed] [Google Scholar]
- 29.Pape HC & Pfeifer R. Safe definitive orthopaedic surgery (SDS): repeated assessment for tapered application of Early Definitive Care and Damage Control?: an inclusive view of recent advances in polytrauma management. Injury 2015461–3. ( 10.1016/j.injury.2014.12.001) [DOI] [PubMed] [Google Scholar]
- 30.Giannoudis PV Giannoudis VP & Horwitz DS. Time to think outside the box: 'Prompt-Individualised-Safe Management' (PR.I.S.M.) should prevail in patients with multiple injuries. Injury 2017481279–1282. ( 10.1016/j.injury.2017.05.026) [DOI] [PubMed] [Google Scholar]
- 31.Pfeifer R, Kalbas Y, Coimbra R, Leenen L, Komadina R, Hildebrand F, Halvachizadeh S, Akhtar M, Peralta R, Fattori L, et al. Indications and interventions of damage control orthopedic surgeries: an expert opinion survey. European Journal of Trauma and Emergency Surgery 2021472081–2092. ( 10.1007/s00068-020-01386-1) [DOI] [PubMed] [Google Scholar]
- 32.Varghese M. Prehospital trauma care evolution, practice and controversies: need for a review. International Journal of Injury Control and Safety Promotion 20202769–82. ( 10.1080/17457300.2019.1708409) [DOI] [PubMed] [Google Scholar]
- 33.World Health Organisation. Prehospital trauma care systems. WHO; 2005. Available at: https://www.who.int/publications/i/item/prehospital-trauma-care-systems (last access date 26 February 2023). [Google Scholar]
- 34.Bouzat P, Ageron FX, Brun J, Levrat A, Berthet M, Rancurel E, Thouret JM, Thony F, Arvieux C, Payen JF, et al. A regional trauma system to optimize the pre-hospital triage of trauma patients. Critical Care 201519 111. ( 10.1186/s13054-015-0835-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuller G Pandor A Essat M Sabir L Buckley-Woods H Chatha H Holt C Keating S & Turner J. Diagnostic accuracy of prehospital triage tools for identifying major trauma in elderly injured patients: a systematic review. Journal of Trauma and Acute Care Surgery 202190403–412. ( 10.1097/TA.0000000000003039) [DOI] [PubMed] [Google Scholar]
- 36.Lockey DJ Healey B Crewdson K Chalk G Weaver AE & Davies GE. Advanced airway management is necessary in prehospital trauma patients. British Journal of Anaesthesia 2015114657–662. ( 10.1093/bja/aeu412) [DOI] [PubMed] [Google Scholar]
- 37.Price J Lachowycz K Steel A Moncur L Major R & Barnard EBG. Intubation success in prehospital emergency anaesthesia: a retrospective observational analysis of the inter-changeable operator model (ICOM). Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine 202230 44. ( 10.1186/s13049-022-01032-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.CRASH-3 Trial Collaborators. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet 20193941713–1723. ( 10.1016/S0140-6736(1932233-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.CRASH-2 Trial Collaborators, Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, El-Sayed H, Gogichaishvili T, Gupta S, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 201037623–32. ( 10.1016/S0140-6736(1060835-5) [DOI] [PubMed] [Google Scholar]
- 40.Coats TJ Fragoso-Iniguez M & Roberts I. Implementation of tranexamic acid for bleeding trauma patients: a longitudinal and cross-sectional study. Emergency Medicine Journal 20193678–81. ( 10.1136/emermed-2018-207693) [DOI] [PubMed] [Google Scholar]
- 41.Giannoudi M & Harwood P. Damage control resuscitation: lessons learned. European Journal of Trauma and Emergency Surgery 201642273–282. ( 10.1007/s00068-015-0628-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harris T Thomas GO & Brohi K. Early fluid resuscitation in severe trauma. BMJ 2012345 e5752. ( 10.1136/bmj.e5752) [DOI] [PubMed] [Google Scholar]
- 43.Kudo D Yoshida Y & Kushimoto S. Permissive hypotension/hypotensive resuscitation and restricted/controlled resuscitation in patients with severe trauma. Journal of Intensive Care 20175 11. ( 10.1186/s40560-016-0202-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brito AMP & Schreiber M. Prehospital resuscitation. Trauma Surgery and Acute Care Open 20216 e000729. ( 10.1136/tsaco-2021-000729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thies KC & Ruetzler K. Prehospital blood transfusion: who benefits? Lancet. Haematology 20229e238–e239. ( 10.1016/S2352-3026(2200074-6) [DOI] [PubMed] [Google Scholar]
- 46.Brenner M Teeter W Hoehn M Pasley J Hu P Yang S Romagnoli A Diaz J Stein D & Scalea T. Use of resuscitative endovascular balloon occlusion of the aorta for proximal aortic control in patients with severe hemorrhage and arrest. JAMA Surgery 2018153130–135. ( 10.1001/jamasurg.2017.3549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore LJ Martin CD Harvin JA Wade CE & Holcomb JB. Resuscitative endovascular balloon occlusion of the aorta for control of noncompressible truncal hemorrhage in the abdomen and pelvis. American Journal of Surgery 20162121222–1230. ( 10.1016/j.amjsurg.2016.09.027) [DOI] [PubMed] [Google Scholar]
- 48.American College of Surgeons’ Committee on Trauma. Advanced trauma life support (ATLS): the ninth edition. Journal of Trauma and Acute Care Surgery 2013741363–1366. ( 10.1097/TA.0b013e31828b82f5) [DOI] [PubMed] [Google Scholar]
- 49.British Orthopaedic Association (BOA). BOA standards for trauma and orthopaedics (BOASTs). British Orthopaedic Association; 2018. Available at: https://www.boa.ac.uk/standards-guidance/boasts.html (date last accessed 26 February 2023). [Google Scholar]
- 50.Knops SP Schep NW Spoor CW van Riel MP Spanjersberg WR Kleinrensink GJ van Lieshout EM Patka P & Schipper IB. Comparison of three different pelvic circumferential compression devices: a biomechanical cadaver study. Journal of Bone and Joint Surgery. American Volume 201193230–240. ( 10.2106/JBJS.J.00084) [DOI] [PubMed] [Google Scholar]
- 51.Tiziani S Janett AS Alkadhi H Osterhoff G Sprengel K & Pape HC. Does the accuracy of prehospital pelvic binder placement affect cardiovascular physiological parameters during rescue? A clinical study in patients with pelvic ring injuries. OTA International 20225(Supplement) e186. ( 10.1097/OI9.0000000000000186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spanjersberg WR Knops SP Schep NW van Lieshout EM Patka P & Schipper IB. Effectiveness and complications of pelvic circumferential compression devices in patients with unstable pelvic fractures: a systematic review of literature. Injury 2009401031–1035. ( 10.1016/j.injury.2009.06.164) [DOI] [PubMed] [Google Scholar]
- 53.Philipsen SPJ Vergunst AA & Tan ECTH. Traction Splinting for midshaft femoral fractures in the pre-hospital and Emergency Department environment-A systematic review. Injury 2022534129–4138. ( 10.1016/j.injury.2022.09.051) [DOI] [PubMed] [Google Scholar]
- 54.Gardner MJ Parada S & Chip Routt ML. Internal rotation and taping of the lower extremities for closed pelvic reduction. Journal of Orthopaedic Trauma 200923361–364. ( 10.1097/BOT.0b013e31819c4a3f) [DOI] [PubMed] [Google Scholar]
- 55.Papakostidis C Kanakaris NK Pretel J Faour O Morell DJ & Giannoudis PV. Prevalence of complications of open tibial shaft fractures stratified as per the Gustilo-Anderson classification. Injury 2011421408–1415. ( 10.1016/j.injury.2011.10.015) [DOI] [PubMed] [Google Scholar]
- 56.Lack WD Karunakar MA Angerame MR Seymour RB Sims S Kellam JF & Bosse MJ. Type III open tibia fractures: immediate antibiotic prophylaxis minimizes infection. Journal of Orthopaedic Trauma 2015291–6. ( 10.1097/BOT.0000000000000262) [DOI] [PubMed] [Google Scholar]
- 57.Roddy E Patterson JT & Kandemir U. Delay of antibiotic administration greater than 2 hours predicts surgical site infection in open fractures. Injury 2020511999–2003. ( 10.1016/j.injury.2020.04.031) [DOI] [PubMed] [Google Scholar]
- 58.Lack W Seymour R Bickers A Studnek J & Karunakar M. Prehospital antibiotic prophylaxis for open fractures: practicality and safety. Prehospital Emergency Care 201923385–388. ( 10.1080/10903127.2018.1514089) [DOI] [PubMed] [Google Scholar]
- 59.American College of Surgeons' Committee on Trauma. ATLS Advanced Trauma Life Support. 10th ed.Chicago: American College of Surgeons' Committee on Trauma. [Google Scholar]
- 60.Saleten M Laitselart P Martinez T Descamps C Debien B Boutonnet M & Pasquier P. Who's who in the trauma bay? Association between wearing of identification jackets and trauma teamwork performance: a simulation study. Journal of Emergencies, Trauma, and Shock 202215139–145. ( 10.4103/jets.jets_168_21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ryb GE Cooper C & Waak SM. Delayed trauma team activation: patient characteristics and outcomes. Journal of Trauma and Acute Care Surgery 201273695–698. ( 10.1097/TA.0b013e31825abf6f) [DOI] [PubMed] [Google Scholar]
- 62.Synnot A, Karlsson A, Brichko L, Chee M, Fitzgerald M, Misra MC, Howard T, Mathew J, Rotter T, Fiander M, et al. Prehospital notification for major trauma patients requiring emergency hospital transport: A systematic review. Journal of Evidence-Based Medicine 201710212–221. ( 10.1111/jebm.12256) [DOI] [PubMed] [Google Scholar]
- 63.Harmsen AMK Geeraedts LMG Giannakopoulos GF Terra M Christiaans HMT Mokkink LB & Bloemers FW. National consensus on communication in prehospital trauma care, the DENIM study. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine 201725 67. ( 10.1186/s13049-017-0414-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Linder F Holmberg L Bjorck M Juhlin C Thorbjornsen K Wisinger J Polleryd P Eklof H & Mani K. A prospective stepped wedge cohort evaluation of the new national trauma team activation criteria in Sweden - the TRAUMALERT study. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine 201927 52. ( 10.1186/s13049-019-0619-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rehn M, Lossius HM, Tjosevik KE, Vetrhus M, Ostebo O, Eken T. & Rogaland Trauma System Study Collaborating Group. Efficacy of a two-tiered trauma team activation protocol in a Norwegian trauma centre. British Journal of Surgery 201299199–208. ( 10.1002/bjs.7794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Curtis K Olivier J Mitchell R Cook A Rankin T Rana A Watson WL & Nau T. Evaluation of a tiered trauma call system in a level 1 trauma centre. Injury 20114257–62. ( 10.1016/j.injury.2010.05.004) [DOI] [PubMed] [Google Scholar]
- 67.Maarseveen OECV Ham WHW Huijsmans RLN & Leenen LPH. The pace of a trauma resuscitation: experience matters. European Journal of Trauma and Emergency Surgery 2022482503–2510. ( 10.1007/s00068-021-01838-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, Filipescu D, Hunt BJ, Komadina R, Nardi G, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Critical Care 201620 100. ( 10.1186/s13054-016-1265-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015313471–482. ( 10.1001/jama.2015.12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.National insitute for health and care excellence (NICE). Guidelines (NG 39): Assessment and Initial Management 2016. Available at: https://www.nice.org.uk/guidance/ng39 (date last accessed 26 February 2023). [Google Scholar]
- 71.Ramesh GH Uma JC & Farhath S. Fluid resuscitation in trauma: what are the best strategies and fluids? International Journal of Emergency Medicine 201912 38. ( 10.1186/s12245-019-0253-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moore EE Moore HB Kornblith LZ Neal MD Hoffman M Mutch NJ Schöchl H Hunt BJ & Sauaia A. Trauma-induced coagulopathy. Nature Reviews. Disease Primers 20217 30. ( 10.1038/s41572-021-00264-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spahn DR, Bouillon B, Cerny V, Duranteau J, Filipescu D, Hunt BJ, Komadina R, Maegele M, Nardi G, Riddez L, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fifth edition. Critical Care 201923 98. ( 10.1186/s13054-019-2347-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wikkelso A Wetterslev J Moller AM & Afshari A. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database of Systematic Reviews 20162016CD007871. ( 10.1002/14651858.CD007871.pub3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Da Luz LT Nascimento B Shankarakutty AK Rizoli S & Adhikari NK. Effect of thromboelastography (TEG(R)) and rotational thromboelastometry (ROTEM(R)) on diagnosis of coagulopathy, transfusion guidance and mortality in trauma: descriptive systematic review. Critical Care 201418 518. ( 10.1186/s13054-014-0518-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spoerke N Underwood S Differding J Van P Sambasivan C Shapiro D & Schreiber M. Effects of ethanol intoxication and gender on blood coagulation. Journal of Trauma 2010681106–1111. ( 10.1097/TA.0b013e3181d86860) [DOI] [PubMed] [Google Scholar]
- 77.Westfall KM Ramcharan RN Shulkosky MM Wahl WL & Hecht JP. The effect of antiplatelet agents on thromboelastography. American Surgeon 202231348221124327. ( 10.1177/00031348221124327) [DOI] [PubMed] [Google Scholar]
- 78.Vela JH Wertz CI Onstott KL & Wertz JR. Trauma imaging: A literature review. Radiologic Technology 201788263–276. [PubMed] [Google Scholar]
- 79.Maghraby NH Alshaqaq HM AlQattan AS Alfaraj AF Alghamdi OA Alzawad MJ & Farcy DA. Negative whole-body computed tomography scans in polytrauma patients: a retrospective cohort study. Open Access Emergency Medicine 202012305–313. ( 10.2147/OAEM.S263754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hansen CK Strayer RJ Shy BD Kessler S Givre S & Shah KH. Prevalence of negative CT scans in a level one trauma center. European Journal of Trauma and Emergency Surgery 20184429–33. ( 10.1007/s00068-016-0741-y) [DOI] [PubMed] [Google Scholar]
- 81.Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, Howe NL, Ronckers CM, Rajaraman P, Sir Craft AW, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 2012380499–505. ( 10.1016/S0140-6736(1260815-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Papakostidis C Kanakaris N Dimitriou R & Giannoudis PV. The role of arterial embolization in controlling pelvic fracture haemorrhage: a systematic review of the literature. European Journal of Radiology 201281897–904. ( 10.1016/j.ejrad.2011.02.049) [DOI] [PubMed] [Google Scholar]
- 83.Vaidya R Waldron J Scott A & Nasr K. Angiography and embolization in the management of bleeding pelvic fractures. Journal of the American Academy of Orthopaedic Surgeons 201826e68–e76. ( 10.5435/JAAOS-D-16-00600) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rhee P Joseph B Pandit V Aziz H Vercruysse G Kulvatunyou N & Friese RS. Increasing trauma deaths in the United States. Annals of Surgery 201426013–21. ( 10.1097/SLA.0000000000000600) [DOI] [PubMed] [Google Scholar]
- 85.Pillai AS Srinivas S Kumar G & Pillai AK. Where does interventional radiology fit in with trauma management algorithm? Seminars in Interventional Radiology 2021383–8. ( 10.1055/s-0041-1725114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singh A Kumar A Kumar P Kumar S & Gamanagatti S. "Beyond saving lives": current perspectives of interventional radiology in trauma. World Journal of Radiology 20179155–177. ( 10.4329/wjr.v9.i4.155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kanakaris NK & Giannoudis PV. Trauma networks: present and future challenges. BMC Medicine 20119 121. ( 10.1186/1741-7015-9-121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davenport RA Tai N West A Bouamra O Aylwin C Woodford M McGinley A Lecky F Walsh MS & Brohi K. A major trauma centre is a specialty hospital not a hospital of specialties. British Journal of Surgery 201097109–117. ( 10.1002/bjs.6806) [DOI] [PubMed] [Google Scholar]
- 89.Cheng M Cheung MT Lee KY Lee KB Chan SC Wu AC Chow YF Chang AM Ho HF & Yau KK. Improvement in institutional protocols leads to decreased mortality in patients with haemodynamically unstable pelvic fractures. Emergency Medicine Journal 201532214–220. ( 10.1136/emermed-2012-202009) [DOI] [PubMed] [Google Scholar]
- 90.Tisherman SA & Stein DM. ICU management of trauma patients. Critical Care Medicine 2018461991–1997. ( 10.1097/CCM.0000000000003407) [DOI] [PubMed] [Google Scholar]
- 91.Johannsen S, Brohi K, Johansson PI, Moore EE, Reinhold AK, Schochl H, Shepherd JM, Slater B, Stensballe J, Zacharowski K, et al. Getting hit by the bus around the world - a global perspective on goal directed treatment of massive hemorrhage in trauma. Current Opinion in Anaesthesiology 202134537–543. ( 10.1097/ACO.0000000000001025) [DOI] [PubMed] [Google Scholar]
- 92.Harvey D Butler J Groves J Manara A Menon D Thomas E & Wilson M. Management of perceived devastating brain injury after hospital admission: a consensus statement from stakeholder professional organizations. British Journal of Anaesthesia 2018120138–145. ( 10.1016/j.bja.2017.10.002) [DOI] [PubMed] [Google Scholar]
- 93.Briggs R O'Shea E de Siun A O'Neill D Gallagher P Timmons S & Kennelly S. Does admission to a specialist geriatric medicine ward lead to improvements in aspects of acute medical care for older patients with dementia? International Journal of Geriatric Psychiatry 201732624–632. ( 10.1002/gps.4501) [DOI] [PubMed] [Google Scholar]
- 94.Roslani AC & Chang NL. Surgical outcome of patients with hyperparathyroidism in a non-specialist surgical ward. Medical Journal of Malaysia 200661410–415. [PubMed] [Google Scholar]
- 95.Groven S Eken T Skaga NO Roise O Naess PA & Gaarder C. Long-lasting performance improvement after formalization of a dedicated trauma service. Journal of Trauma 201170569–574. ( 10.1097/TA.0b013e31820d1a9b) [DOI] [PubMed] [Google Scholar]
- 96.National Insitute for H ealth and Care Excellence (NICE). Major Trauma: Service Delivery (NICE Guideline, No. 40.), 10. A trauma service providing continuity of care 2016. [PubMed] [Google Scholar]
- 97.Woods K, Hayanga JWA, Cannon J, Lemons W, Philips M, Schmidt A, Boh R, Noor K, Fornaresio L, Thibault D, et al. Staffing in a Level 1 trauma center: quantifying capacity for preparedness. Disaster Medicine and Public Health Preparedness 2022161990–1996. ( 10.1017/dmp.2021.269) [DOI] [PubMed] [Google Scholar]
- 98.Cutugno CL. The 'graying' of trauma care: addressing traumatic injury in older adults. American Journal of Nursing 201111140–50. ( 10.1097/01.NAJ.0000407300.77350.03) [DOI] [PubMed] [Google Scholar]
- 99.Reade MC. Perspective: the top 11 priorities to improve trauma outcomes, from system to patient level. Critical Care 202226 395. ( 10.1186/s13054-022-04243-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Herron J Hutchinson R Lecky F Bouamra O Edwards A Woodford M & Eardley WGP. The impact of age on major orthopaedic trauma: an analysis of the United Kingdom Trauma Audit Research Network database. Bone and Joint Journal 201799–B1677–1680. ( 10.1302/0301-620X.99B12.BJJ-2016-1140.R2) [DOI] [PubMed] [Google Scholar]
- 101.Alshibani A Alharbi M & Conroy S. Under-triage of older trauma patients in prehospital care: a systematic review. European Geriatric Medicine 202112903–919. ( 10.1007/s41999-021-00512-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Conroy S Carpenter C & Banerjee J. Silver IIQuality Care for Older People With Urgent Care Needs 2021. (date last accessed 26 February 2023). [Google Scholar]
- 103.De Vincentis A, Behr AU, Bellelli G, Bravi M, Castaldo A, Galluzzo L, Iolascon G, Maggi S, Martini E, Momoli A, et al. Orthogeriatric co-management for the care of older subjects with hip fracture: recommendations from an Italian intersociety consensus. Aging Clinical and Experimental Research 2021332405–2443. ( 10.1007/s40520-021-01898-9) [DOI] [PubMed] [Google Scholar]
- 104.Grigoryan KV Javedan H & Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. Journal of Orthopaedic Trauma 201428e49–e55. ( 10.1097/BOT.0b013e3182a5a045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Coats TJ, Bouamra O, Edwards A, Lecky F, Mirkes E, Sergeant J, et al. Prediction of 6 month Trauma Proms using in-hospital data. Emergency Medicine Journal 202239 A973. [Google Scholar]
- 106.Bull C Teede H Watson D & Callander EJ. Selecting and implementing patient-reported outcome and experience measures to assess health system performance. JAMA Health Forum 20223e220326. ( 10.1001/jamahealthforum.2022.0326) [DOI] [PubMed] [Google Scholar]
- 107.Lotfalla A Halm J Schepers T & Giannakopoulos G. Health-related quality of life after severe trauma and available Proms: an updated review (Part I). European Journal of Trauma and Emergency Surgery 2022. ( 10.1007/s00068-022-02178-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Andrzejowski P Holch P & Giannoudis PV. Measuring functional outcomes in major trauma: can we do better? European Journal of Trauma and Emergency Surgery 2022481683–1698. ( 10.1007/s00068-021-01720-1) [DOI] [PubMed] [Google Scholar]
- 109.Pape HC, Probst C, Lohse R, Zelle BA, Panzica M, Stalp M, Steel JL, Duhme HM, Pfeifer R, Krettek C, et al. Predictors of late clinical outcome following orthopedic injuries after multiple trauma. Journal of Trauma 2010691243–1251. ( 10.1097/TA.0b013e3181ce1fa1) [DOI] [PubMed] [Google Scholar]
- 110.Kamp O Pfeifer R Ritschel M Flohe S & Bieler D. Polytrauma outcome: implementation of health-related quality of life assessment into the German Trauma Registry. European Journal of Trauma and Emergency Surgery 202147869–874. ( 10.1007/s00068-019-01270-7) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a