Abstract
Immune checkpoint inhibitors improve overall survival in advanced urothelial carcinoma, but response rates remain modest. We performed a multi-institutional retrospective cohort study comparing outcomes (observed response rate, progression-free, and overall survival) between patients based on time from initiation of first line platinum-based chemotherapy to second line immune checkpoint inhibitor. This study provides preliminary data that earlier resistance to platinum-based chemotherapy may be associated with shorter survival in those who receive subsequent ICI.
Background:
Early progression on first-line (1L) platinum-based therapy or between therapy lines may be a surrogate of more aggressive disease and poor outcomes in advanced urothelial carcinoma (aUC), but its prognostic role regarding immune checkpoint inhibitor (ICI) response and survival is unclear. We hypothesized that shorter time until start of second-line (2L) ICI would be associated with worse outcomes in aUC.
Patients and Methods:
We performed a retrospective multi-institution cohort study in patients with aUC treated with 1L platinum-based chemotherapy, who received 2L ICI. Patients receiving switch maintenance ICI were excluded. We defined time to 2L ICI therapy as the time between the start of 1L platinum-based chemotherapy to the start of 2L ICI and categorized patients a priori into 1 of 3 groups: less than 3 months versus 3–6 months versus more than 6 months. We calculated overall response rate (ORR) with 2L ICI, progression-free survival (PFS) and overall survival (OS) from the start of 2L ICI. ORR was compared among the 3 groups using multivariable logistic regression, and PFS, OS using cox regression. Multivariable models were adjusted for known prognostic factors.
Results:
We included 215, 215, and 219 patients in the ORR, PFS, and OS analyses, respectively, after exclusions. ORR difference did not reach statistical significance between patients with less than 3 months versus 3–6 months versus more than 6 months to 2L ICI. However, PFS (HR 1.64; 95% CI 1.02–2.63) and OS (HR 1.77; 95% CI 1.10–2.84) was shorter among those with time to 2L ICI less than 3 months compared to those who initiated 2L ICI more than 6 months.
Conclusion:
Among patients with aUC treated with 2L ICI, time to 2L ICI less than 3 months was associated with lower, but not significantly different ORR, but shorter PFS and OS compared to 2L ICI more than 6 months. This highlights potential cross resistance mechanisms between ICI and platinum-based chemotherapy.
Keywords: Bladder cancer, Immunotherapy, Platinum resistance, Checkpoint Inhibitor, Outcomes
Introduction
Standard of care first line (1L) treatment for patients with advanced urothelial carcinoma (aUC), defined as locally advanced/unresectable or metastatic disease, is combination platinum-based chemotherapy for those who are platinum eligible.1 Unfortunately, median progression-free survival on platinum-based combination chemotherapy alone is only 7–8 months (mo).2
ICIs have shown durable responses as second-line (2L) therapy after progression on platinum-based chemotherapy.3,4 The KEYNOTE-045 trial demonstrated significantly longer overall survival with pembrolizumab compared to taxane or vinflunine as 2L therapy in patients with progression on platinum-based chemotherapy providing level I evidence in that setting.5 Nivolumab and avelumab also have Food and Drug Administration (FDA) approval for the treatment of aUC that has progressed during or after receipt of platinum-based chemotherapy but without level I evidence. Atezolizumab has FDA approval in 1L setting in cisplatin-ineligible patients with PD-L1-high status (based on Ventana SP142 assay) or platinum-ineligible patients (irrespective of PD-L1 status).6
Early progression on platinum-based chemotherapy has been identified as a poor prognostic factor regarding outcomes to subsequent chemotherapy for several cancer types, including aUC.7–9 A retrospective of pooled prospective phase II trials showed that shorter time from 1L chemotherapy to 2L treatment (<3 mo vs. >3 mo) was identified as an independent poor prognostic factor in the setting of 2L therapy for aUC. Resistance to platinum, defined as progression on platinum-based chemotherapy within 6 months of initiation, has also been extensively studied in ovarian cancer.10 In a study, the immune tumor microenvironment in platinum-resistant ovarian cancer was shown to be altered to a “cold” tumor, characterized by low infiltration by CD8+ T cells and activated CD4+ T cells, and increased infiltration by regulatory T cells.11 Similarly, in urothelial carcinoma, up to a third of patients may have decreased expression of T cell-related immune genes, which might also possibly impair response to ICI.12
A few studies have investigated the impact of platinum resistance on ICI outcomes in patients with aUC. In our study, we assessed outcomes [observed response rate (ORR), progression-free survival (PFS), and overall survival (OS)] with ICI in patients with aUC and prior progression on and/or after platinum-based chemotherapy. We hypothesized that shorter time until start of 2L ICI would be associated with lower ORR and shorter survival.
Patients and Methods
Patient selection and data collection
After institutional review board approval was attained in concordance with the Declaration of Helsinki, we performed a retrospective cohort study of patients from 26 institutions in the United States and Europe. The cohort has been previously described13,14 but briefly, the cohort collected data on patients with aUC treated with ICI, each institution identified consecutive patients using a combination of provider-driven and electronic health record search algorithms. Patients were included in this analysis, if they had aUC, were treated with 1L platinum-based chemotherapy and then 2L ICI upon progression on and/or after chemotherapy. We calculated the time from start of 1L platinum-based chemotherapy to start of 2L ICI, categorizing the exposure into 3 groups: less than 3 months, 3–6 months and more than 6 months. We were unable to capture the exact timing of completion or discontinuation of 1L platinum-based chemotherapy, hence we calculated the time from start date of 1L chemotherapy to start date of 2L ICI. Given most patients received ICI before approval of switch maintenance avelumab and that such maintenance therapy is different than 2L ICI (ie, initiated prior to platinum progression), patients were excluded if they received switch maintenance ICI. Patients who had stopped 1L chemotherapy for reasons other than progression (eg, toxicity) were excluded as our primary goal was to investigate the impact of putative platinum resistance on ICI outcomes. Additionally, patients were excluded if they had missing data, pure non-UC histology, received multiple lines of ICIs, if ICI was given for a different indication, on a clinical trial or in combination with other systemic therapy (Figure 1). For data collection and storage, we used web-based, secure, and standardized REDCap capture tools hosted at the Institute of Translational Sciences at University of Washington.15,16 Data collected included demographics, clinicopathological factors, including time from start of 1L platinum-based chemotherapy, ICI treatment and outcomes (ORR, PFS, OS). Pathology and radiology results were assessed based on notes in the electronic health record; no central review of either was performed. Timing of imaging and designation of response and progression were investigator designated; although RECIST v1.1 criteria principles were used for the evaluation of best response the latter was determined by the chart abstractor based on best available information in clinical notes and radiographic studies.
Figure 1.
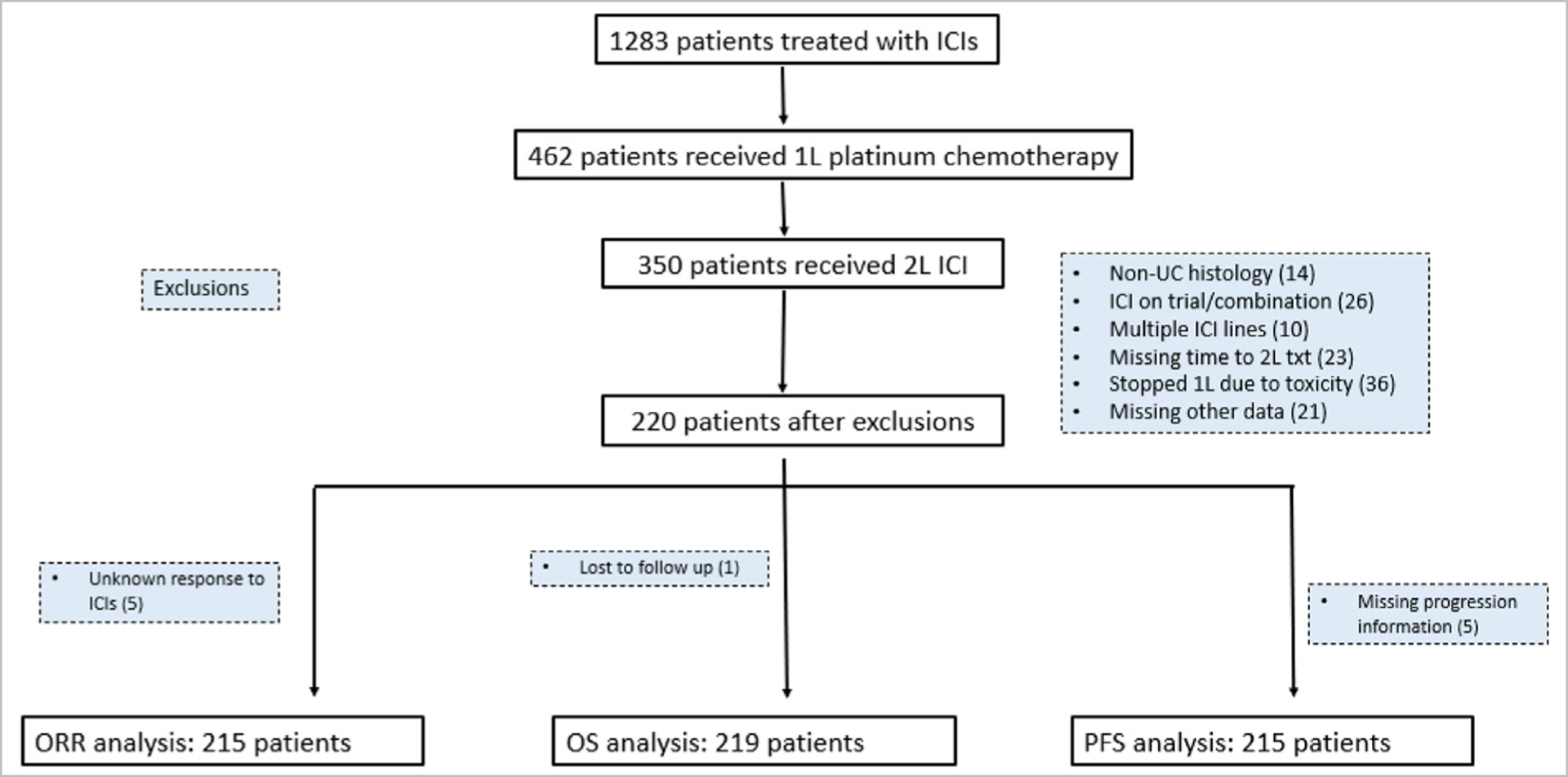
Consort flow diagram of patient selection and exclusion rationale.
Statistical Analysis
Baseline characteristics were summarized using descriptive statistics and compared via chi-square and Student’s t tests, for categorical and continuous variables, respectively. ORR was calculated as the sum of patients with investigator-determined complete or partial response divided by the total number of patients with available data. OS was measured from the date of ICI initiation until death (of any cause) and PFS was measured from the date of ICI initiation until the date of investigator determined radiographic and/or clinical progression, or death. Patients who did not experience death or progression were censored at the date of last follow-up. To assess the follow-up time, we used the reverse Kaplan-Meier method.
We calculated the time from start of 1L platinum chemotherapy to start of 2L ICI, trichotomizing the exposure to less than 3 months, 3–6 months and more than 6 months. Our analysis compared ORR on 2L ICI treatment; and PFS and OS from the start of 2L ICI between the different groups. Multivariable logistic regression was used to estimate the odds ratio (OR) and 95% confidence intervals (CI) of response. Kaplan-Meier method was used for survival curves and to estimate median (m)OS and median (m)PFS. Cox regression was used to determine the effect of time to 2L ICI (<3 months vs. 3–6 months vs. >6 months) on OS and PFS; differences between groups were expressed as hazard ratios (HRs) and 95% confidence interval (CI). For the multivariable analysis, models were adjusted based on individual Bellmunt prognostic risk factors,17 which gives 1 point each for ECOG performance status more than 0, presence of liver metastases and hemoglobin level less than 10g/dL. Statistical significance was determined if 95% CI did not cross 1 and P < .05; all P values were 2 tailed. All analyses were performed using R version 4.1.0 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 1283 patients with aUC treated with an ICI between 2013 and 2021 across 26 different institutions are included in our database, of which 462 had received 1L platinum-based chemotherapy. Of those, 350 patients had subsequently received 2L ICI. After exclusions, 215, 215, and 219 patients were included in the ORR, PFS and OS analyses, respectively (Figure 1). The median follow-up time, from time of 1L ICI initiation, measured by the reverse Kaplan-Meier method was 23 months. In our cohort, the median duration of 2L ICI therapy was 4.8 months Figure 2, Figure 3.
Figure 2.
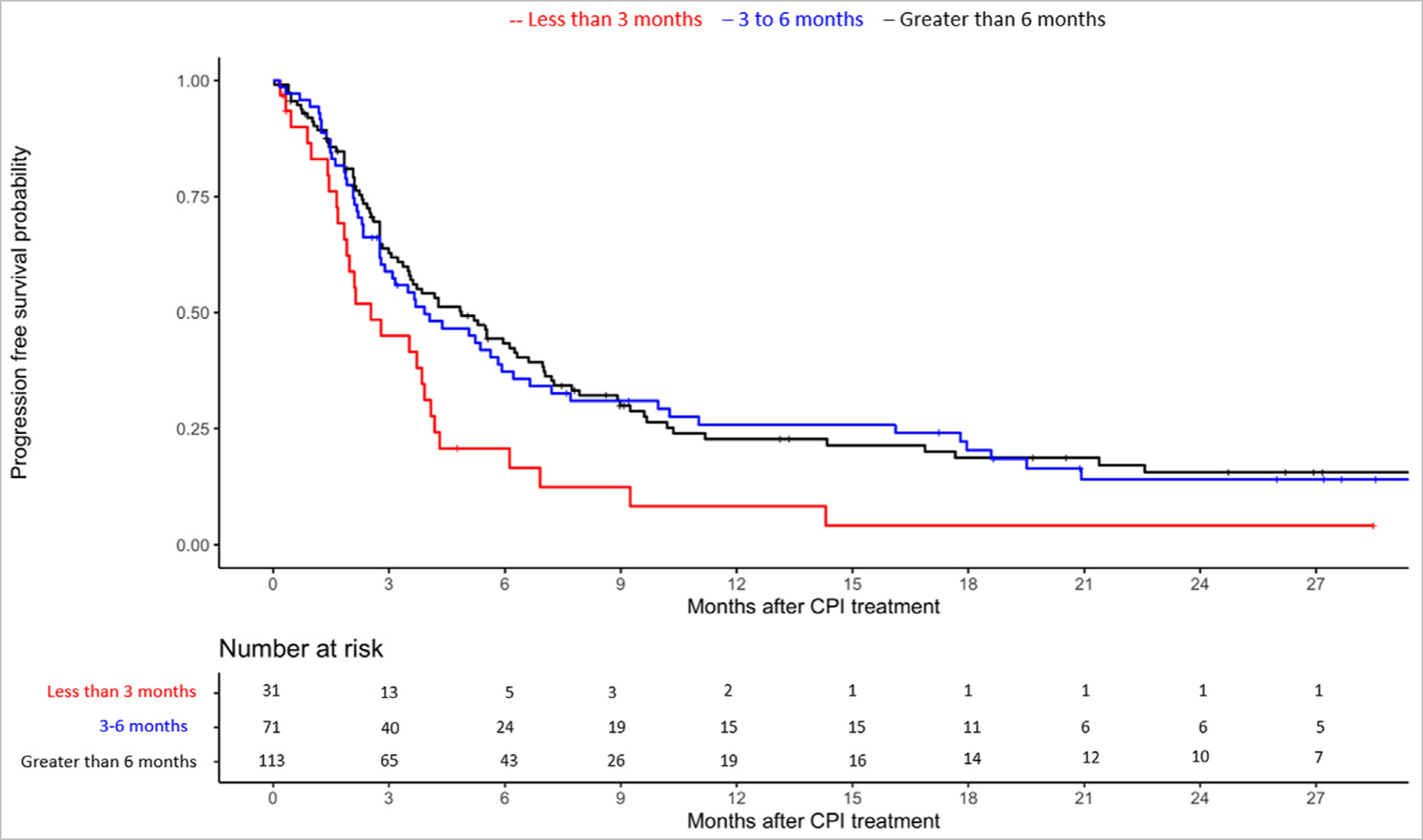
Kaplan Meier curve for progression-free survival with ICI according to time to 2L ICI initiation stratified by less than 3 months versus 3–6 months versus more than 6 months from starting platinum-based chemotherapy.
Figure 3.
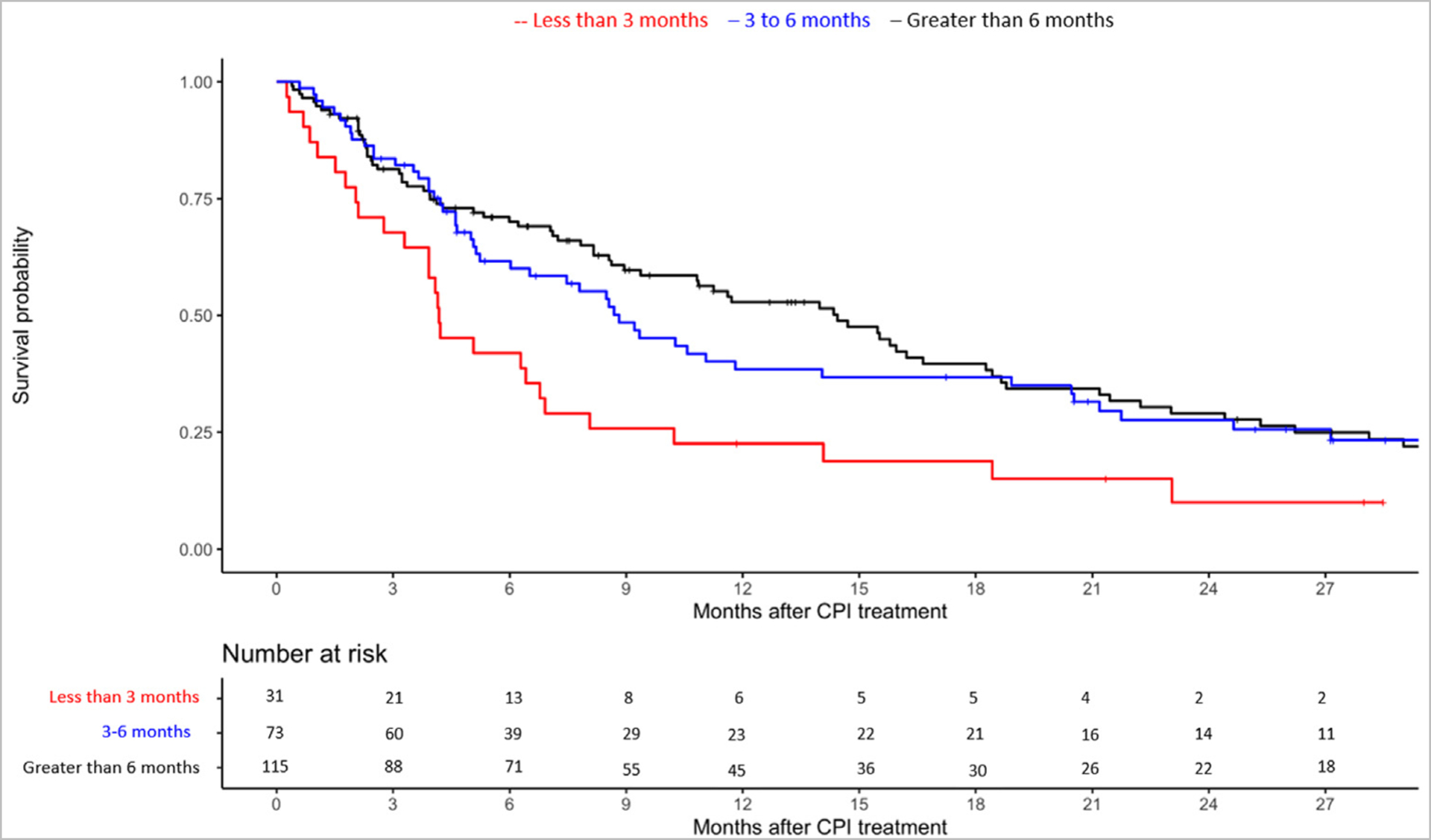
Kaplan Meier curve for overall survival with ICI according to time to 2L ICI initiation stratified by less than 3 months versus 3–6 months versus more than 6 months from starting platinum-based chemotherapy.
Table 1 shows the baseline characteristics for patients who received 2L ICI after progression on 1L platinum-based chemotherapy. Median age at ICI initiation for this cohort was 70 years, 80% were men, 76% were White, 76% had pure urothelial histology, 21% upper tract, and 59% received cisplatin in 1L setting. Patients with time to 2L ICI less than 3 months had significantly higher Bellmunt scores (Table 1).
Table 1.
Baseline Characteristics for Patients Stratified by Time to 2L ICI After 1L Platinum-Based Chemotherapy.
| Time to 2L ICI | < 3 months n = 31 | 3–6 months n = 73 | > 6 months n = 116 | Total n = 220 | P |
|---|---|---|---|---|---|
| Age at ICI initiation, median (IQR) | 70 (62 – 75) | 71 (65–75) | 70 (61 to 75) | 70 (63–75 | 0.863 |
| Sex, n (%) | |||||
| Male | 23 (74.2%) | 57 (78.1%) | 96 (82.8%) | 176 (80.0%) | 0.712 |
| Female | 8 (25.8%) | 16 (21.9%) | 20 (17.2%) | 44 (20.0%) | |
| Smoking History, n (%) | |||||
| Ever Smoker | 8 (25.8%) | 30 (25.9%) | 21 (28.8%) | 59 (26.8%) | 0.959 |
| Never Smoker | 23 (74.2%) | 85 (73.3%) | 50 (68.5%) | 158 (71.8%) | |
| Missing | 0 (0%) | 1 (0.9%) | 2 (2.7%) | 3 (1.4%) | |
| Race/Ethnicity, n (%) | |||||
| White/Caucasian | 23 (74.2%) | 52 (71.2%) | 91 (78.4%) | 166 (75.5%) | 0.930 |
| Hispanic/Latino | 3 (9.7%) | 10 (13.7%) | 14 (12.1%) | 27 (12.3%) | |
| Black/African-American | 1 (3.2%) | 1 (1.4%) | 3 (2.6%) | 5 (2.3%) | |
| Asian | 3 (9.7%) | 4 (5.5%) | 4 (3.4%) | 11 (5.0%) | |
| Native American | 0 (0%) | 1 (1.4%) | 0 (0%) | 1 (0.5%) | |
| Other | 0 (0%) | 0 (0%) | 2 (1.7%) | 2 (0.9%) | |
| Unknown | 1 (3.2%) | 5 (6.8%) | 2 (1.7%) | 8 (3.6%) | |
| Histology, n (%) | |||||
| Pure UC | 21 (67.7%) | 52 (71.2%) | 94 (81.0%) | 167 (75.9%) | 0.299 |
| Mixed UC | 10 (32.3%) | 21 (28.8%) | 22 (19.0%) | 53 (24.1%) | |
| UTUC vs. LTUC, n (%) | |||||
| Lower | 21 (67.7%) | 60 (82.2%) | 91 (78.4%) | 172 (78.2%) | 0.598 |
| Upper | 9 (29.0%) | 13 (17.8%) | 25 (21.6%) | 47 (21.4%) | |
| Missing | 1 (3.2%) | 0 (0%) | 0 (0%) | 1 (0.5%) | |
| De Novo Metastatic, n (%) | |||||
| Yes | 30 (96.8%) | 68 (93.2%) | 108 (93.1%) | 206 (93.6%) | 0.837 |
| No | 0 (0%) | 2 (2.7%) | 3 (2.6%) | 5 (2.3%) | |
| Missing | 1 (3.2%) | 3 (4.1%) | 5 (4.3%) | 9 (4.1%) | |
| Type of platinum chemotherapy in 1L, n (%) | |||||
| Carboplatin-based | 17 (54.8%) | 30 (41.1%) | 45 (38.8%) | 92 (41.8%) | 0.455 |
| Cisplatin-based | 14 (45.2%) | 43 (58.9%) | 71 (61.2%) | 128 (58.2%) | |
| Albumin < 3.5 g/dL at ICI initiation, n (%) | |||||
| No | 15 (48.4%) | 50 (68.5%) | 86 (74.1%) | 151 (68.6%) | 0.0412 |
| Yes | 13 (41.9%) | 15 (20.5%) | 22 (19.0%) | 50 (22.7%) | |
| Missing | 3 (9.7%) | 8 (11.0%) | 8 (6.9%) | 19 (8.6%) | |
| Hgb < 10 g/dL at ICI initiation, n (%) | |||||
| No | 14 (45.2%) | 46 (63.0%) | 98 (84.5%) | 158 (71.8%) | <0.001 |
| Yes | 16 (51.6%) | 24 (32.9%) | 17 (14.7%) | 57 (25.9%) | |
| Missing | 1 (3.2%) | 3 (4.1%) | 1 (0.9%) | 5 (2.3%) | |
| Liver Metastasis at ICI initiation, n (%) | |||||
| No | 19 (61.3%) | 55 (75.3%) | 91 (78.4%) | 165 (75.0%) | 0.278 |
| Yes | 12 (38.7%) | 18 (24.7%) | 25 (21.6%) | 55 (25.0%) | |
| ECOG PS at time of ICI initiation, n (%) | |||||
| 0 | 3 (9.7%) | 15 (20.5%) | 26 (22.4%) | 44 (20.0%) | 0.343 |
| 1 | 17 (54.8%) | 39 (53.4%) | 68 (58.6%) | 124 (56.4%) | |
| 2 | 5 (16.1%) | 12 (16.4%) | 9 (7.8%) | 26 (11.8%) | |
| 3 | 2 (6.5%) | 1 (1.4%) | 1 (0.9%) | 4 (1.8%) | |
| Missing | 4 (12.9%) | 6 (8.2%) | 12 (10.3%) | 22 (10.0%) | |
| ICI Received, n (%) | |||||
| Atezolizumab | 16 (51.6%) | 38 (52.1%) | 56 (48.3%) | 110 (50.0%) | 0.509 |
| Avelumab | 3 (9.7%) | 4 (5.5%) | 3 (2.6%) | 10 (4.5%) | |
| Durvalumab | 0 (0%) | 0 (0%) | 3 (2.6%) | 3 (1.4%) | |
| Nivolumab | 1 (3.2%) | 2 (2.7%) | 13 (11.2%) | 16 (7.3%) | |
| Pembrolizumab | 11 (35.5%) | 29 (39.7%) | 41 (35.3%) | 81 (36.8%) | |
| Bellmunt Risk Score, n (%) | |||||
| 0 | 0 (0%) | 11 (15.1%) | 19 (16.4%) | 30 (13.6%) | 0.0132 |
| 1 | 10 (32.3%) | 26 (35.6%) | 60 (51.7%) | 96 (43.6%) | |
| 2 | 13 (41.9%) | 20 (27.4%) | 22 (19.0%) | 55 (25.0%) | |
| 3 | 4 (12.9%) | 8 (11.0%) | 3 (2.6%) | 15 (6.8%) | |
| Missing | 4 (12.9%) | 8 (11.0%) | 12 (10.3%) | 24 (10.9%) | |
Observed Response Rate
A total of 215 patients were included in ORR analysis: 31, 72, and 112 patients started 2L ICI less than 3 months, 3–6 months and more than 6 months from starting 1L platinum-based chemotherapy, respectively. The ORR for these 3 groups were 13%, 28% and 25%, respectively. Despite the notably lower ORR in the less than 3 month group the odds of response to ICI were not significantly different between groups (Table 2).
Table 2.
Observed Response Rate, Progression-Free Survival, and Overall Survival Based on Time to 2L ICI Initiation Stratified to less than 3 Months Versus 3–6 Months Versus more than 6 Months Since Starting Platinum-Based Chemotherapy.
| <3 months, 3–6 months, >6 months | ||||
|---|---|---|---|---|
| Observed Response Rate (ORR) | ||||
| Time to 2L ICI | N | ORR, % (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
| <3 months | 31 | 13(5–26) | 0.44 (0.12–1.26) | 0.62 (0.15–1.97) |
| 3–6 months | 72 | 28(18–40) | 1.15 (0.59–2.25) | 1.28 (0.61–2.63) |
| >6 months | 112 | 25(18–34) | Reference | Reference |
| Progression-Free Survival (PFS) | ||||
| Time to 2L ICI | N | Med. PFS, months (95% CI) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) |
| <3 months | 31 | 3(2–4) | 1.88 (1.21–2.91) | 1.64 (1.02–2.63) |
| 3–6 months | 71 | 4(3–6) | 1.03 (0.74–1.44) | 1.08 (0.75–1.54) |
| >6 months | 113 | 5(4–7) | Reference | Reference |
| Overall Survival (OS) | ||||
| Time to 2L ICI | N | Med. OS, months (95% CI) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) |
| <3 months | 31 | 4(3–8) | 2.07 (1.33–3.23) | 1.77 (1.10–2.84) |
| 3–6 months | 73 | 9(5–19) | 1.12 (0.79–1.60) | 1.07 (0.74–1.57) |
| >6 months | 115 | 14(9–18) | Reference | Reference |
Progression-Free Survival
A total of 215 patients were included in PFS analysis: 31, 71, and 113 patients started 2L ICI less than 3 months, 3–6 months and more than 6 months from starting 1L platinum-based chemotherapy. Among the 3 groups, mPFS was 3 months (95% CI 2–4), 4 months (95% CI 3–6) and 5 months (95% CI 4–7), respectively. Patients who started 2L ICI less than 3 months had significantly shorter PFS (vs. those with time to 2L ICI>6 months; aHR 1.64; 95% CI 1.02–2.63; Table 2).
Overall Survival
A total of 219 patients were included in OS analysis: 31, 73, and 115 patients started 2L ICIs less than 3 months, 3–6 months and more than 6 months from starting 1L from starting 1L platinum-based chemotherapy. Among the 3 groups, mOS was 4 months (95% CI 3–8 months), 9 months (95% CI 5–19 months) and 14 months (95% CI 9–18 months), respectively. Patients with less than 3 months to 2L ICI had significantly shorter OS versus those with time to 2L ICI more than 6 months (aHR 1.77; 95% CI 1.10–2.84; Table 2).
Discussion
In this retrospective multi-institution cohort study of patients with aUC treated with 2L ICI after progression on 1L platinum-based chemotherapy, patients with time to 2L ICI less than 3 months had shorter PFS and OS compared to those with time to 2L ICI more than 6 months. Our hypothesis-generating study provides relevant data suggesting that putative earlier resistance to platinum-based chemotherapy may be associated with shorter survival in those who receive 2L ICI.
To date, there have only been a few studies investigating the impact of platinum resistance on ICI response in those with aUC. Subset analysis of Keynote-045 showed that those who started therapy on that trial more than or equal to 3 months after last chemotherapy dose had a HR for death of 0.66 (95% CI 0.49–0.89) while those who initiated pembrolizumab less than 3 months had HR of 0.82 (95% CI 0.58–1.15).5 Although this was not a prespecified head-to-head comparison and should be interpreted with caution, this analysis might indirectly raise the hypothesis that patients with primarily platinum-refractory aUC might also have less benefit with subsequent ICI. Indeed, another study reported that primary resistance to platinum-based chemotherapy portends a poor prognosis with anti-PD(L)1 therapy, although a minority and similar fraction of patients have response.18 A prognostic model proposed for patients receiving second-line PD-L1 inhibitors did not identify time from prior platinum-based chemotherapy as an independent prognostic factor, but that study did not evaluate the time from initiation of platinum-based chemotherapy.19 Also, a retrospective analysis of pooled, prospective phase II trials investigating 2L chemotherapy after progression on 1L platinum-based chemotherapy showed that shorter time to progression on 1L therapy was a poor prognostic factor for survival on 2L therapy.9 Moreover, results from phase II trials investigating rucaparib (PARP inhibitor, ATLAS trial) or mocetinostat (a class I/IV histone deacetylase inhibitor) in platinum-refractory aUC have shown poor outcomes with non-ICI therapies in the platinum-refractory setting.20,21 The open-label multi-arm BISCAY trial, which investigated 2L combination durvalumab with biomarker-selected targeted therapies in platinum-refractory aUC did not meet specified threshold of target ORR.22 Those examples, as well as other clinical trials,20–22 may indicate that poor response and early progression on 1L platinum-based chemotherapy can be associated with poor response and outcomes with several 2L therapies, including, but not limited to, ICI.
Patients with early progression on platinum-based chemotherapy have underlying aggressive disease features. In our study, patients with time to 2L ICI less than 3 months had significantly higher Bellmunt risk score at the time of ICI initiation. However, it is notable that after adjusting for Bellmunt factors, our findings for shorter PFS and OS among patients with time to 2L ICI less than 3 months remained significant. Given the poor outcomes among patients with early progression on platinum-based chemotherapy, opportunities for subsequent therapies for this population might sometimes be limited. These patients should be prioritized for clinical trials with novel therapies and combinations to maximize exposure to potentially life-prolonging therapies. Currently, multiple studies investigating combination therapies are underway and may be suitable options for these patients. TROPHY-U-01 cohort 3 investigating Sacituzumab govitecan (SG) plus pembrolizumab in patients with aUC who progressed very early on platinum-based chemotherapy showed encouraging ORR 34% and tumor reduction in 63% of patients with a manageable safety profile.23 Other trials investigating combination ICI and novel agents in aUC who had progressed on 1L platinum-based chemotherapy have shown promising results.24–26 Several other combinations, such as FGFR inhibitor plus ICI, need to be further evaluated in prospective trials in this very hard to treat population. Patients who have disease that progresses quickly on 1L chemotherapy most likely have an underlying aggressive disease, therefore may benefit from upfront combination therapy, but this needs to be further investigated in clinical trials.
It may also be the case that platinum resistance may have overlapping molecular mechanisms of resistance with immunotherapy. It has been shown that platinum-resistant carcinomas have an altered tumor microenvironment and can be considered to be “cold” tumors characterized by low infiltration by CD8+ T cells and activated CD4+ T cells, and increased infiltration by regulatory T cells.11,12 This “cold” immune tumor micro-environment may render patients susceptible to worse outcomes with subsequent ICI. Studies have shown that alterations in DNA damage response (DDR) genes have been associated with longer survival in patients treated with platinum-based chemotherapy and with clinical benefit from ICI therapy.27,28 This highlights possible overlapping mechanisms of response to platinum-based chemotherapy and ICI in aUC, which might possibly explain why concurrent chemotherapy plus ICI have not improved OS in phase III trials. Additional putative molecular biomarkers, such as basal versus luminal versus other subtypes, are also the objective of several studies evaluating response to therapies in aUC.
Notably, while our study did not include patients receiving switch-maintenance avelumab, our findings may indirectly further support this therapeutic approach. The JAVELIN Bladder 100 trial showed that avelumab switch maintenance following combination platinum-based chemotherapy prolongs OS and PFS in patients who have not progressed on first-line platinum-based chemotherapy.29 Given the relatively short duration of response to platinum-based chemotherapy and the outcomes with short time to 2L ICI, it is reasonable to believe that among patients without progression on platinum-based chemotherapy, it is relevant to start avelumab as switch maintenance therapy rather than wait for progression.
Strengths of our study include the use of real-world data and the large sample size from multiple institutions across the United States and Europe. However, our study has limitations inherent to the retrospective study design, lack of randomization, possible selection biases and unmeasured confounding. We could not ascertain the exact time of completion or early discontinuation of 1L platinum chemotherapy, so we used the time from starting chemotherapy in our analysis. We did not include patients receiving switch maintenance avelumab given the different disease setting and available sample size, even though this is now standard of care. In addition, given the multi-institution retrospective study design, we could not conduct central radiology or pathology review or use standardized imaging response criteria (RECIST), and there may have been practice-related variability in therapy administration, disease monitoring and follow up periods, which could affect ascertainment of response and progression. We could not account for dose reductions, dose density/intensity and number of cycles of chemotherapy. We also could not analyze molecular biomarkers or outcomes with antibody drug conjugates or FGFR inhibitor given as 2L therapy. Despite limitations, this analysis provides important preliminary data that early progression on platinum-based chemotherapy seems associated with shorter survival in patients with aUC who receive 2L ICI.
Conclusion
Patients with early resistance to platinum-based chemotherapy represent an unmet need clinically and have poor outcomes; therefore, they may be considered for combination therapies in clinical trials. More studies are needed regarding the drivers and impact of early platinum resistance in patients with aUC treated subsequently with ICI or other therapies, while evaluation of biomarkers and therapy combinations are ongoing in clinical trials.
Clinical Practice Points.
Early progression on platinum-based chemotherapy has been identified as a poor prognostic factor regarding outcomes to subsequent chemotherapy for several cancer types, including locally advanced unresectable and metastatic urothelial carcinoma (aUC)
While immune checkpoint inhibitors (ICI) improve survival in aUC, little is known regarding outcomes in those patients who progress early on first line platinum-based chemotherapy
We compared observed response rate, progression-free and overall survival between patients based on time from initiation of first line platinum-based chemotherapy to second line ICI
Our hypothesis-generating study provides relevant data suggesting that putative earlier resistance to platinum-based chemotherapy may be associated with shorter survival in those who receive 2L ICI.
Acknowledgments
R Talukder and A.R. Khaki were supported by the National Cancer Institute under training grant T32A009515
D. Makrakis and L. N. Diamantopoulos acknowledge the support of Kure It Cancer Research. J.L. Wright, R. B. Montgomery, E. Y. Yu, A.C. Hsieh and P. Grivas acknowledge the support of the Seattle Translational Tumor Research Program at the Fred Hutchinson Cancer Center. D. J. Pinato acknowledges the infrastructure support provided by the Imperial Experimental Cancer Medicine Centre, Cancer Research UK Imperial Centre, the Imperial College Healthcare NHS Trust Tissue Bank and the Imperial College BRC.
Research Electronic Data Capture at the Institute of Translational Health Sciences is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award UL1 TR002319. David J. Pinato is supported by grant funding from the Welcome Trust Strategic Fund (PS3416).
Disclosure
Rafee Talukder has no COI to declare.
Dimitrios Makrakis has no COI to declare.
Genevieve Ihsiu Lin has no COI to declare.
Leonidas N. Diamantopoulos has no COI to declare.
Scott Dawsey has received honoraria from MJH Life Sciences.
Shilpa Gupta has received personal fees from Bristol Myers Squibb, Merck, Janssen, Seattle Genetics, EMD Sorono and Pfizer, and has received grants from Astellas, BMS and Bristol Myers Squibb.
Lucia Carril-Ajuria has no COI to declare.
Daniel Castellano has served as advisor/consultant for Lilly, Pierre-Fabre, Boehringer Ingelheim, Roche/Genentech, AstraZeneca, Ipsen, Bayer, Sanofi, Janssen Oncology, Pfizer, Bristol-Myers Squibb, Astellas Pharma, Novartis, MSD Oncology and Sanofi. His institution has received research funding from Janssen Oncology and has received travel accommodations/expenses from Roche, Pfizer, Astra Zeneca Spain and Bristol-Myers Squibb.
Ivan de Kouchkovsky has no COI to declare.
Tanya Jindal has no COI to declare.
Vadim S. Koshkin has no COI to declare.
Joseph J. Park has no COI to declare.
Ajjai Alva has received grants/contracts from Arcus Biosciences, AstraZeneca Pharmaceuticals, LP Bristol-Myers Squibb Company, Eisai Inc., Esanik, Ionnis, Merck & Co., Inc. and Prometheus, consulting fees from Bristol-Myers Squibb Company, EMD Serono, Merck & Co., Inc., and Pfizer Inc., and had a leadership/fiduciary role in ASCO TAPUR/CRC.
Mehmet A. Bilen has acted as a paid consultant for and/or as a member of the advisory boards of Exelixis, Bayer, BMS, Eisai, Pfizer, AstraZeneca, Janssen, Calithera Biosciences, Genomic Health, Nektar, EMD Serono, SeaGen, and Sanofi, and has received grants to his institution from Merck, Xencor, Bayer, Bristol-Myers Squibb, Genentech/Roche, SeaGen, Incyte, Nektar, AstraZeneca, Tricon Pharmaceuticals, Genome & Company, AAA, Peloton Therapeutics, and Pfizer for work performed as outside of the current study.
Tyler F. Stewart has no COI to declare.
Rana R. McKay has received compensation as advisor/consultant for AstraZeneca, Aveo, Bayer, Bristol Myers Squibb, Calithera, Caris, Dendreon, Exelixis, Janssen, Merck, Myovant, Novartis, Pfizer, Sanofi, Tempus. Financing of Scientific Research: Pfizer, Tempus, Bayer.
Nishita Tripathi has no COI to declare.
Neeraj Agarwal has served as consultant to Astellas, Astra Zeneca, Aveo, Bayer, Bristol Myers Squibb, Calithera, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Foundation Medicine, Genentech, Gilead, Janssen, Merck, MEI Pharma, Nektar, Novartis, Pfizer, Pharma-cyclics, and Seattle Genetics, and has received research funding for his institution from Astellas, Astra Zeneca, Bavarian Nordic, Bayer, Bristol Myers Squibb, Calithera, Celldex, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Genentech, Gilead, Glaxo Smith Kline, Immunomedics, Janssen, Medivation, Merck, Nektar, New Link Genetics, Novartis, Pfizer, Prometheus, Rexahn, Roche, Sanofi, Seattle Genetics, Takeda, and Tracon.
Naomi Vather-Wu has no COI to declare.
Yousef Zakharia has been in the advisory board for Bristol Myers Squibb, Amgen, Roche Diagnostics, Novartis, Janssen, Eisai, Exelixis, Castle Bioscience, Array, Bayer, Pfizer, Clovis and EMD Serono, has received grants for his institution from NewLink Genetics, Pfizer, Exelixis, Eisai, has received honoraria from Pfizer and Novartis.
Rafael Morales-Barrera has served as consultant/advisor for Sanofi, AstraZeneca, Astellas Pharma and MSD, has been in the speaker’s Bureau for Astellas Pharma, Merck/Pfizer and MSD Oncology, and has received travel accommodations from Sanofi, Pfizer, MSD, Astellas Pharma, Astra Zeneca, Bayer, Roche/Genentech.
Michael E. Devitt has no COI to declare.
Alessio Cortellini has served as consultant for Astrazeneca, MSD, BMS and Roche, and has received speaker’s compensation from Astrazeneca, Novartis and Eisai.
Fulgenzi Claudia Angela Maria has no COI to declare.
David J. Pinato received lecture fees from ViiV Healthcare and Bayer Healthcare and travel expenses from BMS and Bayer Healthcare; consulting fees for Mina Therapeutics, EISAI, Roche, and Astra Zeneca, and received research funding to his institution from MSD and BMS.
Ariel Nelson has no COI to declare.
Christopher J. Hoimes has received consulting fees from Merck, Seagen, Astellas, BMS and speaker’s compensation for Eisai, Seagen, Astellas, BMS.
Kavita Gupta has no COI to declare.
Benjamin A. Gartrell has no COI to declare.
Alex Sankin has no COI to declare.
Abhishek Tripathi has received Honoraria from Urology times, grants/contracts from Clovis Oncology, Corvus Pharmaceuticals, Bayer, EMD Serono, Aravive, WindMIL, Exact Sciences, and Pfizer and served as an advisor for Foundation Medicine and Pfizer, Genzyme, EMD Serono, Exelixis, Deka Biosciences and Seattle Genetics.
Roubini Zakopoulou has no COI to declare.
Aristotelis Bamias has received grants/contracts from Pfizer, BMS, Astra Zeneca, Ipsen, and served as advisor and received payment/honoraria for lectures from BMS, Ipsen, MSD.
Jure Murgic has received honoraria from Roche and Astellas Pharma, has served as consultant/advisor for Bristol-Myers Squibb and Sandoz, has received research funding from Astellas Pharma and has received travel expenses from BMS, Roche and Janssen.
Ana Fröbe has served as consultant/advisor for Bayer Germany, Astellas, BMS, Pfizer, Sandoz and Janssen Oncology.
Alejo Rodriguez-Vida has served as advisor for MSD, Pfizer, BMS, Astellas, Janssen, Bayer, Clovis, Ipsen and Roche has received honoraria or travel expenses from Pfizer, MSD, Astellas, BMS, Janssen, Astra Zeneca, Roche, Bayer, Ipsen and Sanofi Aventis, and has received research funding from Takeda, Pfizer, and Merck.
Alexandra Drakaki has served as consultant for Bristol-Myers Squibb, AstraZeneca, RADMETRIX, Seattle Genetics, Janssen, PACT Pharma, Merck, Roche/Genentech, Exelixis, Dyania Health, has received research funding from Kite/Gilead, AstraZeneca, Genentech/Roche, BMS, Merck Sharp & Dohme, Jounce Therapeutics, Infinity Pharmaceuticals, Seattle Genetics/Astellas, and has received travel expenses from Lilly, AstraZeneca and Seattle Genetics.
Sandy Liu has received honoraria from Esai, Exelixis and EMD-Serono.
Eric Lu has no COI to declare.
Vivek Kumar has no COI to declare.
Giuseppe Di Lorenzo has no COI to declare.
Monika Joshi has received research funding to her institution from Astra Zeneca, Eisai and Pfizer, has served as an advisor for Seagen and Sanofi.
Pedro Isaacsson-Velho had received grants from ASCO Conquer Cancer Foundation, consulting fees from Bayer, Astellas and AstraZeneca, payment for lectures and travel expenses from Astellas, Pfizer, AstraZeneca, Merck, MSD, Janssen and BMS, and has served as an advisor for Astellas, Pfizer and AstraZeneca
Lucia Alonso Buznego has no COI to declare.
Ignacio Duran has received honoraria for speaker engagements, advisory roles or funding of continuous medical education from Astellas, Bristol Myers Squibb, EUSA Pharma, Immunomedics Inc., IPSEN, Jansen, Merck, MSD, Novartis, Pfizer, Roche Genentech, Astra Zeneca and Seattle Genetics, and has received research grants from AstraZeneca, Astellas, and Roche-Genentech.
Marcus Moses has no COI to declare.
Pedro Barata has served as consultant for Astellas; Eisai; Janssen, EMD Serono; Dendreon; Pfizer, Seattle Genetics, BMS, Bayer, Guardant Health, has received research grants from BlueEarth Diagnostics, has been in the speaker’s bureau for Bayer, Caris and Myovant, and has contracted institutional research for AstraZeneca and Merck.
Guru Sonpavde has been in the advisory board for BMS, Genentech, EMD Serono, Merck, Sanofi, Seattle Genetics/Astellas, Astrazeneca, Exelixis, Janssen, Bicycle Therapeutics, Pfizer, Gilead, Scholar Rock, G1 Therapeutics, Eli Lilly/Loxo Oncology, Infinity Pharmaceuticals, has received research support from Sanofi, Astrazeneca, Gilead, QED, Lucence, Predicine, BMS, has served in a steering committee of studies for BMS, Bavarian Nordic, Seattle Genetics, QED, G1 Therapeutics (all unpaid), and Astrazeneca, EMD Serono, Debiopharm, has served in the data safety monitoring committee for Mereo Biopharma, has received travel compensation from BMS, AstraZeneca, has received writing/editor fees from UpToDate, Editor of Elsevier Practice Update Bladder Cancer Center of Excellence and received speaking fees from Physicians Education Resource (PER), Onclive, Research to Practice, Medscape, Cancer Network, Masters Lecture Series (MLS).
Jonathan. L. Wright has received grants from Nucleix, Inc, Altor Biosci, Merck, SWOG and National Institutes of Health, royalties/licenses from UpToDate, and consulting fees from Sanofi-Genzyme.
Evan Y. Yu has received research funding to his institution Bayer, Blue Earth, Daiichi Sankyo, Dendreon, Lantheus, Merck, Seagen, Taiho and received consulting fees from Abbvie, Advanced Accelerator Applications, Bayer, Clovis, Exelixis, Janssen, Merck, Sanofi.
Robert Bruce Montgomery has received research funding to his institution from Janssen Oncology, AstraZeneca, Clovis, Astellas Pharma and Beigene.
Andrew C. Hsieh has received research funding from Denali, Inc. Petros Grivas has done paid consulting with AstraZeneca, Astellas Pharma, Boston Gene, Bristol Myers Squibb, Dyania Health, EMD Serono, Exelixis, Fresenius Kabi, Genentech/Roche, Gilead Sciences, GlaxoSmithKline, Guardant Health, Infinity Pharmaceuticals, Janssen, Lucence Health, Merck & Co., Mirati Therapeutics, Pfizer, PureTech, QED Therapeutics, Regeneron Pharmaceuticals, Seattle Genetics, Silverback Therapeutics, 4D Pharma PLC, UroGen. His institution has received grants from Bavarian Nordic, Bristol Myers Squibb, Clovis Oncology, Debiopharm, EMD Serono, G1 Therapeutics, Gilead Sciences, GlaxoSmithKline, Merck & Co., Mirati Therapeutics, Pfizer, QED Therapeutics.
Ali Raza Khaki has received honoraria from OncLive/MJH Life Sciences, has owned stocks of Merck and Sanofi, and has had uncompensated relationships with Seattle Genetics/Astellas.
Abbreviations:
- 1L
first line
- 2L
second line
- auc
advanced urothelial carcinoma
- CI
confidence interval
- FDA
Food and Drug Administration
- HR
hazard ratio
- DDR
DNA damage repair and response
- ICI
immune checkpoint inhibitors
- MIBC
muscle invasive bladder cancer
- Mo
months
- NAC
neoadjuvant chemotherapy
- ORR
observed response rate
- OS
overall survival
- PD-1
programmed cell death protein 1
- PD-L1
programmed death-ligand 1
- PFS
progression-free survival
- SG
sacituzumab govitecan
- TME
tumor microenvironment
- UC
urothelial carcinoma
References
- 1.National Comprehensive Cancer Network. Clinical practice guidelines in oncology: bladder cancer; 2020. version 3 [Internet]. [cited 2022 Jan 20] Available at https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. [Google Scholar]
- 2.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. [DOI] [PubMed] [Google Scholar]
- 3.Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018;19:51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galsky MD, Saci A, Szabo PM, et al. Nivolumab in patients with advanced platinum-resistant urothelial carcinoma: efficacy, safety, and biomarker analyses with extended follow-up from checkmate 275. Clin Cancer Res. 2020;26:5120–5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med. 2017;376:1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicenter, phase II trial. The Lancet. 2017;389:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways. Clin Cancer Res. 2008;14:1291–1295. [DOI] [PubMed] [Google Scholar]
- 8.Bokemeyer C, Kollmannsberger C, Harstrick A, et al. Treatment of patients with cisplatin-refractory testicular germ-cell cancer. German Testicular Cancer Study Group (GTCSG). Int J Cancer. 1999;83:848–851. [DOI] [PubMed] [Google Scholar]
- 9.Sonpavde G, Pond GR, Fougeray R, et al. Time from Prior Chemotherapy Enhances Prognostic Risk Grouping in the Second-line Setting of Advanced Urothelial Carcinoma: A Retrospective Analysis of Pooled, Prospective Phase II Trials. Eur Urol. 2013;63:717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuo K, Lin YG, Roman LD, Sood AK. Overcoming Platinum Resistance in Ovarian Carcinoma. Expert Opin Investig Drugs. 2010;19:1339–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Saux O, Ray-Coquard I, Labidi-Galy SI. Challenges for immunotherapy for the treatment of platinum resistant ovarian cancer. Semin Cancer Biol. 2021;77:127–143. [DOI] [PubMed] [Google Scholar]
- 12.Sweis RF, Spranger S, Bao R, et al. Molecular Drivers of the Non-T Cell-Inflamed Tumor Microenvironment in Urothelial Bladder Cancer. Cancer Immunol Res. 2016;4:563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khaki AR, Li A, Diamantopoulos LN, et al. Impact of performance status on treatment outcomes: A real-world study of advanced urothelial cancer treated with checkpoint inhibitors. Cancer. 2020;126:1208–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khaki AR, Li A, Diamantopoulos LN, et al. A New Prognostic Model in Patients with Advanced Urothelial Carcinoma Treated with First-line Immune Checkpoint Inhibitors. Eur Urol Oncol. 2021;4:464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Minor BL, et al. The REDCap Consortium: Building an International Community of Software Platform Partners. J Biomed Inform. 2019;95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellmunt J, Choueiri TK, Fougeray R, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol. 2010;28:1850–1855. [DOI] [PubMed] [Google Scholar]
- 18.Stewart TF, Kotha NV, Dzimitrowicz HE, et al. Efficacy of anti-PD(L)1 therapy for patients (Pts) with advanced urothelial carcinoma (aUC) with primary resistance to platinum-based chemotherapy (PC). J Clin Oncol. 2021;39:e16515 15_supple16515. [Google Scholar]
- 19.Sonpavde G, Manitz J, Gao C, et al. Five-Factor Prognostic Model for Survival of Post-Platinum Patients with Metastatic Urothelial Carcinoma Receiving PD-L1 Inhibitors. J Urol. 2020;204:1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grivas P, Loriot Y, Morales-Barrera R, et al. Efficacy and safety of rucaparib in previously treated, locally advanced or metastatic urothelial carcinoma from a phase II, open-label trial (ATLAS). BMC Cancer. 2021;21:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grivas P, Mortazavi A, Picus J, et al. Mocetinostat for patients with previously treated, locally advanced/metastatic urothelial carcinoma and inactivating alterations of acetyltransferase genes. Cancer. 2019;125:533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powles T, Carroll D, Chowdhury S, et al. An adaptive, biomarker-directed platform study of durvalumab in combination with targeted therapies in advanced urothelial cancer. Nat Med. 2021;27:793–801. [DOI] [PubMed] [Google Scholar]
- 23.Grivas P, Pouessel D, Park CH, et al. TROPHY-U-01 Cohort 3: Sacituzumab govitecan (SG) in combination with pembrolizumab (Pembro) in patients (pts) with metastatic urothelial cancer (mUC) who progressed after platinum (PLT)-based regimens. J Clin Oncol. 2022;40:434 6_suppl434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadeghi S, Quinn DI, Dorff T, et al. 651O Phase II trial of pembrolizumab (P) in combination with sEphB4-HSA (B4) in previously treated metastatic urothelial carcinoma (mUC). Ann Oncol. 2021;32:S678. [Google Scholar]
- 25.Msaouel P, Siefker-Radtke AO, Sweis R, et al. 705MO Sitravatinib (sitra) in combination with nivolumab (nivo) demonstrates clinical activity in checkpoint inhibitor (CPI) naïve, platinum-experienced patients (pts) with advanced or metastatic urothelial carcinoma (UC). Ann Oncol. 2020;31:S556. [Google Scholar]
- 26.Drakaki A, Rezazadeh Kalebasty A, Lee JL, et al. Phase Ib/II umbrella trial to evaluate the safety and efficacy of multiple 2L cancer immunotherapy (CIT) combinations in advanced/metastatic urothelial carcinoma (mUC): MORPHEUS-mUC. J Clin Oncol. 2020;38:TPS591 6_suppl.33052757 [Google Scholar]
- 27.Teo MY, Bambury RM, Zabor EC, et al. DNA Damage Response and Repair Gene Alterations Are Associated with Improved Survival in Patients with Platinum-Treated Advanced Urothelial Carcinoma. Clin Cancer Res. 2017;23:3610–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teo MY, Seier K, Ostrovnaya I, et al. Alterations in DNA Damage Response and Repair Genes as Potential Marker of Clinical Benefit From PD-1/PD-L1 Blockade in Advanced Urothelial Cancers. J Clin Oncol. 2018;36:1685–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2020;383:1218–1230. [DOI] [PubMed] [Google Scholar]


