Abstract
Background
Infection with Helicobacter pylori as the cause of gastric cancer is a global public health concern. In addition to protecting germs from antibiotics, biofilms reduce the efficacy of H. pylori eradication therapy. The nucleotide polymorphisms (SNPs) related with the biofilm forming phenotype of Helicobacter pylori were studied.
Results
Fifty-six H. pylori isolate from Bangladeshi patients were included in this cross-sectional study. Crystal violet assay was used to quantify biofilm amount, and the strains were classified into high- and low-biofilm formers As a result, strains were classified as 19.6% high- and 81.4% low-biofilm formers. These phenotypes were not related to specific clades in the phylogenetic analysis. The accessories genes associated with biofilm from whole-genome sequences were extracted and analysed, and SNPs among the previously reported biofilm-related genes were analysed. Biofilm formation was significantly associated with SNPs of alpA, alpB, cagE, cgt, csd4, csd5, futB, gluP, homD, and murF (P < 0.05). Among the SNPs reported in alpB, strains encoding the N156K, G160S, and A223V mutations were high-biofilm formers.
Conclusions
This study revealed the potential role of SNPs in biofilm formation and proposed a method to detect mutation in biofilm from whole-genome sequences.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12866-023-02889-8.
Keywords: Whole-genome sequences, SNP, Variants, Biofilm formation, Antibiotic resistance, Infectious disease
Introduction
Helicobacter pylori infection remains a public health problem worldwide, affecting half of the humans and inducing various gastrointestinal tract diseases [1]. The acidic and hostile conditions of the human stomach constitute the natural niche of H. pylori, suggesting high adaptation abilities [2, 3]. Biofilm-forming H. pylori strains have been observed in vivo on the surface of the gastric mucosa [4]. The biofilm is a complex exopolysaccharide structure that protects and maintains life in the presence of external stress [5]. The biofilm also protects bacteria against antibiotics, resulting in a decline in H. pylori eradication [6, 7]. Previous studies reported that high-biofilm formers are likely more resistant to antibiotic exposure [7, 8]. Therefore, the prediction of genetic determinants for biofilm formation is necessary. Understanding the mechanism of biofilm formation is essential for improving H. pylori elimination strategies.
Approaches that enable the investigation of genetic mechanisms correlated to a given phenotype are currently available. In the first approach, the gene responsible for a phenotype is studied by generating a mutant of the target gene from the wild-type strain and observing the phenotype [9]. For example, removing the entire gene of alpB was found to inhibit the adhesion and initiation of biofilm formation [10, 11]. In the second approach, the genetic variation that existed in the natural population was evaluated [9, 12]. Statistical analysis is performed on the dataset of strains with two distinctive traits. Genetic variants associated with traits are candidates for explaining mechanisms of the trait seen in the population. This approach has advanced quickly, particularly with the development of whole-genome sequencing methods. Previous studies assessed the SNPs in gyrA, gyrB, and 23s rRNA that is involved in AMR (Antimicrobial Resistance) in order to decipher the mechanism of AMR. [13] [14]. This similar approach is potentially applied to the other phenotype, such as the bacteria’s ability to form biofilm. Genetic variation, such as insertion, deletion, and SNPs in the high and low biofilm, is a field that deserves further investigation. Therefore, using a similar approach as in antibiotic resistance, this study investigates the potential use of the genomic approach to evaluate biofilm formation.
Biofilm formation levels among the strains varied from low to high, indicating a potential complex mechanism involving a specific genotype or variant [6, 15]. The level of genetic variation can be the present absence of genes or the SNP. SNPs that consist of insertion, deletion, and non-synonymous point mutation could be one possible mechanism for biofilm formation [16]—identifying the point mutation and which genes for the focused evaluation have been challenging. Various tools have been developed to assess single nucleotide variants (SNVs), such as “Antimicrobial Resistance Identification By Assembly” (ARIBA), that can be used to assemble targeted genes and detect the presence or absence of genes and nucleotide variants [17]. Meanwhile, finding new candidate genes among the genomes at the present-absent level is also necessary [18, 19].
We performed the genotype-phenotype evaluation of biofilm on the Helicobacter pylori clinical isolates obtained from Bengali ethnic Bangladesh subjects. We investigated the novel nucleotide variant of a list of genes reported to be involved in biofilm formation as a starting point. The steps included confirming those genes’ presence in the clinical isolates and discovering the nucleotide alteration variant that may involve biofilm phenotype shift. We also assessed the other possible novel genes associated with biofilm phenotypic alterations [18].
Results
Distribution of biofilm formation among H. pylori strains from Bangladesh
Biofilm formation was quantified and classified into two groups: high- and low-biofilm formers (Supplementary Figure S1) were determined using crystal violet assay. The group of high-biofilm formers included 19.6% of strains (11/56) with a mean optical density (OD) of 0.85 (SD 0.4). The distribution of low-biofilm formers was 80.4% (45/56) and a mean OD of 0.24 (SD 0.06). Among the low-biofilm former, 11 strains of the low-biofilm formers had OD below control. One representative high biofilm strain (BGD114) confirmed its dense biofilm formation by observation using the SEM (Fig. 1). The growth curve pattern of the isolates was comparable; therefore, the biofilm formation OD was independent of growth (Supplementary Figures S2a and S2b).
Fig. 1.
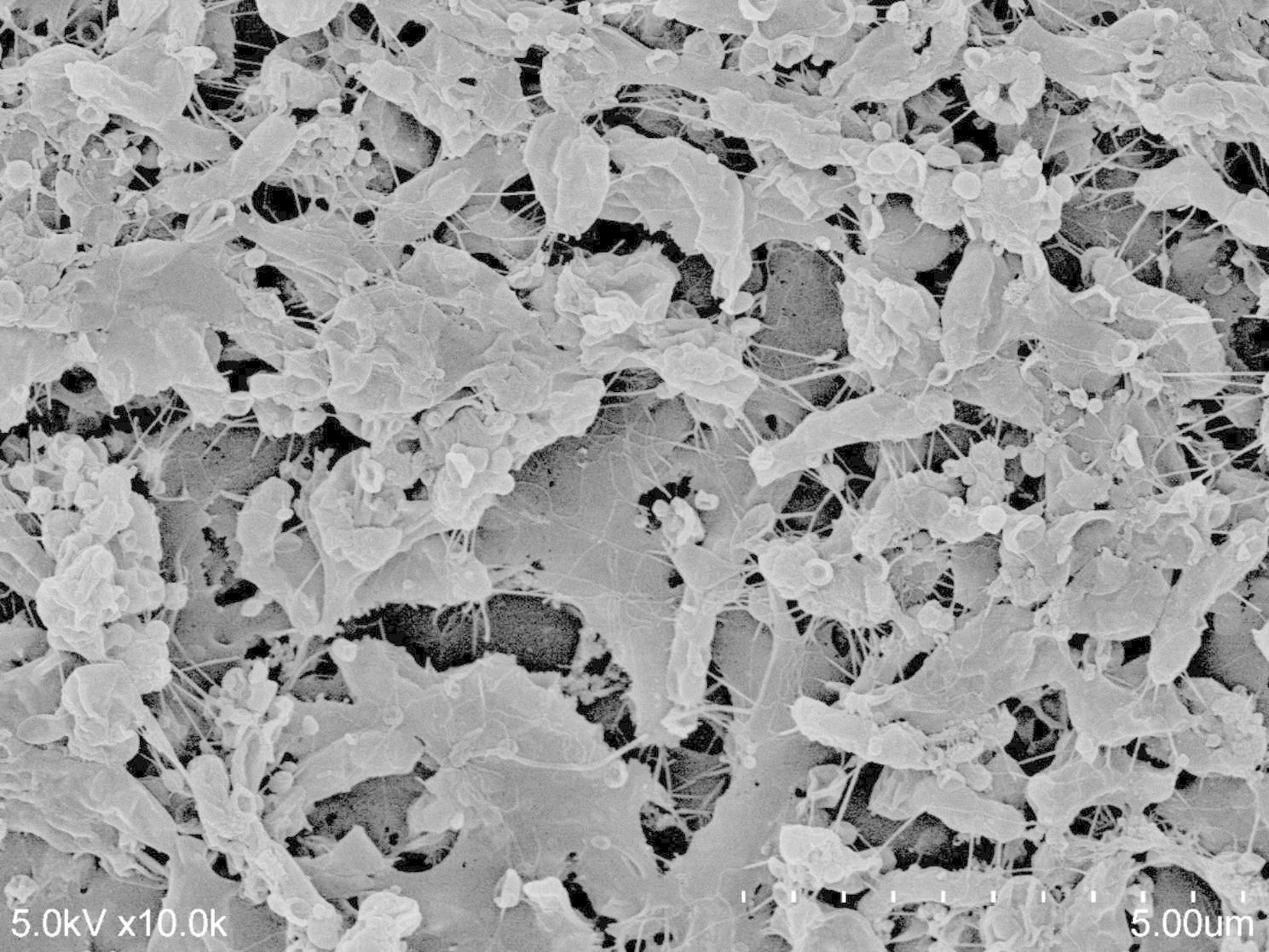
The morphology of biofilm formation on the coverslip surface on the day 4 of culture can be seen in this figure (10,000X magnification). The rod-shaped cells were covered with thick structure
The phylogenetic similarity among the biofilm formers
A phylogenetic tree inferred by the maximum likelihood algorithm was used to assess the eventual association between the biofilm formation phenotype and different genetic H. pylori lineages (Fig. 2). High biofilm was sporadically distributed among the clades; thus, no specific phylogenetic branch was associated with biofilm formation. In the genomic population of H. pylori, the strains were assigned using published reference strains [20]. The H. pylori genomic population has been previously described as hpAfrica1, hpAmerind, hpAsia2, hpEastAsia, and hpEurope. Using the phylogeny approach, the strains in this dataset belong to hpAsia2 and hpEurope. No significant genomic-population association was found between the genome population type and biofilm formation. Among the hpEurope strains, 21.6% (3/19) of the high biofilm formation was observed, and only 15.8% (8/37) was observed in hpAsia2.
Fig. 2.

The phylogenetic tree depicts the population and the biofilm formation
The circle represents the population’s genetic type. The big circle with dark blue and light blue represented the high and low biofilm phenotype, respectively (grey: unknown because biofilm test was unavailable)
The association of the presence and absence of well-known genes to biofilm formation
We compiled the research findings of studies investigating the genes which play roles in biofilm formation. Then we summarized up to 46 genes that will be used as targets for analysis of the Bangladeshi genomes in this dataset (Supplementary Table S2). Using the minimum coverage criteria of 50% and a minimum identity of 90%, 32 of the 46 genes were present in all isolates (Supplementary Table S2). The genes absent in more than 10% of the isolates were vapD, cagD, cagE, and csd5. The Fisher exact test did not give a significant P-value. This result indicated that the absence of the genes above rarely occurred naturally and may not be the only factor contributing to biofilm formation.
The variants associated with biofilm formation
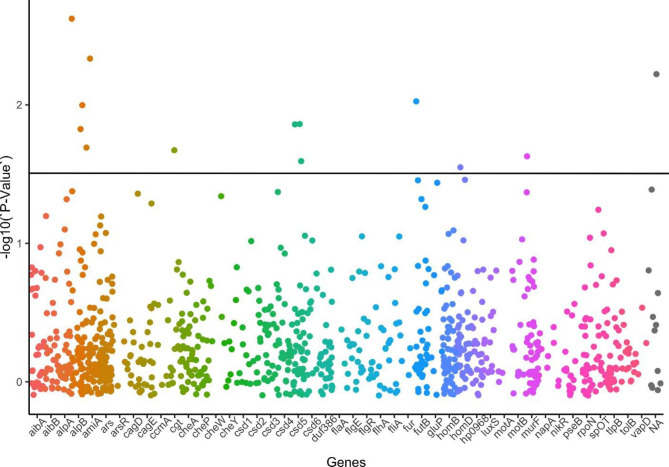
The SNPs were analyzed to find out the mechanism related to biofilm formation (other than the gene absent). This pipeline assembles the gene according to the sequences in the references, ATCC 26695, which has low biofilm formation. The genes investigated in this study played a role in biofilm formation. The total number of polymorphisms extracted from the analysis with the MAF > 10% was 960, including frameshift, insertion, deletion, multiple site polymorphism, and single nucleotide polymorphism, summarised in (Fig. 2). Comparison of the variant found between the high and low biofilm former. A total number of SNPs were observed among the investigated genes, the highest number of SNPs was observed in the homB gene (279 variant), and the lowest was observed in cheY genes (1 variant). The statistically significant variants for the biofilm phenotype are listed in Table 1. The SNPs in adhesion-related genes were included, such as alpA, alpB, homD, cagE, and futB. Other genes that have roles in metabolism and cell shape regulation, such as gluP, cgt, csd4, csd5, murF, and amiA, also showed significant associations. Three SNPs in the alpB gene were significantly associated with the high-biofilm former at A223V, G160S, and N156K. The gene expression of the alpB gene was also higher in the high biofilm former compared to low biofilm former isolates (Supplementary Figure S3).
Table 1.
The significant variants associated with biofilm formation
| Gene | SNP | Proportion in Low Biofilm | Proportion in High Biofilm | Naïve | FDR | Reference |
|---|---|---|---|---|---|---|
| P-value | ||||||
| alpA | G196N | 0.16 | 0.45 | 0.045 | 0.045 | [11] |
| alpB | A223V | 0.31 | 0.73 | 0.017 | 0.045 | [11] |
| G160S | 0.38 | 0.91 | 0.002 | 0.036 | ||
| N156K | 0.09 | 0.45 | 0.01 | 0.045 | ||
| amiA | T168V | 0.07 | 0.36 | 0.022 | 0.045 | [21] |
| cagE | S419C | 0.42 | 0.87 | 0.045 | 0.045 | [15] |
| cgt | V34A | 0.178 | 0.54 | 0.02 | 0.045 | [22] |
| csd5 | M125I | 0.15 | 0.56 | 0.017 | 0.045 | [21] |
| P43S | 0.1 | 0.44 | 0.026 | 0.045 | ||
| V110A | 0.07 | 0.44 | 0.015 | 0.045 | ||
| futB | E98fs | 0.16 | 0.5 | 0.033 | 0.045 | [15] |
| gluP | T85S | 0.27 | 0.64 | 0.032 | 0.045 | [23] |
| homD | A570T | 0.09 | 0.36 | 0.04 | 0.045 | [15] |
| V249fs | 0.13 | 0.45 | 0.029 | 0.045 | ||
| murF | V250I | 0.33 | 0 | 0.026 | 0.045 | [21] |
| Gene | SNP | Proportion in Low Biofilm | Proportion in High Biofilm | P-value | FDR | Reference |
| alpB | T127A | 0.911 | 0.636 | 0.04 | 0.045 | [11] |
| csd4 | C12Y | 0.711 | 0.364 | 0.041 | 0.045 | [21] |
| futB | L317I | 0.432 | 0 | 0.01 | 0.045 | [15] |
The P-values observed in all nucleotide polymorphisms (SNPs) of all genes are summarised in Fig. 3. The x-axis represents the lists of SNPs, and the y-axis represents the p-value from fisher exact tests for the genes and the biofilm phenotype. The straight horizontal line represents the cut-off for the significant P-value (0.05). The dots show the P-value of each variant, and 18 dots above the line have a P-value less than 0.05.
Fig. 3.
Scatter plot of P-values of the nucleotide polymorphisms (SNPs) in the target genes and their association with biofilm formation
The presence of mutation and association to phenotype in the independent dataset
To evaluate whether the mutation is also present in other strains in the population, we randomly selected 20 strains of H. pylori clinical isolates from Indonesia as the validation dataset from the previous study [24]. This dataset comprised the high biofilm former (n = 7) and low biofilm former (n = 13). Each isolate’s genes subjected to the validation (alpB, cgt,gluP, csd4, csd5, murF) were extracted and aligned to 26695. This dataset assessed the presence of mutation among the low and high biofilm former (Supplementary Table S3). Even if these minor number validation data sets, substitution in the cgt genes V34A occurred only in the high biofilm former. A similar tendency was observed in the strains possessing A223V and N156K substitutions in alpB genes. Each SNP and biofilm formation OD was plotted (Supplementary Figure S4). The variation of mutation proportion between high and low biofilm formation was observed between the original and validation dataset and the average biofilm formation among the SNP.
Screening of accessories genes associated with biofilm formation
The whole-genome sequences were annotated, and the pan-genome was constructed. Then the genotype-phenotype association analysis was performed on the accessories genes using a scoary pipeline. The genes naturally absent in certain strains are called accessory genes and can contribute to phenotypic change. The genes presented in < 99% of pan-genome with a minimum identity of 60 and coverage of 50 were used to determine accessory genes. The analysis discovered that 28% of the DUF1524 was absent in the high biofilm former [25]. The results also revealed several genes (Table 2) that functioned in DNA replication (DNA polymerase, DNA primase). Five genes related to gene insertion, such as phages (dB-ParB domain-containing protein of phage, Helicobacter phage FrB41M, putative tail assembly protein of phage), insertion sequence (IS, IS200/IS605 family transposase IS606,) and plasticity gene (VirB4 homolog) were discovered.
Table 2.
Accessories gene associated with biofilm formation
| Gene | Annotation | Present in low biofilm (n) | Present in high biofilm (n) | Proportion of gene present in biofilm level | Naïve P value |
FDR | |
|---|---|---|---|---|---|---|---|
| High | Low | ||||||
| btuB_1 | Vitamin B12 transporter BtuB | 10 | 24 | 0.909 | 0.533 | 0.036 | 1 |
| group_1745 | Phosphoethanolamine transferase CptA | 9 | 21 | 0.818 | 0.467 | 0.047 | 1 |
| group_485 | DUF1524 domain-containing protein | 8 | 45 | 0.727 | 1 | 0.006 | 1 |
| group_824 | G domain-containing protein | 8 | 45 | 0.727 | 1 | 0.006 | 1 |
| group_243 | hypothetical protein | 8 | 16 | 0.727 | 0.356 | 0.041 | 1 |
| group_2979 | VirB4 homolog (VirB4) | 4 | 4 | 0.364 | 0.89 | 0.04 | 1 |
| group_2579 | DNA primase | 3 | 2 | 0.273 | 0.44 | 0.047 | 1 |
| group_2913 | IS200/IS605 family transposase IS606 | 3 | 1 | 0.273 | 0.22 | 0.021 | 1 |
| group_3500 | ddrB-ParB domain-containing protein of phage | 3 | 1 | 0.273 | 0.22 | 0.021 | 1 |
| group_3675 | Putative tail assembly protein of phage | 3 | 2 | 0.273 | 0.44 | 0.047 | 1 |
| group_363 | hypothetical protein | 3 | 2 | 0.273 | 0.44 | 0.047 | 1 |
| dnaE2_1 | Error-prone DNA polymerase | 2 | 0 | 0.182 | 0 | 0.036 | 1 |
| group_4028 | Uncharacterized protein (Helicobacter phage FrB41M) | 2 | 0 | 0.182 | 0 | 0.036 | 1 |
| group_2993 | hypothetical protein | 2 | 0 | 0.182 | 0 | 0.036 | 1 |
| group_290 | conserved hypothetical ATP-binding protein | 0 | 16 | 0 | 0.356 | 0.023 | 1 |
Discussion
Biofilm formation is a beneficial mechanism that requires complex regulation and interaction between bacteria. In H. pylori, in vitro observations of mono-species biofilms showed that high biomass was obtained after 72 h [21, 26]. The evaluation of strains under the same conditions showed that biofilm formation was significantly higher in particular strains, indicating a variation in biofilm formation. These variations were also observed in the present study. Among the strains from the Bangladesh dataset, 19.6% of the strains could be categorized as high-biofilm formers.
In some bacterial species, biofilm formation can be associated with specific lineages or population, e.g., in Staphylococcus aureus [27]. However, this association in H. pylori remains questionable. Therefore, we tried to adress this question by constructing a phylogenetic tree of Bangladeshi isolates using the SNPs-based core genome alignment tree. The result suggested that there was no association of biofilm formation to a specific lineage in H. pylori. This result was supported by other study reported that there is no association between specific lineages or population with biofilm formation [28].
The whole-genome sequence analysis allow researchers to investigate genes and mutations related to biofilm formation. Hence, we evaluated the presence and absence of genes potentially related to biofilm formation (adhesion, shape formation, efflux pump, and even dispersion, as listed in Supplementary Table 2) then obsereved their difference between high-and low biofilm formation group in the Bangladeshi isolates. Our results showed that these genes were present in most isolates, despite variations in the biofilm formation. This condition makes identificatation of the gene marker for the high-biofilm formers in H. pylori remains challenging. While previous study showed that the presence status of several genes such as cagD, futA, and napA, could be associated with the biofilm level [25], this result was not present in this study. Nevertheless, further study should be done to identify polymorphism of the targeted genes that may affect the phenotype.
Next, we focused on amino acid variants analysis, including insertion, deletion, missense mutation, or SNPs. This study is the first to assess the SNPs associated with biofilm formation in H. pylori. Several SNPs have been linked to specific phenotypes of H. pylori, such as diseases like gastric cancer [16]. SNPs or missense mutations are also associated with resistance to clarithromycin, levofloxacin, and rifampicin [29]. The mutation in the targeted gene, such as 23 S rRNA, gyrA, and gyrB, was concordant with antibiotic resistance, and the statistical analysis showed significant results. Therefore, the identification of SNP on the target genes could be informative. We observed that the gene with highest variants is homB. Based on the molecular studies that have been conducted, HomB is an outer membrane appears to be involved in adherence [30]. Outer membrane protein has high variant as a mechanism to adapt to the various host. Although this has high variant, no significant hits were found in this dataset. Meanwhile, the lowest number of variant were present in cheY gene that regulates the direction of flagella motor Due to its critical role in survival, motility related gene has been conserved among archae and bacterial [31]. It has conserved active site residues which are activated by phosphorylation mechanism [32]. The flagella regulation play roles in switching the planktonic and biofilm phenotype. However, the significant variant was not found in this dataset.
We constructed a database of the genes from previous studies associated with biofilm formation to analyze the dataset from Bangladesh. As a result, 11 genes with variants significantly related to biofilm formation were identified. Significant nucleotide polymorphism were found in the genes that are involved in the cell shape regulation ( gluP, cgt, csd4, csd5, murF, and amiA) [33]. These genes regulate the change of helical form to coccoid forms which are commonly observed in biofilm of H. pylori [34, 35]. This finding implies that the cell-shape regulation deserves a spotlight in the biofilm formation mechanism study.
Virulence gene such as cagE and cagD that belong type IV secretion system (T4SS) were also detected. This T4SS secreted CagA and other products were proposed to interplay with luxS ; the quorum sensing gene. As reported in the previous study, the genetic modification on cagE showed a significant alteration of biofilm formation [25, 36, 37]. Furthermore, variants in five genes encode outer membrane proteins (OMPs) of aherewere also discovered [38–40]. The variants in alpA and alpB were significantly associated with the high biofilm formation. Those genes encode OMPs which indicate that adherence and aggregation process is necessary for biofilm formation. The significant variant that we found were adjacent to the variable region of alpB that previously reported to play role in the biofilm formation (locus 121–150) [38]. Therefore, to check the possibility of the gene expression change related to the present of mutation, we performed RT-PCR for the alpB gene. The higher expression of gene were presented by the high biofilm former strains, indicate the increased activity. Because multiple factors could influence the gene expression, mutagenesis on the fragment containing polymorphism is necessary to confirm the result.
After finding the SNPs, validation is essential [41]. For this purpose, we used the data set of H. pylori clinical isolates obtained from Indonesia. A coherent result could be observed in the cgt and alpB, indicating a possible biomarker for biofilm formation. The results also implicate SNP’s contribution in those genes as part of many factors involved in building biofilm phenotype. Nevertheless, we should be cautious with the possible false-positive results, shown by the inconsistent result in other SNPs compared to the primary dataset (Supplementary Figure S3).
The first step of the analysis only targetting the well-known mutation. Thus, we broaden the analysis of all genes throughout the genome. The gene-to-phenotype association study of the accessories gene by the Scoary pipeline discovered a gene annotated DUF1524 associated with the high biofilm formation reported in the previous study [25]. The function of DUF1524 and its roles in biofilm formation is unclear and thus requires further experimental study. The results also found genes related to a mobile genetic element that could be related to the evidence that the biofilm environment enhances the mutation and genetic exchange [42–44].
The main limitation of the genotype-phenotype association of bacteria isolates is the number of samples that could be tested, the high recombination, and geography-specific adaptation [45]. The small number of samples included in this dataset and the statistical approaches applied cannot avoid the possibility of false-positive discovery among the detected SNPs. However, it could be a stepping stone for further molecular studies elucidating the genetic factors involved in biofilm formation and related molecular mechanisms.
Conclusions
The association analysis of the SNPs in the well-known biofilm formation genes proved the ability to screen biofilm-formation capacity from whole-genome sequencing data. We observed a significant association between the SNPs, including alpA, alpB, cagE, and csd4. The next-generation sequence evaluation could be a method to decipher the mechanism of biofilm formation.
Methods
Patient sampling and H. pylori isolates
The isolates used as the primary dataset in this study were obtained from gastric biopsies of patients from a survey involving 133 subjects from Dhaka Medical College Hospital in 2014 [46]. To obtain a single colony of H. pylori isolates, biopsy specimens from the antrum were homogenized in phosphate-buffered saline (PBS). The biopsy was inoculated in H. pylori selective plates and incubated for five days in a 37 °C microaerophilic environment. Each colony was then subcultured in Brucella agar supplemented with 7% horse blood before harvesting for genomic DNA extraction. H. pylori obtained 56 isolates from patients with chronic gastritis (53/56) and peptic ulcers (3/56). The Oita University Faculty of Medicine Japan and the Ethics Committee of the Bangladesh Medical Research Council (BMRC), Dhaka, Bangladesh, approved the protocol of this study.
Biofilm quantification
Biofilm quantification was performed using the crystal violet method for H. pylori with modifications already described in the previous study [44]. Briefly, the blood plate-grown bacteria were pre-cultured for 24 h under microaerophilic conditions in 1 mL Brucella broth supplemented with 10% fetal bovine serum (FBS). Adjusting the bacterial suspension to an optical density of 0.4 (approximately 2.5 × 106/µL), 25 µL of H. pylori suspension was added to 24-well plates containing 1 mL medium. Three days were spent incubating these plates in a microaerophilic environment with shaking (100 rpm). The suspension of planktonic cells was then discarded. The amount of biofilm was determined by measuring the absorbance at 595 nm with a spectrophotometer (Multiskan Go, Thermo Fischer, Japan). The measurement of the well-containing medium devoid of bacteria was used as a negative control. A low biofilm-former had an optical density (OD) 2X the OD of the control sample, whereas a high biofilm-former had an OD 2X the OD of the control sample [47, 48]. The average OD of the control group was 0.2, so 0.4 was determined to be the high biofilm former.
In conclusion, two phenotype groups were identified: low-biofilm formers (low and negative) and high-biofilm formers (high biofilm former strains). Due to the use of reference strain 26695 genes in constructing the reference database, the biofilm formation of reference strain 26695 was also examined but not included in the dataset. The OD of the 26695 strain biofilm was 0.35, less than 0.4, so it was also categorized as a low biofilm producer. Each experiment was conducted in triplicate.
From day 1 to day 4, the bacterial growth was also monitored by spectrophotometry. A 24-well plate is loaded with 1 ml of the same bacterial solution for biofilm quantification. This liquid culture was incubated at 37 degrees Celsius with 10% CO2 and 100 rpm shaking. Each day, the optical density was measured.
Genome sequencing
Genome sequence data were used in a previous study [46]. According to the manufacturer’s instructions, the H. pylori were harvested in PBS, and the DNA was extracted using the Qiagen DNEeasy Kit (Hilden, Germany). The DNA concentration was then measured using the Quantifluor dsDNA System (Madison, USA) and Quantus Fluorometer (Sunnyvale, USA). After standardizing the concentration, whole-genome sequencing was performed using MiSeq Illumina to obtain paired-end reads with a 300 bp length. The quality assessment by QUAST was summarised in Supplementary Table S1.
To analyze the genetic relatedness and the influence of population genetics, we created the whole genome alignment of all Bangladesh sequences and sequences from a previous study that identified the population [20]. The alignment was performed using the Snippy-core (ver 4.6.2) (https://github.com/tseemann/snippy). The alignment was used to construct a maximum likelihood tree using FastTree 2.0, with GTR–nt mode [49]. The tree was then visualized using Microreact [50].
Analysis of the SNP
The analysis of the SNP used in the study was performed using ARIBA pipeline[51]. First, 42 genes of strain 26695 that were related to biofilm formation mentioned in previous studies were collected to create a reference database (Table S2). The metadata for ARIBA was set into coding sequences and new variants. These references are clustered based on the CD-HIT. Subsequently, the raw reads (fastq format) of the 56 strains were mapped to the reference sequence in the cluster and assembled independently. The reads were mapped to the cluster references with Bowtie, and the variants were called with SAMtools. The results of each strain consisted of the assembled genes and the report of variants compared to strain 26695. The reports were then summarised in a text file. The genes were present if the percent coverage was more than 50% and the percent identity was more than 90%. Then, a summary of SNPs with amino acid changes was shown, and the SNPs presented in less than 10% of the strains were excluded. The presence and absence of SNP were calculated to determine the association with biofilm formation. A Fisher exact test result of P-value < 0.05 was considered significant. The other non-parametric correlation was analyzed using Spearman’s rank correlation model. All statistical analyses and graph construction were performed using R (version 3.5.1).
RNA extraction and measurement of the Gene expression
Helicobacter pylori clinical isolates were randomly selected from high (BGD114, BGD112, and BGD104) and low biofilm former strains (BGD96, BGD109). The high biofilm former possessed the mutation in A223V, G160S and N156K in the alpB genes while the low biofilm former isolates does not have any mutations in the loci above. The gene expression levels of alpb and ppa were measured. RNA was isolated from H. pylori cultured for 36 h. Total RNA from the bacteria was isolated using a commercially available kit (PureLinkTM RNA Mini Kit; Invitrogen by Thermo Fisher Scientific Inc.). The total RNA was quantified using the QuantiFluor® RNA System (Promega). A total of 120 ng RNA was converted into cDNA by using the PrimeScript™ RT Master Mix (Perfect Real Time) with the final product volume of 10 µl (Takara Bio Inc.). Real-time PCR was performed using iTaq Universal SYBR Green Supermix (Bio-Rad Laboratories, Inc.). A standard curve was constructed with 5-fold serial dilutions for each gene target. The expression of the genes was analyzed by using absolute method quantification.
Analysis of accessories gene
This study used a set of genes to analyze the SNPs. To investigate if the genomes’ remaining genes (especially accessories genes) could also be associated with biofilm formation, we created a whole genome assembly for each strain. The reads was trimmed (ver 0.32) by trimmomatic and denovo - assembled using Spades (ver 3.1) [52, 53]. The results annotated by Prokka (ver 1.4) and the gff files output are used to construct a pan-genome by Roary (ver 3.11) [54, 55]. The gene –presence-absence result from Roary and the metadata containing high-low biofilm is used as the input for the Scoary (ver 1.6.16 [18].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thanks to Susi Hidayah for the administrative and editing support.
Authors’ contributions
Study conception and design (K.A.F. and Y.Y.). Acquisition of data (K.A.F, H.A, M.M, M.Y, T.M, J.A, P.S), Computational method development (K.A.F, L.A.W, V.P.T, and E.T.K.). Analysis and interpretation of data (K.A.F, E.T.K, V.P.T,). Writing manuscript (K.A.F, Y.Y.), Writing and editing the manuscript (K.A.F, E.T.K, R.I.A, Y.Y.). Critical revision of the manuscript and final approval (all authors).
Funding
This study was supported in part by grants from the National Institutes of Health (DK62813) (YY) and Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan (18KK0266, 19H03473, 21H00346 and 22H02871) (Y.Y.) (YY) and 21K07898 (JA), 21K08010 (TM). The Japan Society also supported this work for the Promotion of Science Institutional Program for Young Researcher Overseas Visits and the Strategic Funds for the Promotion of Science and Technology Agency (JST) for YY. RIA was a PhD student supported by the Japanese Government (MEXT) scholarship program for 2019.
This work was also supported by Japan Agency for Medical Research and Development (AMED) [e-ASIA JRP, Science and Technology Research Partnership for Sustainable Development (SATREPS), Global Alliance for Chronic Diseases (GACD)] (Y.Y.)and Japan International Cooperation Agency (JICA) [SATREPS] (Y.Y.).
This study was also supported by Thailand Science Research and Innovation Fundamental Fund, Bualuang ASEAN Chair Professorship at Thammasat University, and Center of Excellence in Digestive Diseases, Thammasat University, Thailand.
Data Availability
All genome data were stored in Genbank with BioProject accession PRJDB11821 (Available at: https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJDB11821).
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted following the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Oita University Faculty of Medicine Japan with ( No: P-12-10) and the Ethics Committee of the Bangladesh Medical Research Council (BMRC) (Ref: BMRC/NREC/2013–2016/2151), Dhaka, Bangladesh. All participants submitted informed consent after receiving the information.
Consent for Publication
None applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007;133(2):659–72. doi: 10.1053/j.gastro.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 2.Dube C, Tanih N, Ndip R. Helicobacter pylori in water sources: a global environmental health concern. Rev Environ Health. 2009;24(1):1–14. doi: 10.1515/REVEH.2009.24.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Stark RM, Gerwig GJ, Pitman RS, Potts LF, Williams NA, Greenman J, et al. Biofilm formation by Helicobacter pylori. Lett Appl Microbiol. 1999;28(2):121–6. doi: 10.1046/j.1365-2672.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- 4.Carron MA, Tran VR, Sugawa C, Coticchia JM. Identification of Helicobacter pylori biofilms in human gastric mucosa. J Gastrointest Surg Off J Soc Surg Aliment Tract. 2006;10(5):712–7. doi: 10.1016/j.gassur.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Monds RD, O’Toole GA. The developmental model of microbial biofilms: ten years of a paradigm up for review. Trends Microbiol. 2009;17(2):73–87. doi: 10.1016/j.tim.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Yonezawa H, Osaki T, Kurata S, Zaman C, Hanawa T, Kamiya S. Assessment of in vitro biofilm formation by Helicobacter pylori. J Gastroenterol Hepatol. 2010;25(Suppl 1):90–4. doi: 10.1111/j.1440-1746.2009.06213.x. [DOI] [PubMed] [Google Scholar]
- 7.Fauzia KA, Miftahussurur M, Syam AF, Waskito LA, Doohan D, Rezkitha YAA, et al. Biofilm formation and antibiotic resistance phenotype of Helicobacter pylori Clinical isolates. Toxins. 2020;12(8):473. doi: 10.3390/toxins12080473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yonezawa H, Osaki T, Kamiya S. Biofilm formation by Helicobacter pylori and its involvement for Antibiotic Resistance. Biomed Res Int. 2015;2015:914791. doi: 10.1155/2015/914791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falush D. Bacterial genomics: microbial GWAS coming of age. Nat Microbiol. 2016;1(5):1–2. doi: 10.1038/nmicrobiol.2016.59. [DOI] [PubMed] [Google Scholar]
- 10.Cellini L, Grande R, Traini T, Di Campli E, Di Bartolomeo S, Di Iorio D, et al. Biofilm formation and modulation of luxS and rpoD expression by Helicobacter pylori. Biofilms. 2005;2(2):119. doi: 10.1017/S1479050505001845. [DOI] [Google Scholar]
- 11.Yonezawa H, Osaki T, Fukutomi T, Hanawa T, Kurata S, Zaman C et al. Diversification of the AlpB outer membrane protein of Helicobacter pylori affects biofilm formation and cellular adhesion. J Bacteriol. 2017;199(6): e00729-16. [DOI] [PMC free article] [PubMed]
- 12.Simonin-Wilmer I, Orozco-del-Pino P, Bishop DT, Iles MM, Robles-Espinoza CD. An overview of strategies for detecting genotype-phenotype Associations across Ancestrally diverse populations. Front Genet. 2021;2141. [DOI] [PMC free article] [PubMed]
- 13.Lauener FN, Imkamp F, Lehours P. Genetic determinants and prediction of Antibiotic Resistance Phenotypes in Helicobacter pylori. J Clin Med. 2019; 8(1): 53. [DOI] [PMC free article] [PubMed]
- 14.Tuan VP, Narith D, Tshibangu-Kabamba E, Dung HDQ, Viet PT, Sokomoth S et al. A next-generation sequencing-based Approach to identify genetic determinants of Antibiotic Resistance in Cambodian Helicobacter pylori Clinical isolates. J Clin Med. 2019;8(6):858. [DOI] [PMC free article] [PubMed]
- 15.Wong EHJ, Ng CG, Chua EG, Tay ACY, Peters F, Marshall BJ, et al. Comparative Genomics revealed multiple Helicobacter pylori genes Associated with Biofilm formation in Vitro. PLoS ONE. 2016;11(11):e0166835–e. doi: 10.1371/journal.pone.0166835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berthenet E, Yahara K, Thorell K, Pascoe B, Meric G, Mikhail JM, et al. A GWAS on Helicobacter pylori strains points to genetic variants associated with gastric cancer risk. BMC Biol. 2018;16(1):84. doi: 10.1186/s12915-018-0550-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malfertheiner P, Megraud F, O'morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, et al. Management of Helicobacter pylori infection—the Maastricht V/Florence consensus report. Gut. 2017 Jan 1;66(1):6-30. [DOI] [PubMed]
- 18.Brynildsrud O, Bohlin J, Scheffer L, Eldholm V. Rapid scoring of genes in microbial pan-genome-wide association studies with Scoary. Genome Biol. 2016;17(1):1–9. doi: 10.1186/s13059-016-1108-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MT, et al. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics. 2015;31(22):3691–3. doi: 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yahara K, Furuta Y, Oshima K, Yoshida M, Azuma T, Hattori M, et al. Chromosome painting in silico in a bacterial species reveals fine population structure. Mol Biol Evol. 2013;30(6):1454–64. doi: 10.1093/molbev/mst055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hathroubi S, Servetas SL, Windham I, Merrell DS. Helicobacter pylori biofilm formation and its potential role in pathogenesis. Mol Biol Rev. 2018;82(2): e00001-18. [DOI] [PMC free article] [PubMed]
- 22.De la Cruz MA, Ares MA, von Bargen K, Panunzi LG, Martínez-Cruz J, Valdez-Salazar HA, et al. Gene expression profiling of transcription factors of Helicobacter pylori under different environmental conditions. Front Microbiol. 2017;8:615. doi: 10.3389/fmicb.2017.00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blair KM, Mears KS, Taylor JA, Fero J, Jones LA, Gafken PR, et al. The Helicobacter pylori cell shape promoting protein Csd5 interacts with the cell wall, MurF, and the bacterial cytoskeleton. Mol Microbiol. 2018;110(1):114–27. doi: 10.1111/mmi.14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miftahussurur M, Doohan D, Nusi IA, Adi P, Rezkitha YAA, Waskito LA, et al. Gastroesophageal reflux disease in an area with low Helicobacter pylori infection prevalence. PLoS ONE 2018;13(11):e0205644. [DOI] [PMC free article] [PubMed]
- 25.Wong EH, Ng CG, Chua EG, Tay AC, Peters F, Marshall BJ, et al. Comparative Genomics revealed multiple Helicobacter pylori genes Associated with Biofilm formation in Vitro. PLoS ONE. 2016;11(11):e0166835. doi: 10.1371/journal.pone.0166835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yonezawa H, Osaki T, Kurata S, Fukuda M, Kawakami H, Ochiai K, et al. Outer membrane vesicles of Helicobacter pylori TK1402 are involved in biofilm formation. BMC Microbiol. 2009;9:197. doi: 10.1186/1471-2180-9-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tasse J, Trouillet-Assant S, Josse J, Martins-Simões P, Valour F, Langlois-Jacques C, et al. Association between biofilm formation phenotype and clonal lineage in Staphylococcus aureus strains from bone and joint infections. PLoS ONE. 2018;13(8):e0200064. doi: 10.1371/journal.pone.0200064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song M, Li Q, Zhang Y, Song J, Shi X, Shi C. Biofilm formation and antibiotic resistance pattern of dominant Staphylococcus aureus clonal lineages in China. J Food Saf. 2017;37(2):e12304. doi: 10.1111/jfs.12304. [DOI] [Google Scholar]
- 29.Domanovich-Asor T, Motro Y. Genomic analysis of Antimicrobial Resistance genotype-to-phenotype agreement in Helicobacter pylori. Microorganisms. 2020; 9(1): 2. [DOI] [PMC free article] [PubMed]
- 30.Oleastro M, Cordeiro R, Ferrand J, Nunes B, Lehours P, Carvalho-Oliveira I, et al. Evaluation of the clinical significance of homb a novel candidate marker of Helicobacter pylori strains associated with peptic ulcer disease. J Infect Dis. 2008;198(9):1379–87. doi: 10.1086/592166. [DOI] [PubMed] [Google Scholar]
- 31.Quax TEF, Altegoer F, Rossi F, Li Z, Rodriguez-Franco M, Kraus F et al. Structure and function of the archaeal response regulator CheY. Proc Natl Acad Sci U S A. 2018;115(6):E1259-E68. [DOI] [PMC free article] [PubMed]
- 32.Williams SM, Chen YT, Andermann TM, Carter JE, McGee DJ, Ottemann KM. Helicobacter pylori chemotaxis modulates inflammation and bacterium-gastric epithelium interactions in infected mice. Infect Immun. 2007;75(8):3747–57. doi: 10.1128/IAI.00082-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang DC, Blair KM, Taylor JA, Petersen TW, Sessler T, Tull CM et al. A genome-wide Helicobacter pylori morphology screen uncovers a membrane-spanning helical cell shape complex. J Bacteriol. 2019;201(14). [DOI] [PMC free article] [PubMed]
- 34.Attaran B, Falsafi T, Moghaddam AN. Study of biofilm formation in C57Bl/6J mice by clinical isolates of Helicobacter pylori. Saudi J gastroenterology: official J Saudi Gastroenterol Association. 2016;22(2):161–8. doi: 10.4103/1319-3767.178529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rizzato C, Torres J, Kasamatsu E, Camorlinga-Ponce M, Bravo MM, Canzian F, et al. Potential role of Biofilm formation in the development of Digestive Tract Cancer with Special Reference to Helicobacter pylori infection. Front Microbiol. 2019;10:846. doi: 10.3389/fmicb.2019.00846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Toole G, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annu Rev Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 37.Cole SP, Harwood J, Lee R, She R, Guiney DG. Characterization of monospecies biofilm formation by Helicobacter pylori. J Bacteriol. 2004;186(10):3124–32. doi: 10.1128/JB.186.10.3124-3132.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Odenbreit S, Faller G, Haas R. Role of the alpAB proteins and lipopolysaccharide in adhesion of Helicobacter pylori to human gastric tissue. Int J Med Microbiol. 2002;292(3–4):247–56. doi: 10.1078/1438-4221-00204. [DOI] [PubMed] [Google Scholar]
- 39.Zhang ZW, Dorrell N, Wren BW, Farthing MJG. Helicobacter pylori a dherence to gastric epithelial cells: a role for non-adhesin virulence genes. J Med Microbiol. 2002;51(6):495–502. doi: 10.1099/0022-1317-51-6-495. [DOI] [PubMed] [Google Scholar]
- 40.Alm RA, Bina J, Andrews BM, Doig P, Hancock RE, Trust TJ. Comparative genomics of Helicobacter pylori: analysis of the outer membrane protein families. Infect Immun. 2000;68(7):4155–68. doi: 10.1128/IAI.68.7.4155-4168.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.König IR. Validation in Genetic Association Studies. Brief Bioinform. 2011;12(3):253–8. doi: 10.1093/bib/bbq074. [DOI] [PubMed] [Google Scholar]
- 42.Jones JM, Grinberg I, Eldar A, Grossman AD. A mobile genetic element increases bacterial host fitness by manipulating development. Elife. 2021;10:e65924. doi: 10.7554/eLife.65924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Devaraj A, Buzzo JR, Mashburn-Warren L, Gloag ES, Novotny LA, Stoodley P, et al. The extracellular DNA lattice of bacterial biofilms is structurally related to Holliday junction recombination intermediates. Proc Natl Acad Sci U S A. 2019;116(50):25068–77. doi: 10.1073/pnas.1909017116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yonezawa H, Osaki T, Hanawa T, Kurata S, Ochiai K, Kamiya S. Impact of Helicobacter pylori biofilm formation on clarithromycin susceptibility and generation of resistance mutations. PLoS ONE. 2013;8(9):e73301. doi: 10.1371/journal.pone.0073301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Power RA, Parkhill J, de Oliveira T. Microbial genome-wide association studies: lessons from human GWAS. Nat Rev Genet. 2017;18(1):41–50. doi: 10.1038/nrg.2016.132. [DOI] [PubMed] [Google Scholar]
- 46.Ansari S, Kabamba ET, Shrestha PK, Aftab H, Myint T, Tshering L, et al. Helicobacter pylori bab characterization in clinical isolates from Bhutan, Myanmar, Nepal and Bangladesh. PLoS ONE. 2017;12(11):e0187225. doi: 10.1371/journal.pone.0187225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Odeyemi OA. Microtiter plate assay methods of classification of bacterial biofilm formation. Food Control. 2017(73):245–6.
- 48.Reiter KC, Villa B, da Silva Paim TG, de Oliveira CF, d’Azevedo PA. Inhibition of biofilm maturation by linezolid in meticillin-resistant Staphylococcus epidermidis clinical isolates: comparison with other drugs. J Med Microbiol. 2013;62(3):394–9. doi: 10.1099/jmm.0.048678-0. [DOI] [PubMed] [Google Scholar]
- 49.Price MN, Dehal PS, Arkin AP. FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS ONE. 2010;5(3):e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Argimón S, Abudahab K, Goater RJ, Fedosejev A, Bhai J, Glasner C et al. Microreact: visualizing and sharing data for genomic epidemiology and phylogeography. Microb genomics. 2016;2(11): e000093. [DOI] [PMC free article] [PubMed]
- 51.Hunt M, Mather AE, Sánchez-Busó L, Page AJ, Parkhill J, Keane JA et al. ARIBA: rapid antimicrobial resistance genotyping directly from sequencing reads. Microb genomics. 2017;3(10). [DOI] [PMC free article] [PubMed]
- 52.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput biology: J Comput Mol cell biology. 2012;19(5):455–77. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30(14):2068–9. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 55.Page AJ, Taylor B, Delaney AJ, Soares J, Seemann T, Keane JA, et al. Rapid efficient extraction of SNPs from multi-FASTA alignments. Microb genomics. 2016;2(4):e000056. doi: 10.1099/mgen.0.000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All genome data were stored in Genbank with BioProject accession PRJDB11821 (Available at: https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJDB11821).