Abstract
Virulence mechanisms typically evolve through the continual interaction of a pathogen with its host. In contrast, it is poorly understood how environmentally acquired pathogens are able to cause disease without prior interaction with humans. Here, we provide experimental evidence for the model that Legionella pathogenesis in humans results from the cumulative selective pressures of multiple amoebal hosts in the environment. Using transposon sequencing, we identify Legionella pneumophila genes required for growth in four diverse amoebae, defining universal virulence factors commonly required in all host cell types and amoeba-specific auxiliary genes that determine host range. By comparing genes that promote growth in amoebae and macrophages, we show that adaptation of L. pneumophila to each amoeba causes the accumulation of distinct virulence genes that collectively allow replication in macrophages and, in some cases, leads to redundancy in this host cell type. In contrast, some bacterial proteins that promote replication in amoebae restrict growth in macrophages. Thus, amoebae-imposed selection is a double-edged sword, having both positive and negative impacts on disease. Comparing the genome composition and host range of multiple Legionella species, we demonstrate that their distinct evolutionary trajectories in the environment have led to the convergent evolution of compensatory virulence mechanisms.
The evolution of human pathogens is largely dictated by their replication in humans1, with adaptations resulting in a fitness advantage being inherited by progeny. In contrast, it is poorly understood how environmental pathogens acquire virulence mechanisms allowing them to cause disease despite no previous interaction with humans.
Legionella pneumophila is an intracellular bacterial pathogen2 and ubiquitous in the environment3,4. Following inhalation of contaminated water aerosols, L. pneumophila replicates within alveolar macrophages5. Importantly, L. pneumophila is not transmitted from person to person6 and thus has not evolved virulence mechanisms through consecutive passage in humans. While exposure can lead to pneumonia7, this typically occurs in the elderly and individuals who are immunocompromised or on immunosuppressive therapy8,9. Thus, L. pneumophila presumably acquires the necessary virulence factors to cause disease before encountering a human host, but they are only effective when the immune system is impaired.
L. pneumophila replication in macrophages depends on the type IVb secretion system Dot/Icm10,11, which translocates over 300 L. pneumophila proteins into the host cell12–14. While the Dot/Icm system is absolutely required for virulence15,16, the absence of individual Icm/Dot translocated substrates (IDTS) rarely impacts intracellular growth17, which is largely due to redundancy among these proteins18,19. For example, a L. pneumophila mutant lacking 31% of its IDTS is not defective for growth in cultured macrophages20. Thus, L. pneumophila replication in macrophages is mediated by redundant virulence mechanisms that were acquired in the environment.
In nature, amoebae and ciliated protozoa devour bacteria for food21. In contrast, L. pneumophila has established mechanisms to survive and replicate within these organisms20,22,23, leading to the model that the interaction of Legionella spp. with amoebae provides a training ground for the evolution of virulence strategies necessary for growth in macrophages and thus their ability to cause disease in humans20,22–28.
Using a genetic screening strategy, we identify L. pneumophila genes required for growth in four diverse amoebal hosts, with each amoeba responsible for the acquisition and maintenance of distinct sets of genes. We show that L. pneumophila growth in macrophages results from the cumulative selective pressures of multiple amoebal hosts, which in some cases lead to redundancy among virulence proteins, but also imposes constraints on pathogen evolution by selecting for factors that inadvertently restrict growth in macrophages. Taken together, our work demonstrates that individual Legionella species have independently evolved distinct but compensatory virulence mechanisms as a result of their differential adaptations to amoebal predation.
Results
To identify genes required for L. pneumophila growth in amoebae, a library of L. pneumophila transposon mutants was used to individually challenge four amoebal hosts: Acanthamoeba castellanii, Acanthamoeba polyphaga, Hartmannella vermiformis and Naegleria gruberi. These amoebae support L. pneumophila replication in the environment29, are highly prevalent in contaminated water sources responsible for outbreaks of disease30, and exhibit varying degrees of phylogenetic diversity as A. castellanii and A. polyphaga are members of the same genus, H. vermiformis is in a separate class and N. gruberi belongs to a different phylum31. Amoebae were challenged for 24 h, equivalent to a single round of infection (Extended Data Fig. 1), and the fitness contribution of individual genes was determined by transposon sequencing (Tn-seq)32.
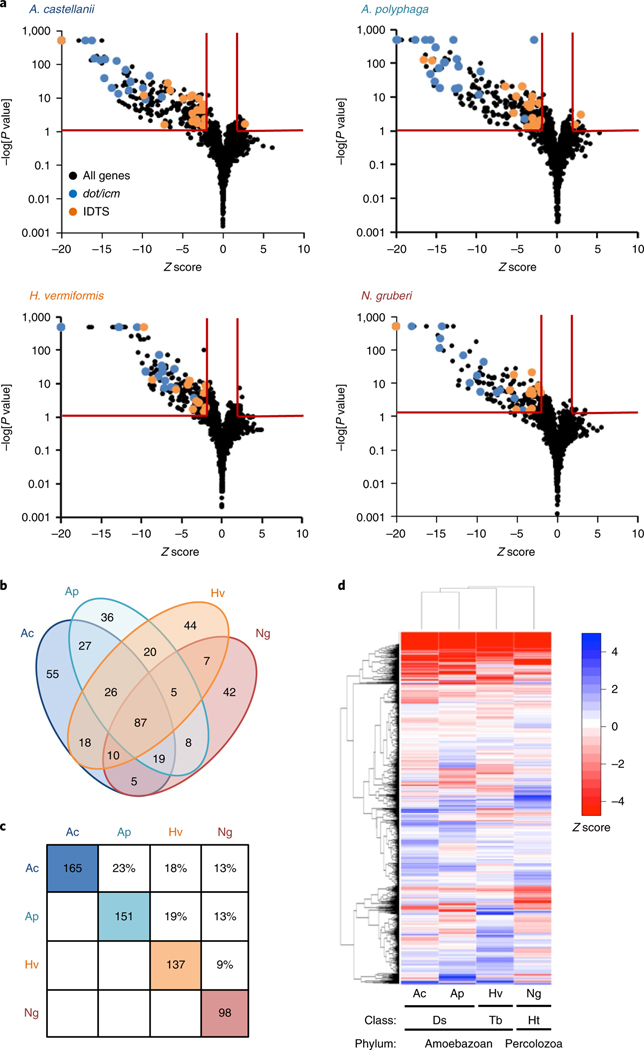
Tn-seq identified 253, 239, 225 and 186 genes as important for L. pneumophila growth in A. castellanii, A. polyphaga, H. vermiformis and N. gruberi, respectively (Fig. 1a and Supplementary Table 1). Eighty-seven genes were required for growth in all amoebae examined (Fig. 1b and Supplementary Table 1) including 18 dot/icm genes, consistent with the critical role of the Dot/Icm translocon15,16. The impact of mutations in most genes, however, was host-specific, with 145 genes required in a subset of amoebae and 177 genes required for growth in only a single host (Fig. 1b). Overall, the gene requirements and fitness profiles between amoebae correlated with their phylogenetic relatedness (Fig. 1c,d). The host-specific requirements for the majority of L. pneumophila genes demonstrate distinct differences between these amoebae that necessitate specific genes for survival and replication.
Fig. 1 |. Tn-seq identifies L. pneumophila genes required for growth in diverse amoebal hosts.
a, Volcano plots62 of the fitness scores (Z scores) of L. pneumophila genes for replication in A. castellanii (Ac), A. polyphaga (Ap), H. vermiformis (Hv) and N. gruberi (Ng) as determined by Tn-seq (Methods). Genes were considered to provide a fitness advantage or disadvantage if the Z score was >2 s.d. from the population mean and was statistically significant based on a two-tailed Student t-test P < 0.05 after Welch modification and Benjamini–Hochberg correction (indicated by red lines) (Supplementary Table 1). Data are based on n = 8 biological replicates of two technical replicates each. dot/icm and Icm/Dot translocated substrate (IDTS) genes that pass these criteria are highlighted. Genes with P = 0 were assigned P = 10−500. b, Common and host-specific gene requirements for L. pneumophila growth in four amoebal hosts. c, Pairwise comparisons of L. pneumophila genes identified by Tn-seq define variable overlap between amoebal hosts. Coloured boxes indicate the number of genes identified as important in the indicated amoebal host when disrupted in a WT strain background, excluding universal genes required in all amoebae. Percentages indicate the percent overlap between paired sets of genes. d, The fitness profile of all L. pneumophila genes examined correlates with the phylogenetic distance of individual amoebal hosts. The hierarchical cluster analysis is based on the fitness scores of 2,069 L. pneumophila genes in A. castellanii, A. polyphaga, H. vermiformis and N. gruberi. Z < −5 or Z > 5 were truncated to −5 and 5, respectively. Ds, Discosea; Tb, Tubulinea; Hb, Heterolobosea.
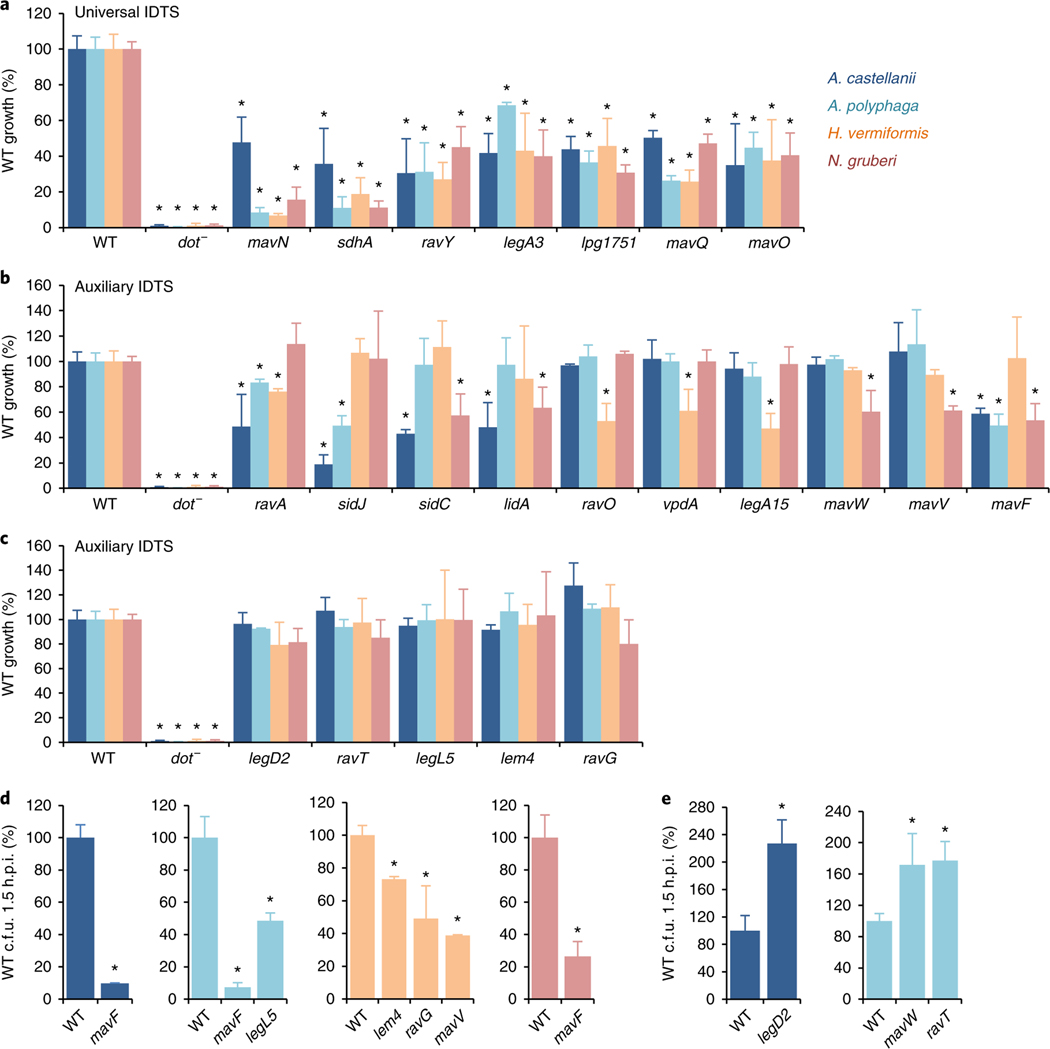
Of the genes identified as important for growth in at least one amoebal host, 44 encoded IDTS (Fig. 1a and Supplementary Table 1). In contrast, individual loss of only four IDTS (MavN/IroT, SdhA, RavY or Lpg2505) severely impairs L. pneumophila growth in macrophages33–36. To confirm the impact of individual IDTS on survival and/or replication in amoebae, null mutants for 22 of the 44 IDTS genes were constructed and their phenotypes were examined. Sixteen null mutants had impaired intracellular growth in at least one amoebal host (Fig. 2a–c). The phenotypes were specific as each mutant grew as well as the wild-type (WT) strain in bacteriological medium (Extended Data Fig. 2a) and mutations in IDTS genes predicted to be neutral did not impair bacterial growth in any host (Extended Data Fig. 2b). For mavB, the phenotype predicted by Tn-seq was due to polar effects of the transposon insertions on lpg1751 (Extended Data Fig. 2c), deletion of which impaired growth in all amoebae (Fig. 2a). For genes encoded in putative operons, in trans complementation demonstrated the phenotypes were due to deletion of the indicated gene (Extended Data Fig. 2d,e). Five IDTS mutants showed reduced numbers 1.5 h after challenging host cells compared to the WT strain (Fig. 2d), indicative of either reduced uptake and/or defects in avoiding digestion. In contrast, three IDTS mutants exhibited increased numbers at 1.5 h (Fig. 2e) but the number of bacteria at 24 h was similar to the WT strain (Fig. 2b,c) suggesting a subpopulation of bacteria failed to generate a replication compartment, kinetic defects in replication vacuole formation, and/or reduced numbers of bacterial divisions per vacuole. These data define several IDTS genes that are differentially required to establish growth in four environmental hosts of L. pneumophila and distinguish IDTS that are redundant in macrophages.
Fig. 2 |. universal and auxiliary IDTS define the host range of L. pneumophila.
a, Phenotypic analysis of L. pneumophila IDTS mutants identify a set of universal IDTS genes important for growth in all four amoebal hosts examined. b, Intracellular growth defects of a subset of IDTS mutants define auxiliary IDTS genes that are important for growth in only one or a subset of amoebal hosts. c, A subset of IDTS mutants predicted by Tn-seq to exhibit a fitness defect are not impaired for intracellular replication. In a–c, WT, a Dot/Icm translocation deficient (dot−) and the indicated IDTS null mutant strains were used to challenge amoebae and bacterial growth, based on recovered c.f.u. on solid media from lysed host cells. They were assessed after 24 h, equivalent to a single round of infection. Plotted is the total bacterial yield 24 h post-infection (h.p.i.) normalized to the WT strain by the number of intracellular bacteria 1–2 h.p.i. d, A subset of IDTS mutants show reduced numbers of intracellular bacteria after challenging host cells, demonstrating defects in uptake and/or survival at early stages of the infection cycle. e, IDTS mutants showing increased numbers of intracellular bacteria 1.5 h after challenging host cells, indicative of enhanced uptake and/or survival following internalization. d,e, The WT and indicated null mutant strains were used to challenge amoebae for 1.5 h. Cells were then treated with gentamycin to kill extracellular bacteria and internal bacteria were enumerated based on recovered c.f.u. from host cell lysates plated on bacteriological medium. Data in a–e are the mean ± s.d. of 2–8 biological replicates, each generated from three technical replicates; *P < 0.05, one-way analysis of variance (ANOVA), relative to the WT strain (Source Data for Fig. 2).
Comparing the behaviour of IDTS null mutants in amoebae revealed two phenotypic trends: IDTS important for growth in all amoebae (Fig. 2a) and IDTS important in only one or a subset of amoebae (Fig. 2b–e), designated universal and auxiliary IDTS, respectively. Universal IDTS are predicted to perform essential functions required for growth in all host cell types. Consistent with this, deletion of sdhA or ravY in the L. pneumophila strains 130b and Paris, or deletion of mavN in strain Paris, impaired replication in all four amoebae (Extended Data Fig. 3), and these IDTS are required for growth in macrophages33–36. In contrast, auxiliary IDTS are likely to allow L. pneumophila to cope with different host restrictions and are thus critical determinants of the broad host range of this pathogen.
Given that humans are accidental hosts of L. pneumophila, it is commonly hypothesized that genes employed by L. pneumophila to replicate in macrophages are acquired to combat predation by amoebae20,22–28. To test this, we examined the importance of the universal and auxiliary IDTS for growth in human monocyte-derived macrophages (U937 cells). Interestingly, all 7 universal IDTS were required to varying degrees for growth within macrophages (Fig. 3a), demonstrating they perform key functions and/or exploit common host cell targets between amoebae and macrophages. Conversely, 14/15 auxiliary IDTS were dispensable in U937 cells (Fig. 3b,c). Similar phenotypes were observed for 18/22 (82%) of the IDTS mutants when examined in primary murine macrophages (Extended Data Fig. 4a,b). Intriguingly, the ΔvpdA, ΔravG and ΔmavW mutants showed enhanced replication in macrophages (Fig. 3b) despite impaired growth in H. vermiformis or N. gruberi (Fig. 2b–e), suggesting their presence restricts replication in macrophages. Thus, the interaction between L. pneumophila and amoebae simultaneously selects for IDTS with beneficial and detrimental effects in macrophages.
Fig. 3 |. IDTS genes selected for in amoebae both promote and restrict L. pneumophila replication in macrophages.
a, Universal IDTS genes are important for optimal L. pneumophila replication in cultured U937 cells. b, Auxiliary IDTS genes are dispensable for L. pneumophila growth in macrophages and, in some cases, restrict L. pneumophila intracellular replication, as mutations in a subset of IDTS genes result in enhanced bacterial growth relative to the WT strain. c, Auxiliary IDTS genes that exhibit altered uptake and/or survival in amoebae generally do not show similar phenotypes in macrophages. The WT and indicated IDTS null mutant strains were used to challenge U937 cells for 1 h. Cells were treated with gentamycin to kill extracellular bacteria and internal bacteria were enumerated based on c.f.u. recovered from host cell lysates plated on bacteriological medium. d, Amoebae-specific IDTS genes are individually dispensable for growth in macrophages but, when deleted in combination, severely impair L. pneumophila intracellular growth. In a,b,d, the WT, dot− and indicated IDTS null mutant strains were used to challenge U937 cells and bacterial growth, based on recovered c.f.u. on solid media from lysed host cells, were monitored 24 h.p.i. and normalized to the WT strain by the number of intracellular bacteria 1 h.p.i. Data are the mean ± s.d. of 2–4 biological replicates, each generated from three technical replicates; *P < 0.05, one-way ANOVA, relative to the WT strain (Source Data for Fig. 3).
One explanation for the lack of phenotypes for certain IDTS mutants in macrophages is that IDTS acquired for growth in amoebae perform compensatory roles in macrophages, thereby resulting in the accumulation of redundant IDTS in higher eukaryotes. Consistent with this idea, 20 of the auxiliary IDTS genes identified in this study as important for growth in at least one amoebal host were shown in a previously published insertional mutagenesis and depletion (iMAD) study to contribute redundant functions for L. pneumophila growth within macrophages18. To test whether auxiliary IDTS are a source of redundancy in macrophages, we examined the effects of deleting pairs of auxiliary IDTS belonging to different iMAD-defined functional groups. lidA was important for growth in A. castellanii and N. gruberi, whereas ravO and vpdA were important for growth in H. vermiformis (Fig. 2b). While all three genes were individually dispensable for growth in U937 cells, replication of ΔlidAΔravO and ΔlidAΔvpdA double mutants was impaired in these cells (Fig. 3d). Similar results were observed in primary murine macrophages (Extended Data Fig. 4c). Thus, the combined selective pressures of distinct amoebal hosts can drive the accumulation of IDTS that are redundant in macrophages, and the identification of amoeba-specific IDTS genes provide a targeted approach to identify sources of this redundancy.
Although the Dot/Icm translocon and its substrates play a central role in Legionella pathogenesis, they comprised only 11% of the genes identified as important for L. pneumophila growth in amoebae. The remaining genes represented numerous cellular processes including transcriptional regulation, signal transduction, transport, cell envelope biosynthesis, protein synthesis, processing, export and degradation, and nucleotide, carbohydrate, amino acid, lipid and vitamin metabolism and acquisition (Supplementary Table 1). For example, transposon insertions in biotin and thiamin biosynthetic genes caused reduced fitness in the Acanthamoeba sp. and N. gruberi, respectively, while neither affected L. pneumophila growth in H. vermiformis. Consistent with this, a L. pneumophila mutant lacking the biotin biosynthetic operon ΔbioABFHD (Δbio) was severely impaired for replication in A. castellanii but grew as well as the WT strain in H. vermiformis and N. gruberi (Fig. 4a,b and Extended Data Fig. 5). Conversely, a thiamin biosynthetic operon mutant ΔthiOGDEF (Δthi) exhibited impaired replication in N. gruberi but not A. castellanii or H. vermiformis (Fig. 4c,d). In contrast, both biosynthetic capabilities were required for optimal bacterial growth in U937 cells. Both the WT and Δbio strains exhibited reduced growth in macrophages cultured in medium depleted of biotin, with the Δbio mutant having a more severe defect (Fig. 4e). The Δthi mutant showed reduced growth relative to the WT strain in complete medium while both strains showed reduced growth in thiamin-depleted medium (Fig. 4f). Thus, two biosynthetic pathways differentially required for L. pneumophila growth in amoebae are collectively important for replication in macrophages. These results demonstrate that the combined selective pressures of distinct amoebal hosts drive the evolution of this human pathogen.
Fig. 4 |. L. pneumophila virulence in macrophages results from the combined selective pressures of multiple amoebal hosts.
a, The L. pneumophila biotin biosynthetic operon bioABFHD (bio) is important for growth in A. castellanii but not H. vermiformis or N. gruberi. b, The Δbio mutant growth defect in A. castellanii can be rescued by supplementing the amoebal culture medium with biotin. c, The L. pneumophila thiamin biosynthetic genes thiOGDEF (thi) support bacterial replication in N. gruberi but are dispensable for growth in A. castellanii and H. vermiformis. d, Exogenous addition of thiamin to the amoebal culture medium restores growth of the Δthi mutant in N. gruberi. e, The L. pneumophila biotin biosynthetic operon is required for optimal growth in U937 cells. f, De novo biosynthesis of thiamin is important for L. pneumophila replication in U937 cells. In a–f, the indicated L. pneumophila strains were used to challenge host cells in the appropriate amoebal infection medium (Methods) or in the presence (+) or absence (−) of biotin or thiamin, as indicated. Intracellular growth, based on recovered c.f.u. from cell lysates, was monitored 24 h.p.i. and normalized to the WT strain by the number of intracellular bacteria 1–2 h.p.i. Data are the mean ± s.d. of 2–4 biological replicates, each generated from three technical replicates; *P < 0.01, one-way ANOVA, relative to the WT strain (Source Data for Fig. 4). g, Left: Sets of genes important for L. pneumophila replication in each amoebal host compared to those identified as important in U937 cells. Right: Venn diagram of the genes overlapping between each pair of data sets (blue box) demonstrate common and amoebaspecific requirements for genes that promote growth in macrophages. h, Lack of complete overlap between L. pneumophila genes important for growth in macrophages (grey) and the collective set of genes required in amoebae (green) suggests that additional amoebal hosts contribute to the evolution of L. pneumophila as a human pathogen.
To examine the contribution of individual amoebae in enabling L. pneumophila to replicate in macrophages, genes important for growth in amoebae were compared with the 265 genes identified as important for growth in U937 cells (Methods) (Supplementary Table 1). Of the 231 genes with fitness scores in amoebae and macrophages, 104 genes were important for growth in macrophages and at least one amoebal host, with similar overlap with all four amoebae (Fig. 4g). Forty-seven genes were universal genes defined in amoebae demonstrating critical functions in both amoebae and mammalian cells. Importantly, the remaining 57 genes were amoeba-specific, thus genes specific to each amoebal host were important for replication in macrophages. However, not all genes important in macrophages were important in amoebae (Fig. 4h). Given the extensive host range of L. pneumophila, it is likely that these 127 macrophage-specific genes were selected for in other protozoan hosts22,23. Collectively, these data demonstrate that the ability of L. pneumophila to grow in macrophages results from the combined selective pressures of multiple amoebal hosts.
The Legionella genus is comprised of over 60 species (www.bacterio.net/legionella.html and www.specialpathogenslab.com/legionella-species.php) with tremendous genetic diversity37,38. To determine whether genome composition was predictive of host range, the growth of 13 Legionella species in A. castellanii, H. vermiformis and N. gruberi was compared with that of L. pneumophila. The different species encode distinct sets of predicted IDTS, are genetically and phylogenetically diverse37 and represent environmental, potable water and clinical isolates (Supplementary Table 2). All 13 species lacked homologues of a subset of the genes (6–14%) important for L. pneumophila growth in each amoebal host (Supplementary Tables 1 and 3). Consistent with this, all 13 species showed marked growth defects in N. gruberi (Fig. 5a) and 5 of these (L. shakespearei, L. tucsonensis, L. anisa, L. dumoffii and L. cherrii) were defective for growth in all three amoebal hosts (Fig. 5a and Extended Data Fig. 6). In contrast, 7 species grew as well as L. pneumophila in A. castellanii and/or H. vermiformis (Fig. 5a). These species must encode genes that compensate for the lack of L. pneumophila virulence genes, either genes absent from the L. pneumophila genome or genes that are differentially regulated. For example, although defective for growth in N. gruberi, L. feeleii replication in A. castellanii and H. vermiformis was comparable to L. pneumophila despite lacking 11% and 14% of the genes that support L. pneumophila growth in these two hosts, including the universal IDTS ravY, sdhA and lpg1751 (Fig. 2a). Introducing ravY into L. feeleii increased its replication in N. gruberi (Fig. 5b). Thus, L. feeleii has a gene that can compensate for lack of ravY in A. castellanii and H. vermiformis but not N. gruberi, suggesting that the efficacy of compensatory genes depends on the host cell type. Collectively, these results demonstrate that individual species employ different sets of proteins for growth in common amoebal hosts.
Fig. 5 |. individual species of Legionella employ different sets of genes for replication in amoebae and macrophages.
a, Growth of 13 Legionella species in A. castellanii, H. vermiformis and N. gruberi. Light coloured bars indicate species that grow as well as L. pneumophila and dark coloured bars indicate a growth defect. The relative growth of a L. pneumophila dot− strain is indicated by a grey dotted line. Species are classified into four phenotypic groups (groups I, II, III and IV) (top) based on the presence or absence of growth defects across the three amoebal hosts compared to L. pneumophila: group I, species defective for growth in all three amoebal hosts; group II, species that grew as well as L. pneumophila in A. castellanii and H. vermiformis but not N. gruberi; group III, species that grew robustly in A. castellanii but not H. vermiformis or N. gruberi; and group IV, species that grew in H. vermiformis but were impaired in A. castellanii and N. gruberi. The phylogenetic tree of the 13 Legionella species (bottom) was generated based on a set of 78 orthologous groups defined previously37 (see Methods). b, Ectopic expression of ravY from the self-replicating vector pJB1806 (pravY) in L. feeleii increases intracellular growth in N. gruberi. c, Replication of 13 Legionella species in U937 cells. Grey bars indicate species that grow as well as L. pneumophila, black bars indicate a growth defect and light grey bars indicate increased growth compared to L. pneumophila. d, Ectopic expression of ravG from pJB1806 (pravG) in L. dumoffii results in increased bacterial numbers in H. vermiformis 1 h.p.i. e, Ectopic expression of vpdA from pJB1806 (pvpdA) in L. dumoffii increases intracellular replication in H. vermiformis. In a,b,c,e, intracellular growth, based on recovered c.f.u. from cell lysates, was monitored 24 h.p.i. and normalized to L. pneumophila (a,c), L. feeleii (b) or L. dumoffii (e) harbouring empty vector by the number of intracellular bacteria 1 h.p.i. In d, H. vermiformis was challenged with the indicated strains for 1.5 h, treated with gentamycin to kill extracellular bacteria and then internal bacteria were enumerated based on recovered c.f.u. from host cell lysates plated on bacteriological medium. Data are the mean ± s.d. of 2–6 biological replicates, each consisting of three technical replicates; *P < 0.05, one-way ANOVA relative to L. pneumophila (a,c), or relative to L. feeleii (b) or L. dumoffii (d,e) harbouring the empty vector (Source Data for Fig. 5).
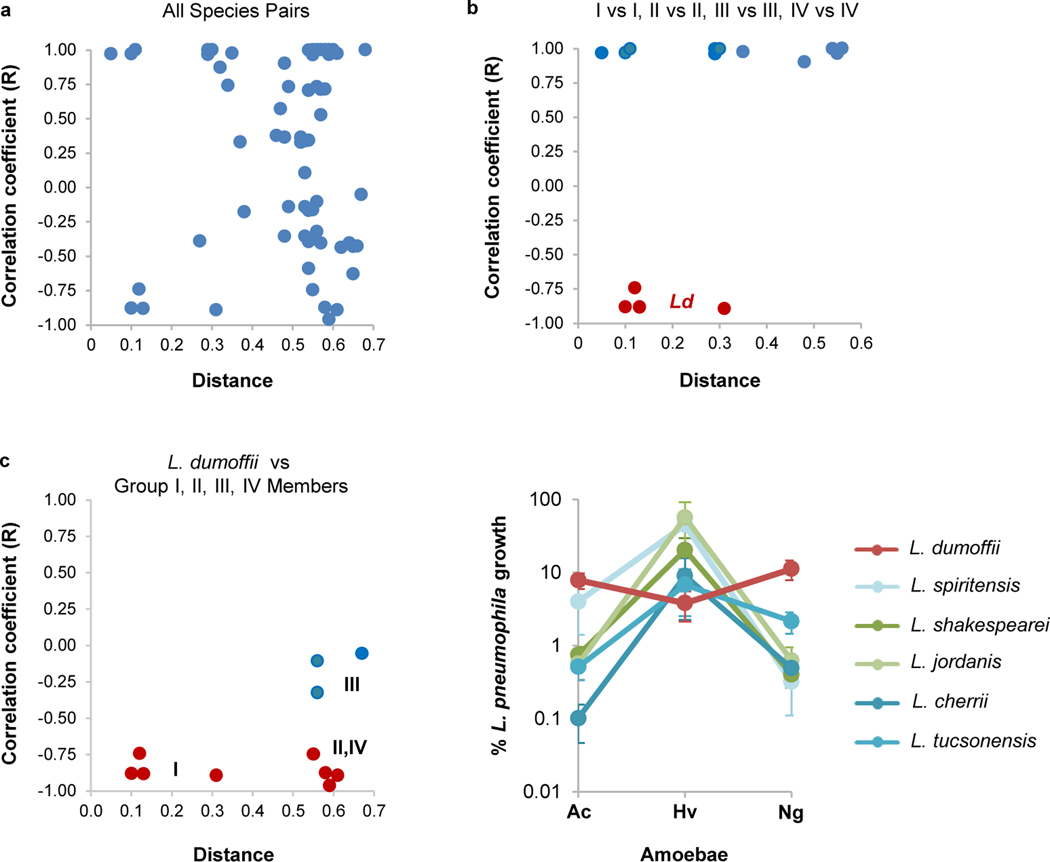
Based on their phenotypes in amoebae, Legionella species were classified into four distinct phenotypic groups (Fig. 5a). Species with defects in all three amoebae (group I) partitioned to one of two major clades in a phylogenetic tree, whereas species exhibiting robust growth in at least one amoeba clustered to the other major clade (groups II, III and IV). Within this clade, however, members of groups II, III and IV were randomly dispersed compared to their phylogenetic clustering (Fig. 5a and Extended Data Fig. 7a). Since L. pneumophila genes important for growth in amoebae support growth in macrophages, we examined whether fitness in amoebae was indicative of the ability to grow in U937 cells. Of the 13 species analysed, 5 exhibited robust replication in macrophages comparable to or better than L. pneumophila, while the other 7 species showed reduced growth (Fig. 5c). Species with similar levels of growth did not partition to any particular phenotypic group (Fig. 5a). Thus, fitness profiles in three prevalent environmental hosts of Legionella were insufficient to predict growth in macrophages. Interestingly, L. dumoffii showed enhanced replication in macrophages compared to L. pneumophila (Fig. 5c). L. dumoffii also exhibited the opposite fitness profile of other species in amoebae, with reduced growth in H. vermiformis relative to A. castellanii and N. gruberi (Extended Data Fig. 7b–d). These observations suggest a possible inverse relationship between fitness in H. vermiformis and macrophages. For example, vpdA and ravG are both important for L. pneumophila growth in H. vermiformis (Fig. 2) but restrict its replication in macrophages (Fig. 3b). Notably, L. dumoffii lacks homologues of both genes (Supplementary Table 1). Introducing ravG or vpdA into L. dumoffii resulted in increased numbers of bacteria 1 h.p.i. (Fig. 5d) and replication (Fig. 5e), respectively, in H. vermiformis, consistent with their roles in L. pneumophila (Fig. 2b,d). This was specific as ravO (also required for L. pneumophila growth in H. vermiformis (Fig. 2b) and absent in L. dumoffii) had no effect on either phenotype (Fig. 5d,e). Thus, L. dumoffii lacks genes important in H. vermiformis, possibly due to lack of exposure to or predation by this amoeba in the environment, which may inadvertently enhance its virulence in macrophages.
Collectively, our results demonstrate that, as a consequence of its passage through multiple amoebae, L. pneumophila has accrued distinct virulence genes that ultimately allow critical expansion of its host range to human macrophages. The acquisition of different sets of virulence factors through adaptations to different combinations of amoebae has led to the convergent evolution of distinct but compensatory virulence mechanisms in individual Legionella species. While some adaptations promote growth in macrophages, others are restrictive, therefore necessitating specific combinations of amoebae-imposed selective pressures and bacterial adaptations to enable the transition from environmental reservoirs to humans (Extended Data 8).
Discussion
L. pneumophila is an environmental organism that is not transmitted from human to human. Thus, it is often speculated that the selective pressure for virulence traits occurs during its encounters with protozoa. These selective forces are multifactorial as L. pneumophila must adapt to both the restrictive environment of individual protozoan hosts20,23 and diverse protozoan populations. Thus, the fitness of L. pneumophila in natural reservoirs depends on its ability to both expand and maintain its virulence gene repertoire.
Here we demonstrate that L. pneumophila growth in different amoebal hosts requires different combinations of genes. We identified seven IDTS that are required for optimal growth in four amoebal hosts (Fig. 2). These universal IDTS are likely to perform vital functions—for example, MavN/IroT mediates acquisition of the essential metal iron33. Not surprisingly, these IDTS were each important for growth in macrophages (Fig. 3). In parallel, the roles of a larger set of 37 IDTS were amoebae-specific. These auxiliary IDTS allow L. pneumophila to adapt to differences between amoebae, such as nutrient availability and/or the presence or absence of specific cellular proteins or pathways. Remarkably, these 37 auxiliary IDTS constitute only 15% of the complete IDTS repertoire of L. pneumophila, suggesting that the remaining IDTS play key roles in other protozoan hosts of this bacterium22.
Through comparative analyses between hosts, we provide evidence that the ability of L. pneumophila to replicate in macrophages arises from its interaction with amoebae in the environment. This cannot be achieved by the adaptation to a single or even a subset of closely related amoebae, but instead requires a compilation of genes selected for by growth within multiple, diverse hosts. At the same time, the transition from amoebae to macrophage hosts requires appropriate combinations of amoebae-imposed selective pressures and bacterial adaptations, as some selective pressures can have adverse effects on L. pneumophila fitness in macrophages (Figs. 2e and 3b). For example, deletion of vpdA, mavW or ravG reduced L. pneumophila growth in amoebae but enhanced replication in macrophages (Figs. 2 and 3). Similar increases in L. pneumophila replication in macrophages have been reported for strains lacking LegS2 and LegC4, the activities of which enhance the innate immune response36,39. This phenomenon is not unique to IDTS. Lysine prototrophy is required for L. pneumophila growth in amoebae but restricts replication in macrophages40, probably by impacting production of peptidoglycan, an antigen of the innate immune system. Selective pressures in amoebae that ensure the maintenance of virulence factors that trigger an immune response may contribute to the inability of L. pneumophila to cause disease unless the immune system is impaired. Thus, amoebae can have both positive and negative effects on the evolution of a human pathogen, by selecting for genes that both promote and restrict growth in macrophages.
Virulence gene repertoires sufficient for growth in amoebae and macrophages are exceedingly complex and highly combinatorial (Extended Data 6). For example, the Legionella pan-genome encompasses as many as 1,600 predicted IDTS, of which only 8 core IDTS are conserved across 58 sequenced species37,38. Since diverse Legionella species overlap in host range (Fig. 5), individual species must have assembled different combinations of virulence factors for growth in common amoebal hosts and, as an inadvertent consequence, macrophages. This is supported by a study that recently reported tremendous genetic diversity among Legionella species able to replicate in human monocytes38. Moreover, of the 8 core IDTS, only 2 were identified here as universal IDTS in L. pneumophila, suggesting that other species employ alternate IDTS with compensatory functions.
The accumulation of diverse repertoires of virulence proteins, including those that promote growth in macrophages, is likely to be driven by variations in the types of amoebae and the frequency with which they are encountered in the environment. In contrast, some species may have limited exposure to certain amoebae, thereby resulting in a virulence gene repertoire insufficient for replication in macrophages, or inadvertently increasing the pathogenic potential of the bacterium by limiting the accumulation of genes that restrict growth in macrophages. Thus, the combined selective pressures of diverse amoebal hosts shape and drive the emergence of a human pathogen from environmental reservoirs.
Methods
Bacterial and cell culture conditions.
All L. pneumophila strains and Legionella species (Supplementary Tables 2 and 4) were cultured in ACES (N-(2-acetamido)-2-aminoethanesulfonic acid) buffered yeast extract (AYE) bacteriological medium or on solid charcoal buffered yeast extract agar medium (CYE), supplemented with 0.4 g l−1 iron(III) nitrate, 0.135 g l−1 cysteine41 and 0.1 mg ml−1 thymidine for thy− auxotroph strains and, when appropriate, 40 μg ml−1 kanamycin or 5% (w/v) sucrose. For growth of strains in amoebae, the chromosomal thyA− allele was replaced with thyA+ by allelic exchange using pJB3395 (a gift from J. Vogel, Washington University) as described previously42. E. coli strains were cultured in LB medium supplemented with 50 μg ml−1 ampicillin or 50 μg ml−1 kanamycin when appropriate.
A. castellanii (ATCC 30234) and A. polyphaga (ATCC 30461) were cultured in PYG medium (ATCC 712) at 25 °C. H. vermiformis (ATCC 50237) and N. gruberi (ATCC 30224) were grown in modified PYNFH medium (ATCC 1034) at 35 °C and 25 °C, respectively. Human monocyte-derived U937 cells (ATCC CRL-1593.2)43 were cultured in RPMI 1640 medium containing 10% fetal bovine serum and terminally differentiated by treating with 16 nM 12-O-tetradecanoylphorbol-13-acetate (TPA) for 48 h before challenge with Legionella. Eukaryotic cells were acquired from the American Type Culture Collection (ATCC) and authenticated using the isoenzyme test and short tandem repeat profiling, and confirmed negative for mycoplasma by ATCC. Primary bone-marrow-derived macrophages from A/J mice were isolated and cultured as previously described15.
Construction of L. pneumophila transposon mutant library for Tn-seq.
L. pneumophila Philadelphia-1 strain Lp0244,45 thy+ was transformed with pTO100MmeI as described previously20. pTO100MmeI (a gift from R. Isberg, Tufts University School of Medicine) is a derivative of pTO10020, a mariner himar1-based transposon consisting of a kanamycin resistance cassette, in which an MmeI restriction sequence has been incorporated in the 3’ himar1 transposon sequence. pTO100MmeI was propagated in an E. coli DH5α λpir F+ strain expressing LacIq20. Twenty pools of 10,000 mutants each were harvested in AYE medium containing 20% (vol/vol) glycerol. Bacterial suspensions were adjusted to a final concentration of approximately 2 × 1010 c.f.u. ml−1 and stored at −80 °C.
Tn-seq screen.
Growth of the L. pneumophila transposon mutants in amoebae.
For A. castellanii and A. polyphaga, 2 × 106 cells were plated in PYG medium and incubated at 25 °C overnight. The PYG medium was replaced with Ac buffer46 and shifted to 37 °C 1 h before challenge with bacteria. For H. vermiformis and N. gruberi, 2 × 107 cells in PYNFH and 4 × 106 cells in Ac buffer, respectively, were plated and immediately challenged with Legionella at 35 °C and 37 °C, respectively. Eight of the L. pneumophila transposon mutant library pools, encompassing ∼80,000 mutants, were independently cultured in AYE at 37 °C to an absorbance at 600 nm (A600) = 3.8–4.0. Aliquots of bacterial cultures were plated on CYE medium containing kanamycin and incubated at 37 °C for 4 days (the input sample), or used to challenge amoebae at a multiplicity of infection (MOI) of 1 for 1 h (A. castellanii and A. polyphaga) or 2 h (H. vermiformis and N. gruberi) in duplicate. Cells were then rinsed twice with fresh medium and incubated at 37 °C for 24 h, equivalent to a single round of infection. The amoebae were then lysed with 0.05% saponin, and diluted lysates were plated on CYE containing kanamycin and incubated at 37 °C for 4 days (two independently generated output samples per input sample subsequently treated as technical replicates). For all input and output samples, approximately 100,000 c.f.u. were harvested into AYE medium and mixed to homogeneity. Genomic DNA was extracted from triplicate samples of 2 × 109 bacteria using a QIAGEN DNeasy Blood and Tissue kit, including proteinase K and lysozyme treatments, then pooled. Aliquots of each input and output sample were stored at −80 °C.
Transposon sequencing and data analysis.
Genomic DNA isolated from input and output samples were processed as previously described32. All samples from a single amoebal host were barcoded, pooled and sequenced using an Illumina HiSeq 2500 at the Tufts University Core Facility (TUCF), Tufts University School of Medicine. Data analysis was performed using analysis tools provided by the TUCF through Galaxy47 and have recently been published as the Tn-seq data analysis tool MAGenTA48. Briefly, sequence reads for each input and output sample were sorted based on barcodes, then trimmed to remove primer and transposon sequences. Sequences were then aligned to the L. pneumophila Philadelphia-1 genome (AE017354)44 using Bowtie for Illumina49. The number of aligned reads at each insertion site was quantified using the Calculate Fitness Score tool, in which corresponding input and output datasets are normalized by total reads before an output/input ratio is calculated for each insertion site. Transposon insertion sites located in the 5’- and 3’-terminal 20% of the open reading frame32 and/or with less than eight reads in the input sample were excluded from further analysis.
The mariner himar1 transposon inserts at TA dinucleotides, which appear on average every 13 nucleotides in the L. pneumophila Philadelphia-1 genome, resulting in roughly 30 insertions sites per 1 kb gene (the average size of a L. pneumophila open reading frame)20. Our collection of ∼80,000 analysed mutants encompasses 17,781 unique insertions sites of the 245,013 total TA dinucleotides in the genome (7% saturation). The number of unique insertion sites per gene ranged from 0 to 44 (Supplementary Table 1). Each gene was represented by an average of six distinct transposition insertion sites, and thus twelve fitness ratio measurements from duplicate technical replicates. Fitness scores were determined for 2,069 of the 2,810 (74%) protein-encoding genes in the genome. Of the 785 genes lacking transposon insertions, including 48 genes encoding ribosomal and transfer RNAs, 470 overlapped with the 597 genes (79%) lacking transposon insertions in an independently generated transposon mutant library to identify L. pneumophila genes important for survival and/or growth in nutrient rich bacteriological medium (Supplementary Table 1) using transposon site hybridization (TraSH)20. The remaining 315 genes were highly enriched for small open reading frames (<400 nucleotides), suggesting a lower probability of an insertion event due to size and incomplete saturation of the transposon mutant library.
Statistical analyses were performed using a custom script that incorporates established statistical analysis methods for TraSH20,50 and Tn-seq32,48 and is available through GitHub (OConnorLab/TnseqSA). Using this script, the log-converted output/input fitness ratios for each pooled library were zero mean normalized. Fitness ratios for individual insertion sites across each gene were averaged to give a mean fitness value, excluding outliers with a modified Z score51, MZi > 3.5. Fitness ratios were converted to Z scores (the number of standard deviations (s.d.) from the mean)52 for comparison between different host cell types. Gene disruptions were considered to provide a fitness advantage or disadvantage if the gene had a minimum of two insertions, or a single insertion represented in two or more library pools and a fitness score greater than 2 s.d. (−2.0 < Z > 2.0) from the population mean (all values within ± 0.5 s.d. from the mean) and statistically significant from the mean population based on a two-tailed Student t test P < 0.05 after Welch modification and Benjamini and Hochberg correction for multiple testing53 using all genes with a calculated fitness score.
Subsequent examination of 25 null mutants (Figs. 2 and 4 and Extended Data 2) demonstrated that 83/99 (84%) of the mutant:amoeba combinations that were analysed were consistent with the phenotypes predicted by Tn-seq. For a subset of genes, phenotypes were observed for the corresponding null mutants in additional amoebae that were not originally predicted by Tn-seq. As these discrepancies were equally distributed across all four data sets, this is not likely to be due to the sensitivity of individual screens. Of these, 10/16 (65%) had statistically significant Z scores ranging between −1.5 and −2.0, consistent with the fitness disadvantage observed for the null mutants. In other cases, insufficient numbers of insertions or variability in fitness scores across replicates that prevented accurately predicting phenotypes were responsible. Using a less stringent Z score criteria of −1.5, the Tn-seq screen was 92% accurate in predicting phenotypes.
The heat map to compare fitness profiles across amoebae was generated in R (The R Project for Statistical Computing, www.r-project.org) using the function heatmap.2 of the gplots package. Hierarchical clustering was performed by calculating the Euclidean distance between each host cell type using the Z score of all L. pneumophila genes with fitness scores across the four hosts using gplot package in R.
Transposon site hybridization screen.
For U937 cells, 1 × 107 TPA-treated cells were infected with a library of 100,000 L. pneumophila transposon mutants18 at MOI = 1 for 1 h. Bacteria used to challenge cells were plated on charcoal yeast extract agar (CYET) medium (input samples). Cells were incubated for 24 h, lysed with 0.02% saponin and plated on CYET medium (output samples). Input and output samples were processed and analysed by transposon site hybridization50 as previously described18. Genes important for growth in U937 cells were defined based on the average fitness score of three biological replicates of two technical replicates each as previously described18, although this list is not likely to be exhaustive given the redundancy among virulence genes observed in this host cell type.
Construction of Legionella deletion mutants.
Individual genes were deleted in L. pneumophila Philadelphia-1 strain Lp02 by a tandem double recombination counter selection strategy using the suicide plasmid pSR47s, as previously described54. sdhA was deleted in L. pneumophila 130b as described above but using 20% sucrose for counter selection. ravY in L. pneumophila 130b and sdhA and ravY in L. pneumophila Paris were replaced with a chloramphenicol resistance marker using a double recombination strategy by natural transformation with linear DNA, as previously described55. Primers used to construct all deletion plasmids are listed in Supplementary Table 5. Plasmids were propagated in E. coli DH5α λpir56. All plasmids were verified by sequencing and are listed in Supplementary Table 6.
Construction of Legionella expression plasmids.
For in trans complementation, IDTS genes were individually introduced into the L. pneumophila self-replicating plasmid pTO228 by Gateway cloning (Invitrogen) from a pDONR221-based IDTS plasmid library (a gift from R. Isberg, Tufts University School of Medicine). pTO228 is a derivative of pJB90857, in which a DNA fragment from pDEST17 (Invitrogen) encoding a 6×His epitope tag, a chloramphenicol resistance gene and ccdB flanked by attR recombination sites was amplified by PCR and cloned as a SacI-KpnI fragment into similarly digested pJB908 to generate a Gateway-compatible destination vector. For ectopic expression, IDTS genes were cloned into the L. pneumophila self-replicating plasmid pTO1362, a derivative of pJB180658 (a gift from J. Vogel, Washington University), in which the 3×FLAG epitope was amplified from pDTI16633 using primers FlagF and FlagR and cloned as a EcoRI-SacI fragment into similarly digested pJB1806 to allow expression of N-terminal 3×FLAG fusion proteins. Primers used to construct all expression plasmids are listed in Supplementary Table 5. Plasmids were propagated in E. coli strain DH5α. All plasmids were confirmed by sequencing and are listed in Supplementary Table 6.
In vitro growth assays.
Patches of L. pneumophila strains grown on solid CYE medium were resuspended in AYE medium and diluted to an absorbance at 600 nm (A600) of 0.2, equivalent to 0.2 × 109 c.f.u. per ml and grown at 37 °C with shaking for 18–24 h. Growth was monitored by measuring A600 every 2 h. The growth of Legionella species was monitored at 33 °C as described above. The doubling time (dt), was determined using the following equation: dt = t/n where t = time of exponential phase growth (t2-t1, where t1 = time at the beginning of exponential growth and t2 = time at the end of exponential growth) and n = (logA2-logA1)/log2, where A1 and A2 = absorbance at 600 nm of the bacterial culture at t1 and t2, respectively.
Intracellular growth assays.
The growth of Legionella within A. castellanii, A. polyphaga, H. vermiformis and A/J bone marrow-derived primary murine macrophages was monitored as previously described15,41,42. For A. castellanii and A. polyphaga, cells were lysed with 0.05% (w/v) saponin or 0.05% (w/v) deoxycholate. For N. gruberi, trophozoites were harvested at 60–70% confluency and resuspended in Ac buffer. 1 × 105 cells were immediately challenged at MOI = 1 at 35 °C with bacteria grown to post-exponential phase. Cells were incubated for 2 h and then rinsed twice with Ac buffer. At the indicated time points, cells were lysed with 0.05% saponin and plated on CYE to enumerate bacterial c.f.u. For nutrient supplementation experiments, cells were plated and maintained in Ac buffer containing 50 µM biotin or 0.04% thiamin. For U937 cells, 1 × 105 TPA-treated cells were incubated overnight at 37 °C and then challenged at MOI = 1 at 37 °C with post-exponential phase bacteria. Cells were incubated for 1 h then rinsed three times with fresh medium. At the indicated time points, cells were lysed with 0.02% (w/v) saponin and bacteria were enumerated based on recovered c.f.u. from lysates plated on CYE medium. For real-time growth assays in amoebae and U937 cells, 2 × 105 cells were challenged with bacterial strains harboring the plasmid pPpacS-EGFP40 (Supplementary Table 6) at MOI = 4 for 1 h. Cells were then rinsed with Ac buffer (amoebae) or RPMI culture medium lacking riboflavin (U937 cells), and bacterial growth was monitored every hour for 36 h by measuring fluorescence in a Tecan M200Pro spectrophotometer as previously described59. For nutrient depletion experiments, RPMI was prepared by omitting either biotin or thiamin in the formulation. For biotin-depleted medium, 50 ml of fetal bovine serum was incubated with 100 μl of high capacity NeutrAvidin agarose resin (ThermoScientific) at 4 °C overnight with shaking, and then removed by centrifugation at 4,000×g for 5 min before addition to the RPMI medium. For thiamin-depleted medium, 10 μM pyrithiamine hydrobromide was added to the RPMI depleted medium containing 10% fetal bovine serum60 just before plating cells.
Gentamycin protection assays.
1–2 × 105 amoebae or TPA-treated U937 cells were challenged with post-exponential phase L. pneumophila at MOI = 1. At 1–2 h.p.i., cells were rinsed once (amoebae) or three times (U937 cells) and then incubated in medium containing 10 µg ml−1 gentamycin for 2 h. Cells were then rinsed twice with medium lacking antibiotics and lysed with 0.05% (w/v) saponin or 0.05% (w/v) deoxycholate. Lysates were plated on CYE medium and bacteria were enumerated based on recovered c.f.u.
Phylogenetic cluster analysis.
The phylogenetic tree of the 13 Legionella species was generated based on a set of 78 orthologous groups defined previously37 using the maximum likelihood approach. A concatenated protein alignment and the LG + Gamma + F evolutionary model with 100 bootstrap resampling were used to generate the tree using the Randomized Axelerated Maximum Likelihood (RAxML) module61 in Geneious (www.geneious.com).
Reporting Summary.
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Tn-seq raw sequence data supporting the findings of this study are available through the NCBI Sequence Read Archive, accession number PRJNA593054. Fitness ratios, Z scores and statistical test values for all genes examined by Tn-seq are presented in Supplementary Table 1. All other data supporting the findings of this study are available in the indicated Source Data. All plasmids and strains developed during the course of this work will be provided to all investigators upon request.
Code availability
The custom script generated in this study for Tn-seq data analysis is available through GitHub at OConnorLab/TnseqSA.
Extended Data
Extended Data Fig. 1 |. L. pneumophila growth in amoebal hosts and macrophages.
a, Intracellular growth cycle of L. pneumophila in A. castellanii, A. polyphaga, H. vermiformis and N. gruberi. Differences in the sets of bacterial genes required for growth in each amoeba were not due to variations in the duration of the intracellular growth cycle, or the cumulative replication, as no correlation between the number of genes identified and the extent of growth was observed and host-specific genes were identified for all hosts examined (Fig. 1). b, The intracellular growth cycle of L. pneumophila in U937 cells is similar to amoebae. In a-b, Host cells were challenged with wild type (WT) or Dot/Icm translocation deficient (dot-) bacteria constitutively expressing the enhanced green fluorescence protein and bacterial growth was monitored by measuring fluorescence over time. Plotted is the relative fluorescence units (RFU) normalized to the WT strain by the RFU at the 1 hour time point (for a, the WT strain in A. castellanii was used). Data are the mean ± standard deviation of 3 biological replicates, each generated from 3 technical replicates (Source Data for Extended Data Fig. 1).
Extended Data Fig. 2 |. Validation of IDTS mutant phenotypes predicated by Tn-seq.
a, Null mutations in IDTS genes do not impair L. pneumophila replication in nutrient rich bacteriological medium. The wild type (WT) and indicated null mutant strains were grown in CYE medium and the absorbance at 600 nm was monitored over time. Data are the mean ± standard deviation of 2 biological replicates, each generated from 2 technical replicates (Source Data 6). b, IDTS genes for which mutations were predicted to be neutral based on Tn-seq do not impair L. pneumophila growth in amoebae when compared to the WT strain. c, The phenotype predicted by Tn-seq for mavB (lgp1752) is due to polar effects of transposon insertion mutations on lpg1751. Top panel: The L. pneumophila genetic locus of lpg1751 and lpg1752 (mavB). Bottom panel: Deletion of lpg1751 but not mavB severely impairs L. pneumophila replication within A. castellanii and a ΔmavBΔlpg1751 double mutant phenocopies the Δlpg1751 single deletion strain. d, The intracellular growth defects of IDTS null mutants can be rescued by reintroducing the corresponding gene on a self-replicating plasmid. Bacterial strains harboring the empty vector pJB908 or the respective complementation plasmid were used to challenge A. castellanii or H. vermiformis. In b,c,d, The WT and indicated IDTS null mutant strains were used to challenge amoebae and bacterial growth, based on recovered colony forming units (cfus) on solid media from lysed host cells, was assessed after 24 hours. Plotted is the total bacterial yield 24 hours post infection (hpi) normalized to the wild type strain by the number of intracellular bacteria 1–2 hpi. e, Uptake and/or survival phenotypes of IDTS mutants can be rescued by reintroducing the corresponding gene on a self-replicating plasmid. Bacterial strains harboring the empty vector pJB908 or the respective complementation plasmid were used to challenge A. castellanii or H. vermiformis for 1.5 hours. Cells were then treated with gentamycin to kill extracellular bacteria and internal bacteria were enumerated based on recovered cfus from host cell lysates plated on bacteriological medium. In b-e, Data are the mean ± standard deviation of 2–5 biological replicates, each generated from 3 technical replicates; *P < 0.02, 1-way ANOVA, relative to the WT strain (Source Data for Extended Data Fig. 2).
Extended Data Fig. 3 |. universal IDTS are required for replication of L. pneumophila strains 130b and Paris in amoebae.
The wild type (WT) and indicated IDTS null mutant strains were used to challenge amoebae and bacterial growth, based on recovered colony forming units (cfus) on solid media from lysed host cells, was assessed after 24 hours. Plotted is the total bacterial yield 24 hours post infection (hpi) normalized to the WT strain by the number of intracellular bacteria 1–2 hpi. Data are the mean ± standard deviation of 2–3 biological replicates, each generated from 3 technical replicates, *P<0.02, 1-way ANOVA, relative to the WT strain (Source Data for Extended Data Fig. 3).
Extended Data Fig. 4 |. IDTS mutants exhibit similar phenotypes in primary bone marrow-derived murine macrophages.
a, Intracellular growth of the wild type (WT) and universal IDTS mutant strains in primary bone-marrow-derived macrophages from A/J mice. b, Intracellular replication of auxiliary IDTS mutants. c, Amoebae-specific IDTS genes are a source of redundancy in primary macrophages. In a-c, Macrophages were challenge with the indicated strains and bacterial replication, based on recovered colony forming units (cfus) on solid media from lysed host cells, was monitored 24 hpi and normalized to the WT strain by the number of intracellular bacteria 1 hpi. Data are the mean ± standard deviation of 2–5 biological replicates, each generated from 3 technical replicates; *P < 0.03, 1-way ANOVA, relative to the WT strain (Source Data for Extended Data Fig. 4).
Extended Data Fig. 5 |. The amoebae-specific growth defect of the Δbio mutant strain is not medium-dependent.
To rule out prior culturing of A. castellanii in PYG medium and H. vermiformis and N. gruberi in PYNFH medium as a source of the amoebae-specific requirement for the biotin biosynthetic genes, growth of the Δbio mutant in A. castellanii pre-cultured in PYNFH was compared to A. castellanii pre-cultured in PYG. Under both conditions, the Δbio mutant show a growth defect. While this does not rule out trace amounts of biotin in PYNFH being sufficient for L. pneumophila growth in H. vermiformis and N. gruberi, this result demonstrates that in the same medium, the intracellular levels of biotin in A. castellanii are sufficiently lower than in N. gruberi or H. vermiformis, defining distinct differences in the availability of biotin between these amoebal hosts. Data are the mean ± standard deviation of 2 biological replicates, each generated from 3 technical replicates; *P<0.01, 1-way ANOVA, relative to the WT strain (Source Data for Extended Data Fig. 5).
Extended Data Fig. 6 |. Growth of 12 Legionella species compared to L. pneumophila in nutrient rich bacteriological medium.
The intracellular growth phenotypes of the 12 Legionella species were not due to differences in growth kinetics as all species grew as well as L. pneumophila in bacteriological medium. L. pneumophila and the indicated species of Legionella were grown in CYE medium and the absorbance at 600 nm was monitored over time. Data are the mean ± standard deviation of 3 biological replicates, each generated from 3 technical replicates. The doubling time (dt) of each species during the exponential growth phase was determined as dt = t/n, where t = time and n = number of generations as describe in Methods. Data are the mean ± standard deviation of 3 biological replicates, each generated from 3 technical replicates; *P < 0.05, two-tailed Student t test relative to L. pneumophila (Source Data for Extended Data Fig. 6).
Extended Data Fig. 7 |. Correlates between the fitness profiles in amoebae and phylogenetic distances of individual Legionella species.
a, Pairwise comparisons define no correlation between the fitness profiles of 12 Legionella species (Fig. 5) and their phylogenetic relatedness. b, Pairwise comparisons between members of the same phenotypic group (Group I, II, III and IV as defined in Fig. 5) show strong positive correlations (blue) with the exception of L. dumoffii (Ld) and other Group I members (red). c, The fitness profile of L. dumoffii negatively correlates with that of all members of Group I, II and IV species (left panel) and is due to the decreased fitness of L. dumoffii in H. vermiformis relative to A. castellanii and N. gruberi (right panel). Plots are based on the phylogenetic distances and percent L. pneumophila intracellular growth reported in Fig. 5.
Extended Data Fig. 8 |. Different evolutionary trajectories of Legionella in the environment result in the convergent evolution of distinct but compensatory virulence mechanisms.
In the environment, a common ancestor of Legionella encounters distinct, possibly overlapping, sets of diverse amoebal hosts. Amoebal heterogeneity in different environmental niches provides the selective pressure driving the diversification of individual Legionella species through the assembly of both common and distinct sets of virulence genes. The resulting virulence gene repertoires collectively determined fitness in the environment and human macrophages. In some cases, the evolutionary trajectory of the bacterium results in a set of virulence factors that is not sufficient to support replication in macrophages (a). In other cases, different evolutionary trajectories lead to distinct sets of virulence factors that each promote replication in macrophages (b, c) and thus distinct but compensatory virulence mechanisms. Conversely, some bacteria accumulate virulence factors that while important for growth in amoebae restrict replication in macrophages (d).
Supplementary Material
Acknowledgements
We thank J. Vogel, M. Hossain and S. Rego for thoughtful review of the manuscript. We are grateful to R. Isberg (Tufts University School of Medicine) for sharing data, R. Isberg, J. Vogel (Washington University School of Medicine) and N. Cianciotto (Northwestern University School of Medicine) for plasmids and/or strains, and H. Shuman (University of Chicago School of Medicine) for Legionella species. This work was supported by the United States National Institutes of Health (grant no. AI119580–01) to T.J.O.
Footnotes
Competing interests
The authors declare no competing interests.
Additional information
Extended data is available for this paper at https://doi.org/10.1038/s41564-019-0663-7.
Supplementary information is available for this paper at https://doi.org/10.1038/s41564-019-0663-7.
Reprints and permissions information is available at www.nature.com/reprints.
References
- 1.Young BC et al. Severe infections emerge from commensal bacteria by adaptive evolution. eLife 6, e30637 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horwitz MA Formation of a novel phagosome by the Legionnaires’ disease bacterium (Legionella pneumophila) in human monocytes. J. Exp. Med. 158, 1319–1331 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fliermans CB et al. Ecological distribution of Legionella pneumophila. Appl. Environ. Microbiol. 41, 9–16 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dilger T, Melzl H & Gessner A. Legionella contamination in warm water systems: a species-level survey. Int. J. Hyg. Environ. Health 221, 199–210 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Horwitz MA & Silverstein SC. Legionnaires’ disease bacterium (Legionella pneumophila) multiplies intracellularly in human monocytes. J. Clin. Invest. 66, 441–450 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu VL, Zuravleff JJ, Gavlik L & Magnussen MH. Lack of evidence for person-to-person transmission of Legionnaires’ disease. J. Infect. Dis. 147, 362 (1983). [DOI] [PubMed] [Google Scholar]
- 7.Edelstein PH, Meyer RD & Finegold SM. Laboratory diagnosis of Legionnaires’ disease. Am. Rev. Resp. Dis. 121, 317–327 (1980). [DOI] [PubMed] [Google Scholar]
- 8.Schlossberg D & Bonoan J. Legionella and immunosuppression. Semin. Resp. Infect. 13, 128–131 (1998). [PubMed] [Google Scholar]
- 9.Lanternier F et al. Incidence and risk factors of Legionella pneumophila pneumonia during anti-tumor necrosis factor therapy: a prospective French study. Chest 144, 990–998 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Segal G & Shuman HA. Intracellular multiplication and human macrophage killing by Legionella pneumophila are inhibited by conjugal components of IncQ plasmid RSF1010. Mol. Microbiol. 30, 197–208 (1998). [DOI] [PubMed] [Google Scholar]
- 11.Vogel JP, Andrews HL, Wong SK & Isberg RR. Conjugative transfer by the virulence system of Legionella pneumophila. Science 279, 873–876 (1998). [DOI] [PubMed] [Google Scholar]
- 12.de Felipe KS et al. Evidence for acquisition of Legionella type IV secretion substrates via interdomain horizontal gene transfer. J. Bacteriol. 187, 7716–7726 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang L et al. The E Block motif is associated with Legionella pneumophila translocated substrates. Cell Microbiol. 13, 227–245 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu W et al. Comprehensive identification of protein substrates of the Dot/Icm type IV transporter of Legionella pneumophila. PLoS ONE 6, e17638 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berger KH & Isberg RR. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol. Microbiol. 7, 7–19 (1993). [DOI] [PubMed] [Google Scholar]
- 16.Brand BC, Sadosky AB & Shuman HA. The Legionella pneumophila icm locus: a set of genes required for intracellular multiplication in human macrophages. Mol. Microbiol. 14, 797–808 (1994). [DOI] [PubMed] [Google Scholar]
- 17.M Luo ZQ & Isberg RR. Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc. Natl Acad. Sci. USA 101, 841–846 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Connor TJ, Boyd D, Dorer MS & Isberg RR. Aggravating genetic interactions allow a solution to redundancy in a bacterial pathogen. Science 338, 1440–1444 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh S & O’Connor TJ. Beyond paralogs: the multiple layers of redundancy in bacterial pathogenesis. Front. Cell Infect. Microbiol. 7, 467 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Connor TJ, Adepoju Y, Boyd D & Isberg RR. Minimization of the Legionella pneumophila genome reveals chromosomal regions involved in host range expansion. Proc. Natl Acad. Sci. USA 108, 14733–14740 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hahn MW & Höfle MG. Grazing of protozoa and its effect on populations of aquatic bacteria. FEMS Microbiol. Ecol. 35, 113–121 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Boamah DK, Zhou G, Ensminger AW & O’Connor TJ. From many hosts, one accidental pathogen: the diverse protozoan hosts of Legionella. Front. Cell Infect. Microbiol. 7, 477 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amaro F, Wang W, Gilbert JA, Anderson OR & Shuman HA. Diverse protist grazers select for virulence-related traits in Legionella. ISME J. 9, 1607–1618 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segal G & Shuman HA. Legionella pneumophila utilizes the same genes to multiply within Acanthamoeba castellanii and human macrophages. Infect. Immun. 67, 2117–2124 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harb OS, Gao LY & Abu Kwaik Y. From protozoa to mammalian cells: a new paradigm in the life cycle of intracellular bacterial pathogens. Envrion. Microbiol. 2, 251–265 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Molmeret M, Horn M, Wagner M, Santic M & Abu Kwaik Y. Amoebae as training grounds for intracellular bacterial pathogens. Appl. Environ. Microbiol. 71, 20–28 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ensminger AW. Legionella pneumophila, armed to the hilt: justifying the largest arsenal of effectors in the bacterial world. Curr. Opin. Microbiol. 29, 74–80 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Gomez-Valero L & Buchrieser C. Intracellular parasitism, the driving force of evolution of Legionella pneumophila and the genus Legionella. Genes Immun. 20, 394–402 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Scheikl U et al. Free-living amoebae (FLA) co-occurring with legionellae in industrial waters. Eur. J. Protistol. 50, 422–429 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marciano-Cabral F, Jamerson M & Kaneshiro ES. Free-living amoebae, Legionella and Mycobacterium in tap water supplied by a municipal drinking water utility in the USA. J. Water Health 8, 71–82 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Ruggiero MA et al. A higher level classification of all living organisms. PLoS ONE 10, e0119248 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Opijnen T, Bodi KL & Camilli A. Tn-seq: high-throughput parallel sequencing for fitness and genetic interaction studies in microorganisms. Nat. Methods 6, 767–772 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isaac DT, Laguna RK, Valtz N & Isberg RR. MavN is a Legionella pneumophila vacuole-associated protein required for efficient iron acquisition during intracellular growth. Proc. Natl Acad. Sci. USA 112, e5208–e5217 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Portier E et al. IroT/mavN, a new iron-regulated gene involved in Legionella pneumophila virulence against amoebae and macrophages. Environ. Microbiol. 17, 1338–1350 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laguna RK, Creasey EA, Li Z, Valtz N & Isberg RRA. Legionella pneumophila-translocated substrate that is required for growth within macrophages and protection from host cell death. Proc. Natl Acad. Sci. USA 103, 18745–18750 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shames SR et al. Multiple Legionella pneumophila effector virulence phenotypes revealed through high-throughput analysis of targeted mutant libraries. Proc. Natl Acad. Sci. USA 114, e10446–e10454 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burstein D et al. Genomic analysis of 38 Legionella species identifies large and diverse effector repertoires. Nat. Genet. 48, 167–175 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomez-Valero L et al. More than 18,000 effectors in the Legionella genus genome provide multiple, independent combinations for replication in human cells. Proc. Natl Acad. Sci. USA 116, 2265–2273 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abu Khweek A, Kanneganti A, Guttridge DDC & Amer AO. The sphingosine-1-phosphate lyase (LegS2) contributes to the restriction of Legionella pneumophila in murine macrophages. PLoS ONE 11, e0146410 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ensminger AW, Yassin Y, Miron A & Isberg RR. Experimental evolution of Legionella pneumophila in mouse macrophages leads to strains with altered determinants of environmental survival. PLoS Pathog. 8, e1002731 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feeley JC et al. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J. Clin. Microbiol. 10, 437–441 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ensminger AW & Isberg RR. E3 ubiquitin ligase activity and targeting of BAT3 by multiple Legionella pneumophila translocated substrates. Infect. Immun. 78, 3905–3919 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sundstrom C & Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int. J. Cancer 17, 565–577 (1976). [DOI] [PubMed] [Google Scholar]
- 44.Chien M et al. The genomic sequence of the accidental pathogen Legionella pneumophila. Science 305, 1966–1968 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Rao C, Benhabib H & Ensminger AW. Phylogenetic reconstruction of the Legionella pneumophila Philadelphia-1 laboratory strains through comparative genomics. PLoS ONE 8, e64129 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moffat JF & Tompkins LS. A quantitative model of intracellular growth of Legionella pneumophila in Acanthamoeba castellanii. Infect. Immun. 60, 296–301 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Afgan E, Baker D, Batut B, van den Beek M & Bouvier D. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 46, W537–W544 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCoy KM, Antonio ML & van Opijnen T. MAGenTA: a Galaxy implemented tool for complete Tn-seq analysis and data visualization. Bioinformatics 33, 2781–2783 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Langmead B, Trapnell C, Pop M & Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sassetti CM, Boyd DH & Rubin EJ. Comprehensive identification of conditionally essential genes in mycobacteria. Proc. Natl Acad. Sci. USA 98, 12712–12717 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iglewicz B & Hoaglin DC How to Detect and Handle Outliers (ASQC, 1993). [Google Scholar]
- 52.Kropp KP. The Rorschah Z score. J. Proj. Tech. 19, 443–452 (1955). [DOI] [PubMed] [Google Scholar]
- 53.Benjamini Y & Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. 57, 289–300 (1995). [Google Scholar]
- 54.Merriam JJ, Mathur R, Maxfield-Boumil R & Isberg RR. Analysis of the Legionella pneumophila fliI gene: intracellular growth of a defined mutant defective for flagellum biosynthesis. Infect. Immun. 65, 2497–2501 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stewart CR, Rossier O & Cianciotto NP. Surface translocation by Legionella pneumophila: a form of sliding motility that is dependent upon type II protein secretion. J. Bacteriol. 191, 1537–1546 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kolter R, Inuzuka M & Helinski DR. Trans-complementation-dependent replication of a low molecular weight origin fragment from plasmid R6K. Cell 15, 1199–1208 (1978). [DOI] [PubMed] [Google Scholar]
- 57.Sexton JA et al. The Legionella pneumophila PilT homologue DotB exhibits ATPase activity that is critical for intracellular growth. J. Bacteriol. 186, 1658–1666 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bardill JP, Miller JL & Vogel JP. IcmS-dependent translocation of SdeA into macrophages by the Legionella pneumophila type IV secretion system. Mol. Microbiol. 56, 90–103 (2005). [DOI] [PubMed] [Google Scholar]
- 59.O’Connor TJ et al. Iron limitation triggers early egress by the intracellular bacterial pathogen Legionella pneumophila. Infect. Immun. 84, 2185–2197 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zera K & Zastre J. Thiamine deficiency activates hypoxia inducible factor-1α to facilitate pro-apoptotic responses in mouse primary astrocytes. PLoS ONE 12, e0186707 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stamatakis A et al. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690 (2006). [DOI] [PubMed] [Google Scholar]
- 62.Cui X & Churchill GA. Statistical tests for differential expression in cDNA microarray experiments. Genome Biol. 4, 210 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Tn-seq raw sequence data supporting the findings of this study are available through the NCBI Sequence Read Archive, accession number PRJNA593054. Fitness ratios, Z scores and statistical test values for all genes examined by Tn-seq are presented in Supplementary Table 1. All other data supporting the findings of this study are available in the indicated Source Data. All plasmids and strains developed during the course of this work will be provided to all investigators upon request.