Abstract
Importance:
Treatment of congenital ichthyoses primarily focuses on reversing skin scaling and is not pathogenesis based. Recent studies showed Th17 immune skewing, as in psoriasis, across the spectrum of ichthyosis, suggesting that targeting this pathway might broadly reduce disease severity.
Objective:
To determine whether secukinumab, an IL-17A inhibitor, can improve ichthyosis across several congenital ichthyosis subtypes.
Design:
Exploratory 16-week double-blind, randomized, placebo-controlled trial comparing secukinumab 300 mg every 4wks to placebo (1:1 randomization) in adults with the four major congenital ichthyosis subtypes (NCT03041038), followed by a 16-week open-label phase to evaluate response of the placebo-first group and a 20-week extension for safety. Significant differences in secukinumab- vs. placebo-treated subjects at Wk16 in the Ichthyosis Area Severity Index (IASI) score and lack of increased mucocutaneous bacterial and/or fungal infections were the co-primary efficacy and safety endpoints, respectively.
Setting:
Two tertiary referral centers: Northwestern University Feinberg School of Medicine, Chicago, and Mount Sinai Icahn School of Medicine, New York.
Participants:
Twenty subjects ≥ 18 yo with genotype-confirmed epidermolytic ichthyosis, Netherton syndrome, lamellar ichthyosis, or congenital ichthyosiform erythroderma with at least moderate erythroderma.
Results:
IL-17A inhibition did not significantly reduce severity or increase mucocutaneous infections among the 18 who completed the 16-week double-blind phase. Five patients with 29–50% clinical improvement at Wk32 requested drug continuation. Th17-related biomarkers were not significantly reduced vs. baseline or placebo-treated levels.
Limitations:
Small sample size; heterogeneous ichthyosis subsets.
Conclusion:
IL-17 inhibition with secukinumab is safe, but not efficacious across the spectrum of adult ichthyoses.
Gov registration number:
NCT03041038; first posted on 02/02/2017.
Keywords: Biologic, IL-17, Ichthyosis, Monoclonal antibody, Secukinumab
Introduction
Congenital ichthyoses are orphan disorders (< 1:200,000 persons) characterized by having a poor epidermal barrier in association with skin thickening, scaling, and inflammation. Epidermolytic ichthyosis (EI), Netherton syndrome (NS), and autosomal recessive congenital ichthyosis (ARCI), including lamellar ichthyosis (LI) and congenital ichthyosiform erythroderma (CIE), are among the most common orphan forms. Therapy is supportive and time-consuming. Oral and topical retinoids often are poorly tolerated, especially by more inflamed subtypes, and risk side effects. Potential systemic absorption of topical corticosteroids and calcineurin inhibitors restricts their use. Although the gene variants underlying congenital ichthyoses are well understood, the mechanism by which these genetic alterations translate into the phenotypic changes of the ichthyoses remains unclear.
Recent skin and blood profiling studies of orphan forms of ichthyosis showed a shared pro-inflammatory Th17 biomarker signature that strongly correlated with overall and erythema severity [1,2,3]. Psoriasis, a common inflammatory disorder with a similar immunophenotype, greatly improves from targeted therapy with IL-17A inhibition for 16 weeks [4], suggesting the possibility that this Th17 skewing shared among the congenital ichthyoses could participate in disease pathogenesis and be amenable to Th17 pathway inhibition. We conducted a two-center, double-blind, randomized, placebo-controlled 16-week exploratory trial to investigate the efficacy and safety of secukinumab, an FDA-approved anti-IL-17 antibody for plaque psoriasis. In this trial, we purposefully tested secukinumab across the spectrum of adults with these four orphan forms of ichthyosis, given their shared skin immune profile, to gain preliminary data about natural disease course and potential response to Th17 inhibition.
Methods
Study design and patient characteristics
Subjects were ≥ 18yo with genotype-confirmed EI, NS, LI or CIE with a total Ichthyosis Area and Severity Index (IASI) score1 of at least 18 (of a possible 48) and an erythema subscore of at least 12 (moderate severity) (Table 1). After providing written, IRB-approved informed consent at Northwestern University, Chicago or Mount Sinai, New York, patients were screened for exclusion criteria, including baseline infection, immunodeficiency, inflammatory bowel disease, or laboratory abnormalities. Eligible subjects underwent computerized randomization for 1:1 placebo:secukinumab 300 mg allocation stratified in random blocks of four by disease subtype to assure the ability to have at least 4 subjects in each group. Randomization was not stratified by site due to the small sample size. Codes were sent from the data coordinating center at Northwestern and distributed to a licensed pharmacist at each study site. Study drug was controlled/coded by the research pharmacy team until allocation, at which point the syringe with “secukinumab 300 mg or placebo” was provided to the blinded study team member and subject for injection.
Table 1.
Baseline characteristics
| Placebo | Secukinumab | Total | ||
|---|---|---|---|---|
|
| ||||
| (n = 9) | (n = 11) | (n = 20) | ||
|
| ||||
| Sex | ||||
| Female | 4 (44.4%) | 8 (72.7%) | 12 (60.0%) | |
| Male | 5 (55.6%) | 3 (27.3%) | 8 (40.0%) | |
| Age | ||||
| Mean (SD) | 35.5 (12.7) | 34.2 (11.7) | 34.7 (12.9) | |
| Median [Min, Max] | 33.0 [18.0, 59.0] | 32.5 [19.0, 56.0] | 32.5 [18.0, 59.0] | |
| Ethnicity | ||||
| Hispanic | 1 (11.1%) | 1 (9.0%) | 2 (10.0%) | |
| Non-Hispanic | 8 (88.9%) | 10 (91.0%) | 18 (90.0%) | |
| Race | ||||
| Black | 0 (0%) | 1 (9.0%) | 1 (5.0%) | |
| White | 8 (88.9%) | 9 (81.8%) | 17 (85.0%) | |
| Missing | 1 (11.1%) | 1 (9.0%) | 2 (10.0%) | |
| Weight (kg) | ||||
| Mean (SD) | 75.5 (30.4) | 69.9 (13.5) | 72.4 (22.0) | |
| Median [Min, Max] | 67.2 [52.3, 148] | 66.8 [55.0, 94.0] | 67.0 [52.3, 148] | |
| Site | ||||
| Mount Sinai | 2 (22.2%) | 4 (36.4%) | 6 (30.0%) | |
| Northwestern | 7 (77.8%) | 7 (63.6%) | 14 (70.0%) | |
| Subtype | ||||
| CIE | 2 (22.2%) | 3 (27.3%) | 5 (25.0%) | |
| EI | 2 (22.2%) | 2 (18.2%) | 4 (20.0%) | |
| LI | 2 (22.2%) | 4 (36.4%) | 6 (30.0%) | |
| NS | 3 (33.3%) | 2 (18.2%) | 5 (25.0%) | |
|
| ||||
| IASI total | Mann– Whitney p value | |||
|
| ||||
| Mean (SD) | 36.2 (4.7) | 33.7 (6.5) | 34.8 (5.9) | |
| Median [Min, Max] | 38.1 [26.4, 43.2] | 34.0 [21.6, 42.0] | 35.8 [21.6, 43.2] | 0.38 |
| IASI-E | ||||
| Mean (SD) | 18.2 (3.1) | 16.9 (3.1) | 17.5 (3.1) | |
| Median [Min, Max] | 18.6 [12.7, 21.9] | 18.9 [11.9, 20.4] | 18.75 [11.9, 21.9] | 0.36 |
| IASI-S | ||||
| Mean (SD) | 18.0 (3.7) | 16.9 (4.9) | 17.4 (4.5) | |
| Median [Min, Max] | 19.2 [12.6, 21.9] | 18.5 [9.0, 23.7] | 18.6 [9.0, 23.7] | 0.56 |
| VIIS | ||||
| Mean (SD) | 23.8 (2.9) | 21.5 (3.6) | 22.6 (3.5) | |
| Median [Min, Max] | 24.5 [20.0, 28.0] | 21.5 [15.0, 28.0] | 21.8 [15.0, 28.0] | 0.22 |
| Bodemer | ||||
| Mean (SD) | 45.6 (12.3) | 40.6 (13.3) | 42.8 (13.1) | |
| Median [Min, Max] | 44.0 [33.0, 74.0] | 35.0 [26.0, 65.0) | 37.5 [26.0, 74.0] | 0.27 |
| TEWL-arm (g/m2/h) | ||||
| Mean (SD) | 32.1 (14.3) | 32.7 (14.5) | 32.4 (14.4) | |
| Median [Min, Max] | 31.7 [12.5, 63.6] | 30.2 [13.2, 66.8] | 31.0 [12.5, 66.8] | > 0.99 |
| TEWL-buttock (g/m2/h) | ||||
| Mean (SD) | 28.3 (12.3) | 33.3 (13.0) | 30.9 (12.9) | |
| Median [Min, Max] | 23.7 [14.7, 52.5] | 31.0 [12.8, 61.5] | 28.8 [12.8, 61.5] | 0.4 |
| DLQI | ||||
| Mean (SD) | 7.9 (4.6) | 9.5 (6.8) | 8.8 (6.0) | |
| Median [Min, Max] | 7.0 [2.0, 18.0] | 10.0 [1.0, 28.0] | 8.0 [1.0, 28.0] | 0.51 |
| iQoL-32 | ||||
| Mean (SD) | 62.3 (9.4) | 73.5 (19.3) | 68.5 (16.6) | |
| Median [Min, Max] | 65.0 [49.0, 76.0] | 72.0 [42.0, 116.0] | 65.5 [42.0, 116.0] | 0.15 |
| 5-D Itch | ||||
| Mean (SD) | 17.1 (3.4) | 15.1 (4.1) | 16.0 (3.9) | |
| Median [Min, Max] | 19.0 [10.0, 20.0] | 15.0 [9.0, 25.0] | 16.0 [9.0, 25.0] | 0.19 |
| Itch NRS | ||||
| Mean (SD) | 5.4 (2.1) | 3.9 (2.8) | 4.6 (2.6) | |
| Median [Min, Max] | 6.0 [2.0, 8.0] | 3.0 [ 1.0, 10.0] | 5.0 [1.0, 10.0] | 0.17 |
| Pain NRS | ||||
| Mean (SD) | 1.6 (1.9) | 2.8 (3.2) | 2.3 (2.8) | |
| Median [Min, Max] | 1.0 [0.0, 6.0] | 2.0 [0.0, 10.0] | 1.5 [0.0, 10.0] | 0.41 |
Subjects continued their routine bathing and emollient use without change throughout the study. However, use of topical retinoids or keratolytics was prohibited beginning one week prior to Baseline. Systemic retinoids were also prohibited starting four weeks prior to Baseline. The 16-week, double-blind, placebo-controlled phase was followed by a 16-week open-label phase (and then a 20-week extension for safety) (Fig. 1).
Fig. 1.
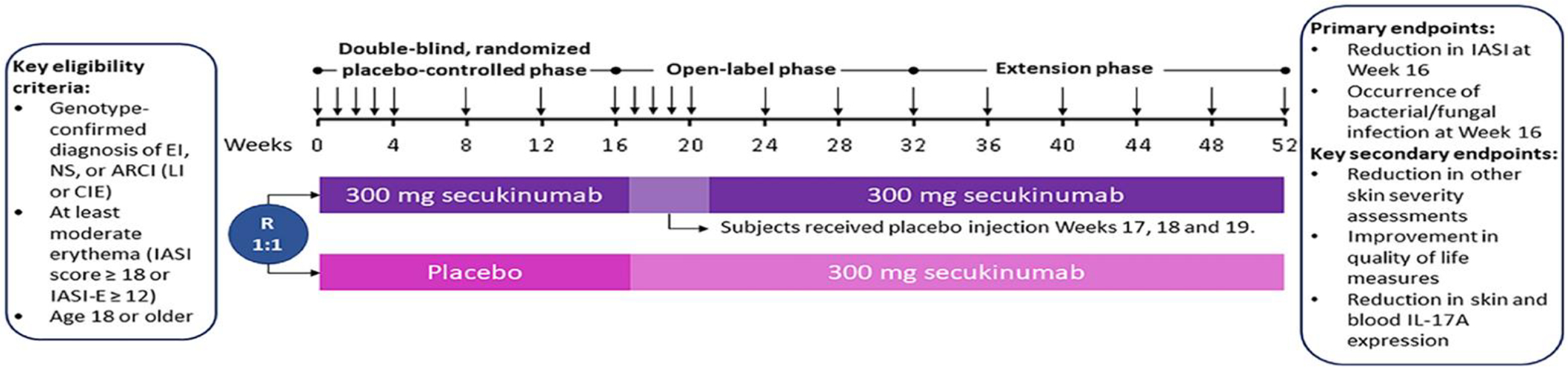
Study design eligible subjects underwent computerized randomization for 1:1 placebo:secukinumab 300 mg allocation, stratified in random blocks of four by disease subtype. Randomization was not stratified by site due to the small sample size. At Baseline, all subjects were given weekly subcutaneous injections of secukinumab or placebo for four weeks, then every four-week dosing through Wk12. At Wk16, this regimen was repeated, but those who had received placebo at baseline received secukinumab weekly for Wks16–19, while those who had received secukinumab continued every 4-week dosing but received placebo injections weekly for Wks17–19. Arrows indicate timepoints of injection
Efficacy and safety assessments
Subjects completed in-person study visits every 4 weeks through Wk24 and then every 8 weeks through Wk48; the final study visit was at Wk52. Supplemental Table 1 provides the Schedule of Assessments for the study. Scores for efficacy assessments throughout the study represented the mean scores of blinded physicians who saw subjects in tandem and without conferring. The primary efficacy endpoint (co-primary endpoint) was reduction in IASI [1] in secukinumab vs. placebo at Wk16. The IASI score was not validated, but was modelled after the Eczema Area and Severity Index (EASI) and Psoriasis Area and Severity Index (PASI), commonly used in clinical trials for atopic dermatitis and psoriasis, respectively. We complemented IASI scores through concurrent use of several other severity and quality of life scores, including the Visual Index for Ichthyosis Severity (VIIS) score, which was validated after initiation of this study for congenital ichthyoses [5]. Other secondary efficacy endpoints included Wk16 IASI-E (erythema subscore), IASI-S (scaling subscore), and the Bodemer scale [6]. Transepidermal water loss (TEWL) [7] was assessed at areas of representative disease activity on the upper arm and upper buttock regions using the AquaFlux AF200 (Biox, London) at Baseline and Weeks 16, 32, and 52. Patient-reported outcomes (PROs) throughout the study included Itch numerical rating scale (NRS) and Pain NRS (both 3-day averages), 5-D Itch scale [8], Dermatology Life Quality Index (DLQI) [9, 10], and ichthyosis-specific Quality of Life score of 32 items (iQol-32) [11].
The co-primary endpoint for safety was number of bacterial and fungal mucocutaneous infections in subjects treated with secukinumab vs. placebo during the first 16 weeks of therapy.
Biomarker analysis
Skin biopsies (4.5 mm) were obtained from an area with representative disease activity on the non-dominant upper outer arm at baseline, 16 wks, and 32 wks for histologic/immunohistologic studies and mRNA expression studies using qRT-PCR. Immunohistochemistry was performed on frozen skin sections using purified mouse monoclonal antibodies. Epidermal thickness and cell counts were quantified using ImageJ V1.42 software (National Institutes of Health, Bethesda, Maryland) (Suppl. Table 2). RNA was extracted for real-time polymerase chain reaction (RT-PCR) using the miRNAeasy Mini Kit (Qiagen, Hilden, Germany). Reverse transcription to complementary DNA (cDNA) from RNA was carried out using the High Capacity cDNA reverse transcription (Thermo Fisher). Pre-amplification was performed on all samples. 100 ng total RNA was used for PreAMP pool. Expression values were normalized to the human acidic ribosomal phosphoprotein (hARP/RPLP0) housekeeping gene (Suppl. Table 3).
Statistical analysis
Descriptive statistics were used to summarize participant demographics, baseline severity, and PRO scores. Frequencies and percentages were recorded for all categorical variables; mean ± SD, median, and range were reported for continuous variables. Differences in baseline severity and PRO measures were assessed by Mann–Whitney U analysis. At study end, clinical endpoints were assessed by comparing Baseline, Wk16 and Wk32 measures in the originally assigned placebo (n = 9) and secukinumab (n = 11) groups using an analysis of variance (ANOVA) with post hoc Sidak multiple comparisons testing. Analyses of skin biopsy data were performed using R-language (R-project.org) and Bioconductor Project packages (www.bioconductor.org). Gene expression profiles were modeled by linear models using R’s lme function. Mean expressions are displayed in a heatmap, in which unsupervised clustering was performed using Euclidean distance and average agglomeration criteria. P < 0.05 considered significant (***p < 0.001, **p < 0.01, *p < 0.05, +p < 0.1).
Results
Twenty-one patients were enrolled, 20 randomized, and 18 completed through Wk16. Seventeen subjects completed Wk32 and 12 finished Wk52. Approximately equal numbers of each ichthyosis subset were enrolled, evenly distributed by arm (Table 1).
No statistically significant difference was found between placebo- and secukinumab-treated groups at Wk16 by ad hoc Sidak’s multiple comparisons in the primary efficacy (total IASI) or secondary severity, PRO, or TEWL endpoints (Table 2). Significant decreases from Baseline to Wk32 in mean IASI-E and VIIS score (p = 0.04 and p = 0.01, respectively) for those treated with secukinumab from baseline were noted and sustained for VIIS at Wk52 (p = 0.01). The placebo-first group had no significant changes in severity or PRO measures at Wk32 or Wk52 (i.e., after more than 16 weeks on secukinumab) (Table 2).
Table 2.
Results of efficacy
| Efficacy scores | Placebo group (n = 9) |
|
Secukinumab group (n = 11) |
|
|
Placebo vs. secukinumab |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Wk 16 mean | % change from baseline | Mean diff. (95% CI) baseline to Wk 16 | Adjusted p value | Wk 16 Mean | % change from baseline | Mean Diff. (95% CI) baseline to Wk 16 | Adjusted p value | Wk 16 mean diff. (95% CI) placebo vs. secukinumab | Adjusted p value | |
|
| ||||||||||
| IASI | 32 | − 11.8 | 4.3 (− 6.5 to 15.1) | 0.61 | 31.3 | − 7.3 | 2.4 (− 7.8 to 12.7) | 0.88 | 0.6 (− 11.6 to 12.9) | > 0.99 |
| IASI− E | 15 | − 17.5 | 3.2 (− 4.0 to 10.4) | 0.53 | 15.6 | − 7.7 | 1.3 (− 4.0 to 6.6) | 0.88 | − 0.6 (− 7.1 to 5.9) | 0.99 |
| IASI− S | 16.9 | − 6.2 | 1.1 (− 3.0 to 5.2) | 0.82 | 15.7 | − 6.8 | 1.1 (− 4.6 to 6.9) | 0.93 | 1.2 (− 5.7 to 8.1) | 0.96 |
| VIIS | 21.1 | − 11.6 | 2.8 (− 2.8 to 8.4) | 0.43 | 19.4 | − 10.2 | 2.2 (− 2.0 to 6.4) | 0.43 | 1.3 (− 4.4 to 7.8) | 0.85 |
| Bodemer Score | 45.7 | 12 | − 0.1 (− 11.3 to 11.1) | > 0.99 | 38 | − 6.5 | 2.6 (− 6.3 to 11.5) | 0.8 | 7.7 (− 2.4 to 17.8) | 0.17 |
| TEWL Arm | 32 | − 0.2 | 0.1 (− 12.1 to 12.2) | > 0.99 | 32.7 | 0 | 0.0 (− 10.1 to 10.1) | > 0.99 | − 0.7 (− 13.2 to 11.8) | > 0.99 |
| TEWL Buttock | 32.8 | 15.8 | − 4.5 (− 14.8 to 5.9) | 0.54 | 33.9 | 1.8 | − 0.6 (− 12.1 to 10.9) | > 0.99 | − 1.2 (− 15.9 to 13.6) | > 0.99 |
| DLQI | 9 | 14.1 | − 1.1 (− 5.8 to 3.5) | 0.87 | 9 | − 5.7 | 0.5 (− 2.8 to 3.9) | 0.96 | 0.0 (− 7.7 to 7.7) | > 0.99 |
| iQoL-32 | 61.7 | − 1.1 | 0.7 (− 13.5 to 14.9) | > 0.99 | 67.8 | − 7.7 | 5.6 (− 1.9 to 13.2) | 0.16 | − 6.2 (− 26.3 to 13.9) | 0.82 |
| 5-D Itch | 16.4 | − 3.9 | 0.7 (− 2.1 to 3.5) | 0.87 | 14.4 | − 4.8 | 0.7 (− 2.5 to 4.0) | 0.9 | 2.1 (− 2.3 to 6.5) | 0.52 |
| Itch NRS | 5.1 | − 6.1 | 0.3 (− 1.5 to 2.2) | 0.94 | 3.7 | − 5.1 | 0.2 (− 1.7 to 2.1) | 0.99 | 1.4 (− 1.4 to 4.2) | 0.5 |
| Pain NRS | 1.8 | 14.3 | − 0.2 (− 1.1 to 0.6) | 0.83 | 1.7 | − 38.7 | 1.1 (− 1.5 to 3.7) | 0.59 | 0.1 (− 2.0 to 2.1) | > 0.99 |
|
| ||||||||||
| Efficacy scores | Placebo-first group—on open-label secukinumab (n = 9) | Secukinumab group (n = 11) | Placebo-first v. secukinumab | |||||||
| Wk 32 Mean | % change from Week 16 | Mean diff. (95% CI) week 16 to week 32 | Adjusted p value | Week 32 mean | % change from baseline | Mean diff. (95% CI) baseline to week 32 | Adjusted p value | Week 32 mean diff. (95% CI) Placebo-First vs. Secukinumab | Adjusted p value | |
| IASI | 30.3 | − 5.0 | 1.6 (− 6.9 to 10.1) | 0.93 | 28.3 | − 16.0 | 5.4 (− 0.9 to 11.7) | 0.1 | 2.0 (− 7.1 to 11.1) | 0.91 |
| IASI-E | 14.8 | − 1.5 | 0.2 (− 5.7 to 6.2) | 0.99 | 13.5 | − 20.0 | 3.4 (0.2 to 6.6) | 0.04* | 1.3 (− 2.7 to 5.3) | 0.78 |
| IASI-S | 15.5 | − 8.1 | 1.4 (− 1.8 to 4.5) | 0.54 | 14.9 | − 11.6 | 2.0 (− 1.8 to 5.7) | 0.43 | 0.6 (− 5.5 to 6.8) | 0.99 |
| VIIS | 21.3 | 1.3 | − 0.3 (− 5.5 to 4.9) | > 0.99 | 17.4 | − 19.2 | 4.1 (1.2 to 7.1) | 0.01* | 3.9 (− 1.2 to 9.0) | 0.16 |
| Bodemer Score | 41.5 | − 9.3 | 4.2 (− 4.0 to 12.4) | 0.41 | 37.1 | − 8.7 | 3.5 (− 6.2 to 13.3) | 0.69 | 4.4 (− 6.7 to 15.5) | 0.67 |
| TEWL Arm | 34.6 | 8.1 | − 2.6 (− 14.6 to 9.4) | 0.9 | 32.6 | − 0.4 | 0.1 (− 12.9 to 13.2) | > 0.99 | 2.0 (− 18.8 to 22.8) | 0.99 |
| TEWL Buttock | 34.5 | 5.3 | − 1.7 (− 13.9 to 10.4) | 0.97 | 32.2 | − 3.0 | 1.0 (− 10.9 to 12.9) | 0.99 | 2.2 (− 19.2 to 23.5) | 0.99 |
| DLQI | 8.4 | − 6.2 | 0.6 (− 2.5 to 3.6) | 0.94 | 7.7 | − 19.0 | 1.8 (− 6.2 to 10.0) | 0.9 | 0.7 (− 6.7 to 8.1) | 0.99 |
| iQoL-32 | 61.7 | 0 | 0.0 (− 4.9 to 4.9) | > 0.99 | 63.1 | − 14.1 | 10.4 (− 11.1 to 31.9) | 0.48 | − 1.4 (− 20.4 to 17.6) | > 0.99 |
| 5-D Itch | 16.8 | 2.1 | 0.3 (− 2.6 to 1.9) | 0.96 | 14.9 | − 1.2 | 0.2 (− 4.2 to 4.6) | > 0.99 | 1.9 (− 2.8 to 6.5) | 0.64 |
| Itch NRS | 4.8 | − 6.5 | 0.3 (− 0.4 to 1.0) | 0.48 | 4 | 2.6 | − 0.1 (− 3.5 to 3.3) | > 0.99 | 0.8 (− 2.2 to 3.8) | 0.87 |
| Pain NRS | 2.3 | 31.2 | − 0.6 (− 2.1 to 1.0) | 0.66 | 1.9 | − 32.3 | 0.9 (− 2.5 to 4.3) | 0.85 | 0.4 (− 2.4 to 3.2) | 0.97 |
Despite the failure to improve ichthyoses in the majority of subjects, five (two with NS; two with EI; one with CIE/NIPAL4) chose to continue secukinumab at trial end because of self-perceived improvement (Fig. 2, Table 3); by Wk32 (16 weeks for two initially on placebo and 32 weeks for three initially on secukinumab), the five each had a decreased total IASI from baseline (median change − 36%; range − 29 to − 50%) and subsets scores [IASI-E (median change − 37%; range − 25 to − 53%) and IASI-S [median change − 37%, range − 27 to − 44%)] (Suppl. Tables 4–6). The best response at Wk32, in an 18-year-old woman with NS, involved slight worsening on placebo at Wk16, but a 53% reduction in IASI at Wk32 (IASI-E 20.4–9.6 and IASI-S 15.6–7.2), with continued improvement thereafter. In addition to reduction in IASI scores, these patients reported reduction in itch and pain (Table 3) and disease burden (less need for emollient application, less desquamation requiring vacuuming, ability to wear dark clothes), which contributed to their decision to continue the secukinumab post-study.
Fig 2.
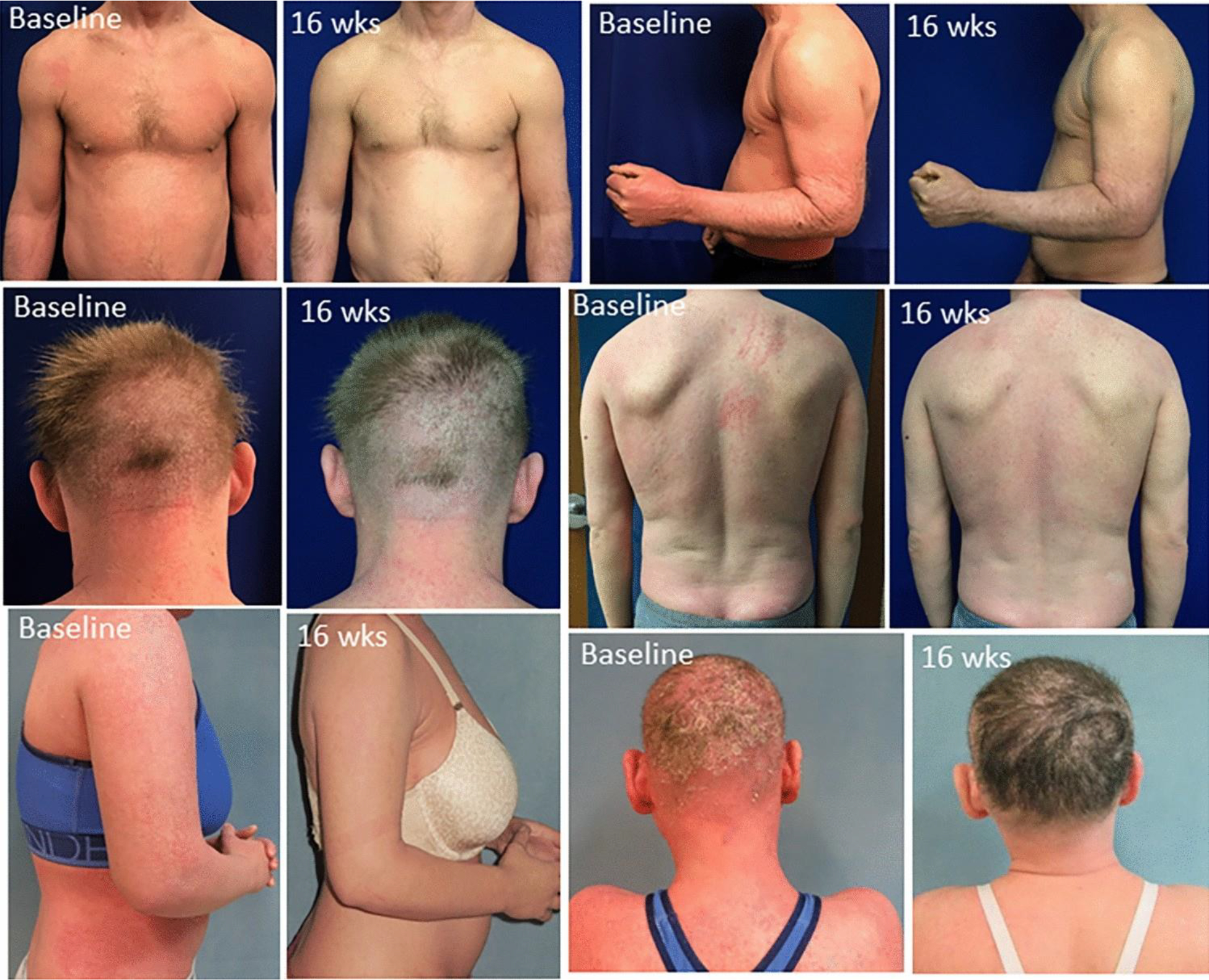
Clinical images from subjects with self-reported improvement Representative images from three subjects (top: CIE (800–6); middle: NS (800–9); bottom: NS (801–1)) at baseline and after 16 weeks on secukinumab. These subjects were among the five who noted reduced erythema and/or scaling and chose to continue secukinumab after trial completion
Table 3.
Individual QoL, itch and pain scores in five patients with self-reported improvement
| DLQI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject number | Subject ichthyosis SUBTYPE-NUMBER | DLQIBASE-LINE subtype | DLQI DLQI Week 16Baseline | DLQI We % Change Baseline to Week 16ek 16 | % Change Baselin DLQI Week 32e to Week 16 | DLQI Week 32% Change Baseline to Week 32 | % Change Ba % Change Week 16 to Week 32seline to Week 32 | % Change Wee DLQI Week 52 k 16 to Week 32 | DLQI Week 52%Change Baseline to Week 52 | |
| 800–4 | EI | 12 | 16 | 33% | 11 | − 8% | − 31% | 13 | 8% | |
| Secukinumab | 800–7 | EI | 9 | 7 | − 22% | 7 | − 22% | 0% | 8 | − 11% |
| 800–6 | CIE | 10 | 7 | − 30% | 4 | − 60% | − 43% | 3 | − 70% | |
| Placebo | 800–9 | NS | 6 | 8 | 33% | 9 | 50% | 13% | 12 | 100% |
| 801–1 | NS | 13 | 12 | − 8% | 7 | − 46% | − 42% | 7 | − 46% | |
| iQoL32 | ||||||||||
| Subject Number | Ichthyosis Subtype | iQoL Baseline | iQoL32 We iQoL32 Week 16ek 16 | % Change Baseli % Change Baseline to Week 16ne to Week 16 | iQoL32 Week 32 iQoL32 Week 32 |
% Change Bas % Change Baseline to Wee
32eline to Week 32 |
% Change Wee % Change Week 16 to Week 32 k 16 to Week 32 | iQoL32 Week 5 iQoL32 Week 522 | % Change Baseline % Change Baseline to Week 52 to Week 52 | |
| 800–4 | EI | 88 | 79 | − 10% | 86 | − 2% | 9% | 75 | − 15% | |
| Secukinumab | 800–7 | EI | 88 | 68 | − 23% | 62 | − 30% | − 9% | 68 | − 23% |
| 800–6 | CIE | 61 | 51 | − 16% | 42 | − 31% | − 18% | 42 | − 31% | |
| Placebo | 800–9 | NS | 49 | 57 | 16% | 58 | 18% | 2% | 62 | − 27% |
| 801–1 | NS | 76 | 49 | − 36% | 47 | − 38% | − 4% | 47 | 38% | |
| NRS Itch | ||||||||||
| Subject Number | Ichthyosis Subtype | NRS Itch Baseline | NRS Itch Week 16 | % Change Baseline to Week | NRS Itch Week 32 | % Change Baseline to Week 32 | % Change Week 16 to Week | NRS Itch Week 52 | % Change Baseline to Week 52 | |
| 800–4 | EI | 6 | 5 | − 17% | 6 | 0% | 20% | 4 | − 33% | |
| Secukinumab | 800–7 | EI | 3 | 2 | − 33% | 1 | − 67% | − 50% | 1 | − 67% |
| 800–6 | CIE | 3 | 2 | − 33% | 2 | − 33% | 0% | 2 | − 33% | |
| Placebo | 800–9 | NS | 2 | 2 | 0% | 2 | 0% | 0% | 2 | 0% |
| 801–1 | NS | 6 | 3 | − 50% | 2 | − 67% | − 33% | 1 | − 83% | |
| Week 52 | 5D-pruritus | |||||||||
| Subject Number | Ichthyosis Subtype | 5D Baseline | 5D Week 16 | % Change Baseline to Week 16 | 5D Week 32 | % Change Baseline to Week 32 | % Change Week 16 to Week 32 | 5D Week 52 | % Change Baseline to Week 52 | |
| 800–4 | EI | 16 | 16 | 0% | 13 | − 19% | − 19% | 16 | 0% | |
| Secukinumab | 800–7 | EI | 14 | 10 | − 29% | 11 | − 21% | 10% | 10 | − 29% |
| 800–6 | CIE | 15 | 10 | − 33% | 13 | − 13% | 30% | 11 | − 27% | |
| Placebo | 800–9 | NS | 14 | 14 | 0% | 19 | 36% | 36% | 12 | − 14% |
| 801–1 | NS | 16 | 16 | 0% | 14 | − 13% | − 13% | 13 | − 19% | |
| NRS Pain | ||||||||||
| Subject Number | Ichthyosis Subtype | NRS Pain Baseline | NRS Pain Week 16 | % Change Baseline to Week 16 | NRS Pain Week 32 | % Change Baseline to Week 32 | % Change Week 16 to Week 32 | Subject Number | Ichthyosis Subtype | |
| 800–4 | EI | 8 | 2 | − 75% | 1 | − 88% | − 50% | 3 | − 63% | |
| Secukinumab | 800–7 | EI | 0 | 0 | n/a | 0 | n/a | n/a | 0 | n/a |
| 800–6 | CIE | 1 | 0 | − 100% | 0 | − 100% | n/a | 0 | − 100% | |
| Placebo | 800–9 | NS | 2 | 2 | 0% | 2 | 0% | 0% | 2 | 0% |
| 801–1 | NS | 1 | 1 | 0% | 1 | 0% | 0% | 1 | 0% | |
Adverse event details are shown in Suppl. Table 7. Documented bacterial or fungal mucocutaneous infections were equivalent in subjects treated with secukinumab (n = 1 Trichophyton tonsurans) vs. placebo (n = 1 Staphylococcus aureus) at Wk16, meeting the co-primary (safety) endpoint. Three hospitalizations occurred, two for gastroesophageal reflux disease (GERD) and one for pyelonephritis; none of these SAEs were deemed related to secukinumab or led to ongoing disability/incapacity or treatment discontinuation.
Skin biopsies taken at Wk16 and Wk32 overall showed no significant decrease in either arm from Baseline in epidermal thickness or KRT16 mRNA expression, nor in numbers of CD3+ T-cells or CD11c+ myeloid dendritic cells (DC) (Suppl. Fig. 1). Quantitative RT-PCR was performed to trace secukinumab-induced inhibition of Th17/IL-17-related biomarkers in skin. All patients had elevated Th17 markers (CXCL1, hBD2, IL17A, LL37, S100A’s, and/or PI3) at baseline without consistently higher levels in either arm (Suppl. Fig. 2). Biomarkers of the Th17 pathway trended down in subjects treated first with secukinumab from Baseline to Wk16, although reductions from baseline failed to reach significance at either Wk16 or Wk32 (Suppl. Figs. 2–3). There was also no difference in reduction in Th17 biomarkers in secukinumab-first vs. placebo-first groups at Wk16 or Wk32 (Suppl. Fig. 2). As such, changes IASI-E and VIIS at Wk32 in secukinumab-first subjects were not accompanied by significant reduction in Th17/IL-17-related biomarkers. However, Th17 (HBD2/DEFB4A, IL-17A, LL37, IL-23A/IL-23p19, IL-12/IL-23p40, PI3) and Th17/Th22 (S100A7/8/9) markers were significantly decreased in the placebo-first group at Wk32 (i.e., after 16 weeks on secukinumab) versus baseline (p < 0.05 to < 0.01; Suppl. Fig. 3), suggesting effective reduction of the Th17 pathway despite lack of overall secukinumab efficacy.
Discussion
The recent discovery that major orphan forms of congenital ichthyosis share significant Th17 immune skewing in skin and blood, as in plaque psoriasis, suggested that repurposing of commercially available biologics targeting the Th17 pathway could improve ichthyoses as a group. Using secukinumab, we tested whether IL-17 antagonism would lead to clinical disease improvement in the group of orphan forms of ichthyosis and whether this improvement would correlate with reductions in cutaneous expression of Th17 biomarkers. Across the entire cohort, secukinumab showed no significant difference in efficacy from placebo in total IASI score reduction at Wk16, the primary efficacy endpoint. Differences were only seen in the secukinumab-first group at Wk32 vs. Baseline in both IASI-E and VIIS scores, but this was not replicated in the placebo-first group at Wk52 (e.g., about 32 Wks on secukinumab). Th17 biomarkers S100A7A, DEFB4A, and PI3, which are response genes within four weeks in psoriasis,11 were also not significantly reduced at Wk16 and were only decreased in the placebo-first group, which showed no significant clinical improvement. Although the failure to show molecular and clinical responses could reflect underpowering, our data provide evidence that IL-17A is likely not pathogenic as a single target across the spectrum of long-standing ichthyosis.
The majority of patients in this study failed to meet the primary endpoint for efficacy (with those treated first with secukinumab achieving a mean increase in IASI score of 1.6 at Wk16 vs. those treated first with placebo having a mean reduction in IASI score of 9.25). While the skewed Th17 immune profile seen across all orphan forms of ichthyosis likely reflects response to changes in the microbiome across the impaired epidermal barrier, the clinically meaningful improvement in response to IL-23/Th17 inhibition in some patients suggests a pathogenic role for these individuals. Indeed, five of our subjects chose to continue secukinumab after trial completion, attesting to benefit. These patients had a 29–50% reduction in IASI by 32 wks, with reductions in both erythema and scaling (Suppl. Table 4). The meaningful benefit, despite having less than 50% improvement (only 1 patient reached IASI 50 on secukinumab at Wk16), provides evidence that the required achievement of EASI 75 or PASI 75 in atopic dermatitis and psoriasis trials, respectively, is far too high for clinical trials using IASI in the future. Patients who responded to secukinumab and continued its use recounted spending less time on ichthyosis care, as well as less itch and pain (Suppl. Table 8). The sample size of this trial was too small to identify clinical or biomarker characteristics indicating increased likelihood for a response to therapy. However, the lack of response in any patient with lamellar ichthyosis suggests that a trial of a Th17 pathway inhibitor for lamellar ichthyosis is unlikely to yield benefit. Anecdotal reports largely describe pediatric and young adult patients with CIE or NS treated successfully with secukinumab, ustekinumab, or dupilumab plus guselkumab [12,13,14,15,16,17,18,19], implying increased responsiveness in younger, more erythrodermic patients. Discovering endotypic or phenotypic differences in responders vs. non-responders could increase our understanding about the heterogeneity of ichthyosis subsets and predict therapeutic response.
Our pilot study was limited by small sample size for each subset of ichthyosis and few validated ichthyosis-specific severity measures. Furthermore, fluctuations in disease activity during placebo treatment (especially one patient each with NS and CIE) led to higher-than-anticipated placebo responses, confirming the value of double-blind, placebo-controlled ichthyosis trials. Not all patients with CIE, EI, or NS had clinically meaningful responses to secukinumab, attesting to the need for other directions in therapy. Upstream molecules, such as IL-23 or IL-36 family/ IL-36 receptor (highly overexpressed in ichthyosis2), may be alternative targets given their broader suppression of the IL-23/Th17 pathway.
Supplementary Material
Funding
Investigator-initiated grant from Novartis CAIN457AUS05T (AP, EG); genotyping was supported by NIH R01AR068392 (KC), biosample management through NIH P30AR075049 (Northwestern’s Skin Biology and Disease Resource-based Center/SBDRC) (AP), and regulatory support and some of the clinical statistical analyses through NIH UL1TR001422 (Northwestern University Clinical and Translational Sciences Institute/NUCATS and its Biostatistics Collaboration Center. Dr. Lefferdink received salary funding from a National Psoriasis Foundation fellowship grant.
Abbreviations
- AE
Adverse event(s)
- ARCI
Autosomal recessive congenital ichthyosis
- CIE
Congenital ichthyosiform erythroderma
- DLQI
Dermatology Life Quality Index
- EI
Epidermolytic ichthyosis
- IASI
Ichthyosis Area Severity Index
- IASI-E
Erythema Subscore of Ichthyosis Area Severity Index
- IASI-S
Scaling Subscore of Ichthyosis Area Severity Index
- IRB
Institutional review board
- iQoL-32
Ichthyosis quality of life-32 items
- LI
Lamellar ichthyosis
- LOCF
Last-observation carried forward
- NRS
Numerical Rating Scale
- NS
Netherton syndrome
- PASI
Psoriasis Area Severity Index
- PRO
Patient-reported outcome
- SAE
Serious adverse event
- SD
Standard deviation
- TEWL
Transepidermal water loss
- VIIS
Visual Index for Ichthyosis Severity
Footnotes
Conflict of interest Drs. Lefferdink, Chima, Ibler, Pavel, Kim, Wu, and Rangel, and Ms. Abu-Zayed, Wu, Jackson, and Singer declare no conflicts. Dr. Choate has been an investigator for Alderya, Mayne, Galderma, and Regeneron and consultant with honorarium for AbbVie, Eli Lilly, Janssen, KrystalBio, Lifemax, Mayne, and Timber. Dr. Guttman-Yassky has been a researcher/consultant for AbbVie, Anacor, AnaptysBio, Asana Biosciences, Botanix, Celgene, DBV, Dermira, DS Biopharma, Escalier, Galderma, Glenmark, Innovaderm, Janssen, Kyowa Kirin, Leo Pharma, Lilly, MedImmune/AstraZeneca, Mitsubishi Tanabe, Novan, Novartis, Pfizer, Promius, Ralexar, Regeneron, Sanofi-Aventis, Stiefel/GlaxoSmithKline (GSK), UCB, and Vitae. Dr. Paller has been an investigator for AbbVie, AnaptysBio, Eli Lilly, Incyte, Janssen, KrystalBio, Novartis, Regeneron, and UCB and a consultant with honorarium for Abbvie, Abeona, Alcimed, Almirall, Amagma, Anaptysbio, Arena, Azitra, BiomX, Boehringer Ingelheim, Castle Biosciences, Catawba, Dermira, Eli Lilly, Exicure, Forte, Kamari, Leo, Lifemax, NAOS, Novartis, Pfizer, Phoenix, Pierre Fabre, Regeneron, Sanofi/Genzyme, Seanergy, Trifecta, and UCB. She has served on Data Safety Monitoring Boards for AbbVie, Bausch, Galderma, and Novan.
Declarations
Ethics approval This study was reviewed and approved by the institutional review boards of Northwestern University and Icahn School of Medicine at Mount Sinai Medical Center. IRB #: STU00202656/ STU00202022 (NU); 17–00126 (MS).
Consent to participate Informed consent was obtained from all individual participants included in the study.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00403-022-02325-3.
Data availability
The minimal dataset that supports the central findings of this study can be requested from the corresponding authors.
References
- 1.Paller AS, Renert-Yuval Y, Suprun M, Esaki H, Oliva M, Huynh TN, et al. (2017) An IL-17–dominant immune profile is shared across the major orphan forms of ichthyosis. J Allergy Clin Immunol 139:152–165. 10.1016/j.jaci.2016.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malik K, He H, Huynh TN, Tran G, Mueller K, Doytcheva K et al. (2019) Ichthyosis molecular fingerprinting shows profound TH17 skewing and a unique barrier genomic signature. J Allergy Clin Immunol 143:604–618. 10.1016/j.jaci.2018.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Czarnowicki T, He H, Leonard A, Malik K, Magidi S, Rangel S et al. (2018) The major orphan forms of ichthyosis are characterized by systemic T-cell activation and Th-17/Tc-17/Th-22/Tc-22 polarization in blood. J Invest Dermatol 138:2157–2167. 10.1016/j.jid.2018.03.1523 [DOI] [PubMed] [Google Scholar]
- 4.Thaçi D, Blauvelt A, Reich K, Tsai TF, Vanaclocha F, Kingo K et al. (2015) Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol 73:400–409. 10.1016/j.jaad.2015.05.013 [DOI] [PubMed] [Google Scholar]
- 5.Marukian NV, Deng Y, Gan G, Ren I, Thermidor W, Craiglow BG et al. (2017) Establishing and validating an ichthyosis severity index. J Invest Dermatol 137:1834–1841. 10.1016/j.jid.2017.04.037 [DOI] [PubMed] [Google Scholar]
- 6.Bodemer C, Bourrat E, Mazereeuw-Hautier J, Boralevi F, Barbarot S, Bessis D, Blanchet-Bardon C et al. (2011) Short- and medium-term efficacy of specific hydrotherapy in inherited ichthyosis. Br J Dermatol 165:1087–1094. 10.1111/j.1365-2133.2011.10510 [DOI] [PubMed] [Google Scholar]
- 7.Erickson TR, Murphrey MB, Abu-Zayed H, Wu B, Ibler E, Rangel SM et al. (2020) Transepidermal water loss in the orphan forms of ichthyosis. Pediatr Dermatol 37:771–773. 10.1111/pde.14221 [DOI] [PubMed] [Google Scholar]
- 8.Elman S, Hynan LS, Gabriel V, Mayo MJ (2009) The 5-D itch scale: a new measure of pruritus. Br J Dermatol 162:587–593. 10.1111/j.1365-2133.2009.09586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basra MK, Fenech R, Gatt RM, Salek MS, Finlay AY (2008) The Dermatology Life Quality Index 1994–2007: a comprehensive review of validation data and clinical results. Br J Dermatol 159:997–1035. 10.1111/j.1365-2133.2008.08832 [DOI] [PubMed] [Google Scholar]
- 10.Dreyfus I, Taïeb C, Barbarot S, Maza A, Galera I, Bourrat E, et al. et al (2013) iQoL-32: a new ichthyosis-specific measure of quality of life. J Am Acad Dermatol 69:82–87. 10.1016/j.jaad.2013.01.022 [DOI] [PubMed] [Google Scholar]
- 11.Krueger JG, Wharton KA Jr, Schlitt T, Suprun M, Torene RI, Jiang X et al. (2019) IL-17A inhibition by secukinumab induces early clinical, histopathologic, and molecular resolution of psoriasis. J Allergy Clin Immunol 144:750–763. 10.1016/j.jaci.2019.04.029 [DOI] [PubMed] [Google Scholar]
- 12.Hernández-Martín A, Kennedy-Batalla R, Cañedo E, González-Sarmiento R, Kennedy-Batalla Rebeca, Martínez-Bonet Marta, et al. (2019) Imbalance in T-helper 17 cells and targeted therapy in an infant with SAM-like syndrome. N Engl J Med 381:2176–2178. 10.1056/NEJMc1908531 [DOI] [PubMed] [Google Scholar]
- 13.Paller AS. (2020) Pathogenesis-based therapy with repurposed biologics for monogenic inflammatory skin disorders. JAMA Dermatol 156:839–41. 10.1001/jamadermatol.2020.1018 [DOI] [PubMed] [Google Scholar]
- 14.Luchsinger I, Knöpfel N, Theiler M, Bonnet des Claustres M, Barbieux C, Schwieger-Briel A, et al. (2020) Secukinumab therapy for Netherton syndrome. JAMA Dermatol156:907–11. 10.1001/jamadermatol.2020.1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanchard SK, Prose NS (2020) Successful use of secukinumab in Netherton syndrome. JAAD Case Rep 6:577–578. 10.1016/j.jdcr.2020.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paller AS, Czarnowicki T, Renert-Yuval Y, Holland K, Huynh T, Sadlier M, et al. (2018) The spectrum of manifestations in desmoplakin gene (DSP) spectrin repeat 6 domain mutations: Immunophenotyping and response to ustekinumab. J Am Acad Dermatol 78:498–505.e2. 10.1016/j.jaad.2017.10.026 [DOI] [PubMed] [Google Scholar]
- 17.Sun Q, Wine Lee L, Hall EK, Choate KA, Elder RW. (2021) Hair and skin predict cardiomyopathies: Carvajal and erythrokeratodermia cardiomyopathy syndromes. Pediatr Dermatol 38:31–38. 10.1111/pde.14478 [DOI] [PubMed] [Google Scholar]
- 18.Frommherz LH, Schempp CM, Has C. (2021) Secukinumab for the treatment of SAM syndrome associated with desmoglein-1 deficiency. Br J Dermatol 184:770–772. 10.1111/bjd.19684 [DOI] [PubMed] [Google Scholar]
- 19.Steinhoff M, Al-Marri F, Al Chalabi R, Gieler U, Buddenkotte J (2021) Recalcitrant erythrodermic ichthyosis with atopic dermatitis successfully treated with dupilumab in combination with guselkumab. Skin Health Dis.e87. 10.1002/ski2.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The minimal dataset that supports the central findings of this study can be requested from the corresponding authors.


