Abstract
Aim
The aim of the study was to explore the efficacy as well as the mechanism of action of Pitongshu (PTS) on rats with functional dyspepsia (FD) induced by iodoacetamide gavage and tail clamping.
Methods
The bioactive components of PTS were obtained from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP), whereas the potential targets of PTS were obtained from the Similarity Ensemble Approach (SEA), TCMSP, and Swiss Target Prediction Database. The disease targets were obtained from the DisGeNET database, whereas Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were performed using the R Software. The method of iodoacetamide gavage combined with tail clamping was used to establish the FD rat model in this study. Body weight, food intake, gastrointestinal motility, gastric acidity and secretion, and the mechanical pain threshold of rats were measured. The open-field test was also performed. The stomach and duodenum were histologically observed. The levels of serotonin (5-HT), Calcitonin Gene-Related Peptide (CGRP), Motilin (MTL), and Gastrin (GAS) in gastric tissues were detected by ELISA.
Results
A total of 139 bioactive components and 17 potential targets of PTS were identified through a network pharmacology approach. The results of GO and KEGG enrichment analyses indicated that PTS could reduce the 5-HT secretion of gastric tissues through the serotonergic synaptic pathway and alleviate the symptoms of FD, indicating that PTS plays a therapeutic role. The results of animal experiments showed that PTS could increase body weight and food intake, improve autonomous activity, and decrease gastric acidity and secretion in FD rats. Furthermore, gastric sensitivity increased in FD rats, and PTS treatment could significantly decrease it. The results of ELISA showed that the overexpression of 5-HT and CGRP was decreased after PTS treatment in FD rats. Lastly, PTS could significantly improve gastrointestinal motility, as well as the levels of GAS and MTL in FD rats.
Conclusion
PTS may reduce 5-HT secretion by regulating the serotonergic synaptic pathway, thereby reducing visceral sensitivity and alleviating the symptoms of FD.
Keywords: Pitongshu, functional dyspepsia, visceral hypersensitivity, serotonin synapse, 5-HT, CGRP
1. INTRODUCTION
Functional Dyspepsia (FD) is a non-organic disease caused by the dysfunction of the stomach and duodenum. The clinical symptoms include upper abdominal pain, upper abdominal distension, nausea, vomiting, belching, loss of appetite, and other uncomfortable symptoms [1, 2]. According to the different clinical symptoms, the Rome type IV standard divides FD into Postprandial Distress Syndrome (PDS), Epigastric Pain Syndrome (EPS), and subtypes with overlapping characteristics of PDS and EPS. A systematic review has revealed that the prevalence of FD is 21.8% [3]. A multicenter study in nine countries and regions in Asia reported that among 1,115 patients with unchecked dyspepsia, 43% of cases were diagnosed with FD after gastroscopy [4]. Although FD is not life-threatening, it affects the quality of life of patients, as well as their physical and mental health and work efficiency. It also requires extensive medical resources [5, 6]. As such, FD has become a major public health problem in developing and developed countries, and currently, the treatment of FD is still a major challenge.
The pathogenesis of FD is relatively complicated, and it is difficult to explain its pathogenesis using the conventional concepts of organic, metabolic, and systemic diseases. Modern medicine does not yet fully understand the pathogenesis of FD, and it is generally believed that the pathogenesis of the disease is caused by a combination of factors. For instance, it may be related to abnormal gastrointestinal motility, visceral hypersensitivity, local environmental stimuli in the stomach, the brain-gut axis, and mental and psychological factors [7, 8].
Increasing research has demonstrated that Traditional Chinese Medicine (TCM) has a variety of characteristics and advantages in terms of relieving symptoms and reducing disease recurrence. Pitongshu (PTS), including Bupleurum (Chai Hu), Citrus aurantium (Zhi Qiao), White Peony (Bai Shao), Licorice (Gan Cao), Magnolia (Hou Po), is derived from Professor Kun-Gen Wang’s clinically effective prescription for the treatment of Functional Dyspepsia (FD), and its safety and efficacy have been confirmed by randomized, controlled, double-blind trials of the early stage of the disease. Based on a previous study, PTS has good therapeutic effects on rats with FD [9], and although PTS has shown significant clinical efficacy in the treatment of FD, its mechanism of action is not clear.
Growing evidence supports the contention that a network pharmacology approach is critical in the research of TCM [10]. Network pharmacology emphasizes the multi-channel regulation of signaling pathways, improves the therapeutic effects of drugs, reduces toxic side effects, and improves the success rates of clinical trials of new drugs. Network pharmacology is based on an understanding of the network of “disease–target–drug” interactions, which can identify new interventions and impacts of drugs on the disease network and analyze the effects of drugs on different nodes in the network so as to better understand the safety, efficacy and potential toxicity of drugs. Network pharmacology fosters new ideas and methods for analyzing the complex field of TCM, promotes the design, research and development of new Chinese medicines, and solidifies the direction for the modernization of Chinese medicines.
The aim of this study is to verify the efficacy of PTS and to examine the mechanism of action of PTS on FD. A network pharmacology approach was used to develop a component-target-disease network, which was combined with animal experiments to explore the mechanism of action of PTS in the treatment of FD and to provide a scientific basis for further research and development of PTS for use in clinical settings.
2. MATERIALS AND METHODS
2.1. Network Pharmacology Prediction
2.1.1. Analysis for Effective Chemical Ingredients in PTS
The bioactive ingredients of PTS were collected from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) database (https://tcmsp-e.com/). The ingredients, whose oral bioavailability (OB) ≥ 30% and drug-likeness (DL) ≥ 0.18, were reserved. The potential targets of the effective chemical ingredients of PTS were obtained from the TCMSP database, SEA database (https://sea.bkslab.org/) and Swiss database (http://www.swisstargetprediction.ch/). Then, the target from three databases was imported into EXCEL, and the duplicated targets were deleted.
2.1.2. Screening for FD-Related Targets
“Dyspepsia” or “functional dyspepsia” was used as a critical term to search for disease targets related to FD in the DisGeNET database (https://www.disgenet.org/).
2.1.3. Correlation Analysis Between PTS and FD-Related Targets
FD-related targets and potential targets for PTS treatment were screened manually, and the interaction network of protein was visualized through the STRING database (https://cn.string-db.org/). A diagram of the relationship between the targets and the bioactive ingredients was created.
2.1.4. GO and KEGG Pathway Enrichment Analyses
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed by R software 3.6.2 (x64).
2.2. PTS Decoction Preparation
The raw herbs, which have been subjected to plant origin characterization, were purchased from Zhejiang University of Chinese Medicine Chinese Herbal Pieces Co., Ltd. The herbs used in this experiment were all dried products processed according to the Chinese Pharmacopoeia, called decoction pieces. Furthermore, the prescription dosage ratio of Pitongshu is Bupleurum: Citrus aurantium: White Peony: Licorice: Magnolia = 6:10:12:3:10. PTS decoction was prepared by Institute of Chinese Medicine and Health Products, Zhejiang University of Technology.
The PTS used in this study had a similar chemical preparation to our previous literature [9]. Briefly, PTS weighed according to the prescription was soaked in 8 times the amount of distilled water, and boiled for 1 h at 100°C by using a Chinese medicine decoction machine (model YJX40-G, Beijing Donghuayuan Medical Equipment Co., Ltd.). Then, it was filtered to obtain the filtrate, and water at 8 times the amount of PTS was added to the medicine residue for obtaining the second decoction; the solution was then again filtered to obtain the filtrate, and lastly, the two filtrates were combined. The extract was concentrated at 65°C by a rotary evaporator. The high concentration extract of Pitongshu is 1.14 g/mL. Pitongshu of medium and low concentration was diluted with purified water, respectively.
2.3. Drugs and Chemicals
Domperidone was purchased from Xi’an Janssen Pharmaceutical Co., Ltd. Sodium carboxymethyl cellulose and H&E stain were purchased from Shanghai Yuanye Biological Technology Co., Ltd. The skimmed milk powder was purchased from Shenggong Biological Engineering (Shanghai) Co., Ltd. Sucrose was purchased from Guangdong Guanghua Technology Co., Ltd. Sodium hydroxide was purchased from Xilong Science Co., Ltd.
2.4. Animals
96 seven-day-old (12-15 g) male Sprague-Dawley rats (SPF grade) were purchased from Hangzhou Medical College and reared in the animal room of Zhejiang University of Technology. They were able to get food and water freely in an environment of temperature at 25 ± 3°C, humidity at 38 ± 5%, and 12 h light/dark cycle. The animal protocol was approved by the ethics committee of Zhejiang University of Technology (SCXK (Zhe) 2019-0002).
2.5. Modeling and Administration
7-day-old male SD rats were used for the experiment. After 3 days of adaptive feeding, 16 rats were randomly selected as the normal control group (NG), according to the body weight. Other 80 rats were used to establish the FD model through iodoacetamide gavage combined with tail clamping [11]. The model rats were given 0.2 mL of a mixed solution of 0.1% iodoacetamide and 2% sucrose for six consecutive days, while NG rats were given 2% sucrose solution only. After the rats were 7 weeks old, the model rats were given tail-clamping stimulation 3 times a day (9:00, 14:00, 18:00) with a duration of 30 mins for 9 days. The NG rats were not given any intervention. Rats may be scratched and injured during tail clamping, and 0.5% iodophor can be given to rub the injured part of rats to prevent wound infection and avoid inflammation.
After modeling, model rats were randomly divided into 5 groups according to their body weight: (1) model group (MG), (2) domperidone-treated group (DOM), (3) PTS-L group (3.8 g/kg, PTS of low dose), (4) PTS-M group (7.6 g/kg, PTS of medium dose) (5) PTS-H group (11.4 g/kg, PTS of high dose). Rats were given intragastric administration at 14:30-15:30 daily for 19 consecutive days. The gavage volume was converted to 1 mL/100 g according to body mass. Except for the NG and MG, which were given an equal volume of distilled water, other administration groups were given corresponding drugs.
2.6. Plantar Mechanical Pain Threshold Test
The plantar mechanical pain threshold test was performed after the administration of PTS for 2 weeks. A 25 cm × 10 cm × 10 cm squirrel cage was prepared, the rats were placed in the suspended squirrel cage in sequence, the bottom of the cage was fully exposed, and testing was started after the rats were quiet. A series of Von Frey filaments were applied to the central part of the plantar surface of the right hind-paw with force increasing in grams (6.0, 8.0, 10, 15, 26, 60, 100, and 180 g) [12]. Each rat was tested three times, and the interval between two tests of the same rat was at least 2 min. The average of the two data points with the smallest difference among the three data points was taken as the final plantar mechanical pain threshold of the rats.
2.7. Open-Field Test
After drug administration for 2 weeks, the experimental rats were randomly placed in the open field box in a quiet and light-isolated environment. After adaptation for 3 min, the rats were put in the lower left corner of the box by grabbing 1/3 of the tail root, and the activity of the rats within 3 min was recorded [13]. Autonomous activity test index included total movement distance (unit: m), time mobile (unit: s), time immobile (unit: s) and track chart.
2.8. Gastric Emptying and Intestinal Propulsion Experiment
After the last administration of PTS, rats in each group were made to fast (20 h). On the 20th day, eight rats in each group were given nutritional semi-solid paste (1 mL/100 g), and the rats were euthanized 30 min later.
The surface mucus and blood were wiped off the whole stomach and weighed. The stomach body was cut along the greater curvature of the organ, and the solid paste in the stomach was washed with normal saline, followed by wiping. The stomach was weighed to obtain the net weight. The gastric emptying rate was calculated.
The gastric remnant rate was calculated as follows [14]:

The intestine from the pylorus to the ileocecal area was cut, placed on a white paper, and pulled into a straight line. The length of the small intestine was measured, which was taken as the total length of the small intestine. The distance from the pylorus to the front of the semi-solid paste was the distance that the semi-solid paste advanced in the small intestine.
The whole small intestine from the pylorus to the ileocecal junction and the distance of movement of the ingested graphite powder from the pylorus were measured to calculate the propulsive as follows:

2.9. Investigation of Gastric Acidity and Secretion
After the final administration of PTS, another eight rats in each group were euthanized. The junction between the gastric cardia and the esophagus was ligated. The stomach contents were collected and weighed to measure gastric acidity and secretion using an acid-base automatic titrator (ZDJ-4A, Shanghai Lei Magnetic Instrument Co., Ltd., China). In brief, the samples were titrated with 0.01mol/L NaOH solution, followed by constant shaking during the titration. The pH of the titration endpoint was set to 7, the total milliliters (mL) of NaOH solution consumed was recorded, and the total acidity of the stomach contents was recorded [15].
(V*0.01)/m*1000
In the formula, X is the gastric acidity (μmol/g).
V is the amount of NaOH solution consumed in milliliters (mL).
The concentration of NaOH solution is 0.01 (mmol/mL).
m is the weight (g) of the stomach contents.
Gastric acid secretion = total mass of stomach contents (g) × gastric acidity (μmol/g)
2.10. Enzyme-Linked Immunosorbent Assay (ELISA)
The gastric tissues were taken out from the -80 °C refrigerator, and 1 g of the gastric tissues of rats in each group was weighed. The PBS was added into the centrifuge tube with the gastric tissues in it according to 1:9 (w/v), and then the mixture of the gastric tissues and PBS was grinded with a tissue homogenizer for a set time of 60 s to get the gastric tissue homogenate. After that, the gastric tissue homogenate was centrifuged at 4 °C and 3600 r/min for 20 minutes by using a high-speed centrifuge (Legend Micro 17, Thermo Fisher Scientific Co., Ltd., China); the supernatant was then taken to obtain the tissue homogenate to be tested.
The concentrations of GAS, MTL, 5-HT and CGRP were detected with Rat GAS, MTL, 5-HT and CGRP ELISA kits (Shanghai Enzyme Link Biotechnology Co., Ltd., China), respectively, according to the manufacturer’s instructions. In brief, GAS, MTL, 5-HT and CGRP antibody and HRP-conjugate were incubated with the above-collected supernatant for 1 h at 37 °C. The signal was detected by a microplate reader (model Epoch2c, Bio-TEK, USA) at 450 nm [16].
2.11. Histopathological Observation of Stomach and Duodenum
Hematoxylin and Eosin (H&E) staining was used to evaluate the histopathology of the stomach and duodenum [17]. The gastric and duodenal specimens were fixed in a 4% formaldehyde solution for 72 hours. Part of the tissue was dehydrated and embedded in paraffin, and then cut into 4 μm-thick paraffin sections with a tissue microtome, and stained with H&E. The histopathological changes were observed under a biological optical electron microscope (Olympus BX43, Japan).
2.12. Statistical Analysis
IBM SPSS Statistics 19.0 was used to analyze data. All values were expressed as mean ± SD. Statistical differences between groups were determined by using Student’s t-test or one-way analysis of variance (ANOVA). P< 0.05 was considered statistically significant. Diagrams were prepared applying the GraphPad Prism 7.0.
3. RESULTS
3.1. Active Ingredients in PTS
A total of 138 active ingredients for PTS were obtained through the TCMSP database, and a total of 139 active substances were found through literature [18] (Table S1 (459.3KB, zip) ; Supplementary Material).
3.2. Targets Acquisition
3022 potential targets of PTS were obtained from the TCMSP database, 3826 potential targets from SEA, and 322 potential targets were obtained from the Swiss database. The targets from the three databases were merged, and duplicate values were removed to obtain a total of 842 potential targets (Table S2 (459.3KB, zip) ; Supplementary Material).
“Dyspepsia” or “functional dyspepsia” were inputted as terms into DisGeNET. A total of 61 non-repeated targets were obtained (Table S3 (459.3KB, zip) ; Supplementary Material). The targets obtained and the disease targets were intersected, and a total of 17 non-repetitive targets were obtained, as shown in Table 1 and Fig. (1a).
Table 1.
The detailed information and parameters of 17 targets in the PPI network.
| NO | Node | Degree | Description |
|---|---|---|---|
| 001 | IL1B | 13 | Interleukin-1 beta |
| 002 | TNF | 12 | Tumor necrosis factor |
| 003 | PTGS2 | 11 | Prostaglandin G/H synthase 2 |
| 004 | IFNG | 8 | Interferon gamma |
| 005 | TP53 | 8 | Cellular tumor antigen p53 |
| 006 | SERPINE1 | 7 | Plasminogen activator inhibitor 1 |
| 007 | MIF | 6 | Macrophage migration inhibitory factor |
| 008 | TGFB1 | 6 | Transforming growth factor beta-1 proprotein |
| 009 | CRH | 4 | Corticotropin-releasing hormone |
| 010 | NFE2L2 | 4 | Corticoliberin |
| 011 | PTGS1 | 4 | Prostaglandin G/H synthase 1 |
| 012 | SLC6A4 | 4 | Sodium-dependent serotonin transporter |
| 013 | TRPV1 | 4 | Transient receptor potential cation channel subfamily V member 1 |
| 014 | CD14 | 3 | Monocyte differentiation antigen CD14 |
| 015 | CYP2C19 | 3 | Cytochrome P450 2C19 |
| 016 | HTR3A | 3 | 5-hydroxytryptamine receptor 3A |
| 017 | GSTK1 | 0 | Glutathione S-transferase kappa 1 |
Fig. (1).
Target prediction of PTS by bioinformatic tools. (A). Venn diagram of the targets of PTS in FD. The blue one is the potential target of PTS, the yellow one is the target related to functional dyspepsia, and the middle one is the common target. (Fig. A was performed from: https://bioinfogp.cnb.csic.es/tools/venny/index.html) (B). Protein-protein interaction network of targets. The greater the radius of the node, the greater the degree. (Fig. B was generated with cytoscape software) (C). Protein-protein interaction (PPI) network of targets from STRING PPI database. (Fig. C was exported from String PPI database: https://cn.string-db.org/cgi/input.pl?input_page_show_search=on&sessionId=Pa502AhiAMp4).
3.3. Protein-Protein Interaction (PPI) Network of Common Targets
After importing the 17 targets obtained into the string PPI database, the disconnected nodes were hid in the network. The result suggests that the number of nodes was 16, the number of edges was 50, the expected number of edges was 12, the average degree was 6.25, and the PPI enrichment p-value <8.86*10-16. Among them, the degrees of IL1B, TNF and PTGS2 were greater than 10, which were the 3 targets with the most degrees. The interaction relationship is shown in Table 1 and Figs. (1B and C.)
3.4. GO and KEGG Enrichment Analysis
A total of 16 GO entries with P < 0.01 were obtained from GO enrichment analysis via R software. PTS may alleviate FD through receptor-ligand activity and signaling receptor activator activity. A total of 58 results were obtained from KEGG enrichment analysis, 46 of which had a p-value less than 0.01. As shown in Figs. (2 and 3), PTS may play a therapeutic role against FD through a serotonergic synapse, inflammatory bowel disease, IL-17 signaling pathway, and so on. In order to discover the core mechanism of the effect of PTS, the first 20 pathways related to 13 key targets in the PPI network were selected to construct an “ingredient - target - pathway” network (Fig. 4).
Fig. (2).
The bubble chart of the result of GO enrichment analysis. (Fig. 2 was generated by R language).
Fig. (3).
The bar graph of the result of GO enrichment analysis. (Fig. 3 was generated by R language).
Fig. (4).
Network of bioactive ingredients-targets-pathways. The green node represents bioactive ingredients in PTS, and the pink cluster represents the FD-related targets; the blue nodes represent pathways from the result of KEGG enrichment analysis. (Fig. 4 was generated with cytoscape software).
3.5. PTS Ameliorates the Symptoms of the Rats with FD
To investigate the effects of PTS on FD, we detected the total weight gain, the total food intake, gastrointestinal motor function, and gastric acid secretion of rats in all groups. Compared to the NG, the total weight gain and the total food intake of the MG were significantly reduced (P < 0.01), while they were increased significantly in each dose group of PTS compared to the MG after administration (P < 0.05, 0.01) (Figs. 5A and B).
Fig. (5).
PTS alleviates the symptoms of the rats with FD. (A). The total weight gain. (B). The total food intake. (C). The gastric emptying rate (D). The intestinal propulsion rate. (E). The gastric acidity. (F). The gastric secretion. Abbreviation: NG-normal group; MG-model group; DOM-domperidone-treated group; PTS-L-low dose of PTS group; PTS-M-middle dose of PTS group; PTS-H-high dose of PTS group. The data were expressed as mean ± SD of 16 rats in each group. *P <0.05; **P <0.01, compared to the NG; #P <0.05; ##P <0.01, compared to the MG.
It was found that the gastric emptying rate and intestinal propulsion rate of the MG were decreased significantly, compared to the NG (P <0.01). While they were increased in the PTS groups by varying degrees compared to the MG (Figs. 5C and D). As shown in Figs. (5E and F), the gastric acidity and secretion of the MG were increased compared to the NG (P <0.01). After drug administration, the gastric acidity and secretion in the PTS groups were lower than that in the MG. The results suggested that PTS can effectively improve the symptoms of the rats with FD (P < 0.05, 0.01).
3.6. Effect of PTS on the Mechanical Pain Threshold of FD Rats
In most related studies, plantar mechanical pain threshold test was performed to explore somatic sensation in order to evaluate visceral hypersensitivity. As shown in Fig. (6), the mechanical pain threshold in the MG was significantly lower than that in the NG (P < 0.01). After drug administration for two weeks, the mechanical pain threshold of each dose group of PTS was increased compared to the MG (P < 0.05, P < 0.01). These findings indicate that PTS can increase the pain threshold of FD rats and improve the visceral hypersensitivity of FD rats.
Fig. (6).
The plantar mechanical pain threshold. Abbreviation: NG-normal group; MG-model group; DOM-domperidone-treated group; PTS-L-low dose of PTS group; PTS-M-middle dose of PTS group; PTS-H-high dose of PTS group. The data were expressed as mean ± SD of 16 rats in each group. *P <0.05; **P <0.01, compared to the NG; #P <0.05; ##P <0.01, compared to the MG.
3.7. Open-Field Test Results
The open-field test was commonly used to assess the autonomous activity of FD rats. The results revealed that the MG rats had a decrease in distance and mobile time, and had an improvement in immobile time, compared to the NG (P < 0.01). While the distance and mobile time were obviously improved, and immobile time decreased in FD rats treated with PTS-L, PTS-M and PTS-H compared to the MG (P < 0.05, 0.01). The open-field test indicated that PTS could improve the autonomous activity of FD rats (Fig. 7).
Fig. (7).
Open-field test in FD rats. (A). The tract plot. (B). Open-field moving distance. (C). Open-field immobile time. (D) Open-field mobile time. Abbreviation: NG-normal group; MG-model group; DOM-domperidone-treated group; PTS-L-low dose of PTS group; PTS-M-middle dose of PTS group; PTS-H-high dose of PTS group. The data were expressed as mean ± SD of 16 rats in each group. *P <0.05; **P <0.01, compared to the NG; #P <0.05; ##P <0.01, compared to the MG.
3.8. Effects of PTS on MTL, GAS, 5-HT and CGRP Levels in Gastric Tissue of FD Rats
To confirm the prediction of Network pharmacology, the results of the molecular biology experiments are listed. It was found that the levels of 5-HT and CGRP were improved rapidly in the MG (P < 0.05, 0.01), with lower MTL and GAS values (P < 0.01), compared to the NG. While the PTS with middle and high dose treatment could reduce the activity of 5-HT and CGRP (P < 0.05, 0.01), and the PTS-L could also reduce the activity of 5-HT (P < 0.05). In addition, the activity of MTL and GAS was increased after the treatment of PTS of FD rats, compared to the MG (P < 0.05, 0.01) (Fig. 8). The results indicated again that PTS could ameliorate visceral hypersensitivity in FD rats, and thus enhance gastrointestinal motility.
Fig. (8).
Effects of PTS on MTL, GAS, 5-HT and CGRP levels in gastric tissue of FD rats. (A). The level of MTL in gastric tissue. (B). The level of GAS in gastric tissue. (C). The level of 5-TH in gastric tissue. (D). The level of CGRP in gastric tissue. Abbreviation: NG-normal group; MG-model group; DOM-domperidone-treated group; PTS-L-low dose of PTS group; PTS-M-middle dose of PTS group; PTS-H-high dose of PTS group. The data were expressed as mean ± SD of 12 rats in each group. *P <0.05; **P <0.01, compared to the NG; #P <0.05; ##P <0.01, compared to the MG.
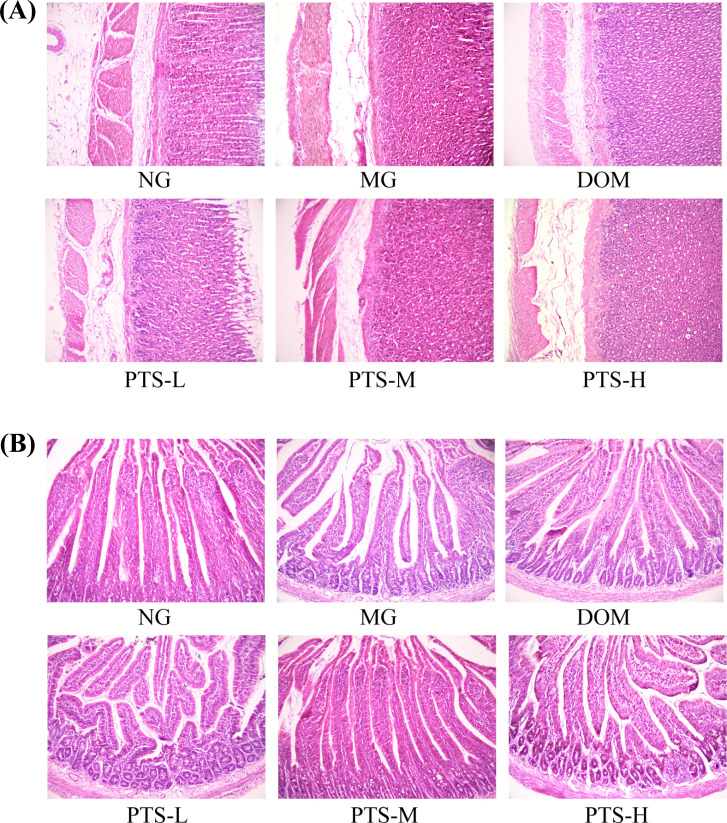
3.9. HE Staining of the Stomach and Duodenum
The histopathological results showed that the mucosa of gastric antrum and duodenum in each group was smooth, and the mucosal epithelium was intact. No chronic inflammation was observed in the gastric histology among the groups (Fig. 9), which perfectly matched the classic theory of FD without organic lesions [19].
Fig. (9).
Effect of PTS on histopathological changes in the stomach and duodenum. (A). The morphology and structure of gastric tissue. (HE staining, 200×). (B). The morphology and structure of duodenum tissue. (HE staining, 200×). Abbreviation: NG-normal group; MG-model group; DOM-domperidone-treated group; PTS-L-low dose of PTS group; PTS-M-middle dose of PTS group; PTS-H-high dose of PTS group.
4. DISCUSSION
Visceral hypersensitivity occurs when the digestive tract is unresponsive to physiological stimuli and/or has a strong response to noxious stimuli, which is an important phenomenon that can clarify many unexplained gastrointestinal symptoms [20, 21]. 160 patients with FD were examined in a clinical study. After applying pressure to the balloon, it was observed that 54% of patients had gastric hypersensitivity. Visceral hypersensitivity has been reported to be related to 5-HT [22]. However, due to the complexity of the bioactive components of TCM and their many targets, the mechanism of action of PTS in the treatment of FD is still unclear.
In this study, we established an FD rat model using iodoacetamide, which was administered by gavage, combined with tail clamping. The model rats showed decreased body weight, poor appetite, and increased gastric acidity and secretion, with no detectable gastric pathological changes, and these findings were consistent with those of patients with FD [23].
Previous studies have revealed that weakened gastric and duodenal motility caused by mechanical or chemical stimulation is a common pathophysiological change that causes FD. Gastric emptying in FD patients is slower compared to healthy individuals [24]. In addition, a study has reported that some gastrointestinal hormones, such as MTL and GAS, are closely related to gastrointestinal motility. MTL regulates digestive movement, stimulates the gastrointestinal tract to perform physiological electrical and mechanical movements, and accelerates gastric emptying [25]. GAS regulates gastrointestinal motility and promotes the secretion of gastric acid and pepsin [26]. In this study, autonomous activity, gastric emptying, and intestinal propulsion were ameliorated in rats with FD, and the levels of MTL and GAS were improved in FD rats after the administration of PTS. However, the mechanisms of action behind these improvements remain unknown.
It is unrealistic to assume that FD can be cured through a single target because the pathogenesis and progression of FD involve multiple pathways, such as the CRF pathway, the NO-cGMP-PKG pathway, and the EC cell-5HT3R pathway [27-29]. To define the mechanism of action of PTS in the treatment of FD, PPI, GO, and KEGG analyses were carried out. The results of GO enrichment analysis revealed that PTS may ameliorate the symptoms of FD through several pathways, including receptor activity and signal transduction. The results of KEGG enrichment analysis indicated that multiple signaling pathways are involved, such as those related to serotonergic synapses, inflammatory bowel disease, and IL-17 activation. The results of PPI analysis revealed that the levels of targets, such as IL1B, TNF, PTGS2, IFNG, TP53, SERPINE1, MIF, TGFB1, CRH, and NFE2L2, were the highest, which was quite different from the results of enrichment analysis. FD pathogenesis involves multiple signaling pathways,and these targets and pathways should be systematically analyzed during analysis. Consideration should also be given from a pathway perspective when performing PTS for the study of FD mechanisms.
Visceral hypersensitivity is an important pathological feature of FD, and in our study, a visceral hypersensitive FD animal model was established using IA and adult tail-clamping [29, 30]. 5-HT has been reported to play an important role in visceral hypersensitivity [31]. Five targets, including HTR3A (5-TH receptor 3A), SLC6A4 (SERT), PTGS1 (COX1), CYP2C19 (cytochrome P450), and PTGS2 (COX2), were components of the 5-HT synapse pathway, and showed a close relationship with 5-HT regulation in the results of KEGG enrichment analysis. The 5-TH3A receptor is a subtype of the 5-TH3 receptor, and it has been reported that the 5-HT3 receptor is an excitatory mediator in visceral sensory pathways and 5-HT3 antagonists may reduce the level of perception of visceral distention [27, 32]. PTGS1 (COX1) and PTGS2 (COX2), which are important enzymes downstream of the 5-TH-related pathway, can oxidize arachidonic acid to PGs. CYP2C19 is also an important enzyme in the 5-HT pathway, whereas SLC6A4 plays an important role in the reuptake of 5-HT, indicating that these targets play important roles in visceral hypersensitivity.
Visceral hypersensitivity is a crucial pathophysiological mechanism in FD [33]. Although the mechanism of visceral paresthesia is not yet fully understood, it is generally believed to be related to the abnormal secretion of gastrointestinal hormones, such as CGRP and 5-HT. We observed that CGRP could delay gastric emptying, inhibit gastrointestinal motility, and suppress gastrointestinal hormone secretion, including GAS and MTL. 5-HT, an indole derivative that is widely distributed in the central nervous and gastrointestinal systems, is mainly produced by enterochromaffin cells in the gastrointestinal tract. Studies have reported that gastric sensitivity is associated with an increase in 5-HT [29, 34], which participates in physiological processes, such as gastrointestinal movement and secretion, by binding to different 5-HT receptors.
It has been reported that visceral hyperesthesia is significantly related to increased 5-HT secretion. The mechanism may involve 5-HT3 receptors, 5-HT4 receptors, and 5-HT1A receptors, as all are involved in visceral hyperesthesia. 5-HT3 receptors and 5-HT4 receptors are involved in transient hyperalgesia, whereas 5-HT1A receptors are involved in long-term severe hyperalgesia [35]. Furthermore, studies have revealed that the plantar mechanical pain threshold is positively correlated with visceral hypersensitivity, and the results of a plantar mechanical pain threshold study reflect the increased visceral hypersensitivity of model rats [36]. In this study, after two weeks of administration of PTS, the levels of 5-HT and CGRP decreased, which may have reduced the visceral sensitivity of FD rats. At the same time, the results of animal experiments are consistent with the predictions of network pharmacology, suggesting that PTS may alleviate the symptoms of FD by regulating serotonergic synapses.
CONCLUSION
In conclusion, the results of this study indicated that PTS may reduce 5-HT secretion by regulating the serotonergic synaptic pathway and reducing visceral sensitivity, which can alleviate the symptoms of FD. At the same time, PTS promoted gastric emptying and intestinal propulsion in rats, inhibited the secretion of gastric acid, and improved autonomous activity, thereby treating the symptoms of FD.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- 5-HT
Serotonin
- ANOVA
Analysis of Variance
- CGRP
Calcitonin Gene Related Peptide
- DOM
Domperidone-Treated Group
- EC
Enterochromaffin
- EPS
Epigastric Pain Syndrome
- FD
Functional Dyspepsia
- GAS
Gastrin
- GO
Gene Ontology
- HE
Hematoxylin and Eosin
- KEG
Kyoto Encyclopedia of Genes and Genomes
- MG
Model Group
- MTL
Motilin
- NG
Control Group
- PDS
Postprandial Distress Syndrome
- PPI
Protein-Protein Interaction
- PTS
Pitongshu
- SEA
Similarity Ensemble Approach
- TCM
Traditional Chinese Medicine
- TCMSP
Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform
AUTHOR’S CONTRIBUTION
Li-Jie Zhu and Han-Song Wu performed all experiments and drafted the manuscript; Yi-Hui Zhi, Shu-Hua Shen and Kun-Gen Wang discussed and revised the manuscript. Lin-Zi Li and Bo Li were responsible for the project management. Gui-Yuan Lv and Su-Hong Chen designed and funded support for this research. All authors carefully revised the whole text of the manuscript and agreed to submit the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The animal protocol was approved by the ethics committee of Zhejiang University of Technology, China (SCXK (Zhe) 2019-0002).
HUMAN AND ANIMAL RIGHTS
No humans were used in this study. All animal research procedures were followed in accordance with the standards set forth in the eighth edition of Guide for the Care and Use of Laboratory Animals (published by the National Academy of Sciences, The National Academies Press, Washington, D.C.).
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The datasets supporting the conclusions of this article are available in a public database from TCMSP, SEA, DisGeNET and the Swiss Target Prediction Database.
FUNDING
This study was supported by the Research and Development of Wang’s empirical formula in hospital (No. KYY-HX-20190884) and the National Key Research and Development Program of China (No. 2017YFC1702202). Pitongshu (PTS) was obtained from Professor Kun-Gen Wang’s clinically effective prescription for the treatment of Functional Dyspepsia (FD).
CONFLICT OF INTEREST
The author(s) declare no conflict of interest, financial or otherwise.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher's website along with the published article.
Table S1 (459.3KB, zip) : Active ingredients of PTS obtained through TCMSP. Table S2 (459.3KB, zip) : Potential targets of PTS obtained from the TCMSP, SEA and Swiss database. Table S3 (459.3KB, zip) : Disease targets of FD obtained from the DisGeNET (Supplementary Materials).
REFERENCES
- 1.Li J., Chen Y., Li Y. Consensus on the diagnosis and treatment of functional dyspepsia with integrated traditional Chinese and western medicine. Chin. J. Integr. Med. 2017;25(12):889–894. doi: 10.19538/j.ek2022010602. [DOI] [Google Scholar]
- 2.Enck P., Azpiroz F., Boeckxstaens G., Elsenbruch S., Feinle-Bisset C., Holtmann G., Lackner J.M., Ronkainen J., Schemann M., Stengel A., Tack J., Zipfel S., Talley N.J. Functional dyspepsia. Nat. Rev. Dis. Primers. 2017;3:17081. doi: 10.1038/nrdp.2017.81. [DOI] [PubMed] [Google Scholar]
- 3.Ford A.C., Marwaha A., Sood R., Moayyedi P. Global prevalence of, and risk factors for, uninvestigated dyspepsia: A meta-analysis. Gut. 2015;64(7):1049–1057. doi: 10.1136/gutjnl-2014-307843. [DOI] [PubMed] [Google Scholar]
- 4.Kwan A.C., Bao T., Chakkaphak S., Chang F.Y., Ke M., Law N.M., Leelakusolvong S., Luo J.Y., Manan C., Park H.J., Piyaniran W., Qureshi A., Long T., Xu G.M., Xu L., Yuen H. Validation of Rome II criteria for functional gastrointestinal disorders by factor analysis of symptoms in Asian patient sample. J. Gastroenterol. Hepatol. 2003;18(7):796–802. doi: 10.1046/j.1440-1746.2003.03081.x. [DOI] [PubMed] [Google Scholar]
- 5.Vakil N., Howden C., Moayyedi P., Tack J. White paper AGA: Functional dyspepsia. Clin. Gastroenterol. Hepatol. 2017;15(8):1191–1194. doi: 10.1016/j.cgh.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Lacy B.E., Weiser K.T., Kennedy A.T., Crowell M.D., Talley N.J. Functional dyspepsia: The economic impact to patients. Aliment. Pharmacol. Ther. 2013;38(2):170–177. doi: 10.1111/apt.12355. [DOI] [PubMed] [Google Scholar]
- 7.Koduru P., Irani M., Quigley E.M.M. Definition, pathogenesis, and management of that cursed dyspepsia. Clin. Gastroenterol. Hepatol. 2018;16(4):467–479. doi: 10.1016/j.cgh.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Miwa H., Watari J., Fukui H., Oshima T., Tomita T., Sakurai J., Kondo T., Matsumoto T. Current understanding of pathogenesis of functional dyspepsia. J. Gastroenterol. Hepatol. 2011;26(Suppl. 3):53–60. doi: 10.1111/j.1440-1746.2011.06633.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhi Y., Shen W., Wang K., Lin Y., Huang L., Shen S., Sun J., Cai L. The mechanism of pitongshu in treating functional dyspepsia. J. Zhejiang Chin. Med. Univ. 2018;42(01):8–16. doi: 10.16466/j.issn1005-5509.2018.01.002. [DOI] [Google Scholar]
- 10.Gao K., Yang R., Zhang J., Wang Z., Jia C., Zhang F., Li S., Wang J., Murtaza G., Xie H., Zhao H., Wang W., Chen J. Effects of Qijian mixture on type 2 diabetes assessed by metabonomics, gut microbiota and network pharmacology. Pharmacol. Res. 2018;130:93–109. doi: 10.1016/j.phrs.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Zhu C., Zhao L., Zhao J., Zhang S. Sini San ameliorates duodenal mucosal barrier injury and low-grade inflammation via the CRF pathway in a rat model of functional dyspepsia. Int. J. Mol. Med. 2020;45(1):53–60. doi: 10.3892/ijmm.2019.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J., Xu L., Lv L., Zeng E., Zhang Z., Wang F., Tang X. Xiangsha liujunzi decoction alleviates symptoms in rats with functional dyspepsia through EGC-derived NGF. BMC Complement. Altern. Med. 2015;15:387. doi: 10.21203/rs.3.rs-818846/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhan L., Dong Y., Yang K., Lei S., Li B., Teng X., Zhou C., Luo R., Yu Q., Jin H., Lv G., Chen S. Soporific effect of modified suanzaoren decoction and its effects on the expression of cck-8 and orexin-A. J. Evid. Based Complementary Altern. Med. 2020;2020:6984087. doi: 10.1155/2020/6984087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu J., Tong H., Ye X., Zhang J., Huang Y., Yang M., Zhong L., Gong Q. The effects of low-dose and high-dose decoctions of Fructus aurantii in a rat model of functional dyspepsia. Med. Sci. Monit. 2020;26:e919815. doi: 10.12659/MSM.919815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W., Chen W., Yang M. Effect of wei fuchun tablet on gastrointestinal function in functional dyspesia rats. Chin. J. Mod. Appl. Pharm. 2019;36(07):829–832. doi: 10.13748/j.cnki.issn1007-7693.2019.07.012. [DOI] [Google Scholar]
- 16.Chen Y.H., Luo R., Lei S.S., Li B., Zhou F.C., Wang H.Y., Chen X., He X., Wang Y.Z., Zhan L.H., Lu T.T., Su J., Yu Q.X., Li B., Lv G.Y., Chen S.H. Anti-inflammatory effect of Ganluyin, a Chinese classic prescription, in chronic pharyngitis rat model. BMC Complement. Med. Ther. 2020;20(1):265. doi: 10.1186/s12906-020-03057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X., Ge H.Z., Lei S.S., Jiang Z.T., Su J., He X., Zheng X., Wang H.Y., Yu Q.X., Li B., Lv G.Y., Chen S.H. Dendrobium officinalis six nostrum ameliorates urate under-excretion and protects renal dysfunction in lipid emulsion-induced hyperuricemic rats. Biomed. Pharmacother. 2020;132:110765. doi: 10.1016/j.biopha.2020.110765. [DOI] [PubMed] [Google Scholar]
- 18.Fan Y., Zhao Y. Research progress of cuttlebone. Chin. J. Ethnomed. Ethnopharm. 2016;25(04):47–48. [Google Scholar]
- 19.Stanghellini V., Chan F.K., Hasler W.L., Malagelada J.R., Suzuki H., Tack J., Talley N.J. Gastroduodenal disorders. Gastroenterology. 2016;150(6):1380–1392. doi: 10.1053/j.gastro.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Xue Z., Wu C., Wei J., Xian M., Wang T., Yang B., Chen M. An orally administered magnoloside A ameliorates functional dyspepsia by modulating brain-gut peptides and gut microbiota. Life Sci. 2019;233:116749. doi: 10.1016/j.lfs.2019.116749. [DOI] [PubMed] [Google Scholar]
- 21.Sayuk G.S., Gyawali C.P. Functional dyspepsia: diagnostic and therapeutic approaches. Drugs. 2020;80(13):1319–1336. doi: 10.1007/s40265-020-01362-4. [DOI] [PubMed] [Google Scholar]
- 22.Camilleri M., Coulie B., Tack J.F. Visceral hypersensitivity: Facts, speculations, and challenges. Gut. 2001;48(1):125–131. doi: 10.1136/gut.48.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomita T., Oshima T., Fukui H., Watari J., Miwa H. Role of acid in functional dyspepsia. Nihon Rinsho Jpn. J. Clin. Med. 2015;73(7):1202–1208. [PubMed] [Google Scholar]
- 24.Haag S., Talley N.J., Holtmann G. Symptom patterns in functional dyspepsia and irritable bowel syndrome: relationship to disturbances in gastric emptying and response to a nutrient challenge in consulters and non-consulters. Gut. 2004;53(10):1445–1451. doi: 10.1136/gut.2003.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitazawa T., Kaiya H. Regulation of gastrointestinal motility by motilin and ghrelin in vertebrates. Front. Endocrinol. (Lausanne) 2019;10:278. doi: 10.3389/fendo.2019.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kazemi M., Eshraghian A., Hamidpour L., Taghavi S. Changes in serum ghrelin level in relation to meal-time in patients with functional dyspepsia. United European Gastroenterol. J. 2015;3(1):11–16. doi: 10.1177/2050640614563373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gershon M.D., Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132(1):397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Wu Z., Lu X., Zhang S., Zhu C. Sini-san regulates the NO-cGMP-PKG pathway in the spinal dorsal horn in a modified rat model of functional dyspepsia. Evid. Based Complement. Alternat. Med. 2020;2020:3575231. doi: 10.1155/2020/3575231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao J., Zhao L., Zhang S., Zhu C. Modified Liu-Jun-Zi decoction alleviates visceral hypersensitivity in functional dyspepsia by regulating EC cell-5HT3r signaling in duodenum. J. Ethnopharmacol. 2020;250:112468. doi: 10.1016/j.jep.2019.112468. [DOI] [PubMed] [Google Scholar]
- 30.Talley N.J., Ford A.C. Functional dyspepsia. N. Engl. J. Med. 2015;373(19):1853–1863. doi: 10.1056/NEJMra1501505. [DOI] [PubMed] [Google Scholar]
- 31.Stanghellini V., De Ponti F., De Giorgio R., Barbara G., Tosetti C., Corinaldesi R. New developments in the treatment of functional dyspepsia. Drugs. 2003;63(9):869–892. doi: 10.2165/00003495-200363090-00003. [DOI] [PubMed] [Google Scholar]
- 32.Moss H.E., Sanger G.J. The effects of granisetron, ICS 205-930 and ondansetron on the visceral pain reflex induced by duodenal distension. Br. J. Pharmacol. 1990;100(3):497–501. doi: 10.1111/j.1476-5381.1990.tb15836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simrén M., Törnblom H., Palsson O.S., van Tilburg M.A.L., Van Oudenhove L., Tack J., Whitehead W.E. Visceral hypersensitivity is associated with GI symptom severity in functional GI disorders: Consistent findings from five different patient cohorts. Gut. 2018;67(2):255–262. doi: 10.1136/gutjnl-2016-312361. [DOI] [PubMed] [Google Scholar]
- 34.Zhang B., Huo C., Li Y., Zhu H. Effect of radix saposhnikoviae extract on mast cells in colonic mucosa of rats with diarrhea predominant irritable bowel syndrome through 5 -hydroxytryptamine signaling axis. J. Zhejiang Chin. Medical Univ. 2021;45(08):857–865. doi: 10.16466/j.issn1005-5509.2021.08.008. [DOI] [Google Scholar]
- 35.Geerts I.S., De Meyer G.R., Bult H. Collar-induced elevation of mRNA and functional activity of 5-HT(1B) receptor in the rabbit carotid artery. Br. J. Pharmacol. 2000;131(8):1723–1731. doi: 10.1038/sj.bjp.0703732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verne N.G., Robinson M.E., Price D.D. Hypersensitivity to visceral and cutaneous pain in the irritable bowel syndrome. Pain. 2001;93(1):7–14. doi: 10.1016/S0304-3959(01)00285-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher's website along with the published article.
Table S1 (459.3KB, zip) : Active ingredients of PTS obtained through TCMSP. Table S2 (459.3KB, zip) : Potential targets of PTS obtained from the TCMSP, SEA and Swiss database. Table S3 (459.3KB, zip) : Disease targets of FD obtained from the DisGeNET (Supplementary Materials).
Data Availability Statement
The datasets supporting the conclusions of this article are available in a public database from TCMSP, SEA, DisGeNET and the Swiss Target Prediction Database.