Abstract
All RNA viruses produce and use RNA-dependent RNA polymerases (RdRPs) for their genome replication. Efficient viral genome replication also requires host factors which often function as co-factors of viral RdRP. To identify host factors required for nodavirus genome replication, we carried out an unbiased genetic screen in Caenorhabditis elegans mutants defective in antiviral RNA interference. This genetic screen utilized a self-replicating GFP-tagged viral replicon, derived from flock house virus, as a reporter for the loss of viral genome replication. Upon completing the screen, 16 candidate alleles were isolated and assigned to 14 candidate genes through genetic complementation. Interestingly, 4 of the candidate genes were also found to be required for the genome replication of Orsay virus, a nodavirus that naturally infects C. elegans. Our unbiased genetic screen therefore has led to the identification of a set of worm genes conserved for nodavirus genome replication.
Introduction
Viruses are obligate intracellular pathogens that absolutely rely on host factors for replication. A complete viral replication cycle involves complex and specific interactions in between viral products and host factors. Since many of these interactions are essential for viruses to survive or to reproduce themselves targeting and disrupting these interactions may slow down or completely inhibit virus replication. Thus, identification and mechanistic study of host factors involved in viral replication may facilitate the development of novel antiviral therapeutics.
Due to their small body size, short life span, hermaphroditic lifestyle and genetic contractability Caenorhabditis elegans nematodes have been successfully used for genetic dissection of important biological pathways (1). Recently, four plus-stranded RNA viruses, Orsay virus, Santeuil nodavirus, Leblanc nodavirus and melnik virus, have been found to naturally infect nematode worms (2–4). Of these four viruses Orsay virus is the only one that can naturally infect C. elegans, making the Orsay virus-C. elegans combination an ideal model system for studying virus replication and antiviral mechanisms using genetic approaches. These nematode infecting viruses are closely related to nodaviruses, such as flock house virus (FHV), although they may differ in gene expression for genomic RNA2 (2, 5). The RNA1 genome of Orsay virus encodes viral replicase, an RNA-dependent RNA polymerase (RdRP). Orsay virus RNA2 encodes coat protein and another structure protein with currently unknown function (5). Orsay virus infection can be easily initiated by feeding C. elegans with virus-containing bacterial food. Using a reporter gene that is inducible to Orsay virus infection the Wang lab at University Washington in St. Louis has successfully identified several host factors required for Orsay virus cell entry, suggesting that the Orsay virus-C. elegans system can serve as powerful genetic system to dissect the genetic pathways mediating virus-host interactions (6–8).
All RNA viruses in classes III, IV and V of Baltimore classification system produce and use RdRP to replicate their genome. Class IV RNA viruses, namely plus-stranded RNA viruses, produce their RdRP through direct translation of the viral genome, making it possible to initiate their infection by delivering viral genome through a transgene strategy (9–15). Interestingly, when the infection of Orsay virus is initiated from a transgene, which bypasses the cell entry step, viral replication is still confined in intestine cells like that during natural infection, suggesting that some host factors absolutely required for Orsay virus replication is missing in non-intestine cells (9). Nevertheless, the success in delivering Orsay virus into C. elegans as a transgene makes it possible to simultaneously inoculate a very large number of worms for random genetic screen. However, an Orsay virus derived replicon tagged for direct readout of viral genome replication is still lacking, making it impossible to use Orsay virus as a reporter to identify host factors specifically required for viral genome replication.
Although an insect origin, FHV replicates efficiently and triggers potent RNAi-mediated antiviral response in C. elegans (10). As illustrated in Figure 1 A, FR1gfp is an FHV RNA1-derived replicon which produces bright green fluorescence in response to heat induction in RNAi defective worm mutants (11). Since the green fluorescence is produced upon translation of a subgenomic RNA, termed RNA3, which can only be produced by replicating FR1gfp, the production of green fluorescence is thus a direct readout of viral genome replication. Thus, RNAi defective C. elegans mutants carrying a heat inducible FR1gfp transgene will serve us well as reporter worm strains in identifying worm genes involved in FHV genome replication through random genetic screen. The fact that FHV RdRP is a homologue of Orsay virus RdRP and both viruses replicate well in C. elegans suggests that worm genes involved in FHV genome replication may also contribute to Orsay virus genome replication.
Figure 1.
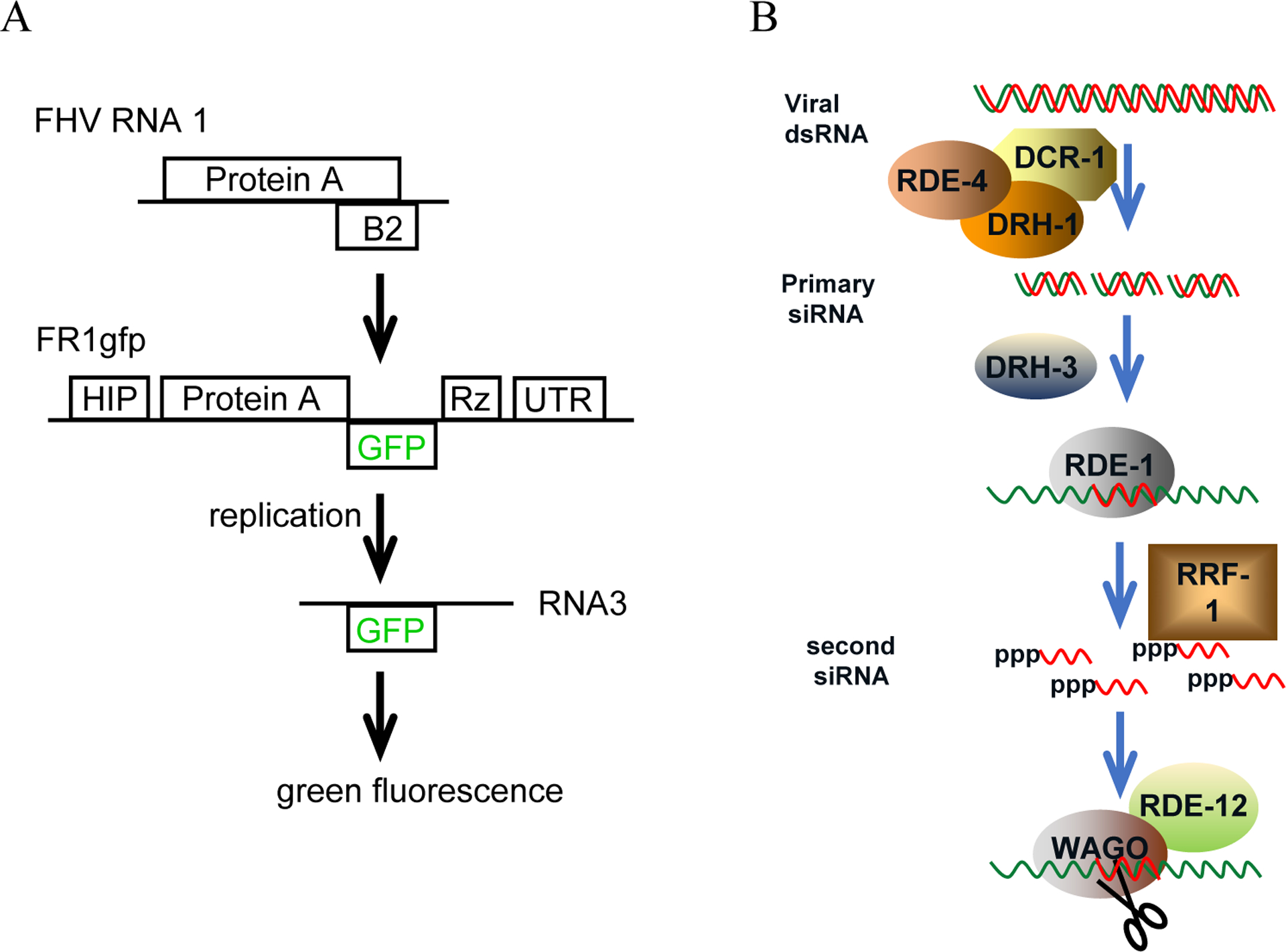
Schematic structure of FR1gfp replicon transgene and current model for antiviral RNAi in C. elegans.
(A) Schematic structure of FR1gfp replicon transgene. Protein A, FHV replicase, an RNA-dependent RNA polymerase. B2, the viral suppressor of RNAi. HIP, heat inducible promoter. GFP, the open reading frame that encodes enhanced green fluorescence protein. Rz, the self-cleaving ribozyme sequence from hepatitis delta virus. UTR, the 3’ end untranslated region of worm gene unc-54. The open reading frames were not drawn to scale. (B) Schematic presentation of current model for antiviral RNAi in C. elegans. DCR-1, the worm dicer. DRH-1, a DEAD box RNA helicase involved in the processing of dsRNA into siRNA by DCR-1. DRH-3, a DEAD box RNA helicase that functions downstream of primary siRNA biogenesis. RDE-1, an Argonaut protein, into which primary siRNAs are loaded. RDE-4, an dsRNA binding protein involved in the processing of dsRNA into siRNA by DCR-1. RRF-1, an RNA-dependent RNA polymerase required for the biogenesis of secondary siRNAs. RDE-12, a DEAD box RNA helicase that promotes the production of secondary siRNAs. WAGO, worms-specific Argonaut proteins that execute the cleavage of RNA transcripts targeted by secondary siRNAs.
To identify worm genes required for FHV and Orsay virus genome replication we have recently carried out a large-scale random genetic screen that utilizing FR1gfp as loss of viral genome replication reporter. This screen led to the identification of 14 worm genes required for FHV genome replication. Importantly, we found that 4 of these candidate genes also contribute to Orsay virus replication initiated through natural infection or heat inducible transgene. Since transgene mediated virus delivery bypasses the cell entry step we believe that most of those 4 candidate genes may play important roles in enabling Orsay virus genome replication. Considering the fact that the RdRP function domains are well conserved in diverse RNA viruses, some of the identified worm genes may encode key factors required for the genome replication of diverse RNA viruses, including those infecting plants and vertebrates. Thus, although the genetic identity of our candidate genes remains to be revealed we believe that function and mechanistic study on these genes will lead to a better understanding of how RNA viruses replicate their genome with the aid of host factors.
Results
1. Development of a reporter worm strain for the identification of genes required for FHV replication.
In C. elegans RNA interference (RNAi) is a major antiviral mechanism that detects and processes virus-produced double-stranded RNAs into primary small interfering RNAs (siRNAs). These primary siRNAs will then activate the production of secondary siRNAs which guide the destruction of virus genome transcripts (11, 16, 17). Accumulating evidence suggests that, as illustrated in Figure 1 B, DRH-1 (Dicer-related RNA helicase 1) and RDE-4 (RNAi defective 4) contribute to the biogenesis of primary viral siRNAs through distinct mechanisms (18) whereas RDE-1, a worm Argonaut protein, recruits primary viral siRNAs and activates the production of secondary siRNAs by RRF-1 in a DCR-1-independent manner (19–21). Secondary siRNAs are 22 nucleotides (nt) in length and differentiate themselves from primary siRNAs by carrying a tri-phosphate group at the 5’ end. Secondary siRNAs may mediate target transcript cleavage executed by secondary Argonaut proteins (22). Previously, it was also shown that a second Ago protein, C04F12.1, also contributes to worm antiviral RNAi through currently unknown mechanism (11). Since multiple factors contribute to the production or function of primary siRNAs, loss of function for drh-1, rde-1 or rde-4 alone does not completely abolish antiviral RNAi. Therefore, in order to enhance the sensitivity of our genetic screen we chose to use FR1gfp-transgenic drh-1;rde-1;rde-4 triple mutant as the reporter of loss of viral genome replication. To this end we first generated an rde-4 null allele through CRISPR mediated genome editing. We then combined this null allele with null alleles corresponding to drh-1 (ok3495) and rde-1 (ok569) and an FR1gfp transgene allele through genetic crosses. To find out whether the triple mutants as reporters will indeed be more sensitive in picking up potential target alleles we compared FR1gfp replication in single, double and triple mutants corresponding to drh-1, rde-1 and rde-4. As shown in Figure 2 A, enhanced FR1gfp replication was readily detected in the triple mutants as compared to the double mutants, which supported higher level of viral replication than the single mutants. Correspondingly, we observed uniform bright green fluorescence in young and fully developed adults (Figure 2 B). These results together suggest that by using the drh-1:rde-1:rde-4 triple mutants as reporter worms we will have better chance in picking up genetic alleles that only partially compromise FR1gfp genome replication.
Figure 2.

Development of worm reporter for the identification of genes required for nodavirus genome replication.
(A) Accumulation of FR1gfp genomic and subgenomic RNAs in single, double or triple mutants corresponding to drh-1, rde-1 and rde-4. All worm strains contain the same FR1gfp replicon transgene. Total RNA samples were prepared 24 hours post heat induction. The FR1gfp genomic RNA, RNA1, and subgenomic RNA, RNA3, were detected using alkaline phosphatase labelled GFP cDNA. Methylene stained ribosomal RNA (rRNA) was detected and used as equal loading control. (B) Green fluorescence produced by FR1gfp in the triple mutant 24 hours post heat induction.
2. Identification of worm genes required for FHV genome replication.
To identify C. elegans genes required for FR1gfp replication we carried out a large-scale random genetic screen using drh-1;rde-1;rde-4 triple mutants carrying the FR1gfp transgene array. The genetic screen began with the treatment of synchronized L4 worms with mutagen ethyl methanesulfonate (EMS). We bleached the F1 worms to get synchronized F2 worms for screen. The F2 adults were then screened for loss of viral replication. Upon completing the screen we isolated altogether 16 candidate mutants with significantly reduced green fluorescence after heat induction (Figure 3, A). To confirm that the loss of GFP production is indeed a result of compromised viral replication we checked the accumulation of FR1gfp transcripts in each of the isolated mutants. As shown in Figure 3, B and C, FR1gfp replication is significantly reduced in all isolated candidate mutants, confirming that the loss of green fluorescence is indeed a result of compromised viral genome replication.
Figure 3.
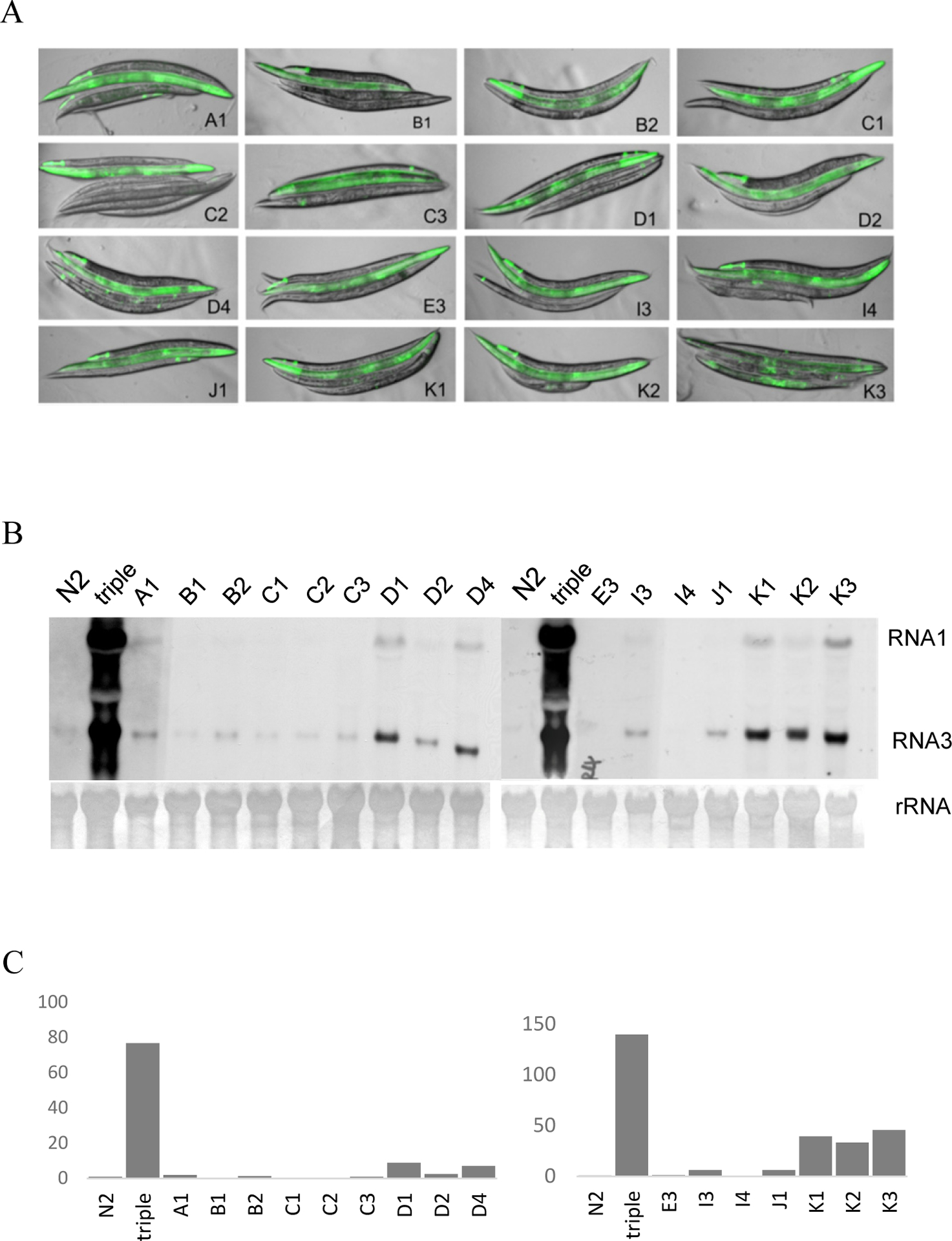
Detection of FR1gfp replication in isolated mutants.
(A) Visualization of FR1gfp replication in candidate mutants corresponding to different alleles as indicated. The worms were stacked up with wildtype N2 on top, drh-1;rde-1;rde-4 triple mutant the second and isolated mutants the rest. The images were produced by merging pictures taken 24 hours post heat induction. All pictures were taken with the same exposure setting. (B) Accumulation of FR1gfp genomic and subgenomic RNAs in worms carrying different alleles as indicated. Triple, the drh-1;rde-1;rde-4 triple mutants. Total RNA samples were prepared 24 hours post heat induction. The FR1gfp genomic RNA, RNA1, and subgenomic RNA, RNA3, were detected using alkaline phosphatase labelled GFP cDNA. Methylene stained ribosomal RNA (rRNA) was detected and used as equal loading control. (C) Quantification of FR1gfp RNA3 accumulation as detected in (B). imageJ was used for this quantification analysis.
3. Characterization of the identified alleles through genetic complementation
To find out whether some of the identified alleles are derived from the same gene we performed genetic complementation assay for all isolated alleles. In this assay, worms containing different alleles were crossed and the F1 worms were examined to see whether FR1gfp replication is compromised like that in parental worms. Theoretically, if two candidate alleles are derived from the same gene the F1 worms will produce the same phenotype as the parental worms. This test successfully assigned the identified alleles, all recessive, to 14 different genes (table 1). We name these candidate genes as viral replication defective (vrd) genes. To find whether any of the identified alleles are temperature sensitive alleles we maintained the corresponding mutants at 25°C to see whether they still breed properly. This test confirmed that none of the identified alleles is temperature sensitive alleles (table 1). However, various developmental defects were observed for some of the mutants (table 1). Probably, some of vrd genes may contribute to important cellular functions and/or worm development.
Table 1.
The sensitivity to skn-1 dsRNA feeding was recorded as whether the eggs laid by the treat worms can hatch.
| Candidate gene | Allele name | Sensitivity to skn-1 dsRNA feeding | Sensitivity to mCherry dsRNA feeding | Temperature sensitivity | Developmental defects |
|---|---|---|---|---|---|
| vrd-1 | A1 | - | - | N/A | Dead eggs |
| vrd-2 | B1 | - | -- | N/A | Egg laying defect |
| vrd-3 | B2 | - | - | N/A | N/A |
| C1 | - | - | N/A | N/A | |
| vrd-4 | C2 | - | - | N/A | Slow growth |
| vrd-5 | C3 | - | - | N/A | Egg laying defect |
| vrd-6 | D1 | - | - | N/A | Dead eggs & slow growth |
| D2 | - | - | N/A | Dead eggs & slow growth | |
| vrd-7 | D4 | - | - | N/A | Egg laying defect |
| vrd-8 | E3 | - | - | N/A | N/A |
| vrd-9 | I3 | - | - | N/A | Slow growth |
| vrd-10 | I4 | - | - | N/A | Egg laying defect |
| vrd-11 | J1 | - | - | N/A | N/A |
| vrd-12 | K1 | - | - | N/A | N/A |
| vrd-13 | K2 | - | - | N/A | dumpy |
| vrd-14 | K3 | - | - | N/A | N/A |
-, not sensitive; + sensitive. The sensitivity to mCherry dsRNA feeding was recorded as whether the red fluorescence can be silenced in the treat worms.
The temperature sensitivity was tested as to whether the worms can breed at 25°C. egg laying defect was defined as inability to lay eggs properly, as a result, some eggs hatch in uterus.
4. The loss of FR1gfp replication is not a result of restored antiviral RNAi in the candidate mutants.
In C. elegans, RNAi can be initiated by feeding worms with E.coli food expressing target gene derived dsRNA (23). To rule out the possibility that the loss of viral replication is due to restored RNAi response in the isolated mutants we fed the candidate mutants with E. coli food expressing skn-1 dsRNA. The function of skn-1 gene is required for intestine development. skn-1 silencing through dsRNA feeding produces dead-egg phenotype. However, we found that eggs laid by all of the candidate mutants hatched normally (table 1). Consistently, silencing of the mCherry transgene triggered by dsRNA feeding was not detected for any of the mutants (table 1), suggesting that the loss of FR1gfp genome replication is not a result of restored RNAi. As reconfirmation, we checked the accumulation of FR1gfp derived siRNAs in the isolated worm mutants by performing northern blotting assay. As shown in Figure 4, we detected abundant FR1gfp derived siRNAs in drh-1;rde-1 double mutants but not in the triple mutants or the isolated mutants. These results together confirmed that the loss of FR1gfp genome replication in the isolated mutants is not a result of restored antiviral RNAi.
Figure 4.

Accumulation of FR1gfp-derived siRNAs in the isolated mutants.
Total worm RNAs were extracted 24 hours post heat induction. Small RNAs were enriched through isopropanol precipitation. 10μg small RNA sample from each worm strain was resolved in denaturing urea PAGE gel. FR1gfp-derived siRNAs of minus polarity were detected using DIG-labelled DNA oligos that cover the entire FR1gfp genome. miRNA-58 was detected and used as equal loading control.
5. Identification of candidate genes are required for Orsay virus replication
Orsay virus RdRP shares high-level global amino acid sequence similarity with RdRP encoded by other worm-infecting viruses, such as Santeuil virus, Le Blanc virus, and Melnik virus, but low-level amino acid sequence homology with FHV RdRP (18% identity, 30% positive). However, within the RdRP domains Orsay virus and FHV share good sequence similarity (24% identity and 39% positive), making it an intriguing question to ask whether some of the vrd genes also contribute to Orsay virus genome replication. To address this question, we performed northern blotting assay to detect Orsay virus replication in the isolated mutants. To this end, we fed the mutants with E. coli food containing Orsay virus particles and checked viral genomic RNA accumulation in total RNA extracted from the mutants. We found that, as shown in Figure 5, Orsay virus replication was significantly reduced in all mutants except those corresponding to vrd-3 (allele C1), vrd-4 and vrd-13, suggesting that some of our candidate genes may also contribute to Orsay virus genome replication.
Figure 5.

Northern blot detection of Orsay virus replication in the isolated mutants.
(A) Accumulation of Orsay virus RNA1 in the candidate mutants, as indicated, was detected using label cDNA corresponding to Orsay virus RNA1. Orsay virus infection was established by feeding worms with OP50 food containing Orsay virus particles. Triple, the drh-1;rde-1;rde-4 triple mutants. ovRNA1, Orsay virus genomic RNA1. Methylene stained ribosomal RNA (rRNA) was detected and used as equal loading control. (B) Quantification of Orsay virus RNA1 accumulation as detected in (A). imageJ was used for this quantification analysis.
6. Identification of candidate genes required for Orsay virus replication initiated from a transgene.
Previously, an Orsay virus replicon has been successfully developed for initiating Orsay virus infection from a transgene in C. elegans (9). Similar to the FR1gfp replicon, this Orsay virus replicon was under the control of a heat inducible promoter and as such the viral infection can be readily initiated by a simple temperature shift. Since viral replication initiated this way does not require a cell entry step, function loss for worm genes involved in virus cell entry should not affect viral replication. Thus, to find out whether any of our candidate genes contribute to the viral genome replication rather than virus cell entry we introduced an Orsay virus replicon transgene into the isolated mutants through genetic crosses and checked Orsay virus replication after heat induction. To find out whether the original mutations that compromises FR1gfp replication are still present in mutants containing the Orsay virus transgene we first checked FR1gfp replication. We found that all, except the vrd-2 worms, mutants showed significant reduction in FR1gfp replication (Figure 6, A and B). As shown in Figure 6, C and D, Orsay virus replication in most of these mutants exhibited clear reduction. Notably, Orsay virus replication in mutants corresponding to vrd-5, vrd-8, vrd-10 and vrd-11 were significantly reduced compared to that in the original reporter worms. Like that for FR1gfp no reduction in Orsay virus replication was detected in vrd-2 worms. This could simply reflect that fact that the original B1 allele was lost during the outcross process. In contrast to the result shown in Figure 5, Orsay virus replication in vrd-4 mutants was also significantly suppressed. Probably, loss of vrd-4 function leads to a suppression on the transcription of Orsay virus replicon transgene. Nevertheless, these results together suggest that some of our candidate genes, such as vrd-5, vrd-8, vrd-10 and vrd-11, are also required for Orsay virus genome replication.
Figure 6.

Detection of Orsay virus replication initiated from a replicon transgene.
(A) Accumulation of FR1gfp genomic and subgenomic RNAs in worms that also contain the same Orsay virus replicon transgene. (B) Quantification of FR1gfp RNA3 accumulation as detected in (A). imageJ was used for this quantification analysis. (C) Accumulation of Orsay virus RNA1 in different mutants as indicated. All worm strains tested contain the same Orsay virus replicon transgene. Triple, the drh-1;rde-1;rde-4 triple mutants. ovRNA1, Orsay virus RNA1. rRNA, ribosomal RNA stained with methylene blue and serves as equal loading control. (D) Quantification of Orsay virus RNA1 accumulation as detected in (C).
Discussion
Both drh-1 and rde-4 are involved in the biogenesis of primary viral siRNAs (24, 25). However, compared to corresponding single mutants, drh-1;rde-4 double mutants accumulated much less virus-derived primary siRNAs and accordingly supported significantly enhanced viral replication. These observations together suggested that drh-1 and rde-4 contribute to the biogenesis of primary viral siRNAs through distinct mechanisms. In supporting this hypothesis, a recent study showed that DRH-1 is required for the production of viral siRNAs derived from the internal regions whereas RDE-4 is mainly responsible for the biogenesis of viral siRNAs covering terminal regions of viral genome (18). Since worm dicer alone is able to efficiently process dsRNA into siRNAs it is possible that primary siRNAs generated in the absence of DRH-1 and RDE-4 can still be loaded into RDE-1 to mediate antiviral silencing (16). Indeed, when we checked viral replication in drh-1;rde-1;rde-4 triple mutants we found that FR1gfp replication is further enhanced in the triple mutants as compared to corresponding double mutants, leading to more uniform green fluorescence in worm bodies (Figure 2, B). This is especially true in younger adult worms, which usually do not produce as bright green fluorescence as fully developed adults. Therefore, by using drh-1;rde-1;rde-4 triple mutants as loss of viral genome replication reporters, we should have further improved the sensitivity of the genetic screen, allowing for the identification of genetic alleles that only partially compromised viral genome replication.
In C. elegans, an endogenous RNAi pathway competes with antiviral RNAi pathway for key factors such as RDE-4 (22, 26, 27). As such genetic mutations that disrupt the endogenous RNAi pathway often causes enhanced antiviral RNAi activity in wildtype worms and single mutants such as those corresponding to drh-1 (11, 28). Thus, when single mutants are used as reporter worms in the genetic screen there will be a higher chance to pick up false positive mutants, in which the reduced viral replication is a result of enhanced antiviral RNAi. However, due to the loss of 3 key antiviral RNAi factors required for primary siRNA biogenesis or function genetic mutations in endogenous RNAi pathway will not be able to cause enhanced antiviral activity in the triple mutants, making the reporter strain we developed an ideal reporter system for the identification of worm genes required for FHV genome replication.
Previously, genetic screens utilizing modified viral replicon and non-natural hosts have led to the identification of numerous host factors required for viral replication (29–31). Currently, however, to most viruses, exactly what and how host factors contribute to viral replication in natural hosts remains poorly explored. Identification of host factors involved in virus replication through random genetic screen requires robust and efficient inoculation of a large number of host organisms, which can be very challenging to achieve to most viruses. However, since all RNA viral viruses use RdRP for replication and closely related RNA viruses may share a common set of host factors for replication it is possible to use alternative model virus as reporter to look for host factors required for viral replication in natural hosts. In this report, we used an FHV-based replicon FR1gfp as a reporter to look for worm genes required for viral genome replication. Since the replication of FR1gfp replicon is initiated from a transgene, which bypasses the cell entry step, our genetic screen was expected to identify worm genes that function in cytoplasm to enable viral genome replication. Since the green fluorescence is produced from a subgenomic RNA, which can only be produced by replicating FR1gfp, most candidate genes may specifically contribute to viral genome replication. Besides, since viral RdRP is the only viral protein product required for the genome replication we believe that most of our candidate genes should directly or indirectly contribute to the viral RdRP function. To our knowledge, this is the first genetic screen that is designed to identify host genes specifically required for viral genome replication. Orsay virus naturally infects C. elegans. Our effort has thus eventually led to the identification of host genes required for a plus-stranded RNA virus to replicate genome in its natural host.
We noticed that Orsay virus replication initiated through natural infection was not compromised to a comparable degree as that for FHV in mutants corresponding to vrd-1, vrd-4 and vrd-13 (Figure 5). This could simply reflect the fact that these genes do not play a major role in Orsay virus replication as they do for FHV. However, when being initiated from a transgene, the replication of both Orsay virus and FR1gfp was significantly reduced in mutants corresponding to vrd-5, vrd-8, vrd-10 and vrd-11 (Figure 6, A and C), suggesting that these two viruses share a common set of worm genes for their genome replication. Although considered as a nodavirus, Orsay virus replicase shares limited sequence homology with FHV replicase (18% identity, 30% positive). This observation and the fact that these two viruses naturally infect distinct organisms suggests that some of our candidate genes may be required for core function of RdRPs encoded by diverse RNA viruses, even those that are not closely related. Thus, identification and function study of the identified genes may not only significantly improve our understanding on RNA virus genome replication in general but also facilitate the development of novel therapeutics that can be used to treat the infection by a wide variety of RNA viruses.
Materials and Methods
Worm genetics and maintenance
The Bristol N2 isolate of C. elegans was used as the reference wild-type strain throughout this study. All the worm strains were maintained at room temperature (22°C) on standard Nematode Growth Medium (NGM). Mutants derived from N2 strains referred to in the text include drh-1(ok3495), rde-1(ok569), and rde-4 (yt4350), which were either acquired from Caenorhabditis Genetics Center (CGC) or created in our lab. The genotypes for these mutants were confirmed by PCR and sanger DNA sequencing. An Orsay virus replicon transgene located on X chromosome was generated through gonad injection and delivered into isolated worm mutants through genetic crosses. For maintenance, all worm stocks maintained with OP50 food on NGM plates.
Feeding RNAi
The skn-1 feeding RNAi assay was performed using a feeding RNAi protocol described previously (32). For mCherry feeding RNAi the full-length coding sequence of mCherry was inserted into L4440 and the resulting construct was transferred into HT115 bacteria which will produce mCherry dsRNA upon IPTG induction. 4 hours of IPTG treatment was used to induce the dsRNA production before the medium being seeded on the standard ampicillin NGM agar plates. 20 gravid hermaphrodites were placed on feeding RNAi plates to lay eggs at the room temperature (RT). Score the fed worm phenotypes until they reach to young or fully developed adults. As negative control, worms were provided with the feeding RNAi bacteria containing empty L4440 vector.
Generation of null allele through CRISPR mediated deletion
Two guide RNAs, namely crRNAs, were used to specify left and right cut sites in the gene. crRNAs were designed using the online tool provided by Integrated DNA Technologies (IDT). Whenever possible, guide RNAs with highest specificity at cut sites and low off-target affinity were selected to produce desired deletion in the gene. ssDNA oligo donor designed for Homology Directed Repair (HDR) consisted of 30 nucleotides upstream of left cut site and annealed to 30 nucleotides downstream of right cut site. If required, the PAM sequence was changed in the donor DNA. S. pyogenes Cas9 3NLS, tracrRNA, guide RNAs (crRNAs), and ssDNA donor were ordered and obtained from IDT. Before injection, 0.5μl Cas9, 5μl tracrRNA and 1.4μl each of the two crRNAs were mixed and incubated at 370C for 15 minutes. Subsequently, 2.2μl ssDNA and 1.6μl PRF4::rol-6(su1006) plasmid from 500ng/μl stock were added. Bring the final volume to 20 μl with nuclease free water. Young adults of N2 worms were injected with this injection mixture and singled out in separate NGM plates containing OP50 food. Two plates that had the highest number of roller phenotypes among F1 offsprings were selected, and around 40 F1 rollers and/or non- rollers from these two plates were singled out in separate NGM plates. The F1 worms were then genotyped through PCR for desired deletion after they have laid eggs. Homozygous deletion was confirmed by further genotyping at least 10 F2 worms from F1 mother that appeared homozygous for the desired deletion.
Worm genetic crosses and genetic complementation
Males are generated by either natural occurrence or immersed in 15% alcohol (room temperature for 15minutes) intending to avoid virus expression if the transgenic worms carry the heat-inducible viral replicon. All genetic crosses were carried out on NGM plate containing 100mM ampicillin. Male to hermaphrodite ratio varied depending on the nature of the transgene and or genetic allele. Homozygous F2 were characterized with PCR genotyping or feeding RNAi or both. Genetic complementation was conducted to find out if some of the isolated alleles are derived from the same gene. Upon the crosses in between mutants carrying distinct alleles being conducted, the production of green fluorescence on F1 adult males were visualized 24 hours post heat induction. Identified alleles that produced fluorescent F1 males upon successful cross were assigned to the same candidate gene.
Ethyl methanesulfonate (EMS) mutagenesis
L4 worms of synchronized drh1;rde1;rde4 triple mutants carrying the FR1gfp replicon transgene were washed off the plates using M9 buffer and collected in a sterile 15ml tube with 2ml M9 buffer and 2 ml of 2x stock EMS solution (100mM). Animals in the tubes were incubate for 6 hours at room temperature with gently shaking every 30mins. The treated worms were then washed 4 times with 10ml M9 buffer to remove trace EMS. The supernatant is aspirated in another tube with 1M NaOH to inactivate EMS. Treated worms were then transferred to three 10cm NGM plates. F1 worms produced from these treated worms were bleached and the eggs were collected and transferred on 10 10cm NGM plates. F2 adults were screened and positive candidates were selected for further confirmation.
FR1gfp induction and Orsay virus infection
Induction of FR1gfp in transgenic worms was conducted by heat shocking synchronized young adult worms at 33°C for 2 hours and then maintained at 25°C up to 48 hours. Green fluorescence production was visualized and recorded using a dissecting microscope coupled with UV illumination. Orsay virus was prepared by washing infected and freshly starved JU1580 isolate with 5ml autoclaved ddH2O per NGM plate. After spinning at 10000g to separate viral particles from worms and bacterial cells the supernatant was filtrated with 0.22μm filter unit. The filtrate can be directly mixed with OP50 food for virus inoculation or stored in 20% glycerol at −80C degree for future use.
RNA preparation and detection
The detection of FR1gfp and Orsay virus genomic and subgenomic RNAs and FR1gfp-derived siRNAs was carried out using protocols described previously (24).
Imaging microscopy
Both the red and green fluorescence images were recorded under the same exposure for each set of images. A Nikon digital camera p7000 mounted on a Nikon SMZ1500 microscope was used to record all of the images.
Acknowledgment
The authors thank the Caenorhabditis Genetics Center for some of the worm strains used in this study; Dr. Erik Jorgensen for the Pmyo-2::mCherry construct; and Dr. Félix, Dr. Miska, and Dr. Wang for the Orsay virus and the C. elegans isolate JU1580. The authors also want to thank Dr.
This work was supported by National Institutes of Health (1R56AI107249-01A1 and 1R01GM119012-01A1).
References
- 1.Jorgensen EM & Mango SE (2002) The art and design of genetic screens: caenorhabditis elegans. Nat Rev Genet 3(5):356–369. [DOI] [PubMed] [Google Scholar]
- 2.Félix M-A, et al. (2011) Natural and Experimental Infection of Caenorhabditis Nematodes by Novel Viruses Related to Nodaviruses. PLoS Biol 9(1):e1000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franz CJ, Zhao G, Félix M-A, & Wang D (2012) Complete Genome Sequence of Le Blanc Virus, a Third Caenorhabditis Nematode-Infecting Virus. J. Virol 86(21):11940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franz CJ, et al. (2014) Orsay, Santeuil and Le Blanc viruses primarily infect intestinal cells in Caenorhabditis nematodes. Virology 448(0):255–264. [DOI] [PubMed] [Google Scholar]
- 5.Jiang H, et al. (2014) Orsay virus utilizes ribosomal frameshifting to express a novel protein that is incorporated into virions. Virology 450–451(0):213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang H, Chen K, Sandoval LE, Leung C, & Wang D (2017) An Evolutionarily Conserved Pathway Essential for Orsay Virus Infection of Caenorhabditis elegans. mBio 8(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandoval LE, Jiang H, & Wang D (2019) The Dietary Restriction-Like Gene drl-1, Which Encodes a Putative Serine/Threonine Kinase, Is Essential for Orsay Virus Infection in Caenorhabditis elegans. J Virol 93(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang H, Sandoval Del Prado LE, Leung C, & Wang D (2020) Huntingtin-interacting protein family members have a conserved pro-viral function from Caenorhabditis elegans to humans. Proc Natl Acad Sci U S A 117(36):22462–22472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang H, Franz CJ, & Wang D (2014) Engineering Recombinant Orsay Virus Directly in the Metazoan Host C. elegans. J. Virol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu R, et al. (2005) Animal virus replication and RNAi-mediated antiviral silencing in Caenorhabditis elegans. Nature 436(7053):1040–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu R, Yigit E, Li WX, & Ding SW (2009) An RIG-I-Like RNA helicase mediates antiviral RNAi downstream of viral siRNA biogenesis in Caenorhabditis elegans. PLoS Pathogens 5(2):e1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinto YM, Kok RA, & Baulcombe DC (1999) Resistance to rice yellow mottle virus (RYMV) in cultivated African rice varieties containing RYMV transgenes. Nat Biotechnol 17(7):702–707. [DOI] [PubMed] [Google Scholar]
- 13.Dalmay T, Hamilton A, Mueller E, & Baulcombe DC (2000) Potato virus X amplicons in arabidopsis mediate genetic and epigenetic gene silencing. Plant Cell 12(3):369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janda M & Ahlquist P (1993) RNA-Dependent Replication, Transcription, and Persistence of Brome Mosaic-Virus Rna Replicons in Saccharomyces-Cerevisiae. Cell 72(6):961–970. [DOI] [PubMed] [Google Scholar]
- 15.Price BD, Roeder M, & Ahlquist P (2000) DNA-Directed Expression of Functional Flock House Virus RNA1 Derivatives in Saccharomyces cerevisiae, Heterologous Gene Expression, and Selective Effects on Subgenomic mRNA Synthesis. J. Virol 74(24):11724–11733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ketting RF, et al. (2001) Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev 15(20):2654–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schott DH, Cureton DK, Whelan SP, & Hunter CP (2005) An antiviral role for the RNA interference machinery in Caenorhabditis elegans. Proc Natl Acad Sci U S A 102(51):18420–18424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coffman SR, et al. (2017) Caenorhabditis elegans RIG-I Homolog Mediates Antiviral RNA Interference Downstream of Dicer-Dependent Biogenesis of Viral Small Interfering RNAs. mBio 8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aoki K, Moriguchi H, Yoshioka T, Okawa K, & Tabara H (2007) In vitro analyses of the production and activity of secondary small interfering RNAs in C. elegans. EMBO J. 26(24):5007–5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pak J & Fire A (2007) Distinct populations of primary and secondary effectors during RNAi in C. elegans. Science 315(5809):241–244. [DOI] [PubMed] [Google Scholar]
- 21.Sijen T, Steiner FA, Thijssen KL, & Plasterk RH (2007) Secondary siRNAs result from unprimed RNA synthesis and form a distinct class. Science 315(5809):244–247. [DOI] [PubMed] [Google Scholar]
- 22.Yigit E, et al. (2006) Analysis of the C. elegans Argonaute family reveals that distinct Argonautes act sequentially during RNAi. Cell 127(4):747–757. [DOI] [PubMed] [Google Scholar]
- 23.Timmons L & Fire A (1998) Specific interference by ingested dsRNA. Nature 395(6705):854–854 [DOI] [PubMed] [Google Scholar]
- 24.Guo X, Zhang R, Wang J, & Lu R (2013) Antiviral RNA silencing initiated in the absence of RDE-4, a double-stranded RNA binding protein, in Caenorhabditis elegans. J. Virol 87(19):10721–10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo X, Zhang R, Wang J, Ding S-W, & Lu R (2013) Homologous RIG-I–like helicase proteins direct RNAi-mediated antiviral immunity in C. elegans by distinct mechanisms. Proceedings of the National Academy of Sciences 110:16085–16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simmer F, et al. (2003) Genome-wide RNAi of C. elegans using the hypersensitive rrf-3 strain reveals novel gene functions. PLoS Biol 1(1):E12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee RC, Hammell CM, & Ambros V (2006) Interacting endogenous and exogenous RNAi pathways in Caenorhabditis elegans. RNA 12(4):589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilkins C, et al. (2005) RNA interference is an antiviral defence mechanism in Caenorhabditis elegans. Nature 436(7053):1044–1047. [DOI] [PubMed] [Google Scholar]
- 29.Kushner DB, et al. (2003) Systematic, genome-wide identification of host genes affecting replication of a positive-strand RNA virus. Proceedings of the National Academy of Sciences 100(26):15764–15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao L, et al. (2008) Drosophila RNAi screen identifies host genes important for influenza virus replication. Nature 454(7206):890–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hao L, et al. (2014) Genome-Wide Analysis of Host Factors in Nodavirus RNA Replication. PLOS ONE 9(4):e95799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo X, Li W-X, & Lu R (2012) Silencing of host genes directed by virus-derived short interfering RNAs in Caenorhabditis elegans. J. Virol 86(21):11645–11653. [DOI] [PMC free article] [PubMed] [Google Scholar]


