Abstract
Allogeneic hematopoietic stem cell transplantation (HCT) has potential to cure hematologic malignancies, but is associated with significant morbidity and mortality. While deaths during the first year after transplant are often attributable to treatment toxicities and complications, death after the first year may be due to sequelae of accelerated aging caused by cellular senescence. Recipients of younger cells tend to have decreased molecular markers of aging and improved survival. Given that umbilical cord blood (UCB) is the youngest donor source available, we studied the outcomes after the first year of UCB transplant vs. matched related donor (MRD) and matched unrelated donor (MUD) transplant in patients with hematologic malignancies over a 20-year period. After adjusting for selected covariates, UCB recipients who survived at least 1 year after HCT had a hazard of death that was 31% lower than that of MRD/MUD recipients. This trend held true in a subset analysis of subjects with diagnosis of acute leukemia. UCB recipients also experienced lower rates of moderate or severe chronic graft-versus-host disease (GVHD) and non-relapse mortality, and slower time to relapse. UCB and MRD/MUD recipients experienced similar rates of grade 2–4 acute GVHD, chronic GHVD, secondary malignancy, and subsequent allogeneic HCT. UCB is already widely used as a donor source in pediatric HCT; however, adult outcomes and adoption have historically lagged behind in comparison. Recent advancements in UCB transplantation such as the implementation of lower intensity conditioning regimens, double unit transplants, and ex-vivo expansion have improved early mortality, making UCB an increasingly attractive donor source for adults; furthermore, our findings suggest that UCB may actually be a preferred donor source for mitigating late effects of HCT.
Keywords: umbilical cord blood, survivorship, allogeneic transplant, mortality
Structured Abstract
Background:
Allogeneic hematopoietic stem cell transplantation (HCT) has the potential to cure hematologic malignancies, but is associated with significant morbidity and mortality. While deaths during the first year after transplant are often attributable to treatment toxicities and complications, death after the first year may be due to sequelae of accelerated aging caused by cellular senescence. Cytotoxic therapies and radiation used in cancer treatments and conditioning regimens for HCT can induce aging at the molecular level; HCT patients experience time-dependent effects, such as frailty and aging-associated diseases, more rapidly than people who have not been exposed to these treatments. Consistent with this, recipients of younger cells tend to have decreased markers of aging and improved survival, decreased GVHD, and lower relapse rates.
Objectives:
Given that umbilical cord blood (UCB) is the youngest donor source available, we studied the outcomes after the first year of UCB transplant vs. matched related donor (MRD) and matched unrelated donor (MUD) transplant in patients with hematologic malignancies over a 20-year period.
Study Design:
In this single center, retrospective study, we examined the outcomes of all adult patients who underwent their first allogeneic HCT through the Duke Adult Bone Marrow Transplant (ABMT) program from January 1, 1996 to December 31, 2015, to allow for at least 3 years of follow-up. Patients were excluded if they died or were lost to follow-up before day 365 post-HCT; received an allogeneic HCT for a disease other than a hematologic malignancy; or received cells from a haploidentical or mismatched adult donor.
Results:
UCB recipients experienced a better unadjusted overall survival than MRD/MUD recipients (log rank p=0.03, Figure 1, median OS: UCB not reached, MRD/MUD 7.4 years). After adjusting for selected covariates, UCB recipients who survived at least 1 year after HCT had a hazard of death that was 31% lower than that of MRD/MUD recipients (HR: 0.69, 95% CI: 0.47–0.99, p=0.049). This trend held true in a subset analysis of subjects with acute leukemia. UCB recipients also experienced lower rates of moderate or severe chronic graft-versus-host disease (GVHD) and non-relapse mortality, and slower time to relapse. UCB and MRD/MUD recipients experienced similar rates of grade 2–4 acute GVHD, chronic GHVD, secondary malignancy, and subsequent allogeneic HCT.
Conclusions:
UCB is already widely used as a donor source in pediatric HCT; however, adult outcomes and adoption have historically lagged behind in comparison. Recent advancements in UCB transplantation such as the implementation of lower-intensity conditioning regimens, double unit transplants, and ex-vivo expansion have improved early mortality, making UCB an increasingly attractive donor source for adults; furthermore, our findings suggest that UCB may actually be a preferred donor source for mitigating late effects of HCT.
Introduction
Allogeneic hematopoietic stem cell transplantation (HCT) has the potential to cure a wide range of malignant and non-malignant pathologies. However, it is also associated with high rates of morbidity and mortality1, the risks of which are greatest during the first year post-HCT and are most commonly attributable to infections, GVHD, relapse, and treatment-related toxicities2–5. Deaths after the first year of HCT are also common, but the causes usually shift to cGVHD, relapse, or long-term adverse effects of the chemotherapies and radiation employed in the HCT process6–8.
Treatment-related toxicities cause accelerated aging, and thus HCT patients experience time-dependent effects, such as frailty and aging-associated diseases, more rapidly than people who have not been exposed to these treatments9. Cytotoxic therapies and radiation used in cancer treatments and conditioning regimens for HCT can induce cellular senescence, telomere attrition, cell growth arrest, mitochondrial dysfunction, amplified proinflammatory response, and increased reactive oxygen species10; in fact, cellular senescence has been implicated in an accelerated aging process that is thought to affect survivors of HCT10.
Related to this, retrospective studies have shown that patients who receive cells from younger donors generally experience more favorable transplant outcomes, such as increased overall survival (OS), decreased GVHD, and lower relapse rates11–14. As the youngest possible donor source, UCB cells have fewer molecular markers of aging compared to other donor options, which may translate to fewer long-term complications and decreased cell senescence post-HCT15. Simultaneously, however, other studies have associated UCB transplants with less favorable early outcomes (e.g., primary graft failure and slower count recovery) and increased mortality within the first year16,17. This has made it difficult to parse out a potential late advantage of UCB from traditional studies that begin at the date of transplant. To better understand long-term outcomes in HCT survivors, we focused on the patients who survived at least 1 year after HCT for a hematologic malignancy, comparing long-term survival in UCB recipients to MRD/MUD recipients.
Methods
Study Design
In this single center, retrospective study, we examined the outcomes of all adult patients who underwent their first allogeneic HCT through the Duke Adult Bone Marrow Transplant (ABMT) program from January 1, 1996 to December 31, 2015, to allow for at least 3 years of follow-up. Patients were excluded if they died or were lost to follow-up before day 365 post-HCT; received an allogeneic HCT for a disease other than a hematologic malignancy; or received cells from a haploidentical or mismatched adult donor. This study was approved by the Duke Health Institutional Review Board.
Data Sources and Measures
Patient demographics and transplant characteristics were extracted from the Duke ABMT database, electronic medical record, and CIBMTR Pre-TED Forms. Data variables of interest included gender, race, ethnicity, transplant diagnosis, transplant year, hematopoietic stem cell transplantation-specific comorbidity index (HCT-CI), disease risk index (DRI), Karnofsky performance score (KPS) at transplant workup, age at transplant, donor cell characteristics, conditioning regimen, date of engraftment, acute GVHD (aGVHD), chronic GVHD (cGVHD), date of relapse, subsequent HCT, secondary malignancy, and date and cause of death. The Duke ABMT program adopted the HCT-CI scoring method in December 2007; therefore, only patients transplanted after December 2007 were assigned an HCT-CI score using the methods described by CIBMTR18. Scores were retrospectively assigned if not previously recorded. DRI was calculated using refined criteria proposed by Armand et al.19 Neutrophil engraftment was defined as the first of three consecutive days that the absolute neutrophil count (ANC) was at least 500/μL and platelet engraftment was defined as the first of three consecutive days that the platelet count was at least 20,000/mm3. Overall survival (OS) was defined as time from transplant to death or last follow-up. Acute and chronic GVHD were graded using standard criteria20,21. For patients whose death information was not available in the electronic medical record, death certificates were procured from local health departments.
Statistics
Baseline characteristics were stratified by donor type (Table 1). Chi-square tests or Fisher’s exact tests were used to compare categorical variables, as appropriate, and Wilcoxon rank-sum tests or t-tests were used to compare continuous variables, as appropriate. Overall survival was estimated using the Kaplan-Meier method and differences between groups were compared using log-rank test. Multivariable analyses were performed using the Cox proportional hazards model. A stepwise selection with significance of entry=0.1 and significance of stay=0.2 was used, and age, gender, decade, and aGVHD were selected into the multivariate model. Other variables that were candidates but not selected for the multivariate model were time from diagnosis to transplant, time to engraftment, donor age, BMI, time to relapse, ethnicity, graft failure, disease status, cGVHD, HCT-CI, and DRI. Causes of death were designated as either death after relapse or death without relapsed disease (NRM) with subsequent categorization of causes of death. We performed a subset analysis excluding patients who relapsed or underwent salvage transplantation prior to 1 year after index transplantation. Because our dataset represents a heterogeneous population, we additionally performed a subset analysis focused on ≥1-year survivors of HCT with a diagnosis of acute leukemia. All p-values were 2-sided. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.5.0.
Table 1.
Demographics for patients surviving more than 1 year after HCT.
| UCB | MRD/MUD | All Patients | ||
|---|---|---|---|---|
| N=102 | N=354 | N=456 | P-Value | |
| (22.40%) | (77.60%) | (100%) | ||
| Age | ||||
| Median (IQR) | 42 (33 – 51) | 49.5 (39 – 58) | 48 (38 – 57) | <0.001 |
| Donor Age | ||||
| Median (IQR) | – | 50 (41–57) | – | – |
| Gender | ||||
| M | 58 (56.9%) | 201 (56.8%) | 259 (56.8%) | 0.99 |
| Race | ||||
| White | 72 (70.6%) | 328 (92.7%) | 400 (87.7%) | <0.001 |
| Black | 25 (24.5%) | 22 (6.2%) | 47 (10.3%) | |
| Asian | 5 (4.9%) | 2 (0.6%) | 7 (1.5%) | |
| American Indian | 0 (0%) | 2 (0.6%) | 2 (0.4%) | |
| Ethnicity | ||||
| Not Hispanic | 96 (94.1%) | 343 (96.9%) | 439 (96.3%) | 0.22 |
| Hispanic | 5 (4.9%) | 7 (2%) | 12 (2.6%) | |
| Unknown | 1 (1%) | 4 (1.1%) | 5 (1.1%) | |
| Pre-HCT BMI | ||||
| Median (IQR) | 27.84 (24.28 – 31.77) | 27.62 (24.25 – 30.96) | 27.63 (24.25 – 31.11) | 0.47 |
| Disease | ||||
| Acute Leukemia | 71 (69.6%) | 174 (49.2%) | 245 (53.7%) | 0.001 |
| MDS/MPN | 7 (6.9%) | 70 (19.8%) | 77 (16.9%) | |
| Lymphoma | 13 (12.7%) | 61 (17.2%) | 74 (16.2%) | |
| Chronic Leukemia | 11 (10.8%) | 49 (13.8%) | 60 (13.2%) | |
| Status at Transplant | ||||
| Complete Remission | 80 (78.4%) | 204 (57.6%) | 284 (62.3%) | 0.0004 |
| Partial Response | 12 (11.8%) | 50 (14.1%) | 62 (13.6%) | |
| Progressive Disease | 4 (3.9%) | 45 (12.7%) | 49 (10.7%) | |
| Stable Disease | 6 (5.9%) | 55 (15.5%) | 61 (13.4%) | |
| Time from diagnosis to HCT | ||||
| Median (IQR) | 390.5 (196–677) | 363.5 (159–872) | 364.5 (166.5–807.5) | 0.22 |
| Decade of HCT | ||||
| 1996–2005 | 24 (23.5%) | 69 (19.5%) | 93 (20.4%) | 0.40 |
| 2006–2015 | 78 (76.5%) | 285 (80.5%) | 363 (79.6%) | |
| TBI | ||||
| n (%) | 85 (83.3%) | 102 (28.8%) | 187 (41%) | <0.001 |
| Conditioning | ||||
| Myeloablative | 62 (60.8%) | 180 (50.8%) | 242 (53.1%) | 0.08 |
| Cell source | ||||
| UCB | 102 (100%) | – | 102 (22.4%) | – |
| Peripheral blood | – | 329 (92.9%) | 329 (72.1%) | |
| Bone marrow | – | 25 (7.1%) | 25 (5.5%) | |
| KPS | ||||
| 80–100 | 89 (87.3%) | 290 (81.9%) | 379 (83.1%) | 0.008 |
| ≤70 | 11 (10.8%) | 64 (18.1%) | 75 (16.4%) | |
| Unknown | 2 (2%) | 0 (0%) | 2 (0.4%) | |
| Time to neutrophil engraftment | ||||
| Median (IQR) | 21.5 (15 – 27) | 16.5 (14 – 20) | 17 (14 – 21) | <.0001 |
| Time to platelet engraftment | ||||
| Median (IQR) | 41 (33 – 52) | 16 (13 – 21) | 19 (14 – 28) | <.0001 |
| Graft failure | ||||
| Y | 12 (11.8%) | 5 (1.4%) | 17 (3.7%) | <.0001 |
| N | 90 (88.2%) | 349 (98.6%) | 439 (96.3%) | |
| Relapse | ||||
| Y | 19 (18.6%) | 86 (24.3%) | 105 (23%) | 0.23 |
| N | 83 (81.4%) | 268 (75.7%) | 351 (77%) | |
| Time to Relapse | ||||
| Median (IQR) | 421 (265 – 1100) | 310.5 (169 – 539) | 342 (189 – 575) | 0.006 |
| aGVHD | ||||
| Y | 65 (63.7%) | 197 (55.6%) | 262 (57.5%) | 0.03 |
| N | 20 (19.6%) | 117 (33.1%) | 137 (30%) | |
| Unknown | 16 (15.7%) | 40 (11.3%) | 56 (12.3%) | |
| aGVHD grade | ||||
| <2 or Unknown | 56 (54.9%) | 224 (63.3%) | 280 (61.4%) | 0.13 |
| ≥2 | 46 (45.1%) | 130 (36.7%) | 176 (38.6%) | |
| cGVHD | ||||
| Y | 31 (30.4%) | 116 (32.8%) | 147 (32.2%) | 0.72 |
| N | 71 (69.6%) | 238 (67.2%) | 309 (67.8%) | |
| cGVHD grade | ||||
| Mild or Unknown | 95 (93.1%) | 289 (81.6%) | 384 (84.2%) | 0.005 |
| Moderate or Severe | 7 (6.9%) | 65 (18.4%) | 72 (15.8%) | |
| HCT-CI | ||||
| ≤3 | 38 (61.3%) | 188 (73.7%) | 226 (71.3%) | 0.052 |
| >3 | 24 (38.7%) | 67 (26.3%) | 91 (28.7%) | |
| HCT before 12/2007 | 40 | 99 | 139 | |
| DRI | ||||
| Low | 20 (19.6%) | 81 (22.9%) | 101 (22.1%) | 0.81 |
| Int | 70 (68.6%) | 222 (62.7%) | 292 (64%) | |
| High | 9 (8.8%) | 38 (10.7%) | 47 (10.3%) | |
| Very High | 3 (2.9%) | 13 (3.7%) | 16 (3.5%) | |
Results
Patient and Transplant Characteristics and Early Transplant Outcomes
Over the 20 years included in this study period, 1066 patients received a first allogeneic HCT from the Duke ABMT program. 219 patients received cells from a mismatched or haploidentical donor and were excluded from the analysis. Of the remaining 847 patients, 224 received UCB and 623 received MRD/MUD cells. 456 of these patients (54%) survived at least 1 year after HCT and were included in this analysis (Figure 1). Of the 102 UCB recipients included, 68 patients received double cord blood transplant, and 12 patients received transplant after ex-vivo expansion.
Figure 1.
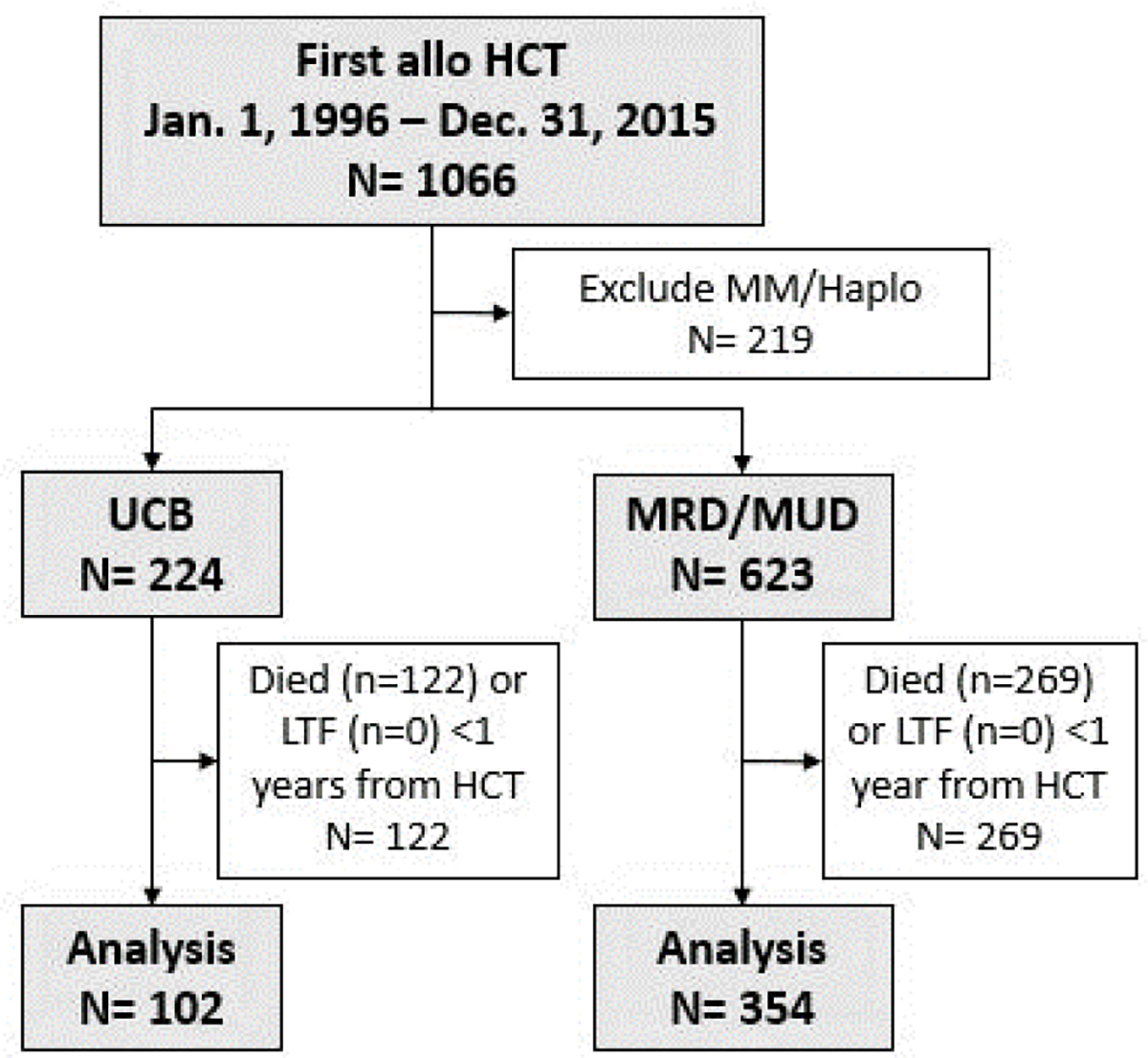
Subject selection.
Among the 456-patient cohort, UCB recipients (n=102, 22%) were more likely to be younger (median age 42 vs. 49.5 years, p<0.001), African American (24.5% vs. 6.2%, p<0.001), diagnosed with acute leukemia (69.6% vs. 49.2%, p=0.001), have better pre-transplant KPS scores (87.3% vs. 81.9% with a score of 80 or above, p=0.008), and be in CR at HCT (78.4% vs. 57.6%, p=0.004) than MRD/MUD recipients. There were no significant differences in gender, ethnicity, pre-HCT BMI, conditioning regimen intensity, HCT-CI, DRI, decade of transplant, or time from diagnosis to transplant between the groups (all p>0.05). The median donor age for MRD/MUD transplants was 49.5 years (Table 1).
UCB recipients experienced slower time to neutrophil engraftment (median: 22 vs. 17 days, p<0.001), slower platelet engraftment (median: 41 vs. 16 days, p<0.001), and increased incidence of primary graft failure (11.8% vs. 1.4%, p<0.001) than MRD/MUD recipients. Both groups experienced similar relapse rates (18.6% vs 24.3%, p=0.23), although earlier relapse was observed in MRD/MUD recipients (median 311 days vs. 421 days, p=0.006). No differences in the rate of grade 2–4 aGVHD (45.1% UCB vs. 36.7% MRD/MUD, p=0.13) or cGVHD were observed (30.4% vs. 32.8%, p=0.72); however, rate of moderate or severe cGVHD was decreased in UCB recipients compared to MRD/MUD recipients (6.9% vs. 18.4%, p=0.005) (Table 1).
Survival and Late Outcomes
UCB recipients presented a better unadjusted overall survival than MRD/MUD recipients (log rank p=0.03, Figure 2, median OS: UCB not reached, MRD/MUD 7.4 years). After selection in a multivariate survival analysis, age, gender, transplant decade, and aGVHD were adjusted for in the final Cox proportional hazard model. UCB recipients again presented with a better adjusted survival outcome than MRD/MUD recipients (HR: 0.69, 95% CI: 0.47–0.99, p=0.049, Table 2). Younger age, female gender, more recent transplant decade, and grade 0–1 aGVHD were also predictive of survival in the multivariate model.
Figure 2.
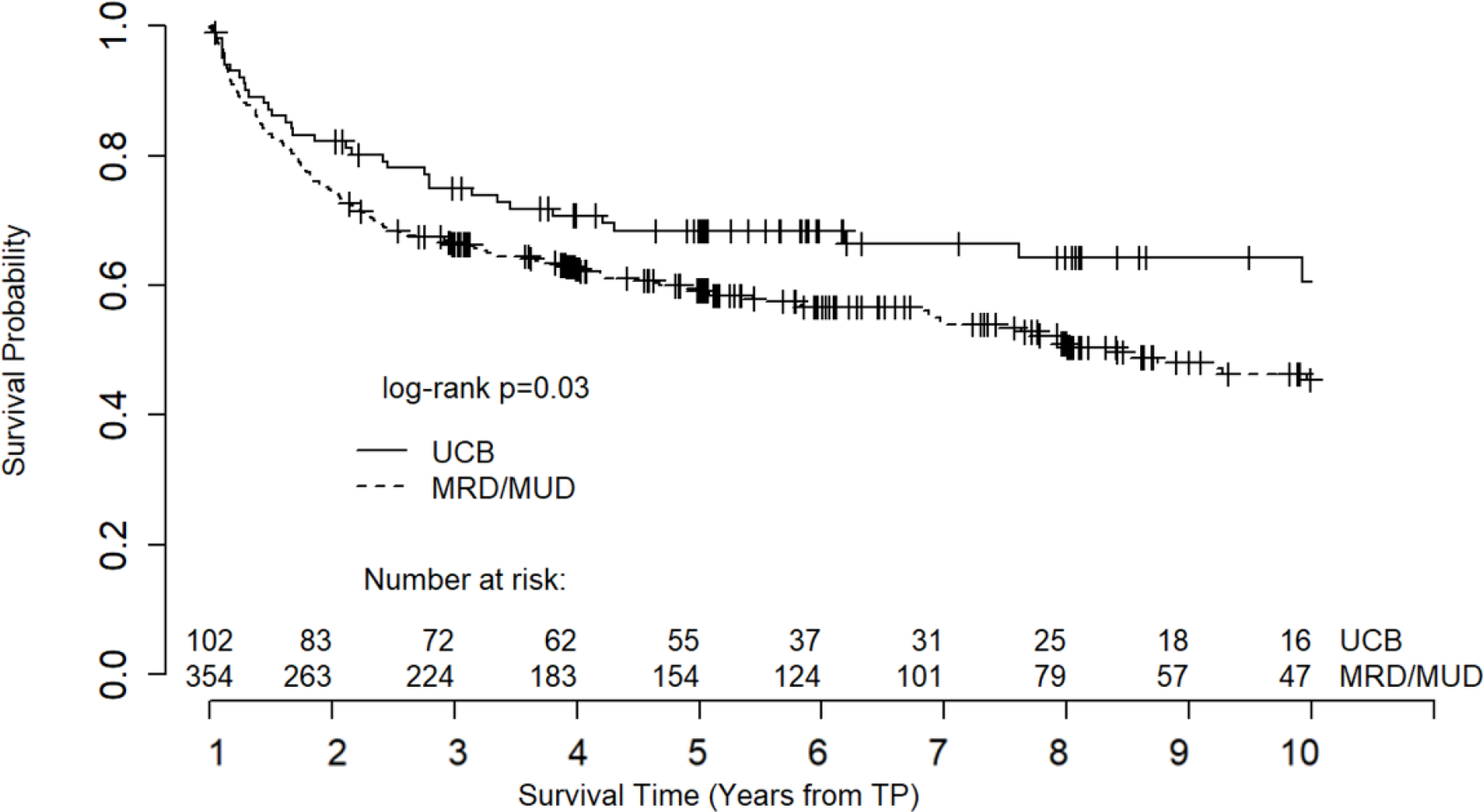
Unadjusted overall survival. Note: Survival curve truncated at 10 years due to declining number at risk.
Table 2.
Cox Proportional Hazard Model on overall survival (covariates after stepwise selection) (N=456, events=202).
| HR (95% CI) | P-Value | Overall P-Value | |
|---|---|---|---|
| Group | |||
| MRD/MUD | -REF- | 0.049 | |
| UCB | 0.69 (0.47 – 0.99) | 0.049 | |
| Age | |||
| Continuous | 1.02 (1.01 – 1.04) | <0.001 | |
| Gender | |||
| M | -REF- | 0.004 | |
| F | 0.65 (0.48 – 0.87) | 0.004 | |
| Decade | |||
| 2006–2015 | -REF- | 0.001 | |
| 1996–2005 | 1.74 (1.24 – 2.43) | 0.001 | |
| aGVHD | |||
| ≥2 | -REF- | 0.02 | |
| <2 or Unknown | 0.71 (0.53 – 0.95) | 0.02 | |
A subset analysis excluding patients who relapsed (n=45) or underwent salvage transplantation (n=10) before 1 year post-transplant found a similar strong trend toward improved survival in UCB recipients compared to MRD/MUD recipients (Supplemental Figure 1). However, this difference was no longer statistically significant (log rank p=0.11), possibly due to sample size.
To further explore the survival trends between the two groups, we performed a subset analysis of ≥1-year HCT survivors with a diagnosis of acute leukemia and found that UCB recipients still showed a strong trend to better overall survival than MRD/MUD recipients, although statistical significance was borderline (log rank p=0.052, Figure 3). The median OS for UCB and MRD/MUD recipients with acute leukemia was 12.9 years and 8.3 years, respectively.
Figure 3.
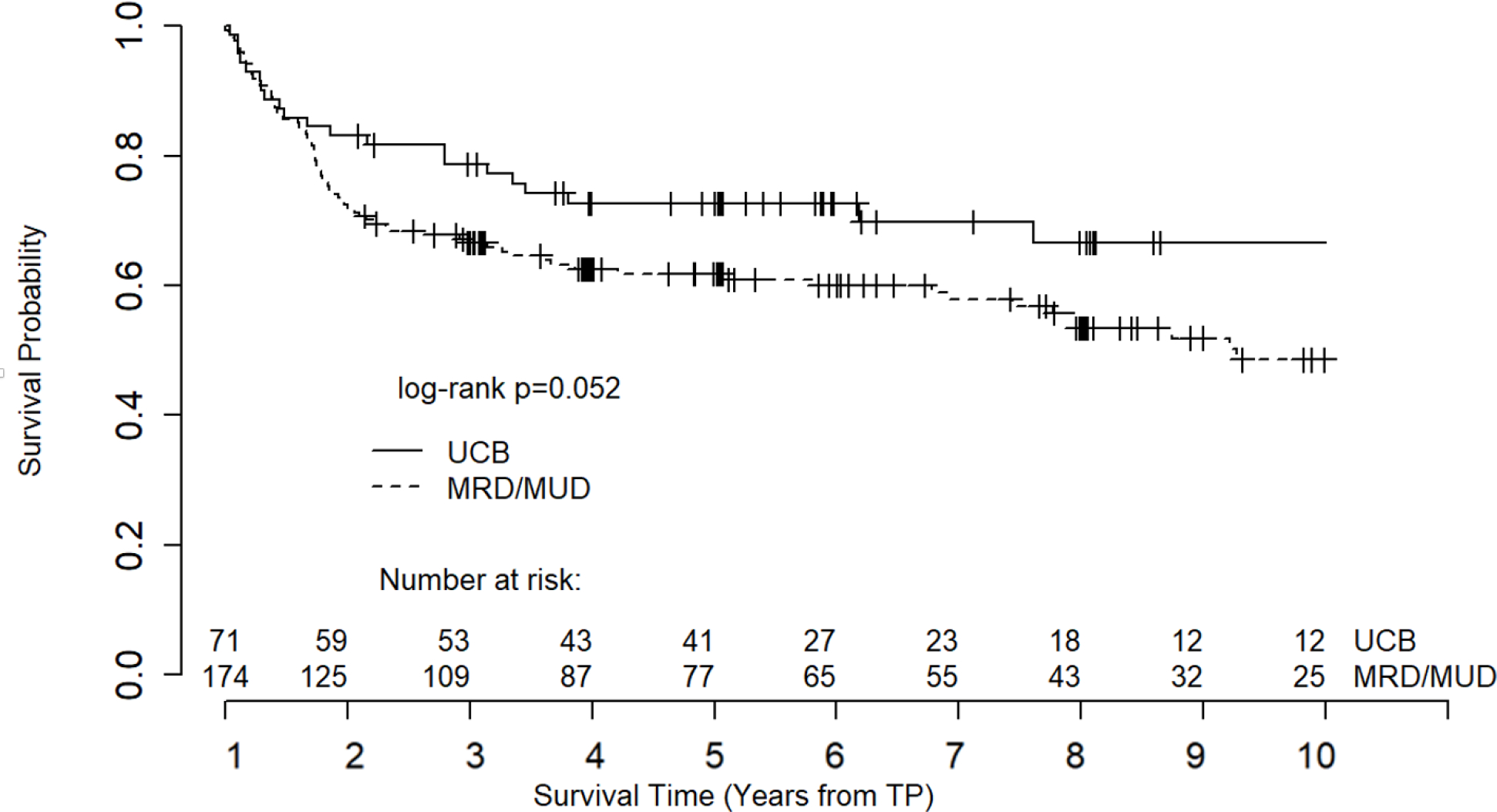
Overall survival of 1-year survivors with diagnosis of acute leukemia. Note: Survival curve truncated at 10 years due to declining number at risk.
Among the 456 patients who lived to 1 year post-HCT, 202 (44%) have since died. No difference in relapse-related mortality (RRM) was observed between MRD/MUD and UCB recipients (19.8% vs. 20.6%, p=0.97). However, the proportion of MRD/MUD recipients who have died of NRM is twice than that of UCB recipients (27.4% vs. 13.7%, p=0.007). The most common causes of NRM included secondary malignancies, infections, organ failure, and GVHD. The specific cause of death could not be determined for 2.2% of the patients, due to incomplete documentation in patient notes preceding 2009 and/or for patients who died outside of North Carolina and whose county of death could not be determined (Table 3).
Table 3.
Cause of Death after 1 year landmark.
| Cause of Death | UCB (n=102) | MRD/MUD (n=354) | Total (n=456) | P-value |
|---|---|---|---|---|
| All-cause mortality | 35 (34.3%) | 167 (47.2 %) | 202 (44.3%) | 0.03 |
| Relapse-related mortality | 21 (20.6%) | 70 (19.8%) | 91 (20%) | 0.97 |
| Non-relapse mortality | 14 (13.7%) | 97 (27.4%) | 111 (24.3%) | 0.007 |
| GVHD | 1 (1%) | 21 (5.9%) | 22 (4.8%) | 0.07 |
| Infection | 6 (5.9%) | 32 (9%) | 38 (8.3%) | 0.42 |
| Organ failure | 5 (4.9%) | 23 (6.5%) | 28 (6.1%) | 0.72 |
| Secondary Malignancy | 1 (1%) | 12 (3.4%) | 13 (2.9%) | 0.34 |
| Unknown/cannot be determined | 1 (1%) | 9 (2.5%) | 10 (2.2%) | 0.57 |
Note: Percentage of all patients who survived ≥1 year
Regarding late effects experienced after HCT, we found that UCB and MRD/MUD recipients underwent subsequent allogeneic HCTs at similar rates (7.8% vs. 7.1%, p=0.96). Recipients of UCB also experienced similar rates of secondary malignancies as recipients of MRD/MUD (6.9% vs. 10.5%, p=0.37).
Although not the primary focus of this study, for completeness we also analyzed survival for all UCB and MRD/MUD recipients, including the 391 who died within 1 year of HCT (Figure 4), as well as death data for these 391 patients. The 1-year mortality rate was 54.4% in UCB recipients and 43.2% in MRD/MUD recipients (Table 4, p=0.004). UCB recipients were more likely to die of NRM (75.4% vs. 54.6% MRD/MUD, p<0.001). This was mostly due to increased infection-related mortality in UCB recipients 32.0% vs. 20.8%, p=0.02). On the other hand, UCB recipients had decreased RRM compared to MRD/MUD recipients (24.6% vs. 45.4%, p<0.001). No differences in death due to GVHD, organ failure, or secondary malignancy were seen between donor sources (Table 4).
Figure 4.
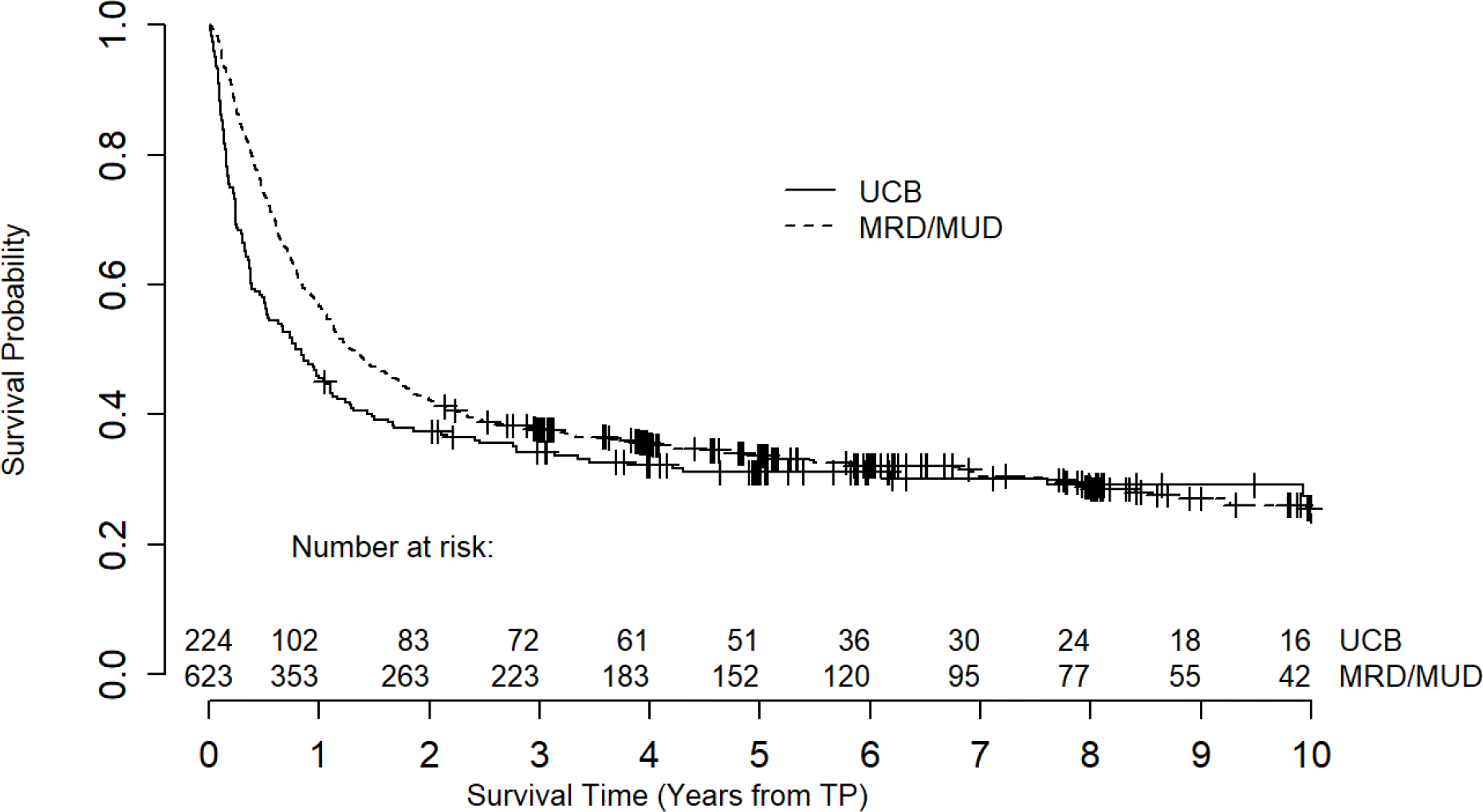
Overall survival of all UCB and MRD/MUD recipients, including those who died before the 1-year landmark.
Table 4.
Cause of death before 1 year landmark.
| Cause of Death before 1 year landmark | UCB (n=122) | MRD/MUD (n=269) | Total (n=391) | P-value |
|---|---|---|---|---|
| All-cause mortality | 122 (54.4%) | 269 (43.2%) | 391 (46.2%) | 0.004 |
| Relapse-related mortality | 30 (24.6%) | 122 (45.4%) | 152 (38.9%) | <0.001 |
| Non-relapse mortality | 92 (75.4%) | 147 (54.6%) | 239 (61.1%) | <0.001 |
| Graft Rejection or Failure | 11 (9%) | 0 (0%) | 11 (2.8%) | (<0.001) |
| GVHD | 6 (4.9%) | 24 (8.9%) | 30 (7.7%) | 0.24 |
| Infection | 39 (32%) | 56 (20.8%) | 95 (24.3%) | 0.02 |
| Organ failure | 35 (28.7%) | 56 (20.8%) | 91 (23.3%) | 0.11 |
| Secondary Malignancy | 1 (0.8%) | 3 (1.1%) | 4 (1%) | >0.99 |
| Unknown/cannot be determined | 0 (0%) | 8 (3%) | 8 (2%) | (0.12) |
Discussion
Umbilical cord blood was first pioneered as a donor option for allogeneic HCT in 198822, and has since become a standard donor source. Public banks maintain inventories of donated cryopreserved UCB cells that are available for patients in need of allogeneic HCT23. Existing literature on UCB outcomes is heterogeneous; while some studies correlate UCB with increased time to engraftment, graft failure, and death within the first year16,17, others report comparable survival between UCB and other donor sources and decreased incidence of severe GVHD in UCB recipients versus MRD recipients16,24,25.
It is well known that the highest risk of transplant-related complications and relapse occurs early after HCT and that the longer after HCT a patient survives, the lower their risk becomes26,27. Given improvements in supportive care transplant approaches, an increasing number of patients now survive to 1 year after HCT; therefore, there is growing interest in the long-term outcomes and prognosis beyond the first year. In this study, we demonstrated that among patients who survived at least 1 year after HCT, UCB recipients had significantly improved subsequent long-term survival compared to MUD/MRD recipients, after adjusting for other clinical factors.
The improved survival observed in UCB recipients appeared to be driven by decreased GVHD-related death (1% vs. 5.9%, p=0.07), though this did not reach statistical significance likely due to sample size. Notably, while the overall instance of cGVHD was similar between groups, UCB recipients in our study experienced a significantly lower rate of moderate or severe cGVHD (6.9% vs. 18.4%, p=0.005), consistent with other reports16,25. Similarly, other studies have associated older donor age with increased cGVHD28,29, suggesting that use of younger donor cells may mitigate cGVHD. Decreased severity of cGVHD may, in turn, be related to decreased frailty: Arora et al. found that compared to autologous HCT recipients, recipients of allogeneic HCT with active cGVHD were at greatly increased risk of frailty (OR 15.02, p<0.001); this difference persisted, at a smaller magnitude, for allogeneic HCT recipients with resolved cGVHD (OR 2.7, p=0.04)30. Thus, extent of cGVHD seems to correlate with frailty, which may be due to the pro-inflammatory pathways in cGVHD. They also found that frailty was associated with a 2.7-fold higher risk of subsequent mortality30. Although aging and frailty do not correlate exactly, there is sufficient clinical overlap to consider frailty an appropriate indicator of aging31.
At the same time, and as discussed in the introduction section, bone marrow transplant recipients have increased susceptibility to accelerated aging processes independent of aGVHD and before the onset of cGVHD32, and multiple studies have shown that use of younger donor cells improves long term outcomes11–14. Bresters et al. found that at a median of 7 years post-pediatric HCT, 93% of patients had at least one late effect of transplant and 24% had more than one late effect of transplant10,33, while another study found that DNA methylation in HCT recipients (i.e., the “epigenetic clock”34) was accelerated to a striking rate of 2.2 years per chronological year post-transplant32. UCB may offer an advantage since using cells from younger donors lowers post-HCT DNA methylation age, even if the subsequent rate of aging remains accelerated.32 Therefore, it is important to highlight that UCB may be improving mortality independent of GVHD. Overall, we theorize that the survival advantage conferred by UCB donor source may be related to attenuation of aging-related late effects through a combination of decreased cGVHD and “resetting the epigenetic clock” with a young donor source. While this paper focuses on survival and does not include detailed reviews of late complications (due to limited records from earlier transplants) or laboratory measures of accelerated aging (due to limited availability of samples) for this cohort of patients, those data would be important for future prospective studies.
In addition to these limitations, as a single center, retrospective study, there are imbalances between study groups. For example, the lower median age of UCB recipients may help explain a survival advantage for UCB, though that advantage persisted even after adjusting for age in the multivariate analysis. Likewise, while UCB recipients had better pre-transplant KPS than MRD/MUD recipients, UCB and MRD/MUD recipients had similar DRI and HCT-CI, which are more objective measures of health status than KPS given that they are based on comorbidities, cytogenetics, and other documented clinical characteristics19,35. UCB recipients were noted to experience greater mortality within 365 days of transplant; it is difficult to definitively determine whether this difference in early mortality reflects increased complications of UCB transplant, such as graft failure, or rather selective attrition of less “fit” UCB patients in the first year. However, in several other studies16,17,36 that have noted increased early mortality for UCB recipients, the difference is thought to be due to increased primary graft failure and slower count recovery leading to infections and other causes of early treatment-related mortality, rather than a baseline survival disadvantage. For example, even after adjusting for patient and disease variables, UCB is associated with delayed hematopoietic recovery (HR = 0.37; 95CI: 0.27–0.52; p<0.001) and increased 100 day transplant-related mortality (HR = 2.13; 95CI: 1.20–3.76;P < .01).36 In this vein, greater rates of complete remission (CR) at the start of HCT in UCB recipients could also be construed as a confounding factor; however, the absence of a difference in relapse-related mortality between the groups suggests that disease status at time of HCT did not appreciably affect outcomes. Likewise, as our cohort of patients experienced similar rates of grade 2–4 aGVHD and relapse, we may postulate that MRD/MUD recipients had worse survival outcomes than UCB recipients due to other factors such as cGVHD severity and late effects of HCT. However, while delayed hematopoietic recovery has not traditionally been associated with fitness, it impossible to exclude a selection bias in this analysis, and thus conclusions from this analysis are only generalizable to subjects who have survived to the landmark timepoint.
Further, we note that despite the limitations of the retrospective and landmark design, it would be unethical to do the gold standard of a randomized clinical trial of UCB vs. MRD/MUD given that MRD has always been the preferred donor source, followed closely by MUD. Therefore, it is only through observational or retrospective studies such as this that we can derive insights into the effects of UCB on accelerated aging and long-term survival. While there are many confounding variables in this retrospective design, our findings of better survival with UCB remain statistically significant upon multivariate analysis. Finally, while the 20+ years of follow-up may be a limitation, it is also a strength in that it allows us to understand the long-term effects of UCB.
There is increasing interest in strategies to improve and prevent late effects of HCT and long-term survival. Our findings suggest a potential long-term advantage to UCB, most likely due to decreased GVHD-related death, that may increase its attractiveness as a donor source. This is especially promising as recent advancements, such as the implementation of lower-intensity conditioning regimens, double unit transplants, and ex-vivo expansion, may help decrease time to engraftment and graft failure rates and have improved early mortality for UCB recipients37. Additional prospective research across larger populations would be beneficial to further explore the long-term outcomes and survival between recipients of UCB and MRD/MUD transplants.
Supplementary Material
Acknowledgements:
This research was supported by National Institute of Aging (NIA) Mini #6 of P30-AG028716-13 (A.D.S.) and the ASH Scholar Award (A.D.S.).
Footnotes
Financial Disclosure Statement: The authors declare no conflict(s) of interest.
References:
- 1.Horan JT, Logan BR, Agovi-Johnson MA, et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made? J Clin Oncol. 2011;29(7):805–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett AJ, Battiwalla M. Relapse after allogeneic stem cell transplantation. Expert Rev Hematol. 2010;3(4):429–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gooley TA, Chien JW, Pergam SA, et al. Reduced Mortality after Allogeneic Hematopoietic-Cell Transplantation. New England Journal of Medicine. 2010;363(22):2091–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohty B, Mohty M. Long-term complications and side effects after allogeneic hematopoietic stem cell transplantation: an update. Blood Cancer J. 2011;1(4):e16–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton BK. Current approaches to prevent and treat GVHD after allogeneic stem cell transplantation. Hematology Am Soc Hematol Educ Program. 2018;2018(1):228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110(10):3784–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29(16):2230–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Styczyński J, Tridello G, Koster L, et al. Death after hematopoietic stem cell transplantation: changes over calendar year time, infections and associated factors. Bone Marrow Transplant. 2020;55(1):126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cupit-Link MC, Kirkland JL, Ness KK, et al. Biology of premature ageing in survivors of cancer. ESMO Open. 2017;2(5):e000250–e000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skinner R, von Zglinicki T. Accelerated Aging in Bone Marrow Transplant Survivors. JAMA Oncology. 2016;2(10):1267–1268. [DOI] [PubMed] [Google Scholar]
- 11.Bastida JM, Cabrero M, Lopez-Godino O, et al. Influence of donor age in allogeneic stem cell transplant outcome in acute myeloid leukemia and myelodisplastic syndrome. Leukemia Research. 2015;39(8):828–834. [DOI] [PubMed] [Google Scholar]
- 12.Shaw BE, Logan BR, Spellman SR, et al. Development of an Unrelated Donor Selection Score Predictive of Survival after HCT: Donor Age Matters Most. Biol Blood Marrow Transplant. 2018;24(5):1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sachiko Seo JK, Atsuta Yoshiko, et al. The Impact of Donor Age on Outcome after Unrelated Bone Marrow Transplantation: Comparison with Unrelated Cord Blood Transplantation. 2015.
- 14.DeZern AE, Franklin C, Tsai H-L, et al. Relationship of donor age and relationship to outcomes of haploidentical transplantation with posttransplant cyclophosphamide. Blood Adv. 2021;5(5):1360–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pipes BL, Tsang T, Peng S-X, Fiederlein R, Graham M, Harris DT. Telomere length changes after umbilical cord blood transplant. Transfusion. 2006;46(6):1038–1043. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi S, Ooi J, Tomonari A, et al. Comparative single-institute analysis of cord blood transplantation from unrelated donors with bone marrow or peripheral blood stem-cell transplants from related donors in adult patients with hematologic malignancies after myeloablative conditioning regimen. Blood. 2006;109(3):1322–1330. [DOI] [PubMed] [Google Scholar]
- 17.Rocha V, Cornish J, Sievers EL, et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. 2001;97(10):2962–2971. [DOI] [PubMed] [Google Scholar]
- 18.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 21.Pidala J, Kim J, Anasetti C, et al. The global severity of chronic graft-versus-host disease, determined by National Institutes of Health consensus criteria, is associated with overall survival and non-relapse mortality. Haematologica. 2011;96(11):1678–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtzberg J, Lyerly AD, Sugarman J. Untying the Gordian knot: policies, practices, and ethical issues related to banking of umbilical cord blood. J Clin Invest. 2005;115(10):2592–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ACOG Committee Opinion No. 771: Umbilical Cord Blood Banking. Obstet Gynecol. 2019;133(3):e249–e253. [DOI] [PubMed] [Google Scholar]
- 24.Ballen K Update on umbilical cord blood transplantation. F1000Res. 2017;6:1556–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma P, Purev E, Haverkos B, et al. Adult cord blood transplant results in comparable overall survival and improved GRFS vs matched related transplant. Blood Adv. 2020;4(10):2227–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SJ, Storer B, Wang H, et al. Providing personalized prognostic information for adult leukemia survivors. Biol Blood Marrow Transplant. 2013;19(11):1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho C, Hsu M, Barba P, et al. Long-term prognosis for 1-year relapse-free survivors of CD34+ cell-selected allogeneic hematopoietic stem cell transplantation: a landmark analysis. Bone marrow transplantation. 2017;52(12):1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paz A, Rigoni L, Fischer G, et al. Donor characteristics and hematopoietic stem cell transplantation outcome: experience of a single center in Southern Brazil. Hematology, Transfusion and Cell Therapy. 2018;40(2):136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watkins BK, Horan J, Storer B, Martin PJ, Carpenter PA, Flowers MED. Recipient and donor age impact the risk of developing chronic GvHD in children after allogeneic hematopoietic transplant. Bone Marrow Transplant. 2017;52(4):625–626. [DOI] [PubMed] [Google Scholar]
- 30.Arora M, Sun C-L, Ness KK, et al. Physiologic Frailty in Nonelderly Hematopoietic Cell Transplantation Patients: Results From the Bone Marrow Transplant Survivor Study. JAMA Oncology. 2016;2(10):1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeda C, Angioni D, Setphan E, et al. Age-Related Frailty: A Clinical Model for Geroscience? J Nutr Health Aging. 2020;24(10):1140–1143. [DOI] [PubMed] [Google Scholar]
- 32.Stölzel F, Brosch M, Horvath S, et al. Dynamics of epigenetic age following hematopoietic stem cell transplantation. Haematologica. 2017;102(8):e321–e323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bresters D, van Gils ICM, Kollen WJW, et al. High burden of late effects after haematopoietic stem cell transplantation in childhood: a single-centre study. Bone Marrow Transplantation. 2010;45(1):79–85. [DOI] [PubMed] [Google Scholar]
- 34.Horvath S DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115–R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaw BE. HCT-CI: Hematopoietic Cell Transplantation-Comorbidity Index ‘Sorror Score’. 2016.
- 36.Rocha V, Cornish J, Sievers EL, et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. 2001;97(10):2962–2971. [DOI] [PubMed] [Google Scholar]
- 37.Gutman JA, Ross K, Smith C, et al. Chronic graft versus host disease burden and late transplant complications are lower following adult double cord blood versus matched unrelated donor peripheral blood transplantation. Bone Marrow Transplant. 2016;51(12):1588–1593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


