Summary
Adaptive immunity to intracellular pathogens and tumors is mediated by antigen-experienced CD8 T cells. Individual naïve CD8 T cells have the potential to differentiate into a diverse array of antigen-experienced subsets that exhibit distinct effector functions, life-spans, anatomic positioning and potential for regenerating an entirely new immune response during iterative pathogenic exposures. The developmental process by which activated naïve cells undergo diversification involves regulation of chromatin structure and transcription but is not entirely understood. This review examines how alterations in chromatin structure, transcription factor binding, extracellular signals and single cell gene expression explain the differential development of distinct effector (TEFF) and memory (TMEM) CD8 T cell subsets that arise after infections. Special emphasis is placed on how Runx-proteins function with additional transcription factors to pioneer changes in chromatin accessibility and drive transcriptional programs that establish the core attributes of cytotoxic T lymphocytes, subdivide circulating and non-circulating TMEM cell subsets, and govern terminal differentiation. The discussion integrates the roles of specific cytokine signals, transcriptional circuits and how regulation of individual nucleosomes and RNA Polymerase II activity can contribute to the process of differentiation. A model that integrates many of these features is discussed to conceptualize how activated CD8 T cells arrive at their fates.
Keywords: Runx transcription factors, nucleosome, chromatin remodeling, transcriptional elongation, CD8 T cell memory, viral infection
Introduction
During intracellular infections or malignancies, individual antigen specific naïve CD8 T cells that become activated have the potential to develop into a wide array of ‘effector’ (TEFF) and ‘memory’ (TMEM) CD8 T cell populations 1,2. Protective TEFF and TMEM cells develop in response to various model intracellular pathogens in mice 3,4 and live viral vaccines in humans 5,6 that cause transient infections, which are efficiently cleared (‘acute infection’). These antigen-experienced CD8 T cell subsets manifest differences in their lifespans, effector functions, capacities for self-renewal and potential for proliferation upon secondary antigen encounter, and their ability to traffic between or localize within distinct lymphoid and non-lymphoid tissue and micro-anatomic locales 7,8. Transcriptional regulation of gene expression governs the differential development of these cells but is incompletely understood and is of longstanding interest 9–12. The central focus of this review is to consider how regulation of chromatin structure, transcription and gene expression during and after the activation of naïve CD8 T cells promotes their progenies to form specific subclasses of long-lived TMEM cells, or to differentiate into TEFF cells that are short-lived.
The CD8 T cell response to an acute infection derives from a small number of naïve antigen-specific CD8 T cells that undergo geometric expansion into a large and heterogeneous population of TEFF cells. During this burst, a comparatively small number of TMEM cells are generated that emerge as the infection resolves. This response follows a categorical pattern of TEFF cell population accumulation, contraction, and TMEM cell formation, but how cells select distinct TEFF and TMEM cell fates is still unclear. To gain insight into how transcriptional control contributes to the process, this review describes multiple established developmental relationships between phenotypically defined antigen-experienced CD8 T cell subsets, and examines how differential gene expression develops within nascent and definitive TEFF and TMEM cell populations. The roles of cytokines that differentially affect the formation of TEFF and TMEM subsets are redefined in the context of the transcriptional circuits and gene expression programs they promote. In addition, the regulation of chromatin structure that occurs as naïve CD8 T cells become activated, the similarities and differences in chromatin structure landscapes that manifest later in distinct TEFF and TMEM cell subsets, and several of the transcription factors (TFs) and chromatin regulatory factors (CRFs) that appear to be involved are discussed. Emphasis is placed on the role of individual nucleosomes and how Runx-family TFs cooperate with additional TF families to pioneer the reprogramming of chromatin accessibility during initial T cell receptor (TCR) stimulation of naïve cells, and how they establish transcriptional circuits that lead to TEFF and TMEM cell differentiation. The function of distal enhancers is discussed, and how regulation of RNA Polymerase II (Pol II) elongation activity might contribute to reinforcing the specific transcriptional programs that differentially drive formation of TEFF and TMEM cells. Finally, a model that integrates observations from all of these aspects is used to conceptualize how naïve CD8 T cells diversify into TEFF and TMEM populations.
The diversity of antigen-experienced CD8 T cells and their putative origins
Antigen-experienced CD8 T cell subsets
An extensive taxonomy of antigen-experienced CD8 T cells that arise during infections in vivo has been defined, and these cell subsets have been reviewed in detail 7,8,13. Only salient points are emphasized here in order to provide the framework for conceptualizing how transcriptional regulation contributes to the diversification process.
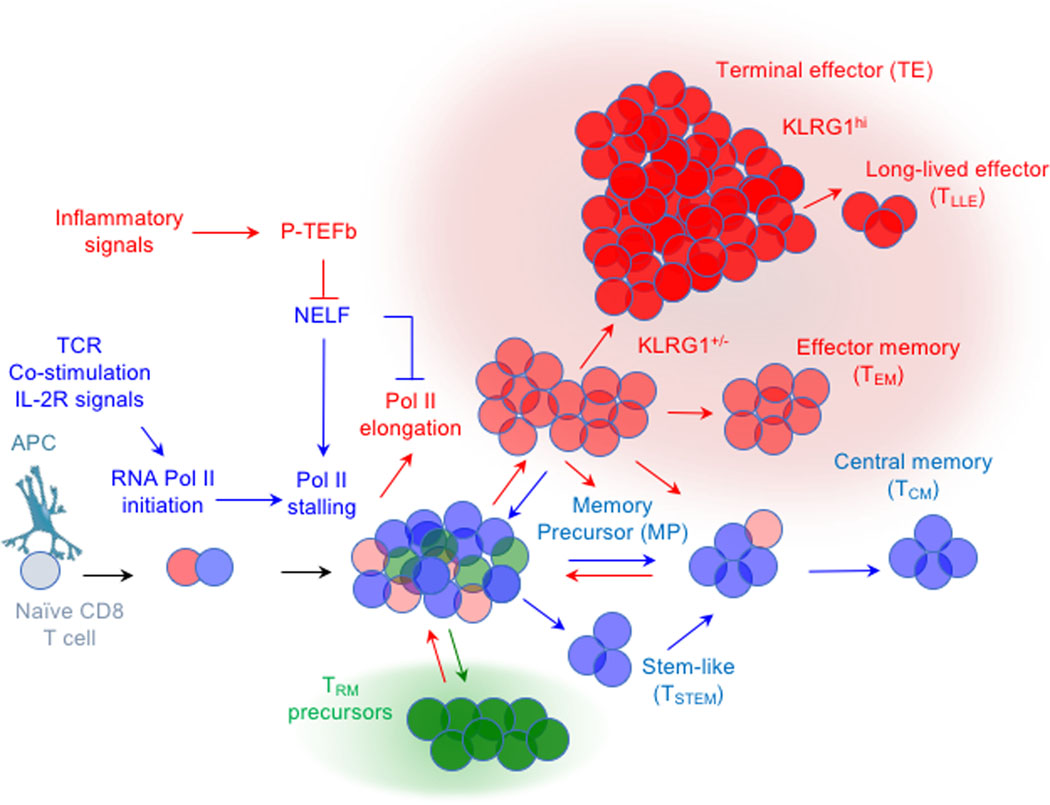
Referring to antigen-experienced CD8 T cells as TEFF and TMEM cells is an imprecise but handy terminology that has its origins in the temporal pattern of a prototypical T cell response to acute infection of mice, which is categorized into effector, contraction and memory phases 11,14,15. The effector phase generally encompasses the time from primary infection to the peak in accumulation of the responding cell population and roughly coincides with the time of pathogen clearance. Cells during this period are termed TEFF cells, although it turns out there is substantial differences in the nature of TEFF cells within the population 11. As the pathogen is cleared, the expanded TEFF cell population ‘contracts’ because many TEFF cells are terminally differentiated and short-lived 16. Over the next few weeks, the declining numbers of TEFF cells ultimately stabilize, which marks the beginning of the memory phase, generally about 30 days after a prototypical acute infection in mice. Cells that persist to this time are considered TMEM cells 16. Although TMEM cells have their origins in the effector phase 17, they are not considered completely manifest in vivo until late times. The identity of TEFF cells early in the response that serve as precursors to TMEM cell subsets later, and the transcriptional regulation that controls whether TEFF cells terminally differentiate or develop into long-lived TMEM cells, are still incompletely understood. Exactly how the entire process occurs remains a matter of intense interest 9,10,12.
Protective TMEM cell subsets that arise after an acute infection resolves are broadly subdivided into circulating (TCIRC) memory cells and tissue-resident memory (TRM) cells 18,19. Within these broad classifications, cells can be further delineated based on phenotype, function, migratory properties and anatomic distribution 8,13. Cells in these subclasses manifest distinct patterns of gene expression and chromatin structure, which suggests that they could be phylogenetically distinct subsets. TCIRC cells consist of effector memory (TEM) and long-lived effector (TLLE) cells (also referred to as terminal-TEM, or effector-like TMEM cells) that patrol the vasculature 20–22, peripheral memory (TPM) cells that recirculate through peripheral tissues and secondary lymphoid organs 23, and central memory (TCM) cells that localize within T cell areas of secondary lymphoid organs 20,23. In addition, memory cells with stem cell like qualities (TSTEM) also localize in lymphoid organs, exhibit overlapping features with TCM cells and some exhibit features of T cells that interact with B cell follicles 24–29. In contrast, TRM cells enter, mature and establish residence in multiple non-lymphoid tissues, wherein they remain largely positioned without re-circulating 13,30. There is additional heterogeneity among TRM cells that is partly related to the tissue in which they reside, and their persistence at different times after infection within the same tissues, implying tissue and temporal dependent regulation 13,31.
Theories of initial TMEM cell development
Despite this taxonomy, and although many population-level precursor-progeny relationships have now been defined, the developmental process by which naïve cells differentiate into distinct TMEM cells is still unclear. Results from longitudinal analysis of cell phenotypes throughout the course of infection, studies using adoptive transfer of phenotypically defined populations, and mathematical modeling have led to linear models of differentiation in which naïve cells initially pass through a TEFF cell stage, before some cells differentiate into TMEM cells 4,11,15,32,33. In contrast, results from lineage tracing using DNA barcodes and single cell transfers support an alternative linear differentiation model in which TCM cells develop directly from activated naïve cells, some of which proliferate more than others and undergo differentiation into TEM and ultimately terminally differentiated TEFF cells 34–37. Separate lineage models have also been described 11,38, and imply that cells early in the response select distinct developmental pathways that lead to the alternative fates of terminally differentiated TEFF cells or long-lived self-renewing TMEM cells 38,39. The separate lineage models provide a solution for the obvious heterogeneity among TEFF cells, and also account for naïve and activated cells that undergo asymmetric cell division 39,40, but do not clarify when divergence into these pathways occurs. More recently, another model leaves behind the notion of defined ‘lineages’, and describes TMEM cell formation as a continuum of cell states that are subdivided into TRM and TCIRC realms that are both arrayed in a tiered descent whereby cells that move into lower tiers lose potential to form or re-form other cells in the continuum 19. Thus, a unifying framework is still outstanding, but will likely include attributes of all of these concepts. In any case, accurately defining this developmental framework will be instrumental for clarifying how gene regulation governs the process in vivo. Insight on both fronts is likely to serve as the basis for more effective vaccination and immunotherapeutic approaches that provide durable immunity against infections and cancers.
CD8 TEFF cell heterogeneity near the peak response to acute infection
Following several model systemic acute infections, the TEFF cell population generally peaks numerically around one week after infection. At this time, TEFF cells with distinguishing phenotypes can be discerned that exhibit distinct proclivities for contributing to formation of TCIRC cell subsets 10,11. During primary TCR stimulation, all naïve CD8 T cells initially downregulate expression of IL-7Rα (CD127), a cytokine receptor that is essential for naïve and TMEM cell survival 41–43. Only some cells re-express CD127 as the infection clears, and these cells preferentially give rise to long-lived TCIRC cells 41,44. Differential expression of both KLRG1 and CD127 on cells has become one of the most extensively used experimental schemes for delineating cells late in the effector phase that possess different potentials to form TMEM cells 45.
Most KLRG1hi CD127lo cells inefficiently persist into the memory phase and have been termed short-lived effector cells (SLEC) or terminal effector (TE) cells 3,41,45–47. Some KLRG1hi cells can give rise to TLLE cells, although most KLRG1hi cells do not survive contraction 20,22. In contrast, KLRG1lo CD127hi cells are termed memory precursor (MP) effector cells, because they efficiently give rise to TEM (CD62Llo CD127hi) and TCM (CD62Lhi CD127hi) subsets 3,41,45–47. In addition, KLRG1hi CD127hi ‘double positive’ effector cells (DPECs) also contribute to several subsets in the memory compartment 3,21,48. Finally, KLRG1lo CD127lo cells can give rise to all KLRG1/CD127 subsets and are referred to as early effector (EE) cells 3,45,46. The differential expression of multiple additional markers including CD27, CD43, CD62L, CXCR3 and CX3CR1 on TEFF cells near the peak response to infection has also been correlated with the development of TCM, TEM and TPM subsets 21,23,34,36,47. Classifying whether each of these phenotypically defined TEFF and TMEM cell subsets are variations along a continuum 7, or are separate cell lineages, is probably not as important as clarifying the regulation of gene expression that defines their development because they each contribute to primary and recall immunity depending on the context.
The point at which TEFF cells become specified into distinct subsets with differential TMEM cell potential prior to the peak response is still unresolved, but there is evidence that lineage-bias develops well before maximum population expansion. The KLRG1/CD127 paradigm is less informative at early times because CD127 is not re-expressed until late in the effector phase, and KLRG1 expression is not an exclusive feature of terminal differentiation 41,48. KRLG1hi and KLRG1lo cells isolated 4.5 days after infection with the Armstrong strain of lymphocytic choriomeningitis virus (LCMVArm) manifest both effector cell qualities and substantial TMEM cell formation potential, although KLRG1hi cells preferentially acquire a TE phenotype and have less TMEM potential 45,49. Differential expression of the interleukin-2 receptor alpha (IL-2Rα), as well as the transcriptional regulators Id2 and Id3, can demarcate cells at early time points that have differential potentials for developing into TE and TEM, or TMEM cells. IL-2Rαlo cells isolated on day 5 after infection preferentially form TCM cells, whereas IL-2Rαhi cells preferentially become TEM cells after adoptive transfer 50–52, which suggests that differential IL-2R signals might contribute to the early heterogeneity in TMEM cell potential 42. The source of this differential developmental potential was further defined using mice with gene-targeted Id2-YFP and Id3-GFP reporter alleles 53. A subset of IL-2Rαlo, KLRG1lo, and CD62Llo cells responding to either Listeria monocytogenes or Vesicular Stomatitis Virus on day 5 more highly express Id3-GFP and slightly less Id2-YFP than others. These Id3hi/Id2lo cells preferentially form MP cells several days later based on KLRG1/CD127 expression, and ultimately give rise to a TCIRC cell population that develops into more secondary TEFF cells upon rechallenge, compared to their Id2hi/Id3lo counterparts 53. Likewise, CD8 T cells from mice with a targeted Tcf7-GFP allele have been used to trace cells expressing the transcription factor (TF) TCF1 (encoded by Tcf7) 40,54. Tcf7-GFPhi cells early in the response express less KLRG1 and other features of terminally differentiated cells, and preferentially give rise to self-renewing TCM subsets after adoptive transfer 28,40,55. The deficiency in either Id3 or Tcf7 compromises the formation of TCM cells 53,55, whereas deficiency in Il2ra (IL-2Rα /CD25) impairs TE and TEM cell formation 42,52,56,57. Thus, cells that express differential amounts of these factors early in the response correlates with their capacity to form TEFF and TCIRC cells, which suggests that cells become lineage-biased prior to formation of canonical TE and MP cells defined later by KRLG1 and CD127 expression.
The origins of TRM and TCIRC subsets are likely to be different
TRM cells and TCIRC cells might develop from distinct TEFF cell precursors. TRM cells that develop in the skin following herpes simplex virus infection in mice derive from a KLRG1lo TEFF cell population 58, which could imply that canonical KLRG1lo CD127hi MP cells are the source of TRM cells. However, a substantial fraction of TRM cells in multiple non-lymphoid tissues develop from KLRG1hi CD127hi DPECs that downregulate KLRG1 48. In addition, MP cells isolated from the spleen, and TRM precursors isolated from non-lymphoid tissues, one week after infection with LCMVArm each exhibit distinct gene expression and chromatin accessibility profiles, which suggests that these subsets are distinct 12,31,59,60. Consistent with this interpretation, TEFF cells that seed TRM cells in the intestinal epithelium are most frequent in the spleen ~4 days after systemic LCMVArm infection 61, substantially earlier than when canonical MP cells become evident 45. Finally, TEFF cells that exhibit increased TRM-associated gene expression while in the circulation preferentially form TRM cells, and lineage tracing studies using DNA barcodes showed their formation is imprinted at the clonal level before entering the skin 62. These results indicate that the precursors of TRM and TCIRC cells might arise differentially at early times.
Additional evidence that supports this conclusion is the fact that the TFs Runx3 (and its obligatory partner, Cbfb) and Blimp1 (encoded by Prdm1), which are each essential for normal TRM formation 59,63, are most highly expressed by TEFF cells on day 5 compared to day 8 after LCMVArm infection 64. Depletion of either Runx3 or Cbfb results in a near complete loss in formation of KLRG1hi CD127hi DPECs, but only a partial reduction in formation of MP cells, while reciprocally, enforced Runx3 expression reduces TE cell frequencies and drives increased formation of both DPECs and TRM cells 59,64. These studies provide molecular evidence for distinct, early transcriptional programming of TCIRC and TRM precursors during systemic infection. Additional analyses using single cell genomics, lineage tracing, and the identification of additional differentially expressed markers on TEFF cells that correlate with the development of TRM cells could help to continue defining how and when responding CD8 T cells become subdivided into TRM and TCIRC subsets 31,60.
Flexibility in the differentiated states of some TEFF and TMEM cells
To what extent antigen-experienced CD8 T cells that have adopted specific phenotypic characteristics have actually ‘fixed’ their differentiation status remains an open question 19,65. Several examples exist in which cells isolated on the basis of one cell phenotype undergo a transition into an alternative type. For example, some mature TRM cells isolated from the small intestinal epithelium differentiate into TCM, TEM and new TRM upon secondary challenge 66. In addition, although virtually all TEFF cells downregulate CD62L expression during the effector phase by the peak response, transfer studies have shown that CD62Llo MP cells, but not CD62Llo TE cells, re-induce CD62L expression prior to their initial homeostatic cell division as a consequence of DNA demethylation in the Sell (CD62L) locus 47. Reciprocally, but in a similar vein, not all cells during the effector phase that initially upregulate expression of effector-associated genes sustain their expression and commit to terminal differentiation at later times. Analysis of mice in which Cre recombinase expression is driven by either the Klrg1 (KLRG1) locus or a BAC-transgenic Gzmb (Granzyme B) locus, to permanently mark cells having expressed these genes by using Cre expression to activate constitutive reporter gene expression, demonstrated that many cells which initially expressed Klrg1 or Gzmb downregulate their expression later, and ultimately become TMEM cells 48,67. Thus, gene expression in many TEFF cells is not permanently stabilized, and appears to be reversible given the appropriate circumstances, which is consistent with the natural reversibility inherent to transcriptional control in response to fluctuating levels of transcriptional regulatory proteins 68. For example, a population of cells with an apparently uniform phenotype might appear similar at the protein level (e.g., KLRG1hi), but be composed of individual cells that manifest distinct underlying metastable transcriptional states that ultimately favor alternative developmental outcomes (e.g., TE or TMEM cells) 69. Defining how regulation of chromatin structure and transcription govern the stability and flexibility of these gene expression states is central to understanding how distinct CD8 T cell subsets are initially established and then maintained.
Regulation of gene expression during the formation of TEFF and TMEM CD8 T cell subsets
Heterogeneous gene expression in activated CD8 T cells leads to lineage-bias early during infection
CD8 T cells begin manifesting gene expression that is biased toward one or another TEFF or TMEM cell lineage early during their response to infection, but do not solidify these gene expression programs until later during differentiation. Recent single cell RNA-seq (scRNA-seq) analysis has shown that distinct groups of cells within the overall TEFF population early after LCMVArm infection are enriched with gene expression signatures specific to TEFF or TMEM cell subsets that develop later 31,60. This suggests that early developmental decisions could be made that set some cells on distinct trajectories.
Consistent with this, bulk gene expression in FACS-purified subsets that are ‘TEFF-like’ based on being KLRG1hi or Id2hi at early times after infection are more enriched with gene expression from mature TE cells, whereas those that are more ‘TMEM-like’ based on being KLRG1lo, or Id3hi, are more enriched with gene expression characteristic of TMEM or MP cells 49,53,64,70. However, discerning the cells that are actually ‘differentiated’ at early times on the basis of only a handful of surface receptors or reporter genes is likely limited, because of transcriptomic variation between single cells 69, and gene expression patterns that do not stabilize until later. For example, gene expression on day 5 after infection is more similar between KRLG1hi and KLRG1lo cells than it is between either subset and naïve cells, or between either subset and mature EE, TE or MP phenotypic cells on day 8 post LCMVArm infection 49,64. In addition, unsupervised clustering of genes based on the kinetics of their expression throughout the CD8 T cell response has shown that the expression of groups of genes associated with mature TEFF and TMEM cell subsets is dynamic at early times, before their expression is consolidated at later times 71. Thus, gene expression programs become more differentiated over time and gene expression of mature TEFF and TMEM cells develops progressively at the population level.
At the single cell level, gene expression in responding CD8 T cells is heterogeneous and also appears to be unstable at early times after infection. This attribute is a general characteristic of cells undergoing lineage choice and probably facilitates multilineage differentiation potential in the early TEFF cell population 69. Single cell analysis of gene expression in daughter cells resulting from the first naïve CD8 T cell division after infection found they clustered in two distinct groups 72. One group is characterized by TCM-like gene expression whereas the other TE-like, a result that is consistent with asymmetric cell division upon naïve cell activation 39. However, this stark dichotomy in gene expression is not evident in TEFF cells on day 4 after infection, and their gene expression is distinct from those after their first cell division 72, which suggests the initial gene expression patterns might not have been inherited. However, expression of multiple ‘fate-classifier’ genes that are associated with either TEFF or TMEM states could be used to categorize the day 4 TEFF cells, suggesting that lineage-bias exists at this time 72. An important unanswered question is whether TEFF cells with a particular bias in gene expression on day 4 were the specific progeny of one category of daughter cells that were TCM or TE cell biased after their first division, which would imply that the initially divergent gene expression patterns were maintained within a lineage of cells at later times. Given that lineage-tracing studies suggest individual naïve cells stochastically give rise to progenies comprising either terminal TEFF, or TMEM cell fates 35, it is likely that the initial divergence in gene expression among first generation daughter cells is not specifically retained in most of their progenies. Thus, naïve cells might adopt alternative transcriptional states after their first division, but it is unclear how frequently these states are preserved and account for distinct early differentiation trajectories.
Another possible interpretation that could explain cells exhibiting either TCM or TE-biased gene expression after their first division in vivo is that they comprise daughter cells in different temporal stages of the same gene expression program, rather than qualitatively distinct programs. A separate scRNA-seq analysis using carefully controlled in vitro conditions demonstrated that all naïve cells activate a categorical gene expression program within the first 6 hours of stimulation, regardless of the initiating ligand’s ‘strength’ 73. The signal strength governs the rate at which cells in the population activate the program but does not change qualitatively the induced program’s nature. Together, these studies demonstrate that early gene expression is dynamic and unstable and that differences observed at singular time points in vivo could relate to asynchrony. Future studies that link the kinships of single responding cells and their individual transcriptomes to trace how transcriptional states are propagated within lineages will likely provide insight as to when and how gene expression programs are initially established and stabilized in vivo.
Extracellular signals and transcriptional circuits that differentially regulate formation of TE and TMEM cells
A single brief period of TCR and co-stimulatory receptor stimulation experienced by naïve CD8 T cells is sufficient to induce a complete program of memory cell differentiation 74,75. Additional signals are necessary to drive terminal differentiation of TEFF cells 3,42,45,51,76–79. However, inferences from lineage tracing studies indicate that the differentiation fates of CD8 T cells are established before extensive population expansion 34,36, suggesting that signals delivered early in the response ‘imprint’ whether some activated cells will ultimately stabilize gene expression that preserves formation of TMEM cells or drives terminal TEFF cell differentiation. Described below are multiple signals and transcriptional networks that promote either terminal or TMEM cell differentiation. The integration of these opposing signals early during infection is likely to foster the heterogeneity and instability of gene expression among responding cells in the effector phase at early times, before alternative transcriptional programs become dominant within distinct cell populations.
Signals and transcriptional circuits that promote terminal differentiation
Lineage tracing studies indicate that there is a positive correlation between protracted cell division history and terminal differentiation 34,36. One part of the explanation for how activated cells might ‘commit’ at early times to terminal differentiation is that naïve cells integrate signals during activation that are delivered through the TCR, co-stimulatory receptors and receptors for inflammatory cytokines, such as IL-12 and IL-2, and translate their sum into the extent of cell division; greater sums predict more extended cell division 80. Stimulation with type I IFNs, IL-12 and IL-2 determines the magnitude and duration of IL-2Rα expression, which is required for high affinity binding of IL-2 42,45,51,77,78,81. IL-2Rα expression is positively regulated by the continued presence of IL-2 82, which promotes the proliferation and survival of stimulated cells by inactivating FoxO-family TFs and other mechanisms 83–85. Thus, early inflammatory cytokine signals establish a positive feedback loop that regulates expansion of the TEFF cell population in response to the availability of IL-2, whose concentrations rise transiently and are sustained while it is produced by CD4 and CD8 T cells responding to the presence of antigen 51,52,86. A second part of the explanation is that inflammatory cytokines (type I IFN, IL-12, and IL-2) enhance expression of TFs such as T-bet, Zeb2, Id2, and Blimp1, which each directly promote transcription of genes encoding factors that underlie terminal differentiation 42,45,51,53,79,87. Thus, signals from multiple cytokines received early in the response that program extended proliferation also drive gene expression characteristic of TE cells. Coupling both of these features in activated cells is likely to increase the probability that cells stabilize gene expression underlying TE cells and become terminally differentiated.
IL-2 and IL-12 mediate distinct and overlapping effects that promote terminal differentiation 42,79. Deficiency in IL-2Rα results in impaired formation of TE cells 42,56,79. IL-2 stimulation positively regulates expression Irf4 and Batf after TCR stimulation (M.E. Pipkin unpublished observations), which encode TFs that co-bind composite bZIP-IRF motifs in genes whose expression promotes overall TEFF cell accumulation and terminal differentiation 88. Deficiency in either of these TFs impairs TE cell differentiation 88–91. In addition, T-bet expression is impaired in activated CD8 T cells lacking IL-2Rα, however, increased IL-2 stimulation of wildtype cells does not increase Tbx21 (T-bet) mRNA expression 42. Thus, IL-2R stimulation is required for terminal differentiation, but sustained IL-2R signals most likely promote TE cell differentiation by mechanisms other than increasing Tbx21 gene expression. In contrast, IL-12 potently induces Tbx21 gene expression, which drives terminal differentiation 14,45. T-bet cooperatively induces TE cell development by functioning with additional TFs, including Zeb2 and Blimp1 9,10,79,92. T-bet directly binds the Zeb2 gene locus and enhances its expression. Zeb2 is required for optimal T-bet binding to cis-regulatory regions in other downstream genes that both TFs control 92. Thus, T-bet activates its own facilitator (Zeb2), which creates a feed-forward circuit that crystalizes gene expression comprising the terminally differentiated state 92,93. In addition, both IL-2 and IL-12 positively regulate Prdm1 mRNA expression, and Blimp1 is essential for terminal differentiation 87,94,95. Thus, both cytokines cooperatively promote terminal differentiation. However, Blimp1 represses Il2ra expression late in the effector phase, suggesting it is part of a negative feedback circuit that regulates the magnitude of the TEFF cell population by desensitizing KLRG1hi cells to IL-2 signals during the contraction phase 56,96.
Although IL-2R signals are necessary for terminal differentiation, they also ensure the normal programming of TCM cells, which indicates that IL-2 functions as a formatting factor that facilitates multiple differentiation outcomes, rather than only instructing one fate over the other. Although CD8 T cells deficient in IL-2Rα preferentially develop a phenotype resembling TCM cells, these cells are not normal, and do not undergo robust recall proliferation during secondary infections 52,57,86. The rapid kinetics of IL-2Rα expression early during infection suggest that the intensity or duration of transient IL-2 stimulation programs TCM cell formation and regulates terminal differentiation early in the response 51. Notably, gene expression has not been analyzed in IL-2Rα-deficient cells at early times during infection while IL-2 stimulation is normally underway. Future studies of IL-2R-dependent nascent mRNA expression (in addition to mature mRNA expression), is likely to be important for determining how transcription-dependent regulation by IL-2 initially establishes both TMEM and TEFF cell populations. In addition, the transient and dynamic nature of IL-2Rα expression during T cell responses suggests IL-2 stimulation could function at multiple stages, and future studies using methods to conditionally control IL-2Rα expression could be important for delineating the physiological roles of IL-2 throughout TMEM cell formation and homeostasis.
Signals and transcriptional circuits that establish and preserve TMEM cell differentiation
The cytokines IL-10 and IL-21 each activate the TF STAT3 and are important for formation of MP cells and establishing the TCM compartment, in part by counteracting IL-12 signals. Cells lacking STAT3 aberrantly develop a terminally differentiated phenotype, form fewer MP cells and fail to form or sustain TCM cells during viral infection 97. Blocking IL-10 in the context of IL-21 deficiency also reduces formation of TCM cells, suggesting these cytokines are responsible for activating the STAT3 that promotes TCM development 97. STAT3-deficient cells express less of the inhibitory molecule suppressor of cytokine signalling 3 (SOCS3), and exhibit enhanced IL-12 responsiveness, resulting in reduced expression of the TFs Bcl6, Blimp1 and Eomes 97. Disruption of Bcl6 also results in reduced frequencies of MP cells and fewer TCM cells in the memory phase 97. In addition, Eomes deficient cells contribute poorly to the TCM compartment, and its expression in wildtype cells is repressed by IL-12 stimulation 98,99. Thus, these TFs appear to function downstream of STAT3 during infection, probably in response to both IL-10 or IL-21, and perhaps other cytokines. In addition, the stability of the IL-10 pathway appears to be sustained by a positive feedback loop involving Eomes 100. In the absence of Eomes, TEFF cells express less IL-10, Bcl6 and CD62L. Conversely, enforced Eomes expression is able to induce expression of Bcl6, IL-10 and CD62L, both in wildtype and IL-10-deficient TEFF cells 100. These results indicate that the ability of activated CD8 T cells to sense cytokines that activate STAT3 (e.g., IL-10 and IL-21, and potentially others), and retard signals from IL-12, is important for establishing or maintaining TCM cell developmental potential during the effector phase.
Given that STAT5 and STAT3 can compete for occupying overlapping binding sites to produce distinct differentiation outcomes during CD4 T cell differentiation 101, it is possible that IL-2R stimulation, which activates STAT5, directly influences STAT3-dependent programs during the differentiation of activated CD8 T cells. In line with this hypothesis there is strong negative regulation of Bcl6 expression in response to IL-2R stimulation. Bcl6 is expressed in naïve CD8 T cells, and its expression is maintained throughout TCR stimulation 42,51. Upon cessation of TCR stimulation and provision of exogenous IL-2, Bcl6 is quickly repressed, and reciprocally Prdm1 is induced 42. Bcl6 is re-expressed in conjunction with Il7ra after several days, in a manner that is inversely related to the concentration of IL-2 in culture 42. Bcl6 is not repressed normally in IL-2Rα deficient CD8 T cells during LCMV infection, confirming that IL-2R signals are essential for repressing Bcl6 in vivo 42,51,56. These results suggest that IL-2R signals might promote the antagonistic regulation of both Bcl6 and Blimp1 in CD8 T cells, analogous to what occurs in CD4 T cells, which requires STAT5 102–107. Thus, titration of Bcl6 and Blimp1 expression in response to IL-2R signals and STAT5, and other cytokines that activate STAT3, could be important for establishing divergent gene expression programs that bias differentiation of distinct TEFF and TMEM cell subsets. Additional studies are needed to determine the order of operations in how this occurs downstream of TCR and IL-2R signals in CD8 T cells. One intriguing possibility is that variation in Bcl6 and Blimp1 expression influences the type of TMEM cells that develop, because both factors are implicated in distinct TMEM subsets. Although disruption of Prdm1 clearly impairs terminal differentiation, and results in an increase in TCM-like cells 87,94–96, its deficiency also reduces TRM cell formation 12,63,108, suggesting it has roles in multiple TMEM subsets. Reciprocally, whereas Bcl6 deficiency impairs formation of TCM cells 97,109, it is also expressed in some TRM cells at late memory time points 31. In addition, its high expression in TCM cells and cells that exhibit features of follicular CD4 T cells and stem cells suggests it might contribute those that are memory stem (TSTEM) cell-like 28,29,110. Thus, the roles of Bcl6 and Blimp1 in the regulation of TEFF and TMEM cells is likely more complex than currently appreciated. Clarifying their regulation by IL-2R signals is likely to lead to a better understanding of how distinct TMEM cell subsets develop.
In addition to cytokines that promote STAT3-mediated signals, TGF-β signals also counteract terminal differentiation, induce aspects of both TCIRC and TRM cells, and have tissue-specific effects. In the spleen, TGF-β promotes development of TCIRC cells by inducing and perpetuating the expression of the TF Zeb1 during TEFF and TMEM cell maturation. Disruption of Zeb1 in activated T cells in vivo using Gzmb-Cre (which becomes expressed after T cell activation) reduces the number of TCM cells that form after LCMVArm infection 111. Zeb1-deficient cells that persist into the memory phase express reduced amounts of genes encoding factors that normally ensure TCM homeostasis, suggesting Zeb1 promotes TCM gene expression. TGF-β interferes with terminal differentiation because Zeb1 and the mir-200 family microRNAs both repress Zeb2 expression, which uncouples the T-bet/Zeb2 driven pathway that otherwise drives the terminal differentiation program 111. Accordingly, post-thymic disruption of TGF-β receptor II (Tgfbr2fl/fl dLck-Cre+) causes increased fractions of KLRG1hi cells in the spleen during acute LCMV infection, and culture of human CD8 T cells with TGF-β represses KLRG1 expression 112.
In non-lymphoid tissues, TGF-β signals are essential for maturation of TRM cells. The TRM phenotype becomes fully manifest after establishing residence in particular non-lymphoid sites 58, and a key step in TRM formation is their retention within particular non-lymphoid tissues, where TGF-β expression is plentiful. Expression of the E-cadherin-binding integrin CD103 (Itgae) on T cells is essential for TRM cell retention in certain epithelial tissues, and is upregulated on TRM cells that establish residency at these sites 113. In the skin, salivary gland and in the intestinal epithelia, CD8 T cells lacking TGF-β receptor II do not upregulate CD103, and overexpress KLRG1 following LCMV infection 112. Likewise, expression of a dominant negative form of TGF-βRII in LCMV-specific CD8 T cells also prevents normal upregulation of CD103 expression 113. TEFF cells from day 4 after LCMVArm infection, which are enriched with TRM precursors 61, induce CD103 expression when cultured with TGF-β, whereas splenic TCIRC cells from later times do not 113. Collectively, these results suggest a model in which TGF-β signals in the spleen retard terminal differentiation, and perhaps maintain competence of some TEFF cells that will emigrate to certain distal non-lymphoid tissues where they receive additional TGF-β stimulation in situ and complete TRM maturation.
Runx-family proteins establish core transcriptional programs of CD8+ cytotoxic T lymphocytes
Transcriptional control of the Prf1 locus by Runx3 initially implicated Runx-TFs in the programming of TEFF cell differentiation
A unique function of antigen-experienced CD8 T cells is their capacity to lyse infected or malignant cells using cytotoxic granules that contain the pore-forming protein perforin, and a family of serine esterases (the granzymes) that activate multiple cell death pathways following perforin-mediated delivery into target cells 114,115. Prf1 (encodes perforin) is specifically expressed in antigen-experienced CD8 T cells and regulation of the Prf1 locus of humans and mice has served as a model to study in order to define molecules that could be critical for the differentiation of naïve CD8 T cells into TEFF and TMEM cytotoxic T lymphocytes (CTLs) 116,117. Early transgenic analyses indicated cis-regulatory regions in the Prf1 locus might become active in developing T cells as early as the double negative stage in the thymus (reviewed in 116). However, the earliest point in which endogenous Prf1 transcripts become detectable occurs as double positive thymocytes undergo positive selection into the CD8 lineage, and is conspicuously coordinated with upregulation of the TF Runx3 118. Runx-family TFs are essential for T cell development in the thymus, and Runx3 is essential for specification of the CD8 T cell lineage 119–121. These studies formed the premise of a hypothesis that Runx3 would be critical for programming transcriptional control of the Prf1 locus, and perhaps the differentiation of TEFF and TMEM cells.
Runx3 drives the transcriptional program of CTLs following naïve CD8 T cell activation
The Runx-family of TFs is encoded by three genes (Runx1, Runx2 and Runx3) in mammals 122. High affinity DNA recognition by each of the Runx proteins results from an allosteric change in their Runt domain that is induced by binding of their common partner Cbfb (encoded by Cbfb) 123,124. Analysis of CD8 T cells from Runx3 deficient mice showed that Runx3 is essential for inducing Prf1 and multiple additional key effector functions characteristic of CTLs (Fig. 1). Initially, the requirement for Runx3 during the development of antigen-experienced CD8 T cells was examined using purified CD8 T cells from an outbred strain of mice (ICR) that survive germline inactivation of Runx3 122,125. Thymic CD8 T cell development is substantially impaired in the absence of Runx3, but residual CD8 T cells with a mature phenotype exist, although most fail to repress Cd4 expression 120,121. Runx1 is strongly upregulated in Runx3 deficient CD8 T cells 125, and partial redundancy between Runx1 and Runx3 accounts for the incomplete block in CD8 T cell development in the absence of Runx3 119,121. In wildtype CD8 T cells, Prf1 transcripts are strongly upregulated after naïve cells stimulated with TCR and co-stimulatory signals are cultured with IL-2 at concentrations that sustain IL-2R signals in the context of intermediate affinity receptors (i.e., IL-2Rβ and the common gamma chain (γc))42,125,126. In contrast, Runx3 deficient cells do not induce expression of either Prf1 or Gzmb (encodes Granzyme B) under these conditions, and also inefficiently produce IFNγ, TNF and IL-2 upon restimulation, despite initially becoming activated and accumulating (albeit less strongly) 125. Thus, Runx3 is essential for establishing hallmark effector functions of cytotoxic lymphocytes in antigen-receptor stimulated CD8 T cells.
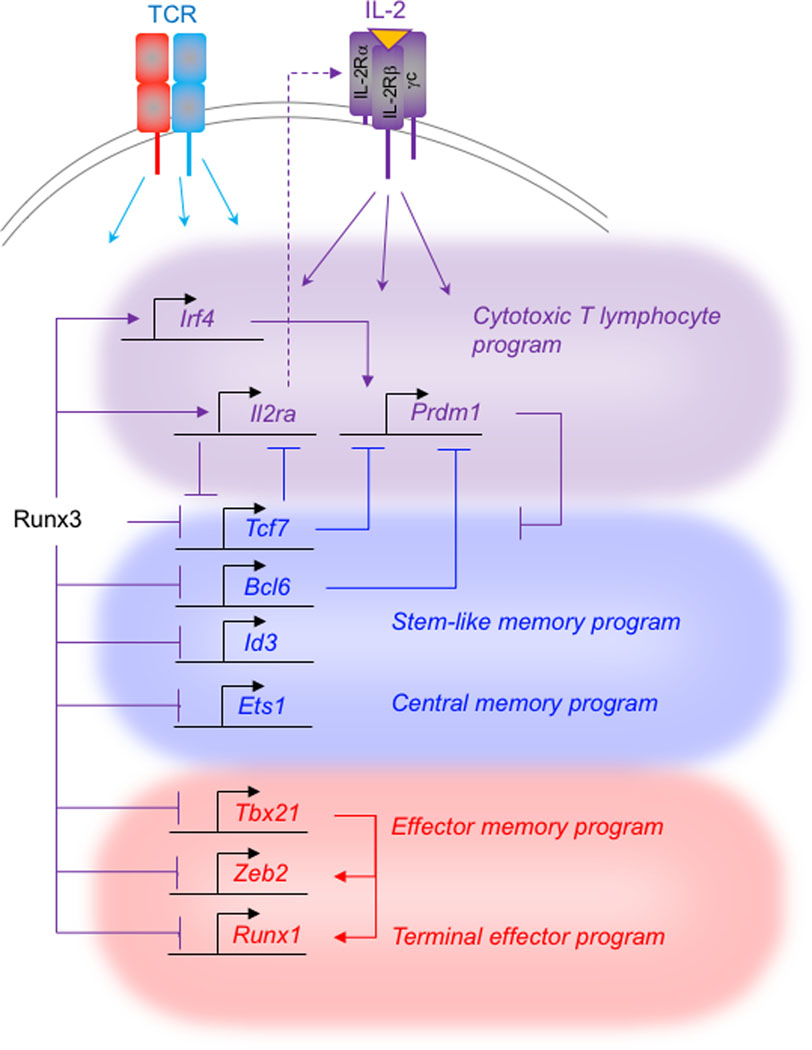
Fig. 1. Runx3 and Tcf1 titrate IL-2R expression and regulate multiple transcriptional circuits that promote development of distinct TEFF and TMEM cell programs.
Runx3 activates transcription and binds to multiple genes encoding regulatory factors that drive transcription of genes underlying the core cytotoxic T lymphocyte differentiation program (purple). IL-2 receptor driven feedback resulting from increased or sustained Il2ra (IL-2Rα) expression creates signals that activate and repress multiple genes encoding regulatory factors. Runx3 directly represses Tcf7 and multiple additional genes that promote differentiation of TCM and TSTEM cells (blue). Tcf1 negatively regulates Il2ra expression. Reduced Il2ra expression in Runx3 deficient cells is restored upon concomitant ablation of Tcf7, and this partially restores certain IL-2R-dependent gene expression events. Runx3 reduces expression of multiple TFs that promote terminal differentiation (red).
Runx3 functions in conjunction with proteins from the T-box family of TFs. The phenotype of Runx3 deficient cells is somewhat analogous to CD8 T cells deficient in the T-box TFs T-bet and Eomes, in that they are also unable to differentiate into bona fide CTLs 127,128, which suggested potential genetic interactions between Runx3 and these T-box TFs could promote CTL differentiation. Analysis of Runx3 deficient cells showed they upregulate T-bet after differentiation in vitro, but do not induce Eomes expression 125. Complementation of Runx3 deficient CD8 T cells by retroviral transduction with a hyperactive form of Eomes (Eomes-VP16) that transactivates Prf1 in wildtype cells is unable to do so in Runx3 deficient cells, but is able restore their capacity to produce IFNγ. In contrast, Runx3 complementation restores both Eomes expression and the CTL gene expression program 125. In addition, Runx3 binds directly to cis-regulatory regions of both Prf1 and Eomes, suggesting it functions at these genes directly 125,129. These results suggest that both Runx3 and Eomes are important for inducing the CTL program, with Runx3 being located upstream of Eomes. It also indicates that T-bet is insufficient to activate Prf1 and Gzmb expression in the absence of Runx3. Consistent with this, CD8 T cells lacking only Tbx21 (encodes T-bet) do not exhibit a defect in Prf1 mRNA upregulation during differentiation in cell culture, although they inefficiently induce IFNγ, prior to upregulation of Eomes 42,125. These studies collectively establish Runx3 as a cornerstone TF that in conjunction with T-box proteins drives the gene expression program of cytotoxic lymphocytes in activated CD8 T cells.
Multiple Runx-TFs regulate terminal differentiation and formation of TCIRC subsets
Runx1, Runx3 and Cbfb are expressed in naïve CD8 T cells, but all three Runx proteins (including Runx2) are expressed in antigen-experienced CD8 T cells 64, and appear to govern TEFF and TMEM cell differentiation in vivo 59,64,130,131. Conditional Runx3 gene deficiency in T cells demonstrated it is essential for development of genuine CTLs, and the clearance of viral pathogens and tumors 59,64,131. At early times after LCMVArm infection, Runx3-null CD8 T cells exhibit dramatically reduced accumulation, delayed upregulation of KLRG1 and defective upregulation of multiple genes that are characteristic of TEFF cells 64,131. However, the phenotype is more complex than a simple defect in TEFF cell generation. RNA interference (RNAi) of either Runx3 or Cbfb in adoptively transferred LCMV-specific P14 TCR transgenic CD8 T cells during LCMVArm infection demonstrated that insufficiency in either factor increases the fractions of KLRG1hi CD127lo TE-like cells, while reducing the frequencies of both KLRG1hi CD127hi DPECs and classical KLRG1lo CD127hi MP cells at the peak of the response to acute viral infection 64. Conditional Runx3 disruption in post-thymic T cells confirmed these phenotypes in polyclonal endogenous LCMV-specific CD8 T cells, and showed that loss of only one Runx3 allele elicits the same phenotype without dramatically impairing cell accumulation 64. Moreover, gene-disruption and RNAi each also demonstrated that Runx3- or Cbfb-deficient CD8 T cells form fewer normal TCIRC memory cells. Runx3 deficient cells at early memory time points comprise reduced frequencies of KLRG1hi and CD127hi (TEM-like) cells, increased fractions of KLRG1hi CD127lo (TLLE-like) cells, and equivalent frequencies of KLRG1lo and CD127hi (TCM-like) cells, compared to control cells 64. Analogously, conditional disruption of Runx2 also results in reduced formation of MP cells, and impaired persistence of TCIRC cells 130,132. These results support the conclusion that Runx2 and Runx3 promote TMEM cell development and negatively regulate terminal differentiation.
In contrast, Runx1 might promote terminal differentiation of TEFF cells. Runx1 expression in activated CD8 T cells is lost upon T-bet depletion, but is strongly upregulated in Runx3 deficient or Cbfb-deficient cells, and reciprocally, Runx3 overexpression represses both Runx1 and T-bet expression in vivo 64. However, RNAi-mediated depletion of Runx1 does not elicit clear phenotypes based on KLRG1 and CD127 staining at the peak of the response to LCMV infection 64. Therefore, Runx1 might not be crucial for TEFF cell differentiation. However, another possibility is that discerning a Runx1 loss-of-function phenotype is complicated by compensation from other Runx factors, and might only manifest in the context of Runx3 deficiency. Consistent with this viewpoint, the TE-like phenotype of cells lacking Runx3 (which overexpress Runx1) is strongly correlated with genes linked to cis-regulatory regions that are more accessible in Runx3 deficient cells, and that are enriched with motifs recognized by Runx1 64. Further experiments that analyze compound deficiency in both Runx1 and Runx3 could clarify whether the phenotype of Runx3 deficiency requires Runx1 upregulation; and, overexpression of Runx1 cDNA in activated CD8 T cells could provide gain-of-function evidence for how Runx1 might affect TEFF and TMEM cell formation.
Runx3 orchestrates transcription that ensures TMEM cells differentiate from terminal TEFF cells
Runx3 has a complex effect on gene expression during T cell activation that weaves CTL effector functions into TMEM cell development, while preventing terminal differentiation (Fig. 1). At early times after infection, Runx3 deficient cells are less frequently KLRG1hi and inefficiently induce multiple genes whose expression is normally upregulated in both TE and MP cells compared to naïve cells, including genes associated with TEFF cells such as Irf4, Prdm1, Id2, Il2ra and Prf1, and this explains the failure of Runx3 deficient cells to become bona fide CTLs that are protective 64,131. Simply speaking, defective expression of these genes suggests terminal differentiation should be impaired, because gene deficiency in either Irf4, Prdm1, Id2, or Il2ra impairs development of TE cells 9,10. However, near the peak response to infection Runx3 deficient cells preferentially acquire a TE-like cell surface phenotype and this correlates with overexpression of Tbx21, Zeb2 and Runx1 64 (Fig. 1). This phenotype is suppressed by Tbx21 RNAi, indicating that the TE-like phenotype of Runx3 deficient cells is still dependent on T-bet 45. Runx3 overexpression in wildtype cells represses both Tbx21 and Runx1 and the TE-like phenotype in vivo, whereas neither Tbx21 deficiency nor its overexpression alters Runx3 expression 59,64. These results suggest that Runx3 restrains terminal differentiation upstream of Tbx21 and Runx1 and ensures that cells with cytotoxic effector functions develop into TMEM cells. It is notable that Runx3 also positively regulates Eomes expression during differentiation of CD8 T cells in cell culture, suggesting it could be important for promoting the fitness of TMEM subsets 98,125.
Although Runx3 deficient cells skew toward a KRLG1hi phenotype near the peak response to infection, their complex phenotype also involves overexpression of Tcf7, Bach2, and Id3 compared to wildtype cells 64,131. These genes encode transcriptional regulatory factors whose expression and functions normally promote TCM formation 9,53,55,133 (Fig. 1). Correlatively, Runx3 deficient cells exhibit certain features of TCM cells (e.g., Sell (CD62L) overexpression) but are likely defective, because the effects of TCM-specific regulatory factors might differ in the context of Runx3 deficiency. For example, Bach2 encodes a TF from the bZIP family that can compete with other bZIP family factors, such as Jun-proteins, and inhibit AP-1 binding during TCR stimulation 132,133. Bach2-deficient cells accumulate less efficiently after infection, do not re-express CD62L and fail to downregulate KLRG1 on some TEFF cells that develop into TMEM cells (i.e., ‘Ex-KLRG1’ cells) 48,133. However, Runx3 deficient cells fail to establish chromatin accessibility to Bach2 binding elements during TCR stimulation 64. Thus, it is possible that dysregulated KLRG1 expression and non-canonical TMEM cell formation in Runx3 deficient cells relates to defective Bach2 dependent transcriptional control, despite its overexpression. These studies indicate that Runx-family TFs are important for properly delineating development of TMEM cells from terminal differentiated TEFF cells, and that the underlying transcriptional networks are complex.
Runx3 drives transcription of a core set of genes that promote tissue residency and formation of TRM cells
Runx3 differentially controls the formation of TRM and TCIRC subsets. A role for Runx3 in TRM cells was identified in a pooled RNAi screen that focused on candidate genes encoding TFs selected computationally as potential TRM regulators 59,134,135. Initially, the PageRank algorithm was used to identify potentially important TFs by ranking them according to enrichment of their binding motifs within chromatin accessible cis-regulatory regions annotated to a network of genes expressed in intestinal intraepithelial lymphocyte (IEL) TRM cells 59,136. The RNAi screen showed that cells carrying short hairpin RNAs in microRNA contexts (shRNAmirs) specific for Runx3 were strongly depleted from IEL TRM cells compared to splenic TCM cells following LCMV infection 59. Additional loss-of-function approaches demonstrated that Runx3 deficient cells inefficiently initiate TRM differentiation at early times, and also fail to sustain TRM cell homeostasis in situ at later times, whereas enhanced Runx3 expression after retroviral transduction increased the number and differentiation of TRM cells in multiple tissues, and solid tumors 59. Runx3 deficient cells appear to initially enter non-lymphoid tissues at early times equivalently to wildtype cells, but fail to differentiate into TRM cells and persist in the intestinal epithelium.
RNA-seq studies demonstrated that Runx3 drives transcription that underlies the differential development of TRM and TCIRC cells. Runx3 overexpression induces expression from a large fraction of genes in a ‘core residency signature’ that comprises genes more highly expressed in TRM cells from five different non-lymphoid tissues compared to splenic TEM and TCM cells (TCIRC); and represses expression of many genes in a ‘core circulating signature’ that comprises genes more highly expressed in TCIRC cells relative to TRM cells. Conversely, Runx3 deficient cells fail to upregulate genes in the core residency signature, and instead upregulate those in the core circulating signature 59. Analysis of ChIP-seq data showed that Runx3 binds directly to genes whose transcription it activates, such as Itgae (CD103), Tgfbr1 and Tgfbr2, which are functionally required for tissue residence at epithelial sites 59,113,129. Runx3 also activates and binds directly to genes that encode TFs, such as Prdm1 and the Nr4a-family 63,137, which are required for TRM cell formation 12,59. Thus, Runx3 dependent gene activation directly accounts for the TRM cell phenotype, and also positively regulates expression of additional TFs that induce TRM development. The effect of Runx3 on these transcriptional programs is manifest in CD8 T cells during differentiation under reductionist conditions in cell culture 42,59, which indicates that Runx3 can promote central features of the TRM gene expression program independent of tissue-specific contexts 138. These results imply that Runx3 is an important initiator of the TRM differentiation program, which might occur prior to entry into target non-lymphoid tissues. Consistent with this, Runx3 deficient cells are preferentially retained in the splenic white pulp and aberrantly manifest features of TCM cells 59,64,131. These studies suggest that Runx3 antagonizes aspects of TCIRC formation by repressing transcription of genes encoding TFs that positively induce TCM formation.
Runx3 integrates with IL-2R signals to govern Tcf7 expression and TSTEM differentiation
The higher expression of Tcf7, Bach2, Id3 and Sell in MP-like cells that lack Runx3 could also suggest that distinct developmental programs are invoked in the absence of Runx3 (Fig. 1). In line with this perspective, Runx3 deficient cells enter B cell follicles and are able to provide help to B cells, akin to follicular T helper (TFH) cells 131. In addition, Runx3 deficient MP-like cells overexpress Cxcr5, Maf, Icos and Bcl6 genes, which are hallmarks of both TFH 64,131,139 and progenitor TSTEM-like cells 27,28, suggesting that Runx3 deficient CD127hi cells in the spleen at the peak of acute infection are not canonical MP cells. The development of normal TSTEM cells requires the TF Tcf1 (encoded by Tcf7) and they are a prominent feature of chronic viral infections, where they sustain production of terminally exhausted T cells 27,28. Enhanced Tcf7 expression in Runx3 deficient cells could indicate that Runx3 normally represses formation of TSTEM cells. During acute LCMVArm infection of mice with T cell specific Runx3 deficiency, the formation of follicular ‘TSTEM-like’ cells correlates with clonal deletion of NP396-reactive CD8 T cells, and failure to clear the virus 64,131, which are de facto features of chronic infection and T cell exhaustion 28,140,141. Thus, one potential hypothesis is that Runx3 could be important for negatively regulating development of T cell exhaustion by repressing development of TSTEM-like cells.
Runx3 and its regulation of Tcf7 could be important for controlling IL-2Rα expression, titrating the levels of Bcl6 and Blimp1 and governing differential origins of TRM, TCM and TSTEM cells (Fig. 1). Runx3 directly regulates Bcl6 and Prdm1 but also regulates their expression indirectly through IL-2R signals and Tcf1. Runx3 directly represses Tcf7 and Bcl6, and this involves Runx3-dependent deposition of histone H3 lysine 27 trimethylation (H3K27me3) at Runx-Cbfb-binding sites in the Tcf7 and Bcl6 genes 131. At the same time, Runx3 promotes transcription of Il2ra and Prdm1 and increases chromatin accessibility of cis-regulatory regions where it binds in both loci, indicating that Runx3 activates these genes directly 64,129,131. However, a key event in this regulatory network might be downregulation of Tcf7 by Runx3, because disruption of Tcf7 is sufficient to restore expression of IL-2Rα and Prdm1, even when Runx3 is also simultaneously inactivated 131 (Fig. 1). Given that IL-2R stimulation positively regulates Prdm1 expression and both factors negatively regulate Bcl6 expression 42,79, the control of Il2ra transcription by Runx3 and Tcf1 could be a very early event that regulates diversification of circulating and non-circulating TEFF and TMEM cell lineages. The regulation of Bcl6 might be even more complicated, because its expression is more strongly reduced in cells lacking both Runx3 and Tcf7, compared to those lacking only Tcf7 131, which suggests that both Runx3 and Tcf1 are necessary at some level for Bcl6 expression. It is also worth noting that Bcl6 represses Runx3 expression in CD4 T cells 142, raising the question of whether this regulation might also operate in CD8 T cells under certain circumstances. Future studies that discern the expression, epistatic relationships and kinetic binding activity of these factors at early times during T cell activation is likely to clarify how IL-2R signals integrate with these TFs to establish the differential origins of distinct TMEM cell populations.
Chromatin structure provides both stability and flexibility to gene expression programs and is governed at the level of the nucleosome
Nucleosomes are the fundamental repeating subunits of chromatin structure
Chromatin structure regulates the transcriptional control of genes by governing accessibility to its underlying DNA sequences, and by functioning as a scaffold for docking enzymatic complexes that regulate multiple genome functions. Nucleosome core particles are the fundamental repeating subunits of chromatin structure and consist of 147bp of DNA wrapped around a protein octamer formed by two copies of core histones H2A, H2B, H3 and H4 143,144. Adjacent nucleosomes are separated by 20–50bp of ‘linker’ DNA, and the DNA entering and exiting some nucleosomes is also associated with linker histone H1 145. Multiple amino acid residues in the N-terminal tails of the core histones, and in their globular regions, can be covalently modified by the addition or removal of various chemical groups (e.g., acetyl, methyl, ubiquitin or phosphate). Histone modifications can alter the nature of histone-DNA interactions and serve as specific docking sites of chromatin regulatory factors (CRFs) and other DNA modifying complexes that govern genome functions, including transcription 146,147. Furthermore, 5’ cytosine methylation (5mC) and hydroxymethylation (5hmC) of DNA also govern the nature of histone-DNA interactions, as well as the recruitment of protein complexes to specific chromatin regions with these modifications 148,149.
DNA from approximately 80% of the mammalian genome is wrapped in nucleosomes, thus occluding the majority of the genome. Their organization is defined by their translational location on the DNA (position), and by the frequency with which a defined DNA segment is bound by a nucleosome in a population of cells (occupancy). Nucleosomes are not randomly distributed on the genome but adopt preferred positions because the affinity of histones for particular DNA sequences varies, analogous to conventional sequence specific DNA binding TFs 150–153. Thus, histones, TFs and other DNA-binding proteins must compete for genome occupancy. In addition, CRFs that use the energy released from hydrolysis of ATP, can mechanically alter the topology of histones on DNA and change their locations and occupancy 154,155. The sequence of underlying DNA, the competitive binding activity of multiple proteins for overlapping DNA sequences, and the action of chromatin remodeling machines govern the nucleosome organization landscape (Fig. 2).
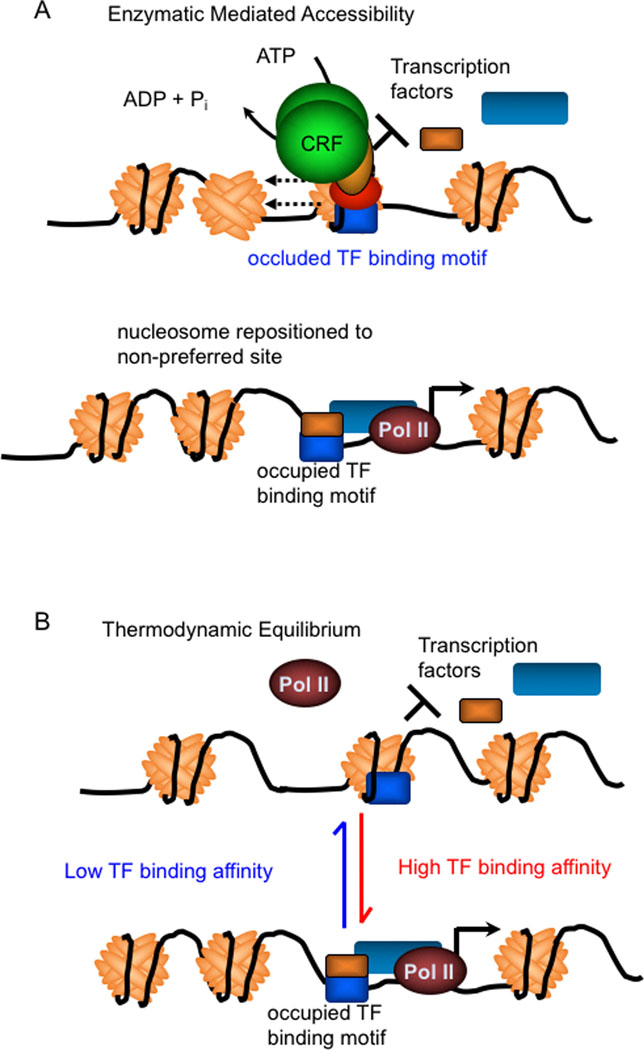
Fig. 2. Nucleosome organization in the genome is established by chromatin remodelling machines and competition with other DNA-binding regulatory factors.
(A) Nucleosome organization, especially near TSSs and other cis-regulatory regions is constrained by chromatin remodelling factor (CRF) enzymes that use the energy released from hydrolysis of ATP to catalyze repositioning of nucleosomes on DNA. Diverse assemblies of chromatin remodelling complexes built around one of four families of ATPases provide specificity. However, most subunits with these chromatin remodelling complexes lack DNA-binding domains that provide sequence-specificity, and require conventional sequence specific TFs to guide CRFs to their appropriate targets. (B) The histones in nucleosomes bind to DNA with varying affinities, and nucleosomes prefer to occupy certain sequences more than others. Competition between nucleosomes and other DNA-binding factors influences the positioning and occupancy of nucleosomes on the genome. TF-directed recruitment of ATPase-dependent remodellers to specific genes probably facilitates formation of nucleosomes on non-preferred sequences instead of regulatory sequences actively bound by their cognate TFs.
‘Epigenetic’ regulation of transcription is governed at the level of individual nucleosomes
Individual nucleosomes are key determinants of genome function, and both positive and negative regulation of transcription most likely occurs in the context of ‘de-condensed’ chromatin, rather than in the context of progressively higher-order chromatin fibers. Recent direct visualization of chromatin in fixed cells using electron microscopy tomography (EMT) has provided compelling evidence that the extent of nuclear chromatin is comprised of 5–25 nm fibers – roughly the diameter of one or two nucleosomes -- that exhibit different packing densities in 3-D space, rather than forming higher-order chromatin fibers 156. The ordered stacking of nucleosomes and folding into hierarchically compacted, inaccessible chromatin fibers, which have been observed microscopically, and analyzed structurally under highly ionic conditions in vitro might not reflect the majority of chromatin in vivo 156,157. One important conceptual implication from this discovery is that the roles of nucleosomes and their posttranslational modifications might relate more to controlling activity of discrete regulatory sequences, establishing the architecture of active TSSs 155,158, and governing the trafficking of RNA polymerase II (Pol II) through transcribed genes 159,160, rather than to promoting hierarchical folding of higher order chromatin fibers. From this perspective, it is notable that high resolution mapping of nucleosomes in T cells at a subset of genes that are important for T cell differentiation showed that specific nucleosomes were distinctly organized in key cis-regulatory regions between disparate T cell subtypes, whereas 95% of nucleosomes in non-regulatory DNA were positioned similarly between T cell subtypes 161 (and M.E. Pipkin unpublished observations). Thus, continuous competition between DNA-sequence specific TFs and nucleosomes that recruit distinct histone modifying enzymes and chromatin remodeling machines that govern the stability of nucleosomes in specific locations within cis-regulatory regions and transcribed regions, as well as the differential recruitment and activity of Pol II complexes at promoters and in gene-bodies, as they traverse nucleosomes, could be the important chromatin-level mechanisms that govern the ‘epigenetic’ control of cell differentiation 68. The differentiation of naïve CD8 T cells into distinct antigen-experienced CD8 T cell subsets provides a great model to elucidate how chromatin regulation establishes and remodels transcriptional programs during cell differentiation in vivo.
Naïve CD8 T cell activation results in chromatin accessibility changes that establishes a core network of accessible cis-regulatory regions that are common to all antigen-experienced CD8 T cells, and a smaller number of regions that are more, or less, accessible in specific TEFF or TMEM subsets compared to the others. In regions where TFs bind, nucleosomes are disrupted and the local DNA is hypersensitive to cleavage by endonucleases such as DNase I, or transposase 162. These regions can be mapped genome-wide by using high-throughput sequencing (e.g., ATAC-seq, DNase-seq) 163,164. Inspection of these regions using computational methods has been an important method for identifying potentially functional TF binding sites 136,165. In addition, protection of the protein-bound sequences within these regions results in DNA cleavage patterns that reveal ‘footprints’ which can be used to infer the occupancy of transcription factors on their cognate sites 164. These approaches have been used to define accessible cis-regulatory regions in multiple purified subsets of antigen-experienced CD8 T cells, and to infer TFs that are likely critical for their function.
Pioneering chromatin accessibility during initial TCR stimulation of naïve CD8 T cells
Chromatin accessibility develops rapidly during TCR stimulation
The analysis of naïve CD8 T cells during initial activation suggests that stimulated cells undergo extensive chromatin remodeling at genes associated with mature TMEM cells, and both TE and MP cells. TCR and co-stimulation induces de novo accessibility of ~15% of all regions that are accessible in mature TMEM subsets, and similar percentages of regions that are specific to TE and MP cells, within the first 24 hours of naive CD8 T cell activation 64. The accessibility of many of these de novo accessible regions appear to be maintained stably accessible in mature TMEM CD8 T cells 64,166, which demonstrates that essential aspects of chromatin remodeling that is specific to TMEM cells are programmed prior to the first cell division. Induction of chromatin accessibility found in TE cells at the same time also suggests many features required for terminal differentiation are co-established. A reasonable way to interpret these observations is that naïve cells initially differentiate into a state that acquires multilineage potential, and that later events reinforce the specific chromatin accessibility landscapes that are ultimately unique to TEFF or TMEM cell subsets. This likely includes reforming nucleosomes in regions that were initially opened upon stimulation, re-opening regions that were initially closed, and opening additional de novo regions that were not initially accessed. The dynamics of chromatin accessibility at these regions throughout differentiation have yet to be systematically explored.
Transcription factors involved in establishing chromatin accessibility of TEFF and TMEM cells
The mechanisms by which nucleosome-occupied chromatin is remodeled to a stable accessible state is incompletely understood but is thought to involve specialized transcription factors that can invade nucleosome-bound DNA 167,168. In naïve CD8 T cells, the de novo chromatin opening of cis-regulatory regions during TCR stimulation likely depends on the concerted activity of multiple TFs (Fig. 3). DNA sequences within the regions that gain accessibility during initial TCR stimulation are enriched with motifs that can be bound by TFs from multiple different families. Those that are recognized by the RUNX, ETS, bZIP, T-BOX, IRF, RHD, PRDM1 and KLF family of TFs are likely to be of particular importance because their binding motifs are not only statistically enriched, but also highly frequent among all of the de novo accessible regions 64,166,169,170. These motifs fall into three general categories in terms of the pattern of their frequencies within accessible regions at different times after TCR stimulation 64. The first are those whose motif frequencies are greatest within regions that become accessible in the first two hours of TCR stimulation but that are comparatively less frequent at later times in mature differentiated CD8 T cell subsets. These mainly include TF motifs recognized by basic leucine zipper (bZIP, e.g., Fos and Jun [AP-1]) proteins, and Rel homology domain (RHD) family (e.g., NFAT or NFκB) of proteins, which are transiently activated upon TCR stimulation 171. The second category includes motifs that are frequent throughout initial TCR stimulation and whose high frequencies are maintained among accessible regions of mature differentiated CD8 T cell subsets. Notably, these motifs increase in absolute number with increasing time after stimulation, because progressively more cis-regulatory regions become accessible during this time (Fig. 3A, right), and likely reflect positive feedback from cytokine pathways activated by TCR stimulation. These regions mainly include RUNX and ETS motifs 123,124,172. A third category comprises motifs that increase in frequency later during TCR stimulation, and are maintained in mature TEFF and TMEM cells, but are comparatively less frequent overall compared to the previous two categories (Fig. 3A and B). These include interferon regulatory factor (IRF) motifs, and their composite derivatives with ETS or bZIP factors 173,174, and positive regulatory domain (PRDM1) motifs 175. Multiple members of the TF families that recognize these DNA motifs have established requirements for the formation of TEFF and TMEM cells 10–12, which argues these TFs are critical within the first few hours of naïve CD8 T cell activation for initiating and then sustaining multiple avenues of T cell differentiation.
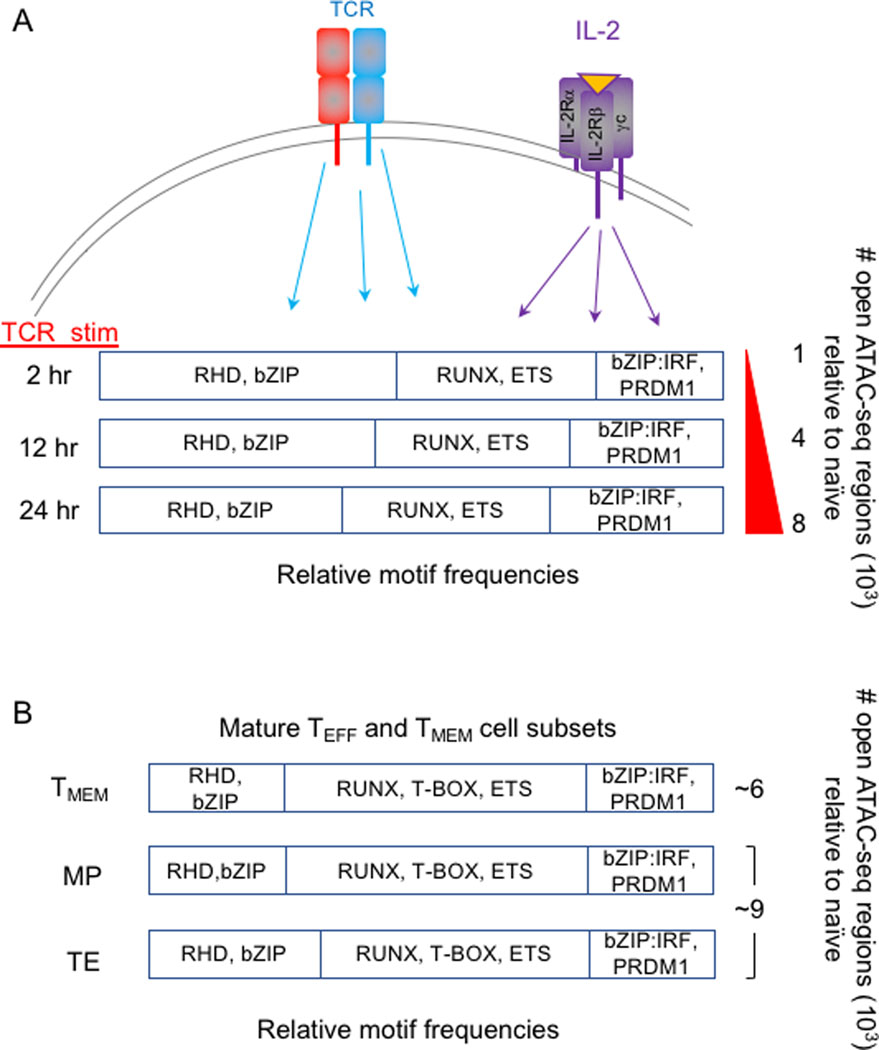
Fig. 3. TCR stimulation induces stable chromatin accessibility of cis-regulatory regions encoding binding sites for multiple TF families.
(A) TCR signals and co-stimulation (not depicted) progressively increases the number of accessible ATAC-seq regions before naïve cells undergo their first cell division. The relative proportions of the most frequently occurring enriched TF motifs within accessible ATAC-seq regions at each time point are depicted. (B) The relative proportions of the most frequently occurring enriched TF motifs within accessible ATAC-seq regions (number of regions shown to right) of mature TEFF and TMEM cell subsets are depicted. Runx, ETS and bZIP motifs are highly frequent in all cases, suggesting their importance for initiating and maintaining the differentiated states of TEFF and TMEM cells.
Runx3 and AP-1 are essential for initial chromatin accessibility during TCR stimulation of naïve cells
Initial chromatin opening of many cis-regulatory sites during TCR stimulation of naïve CD8 T cells involves concerted actions of NFAT, AP-1, RUNX and ETS TFs. In both CD4 and CD8 T cells, TCR stimulation acutely activates NFAT and AP-1 TFs, and chromatin accessibility develops transiently in cis-regulatory regions encoding composite NFAT/AP-1 binding sites. Many of these inducible sites reside adjacent to cis-regulatory regions enriched with RUNX and ETS binding sites that also develop accessibility, but which remain persistently accessible after cessation of TCR signals 64,176–178. Exactly how these TFs modify nucleosomes to establish accessibility is unresolved, but regulatory regions bound by AP-1 or Runx3 that normally gain chromatin accessibility during initial TCR stimulation are not remodelled when AP-1 TF activity is blocked using a dominant negative FOS protein 179 or if cells are Runx3 deficient 64. Runx3 occupancy substantially overlaps with binding of the bZIP TFs BATF, Jun and Jund 64,88,129. Regions of chromatin accessibility in Runx3 deficient cells following TCR stimulation almost entirely lack those that harbor NFAT, AP-1 and other bZIP motifs, (among several others) 64. These results suggest that NFAT and bZIP TFs are insufficient for establishing chromatin accessibility in most of their sites in the absence of Runx3. Thus, multiple classes of factors are critical for early chromatin accessibility, although it is still unclear how each TF is important, whether they act simultaneously at the same sites, or whether there is a specific order of operations.
Concerted action of AP-1, other bZIP dimers and Runx3 TFs during TCR stimulation are likely to initiate chromatin accessibility at a large number of cis-regulatory regions that remain accessible in mature TMEM cells (Fig. 4). A substantial fraction of Runx3 protein in naive cell nuclei is not liberated into nuclear extracts unless salt concentrations that completely extract histones are applied, which indicates Runx3 strongly associates with bulk chromatin prior to TCR stimulation 64. In addition, ChIP-seq analyses indicate that a substantial number of Runx3 binding sites are present in ex vivo CD8 T cells from naïve mice, which suggests Runx3 could be pre-loaded at some target regulatory regions in naïve cells that become stably remodelled following TCR stimulation 129. Consistent with this, the intensity of binding at many of these sites increases after cells differentiate in response to TCR stimulation and culture with IL-2 129. Nuclear Fos and Jun (AP-1) expression in CD4 T cells is induced by CD28-mediated co-stimulation during TCR activation, and is required for chromatin accessibility at AP-1 sites 179,180. Thus, TCR and co-stimulation dependent signals induce increased Runx3 binding and chromatin remodelling, which are likely stimulated by AP-1 activity (Fig. 4), at a large number of cis-regulatory regions in naïve CD8 T cells. It will be interesting to determine the requirement of NFAT proteins in coordinating chromatin opening at AP-1 and Runx3 controlled sites 181.
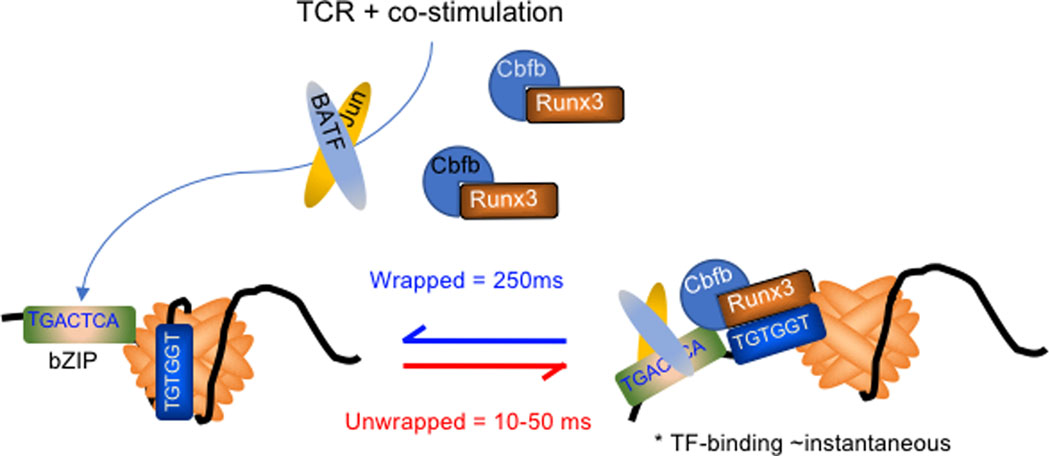
Fig. 4. Spontaneous topological deformations of nucleosomes might facilitate passive entry of TFs into occluded cis-regulatory DNA.
(A) The fully wrapped state of DNA in a nucleosome is transient (left). Spontaneous unwrapping that begins at the DNA entry and exit locations on the nucleosome occurs ~ 4 times per second. The DNA rewraps very fast, as the unwrapped lifespan is only 10–50 ms. However, transcription factor binding is considered nearly instantaneous, allowing TFs to have many opportunities to access their recognition sites in otherwise nucleosome-occluded sequences (right). Transient activation of bZIP family member TFs (and other TFs, e.g., RHD) in response to TCR stimuli facilitates bZIP and Runx-family TFs to capture transiently accessible nucleosome-DNA and prevent rewrapping. Stable remodelling might require additional chromatin remodelling activities that are delivered by the TFs, or might only depend on cooperative binding by multiple TFs.
Potential mechanisms for de novo TF access into nucleosomes in activated CD8 T cells
Multiple mechanisms could account for the ability of Runx3 and other TFs to gain access to nucleosomes during TCR stimulation (Figs. 2 and 4). Certainly, this process could involve the activity of ATP-dependent chromatin remodelling machines that alter histone-DNA contacts and mobilize nucleosomes 154 (Fig. 2A). Consistent with this possibility, multiple essential subunits of two ATP-dependent chromatin remodelling complexes (SWI/SNF- and CHD-families) are highly represented in co-immunoprecipitates after tandem-affinity purification of an epitope-tagged version of Runx3 expressed after stimulation and transduction of naïve CD8 T cells cultured in vitro (D. Wang and M.E. Pipkin, unpublished observations). Thus, chromatin remodelling machines could be recruited to Runx3 during TCR stimulation in response to increased expression or posttranslational modifications of remodeller subunits that increases their affinity for Runx3 in stimulated cells 182, or by interactions with additional TFs, that deliver them to Runx3 bounds sites.
However, enzymatically manufactured chromatin accessibility might not be the incipient event that facilitates access of these TFs to their binding sites. Nucleosomes located in cis-regulatory regions turn over rapidly in vivo and are exchanged many times within a cell generation 183, which suggests that TFs might have multiple opportunities to sample sequences embedded in otherwise nucleosome-occupied DNA. This process could be the result of spontaneous topological transitions that nucleosomes undergo wherein the DNA partially unwraps (~ 4 times per second), and suddenly rewraps 160 (Fig. 4). The rewrapping kinetic is relatively fast, occurring in ~ 20 ms, but the binding of TFs to their sites is known to be fast enough (i.e., nearly instantaneous) to achieve occupancy on their sites prior to the rewrapping event, given sufficient TF concentration and affinity 160. In this way, TFs might first gain access to their binding sites passively according to thermodynamic parameters, and then employ recruited chromatin remodeling machines to enzymatically aid the competing nucleosome(s) to reform on adjacent lower affinity sequences that are otherwise non-preferred 151–153.
Specificity and stability of chromatin accessibility at Runx-TF dependent regulatory regions
Cooperative recognition of cis-regulatory regions by Runx-TFs and several additional TF families are likely to enhance competition with nucleosomes and contribute both to the specificity and stability of cis-regulatory regions that undergo remodeling at Runx-bound sites. Both direct and indirect cooperative mechanisms might account for how Runx-TFs could gain access to their binding sites during TCR stimulation 184. Direct interactions between the Runt-domain of Runx- and ETS-family TFs facilitate their dimerization on DNA and could be important for the specificity and/or increased binding affinity of Runx-TFs at particular sites during TCR stimulation 185. Sequences encoding ETS-family binding sites are some of the most highly enriched motifs in Runx3 ChIP-seq binding sites of TEFF CD8 T cells after differentiation in cell culture 129. In addition, Runx3 deficiency differentially affects the frequency of cis-regulatory regions encoding distinct ETS-family motifs during TCR stimulation. Thus, multiple different TF-TF interactions between different Runx- and ETS-family TFs are likely to shape the accessible chromatin landscape upon TCR stimulation 64. The ETS-family of TFs is encoded by 27 different genes in mice 186, which implies that extensive TF-TF diversity could account for specificity of Runx-dependent gene expression. In addition, Runx-proteins are also known to indirectly activate transcription with bZIP-family TFs on adjacent elements 124,187. The accessibility of cis-regulatory regions encoding motifs recognized by multiple bZIP-family TFs, including BATF and Bach2, are strongly reduced in Runx3 deficient cells during TCR stimulation, and Runx3 binding strongly overlaps BATF and Bach2 ChIP-seq binding sites 64,129. Thus, specificity in Runx3-dependent transcription is likely also conferred by distinct bZIP family TFs. Finally, cooperation between Runx and T-box TFs appear to account for stable remodelling of cis-regulatory regions. Runx and T-box motifs co-occur frequently in sequences that are stably remodelled in memory CD8 T cells, many of which are co-occupied by Runx-Cbfb, T-bet and Eomes proteins 166. The accessibility of cis-regulatory regions encoding T-box motifs are strongly impaired in Runx3 deficient CD8 T cells during TCR stimulation 64, and both sets of TFs cooperatively drive the transcriptional program of effector and memory CD8 T cells after naive cell stimulation 99,125.
Extensive differences in chromatin accessibility develop in TEFF and TMEM cells after naïve CD8 T cell activation
ATAC-seq analyses of bulk TEFF cells, day 35 KLRG1lo CD127hi cells (i.e., a mixture of TEM and TCM, with TLLE excluded), as well as TE and MP CD8 T cell populations that arise following acute infection with the LCMVArm have shown that each of these subsets develop accessibility in a large number of putative cis-regulatory regions that are distinct from those that are accessible in naïve CD8 T cells 136,169,170. The majority of regions that become accessible after naïve cell activation are shared between both TEFF and TMEM cells. There is also extensive overlap between the accessible regions from bulk TEFF cells from acute infection, and TEX cells that develop following chronic infection with the clone 13 strain of LCMV (LCMVCl13) 169,170,188–190. Differences also develop between each of these subsets (discussed below). However, the extensive commonality of accessible chromatin regions that develops after naïve cell activation in all antigen-experienced CD8 T cell subsets suggests all cells might initially share a common developmental history.
Differential chromatin accessibility in subset-specific TEFF and TMEM cells
Differential chromatin accessibility of cis-regulatory regions in antigen-experienced CD8 T cell subsets partly explains their subset-specific gene expression profiles. After an acute LCMVArm infection, bulk TEFF cells from the peak response exhibit a larger number of accessible cis-regulatory regions compared to TMEM cells at the beginning of the memory phase. Depending on the study, which each used distinct criteria for calling accessible regions, ~6,000–13,000 regions are more accessible in TEFF cells on day 8 post infection compared to naïve cells, whereas ~3,000–9,000 regions are more accessible in TMEM cells, compared to naïve cells 169,170. These patterns of chromatin accessibility develop progressively, because only ~2,000 regions were more accessible in TEFF cells from day 4 after infection compared to naïve cells 169. Bulk TEFF and TMEM cells after LCMVArm infection are more similar to each other than either is to naïve cells. Only ~1,100 regions are uniquely accessible in TEFF cells, and only ~140 regions are uniquely accessible in TMEM cells, because many of the regions that are more accessible in either subset compared to naïve cells are accessible in both TEFF and TMEM cells 170. The smaller number of memory-specific regions is because TMEM cells are more closely related to naïve cells and ‘re-develop’ accessibility in regions that are initially accessible in naïve cells, which are inaccessible in TEFF cells. Thus, bulk circulating TEFF and TMEM cells are similar at the level of chromatin accessibility.
Chromatin accessibility in TE and MP cells from the peak response is more similar between each other than MP cells are with mature TMEM cells 170. This emphasizes overall developmental similarity between TE and MP cells, even though more than 1,000 genes are differentially expressed between the two 64. However, consistent with the propensity of MP cells to give rise to circulating TMEM cells, the regions that are accessible in MP cells are slightly biased toward those found in TMEM cells 170. In addition, the regions that are more accessible in mature TMEM cells compared to TEFF cells also show increased accessibility in MP cells compared to TE cells, suggesting that cis-regulatory regions underlying TMEM cell gene expression are more active in MP cells compared to TE cells. In contrast, the regions of accessibility in both TE and MP cells isolated from the spleen differ substantially from putative TRM precursors isolated from non-lymphoid tissues near the peak response to LCMVArm infection 59. Differential chromatin accessibility in these different T cell subsets positively correlates with the specific gene expression patterns in the different subsets 59,136,169,170. Thus, there are cell type specific patterns in chromatin accessibility that develop and correlate with transcriptional changes which drive gene expression profiles specific to distinct TEFF and TMEM cell subsets, and there is substantial similarity between TEFF subpopulations, compared to naïve cells. It is still unknown exactly how these differential patterns of accessibility develop, however, a substantial portion of de novo accessible sites found in both TEFF and TMEM subsets are induced rapidly upon TCR stimulation 64, which suggests they are selectively stabilized in one or the other subset at later times.
Differential enhancer activity correlates with the differentiated states of TEFF and TMEM CD8 T cells
The specific transcriptional activity that defines TE, MP and TMEM cells positively correlates with increased chromatin accessibility, histone modifications that are indicative of active enhancers, and three-dimensional contacts between distal putative cis-acting regulatory regions and target gene promoters within each specific cell subset. Accessible ATAC-seq regions that are shared between all antigen-experienced subsets of CD8 T cells are located in distal regions and near gene transcription start sites (TSSs) 170. In contrast, ATAC-seq regions that are specifically accessible in TEFF or TMEM cells are located in regions distal to TSSs, suggesting that distal cis-regulatory sequences account for lineage-specific gene expression 170. Many of these distal regions appear to be transcriptional enhancers as judged by ChIP-seq analyses of histone modifications (H3K4me3, H3K4me1, H3K27me3, and H3K27Ac) and their specific patterns that can be used to distinguish TSSs from enhancers, and predict their activity 136,191–195. Based on these criteria, distinct TEFF and TMEM subsets manifest specific enhancer repertoires, and TE cells gain nearly 2-fold more active enhancers relative to naive cells, as compared to MP and TMEM cells 136,192,195. This suggests that TE cells might develop from TEFF cells that are more transcriptionally active than MP cells, and is consistent with greater expression of genes in TE cells compared to MP cells that are otherwise upregulated in both cell populations after naïve cell activation. Conversely, MP cells re-engage multiple enhancers that were active in naïve cells and give rise to TMEM cells.
The apparent activity of distal enhancers correlates with increased binding activity of specific sets of TFs and is defined by the enhancer’s motif composition. TE-specific enhancer sequences are enriched with motifs recognized by both T-box and bZIP family TF motifs (as well as others), and are more strongly occupied by T-bet and BATF TFs, compared to putative enhancers that are more active in MP or TMEM cells 88,136,170. This coincides with the requirement for both TFs in terminal differentiation 45,88. However, certain TMEM (TEM and TCM)-specific enhancers are also enriched with T-box motifs, but are less enriched with bZIP motifs 136,170. This correlates with observations that regions of stable chromatin accessibility are bound by T-bet and Eomes 166, and that both TFs are necessary to establish and maintain TMEM cells, in addition to their requirements for the development of terminally differentiated cells 99,136. Notably, a significant fraction of these sites is also occupied by Runx-Cbf complexes 166. Reciprocally, motifs recognized by distinct sets of TFs are enriched in enhancers that are specifically active in TCM cells relative to TE cells 136,192, and include Tcf1, Foxo1, Foxp1, Eomes, Gabpa, Gfi1 and Nr3c1 (as well as others), several of which have essential roles in promoting either MP cell or TCM formation by inducing T cell quiescence, lymphoid tissue homing, and homeostatic self-renewal potential 40,55,98,196–198. Therefore, concerted activity of TFs that are expressed in a lineage or cell type-specific fashion appear to drive the activity of accessible cis-regulatory regions and transcription of genes that promote the respective cellular phenotype.
Differential chromatin modifications at these cell type specific cis-regulatory regions argues that their associated cell types are ‘epigenetically’ distinct. However, maintaining the lineage-specific accessibility and active histone modification profiles probably requires ongoing binding by the relevant TFs to their cognate sites in these regions, as opposed to being maintained passively. For example, the stability of gene expression and the phenotypic characteristics of TE cells and TLLE memory cells depends on the continued function of Zeb2 and Id2 93,199, and probably T-bet (H. Diao and M.E. Pipkin unpublished observations). Conditional disruption of either Zeb2 or Id2 in the memory phase results in KLRG1hi TLLE cells ‘converting’ into those resembling TCM cells 93,199. It is possible that part of this maintenance involves IL-2R signals, because IL-2R stimulation promotes Id2 expression 53, and the persistence of KLRG1hi TLLE cells after viral infection is severely impaired in cells that lack IL-2Rα 56, resulting in enhanced frequencies of TCM-like cells 56,57. These examples reaffirm that the differentiated states of cells at both the epigenetic and transcriptional level are actively, rather than passively, maintained by the continued actions of cell type specific TFs 68. Thus, chromatin accessibility landscapes that are established in human TEFF CD8 T cells during the first week of viral infection, which are maintained for a decade or more in the resulting TMEM populations 5, are most likely the result of continued activity of specific TFs in those cells rather than latent epigenetic modifications. These observations emphasize the importance of the basic mechanics of transcriptional regulation itself, in addition to alterations in chromatin structure that produce ‘epigenetic’ effects.
Basic transcriptional mechanics account for differential gene expression in distinct TEFF cell subsets.
Regulation of transcriptional elongation by pre-recruited Pol II is a major regulatory mechanism
A key feature of TMEM cells is differential chromatin remodeling that creates stable chromatin accessibility in lineage-specific genes that are only transcribed at basal levels in resting cells, as in the case of cytokine genes in T helper cells 200, and the Prf1 gene in TCM cells 5,42,201,202. However, additional transcriptional mechanics account for the induced transcriptional output of these genes. In CD8 T cells, the TSSs of the Ifng, Prf1, and Gzmb genes are persistently accessible in both TEFF and TMEM cells after LCMV infection of mice, and yellow fever virus vaccination of humans 5,203. Pol II is present at the Ifng and Prf1 TSSs, but not at the Gzmb TSS, and this generally correlates with the level of basal expression of mRNA from each of these genes 203. The cis-regulatory regions that control the Prf1 locus are similarly accessible in both TEFF and TMEM cells; however, Prf1 is highly expressed in TEFF cells, but not in TCM cells 42,116,170,202. IL-2R stimulation enhances Prf1 gene expression during the differentiation of activated CD8 T cells in cell culture, and this positively correlates with increased Pol II occupancy at the TSS, but not within the gene body. In contrast, restimulation of TCM-like cells with TCR like signals induces increased Pol II occupancy at the Prf1 TSS and in the gene body (M.E. Pipkin unpublished observations), which correlates with dramatically increased Prf1 mRNA expression 42. Thus, initial establishment of chromatin accessibility, RNA Pol II loading at TSSs and subsequent transcriptional elongation of genes are each steps of transcription that are regulated in CD8 T cells.
There is extensive overlap in the TSSs of genes that become accessible in both TEFF and TMEM cells following initial TCR stimulation 136,170, and this most likely correlates with loading of Pol II at these TSSs and basal transcription. In resting CD4 T cells, the promoters of many genes are chromatin accessible and loaded with Pol II, but their gene bodies lack Pol II and the genes are not expressed highly 204. In contrast, increased lineage-specific gene expression correlates with specific activity of distal enhancers in defined TEFF and TMEM subsets, and predicted interactions with their cognate promoters 192. One hypothesis is that lineage-specific enhancer-promoter interactions activate transcriptional elongation of ‘poised’ Pol II that has already been recruited (Fig. 5).
Fig. 5. Terminal differentiation of TEFF CD8 T cells might require induced positive regulation of transcriptional elongation.
Initial TCR, co-stimulatory and IL-2R dependent signals might drive chromatin remodelling that promotes binding of general transcription factors (GTFs), Pol II recruitment and pre-initiation to generally activate promoters of genes that are important in both TEFF and TMEM cells. However, Pol II that escapes the promoter stalls downstream of the transcription start site (TSS) because its processivity is inhibited by negative elongation factors NELF and DSIF. Successful elongation of Pol II depends on P-TEFb. TE-specific distal enhancers could recruit P-TEFb, which phosphorylates DSIF and NELF, dissociating NELF and converting DSIF into a positive elongation factor. Pol II elongates full length nascent mRNAs (green). This process also induces extensive phosphorylation of the Pol II CTD, which recruits additional mRNA processing activities, and CRFs (e.g., the Set2 family) that deposit histone modifications which demarcate transcriptionally active genes (e.g., H3K36me3) (not depicted). Differential association of lineage-specific enhancers with distinct super elongation complexes that incorporate P-TEFb could explain differential dependence of TE and MP cells on P-TEFb.
Although it has yet to be analyzed directly in T cells, initiated Pol II complexes stall very quickly after departing the promoter, generally within ~100bp of the transcription start site (TSS), and only some paused Pol II complexes elongate successfully 205. Photobleaching studies using fluorescently-tagged RNA Pol II in a transgenic system suggests that 99% of promoter-recruited Pol II complexes do not successfully elongate 206, implying that Pol II elongation is the limiting step in high-level gene expression. Thus, regulation of Pol II pausing and transcriptional elongation is a central mechanism that determines a gene’s rate of transcription in many developmental and acute stimulation conditions, a revised view compared to the original perspective that RNA Pol II recruitment and initiation were the major determinants 205.
Regulating transcription by relieving Pol II pausing fits logically into the biology of TMEM cells which harbor remodeled chromatin structure at many genes that are poised for rapid transcriptional activation. Multiple mechanisms cause Pol II to arrest after it initiates. These include the intrinsic properties of RNA Pol II and its interaction with the underlying DNA, as well as, extrinsic factors including nascent RNA processing, abutment against positioned nucleosomes, and two regulatory protein complexes, NELF (Negative Elongation Factor) 207 and DSIF (5,6-dichloro-1-b-D-ribofuranosylbenzimidazole Sensitivity Inducing Factor; encoded by Spt4 and Spt5) 208 (Fig. 5). The Spt5 subunit of DSIF captures the 3’ end of nascent transcripts, recruits NELF and both factors trap Pol II within its pause sites 209. Both NELF and DSIF are sufficient to induce Pol II pausing in purified in vitro systems, and each are necessary for Pol II pausing in mammalian cells 205,209,210 (Fig. 5). Recruitment of the positive transcription elongation factor b (P-TEFb), induces the release of paused Pol II into productive elongation. Canonical P-TEFb is composed of Cyclin T1 and Cdk9, and its recruitment induces DSIF phosphorylation, which causes dissociation of NELF from the stalled Pol II complex 205,209,210 (Fig. 5), converts DSIF into a positive elongation factor, and induces Pol II elongation 205,209. These events also prime Pol II for intense phosphorylation of its C-terminal domain (CTD), which becomes the binding scaffold that recruits multiple additional RNA-processing and chromatin modifying complexes that facilitate transcription through nucleosomes, mRNA splicing and polyadenylation 205,209,211, resulting in mature mRNAs (Fig. 5).
P-TEFb positively regulates formation of TE cells and Th1 cells during viral infection. Parallel in vivo, pooled RNAi screens of candidate transcriptional regulators identified Cyclin T1 (Ccnt1) as one factor that was required for Th1 cell formation in CD4 T cells, and TE (KLRG1hiCD127lo) cell formation in CD8 T cells, during LCMV infection 134. CD8 T cells depleted of either Cyclin T1 or Cdk9 accumulate normally during LCMV infection, but inefficiently differentiate into TE cells and preferentially acquire a MP phenotype. These cells do not upregulate Prf1 or Gzmb expression and less efficiently control viral titers in hosts after adoptive transfer 134. These results suggest that P-TEFb could be required to increase transcription of genes that encode effector CTL capabilities, and that drive terminal differentiation. In line with this, Cyclin T1-depleted cells inefficiently upregulate T-bet and Blimp1 expression, which are normally expressed in both TE and MP cells, but are more highly expressed in TE cells and required for their terminal differentiation 45,87,99,136,212. One possibility is that activation of distal TE-specific enhancers recruits P-TEFb to a wide array of poised promoters that harbor stalled RNA Pol II at genes that define TE and Th1 cells (Fig. 5), or only at key positive regulators, such as Tbx21 and Prdm1. Conversely, enhancers and TFs that promote Pol II pausing in TEFF cells and do not recruit P-TEFb could favor the differentiation of MP and TMEM cells by maintaining chromatin accessibility at effector-type genes while restricting high rates of their transcription. It is possible that transcription of genes necessary to generate MP cells might not require P-TEFb or might be less dependent on its activity.
Although many of these processes are considered ‘general transcriptional mechanisms’, they are employed in context-dependent fashions, as in the case of lineage-specific enhancer repertoires that are controlled by specific assemblies of TFs 136,192,195. For example, an interaction between Runx1 and P-TEFb is necessary for CD4 silencing during thymic T cell development 213. Thus, regulation of transcriptional elongation has specific developmental consequences. Notably, P-TEFb is incorporated into four related super elongation complexes (SECs) defined biochemically by distinct subunit compositions that each possess P-TEFb catalytic activity against the Pol II CTD in vitro, but exhibit distinct transcriptional effects and gene-specific binding patterns in cells 214. Future studies that map the occupancy of P-TEFb and the distributions of unmodified relative to CTD-phosphorylated forms of Pol II in developing TEFF cell subsets will likely resolve how differential enhancer landscapes specifically control gene activity by regulating P-TEFb recruitment and transcriptional elongation during differentiation of TEFF and TMEM cells.
Chromatin modifications that regulate transcriptional elongation and enforce terminal differentiation
Naïve CD8 T cells express multiple genes that are considered pro-memory (e.g. Il7ra, Sell, and Tcf7) that are initially downregulated following activation and stably repressed in TE cells, but are re-expressed in some TEFF cells that develop into TMEM cells. The initial and stable repression of these genes in TEFF cells is the result of several CRFs with enzymatic activities that methylate specific residues in histones and DNA. CD8 T cells lacking the H3K9 methyltransferase Suppressor Of Variegation 3–9 Homolog 1 (Suv39h1) 215, the H3K27 histone methyltransferase subunits enhancer of zeste subunit 2 (Ezh2) and embryonic ectoderm development (Eed) 72,216, or the de novo DNA methyltransferase 3 Dnmt3a 47,217 each exhibit defects in repression of pro-memory genes in developing TEFF cells.
Normally, naïve CD8 T cells develop islands of H3K9 trimethylation (H3K9me3) at pro-memory genes including Il7r and Sell within 3 days of initial TCR stimulation in vitro 215, but Suv39h1-deficient CD8 T do not develop H3K9me3 density at these and other TCIRC and TSTEM cell-expressed genes in TEFF cells in vivo 215. Likewise, DNA sequences in a similar set of pro-memory genes develop de novo 5mC in TEFF cells by 4 days after viral infection, but these regions are much less methylated in cells lacking the de novo methyltransferase Dnmt3a, and these genes are reexpressed with faster kinetics as the infection resolves compared to control cells 47. In addition, deposition of H3K27me3 at pro-memory genes (e.g., Bach2, Id3, Tcf7) appears to ensure commitment to terminal differentiation by preventing their re-expression near the end of the effector phase 72,216. Initially, downregulation of these genes is independent of H3K27me3 deposition. Locus-specific H3K27me3 deposition is relatively low in both KLRG1hi and KLRG1lo TEFF cells on day 4.5 after infection, but is pronounced in these genes in TE cells on day 10 after infection 216. CD8 T cells in which Ezh2 is conditionally ablated using CD4-Cre are devoid of bulk H3K27me3 in cells after 3 days of TCR stimulation 216. These cells accumulate less and have higher fractions of CD62L positive cells by day 4.5 after infection, despite normal frequencies of KRLG1hi cells at this time 72,216. However, Ezh2-deficient CD8 T cells on day 8 after infection comprise increased frequencies of MP-like cells and reduced frequencies of TE cells compared to wildtype cells. Accordingly, the bulk Ezh2-deficient CD8 T cell population more highly expresses pro-memory genes, and these genes exhibit reduced H3K27me3 deposition 72,216. Thus, Suv39h1 and Ezh2 appear to function sequentially to initiate and then stabilize repression of pro-memory genes, which promotes terminal differentiation.
H3K9me3 and H3K27me3 are found in both promoter proximal and distal cis-acting regions in T cells 215,216,218. However, both modifications are most heavily concentrated in the transcribed aspects of repressed genes, skewed mainly toward the 5’ ends of genes 219. This suggests that the main effects of these histone modifications in activated CD8 T cells could be to govern transcription itself, rather than directly controlling the accessibility or activity of cis-regulatory regions. In other cell types, both H3K9me3 and H3K27me3 in gene bodies negatively regulate transcription by reducing transcriptional elongation and inducing Pol II pausing 220–222. For example, RNAi of the histone H3K9 methyltransferase SETDB1 in preadipocytes reduces H3K9me3 deposition and causes premature release of Pol II from promoter proximal regions and elongation into its target genes, prior to when transcription normally engages in definitive adipocytes 221. Analogously, RNAi of the H3K27me3 de-methylase JMJD3/KDM6B in HL-60 cells increases H3K27me3 at its target genes, which impairs Pol II occupancy in gene bodies, but not at promoter regions 220. This also impairs recruitment of the positive elongation factors SUPT6, which normally associates with elongating Pol II and DSIF, and SUPT16H, a member of the FACT complex that facilitates Pol II elongation by unraveling nucleosomes and re-assembling them in the wake of Pol II 159. Thus, one hypothesis is that recruitment of Suv39h1 and Ezh2-EED and deposition of H3K9me3 and H3K27me3 at pro-memory genes in TEFF cells counteracts the activity of P-TEFb at these genes in TE cells. Reciprocally, because T-bet and JMJD3/KDM6B have been shown to interact in CD4 T cells 223, it stands to reason that T-bet-dependent recruitment of demethylase activity that prevents accumulation of H3K27me3 could account for increased P-TEFb activity at T-bet activated genes that drive the differentiation of Th1 and TE cells.
Concluding remarks
A model for the differential development of TEFF and TMEM cells after naïve cell activation
During hematopoiesis, progenitor cells promiscuously express intermediate amounts of lineage-determining factors and manifest mixed gene expression programs that account for multilineage developmental potential 224. This variability arises within the progenitor population at the level of individual transcriptomes and leads some cells to transiently manifest increased expression of specific regulatory factors that creates lineage-bias and the propensity to activate one developmental pathway instead of another 69. Activated CD4 T cells manifest analogous behavior as they undergo lineage-determination into particular effector T helper cell lineages 225. A similar regulatory logic might also explain how cell fate determination of TEFF and TMEM cell subsets occurs, whereby activation of naïve CD8 T cells induces a general programming phase that initially generates progenitor cells with fluctuating transcriptomes and distinct metastable states that create lineage-bias in some cells (Fig. 6). Extracellular signals that favor differentiation along one or another pathway ‘captures’ these early stochastic deviations and reinforces them. Because CD8 T cells are not sessile and readily migrate through different tissues or microanatomic locales composed of distinct tissue-specific extracellular signals 138, the differentiation pathways that are initially selected can be more or less reinforced in a spatially formatted pattern. Terminal differentiation most likely involves cells that develop TE cell bias and then positively regulate transcriptional elongation of TE-specific genes while preventing transcription elongation of pro-memory genes. Conversely, cells whose regimes favor Pol II pausing at TE-related genes, and that fail to repress pro-memory genes retain the capacity to reactivate aspects of the naïve cell program and develop into TMEM cells. At any stage, cells are likely to retain some potential to revert or revise their developmental program (often referred to as ‘plasticity’), but these revisions are likely to be constrained by their developmental history and their capacity to experience signals that would reinvest in a distinct developmental program. Thus, TMEM CD8 T cell ontogeny might be arranged in an initial common differentiation phase that generates uncommitted progenitors with multilineage potential, followed by stochastic sampling of distinct differentiation paths toward alternative TEFF and TMEM cell subsets that are ultimately reinforced in some cells, as opposed to developing in an exclusively linear continuum, or divergent paths that are specified deterministically 11,35,38. Given that virtually all chromatin modifications are fully reversible, the apparent ‘fates’ of differentiated TEFF cells can be ‘undone’, which explains the reexpression of genes such Sell in MP cells as they transition into the memory phase 47, and the repression of genes such as Klrg1 and Gzmb that are initially expressed but then de-activated in some TEFF cells that become TMEM cells 48,67.
Fig. 6. A multilineage priming model to conceptualize the origins of distinct TEFF and TMEM cells.
Activation of naïve CD8 T cells might initially induce a common programming phase that establishes competence at genes necessary for both TEFF and TMEM cell formation, followed by divergence into distinct TEFF and TMEM pathways that are self-reinforcing. In such a scenario, initial TCR, co-stimulation and integration of multiple extracellular signals induces widespread chromatin remodeling and a population of TEFF cells comprising heterogeneous transcriptomes that develop lineage-bias because of persistent variations in TF protein concentrations in some cells (differently colored cells). These fluctuations activate cell-type specific enhancers that act on previously poised genes, which reinforces and extends the biased transcriptional states. For example, TFs that activate TE-specific enhancers which cause enhanced transcription elongation of genes encoding TFs that also bind TE-specific enhancers creates a feed-forward loop that promotes TE cell differentiation. Simultaneous deposition of chromatin modifications that prevents Pol II elongation in pro-memory genes reduces the survival potential of certain TEFF cells, ensuring terminal differentiation. Conversely, cells that favor TFs and chromatin modifications that enforce Pol II pausing in genes that otherwise drive TE cell differentiation, preserves the potential to develop into distinct TMEM cell subsets. Arrows imply the overall differentiation framework. However, the developmental changes are likely open to revision given the appropriate environmental signals and capacity to receive the signals (bi-directional arrows), because transcriptional states are dynamic, continuously influenced by TF binding activity and virtually all chromatin modifications are known to be reversible.
Future perspectives
Deciphering how transcriptional regulation drives cell differentiation in vivo is complicated technically because the fates of individual cells are not easily traceable, which prevents discerning how the historical experiences of individual cells influence their transcriptional programs and that of their descendants. A key future challenge that will partially overcome this complication is applying single cell genomics and computational strategies to link the genealogies of individual cells to their respective transcriptomes and chromatin structure states. Furthermore, work to date has mainly focused on defining regulatory factors using bulk mRNA analyses in comparative analyses of phenotypically defined subsets and inferring potentially important factors based on differential expression 9,10,12. Dozens of regulatory factors have been defined in this manner, but the mammalian genome encodes ~ 2,000 conventional sequence specific DNA-binding TFs 226, and more than 300 chromatin regulatory factors 146,227. Many of these are likely essential for controlling the differentiation of activated CD8 T cells but are not appreciated because of minor deviations in expression that are not discernable in bulk mRNA analyses. Still others might not be differentially expressed, but are modified posttranslationally, or function specifically due to combinatorial assembly with other factors that are differentially expressed. These are biological themes epitomized by how distinct subunits of CRFs are assembled in unique fashions to provide regulatory specificity 182, and how TFs cooperatively recognize specific cis-regulatory sequences 165,184. Thus, the majority of regulatory factors which establish and maintain the differentiated states of antigen-experienced CD8 T cells might yet to be discovered. The application of both RNAi and CRISPR-based tools in strategies to individually perturb the expression of hundreds or thousands of genes in parallel while analyzing their resulting phenotypes individually in an unsupervised fashion are likely to enable rapid and comprehensive definition of gene functions in myriad of in vivo settings 135,228.
Finally, future studies that map TF binding and nucleosome organization at very high-resolution will be instrumental in determining how competition between TFs and histones might drive chromatin reorganization that underlies CD8 T cell differentiation during immune reactions. One could imagine that cis-regulatory networks are organized in affinity landscapes that sense fluctuations in TF binding activities and TF partnerships to engage distinct TMEM cell differentiation outputs in response to specific T cell stimulation conditions. In this way, specific cis-regulatory region ensembles that become occupied under particular TF activity regimes would respond accordingly with defined transcriptional responses. Nucleosomes can now be mapped at sufficient resolution to detect reliable changes in their occupancy and positioning 161,229, and ChIP-seq methods can map TF-protected DNA at near base-pair resolution 230,231. When these assays are combined with competitive ChIP 232, and the analyses incorporate experimental time series, it is likely that both architectural and kinetic understanding of how specific sets of TFs engage chromatin remodelling and alter transcription during CD8 T cell differentiation. By compiling functional atlases of these factors in T cells, entirely new avenues in which to engineer durable immunity against chronic infections and tumors are likely to be devised.
Acknowledgements
NIH grants R01 AI095634, U19 AI109976, P01AI145815 and DoD grant W81XWH-16-1-0006, and Frenchman’s Creek Women for Cancer Research to MEP supported this work.
References
- 1.Gerlach C, van Heijst JW, Swart E, et al. One naive T cell, multiple fates in CD8+ T cell differentiation. J Exp Med. 2010;207(6):1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stemberger C, Huster KM, Koffler M, et al. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity. 2007;27(6):985–997. [DOI] [PubMed] [Google Scholar]
- 3.Obar JJ, Jellison ER, Sheridan BS, et al. Pathogen-induced inflammatory environment controls effector and memory CD8+ T cell differentiation. J Immunol. 2011;187(10):4967–4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wherry EJ, Teichgraber V, Becker TC, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nature immunology. 2003;4(3):225–234. [DOI] [PubMed] [Google Scholar]
- 5.Akondy RS, Fitch M, Edupuganti S, et al. Origin and differentiation of human memory CD8 T cells after vaccination. Nature. 2017;552(7685):362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller JD, van der Most RG, Akondy RS, et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28(5):710–722. [DOI] [PubMed] [Google Scholar]
- 7.Jameson SC, Masopust D. Understanding Subset Diversity in T Cell Memory. Immunity. 2018;48(2):214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–161. [DOI] [PubMed] [Google Scholar]
- 9.Chang JT, Wherry EJ, Goldrath AW. Molecular regulation of effector and memory T cell differentiation. Nat Immunol. 2014;15(12):1104–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nature reviews Immunology. 2012;12(11):749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27(3):393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milner JJ, Goldrath AW. Transcriptional programming of tissue-resident memory CD8(+) T cells. Curr Opin Immunol. 2018;51:162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masopust D, Soerens AG. Tissue-Resident T Cells and Other Resident Leukocytes. Annu Rev Immunol. 2019;37:521–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8(2):107–119. [DOI] [PubMed] [Google Scholar]
- 15.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2(4):251–262. [DOI] [PubMed] [Google Scholar]
- 16.Badovinac VP, Harty JT. Programming, demarcating, and manipulating CD8+ T-cell memory. Immunol Rev. 2006;211:67–80. [DOI] [PubMed] [Google Scholar]
- 17.Busch DH, Kerksiek KM, Pamer EG. Differing roles of inflammation and antigen in T cell proliferation and memory generation. J Immunol. 2000;164(8):4063–4070. [DOI] [PubMed] [Google Scholar]
- 18.Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. 2014;41(6):886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosato PC, Wijeyesinghe S, Stolley JM, Masopust D. Integrating resident memory into T cell differentiation models. Curr Opin Immunol. 2020;63:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milner JJ, Nguyen H, Omilusik K, et al. Delineation of a molecularly distinct terminally differentiated memory CD8 T cell population. Proc Natl Acad Sci U S A. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olson JA, McDonald-Hyman C, Jameson SC, Hamilton SE. Effector-like CD8(+) T cells in the memory population mediate potent protective immunity. Immunity. 2013;38(6):1250–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Renkema KR, Huggins MA, Borges da Silva H, Knutson TP, Henzler CM, Hamilton SE. KLRG1(+) Memory CD8 T Cells Combine Properties of Short-Lived Effectors and Long-Lived Memory. J Immunol. 2020;205(4):1059–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerlach C, Moseman EA, Loughhead SM, et al. The Chemokine Receptor CX3CR1 Defines Three Antigen-Experienced CD8 T Cell Subsets with Distinct Roles in Immune Surveillance and Homeostasis. Immunity. 2016;45(6):1270–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gattinoni L, Lugli E, Ji Y, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17(10):1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gattinoni L, Zhong XS, Palmer DC, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15(7):808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graef P, Buchholz VR, Stemberger C, et al. Serial transfer of single-cell-derived immunocompetence reveals stemness of CD8(+) central memory T cells. Immunity. 2014;41(1):116–126. [DOI] [PubMed] [Google Scholar]
- 27.He R, Hou S, Liu C, et al. Follicular CXCR5- expressing CD8(+) T cells curtail chronic viral infection. Nature. 2016;537(7620):412–428. [DOI] [PubMed] [Google Scholar]
- 28.Im SJ, Hashimoto M, Gerner MY, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537(7620):417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pais Ferreira D, Silva JG, Wyss T, et al. Central memory CD8(+) T cells derive from stem-like Tcf7(hi) effector cells in the absence of cytotoxic differentiation. Immunity. 2020;53(5):985–1000 e1011. [DOI] [PubMed] [Google Scholar]
- 30.Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol. 2016;16(2):79–89. [DOI] [PubMed] [Google Scholar]
- 31.Milner JJ, Toma C, He Z, et al. Heterogenous Populations of Tissue-Resident CD8(+) T Cells Are Generated in Response to Infection and Malignancy. Immunity. 2020;52(5):808–824 e807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crauste F, Mafille J, Boucinha L, et al. Identification of Nascent Memory CD8 T Cells and Modeling of Their Ontogeny. Cell Syst. 2017;4(3):306–317 e304. [DOI] [PubMed] [Google Scholar]
- 33.Opferman JT, Ober BT, Ashton-Rickardt PG. Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science. 1999;283(5408):1745–1748. [DOI] [PubMed] [Google Scholar]
- 34.Buchholz VR, Flossdorf M, Hensel I, et al. Disparate individual fates compose robust CD8+ T cell immunity. Science. 2013;340(6132):630–635. [DOI] [PubMed] [Google Scholar]
- 35.Buchholz VR, Schumacher TN, Busch DH. T Cell Fate at the Single-Cell Level. Annu Rev Immunol. 2016;34:65–92. [DOI] [PubMed] [Google Scholar]
- 36.Gerlach C, Rohr JC, Perie L, et al. Heterogeneous differentiation patterns of individual CD8+ T cells. Science. 2013;340(6132):635–639. [DOI] [PubMed] [Google Scholar]
- 37.Restifo NP, Gattinoni L. Lineage relationship of effector and memory T cells. Curr Opin Immunol. 2013;25(5):556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reiner SL, Adams WC. Lymphocyte fate specification as a deterministic but highly plastic process. Nat Rev Immunol. 2014;14(10):699–704. [DOI] [PubMed] [Google Scholar]
- 39.Chang JT, Palanivel VR, Kinjyo I, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315(5819):1687–1691. [DOI] [PubMed] [Google Scholar]
- 40.Lin WW, Nish SA, Yen B, et al. CD8(+) T Lymphocyte Self-Renewal during Effector Cell Determination. Cell Rep. 2016;17(7):1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4(12):1191–1198. [DOI] [PubMed] [Google Scholar]
- 42.Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32(1):79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Surh CD, Sprent J. Regulation of mature T cell homeostasis. Semin Immunol. 2005;17(3):183–191. [DOI] [PubMed] [Google Scholar]
- 44.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nature immunology. 2000;1(5):426–432. [DOI] [PubMed] [Google Scholar]
- 45.Joshi NS, Cui W, Chandele A, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27(2):281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plumlee CR, Obar JJ, Colpitts SL, et al. Early Effector CD8 T Cells Display Plasticity in Populating the Short-Lived Effector and Memory-Precursor Pools Following Bacterial or Viral Infection. Sci Rep. 2015;5:12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Youngblood B, Hale JS, Kissick HT, et al. Effector CD8 T cells dedifferentiate into long-lived memory cells. Nature. 2017;552(7685):404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herndler-Brandstetter D, Ishigame H, Shinnakasu R, et al. KLRG1(+) Effector CD8(+) T Cells Lose KLRG1, Differentiate into All Memory T Cell Lineages, and Convey Enhanced Protective Immunity. Immunity. 2018;48(4):716–729 e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. The Journal of experimental medicine. 2008;205(3):625–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arsenio J, Kakaradov B, Metz PJ, Kim SH, Yeo GW, Chang JT. Early specification of CD8+ T lymphocyte fates during adaptive immunity revealed by single-cell gene-expression analyses. Nat Immunol. 2014;15(4):365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32(1):91–103. [DOI] [PubMed] [Google Scholar]
- 52.Obar JJ, Molloy MJ, Jellison ER, et al. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc Natl Acad Sci U S A. 2010;107(1):193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang CY, Best JA, Knell J, et al. The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8+ T cell subsets. Nature immunology. 2011;12(12):1221–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi YS, Gullicksrud JA, Xing S, et al. LEF-1 and TCF-1 orchestrate T(FH) differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6. Nat Immunol. 2015;16(9):980–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou X, Yu S, Zhao DM, Harty JT, Badovinac VP, Xue HH. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 2010;33(2):229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mitchell DM, Ravkov EV, Williams MA. Distinct roles for IL-2 and IL-15 in the differentiation and survival of CD8+ effector and memory T cells. J Immunol. 2010;184(12):6719–6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441(7095):890–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mackay LK, Rahimpour A, Ma JZ, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol. 2013;14(12):1294–1301. [DOI] [PubMed] [Google Scholar]
- 59.Milner JJ, Toma C, Yu B, et al. Runx3 programs CD8(+) T cell residency in non-lymphoid tissues and tumours. Nature. 2017;552(7684):253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kurd NS, He Z, Louis TL, et al. Early precursors and molecular determinants of tissue-resident memory CD8(+) T lymphocytes revealed by single-cell RNA sequencing. Sci Immunol. 2020;5(47). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Masopust D, Choo D, Vezys V, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207(3):553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kok L, Dijkgraaf FE, Urbanus J, et al. A committed tissue-resident memory T cell precursor within the circulating CD8+ effector T cell pool. J Exp Med. 2020;217(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mackay LK, Minnich M, Kragten NA, et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science. 2016;352(6284):459–463. [DOI] [PubMed] [Google Scholar]
- 64.Wang D, Diao H, Getzler AJ, et al. The Transcription Factor Runx3 Establishes Chromatin Accessibility of cis-Regulatory Landscapes that Drive Memory Cytotoxic T Lymphocyte Formation. Immunity. 2018;48(4):659–674 e656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diao H, Pipkin M. Stability and flexibility in chromatin structure and transcription underlies memory CD8 T-cell differentiation. F1000Res. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fonseca R, Beura LK, Quarnstrom CF, et al. Developmental plasticity allows outside-in immune responses by resident memory T cells. Nat Immunol. 2020;21(4):412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bannard O, Kraman M, Fearon DT. Secondary replicative function of CD8+ T cells that had developed an effector phenotype. Science. 2009;323(5913):505–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ptashne M. Epigenetics: core misconcept. Proc Natl Acad Sci U S A. 2013;110(18):7101–7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chang HH, Hemberg M, Barahona M, Ingber DE, Huang S. Transcriptome-wide noise controls lineage choice in mammalian progenitor cells. Nature. 2008;453(7194):544–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Utzschneider DT, Gabriel SS, Chisanga D, et al. Early precursor T cells establish and propagate T cell exhaustion in chronic infection. Nat Immunol. 2020;21(10):1256–1266. [DOI] [PubMed] [Google Scholar]
- 71.Best JA, Blair DA, Knell J, et al. Transcriptional insights into the CD8(+) T cell response to infection and memory T cell formation. Nature immunology. 2013;14(4):404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kakaradov B, Arsenio J, Widjaja CE, et al. Early transcriptional and epigenetic regulation of CD8(+) T cell differentiation revealed by single-cell RNA sequencing. Nat Immunol. 2017;18(4):422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richard AC, Lun ATL, Lau WWY, Gottgens B, Marioni JC, Griffiths GM. T cell cytolytic capacity is independent of initial stimulation strength. Nat Immunol. 2018;19(8):849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2(5):415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2(5):423–429. [DOI] [PubMed] [Google Scholar]
- 76.Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nat Immunol. 2004;5(8):809–817. [DOI] [PubMed] [Google Scholar]
- 77.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174(8):4465–4469. [DOI] [PubMed] [Google Scholar]
- 78.Starbeck-Miller GR, Xue HH, Harty JT. IL-12 and type I interferon prolong the division of activated CD8 T cells by maintaining high-affinity IL-2 signaling in vivo. J Exp Med. 2014;211(1):105–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xin A, Masson F, Liao Y, et al. A molecular threshold for effector CD8(+) T cell differentiation controlled by transcription factors Blimp-1 and T-bet. Nat Immunol. 2016;17(4):422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marchingo JM, Kan A, Sutherland RM, et al. T cell signaling. Antigen affinity, costimulation, and cytokine inputs sum linearly to amplify T cell expansion. Science. 2014;346(6213):1123–1127. [DOI] [PubMed] [Google Scholar]
- 81.Agarwal P, Raghavan A, Nandiwada SL, et al. Gene regulation and chromatin remodeling by IL-12 and type I IFN in programming for CD8 T cell effector function and memory. J Immunol. 2009;183(3):1695–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim HP, Kelly J, Leonard WJ. The basis for IL-2-induced IL-2 receptor alpha chain gene regulation: importance of two widely separated IL-2 response elements. Immunity. 2001;15(1):159–172. [DOI] [PubMed] [Google Scholar]
- 83.Hess Michelini R, Doedens AL, Goldrath AW, Hedrick SM. Differentiation of CD8 memory T cells depends on Foxo1. J Exp Med. 2013;210(6):1189–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ouyang W, Beckett O, Flavell RA, Li MO. An essential role of the Forkhead-box transcription factor Foxo1 in control of T cell homeostasis and tolerance. Immunity. 2009;30(3):358–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stahl M, Dijkers PF, Kops GJ, et al. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol. 2002;168(10):5024–5031. [DOI] [PubMed] [Google Scholar]
- 86.Feau S, Arens R, Togher S, Schoenberger SP. Autocrine IL-2 is required for secondary population expansion of CD8(+) memory T cells. Nature immunology. 2011;12(9):908–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rutishauser RL, Martins GA, Kalachikov S, et al. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31(2):296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kurachi M, Barnitz RA, Yosef N, et al. The transcription factor BATF operates as an essential differentiation checkpoint in early effector CD8+ T cells. Nat Immunol. 2014;15(4):373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Man K, Miasari M, Shi W, et al. The transcription factor IRF4 is essential for TCR affinity-mediated metabolic programming and clonal expansion of T cells. Nat Immunol. 2013;14(11):1155–1165. [DOI] [PubMed] [Google Scholar]
- 90.Nayar R, Schutten E, Bautista B, et al. Graded levels of IRF4 regulate CD8+ T cell differentiation and expansion, but not attrition, in response to acute virus infection. J Immunol. 2014;192(12):5881–5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Raczkowski F, Ritter J, Heesch K, et al. The transcription factor Interferon Regulatory Factor 4 is required for the generation of protective effector CD8+ T cells. Proc Natl Acad Sci U S A. 2013;110(37):15019–15024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dominguez CX, Amezquita RA, Guan T, et al. The transcription factors ZEB2 and T-bet cooperate to program cytotoxic T cell terminal differentiation in response to LCMV viral infection. J Exp Med. 2015;212(12):2041–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Omilusik KD, Best JA, Yu B, et al. Transcriptional repressor ZEB2 promotes terminal differentiation of CD8+ effector and memory T cell populations during infection. J Exp Med. 2015;212(12):2027–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity. 2009;31(2):283–295. [DOI] [PubMed] [Google Scholar]
- 95.Shin H, Blackburn SD, Intlekofer AM, et al. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity. 2009;31(2):309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shin HM, Kapoor V, Guan T, Kaech SM, Welsh RM, Berg LJ. Epigenetic modifications induced by Blimp-1 Regulate CD8(+) T cell memory progression during acute virus infection. Immunity. 2013;39(4):661–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cui W, Liu Y, Weinstein JS, Craft J, Kaech SM. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity. 2011;35(5):792–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Banerjee A, Gordon SM, Intlekofer AM, et al. Cutting edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J Immunol. 2010;185(9):4988–4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Intlekofer AM, Takemoto N, Wherry EJ, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nature immunology. 2005;6(12):1236–1244. [DOI] [PubMed] [Google Scholar]
- 100.Reiser J, Sadashivaiah K, Furusawa A, Banerjee A, Singh N. Eomesodermin driven IL-10 production in effector CD8(+) T cells promotes a memory phenotype. Cell Immunol. 2019;335:93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang XP, Ghoreschi K, Steward-Tharp SM, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12(3):247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Botta D, Fuller MJ, Marquez-Lago TT, et al. Dynamic regulation of T follicular regulatory cell responses by interleukin 2 during influenza infection. Nat Immunol. 2017;18(11):1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Choi YS, Kageyama R, Eto D, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34(6):932–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Choi YS, Yang JA, Yusuf I, et al. Bcl6 Expressing Follicular Helper CD4 T Cells Are Fate Committed Early and Have the Capacity To Form Memory. Journal of immunology. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. The Journal of experimental medicine. 2012;209(2):243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oestreich KJ, Mohn SE, Weinmann AS. Molecular mechanisms that control the expression and activity of Bcl-6 in TH1 cells to regulate flexibility with a TFH-like gene profile. Nat Immunol. 2012;13(4):405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oestreich KJ, Read KA, Gilbertson SE, et al. Bcl-6 directly represses the gene program of the glycolysis pathway. Nat Immunol. 2014;15(10):957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Behr FM, Kragten NAM, Wesselink TH, et al. Blimp-1 Rather Than Hobit Drives the Formation of Tissue-Resident Memory CD8(+) T Cells in the Lungs. Front Immunol. 2019;10:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ichii H, Sakamoto A, Hatano M, et al. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells. Nat Immunol. 2002;3(6):558–563. [DOI] [PubMed] [Google Scholar]
- 110.Wu T, Ji Y, Moseman EA, et al. The TCF1-Bcl6 axis counteracts type I interferon to repress exhaustion and maintain T cell stemness. Sci Immunol. 2016;1(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guan T, Dominguez CX, Amezquita RA, et al. ZEB1, ZEB2, and the miR-200 family form a counterregulatory network to regulate CD8(+) T cell fates. J Exp Med. 2018;215(4):1153–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schwartzkopff S, Woyciechowski S, Aichele U, et al. TGF-beta downregulates KLRG1 expression in mouse and human CD8(+) T cells. Eur J Immunol. 2015;45(8):2212–2217. [DOI] [PubMed] [Google Scholar]
- 113.Casey KA, Fraser KA, Schenkel JM, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol. 2012;188(10):4866–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pipkin ME, Lieberman J. Delivering the kiss of death: progress on understanding how perforin works. Curr Opin Immunol. 2007;19(3):301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Voskoboinik I, Smyth MJ, Trapani JA. Perforin-mediated target-cell death and immune homeostasis. Nature reviews Immunology. 2006;6(12):940–952. [DOI] [PubMed] [Google Scholar]
- 116.Pipkin ME, Rao A, Lichtenheld MG. The transcriptional control of the perforin locus. Immunol Rev. 2010;235(1):55–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Podack ER, Hengartner H, Lichtenheld MG. A central role of perforin in cytolysis? Annu Rev Immunol. 1991;9:129–157. [DOI] [PubMed] [Google Scholar]
- 118.Liu X, Taylor BJ, Sun G, Bosselut R. Analyzing expression of perforin, Runx3, and Thpok genes during positive selection reveals activation of CD8-differentiation programs by MHC II-signaled thymocytes. J Immunol. 2005;175(7):4465–4474. [DOI] [PubMed] [Google Scholar]
- 119.Egawa T, Tillman RE, Naoe Y, Taniuchi I, Littman DR. The role of the Runx transcription factors in thymocyte differentiation and in homeostasis of naive T cells. J Exp Med. 2007;204(8):1945–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Taniuchi I, Osato M, Egawa T, et al. Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development. Cell. 2002;111(5):621–633. [DOI] [PubMed] [Google Scholar]
- 121.Woolf E, Xiao C, Fainaru O, et al. Runx3 and Runx1 are required for CD8 T cell development during thymopoiesis. Proc Natl Acad Sci U S A. 2003;100(13):7731–7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Levanon D, Bettoun D, Harris-Cerruti C, et al. The Runx3 transcription factor regulates development and survival of TrkC dorsal root ganglia neurons. EMBO J. 2002;21(13):3454–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bartfeld D, Shimon L, Couture GC, et al. DNA recognition by the RUNX1 transcription factor is mediated by an allosteric transition in the RUNT domain and by DNA bending. Structure. 2002;10(10):1395–1407. [DOI] [PubMed] [Google Scholar]
- 124.Tahirov TH, Inoue-Bungo T, Morii H, et al. Structural analyses of DNA recognition by the AML1/Runx-1 Runt domain and its allosteric control by CBFbeta. Cell. 2001;104(5):755–767. [DOI] [PubMed] [Google Scholar]
- 125.Cruz-Guilloty F, Pipkin ME, Djuretic IM, et al. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. The Journal of experimental medicine. 2009;206(1):51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang J, Scordi I, Smyth MJ, Lichtenheld MG. Interleukin 2 receptor signaling regulates the perforin gene through signal transducer and activator of transcription (Stat)5 activation of two enhancers. J Exp Med. 1999;190(9):1297–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Intlekofer AM, Banerjee A, Takemoto N, et al. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 2008;321(5887):408–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pearce EL, Mullen AC, Martins GA, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302(5647):1041–1043. [DOI] [PubMed] [Google Scholar]
- 129.Lotem J, Levanon D, Negreanu V, Leshkowitz D, Friedlander G, Groner Y. Runx3-mediated transcriptional program in cytotoxic lymphocytes. PLoS One. 2013;8(11):e80467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Olesin E, Nayar R, Saikumar-Lakshmi P, Berg LJ. The Transcription Factor Runx2 Is Required for Long-Term Persistence of Antiviral CD8(+) Memory T Cells. Immunohorizons. 2018;2(7):251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shan Q, Zeng Z, Xing S, et al. The transcription factor Runx3 guards cytotoxic CD8(+) effector T cells against deviation towards follicular helper T cell lineage. Nat Immunol. 2017;18(8):931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hu G, Chen J. A genome-wide regulatory network identifies key transcription factors for memory CD8(+) T-cell development. Nat Commun. 2013;4:2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Roychoudhuri R, Clever D, Li P, et al. BACH2 regulates CD8(+) T cell differentiation by controlling access of AP-1 factors to enhancers. Nat Immunol. 2016;17(7):851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen R, Belanger S, Frederick MA, et al. In vivo RNA interference screens identify regulators of antiviral CD4(+) and CD8(+) T cell differentiation. Immunity. 2014;41(2):325–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Crotty S, Pipkin ME. In vivo RNAi screens: concepts and applications. Trends Immunol. 2015;36(5):315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yu B, Zhang K, Milner JJ, et al. Epigenetic landscapes reveal transcription factors that regulate CD8(+) T cell differentiation. Nat Immunol. 2017;18(5):573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Boddupalli CS, Nair S, Gray SM, et al. ABC transporters and NR4A1 identify a quiescent subset of tissue-resident memory T cells. J Clin Invest. 2016;126(10):3905–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Seo YJ, Jothikumar P, Suthar MS, Zhu C, Grakoui A. Local Cellular and Cytokine Cues in the Spleen Regulate In Situ T Cell Receptor Affinity, Function, and Fate of CD8(+) T Cells. Immunity. 2016;45(5):988–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Crotty S. Follicular helper CD4 T cells (TFH). Annual review of immunology. 2011;29:621–663. [DOI] [PubMed] [Google Scholar]
- 140.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77(8):4911–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zajac AJ, Blattman JN, Murali-Krishna K, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188(12):2205–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Choi J, Diao H, Faliti CE, et al. Bcl-6 is the nexus transcription factor of T follicular helper cells via repressor-of-repressor circuits. Nat Immunol. 2020;21(7):777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Davey CA, Sargent DF, Luger K, Maeder AW, Richmond TJ. Solvent mediated interactions in the structure of the nucleosome core particle at 1.9 a resolution. J Mol Biol. 2002;319(5):1097–1113. [DOI] [PubMed] [Google Scholar]
- 144.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389(6648):251–260. [DOI] [PubMed] [Google Scholar]
- 145.Bednar J, Garcia-Saez I, Boopathi R, et al. Structure and Dynamics of a 197 bp Nucleosome in Complex with Linker Histone H1. Mol Cell. 2017;66(3):384–397 e388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. [DOI] [PubMed] [Google Scholar]
- 147.Tessarz P, Kouzarides T. Histone core modifications regulating nucleosome structure and dynamics. Nat Rev Mol Cell Biol. 2014;15(11):703–708. [DOI] [PubMed] [Google Scholar]
- 148.Rose NR, Klose RJ. Understanding the relationship between DNA methylation and histone lysine methylation. Biochim Biophys Acta. 2014;1839(12):1362–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li E, Zhang Y. DNA methylation in mammals. Cold Spring Harb Perspect Biol. 2014;6(5):a019133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kaplan N, Moore I, Fondufe-Mittendorf Y, et al. Nucleosome sequence preferences influence in vivo nucleosome organization. Nat Struct Mol Biol. 2010;17(8):918–920; author reply 920–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Segal E, Fondufe-Mittendorf Y, Chen L, et al. A genomic code for nucleosome positioning. Nature. 2006;442(7104):772–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Segal E, Widom J. From DNA sequence to transcriptional behaviour: a quantitative approach. Nat Rev Genet. 2009;10(7):443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Segal E, Widom J. What controls nucleosome positions? Trends Genet. 2009;25(8):335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Clapier CR, Cairns BR. The biology of chromatin remodeling complexes. Annu Rev Biochem. 2009;78:273–304. [DOI] [PubMed] [Google Scholar]
- 155.Zhang Z, Wippo CJ, Wal M, Ward E, Korber P, Pugh BF. A packing mechanism for nucleosome organization reconstituted across a eukaryotic genome. Science. 2011;332(6032):977–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ou HD, Phan S, Deerinck TJ, Thor A, Ellisman MH, O’Shea CC. ChromEMT: Visualizing 3D chromatin structure and compaction in interphase and mitotic cells. Science. 2017;357(6349). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Woodcock CL, Ghosh RP. Chromatin higher-order structure and dynamics. Cold Spring Harb Perspect Biol. 2010;2(5):a000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.de Dieuleveult M, Yen K, Hmitou I, et al. Genome-wide nucleosome specificity and function of chromatin remodellers in ES cells. Nature. 2016;530(7588):113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Belotserkovskaya R, Reinberg D. Facts about FACT and transcript elongation through chromatin. Curr Opin Genet Dev. 2004;14(2):139–146. [DOI] [PubMed] [Google Scholar]
- 160.Li G, Levitus M, Bustamante C, Widom J. Rapid spontaneous accessibility of nucleosomal DNA. Nat Struct Mol Biol. 2005;12(1):46–53. [DOI] [PubMed] [Google Scholar]
- 161.Yigit E, Zhang Q, Xi L, et al. High-resolution nucleosome mapping of targeted regions using BAC-based enrichment. Nucleic acids research. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Weintraub H, Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976;193(4256):848–856. [DOI] [PubMed] [Google Scholar]
- 163.Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods. 2013;10(12):1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hesselberth JR, Chen X, Zhang Z, et al. Global mapping of protein-DNA interactions in vivo by digital genomic footprinting. Nat Methods. 2009;6(4):283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Heinz S, Benner C, Spann N, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38(4):576–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.van der Veeken J, Zhong Y, Sharma R, et al. Natural Genetic Variation Reveals Key Features of Epigenetic and Transcriptional Memory in Virus-Specific CD8 T Cells. Immunity. 2019;50(5):1202–1217 e1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Iwafuchi-Doi M, Zaret KS. Cell fate control by pioneer transcription factors. Development. 2016;143(11):1833–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zaret KS. Pioneering the chromatin landscape. Nat Genet. 2018;50(2):167–169. [DOI] [PubMed] [Google Scholar]
- 169.Scharer CD, Bally AP, Gandham B, Boss JM. Cutting Edge: Chromatin Accessibility Programs CD8 T Cell Memory. J Immunol. 2017;198(6):2238–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Scott-Browne JP, Lopez-Moyado IF, Trifari S, et al. Dynamic Changes in Chromatin Accessibility Occur in CD8(+) T Cells Responding to Viral Infection. Immunity. 2016;45(6):1327–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jain J, McCaffrey PG, Valge-Archer VE, Rao A. Nuclear factor of activated T cells contains Fos and Jun. Nature. 1992;356(6372):801–804. [DOI] [PubMed] [Google Scholar]
- 172.Nye JA, Petersen JM, Gunther CV, Jonsen MD, Graves BJ. Interaction of murine ets-1 with GGA-binding sites establishes the ETS domain as a new DNA-binding motif. Genes Dev. 1992;6(6):975–990. [DOI] [PubMed] [Google Scholar]
- 173.Escalante CR, Brass AL, Pongubala JM, et al. Crystal structure of PU.1/IRF-4/DNA ternary complex. Mol Cell. 2002;10(5):1097–1105. [DOI] [PubMed] [Google Scholar]
- 174.Panne D, Maniatis T, Harrison SC. An atomic model of the interferon-beta enhanceosome. Cell. 2007;129(6):1111–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Keller AD, Maniatis T. Only two of the five zinc fingers of the eukaryotic transcriptional repressor PRDI-BF1 are required for sequence-specific DNA binding. Mol Cell Biol. 1992;12(5):1940–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bevington SL, Cauchy P, Cockerill PN. Chromatin priming elements establish immunological memory in T cells without activating transcription: T cell memory is maintained by DNA elements which stably prime inducible genes without activating steady state transcription. Bioessays. 2017;39(2). [DOI] [PubMed] [Google Scholar]
- 177.Bevington SL, Cauchy P, Piper J, et al. Inducible chromatin priming is associated with the establishment of immunological memory in T cells. EMBO J. 2016;35(5):515–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bevington SL, Cauchy P, Withers DR, Lane PJ, Cockerill PN. T Cell Receptor and Cytokine Signaling Can Function at Different Stages to Establish and Maintain Transcriptional Memory and Enable T Helper Cell Differentiation. Front Immunol. 2017;8:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yukawa M, Jagannathan S, Vallabh S, et al. AP-1 activity induced by co-stimulation is required for chromatin opening during T cell activation. J Exp Med. 2020;217(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben-Neriah Y. JNK is involved in signal integration during costimulation of T lymphocytes. Cell. 1994;77(5):727–736. [DOI] [PubMed] [Google Scholar]
- 181.Xu T, Keller A, Martinez GJ. NFAT1 and NFAT2 Differentially Regulate CTL Differentiation Upon Acute Viral Infection. Front Immunol. 2019;10:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wu JI, Lessard J, Crabtree GR. Understanding the words of chromatin regulation. Cell. 2009;136(2):200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Deal RB, Henikoff JG, Henikoff S. Genome-wide kinetics of nucleosome turnover determined by metabolic labeling of histones. Science. 2010;328(5982):1161–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Morgunova E, Taipale J. Structural perspective of cooperative transcription factor binding. Curr Opin Struct Biol. 2017;47:1–8. [DOI] [PubMed] [Google Scholar]
- 185.Ito Y. Molecular basis of tissue-specific gene expression mediated by the runt domain transcription factor PEBP2/CBF. Genes Cells. 1999;4(12):685–696. [DOI] [PubMed] [Google Scholar]
- 186.Findlay VJ, LaRue AC, Turner DP, Watson PM, Watson DK. Understanding the role of ETS-mediated gene regulation in complex biological processes. Adv Cancer Res. 2013;119:1–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tominaga H, Maeda S, Hayashi M, et al. CCAAT/enhancer-binding protein beta promotes osteoblast differentiation by enhancing Runx2 activity with ATF4. Mol Biol Cell. 2008;19(12):5373–5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jadhav RR, Im SJ, Hu B, et al. Epigenetic signature of PD-1+ TCF1+ CD8 T cells that act as resource cells during chronic viral infection and respond to PD-1 blockade. Proc Natl Acad Sci U S A. 2019;116(28):14113–14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pauken KE, Sammons MA, Odorizzi PM, et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science. 2016;354(6316):1160–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sen DR, Kaminski J, Barnitz RA, et al. The epigenetic landscape of T cell exhaustion. Science. 2016;354(6316):1165–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Calo E, Wysocka J. Modification of enhancer chromatin: what, how, and why? Mol Cell. 2013;49(5):825–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.He B, Xing S, Chen C, et al. CD8(+) T Cells Utilize Highly Dynamic Enhancer Repertoires and Regulatory Circuitry in Response to Infections. Immunity. 2016;45(6):1341–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Firpi HA, Ucar D, Tan K. Discover regulatory DNA elements using chromatin signatures and artificial neural network. Bioinformatics. 2010;26(13):1579–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rajagopal N, Xie W, Li Y, et al. RFECS: a random-forest based algorithm for enhancer identification from chromatin state. PLoS Comput Biol. 2013;9(3):e1002968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Russ BE, Olshansky M, Li J, et al. Regulation of H3K4me3 at Transcriptional Enhancers Characterizes Acquisition of Virus-Specific CD8(+) T Cell-Lineage-Specific Function. Cell Rep. 2017;21(12):3624–3636. [DOI] [PubMed] [Google Scholar]
- 196.Chandele A, Joshi NS, Zhu J, Paul WE, Leonard WJ, Kaech SM. Formation of IL-7Ralphahigh and IL-7Ralphalow CD8 T cells during infection is regulated by the opposing functions of GABPalpha and Gfi-1. J Immunol. 2008;180(8):5309–5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kerdiles YM, Beisner DR, Tinoco R, et al. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol. 2009;10(2):176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Feng X, Wang H, Takata H, Day TJ, Willen J, Hu H. Transcription factor Foxp1 exerts essential cell-intrinsic regulation of the quiescence of naive T cells. Nat Immunol. 2011;12(6):544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Omilusik KD, Nadjsombati MS, Shaw LA, Yu B, Milner JJ, Goldrath AW. Sustained Id2 regulation of E proteins is required for terminal differentiation of effector CD8(+) T cells. J Exp Med. 2018;215(3):773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Agarwal S, Rao A. Modulation of chromatin structure regulates cytokine gene expression during T cell differentiation. Immunity. 1998;9(6):765–775. [DOI] [PubMed] [Google Scholar]
- 201.Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171(1):27–31. [DOI] [PubMed] [Google Scholar]
- 202.Pipkin ME, Ljutic B, Cruz-Guilloty F, et al. Chromosome transfer activates and delineates a locus control region for perforin. Immunity. 2007;26(1):29–41. [DOI] [PubMed] [Google Scholar]
- 203.Zediak VP, Johnnidis JB, Wherry EJ, Berger SL. Cutting edge: persistently open chromatin at effector gene loci in resting memory CD8+ T cells independent of transcriptional status. J Immunol. 2011;186(5):2705–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Schones DE, Cui K, Cuddapah S, et al. Dynamic regulation of nucleosome positioning in the human genome. Cell. 2008;132(5):887–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Adelman K, Lis JT. Promoter-proximal pausing of RNA polymerase II: emerging roles in metazoans. Nat Rev Genet. 2012;13(10):720–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Darzacq X, Shav-Tal Y, de Turris V, et al. In vivo dynamics of RNA polymerase II transcription. Nat Struct Mol Biol. 2007;14(9):796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yamaguchi Y, Takagi T, Wada T, et al. NELF, a multisubunit complex containing RD, cooperates with DSIF to repress RNA polymerase II elongation. Cell. 1999;97(1):41–51. [DOI] [PubMed] [Google Scholar]
- 208.Wada T, Takagi T, Yamaguchi Y, et al. DSIF, a novel transcription elongation factor that regulates RNA polymerase II processivity, is composed of human Spt4 and Spt5 homologs. Genes Dev. 1998;12(3):343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Levine M. Paused RNA polymerase II as a developmental checkpoint. Cell. 2011;145(4):502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Rahl PB, Lin CY, Seila AC, et al. c-Myc regulates transcriptional pause release. Cell. 2010;141(3):432–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yoh SM, Cho H, Pickle L, Evans RM, Jones KA. The Spt6 SH2 domain binds Ser2-P RNAPII to direct Iws1-dependent mRNA splicing and export. Genes Dev. 2007;21(2):160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Johnston RJ, Poholek AC, DiToro D, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325(5943):1006–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jiang H, Zhang F, Kurosu T, Peterlin BM. Runx1 binds positive transcription elongation factor b and represses transcriptional elongation by RNA polymerase II: possible mechanism of CD4 silencing. Mol Cell Biol. 2005;25(24):10675–10683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Luo Z, Lin C, Guest E, et al. The super elongation complex family of RNA polymerase II elongation factors: gene target specificity and transcriptional output. Mol Cell Biol. 2012;32(13):2608–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Pace L, Goudot C, Zueva E, et al. The epigenetic control of stemness in CD8(+) T cell fate commitment. Science. 2018;359(6372):177–186. [DOI] [PubMed] [Google Scholar]
- 216.Gray SM, Amezquita RA, Guan T, Kleinstein SH, Kaech SM. Polycomb Repressive Complex 2-Mediated Chromatin Repression Guides Effector CD8(+) T Cell Terminal Differentiation and Loss of Multipotency. Immunity. 2017;46(4):596–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ladle BH, Li KP, Phillips MJ, et al. De novo DNA methylation by DNA methyltransferase 3a controls early effector CD8+ T-cell fate decisions following activation. Proc Natl Acad Sci U S A. 2016;113(38):10631–10636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Russ BE, Olshanksy M, Smallwood HS, et al. Distinct epigenetic signatures delineate transcriptional programs during virus-specific CD8(+) T cell differentiation. Immunity. 2014;41(5):853–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Barski A, Cuddapah S, Cui K, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129(4):823–837. [DOI] [PubMed] [Google Scholar]
- 220.Chen S, Ma J, Wu F, et al. The histone H3 Lys 27 demethylase JMJD3 regulates gene expression by impacting transcriptional elongation. Genes Dev. 2012;26(12):1364–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Matsumura Y, Nakaki R, Inagaki T, et al. H3K4/H3K9me3 Bivalent Chromatin Domains Targeted by Lineage-Specific DNA Methylation Pauses Adipocyte Differentiation. Mol Cell. 2015;60(4):584–596. [DOI] [PubMed] [Google Scholar]
- 222.Min IM, Waterfall JJ, Core LJ, Munroe RJ, Schimenti J, Lis JT. Regulating RNA polymerase pausing and transcription elongation in embryonic stem cells. Genes Dev. 2011;25(7):742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Miller SA, Weinmann AS. An essential interaction between T-box proteins and histone-modifying enzymes. Epigenetics. 2009;4(2):85–88. [DOI] [PubMed] [Google Scholar]
- 224.Laslo P, Spooner CJ, Warmflash A, et al. Multilineage transcriptional priming and determination of alternate hematopoietic cell fates. Cell. 2006;126(4):755–766. [DOI] [PubMed] [Google Scholar]
- 225.Pipkin ME, Rao A. SnapShot: effector and memory T cell differentiation. Cell. 2009;138(3):606 e601–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Gray PA, Fu H, Luo P, et al. Mouse brain organization revealed through direct genome-scale TF expression analysis. Science. 2004;306(5705):2255–2257. [DOI] [PubMed] [Google Scholar]
- 227.Rando OJ, Chang HY. Genome-wide views of chromatin structure. Annu Rev Biochem. 2009;78:245–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wucherpfennig KW, Cartwright AN. Genetic screens to study the immune system in cancer. Curr Opin Immunol. 2016;41:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Brogaard K, Xi L, Wang JP, Widom J. A map of nucleosome positions in yeast at base-pair resolution. Nature. 2012;486(7404):496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Meers MP, Bryson TD, Henikoff JG, Henikoff S. Improved CUT&RUN chromatin profiling tools. Elife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Rossi MJ, Lai WKM, Pugh BF. Simplified ChIP-exo assays. Nat Commun. 2018;9(1):2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Lickwar CR, Mueller F, Hanlon SE, McNally JG, Lieb JD. Genome-wide protein-DNA binding dynamics suggest a molecular clutch for transcription factor function. Nature. 2012;484(7393):251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]