Abstract
Identification of biological risk factors that contribute to the development of complex neuropsychiatric disorders such as psychosis and autism spectrum disorders (ASD) is key for early intervention and detection. Furthermore, parsing apart the biological heterogeneity associated with these neuropsychiatric syndromes will help us understand the neural mechanisms underlying psychiatric symptom development. 22q11.2 Microdeletion syndrome (22q11DS) is caused by a recurrent genetic mutation that carries significantly increased risk for developing psychosis and/or ASD. In this review, I provide an brief introduction to 22q11DS and discuss common phenotyping strategies that are used to assess psychosis and ASD in this population. I then summarize neuroimaging phenotypes associated with psychosis and ASD in 22q11.DS. Next, I discuss challenges within the field and provide practical suggestions to overcome these obstacles. Finally, I end the review with a discussion of future directions for moving 22q11DS risk and resilience research forward.
Psychosis and autism spectrum disorders (ASD) are neuropsychiatric syndromes that share many features, including deficits in cognition, altered language processing and production, and social impairments (1,2). ASD and psychosis also share underlying genetic contributions (3–7). Despite similarities, ASD and psychosis have different developmental onsets. ASD typically presents in early childhood while psychosis onset usually occurs in late adolescence and early adulthood. Gaining a better understanding of biological risk factors contributing these differential trajectories will help identify causes of the illness and treatment targets, facilitating early detection and intervention.
An approach providing valuable insights into the causes of psychosis and ASD is the detailed examination of a more homogeneous subtype, with a known genetic cause. The 22q11.2 Microdeletion Syndrome (Velocardiofacial/ DiGeorge syndrome; 22q11DS) is a compelling model, as 14–50% of 22q11DS youth meet criteria for ASD (22q11DS-ASD+; 8–11) and 23–41% of 22q11DS adolescents and adults meet criteria for a psychotic disorder (22q11DS-psychosis+; 12–18). 22q11-ASD+ individuals do not have increased risk of later developing psychosis (19,20), suggesting that separate neurobiological mechanisms contribute to the manifestation these syndromes in 22q11DS. The purpose of this review is to examine structural and functional neuroimaging factors that contribute to psychosis and ASD in 22q11DS. First, I provide a brief introduction to 22q11DS and discuss phenotyping strategies used to assess psychosis and ASD in 22q11DS. Next, I summarize how brain alterations in gray matter, diffusion-weighted imaging, and resting-state functional magnetic resonance imaging (rsfMRI) in 22q11DS are related to psychotic and ASD symptoms and social impairments. Third, I discuss current challenges faced in field and provide suggestions to overcome these obstacles. I conclude with future directions for 22q11DS risk and resilience research.
An Introduction to 22q11.2 Microdeletion Syndrome
22q11DS is caused by a hemizygous deletion at chromosome 22q11.2, an area that encompasses ~90 genes (~46 protein-coding genes), including those that play a role in neuronal migration, myelination, and brain development (21,22). 22q11DS is the second most common neurogenetic disorder, affecting approximately 1 in 4000 live births (23). 22q11.2 microdeletions range from 1–3 megabases (Mb) in size (24,25), with the most common deletion size being 3 Mb. 22q11DS is most frequently caused by a de novo mutation, although approximately 10% of the cases are inherited from parents (26). While the phenotype of 22q11DS is highly variable, there are common physical characteristics, including craniofacial anomalies, cardiovascular abnormalities, and immune deficiency (27–29). 22q11DS is also distinguished by a characteristic neurocognitive profile: 22q11DS individuals show visuospatial deficits relative to their verbal performance, and often have lower than average full-scale IQ (30,31). While 22q11DS is considered an ideal “human-knock out” model for understanding psychiatric disorders, unique aspects of 22q11DS may also reflect distinct risk and resilience factors within this population.
Psychosis and ASD phenotyping strategies commonly used in 22q11DS
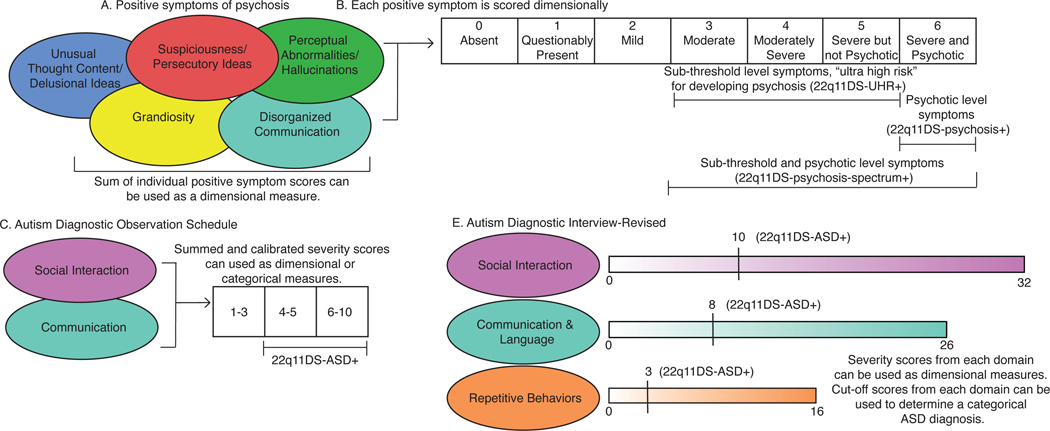
It is important to understand how psychosis and ASD phenotypes are typically characterized in 22q11DS. The Structured Interview for Prodromal Syndromes (SIPS; 32,33), is commonly used to assess psychosis risk in 22q11DS (Figure 1A). Positive symptoms are assessed dimensionally, ranging from no symptoms to psychotic-level symptom severity (Figure 1B). A 22q11DS participant is usually considered to be at “ultra-high risk” for developing psychosis (22q11DS-UHR+) when symptom severity falls within a sub-threshold range. Researchers also compare 22q11DS individuals with a diagnosed psychotic disorder (22q11DS-psychosis+) to 22q11DS individuals who do not meet criteria for psychosis (22q11DS-psychosis-). Psychotic disorder diagnosis is determined from level-6 severity scores on individual positive symptoms and/or through a semi-structured clinical interview. Others combine individuals with sub-threshold and psychotic level severity symptoms (score ≥ 3 on any positive symptom measure), creating a “psychosis-spectrum” group (22q11DS-pychosis-spectrum+) and compare them to 22q11DS individuals without clinically significant positive psychotic symptoms (22q11DS-psychosis-spectrum-). All positive symptom severity scores on the SIPS can be summed to obtain a dimensional measure of psychotic symptoms.
There are important differences in how 22q11DS-UHR+ are assessed in comparison to UHR youth without a known 22q11.2 deletion (idiopathic). Most idiopathic UHR participants meet criteria for “Attenuated Positive Symptom Syndrome”, which means they endorse subthreshold positive symptoms that have started or gotten worse in the past year and symptoms are present at least once per week in the past month. In contrast, frequency and onset criteria are not typically used when assessing positive symptoms in 22q11DS. Despite these differences, multiple studies find that positive symptom presentation is similar in 22q11DS in comparison to idiopathic forms of psychotic symptom presentation (14,34,35).
There are also detailed phenotyping measures used to characterize ASD in 22q11DS. ASDs in 22q11DS are commonly determined through: 1) the Autism Diagnostic Observation Schedule (ADOS; 36), and/or 2) the Autism Diagnostic Interview-Revised (ADI-R; 37). The ADOS is a semi-structured interview administered to the youth and elicits behaviors associated with social interactions and communication (Figure 1C). ADOS scores can be used to create a categorical diagnosis (22q11DS-ASD+ vs. 22q11DS-ASD-) or as a dimensional measure of symptom severity (Figure 1C). The ADI-R is a semi-structured interview administered to the youth’s primary caretaker; dimensional and categorical approaches in the three domains of social interactions, communication, and repetitive behaviors are also used (Figure 1D). Typically, in idiopathic ASD research settings, an individual must meet the identified cut-offs in all three domains of the ADOS and ADI-R to receive an ASD diagnosis, though there is variability in cut-off criteria for an ASD diagnosis, as the Collaborative Programs for Excellence in Autism (CPEA)-defined ASD diagnostic criteria required social communication deficits on the ADI-R and ADOS, but does not require repetitive behavioral impairments (38). Like the CPEA guidelines, in many 22q11DS-ASD studies, if 22q11DS individuals score below the diagnostic threshold on the repetitive domain, but meet the cut-offs for the other two domains, they are still given an ASD diagnosis (e.g., 39,40).
Two studies have directly compared the 22q11DS-ASD+ phenotype to the idiopathic ASD phenotype (41,42). Both studies found that the severity of repetitive behaviors was similar in the two groups, but 22q11DS-ASD+ had less severe communication impairments in comparison to individuals with idiopathic ASD (41,42). Impairments in joint attention, gestural communication, initiating conversation, as well circumscribed interests are characteristic phenotypes associated with 22q11DS individuals, independent of ASD diagnosis (41,42). Taken together, these findings suggest that a “broader autism phenotype” may be more applicable to the 22q11DS population.
Neuroimaging Factors associated with psychosis and ASD in 22q11DS
A brief description of the neuroimaging metrics covered in this review, as well as possible biological interpretations of these measures, is reported in Table 1.
Table 1.
Definitions and possible biological interpretations of the neuroimaging metrics covered in this review.
| T1-weighted (Gray matter) neuroimaging: morphometry of brain structure is measured. | ||
|---|---|---|
| Measure | Definition | Possible Biological Interpretation |
| Cortical Thickness | The thickness of the cerebral cortex, measured from the pial surface to the white/gray matter boundary in the cortex. | Number of neurons in ontogenetic columns (139). |
| Surface Area | Total area of a particular brain region | Measure of proliferation of radial unit progenitors (139). |
| Local Gyrification Index | Amount of cortex buried within the sulcal folds as compared with the amount of cortex on the outer visible cortex. | Believed to reflect early brain development, as cortical folding is determined in the first months of life. |
| Diffusion-weighted neuroimaging: measures how fast water diffuses across a space; greater diffusion indicates that there is more restriction in the opposing directions. | ||
| Measure | Definition | Possible Biological Interpretation |
| Fractional anisotropy | a general metric of overall white matter integrity | Greater fractional anisotropy is typically associated with higher white matter integrity. |
| Axial diffusivity | diffusion of water parallel to white matter fibers | Decreased axial diffusivity is associated with axonal damage (140). |
| Radial diffusivity | diffusion of water perpendicular to white matter tracts | Increased radial diffusivity is associated with demyelination (84,141,142). |
| Mean diffusivity | measure of the magnitude of diffusion | Greater mean diffusivity is typically associated with greater white matter pathology. |
| Resting-state functional magnetic resonance imaging: measures spontaneous fluctuations in neural activity. A functional MRI is acquired in the absence of a task (i.e., participant lies in the scanner while awake but does not engage in a task). | ||
| Measure | Definition | Possible Biological Interpretation |
| Network-Based Connectivity | Multiple individual brain regions that are more likely to correlate with each other are in the same network. | Varies based on network connectivity measure being assessed. |
| Seed-Based Connectivity | One region of the brain is chosen as the “seed” region (e.g., thalamus). Correlations between the time series extracted from this region and other regions of the brain are assessed. “Other regions” may consist of specific regions of interest or the entire rest of the brain. | Higher levels of correlation between two brain areas suggests that there is greater coupling between the two regions. |
Grey matter alterations that contribute to psychosis vulnerability and ASD in 22q11DS
Cross-sectional neuroimaging work in 22q11DS provided proof of concept that cortical regions implicated in idiopathic psychosis (43–49) were also altered in 22q11DS-psychosis+ vs. 22q11DS-psychosis- (50,51). Subsequent longitudinal investigations found that progressive structural changes, particularly in temporal and frontal regions, predicted severity of positive symptoms at follow-up, in those who have or develop sub-threshold psychosis symptoms (52,53) and in 22q11DS-psychosis+ (54,55). Simialrly, a large-scale study compared cortical measures in 22q11DS-psychosis+ vs. 22q11DS-psychosis- and found largest group differences in lower frontal and temporal cortical thickness (56). Effect sizes for these group differences were similar to the cortical thickness differences observed in idiopathic schizophrenia (49). These findings suggest that there are convergent biological mechanisms contributing to progressive cortical gray matter changes in those who develop psychosis, both with and without 22q11DS.
There are also deficits in subcortical regions associated with 22q11DS-psychosis-specturm+. A longitudinal investigation found that, in comparison to 22q11DS-psychosis-spectrum-, 22q11DS-psychosis-spectrum+ exhibited reduced overall hippocampal volumes, an effect driven by CA2/3, CA4, and dentate gyrus subregions (57). In 22q11DS-psychosis-spectrum+, these regions exhibited had a steeper age-related slope, suggesting aberrant hippocampal development contributes to the psychosis onset. Another study found that 22q11DS individuals who endorsed clinically significant auditory hallucinations compared to those who did not had lower baseline volume and steeper age-related slopes in the medial geniculate nuclei of the thalamus (58). Both studies only assessed the sub-regions of one subcortical region, and neither study assessed how change of these metrics (e.g., Visit 2-Visit 1) was associated with psychotic symptoms at follow-up. The ENIGMA 22q11DS Working Group also found subcortical alterations in 22q11Ds-psychosis+, with 22q11DS-psychosis+ exhibiting lower hippocampal, thalamic, and amygdala volumes in comparison to those with 22q11DS-psychosis- (59). In this study, the effect sizes associated with 22q11DS-psychosis+ were similar to multiple psychiatric disorders, including schizophrenia, bipolar disorder, major depressive disorder, and obsessive-compulsive disorder (59). Thus, subcortical aberrations in 22q11DS may reflect a general vulnerability for psychiatric disorders and/or illness severity.
Table 1 summarizes published longitudinal investigations relating gray matter changes to 22q11DS psychosis vulnerability. STable 1 summarizes single site cross-sectional grey matter neuroimaging studies of 22q11DS-psychosis-spectrum+. Importantly, the 22q11DS general neuroanatomic phenotype is unlike idiopathic psychosis. Compared to healthy controls, 22q11DS individuals have overall greater cortical thickness and pervasive surface area reductions (56). It is the 22q11DS-psychosis+ vs. 22q11DS-psychosis- comparisons that are similar to idiopathic psychosis vs. controls.
Cross-sectional studies have examined structural grey matter differences in 22q11DS-ASD+ vs. 22q11DS-ASD-. Compared to 22q11DS-ASD-, 22q11DS-ASD+ exhibited increased cortical volume and surface area in the dorsolateral prefrontal regions, posterior cingulate cortices, and temporal areas (39). 22q11DS-ASD+ also had reduced entorhinal volume and surface area (39). An earlier study (which included a subset of participants examined in (39)) found that 22q11DS-ASD+ had thinner parahippocampal cortices and smaller right amygdala volumes than 22q11DS-ASD- (60). However, there is also evidence of increased amygdala volumes in children with 22q11DS-ASD+ vs. 22q11DS-ASD- (8). In contrast, the largest-scale study of idiopathic ASD found increased frontal and decreased temporal cortical thickness in ASD vs. healthy controls, suggesting that different neurobiological mechanisms may underlie symptom presentation in idiopathic ASD (61).
One study compared cortical measures in 22q11DS-ASD+, 22q11DS-ASD-, idiopathic ASD, and healthy controls. An ASD diagnosis (regardless of deletion status) was associated with increased cortical surface area in insula, superior temporal, fusiform, parahippocampal, lingual, and supramarginal regions, and increased cortical thickness in the isthmus cingulate and the superior temporal gyrus (62). This study conducted a dimensional analysis of ASD phenotypes and found that distinct patterns of neuroanatomic variability were associated with clinical profiles in 22q11DS-ASD+ and idiopathic autism (62). Consistent with these dimensional findings, an investigation of local gyrification indices that differing patterns of abnormalities in 22q11DS-ASD+ vs. individuals with idiopathic autism (63). These findings highlight the importance of comparing 22q11DS-psychosis-spectrum+ and 22q11DS-ASD+ to idiopathic forms of the respective illness.
Diffusion-weighted imaging metrics are related to psychosis and social impairments in 22q11DS
Single site cross-sectional findings of diffusion-weighted abnormalities in 22q11DS-psychosis-spectrum are inconsistent (50,51,56,59,64–77,78, reviewed in STable 2). However, a recent multisite study examined diffusion-weighted imaging measures in 22q11DS-psychosis+ vs. 22q11DS-psychosis- (75), providing a ‘ground truth’ for future investigations of 22q11DS psychosis risk. In comparison to 22q11DS-psychosis-, 22q11DS-psychosis+ had reduced axial diffusivity in multiple white matter tracts, namely those consisting of subcortical-cortical connections (75; reviewed in Table 2). Multiple neurobiological mechanisms could contribute to reduced axial diffusivity in 22q11DS-psychosis+, including axonal damage (79), reduced axonal diameter (80), and/or greater tortuosity in axons (81). A histological examination of postmortem brain tissue of an 22q11DS infant found an increased number of interstitial neurons in comparison to a control group (82). Interstitial neurons are believed to be fetal subplate remnants, and the fetal subplate is involved in guidance and morphogenesis of early development of subcortical-cortical connections (83); disruption of this instruction may contribute to reduced axial diffusivity in 22q11DS-psychosis+.
Table 2.
Summary of studies to date that use a longitudinal framework to examine relationships between neuroimaging metrics and psychotic symptoms in 22q11DS. The majority of studies also studied a control sample; however, this table presents comparisons within the 22q11.2 Microdeletion Syndrome (22q11DS) sample because this review focuses on psychosis risk within this group. N=Number; T1=time point 1; T2=time point 2; T3=time point 3; T4=time point 4; 22q11DS-psychosis+=22q11DS individuals with a psychotic disorder diagnosis; 22q11DS-psychosis-=22q11DS individuals who do not have a psychotic disorder diagnosis; 22q11DS-psychosis-spectrum+=22q11DS individuals that had subthreshold and/or psychotic level positive symptoms; 22q11DS-psychosis-spectrum-=22q11DS individuals who did not have clinically significant positive symptoms; CSF=cerebrospinal fluid; ROI=region of interest; SIPS=Structured Interview for Prodromal Syndromes; *Berhanu et. al. 2016 was a study of 22q11DS participants who had four time points but T1 and T4 were the focus of the study.
| Author and year | Total N 22q11DS | Neuroimaging predictors examined | Analysis conducted | Results | |
|---|---|---|---|---|---|
| N 22q11DS-psychosis+ at T1 | N 22q11DS-psychosis+ at final follow-up | ||||
| Age Information (e.g., range, mean ± SD) | |||||
| Number of time points Approximate length of time between each time point | |||||
| Gothelf et al. 2007 (16) | Total N 22q11DS=19 | Semi-automated segmentation: cortical lobes (frontal, parietal, temporal, occipital), cerebellum, ventricular CSF (143) Manually delineated ROIs: caudate, amygdala, hippocampus, superior temporal gyrus (144–146) | Analysis: Repeated measures analysis of variance Between-subjects factor: Group; Within-subjects factor: brain volumes at T1 and T2 | No significant differences in developmental trajectories between 22q11DS-psychosis+ vs. 22q11DS-psychosis- | |
| N 22q11DS-psychosis+ at T1=0 | N 22q11DS-psychosis+ at T2=7 | ||||
| Age range: 5–20 years Mean age (±SD) 12.3±4.0 years | |||||
| 2 time points Mean 4.9 (± 0.7) years between time points | |||||
| Kates et al., 2011 (52) | Total N 22q11DS=72 | Semi-automated segmentation (147–150) Manually delineated ROIs: frontal lobe subregions, amygdala, hippocampus, superior temporal gyrus, cerebellum, ventricles (145,146,151–155) | Analysis: Poisson zero-inflated regression Predictors: ((T2 volume-T1 volume) * 100) / (T1 volume * interval between scans in years) for each segmentation or ROI Outcome: Total SIPS positive symptom score at T2 | Greater decreases in superior temporal gyrus volume (T1-T2) predicted higher positive symptom scores at T2 | |
| N 22q11DS-psychosis+ at T1=0 | N 22q11DS-psychosis+ at T2=0 | ||||
| Age range: 9–15; Mean age (±SD): 11.9 (±2.1) years | |||||
| 2 time points ~3 years between time points | |||||
| Kunwar et al., 2012 (156) | Total N 22q11DS=58 | Gyrification index of frontal, temporal, parietal, and occipital lobar ROIs | Analysis: Poisson zero-inflated regression Predictors: T2 gyrification index-T1 gyrification index for each ROI Outcome: Total SIPS positive symptom score at T2 | Greater decreases in occipital gyrification index (T2-T1) predicted higher positive symptom scores at T2 | |
| N 22q11DS-psychosis+ at T1=0 | N 22q11DS-psychosis+ at T2=0 | ||||
| Age range: 9–15 years Mean age: 14.9±2.5 years | |||||
| 2 time points ~3 years between time points | |||||
| Berhanu et al., 2016 (157) | Total N 22q11DS=73 | Cortical volume-to-amygdala ratios for 68 ROIs from Desikan atlas (158) | Analysis: Poisson zero-inflated regression Predictors: Cortical volume-amygdala ratios at T1; Outcome: Total SIPS positive symptom score at T4 | Lower anterior cingulate to amygdala volume ratios at T1 predicted higher levels of positive symptoms at T4 Higher occipital to amygdala volume ratios and parietal to amygdala volume ratios at T1 baseline predicted higher positive symptom scores at T4 | |
| N 22q11DS-psychosis+ at T1=0 | N 22q11DS-psychosis+ at T4=Not reported. | ||||
| Age range: 9–15 years Mean age (±SD) 12.1 ± 2.2 years | |||||
| 2 time points* ~12 years between time points examined | |||||
| Ramanathan et al., 2017 (54) | Total N 22q11DS=72 | Cortical thickness and surface area for 5 lobes: frontal, parietal, temporal, occipital and cingulate. Followed up with examination of individual brain regions in any lobe that showed a differential trajectory in 22q11DS vs. controls. | Analysis: Mixed model regressions Model 1: Age*Group (22q11DS-psychosis-spectrum+ vs. 22q11DS-psychosis-spectrum-) Model 2: Age*Group (22q11Ds-psychosis+ vs. 22q11DS-UHR+ vs. 22q11DS-psychosis-spectrum) Model 3: Age*Total SIPS positive symptom score at T3 Outcomes: neuroimaging ROIs | Model 1: Accelerated decreases in superior frontal cortical thickness in 22q11DS-psychosis-spectrum+ vs. 22q11DS-psychosis-spectrum- Model 2: Accelerated decreases in frontal cortical thickness in 22q11DS-psychosis+ vs. others Model 3: steeper linear trajectories in occipital and parietal cortical thickness are associated with higher positive symptoms at T3 | |
| N 22q11DS-psychosis+ at T1=0 | N 22q11DS-psychosis+ at T3=6 | ||||
| Age range: 9–18 years Mean age: 11.8 years | |||||
| 2–3 time points ~3 years between each time point | |||||
| Mancini et al., 2019 (57) | Total N 22q11DS=107 | whole hippocampal volume; hippocampal subfields | Analysis: Mixed model regression Predictors: Age*Group (22q11DS-psychosis-spectrum+ vs. 22q11DS-psychosis-spectrum-) Outcomes: hippocampal ROIs | Group effect: reduced bilateral whole hippocampal volume, reduced bilateral subregions: CA2/CA3, CA4, dentate gyrus, Group x age effect: Steeper decrease in left whole hippocampus in 22q11DS-psychosis-spectrum+ Effect driven by sub-regions: CA2/CA3, CA4, and dentate gyrus | |
| N 22q11DS-psychosis+ at T1=not reported. | N 22q11DS-psychosis+ any time point=20 | ||||
| Age range: 6–35 years Mean age (±SD): 13.5 ± 6.4 years | |||||
| 1–5 time points Mean 3.8 (± 1.07) years between each time point | |||||
| Mancini et al., 2020 (58) | Total N 22q11DS=120 | whole thalamus volume, thalamic sub-regions | Analysis: Mixed model regression Predictors: Age*Group (22q11DS-hallucinations+ vs. 22q11DS-hallucinations-) Outcome: thalamic ROIs | Group effect: reduced bilateral medial geniculate nuclei in 22q11DS-hallucinations+ vs. 22q11DS-hallucinations- Group x age effect: Steeper decrease in medial geniculate nuclei 22q11DS- hallucinations+ | |
| N 22q11DS-psychosis+ at T1=not reported. | N 22q11DS-psychosis+ any time point=20 | ||||
| Age range: 8–35 years Mean age (±SD): 17.7 ± 6.0 years | |||||
| 1–4 time points ~3 years between each time point | |||||
Unlike the grey matter findings, these 22q11DS-psychosis+ white alterations diverged from ENIGMA Schizophrenia-DTI Working group, which found widespread reduced fractional anisotropy in idiopathic schizophrenia (74). The reduced fractional anisotropy in idiopathic schizophrenia is most often driven by increased radial diffusivity (75, presented in Table 2), possibility reflecting reduced myelination (84). These divergent findings suggest that different white matter neurobiological alterations may lead to similar, downstream phenotypes in 22q11DS and idiopathic forms of the illness.
Though there are no published studies comparing diffusion-weighted imaging measures in 22q11DS-ASD+ vs. 22q11DS-ASD-, impaired social cognition is a core feature of idiopathic ASD and psychosis. Relationships between diffusion-weighted imaging metrics and social cognition have been observed in 22q11DS. Increased axial diffusivity, which may reflect greater axonal coherence, in two association tracts (uncinate fasciculus and inferior fronto-occipital fasciculus) was associated with better emotion recognition and theory of mind performance (85), features impaired in idiopathic ASD and psychosis (86–89). Future studies should examine to what extent the neural mechanisms underlying social cognition in 22q11DS converge or diverge with risk factors for psychosis and ASD phenotypes.
Altered functional connectivity is related to psychotic symptoms and social impairments in 22q11DS
Multiple cross-sectional studies have investigated effects of psychotic symptoms on rsfMRI in 22q11DS (76,77,90–92) and one study examined the relationship between functional connectivity and a dimensional measure of ASD in 22q11DS (93). The first rsfMRI study of 22q11DS and psychosis found that reduced activation in superior frontal regions was associated with increased total positive symptoms (90). Later, others found that increased positive symptoms in young adults with 22q11DS were associated with increased connectivity between the precuneus and superior frontal cortex (77), brain regions typically believed to be part of the default mode network (DMN). Still, others found no relationship between rsfMRI DMN connectivity and positive symptoms in 22q11DS (91). Support vector machine (SVM) approaches have been used to distinguish between 22q11DS participants with and without psychotic symptoms; variability in within-network connectivity of DMN regions differentiated 22q11DS-psychosis-spectrum+ from 22q11DS-psychosis-spectrum- with a high-degree of accuracy, in both the training sample and an independent validation data set (92). Finally, increased connectivity between DMN regions (e.g., posterior cingulate cortex, medial prefrontal regions and the anterior cingulate cortex) was associated with fewer autism-spectrum behaviors in 22q11DS (93). Taken together, these findings suggest that altered DMN connectivity contributes to psychosis and autism-spectrum symptoms in 22q11DS.
To date, seed-based approaches have not achieved the same level of success as network-based rsfMRI analyses. Consistent with previous studies of idiopathic schizophrenia and individuals at clinical high risk for developing psychosis (94,95), a recent study found over-connectivity between the thalamus and somatosensory regions and under-connectivity between the thalamus and cerebellum in 22q11DS vs. controls. However, machine-learning techniques did not differentiate 22q11DS-UHR+ vs. 22q11DS-UHR with sufficient accuracy, nor were there significant relationships between thalamic connectivity patterns and psychotic symptoms (78).
Novel approaches to rsfMRI connectivity analyses highlight their potential for understanding 22q11DS psychosis and ASD risk. An examination of dynamic “brain states” across a rsfMRI session found that longer anti-coupling (i.e., one region exhibits increased activation while another shows decreased activation) between the dorsolateral prefrontal cortex and anterior cingulate was associated with higher positive symptoms in 22q11DS (96). Studies have also identified relationships between altered graph theory metrics, which measure network connectivity, and increased psychotic symptoms in 22q11DS (96,97). Future studies should examine these metrics in multisite, longitudinal samples and determine to what extent group differences are observed in 22q11DS-ASD+ vs. 22q11DS-ASD-.
Challenges faced in 22q11DS risk research
This review highlights some of the challenges we face in understanding psychosis and ASD in 22q11DS, including: 1) phenotyping issues and 2) the need to incorporate the role of development into future studies.
Phenotyping challenges in psychosis and ASD in 22q11DS
First, there is no consistent method for determining 22q11DS psychosis-spectrum status. Some studies restrict comparisons to 22q11DS-psychosis+ vs. 22q11DS-psychosis- (56), some include individuals experiencing subthreshold symptoms and those with full-blown psychotic symptoms (57), and some focus specifically on individuals experiencing sub-threshold psychotic symptoms (53). Whilst all approaches have value, I propose that, in future, researchers specify their main approach to psychosis-classification in the primary analyses, and report alternative approaches in supplemental, exploratory analyses. Also, because 22q11DS-ASD neuroimaging research has found that categorical and dimensional approaches provide complementary information (40), it will be important to assess psychosis dimensionally.
Regardless of classification strategy, psychotic symptoms are not always stable in 22q11DS (98,99). In 22q11DS participants who presented with psychotic symptoms at the baseline assessment, 55–61% continued to endorse psychotic symptoms at follow-up, while the remainder experienced symptom remission (98,99). In 22q11DS youth who did not endorse psychotic symptoms at baseline, 13–39% adolescents endorsed emergent symptoms at follow-up (98,99). Researchers must fully characterize psychotic symptoms at baseline and follow-up assessments in 22q11DS. It is important to include variation in symptom severity at the baseline assessments as a predictor when assessing symptom severity at follow-up. This approach is not typically employed in longitudinal studies 22q11DS-psychosis-spectrum+; many studies only include symptom measures at follow-up. Omitting this measure from statistical models is likely leaving out an important source of variation. Finally, other assessment methods may better capture psychotic symptom fluctuations in 22q11DS, like ecological momentary assessment.
We also face phenotyping challenges in understanding neurobiological risk factors for 22q11DS-ASD+. The reported prevalence 22q11DS-ASD+ is highly variable across studies, which may be due to: 1) wide-ranging methodological differences in clinical assessment, 2) ASD diagnosis in 22q11DS possibly reflecting a generalized social impairment, distinct from idiopathic autism (103), and/or 3) core autism behaviors often being recognized as characteristic phenotypes in individuals with 22q11DS regardless of ASD diagnosis (42). One way to mitigate this challenge is with a detailed phenotyping study that includes social cognition, social functioning, and comprehensive ASD assessments, examining how these metrics are related to and/or distinct from each other. Additionally, the temporal stability of ASD symptoms in 22q11DS is not known; it will be essential to examine 22q11DS-ASD symptom fluctuations over time.
Finally, studies rarely report both ASD and psychotic symptoms. Previous 22q11DS-ASD+ neuroimaging results remained significant when 22q11DS-psychosis+ were removed from analyses and when psychotic symptoms were included as a covariate; furthermore, ASD and psychotic symptoms were not correlated (40). Similar approaches are necessary for future investigations. It will also be important to determine what extent of the variance is shared in categorical and dimensional measures of ASD and psychotic symptoms in 22q11DS.
It is essential to incorporate development into 22q11DS risk and resilience research
Another methodological challenge facing 22q11DS research is understanding how development affects onset of symptoms. Most studies report on participants that fall within a large age range, and well-established work shows significant age-associated brain changes across development (104–106). Though most published research covaried for age in analyses, neurobiological factors may exert differential influences on symptomatology at distinct points in development (60,107), resulting in developmentally-specific risk markers. One solution is to use a time-varying analytic approach to characterize how connectivity measures relate to individual differences at different stages of development (60,108). Furthermore, it is important to obtain an accurate estimate of normative age-effects and to identify how 22q11DS neuroimaging profiles deviate from them. Using growth charting methods, deviations from normative age-associated trajectories were linked to psychopathology, suggesting that age-deviation phenotypes provide meaningful information (109,110). Tracking within-subject deviations from normative development in 22q11DS, in reference to an accurately quantified neurodevelopmental growth chart (111), will be informative for understanding risk and resilience to ASD and psychosis in 22q11DS.
Future Directions
I conclude with a discussion of key areas of future focus for 22q11DS risk and resilience research. I discuss the necessity of investigating links between neuroimaging metrics and transdiagnostic psychiatric conditions, the need to conduct resilience research, the importance of large-scale, longitudinal investigations, and the need to link genes to risk and resilience factors.
Importance of transdiagnostic research in 22q11.2 Microdeletion Syndrome
Most 22q11DS individuals meet criteria for at least one psychiatric disorder (47–85%; 53,57,58,112,113). Despite this finding, the transdiagnostic neuroimaging alterations common across psychiatric disorders in 22q11DS are unknown. This is important for earlier identification of 22q11DS high-risk youth and can be probed with existing longitudinal neuroimaging data sets. In addition, future studies of risk factors in 22q11DS could use a data-driven transdiagnostic approach derived from empirical investigations of psychological symptom co-occurrence (114,115). Indeed, elevated levels of mood and anxiety predispose 22q11DS youth for later developing psychosis (as reviewed in 116). Others have highlighted the importance of studying constructs that cut across psychiatric diagnostic boundaries, such as emotion regulation, in 22q11DS (117).
A need to focus on resilience in 22q11DS research
Given that many 22q11DS participants have a psychiatric disorder diagnosis, this deleted suite of genes clearly confers increased risk for psychiatric disorders. However, how do we assess resilience within 22q11DS? Resilience processes are increasingly framed as ‘the dynamic outcome of a dynamic process of successful adaptation to adversity” (118). Is the absence of a psychiatric disorder sufficient to determine that a 22q11DS individual is “resilient”? Researchers must assess perspectives of individuals with lived experience of 22q11DS to understand how they view resilience. Additionally, resilience is a complex construct with temporal aspects that can be measured on multiple levels (119,120). Furthermore, evidence suggests that that one-time measurements of responses to resilience questionnaires are poor predictors of mental health outcome (121). To accurately characterize resilience in 22q11DS, large, longitudinal studies must be implemented to assess the dynamic and multidimensional nature of resilience. Furthermore, consistent with the need for a transdiagnostic approach, neurobiological mechanisms underlying resilience must be “dysfunction specific”, not “disorder-specific’ (121).
To date, there are no published studies linking resilience to neuroimaging phenotypes \in 22q11DS. There is evidence that the reciprocal condition, duplication at the 22q11.2 locus, protects against schizophrenia (122,123). One neuroimaging study found, compared to controls, individuals with 22q11.2 deletions and 22q11.2 duplications had opposing structural brain alterations (124). Protection against psychosis may have neurobiological underpinnings in brain regions with a 22q11.2 gene dosage effect and should be related to measures of resilience.
It is also necessary to characterize how modifiable factors protect against psychopathology in 22q11DS. Higher socioeconomic status is associated with improved psychological functioning in 22q11DS (127). High quality peer relationships, greater parental warmth, and lower levels of neuroticism all contribute to resilience to psychopathology in other high-risk cohorts (128–130), and are potential targets for future investigation to better understand resilience in 22q11DS. Finally, the presence or absence of unique features associated with 22q11DS (e.g., craniofacial or cardiovascular anomalies) may contribute resilience (and/or risk) within this population.
Longitudinal approaches must become the norm in 22q11DS risk and resilience research
22q11DS neuroimaging studies must continue to shift to prospective, risk- and resilience-based studies. Longitudinal investigations of 22q11DS-ASD risk factors have not been conducted. Prospective fetal and infant studies found that distinct neurobiological abnormalities reflect an increased likelihood of later developing idiopathic ASD (131; see 132 this issue). It will be important to conduct prospective neuroimaging studies of 22q11DS infants to see if a similar pattern emerges. Retrospective analyses of fetal MRI may prove useful, as existing evidence shows that 22q11DS-psychosis-spectrum+ are more likely to have incidental radiological findings than 22q11DS-psychosis-spectrum- (133). It may be possible to use fetal and infant clinical MRIs paired with later ASD evaluations to conduct similar investigations in 22q11DS.
As longitudinal designs become more prevalent in 22q11DS research, it is important to focus on statistical rigor. To identify a true risk factor, a study must assess if a baseline predictor contributes to a later outcome. Neurobiological change over time is an important question; however, this question addresses how brain changes shift in concordance with clinical symptomatology, or how neural changes drive later behavioral change. Second, when assessing many neuroimaging variables, correcting for multiple comparisons is essential. If many variables are assessed, it may be more fruitful to focus on feature selection techniques or dimension reduction techniques. Finally, to ensure sufficient power in analyses, we must conduct multisite, prospective investigations of 22q11DS neurobiological risk and resilience factors, similar to the work conducted in UHR psychosis(135,136).
Linking genes within the 22q11.2 locus to neuroimaging phenotypes associated with risk and resilience
If separate neurobiological risk factors underlie psychosis and ASD phenotypes in 22q11DS, then, presumably differing genes within the 22q11.2 locus contribute to these phenotypes. Preliminary evidence suggests that 22q11 deletion size influences clinical and neurobiological phenotypes (56),(137), while another investigation of the 22q11.2 transcriptome found that separate gene co-expression networks were associated with ASD and psychosis (138). However, these networks consisted of genes outside the 22q11.2 locus, suggesting that downstream genomic interactions may drive these phenotypic differences (138). Future research is necessary to understand how genes inside and outside the 22q11.2 locus are related to risk and resilience in 22q11DS.
Conclusions
To date, studies find reduced fronto-temporal cortical thickness in 22q11DS-psychosis+ and idiopathic psychosis, suggesting convergence of grey matter neural mechanisms underlying psychosis onset. Preliminary work suggests that 22q11DS-ASD+ have increased surface area in specific brain regions compared to 22q11DS-ASD-. Together, these findings provide support for differential neurobiological mechanisms underlying risk for psychosis and ASD in 22q11DS. However, white matter alterations in 22q11DS-psychosis+ are markedly different from the idiopathic psychosis, suggesting that distinct white matter neural mechanisms contribute to psychosis onset in these groups. Finally, rsfMRI work suggests that DMN alterations are important for both psychosis and social impairments in 22q11DS. In the future, research must focus on development, longitudinal investigations, statistical rigor, and transdiagnostic risk factors, as well as resilience, in 22q11DS psychiatric research.
Supplementary Material
Table 3.
A). Names of white matter tracts that were disrupted in the largest diffusion-weighted imaging study to date of 22q11DS-psychosis+ vs. 22q11DS-psychosis- (75) B). The known anatomical region connected by each white matter tract. C). Direction of the effects in 22q11DS-psychosis+ vs. 22q11DS-psychosis- study and type of diffusivity measures altered. D). Direction of effects for largest diffusion-weighted imaging study to date of idiopathic schizophrenia vs. healthy controls and type of diffusivity measures altered (159).
| A. Region | B. Anatomical brain structures known to be connected by this white matter tract | C. 22q11DS-psychosis+ vs. 22q11Ds-psychosis- | D. Idiopathic schizophrenia vs. healthy controls |
|---|---|---|---|
| Anterior limb of internal capsule | anterior and medial thalamic nuclei⇔ prefrontal cortex (160) pons⇔frontal cortex | ↓ AD | ↓ FA, ↓ AD, ↑ RD |
| Posterior limb of internal capsule | Pons/cerebellum⇔premotor and primary motor cortex Ventral thalamus⇔premotor and primary motor cortex | ↓ MD | No measures statistically significant |
| posterior thalamic radiation | pulvinar and lateral geniculate thalamic nuclei ⇔ posterior parietal and occipital cortex (161) | ↓ AD | ↓ FA, ↑ MD, ↑ RD |
| sagittal stratum | Thalamus and brainstem ⇔ parietal, occipital, cingulate, and temporal regions of cortex (162) | ↓ AD | ↓ FA, ↑ MD, ↑ RD |
| cingulum of cingulate gyrus | Orbitofrontal cortex ⇔ cingulate gyrus ⇔ temporal lobes (163) | ↓ AD | ↓ FA, ↑ MD, ↑ RD |
| superior longitudinal fasciculus | Parietal lobes ⇔ frontal lobes (164) | ↓ AD | ↓ FA, ↑ MD, ↑ RD |
| Genu of the corpus callosum | Callosal fibers (interhemispheric) | ↓ RD, ↓ MD |
Acknowledgements
The project described was supported by the National Institutes of Health through grants K01 MH112774 (Maria Jalbrzikowski, PI) and from pilot grant funding from the Competitive Medical Research Fund at the University of Pittsburgh (Maria Jalbrzikowski, PI). The author would like to thank Rebecca Hayes, Ph.D. and Leah Vines for reading and providing feedback on earlier versions of this draft. The author would also like to thank Rebecca Hayes for formatting the appropriate references.
Footnotes
Financial Disclosures
Dr. Maria Jalbrzikowski has no financial disclosures or potential conflicts of interest to report.
References
- 1.Eack SM, Bahorik AL, McKnight SAF, Hogarty SS, Greenwald DP, Newhill CE, et al. (2013): Commonalities in Social and Non-Social Cognitive Impairments in Adults with Autism Spectrum Disorder and Schizophrenia. Schizophr Res 148: 24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy E, Benítez-Burraco A (2017): Language deficits in schizophrenia and autism as related oscillatory connectomopathies: An evolutionary account. Neurosci Biobehav Rev 83: 742–764. [DOI] [PubMed] [Google Scholar]
- 3.McCarthy SE, Gillis J, Kramer M, Lihm J, Yoon S, Berstein Y, et al. (2014): De novo mutations in schizophrenia implicate chromatin remodeling and support a genetic overlap with autism and intellectual disability [no. 6]. Molecular Psychiatry 19: 652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Connell KS, McGregor NW, Lochner C, Emsley R, Warnich L (2018): The genetic architecture of schizophrenia, bipolar disorder, obsessive-compulsive disorder and autism spectrum disorder. Molecular and Cellular Neuroscience 88: 300–307. [DOI] [PubMed] [Google Scholar]
- 5.Carroll LS, Owen MJ (2009): Genetic overlap between autism, schizophrenia and bipolar disorder. Genome Medicine 1: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gandal MJ, Haney JR, Parikshak NN, Leppa V, Ramaswami G, Hartl C, et al. (2018): Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science 359: 693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khanzada NS, Butler MG, Manzardo AM (2017): GeneAnalytics Pathway Analysis and Genetic Overlap among Autism Spectrum Disorder, Bipolar Disorder and Schizophrenia [no. 3]. International Journal of Molecular Sciences 18: 527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antshel KM, Aneja A, Strunge L, Peebles J, Fremont WP, Stallone K, et al. (2007): Autistic spectrum disorders in velo-cardio facial syndrome (22q11.2 deletion). J Autism Dev Disord 37: 1776–1786. [DOI] [PubMed] [Google Scholar]
- 9.Vorstman JAS, Morcus MEJ, Duijff SN, Klaassen PWJ, Heineman-de Boer JA, Beemer FA, et al. (2006): The 22q11.2 deletion in children: high rate of autistic disorders and early onset of psychotic symptoms. J Am Acad Child Adolesc Psychiatry 45: 1104–1113. [DOI] [PubMed] [Google Scholar]
- 10.Niklasson L, Rasmussen P, Oskarsdóttir S, Gillberg C (2009): Autism, ADHD, mental retardation and behavior problems in 100 individuals with 22q11 deletion syndrome. Res Dev Disabil 30: 763–773. [DOI] [PubMed] [Google Scholar]
- 11.Niklasson L, Rasmussen P, Oskarsdóttir S, Gillberg C (2001): Neuropsychiatric disorders in the 22q11 deletion syndrome. Genet Med 3: 79–84. [DOI] [PubMed] [Google Scholar]
- 12.Green T, Gothelf D, Glaser B, Debbané M, Frisch A, Kotler M, et al. (2009): Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11.2 deletion) syndrome. J Am Acad Child Adolesc Psychiatry 48: 1060–1068. [DOI] [PubMed] [Google Scholar]
- 13.Pulver AE, Nestadt G, Goldberg R, Shprintzen RJ, Lamacz M, Wolyniec PS, et al. (1994): Psychotic illness in patients diagnosed with velo-cardio-facial syndrome and their relatives. J Nerv Ment Dis 182: 476–478. [DOI] [PubMed] [Google Scholar]
- 14.Murphy KC, Jones LA, Owen MJ (1999): High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry 56: 940–945. [DOI] [PubMed] [Google Scholar]
- 15.Gothelf D, Presburger G, Zohar AH, Burg M, Nahmani A, Frydman M, et al. (2004): Obsessive-compulsive disorder in patients with velocardiofacial (22q11 deletion) syndrome. Am J Med Genet B Neuropsychiatr Genet 126B: 99–105. [DOI] [PubMed] [Google Scholar]
- 16.Gothelf D, Feinstein C, Thompson T, Gu E, Penniman L, Van Stone E, et al. (2007): Risk factors for the emergence of psychotic disorders in adolescents with 22q11.2 deletion syndrome. Am J Psychiatry 164: 663–669. [DOI] [PubMed] [Google Scholar]
- 17.Fung WLA, McEvilly R, Fong J, Silversides C, Chow E, Bassett A (2010): Elevated prevalence of generalized anxiety disorder in adults with 22q11.2 deletion syndrome. Am J Psychiatry 167: 998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider M, Debbané M, Bassett AS, Chow EWC, Fung WLA, van den Bree M, et al. (2014): Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. Am J Psychiatry 171: 627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiksinski AM, Breetvelt EJ, Duijff SN, Bassett AS, Kahn RS, Vorstman JAS (2017): Autism Spectrum and psychosis risk in the 22q11.2 deletion syndrome. Findings from a prospective longitudinal study. Schizophrenia Research 188: 59–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vorstman JAS, Breetvelt EJ, Thode KI, Chow EWC, Bassett AS (2013): Expression of autism spectrum and schizophrenia in patients with a 22q11.2 deletion. Schizophr Res 143: 55–59. [DOI] [PubMed] [Google Scholar]
- 21.Maynard TM, Haskell GT, Peters AZ, Sikich L, Lieberman JA, LaMantia AS (2003): A comprehensive analysis of 22q11 gene expression in the developing and adult brain. Proc Natl Acad Sci U S A 100: 14433–14438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guna A, Butcher NJ, Bassett AS (2015): Comparative mapping of the 22q11.2 deletion region and the potential of simple model organisms. J Neurodev Disord 7. 10.1186/s11689-015-9113-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodship J, Cross I, LiLing J, Wren C (1998): A population study of chromosome 22q11 deletions in infancy. Arch Dis Child 79: 348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edelmann L, Pandita RK, Morrow BE (1999): Low-copy repeats mediate the common 3-Mb deletion in patients with velo-cardio-facial syndrome. Am J Hum Genet 64: 1076–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaikh TH, Kurahashi H, Saitta SC, O’Hare AM, Hu P, Roe BA, et al. (2000): Chromosome 22-specific low copy repeats and the 22q11.2 deletion syndrome: genomic organization and deletion endpoint analysis. Hum Mol Genet 9: 489–501. [DOI] [PubMed] [Google Scholar]
- 26.Demczuk S, Aurias A (1995): DiGeorge syndrome and related syndromes associated with 22q11.2 deletions. A review. Ann Genet 38: 59–76. [PubMed] [Google Scholar]
- 27.Lindsay EA (2001): Chromosomal microdeletions: dissecting del22q11 syndrome. Nat Rev Genet 2: 858–868. [DOI] [PubMed] [Google Scholar]
- 28.McDonald-McGinn DM, LaRossa D, Goldmuntz E, Sullivan K, Eicher P, Gerdes M, et al. (1997): The 22q11.2 deletion: screening, diagnostic workup, and outcome of results; report on 181 patients. Genet Test 1: 99–108. [DOI] [PubMed] [Google Scholar]
- 29.Robin NH, Shprintzen RJ (2005): Defining the clinical spectrum of deletion 22q11.2. J Pediatr 147: 90–96. [DOI] [PubMed] [Google Scholar]
- 30.Bearden CE, Woodin MF, Wang PP, Moss E, McDonald-McGinn D, Zackai E, et al. (2001): The neurocognitive phenotype of the 22q11.2 deletion syndrome: selective deficit in visual-spatial memory. J Clin Exp Neuropsychol 23: 447–464. [DOI] [PubMed] [Google Scholar]
- 31.Moss EM, Batshaw ML, Solot CB, Gerdes M, McDonald-McGinn DM, Driscoll DA, et al. (1999): Psychoeducational profile of the 22q11.2 microdeletion: A complex pattern. J Pediatr 134: 193–198. [DOI] [PubMed] [Google Scholar]
- 32.McGlashan TH (2001): Structured Interview for Prodromal Syndromes (SIPS). New Haven: Yale University. [Google Scholar]
- 33.Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, et al. (2003): Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull 29: 703–715. [DOI] [PubMed] [Google Scholar]
- 34.Bassett AS, Chow EWC, AbdelMalik P, Gheorghiu M, Husted J, Weksberg R (2003): The Schizophrenia Phenotype in 22q11 Deletion Syndrome. Am J Psychiatry 160: 1580–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armando M, Girardi P, Vicari S, Menghini D, Digilio MC, Pontillo M, et al. (2012): Adolescents at ultra-high risk for psychosis with and without 22q11 deletion syndrome: A comparison of prodromal psychotic symptoms and general functioning. Schizophr Res 1–6. [DOI] [PubMed] [Google Scholar]
- 36.Lord C, Risi S, Lambrecht L, Cook EH Jr, Leventhal BL, DiLavore PC, et al. (2000): The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 30: 205–223. [PubMed] [Google Scholar]
- 37.Lord C, Rutter M, Le Couteur A (1994): Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24: 659–685. [DOI] [PubMed] [Google Scholar]
- 38.Lainhart JE, Bigler ED, Bocian M, Coon H, Dinh E, Dawson G, et al. (2006): Head Circumference and Height in Autism. Am J Med Genet A 140: 2257–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gudbrandsen M, Daly E, Murphy CM, Wichers RH, Stoencheva V, Perry E, et al. (2019): The Neuroanatomy of Autism Spectrum Disorder Symptomatology in 22q11.2 Deletion Syndrome. Cerebral Cortex 29: 3655–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jalbrzikowski M, Ahmed KH, Patel A, Jonas R, Kushan L, Chow C, Bearden CE (2017): Categorical versus dimensional approaches to autism-associated intermediate phenotypes in 22q11.2 microdeletion syndrome. Biol Psychiatry Cogn Neurosci Neuroimaging 2: 53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serur Y, Frumber DS, Daon K, Sobal-Havia D, Weinberger R, Shulman C, Gothelf D (2019): Psychiatric disorders and autism in young children with 22q11.2 deletion syndrome compared to children with idiopathic autism. Eur psychiatr 55: 116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kates WR, Antshel KM, Fremont WP, Shprintzen RJ, Strunge LA, Burnette CP, Higgins AM (2007): Comparing phenotypes in patients with idiopathic autism to patients with velocardiofacial syndrome (22q11 DS) with and without autism. Am J Med Genet B Neuropsychiatr Genet 143A: 2642–2650. [DOI] [PubMed] [Google Scholar]
- 43.Arango C, Kahn R (2008): Progressive brain changes in schizophrenia. Schizophrenia bulletin 34: 310–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hulshoff Pol HE, Kahn RS (2008): What Happens After the First Episode? A Review of Progressive Brain Changes in Chronically Ill Patients With Schizophrenia. Schizophr Bull 34: 354–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shepherd AM, Matheson SL, Laurens KR, Carr VJ, Green MJ (2012): Systematic meta-analysis of insula volume in schizophrenia. Biol Psychiatry 72: 775–784. [DOI] [PubMed] [Google Scholar]
- 46.van Erp TGM, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, et al. (2016): Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium [no. 4]. Molecular Psychiatry 21: 547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E (2008): The Anatomy of First-Episode and Chronic Schizophrenia: An Anatomical Likelihood Estimation Meta-Analysis. Am J Psychiatry 165: 1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, et al. (2008): Meta-Analysis of Gray Matter Anomalies in Schizophrenia: Application of Anatomic Likelihood Estimation and Network Analysis. Biol Psychiatry 64: 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Erp TGM, Walton E, Hibar DP, Schmaal L, Jiang W, Glahn DC, et al. (2018): Cortical Brain Abnormalities in 4474 Individuals With Schizophrenia and 5098 Control Subjects via the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) Consortium. Biological Psychiatry 84: 644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Amelsvoort T, Daly E, Henry J, Robertson D, Ng V, Owen M, et al. (2004): Brain Anatomy in Adults With Velocardiofacial Syndrome With and WithoutSchizophrenia: Preliminary Results of a Structural Magnetic Resonance Imaging Study. Arch Gen Psychiatry 61: 1085. [DOI] [PubMed] [Google Scholar]
- 51.Chow EWC (2011): Association of Schizophrenia in 22q11.2 Deletion Syndrome and Gray Matter Volumetric Deficits in the Superior Temporal Gyrus. Am J Psychiatry 168: 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kates WR, Antshel KM, Faraone SV, Fremont WP, Higgins AM, Shprintzen RJ, et al. (2011): Neuroanatomic Predictors to Prodromal Psychosis in Velocardiofacial Syndrome (22q11.2 Deletion Syndrome): A Longitudinal Study. Biological Psychiatry 69: 945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Padula MC, Scariati E, Schaer M, Eliez S (2018): A Mini Review on the Contribution of the Anterior Cingulate Cortex in the Risk of Psychosis in 22q11.2 Deletion Syndrome. Front Psychiatry 9. 10.3389/fpsyt.2018.00372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramanathan S, Mattiaccio LM, Coman IL, Botti J-AC, Fremont W, Faraone SV, et al. (2017): Longitudinal trajectories of cortical thickness as a biomarker for psychosis in individuals with 22q11.2 deletion syndrome. Schizophrenia Research 188: 35–41. [DOI] [PubMed] [Google Scholar]
- 55.Gothelf D, Hoeft F, Ueno T, Sugiura L, Lee AD, Thompson P, Reiss AL (2011): Developmental changes in multivariate neuroanatomical patterns that predict risk for psychosis in 22q11.2 deletion syndrome. Journal of Psychiatric Research 45: 322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun D, Ching CRK, Lin A, Forsyth JK, Kushan L, Vajdi A, et al. (2018): Large-scale mapping of cortical alterations in 22q11.2 deletion syndrome: Convergence with idiopathic psychosis and effects of deletion size. Mol Psychiatry. 10.1038/s41380-018-0078-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mancini V, Sandini C, Padula MC, Zöller D, Schneider M, Schaer M, Eliez S (2019): Positive psychotic symptoms are associated with divergent developmental trajectories of hippocampal volume during late adolescence in patients with 22q11DS. Mol Psychiatry. 10.1038/s41380-019-0443-z [DOI] [PubMed] [Google Scholar]
- 58.Mancini V, Zöller D, Schneider M, Schaer M, Eliez S (2020): Abnormal development and dysconnectivity of distinct thalamic nuclei in patients with 22q11.2 deletion syndrome experiencing auditory hallucinations. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging S2451902220301099. [DOI] [PubMed] [Google Scholar]
- 59.Ching CRK, Gutman BA, Sun D, Villalon Reina J, Ragothaman A, Isaev D, et al. (2020): Mapping Subcortical Brain Alterations in 22q11.2 Deletion Syndrome: Effects of Deletion Size and Convergence With Idiopathic Neuropsychiatric Illness. AJP appi.ajp2019.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jalbrzikowski M, Larsen B, Hallquist MN, Foran W, Calabro F, Luna B (2017): Development of White Matter Microstructure and Intrinsic Functional Connectivity Between the Amygdala and Ventromedial Prefrontal Cortex: Associations With Anxiety and Depression. Biol Psychiatry 82: 511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Rooij D, Anagnostou E, Arango C, Auzias G, Behrmann M, Busatto GF, et al. (2018): Cortical and Subcortical Brain Morphometry Differences Between Patients With Autism Spectrum Disorder and Healthy Individuals Across the Lifespan: Results From the ENIGMA ASD Working Group. Am J Psychiatry 175: 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gudbrandsen M, Bletsch A, Mann C, Daly E, Murphy CM, Stoencheva V, et al. (2020): Neuroanatomical underpinnings of autism symptomatology in carriers and non-carriers of the 22q11.2 microdeletion. Mol Autism 11. 10.1186/s13229-020-00356-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gudbrandsen M, Mann C, Bletsch A, Daly E, Murphy CM, Stoencheva V, et al. (2020): Patterns of Cortical Folding Associated with Autistic Symptoms in Carriers and Noncarriers of the 22q11.2 Microdeletion. Cerebral Cortex bhaa 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Campbell LE, Daly E, Toal F, Stevens A, Azuma R, Catani M, et al. (2006): Brain and behaviour in children with 22q11.2 deletion syndrome: a volumetric and voxel-based morphometry MRI study. Brain 129: 1218–1228. [DOI] [PubMed] [Google Scholar]
- 65.Dufour F, Schaer M, Debbané M, Farhoumand R, Glaser B, Eliez S (2008): Cingulate gyral reductions are related to low executive functioning and psychotic symptoms in 22q11.2 deletion syndrome. Neuropsychologia 46: 2986–2992. [DOI] [PubMed] [Google Scholar]
- 66.Jalbrzikowski M, Jonas R, Senturk D, Patel A, Chow C, Green MF, Bearden CE (2013): Structural abnormalities in cortical volume, thickness, and surface area in 22q11.2 microdeletion syndrome: Relationship with psychotic symptoms. Neuroimage Clin 3: 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmitt JE, Vandekar S, Yi J, Calkins ME, Ruparel K, Roalf DR, et al. (2015): Aberrant Cortical Morphometry in the 22q11.2 Deletion Syndrome. Biol Psychiatry 78: 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sandini C, Scariati E, Padula MC, Schneider M, Schaer M, Van De Ville D, Eliez S (2018): Cortical Dysconnectivity Measured by Structural Covariance Is Associated With the Presence of Psychotic Symptoms in 22q11.2 Deletion Syndrome. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 3: 433–442. [DOI] [PubMed] [Google Scholar]
- 69.Jalbrzikowski M, Sugar CA, Zinberg J, Bachman P, Cannon TD, Bearden CE (2014): Coping styles of individuals at clinical high risk for developing psychosis. Early Interv Psychiatry 8: 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perlstein MD, Chohan MR, Coman IL, Antshel KM, Fremont WP, Gnirke MH, et al. (2014): White matter abnormalities in 22q11.2 deletion syndrome: Preliminary associations with the Nogo-66 receptor gene and symptoms of psychosis. Schizophrenia Research 152: 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kates WR, Olszewski AK, Gnirke MH, Kikinis Z, Nelson J, Antshel KM, et al. (2015): White matter microstructural abnormalities of the cingulum bundle in youths with 22q11.2 deletion syndrome: Associations with medication, neuropsychological function, and prodromal symptoms of psychosis. Schizophrenia Research 161: 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tylee DS, Kikinis Z, Quinn TP, Antshel KM, Fremont W, Tahir MA, et al. (2017): Machine-learning classification of 22q11.2 deletion syndrome: A diffusion tensor imaging study. NeuroImage: Clinical 15: 832–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roalf DR, Eric Schmitt J, Vandekar SN, Satterthwaite TD, Shinohara RT, Ruparel K, et al. (2017): White matter microstructural deficits in 22q11.2 deletion syndrome. Psychiatry Research: Neuroimaging 268: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kikinis Z, Cho KIK, Coman IL, Radoeva PD, Bouix S, Tang Y, et al. (2017): Abnormalities in brain white matter in adolescents with 22q11.2 deletion syndrome and psychotic symptoms. Brain Imaging and Behavior 11: 1353–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Villalón-Reina JE, Martínez K, Qu X, Ching CRK, Nir TM, Kothapalli D, et al. (2019): Altered white matter microstructure in 22q11.2 deletion syndrome: a multisite diffusion tensor imaging study. Mol Psychiatry. 10.1038/s41380-019-0450-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scariati E, Schaer M, Richiardi J, Schneider M, Debbané M, Van De Ville D, Eliez S (2014): Identifying 22q11.2 Deletion Syndrome and Psychosis Using Resting-State Connectivity Patterns. Brain Topogr 27: 808–821. [DOI] [PubMed] [Google Scholar]
- 77.Mattiaccio LM, Coman IL, Thompson CA, Fremont WP, Antshel KM, Kates WR (2018): Frontal dysconnectivity in 22q11.2 deletion syndrome: an atlas-based functional connectivity analysis. Behav Brain Funct 14: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schleifer C, Lin A, Kushan L, Ji JL, Yang G, Bearden CE, Anticevic A (2019): Dissociable Disruptions in Thalamic and Hippocampal Resting-State Functional Connectivity in Youth with 22q11.2 Deletions. J Neurosci 39: 1301–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Song S-K, Sun S-W, Ju W-K, Lin S-J, Cross AH, Neufeld AH (2003): Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20: 1714–1722. [DOI] [PubMed] [Google Scholar]
- 80.Schwartz ED, Cooper ET, Fan Y, Jawad AF, Chin C-L, Nissanov J, Hackney DB (2005): MRI diffusion coefficients in spinal cord correlate with axon morphometry. Neuroreport 16: 73–76. [DOI] [PubMed] [Google Scholar]
- 81.Takahashi M, Ono J, Harada K, Maeda M, Hackney DB (2000): Diffusional anisotropy in cranial nerves with maturation: quantitative evaluation with diffusion MR imaging in rats. Radiology 216: 881–885. [DOI] [PubMed] [Google Scholar]
- 82.Wu P, Teot L, Murdoch G, Monaghan-Nichols AP, McFadden K (2014): Neuropathology of 22q11 deletion syndrome in an infant. Pediatr Dev Pathol 17: 386–392. [DOI] [PubMed] [Google Scholar]
- 83.Kanold PO, Luhmann HJ (2010): The subplate and early cortical circuits. Annu Rev Neurosci 33: 23–48. [DOI] [PubMed] [Google Scholar]
- 84.Song S-K, Yoshino J, Le TQ, Lin S-J, Sun S-W, Cross AH, Armstrong RC (2005): Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 26: 132–140. [DOI] [PubMed] [Google Scholar]
- 85.Jalbrzikowski M, Villalon-Reina JE, Karlsgodt KH, Senturk D, Chow C, Thompson PM, Bearden CE (2014): Altered white matter microstructure is associated with social cognition and psychotic symptoms in 22q11.2 microdeletion syndrome. Front Behav Neurosci 8: 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bora E, Yucel M, Pantelis C (2009): Theory of mind impairment in schizophrenia: Meta-analysis. Schizophrenia Research 109: 1–9. [DOI] [PubMed] [Google Scholar]
- 87.Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ (2010): Facial Emotion Perception in Schizophrenia: A Meta-analytic Review. Schizophr Bull 36: 1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harms MB, Martin A, Wallace GL (2010): Facial Emotion Recognition in Autism Spectrum Disorders: A Review of Behavioral and Neuroimaging Studies. Neuropsychol Rev 20: 290–322. [DOI] [PubMed] [Google Scholar]
- 89.Kimhi Y (2014): Theory of Mind Abilities and Deficits in Autism Spectrum Disorders. Topics in Language Disorders 34: 329–343. [Google Scholar]
- 90.Debbané M, Lazouret M, Lagioia A, Schneider M, Van De Ville D, Eliez S (2012): Resting-state networks in adolescents with 22q11.2 deletion syndrome: Associations with prodromal symptoms and executive functions. Schizophrenia Research 139: 33–39. [DOI] [PubMed] [Google Scholar]
- 91.Padula MC, Schaer M, Scariati E, Schneider M, Van De Ville D, Debbané M, Eliez S (2015): Structural and functional connectivity in the default mode network in 22q11.2 deletion syndrome. J Neurodevelop Disord 7: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schreiner M, Forsyth JK, Karlsgodt KH, Anderson AE, Hirsh N, Kushan L, et al. (2017): Intrinsic Connectivity Network-Based Classification and Detection of Psychotic Symptoms in Youth With 22q11.2 Deletions. Cerebral Cortex 27: 3294–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schreiner MJ, Karlsgodt KH, Uddin LQ, Chow C, Congdon E, Jalbrzikowski M, Bearden CE (2014): Default mode network connectivity and reciprocal social behavior in 22q11.2 deletion syndrome. Soc Cogn Affect Neurosci 9: 1261–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, et al. (2015): Association of Thalamic Dysconnectivity and Conversion to Psychosis in Youth and Young Adults at Elevated Clinical Risk. JAMA Psychiatry 72: 882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, Winkler AM, et al. (2014): Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex 24: 3116–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zöller D, Sandini C, Karahanoğlu FI, Padula MC, Schaer M, Eliez S, Van De Ville D (2019): Large-Scale Brain Network Dynamics Provide a Measure of Psychosis and Anxiety in 22q11.2 Deletion Syndrome. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 4: 881–892. [DOI] [PubMed] [Google Scholar]
- 97.Scariati E, Schaer M, Karahanoglu I, Schneider M, Richiardi J, Debbané M, et al. (2016): Large-scale functional network reorganization in 22q11.2 deletion syndrome revealed by modularity analysis. Cortex 82: 86–99. [DOI] [PubMed] [Google Scholar]
- 98.Schneider M, Schaer M, Mutlu AK, Menghetti S, Glaser B, Debbané M, Eliez S (2014): Clinical and cognitive risk factors for psychotic symptoms in 22q11.2 deletion syndrome: a transversal and longitudinal approach. Eur Child Adolesc Psychiatry 23: 425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tang SX, Moore TM, Calkins ME, Yi JJ, McDonald-McGinn DM, Zackai EH, et al. (2017): Emergent, remitted and persistent psychosis-spectrum symptoms in 22q11.2 deletion syndrome. Transl Psychiatry 7: e1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Michel C, Ruhrmann S, Schimmelmann BG, Klosterkötter J, Schultze-Lutter F (2018): Course of clinical high-risk states for psychosis beyond conversion. Eur Arch Psychiatry Clin Neurosci 268: 39–48. [DOI] [PubMed] [Google Scholar]
- 101.Calkins ME, Moore TM, Satterthwaite TD, Wolf DH, Turetsky BI, Roalf DR, et al. (2017): Persistence of psychosis spectrum symptoms in the Philadelphia Neurodevelopmental Cohort: a prospective two-year follow-up. World Psychiatry 16: 62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Angkustsiri K, Goodlin-Jones B, Deprey L, Brahmbhatt K, Harris S, Simon TJ (2014): Social Impairments in Chromosome 22q11.2 Deletion Syndrome (22q11.2DS): Autism Spectrum Disorder or a Different Endophenotype? J Autism Dev Disord 44: 739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eliez S (2007): Autism in children with 22q11.2 deletion syndrome. J Am Acad Child Adolesc Psychiatry 46: 433–4– author reply 434–4. [DOI] [PubMed] [Google Scholar]
- 104.Lebel C, Treit S, Beaulieu C (2019): A review of diffusion MRI of typical white matter development from early childhood to young adulthood. NMR in Biomedicine 32: e3778. [DOI] [PubMed] [Google Scholar]
- 105.Grayson DS, Fair DA (2017): Development of large-scale functional networks from birth to adulthood: A guide to the neuroimaging literature. NeuroImage 160: 15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vijayakumar N, Mills KL, Alexander-Bloch A, Tamnes CK, Whittle S (2018): Structural brain development: A review of methodological approaches and best practices. Developmental Cognitive Neuroscience 33: 129–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gruskin DC, Rosenberg MD, Holmes AJ (2019): Relationships between depressive symptoms and brain responses during emotional movie viewing emerge in adolescence. NeuroImage 116217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tan X, Shiyko MP, Li R, Li Y, Dierker L (2012): A time-varying effect model for intensive longitudinal data. Psychol Methods 17: 61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kessler D, Angstadt M, Sripada C (2016): Growth Charting of Brain Connectivity Networks and the Identification of Attention Impairment in Youth. JAMA Psychiatry 73: 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jalbrzikowski M, Murty VP, Tervo-Clemmens B, Foran W, Luna B (2019): Age-Associated Deviations of Amygdala Functional Connectivity in Youths With Psychosis Spectrum Disorders: Relevance to Psychotic Symptoms. Am J Psychiatry 176: 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marquand AF, Kia SM, Zabihi M, Wolfers T, Buitelaar JK, Beckmann CF (2019): Conceptualizing mental disorders as deviations from normative functioning. Molecular Psychiatry 24: 1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tang SX, Yi JJ, Calkins ME, Whinna DA, Kohler CG, Souders MC, et al. (2014): Psychiatric disorders in 22q11.2 deletion syndrome are prevalent but undertreated. Psychol Med 44: 1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Olszewski AK, Kikinis Z, Gonzalez CS, Coman IL, Makris N, Gong X, et al. (2017): The social brain network in 22q11.2 deletion syndrome: a diffusion tensor imaging study. Behav Brain Funct 13: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Conway CC, Forbes MK, Forbush KT, Fried EI, Hallquist MN, Kotov R, et al. (2019): A Hierarchical Taxonomy of Psychopathology Can Transform Mental Health Research. Perspect Psychol Sci 14: 419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ruggero CJ, Kotov R, Hopwood CJ, First M, Clark LA, Skodol AE, et al. (2019): Integrating the Hierarchical Taxonomy of Psychopathology (HiTOP) into Clinical Practice. J Consult Clin Psychol 87: 1069–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tang SX, Gur RE (2018): Longitudinal perspectives on the psychosis spectrum in 22q11.2 deletion syndrome. Am J Med Genet 176: 2192–2202. [DOI] [PubMed] [Google Scholar]
- 117.Jonas RK, Montojo CA, Bearden CE (2014): The 22q11.2 deletion syndrome as a window into complex neuropsychiatric disorders over the lifespan. Biol Psychiatry 75: 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kalisch R, Baker DG, Basten U, Boks MP, Bonanno GA, Brummelman E, et al. (2017): The resilience framework as a strategy to combat stress-related disorders. Nat Hum Behav 1: 784–790. [DOI] [PubMed] [Google Scholar]
- 119.Ioannidis K, Askelund AD, Kievit RA, van Harmelen A-L (2020): The complex neurobiology of resilient functioning after childhood maltreatment. BMC Med 18: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ungar M, Theron L (2020): Resilience and mental health: how multisystemic processes contribute to positive outcomes. The Lancet Psychiatry 7: 441–448. [DOI] [PubMed] [Google Scholar]
- 121.Kalisch R, Müller MB, Tüscher O (2015): A conceptual framework for the neurobiological study of resilience. Behav Brain Sci 38: e92. [DOI] [PubMed] [Google Scholar]
- 122.Marshall CR, Howrigan DP, Merico D, Thiruvahindrapuram B, Wu W, Greer DS, et al. (2017): Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects. Nat Genet 49: 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rees E, Kirov G, Sanders A, Walters JTR, Chambert KD, Shi J, et al. (2014): Evidence that duplications of 22q11.2 protect against schizophrenia. Mol Psychiatry 19: 37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lin A, Ching CRK, Vajdi A, Sun D, Jonas RK, Jalbrzikowski M, et al. (2017): Mapping 22q11.2 Gene Dosage Effects on Brain Morphometry. J Neurosci 37: 6183–6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D, et al. (2011): Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron 70: 863–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lin A, Vajdi A, Kushan-Wells L, Helleman G, Hansen LP, Jonas RK, et al. (2020): Reciprocal Copy Number Variations at 22q11.2 Produce Distinct and Convergent Neurobehavioral Impairments Relevant for Schizophrenia and Autism Spectrum Disorder. Biological Psychiatry S0006322320300068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shashi V, Keshavan M, Kaczorowski J, Schoch K, Lewandowski KE, McConkie-Rosell A, et al. (2010): Socioeconomic Status and Psychological Function in Children with Chromosome 22q11.2 Deletion Syndrome: Implications for Genetic Counseling. J Genet Couns 19: 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Collishaw S, Pickles A, Messer J, Rutter M, Shearer C, Maughan B (2007): Resilience to adult psychopathology following childhood maltreatment: Evidence from a community sample. Child Abuse & Neglect 31: 211–229. [DOI] [PubMed] [Google Scholar]
- 129.Lind MJ, Brown RC, Sheerin CM, York TP, Myers JM, Kendler KS, Amstadter AB (2018): Does Parenting Influence the Enduring Impact of Severe Childhood Sexual Abuse on Psychiatric Resilience in Adulthood? Child Psychiatry Hum Dev 49: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bick J, Zajac K, Ralston ME, Smith D (2014): Convergence and Divergence in Reports of Maternal Support Following Childhood Sexual Abuse: Prevalence and Associations with Youth Psychosocial Adjustment. Child Abuse Negl 38: 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hazlett HC, Gu H, Munsell BC, Kim SH, Styner M, Wolff JJ, et al. (2017): Early brain development in infants at high risk for autism spectrum disorder [no. 7641]. Nature 542: 348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Molnar-Szakacs I, Kupis L, Uddin LQ (in press): Neuroimaging markers of risk and resilience in autism spectrum disorders. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schmitt JE, Yi JJ, Roalf DR, Loevner LA, Ruparel K, Whinna D, et al. (2014): Incidental Radiologic Findings in the 22q11.2 Deletion Syndrome. AJNR Am J Neuroradiol 35: 2186–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nielsen AN, Barch DM, Petersen SE, Schlaggar BL, Greene DJ (2019): Machine Learning With Neuroimaging: Evaluating Its Applications in Psychiatry. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging S2451902219303040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Addington J, Liu L, Brummitt K, Bearden CE, Cadenhead KS, Cornblatt BA, et al. (2020): North American Prodrome Longitudinal Study (NAPLS 3): Methods and baseline description. Schizophrenia Research. 10.1016/j.schres.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Addington J, Cadenhead KS, Cornblatt BA, Mathalon DH, McGlashan TH, Perkins DO, et al. (2012): North American Prodrome Longitudinal Study (NAPLS 2): Overview and recruitment. Schizophr Res 142: 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Clements CC, Wenger TL, Zoltowski AR, Bertollo JR, Miller JS, de Marchena AB, et al. (2017): Critical region within 22q11.2 linked to higher rate of autism spectrum disorder. Mol Autism 8. 10.1186/s13229-017-0171-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jalbrzikowski M, Lazaro MT, Gao F, Huang A, Chow C, Geschwind DH, et al. (2015): Transcriptome Profiling of Peripheral Blood in 22q11.2 Deletion Syndrome Reveals Functional Pathways Related to Psychosis and Autism Spectrum Disorder. PLoS One 10: e0132542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rakic P (1988): Specification of cerebral cortical areas. Science 241: 170–176. [DOI] [PubMed] [Google Scholar]
- 140.Budde MD, Xie M, Cross AH, Song S-K (2009): Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. J Neurosci 29: 2805–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Song S-K, Sun S-W, Ju W-K, Lin S-J, Cross AH, Neufeld AH (2003): Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20: 1714–1722. [DOI] [PubMed] [Google Scholar]
- 142.Song S-K, Sun S-W, Ramsbottom MJ, Chang C, Russell J, Cross AH (2002): Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17: 1429–1436. [DOI] [PubMed] [Google Scholar]
- 143.Reiss AL, Hennessey JG, Rubin M, Beach L, Abrams MT, Warsofsky IS, et al. (1998): Reliability and validity of an algorithm for fuzzy tissue segmentation of MRI. J Comput Assist Tomogr 22: 471–479. [DOI] [PubMed] [Google Scholar]
- 144.Eliez S, Barnea-Goraly N, Schmitt JE, Liu Y, Reiss AL (2002): Increased basal ganglia volumes in velo-cardio-facial syndrome (deletion 22q11.2). Biol Psychiatry 52: 68–70. [DOI] [PubMed] [Google Scholar]
- 145.Kates WR, Abrams MT, Kaufmann WE, Breiter SN, Reiss AL (1997): Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatry Res 75: 31–48. [DOI] [PubMed] [Google Scholar]
- 146.Kesler SR, Blasey CM, Brown WE, Yankowitz J, Zeng SM, Bender BG, Reiss AL (2003): Effects of X-Monosomy and X-Linked Imprinting on Superior Temporal Gyrus Morphology in Turner Syndrome. Biol Psychiatry 54: 636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kaplan DM, Liu AM, Abrams MT, Warsofsky IS, Kates WR, White CD, et al. (1997): Application of an automated parcellation method to the analysis of pediatric brain volumes. Psychiatry Res 76: 15–27. [DOI] [PubMed] [Google Scholar]
- 148.Kates WR, Warsofsky IS, Patwardhan A, Abrams MT, Liu AM, Naidu S, et al. (1999): Automated Talairach atlas-based parcellation and measurement of cerebral lobes in children. Psychiatry Res 91: 11–30. [DOI] [PubMed] [Google Scholar]
- 149.Eliez S, Schmitt JE, White CD, Reiss AL (2000): Children and adolescents with velocardiofacial syndrome: a volumetric MRI study. Am J Psychiatry 157: 409–415. [DOI] [PubMed] [Google Scholar]
- 150.Kates WR, Burnette CP, Jabs EW, Rutberg J, Murphy AM, Grados M, et al. (2001): Regional cortical white matter reductions in velocardiofacial syndrome: a volumetric MRI analysis. Biol Psychiatry 49: 677–684. [DOI] [PubMed] [Google Scholar]
- 151.Kates WR, Miller AM, Abdulsabur N, Antshel KM, Conchelos J, Fremont W, Roizen N (2006): Temporal lobe anatomy and psychiatric symptoms in velocardiofacial syndrome (22q11.2 deletion syndrome). J Am Acad Child Adolesc Psychiatry 45: 587–595. [DOI] [PubMed] [Google Scholar]
- 152.Kates WR, Antshel K, Willhite R, Bessette BA, AbdulSabur N, Higgins AM (2005): Gender-moderated dorsolateral prefrontal reductions in 22q11.2 Deletion Syndrome: implications for risk for schizophrenia. Child Neuropsychol 11: 73–85. [DOI] [PubMed] [Google Scholar]
- 153.Kates WR, Antshel KM, AbdulSabur N, Colgan D, Funke B, Fremont W, et al. (2006): A Gender-Moderated Effect of a Functional COMT Polymorphism on Prefrontal Brain Morphology and Function in Velo-cardio-facial Syndrome (22q11.2 Deletion Syndrome). Am J Med Genet B Neuropsychiatr Genet 141B: 274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gur RE, Cowell PE, Latshaw A, Turetsky BI, Grossman RI, Arnold SE, et al. (2000): Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Arch Gen Psychiatry 57: 761–768. [DOI] [PubMed] [Google Scholar]
- 155.Strasser HC, Lilyestrom J, Ashby ER, Honeycutt NA, Schretlen DJ, Pulver AE, et al. (2005): Hippocampal and ventricular volumes in psychotic and nonpsychotic bipolar patients compared with schizophrenia patients and community control subjects: a pilot study. Biol Psychiatry 57: 633–639. [DOI] [PubMed] [Google Scholar]
- 156.Kunwar A, Ramanathan S, Nelson J, Antshel KM, Fremont W, Higgins AM, et al. (2012): Cortical gyrification in velo-cardio-facial (22q11.2 deletion) syndrome: A longitudinal study. Schizophrenia Research 137: 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Berhanu D, Mattiaccio LM, Antshel KM, Fremont W, Middleton FA, Kates WR (2017): Cortical-amygdala volumetric ratios predict onset of symptoms of psychosis in 22q11.2 deletion syndrome. Psychiatry Research: Neuroimaging 259: 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. (2006): An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31: 968–980. [DOI] [PubMed] [Google Scholar]
- 159.Kelly S, Jahanshad N, Zalesky A, Kochunov P, Agartz I, Alloza C, et al. (2017): Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mithani K, Davison B, Meng Y, Lipsman N (2020): The anterior limb of the internal capsule: Anatomy, function, and dysfunction. Behavioural Brain Research 387: 112588. [DOI] [PubMed] [Google Scholar]
- 161.Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PCM, Mori S (2004): Fiber tract-based atlas of human white matter anatomy. Radiology 230: 77–87. [DOI] [PubMed] [Google Scholar]
- 162.Di Carlo DT, Benedetto N, Duffau H, Cagnazzo F, Weiss A, Castagna M, et al. (2019): Microsurgical anatomy of the sagittal stratum. Acta Neurochir 161: 2319–2327. [DOI] [PubMed] [Google Scholar]
- 163.Bubb EJ, Metzler-Baddeley C, Aggleton JP (2018): The cingulum bundle: Anatomy, function, and dysfunction. Neuroscience & Biobehavioral Reviews 92: 104–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nakajima R, Kinoshita M, Shinohara H, Nakada M (2019): The superior longitudinal fascicle: reconsidering the fronto-parietal neural network based on anatomy and function. Brain Imaging and Behavior. 10.1007/s11682-019-00187-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.