Abstract
In this large (n = 396), multicenter (n = 13 sites) retrospective chart review of patients with KRAS G12C mutant NSCLC we observed a relatively short median rwPFS of 4.6 months among patients with KRAS G12C mutant NSCLC treated with docetaxel with or without ramucirumab in the second-line setting, which aligns with the recently reported CodeBreak 200 dataset.
Background:
On May 28, 2021, the United States Food and Drug Administration (FDA) granted accelerated approval to sotorasib for second-line or later treatment of patients with locally advanced or metastatic KRAS G12C mutant non–small cell lung cancer (NSCLC). This was the first FDA-approved targeted therapy for this patient population. Due to a paucity of real world data describing clinical outcomes in patients with locally advanced or metastatic KRAS G12C mutated NSCLC in the second-line or later, we sought to compile a large, academic medical center-based historical dataset to clarify clinical outcomes in this patient population.
Materials and Methods:
The clinical outcomes of 396 patients with stage IV (n = 268, 68%) or recurrent, metastatic (n = 128, 32%) KRAS G12C mutant NSCLC were evaluated in this multicenter retrospective chart review conducted through the Academic Thoracic Oncology Medical Investigator’s Consortium (ATOMIC). Patients treated at 13 sites in the United States and Canada and diagnosed between 2006 and 2020 (30% 2006–2015, 70% 2016–2020) were included. Primary outcomes included real-world PFS (rwPFS) and overall survival (OS) from time of stage IV or metastatic diagnosis, with particular interest in patients treated with second-line docetaxel-containing regimens, as well as clinical outcomes in the known presence or absence of STK11 or KEAP1 comutations.
Results:
Among all patients with stage IV or recurrent, metastatic KRAS G12C mutant NSCLC (n = 201 with KRAS G12C confirmed prior to first line systemic therapy), the median first-line rwPFS was 9.3 months (95% CI, 7.3–11.8 months) and median OS was 16.8 months (95% CI, 12.7–22.3 months). In this historical dataset, first line systemic therapy among these 201 patients included platinum doublet alone (44%), PD-(L)1 inhibitor monotherapy (30%), platinum doublet chemotherapy plus PD-(L)1 inhibitor (18%), and other regimens (8%). Among patients with documented second-line systemic therapy (n = 123), the second-line median rwPFS was 8.3 months (95% CI, 6.1–11.9 months), with median rwPFS 4.6 months (95% CI, 1.4-NA) among 10 docetaxel-treated patients (9 received docetaxel and 1 received docetaxel plus ramucirumab). Within the total study population, 49 patients (12%) had a co-occurring STK11 mutation and 3 (1%) had a co-occurring KEAP1 mutation. Among the 49 patients with a co-occurring KRAS G12C and STK11 mutation, median rwPFS on first-line systemic therapy (n = 23) was 6.0 months (95% CI, 4.7-NA), and median OS was 14.0 months (95% CI, 10.8–35.3 months).
Conclusion:
In this large, multicenter retrospective chart review of patients with KRAS G12C mutant NSCLC we observed a relatively short median rwPFS of 4.6 months among 10 patients with KRAS G12C mutant NSCLC treated with docetaxel with or without ramucirumab in the second-line setting, which aligns with the recently reported CodeBreak 200 dataset.
Keywords: KRAS G12C mutation, Non-small cell lung cancer
Background
Oncogenic mutations in KRAS occur in approximately 26% and 11% of patients with lung adenocarcinoma in Western and Asian populations, respectively.1–3 Until recently, there has been no licensed therapeutic targeting KRAS in patients with non–small cell lung cancer (NSCLC). That changed on May 28, 2021, when the United States Food and Drug Administration (FDA) granted accelerated approval to sotorasib for second-line or later treatment of patients with locally advanced or metastatic KRAS G12C mutant NSCLC.
This was the first FDA-approved targeted therapy for patients with KRAS mutant NSCLC, and it was based on a single-arm study demonstrating a promising objective response rate (37.1%), with a median duration of response of 11.1 months and median progression-free survival (PFS) of 6.8 months4 among patients predominantly treated in the third line or later.
There is a paucity of real-world data describing clinical outcomes in patients with locally advanced or metastatic KRAS G12C mutated NSCLC in the second-line or later, as most prior studies featured outcomes in the first line setting, evaluated specific patient subgroups, or evaluated the prognostic vs. predictive value of KRAS G12C compared to non-G12C genotypes.5–12 We sought to compile a large, academic medical center-based historical dataset to clarify clinical outcomes in the second line or later among patients with KRAS G12C mutant NSCLC.
Materials and Methods
Study Population and Data Collection
The clinical characteristics and outcomes (including demographics, performance status, tumor biomarker testing, treatment types and duration, radiographic results, and follow-up including death) of 396 patients with stage IV (n = 268, 68%) or recurrent, metastatic (n = 128, 32%) KRAS G12C mutant NSCLC were evaluated in this multi-center retrospective chart review conducted through the Academic Thoracic Oncology Medical Investigator’s Consortium (ATOMIC). Patients treated at 13 sites in the United States and Canada and diagnosed between 2006 and 2020 (30% 2006–2015, 70% 2016–2020) were included. Data abstraction occurred between August 2020 and June 2021.
Statistical Analysis
Primary outcomes included real-world PFS (rwPFS) with each line of therapy, with particular interest in the rwPFS when treated with second-line docetaxel, as well as clinical outcomes in the presence of STK11 or KEAP1 comutations. The rwPFS was defined as the time between initiation of systemic therapy within the specific line of therapy and documented progression event (radiographic and/or clinical) or death, with censoring at last clinical assessment if subsequently lost to follow-up and no progression event or death was noted upon medical record review. Overall survival (OS) was defined as the time from initiation of systemic therapy to death, with censoring at last clinical assessment if subsequently lost to follow-up and no progression event or death was noted upon medical record review. In order to minimize immortal time bias from molecular testing results (thus excluding potential cases of acquired KRAS G12C mutations in subsequent lines of therapy), patient inclusion in any line of treatment rwPFS and OS analysis was restricted to those who had KRAS G12C documented any time prior to line of treatment initiation or within 21 days after initiation of the respective line of treatment.
Results
Patient Demographics and Follow-Up
Among the 396 patients, 110 (28%) had a KRAS G12C mutation identified at the time of initial NSCLC diagnosis, and 286 (72%) had a KRAS G12C mutation identified later in their disease course. There were no major demographic differences between patients who had their mutation identified at diagnosis compared to later in their disease course. The median duration of follow-up for the full study cohort was 455 days (15 months). The study cohort included 235 (59%) women and 289 (73%) white patients. Among the study cohort, 371 patients (94%) were current or former smokers (median of 30 pack-year history), and the median age at metastatic disease diagnosis was 67 years old. At the time of metastatic diagnosis, 225 (57%) of patients were classified as Eastern Co-operative Oncology Group Performance Status (ECOG PS) 0 to 1. Patient demographic information is listed in Table 1.
Table 1.
Patient Demographics of Full Cohort of Patients With KRAS G12C Mutant Non–Small Cell Lung Cancer
| Demographic | Patients (N = 396) |
|---|---|
| Sex | |
| Male | 161 (40.66%) |
| Female | 235 (59.34%) |
| Age at metastatic NSCLC diagnosis | |
| 18–64 y | 169 (42.68%) |
| 65–74 y | 139 (35.10%) |
| 75–84 y | 77 (19.44%) |
| > = 85 y | 11 (2.78%) |
| Age at metastatic diagnosis | |
| Median (IQ range) | 67.35 (59.64–74.36) |
| Range | 26.30–93.20 |
| Race | |
| White | 289 (72.98%) |
| Black | 57 (14.39%) |
| Asian Indian | 1 (0.25%) |
| Chinese | 5 (1.26%) |
| Korean | 2 (0.51%) |
| Other Asian | 3 (0.76%) |
| Other—not listed above | 5 (1.26%) |
| Unknown | 34 (8.59%) |
| Smoking status | |
| Current smoker | 85 (21.46%) |
| Former smoker | 286 (72.22%) |
| Never smoker | 18 (4.55%) |
| Unknown | 7 (1.77%) |
| ECOG score at metastatic disease | |
| 0 | 71 (17.93%) |
| 1 | 154 (38.89%) |
| 2 | 41 (10.35%) |
| > = 3 | 130 (32.83%) |
| Not available | 0 (0 ·00%) |
Patient Clinical Characteristics
Among the study cohort, 268 (68%) patients had stage IV disease at diagnosis, with step wise changes corresponding to initial stage among the recurrent, metastatic cohort—57 patients (14%) with stage III disease at diagnosis, 36 patients (8%) with stage II disease initially, and 29 patients (7%) with initially stage I disease. Among patients with tumor PD-L1 score (TPS) recorded (n = 211 [53%]; due to the historical nature of the dataset, PD-L1 testing was not done in 117 patients [30%] and unknown in the remaining 68 patients [17%]), 57 (27%) had <1% expression, 49 (23%) had 1% to 49% expression, and 105 (49%) had 50% or greater expression. Among the full study cohort, 37 (9%) developed brain metastases at any time (OS for brain metastasis cohort included in Supplemental Figure 1) and 28 (7%) developed liver metastases at any time. Detailed clinical characteristics for the full patient cohort are listed in Table 2.
Table 2.
Clinical Characteristics of Full Cohort of Patients With KRAS G12C Mutant Non–Small Cell Lung Cancer
| Clinical Characteristic | Patients (N = 396) |
|---|---|
| Year of metastatic NSCLC diagnosis | |
| <2011 | 15 (3.79%) |
| 2011 | 18 (4.55%) |
| 2012 | 9 (2.27%) |
| 2013 | 6 (1.52%) |
| 2014 | 16 (4.04%) |
| 2015 | 33 (8.33%) |
| 2016 | 50 (12.63%) |
| 2017 | 56 (14.14%) |
| 2018 | 68 (17.17%) |
| 2019 | 83 (20.96%) |
| 2020 | 42 (10.61%) |
| Stage at initial NSCLC diagnosis | |
| Unknown | 6 (1.52%) |
| Stage Ia | 29 (7.32%) |
| Stage IIa | 13 (3.28%) |
| Stage IIb | 23 (5.81%) |
| Stage IIIa | 40 (10.10%) |
| Stage IIIb | 15 (3.79%) |
| Stage IIIc | 2 (0.51%) |
| Stage IV | 268 (67.68%) |
| Histology | |
| Adenocarcinoma | 217 (54.80%) |
| Squamous cell carcinoma | 5 (1.26%) |
| Adenosquamous carcinoma | 4 (1.01%) |
| Large cell carcinoma | 2 (0.51%) |
| Non–small cell carcinoma, NOS | 24 (6.06%) |
| Other | 4 (1.01%) |
| Not specifically noted | 140 (35.35%) |
| PD-L1 grouping | |
| <1% | 57 (27.01%) |
| 1%−49% | 49 (23.22%) |
| ≥50% | 105 (49.76%) |
| Data not available | 185 (46.72%) |
| TMB | |
| <10 mutations/megabase | 26 (60.47%) |
| ≥10 mutations/megabase | 17 (39.53%) |
| Data not available | 353 (89.14%) |
| CoMutations | |
| TP53 | 152 (38%) |
| STK11 | 49 (12%) |
| KEAP1 | 3 (1%) |
| EGFR | ∗n = 1 with L858R, n = 1 with T790M |
| ALK rearrangement | 1 (0.3%) |
| Brain metastases at diagnosis | |
| Yes | 28 (7.1%) |
| No | 368 (92.9%) |
| Brain metastases at any time | |
| Yes | 37 (9.34%) |
| No | 359 (90.66%) |
| Adrenal metastases at any time | |
| Yes | 38 (9.60%) |
| No | 358 (90.40%) |
| Bone metastases at any time | |
| Yes | 76 (19.19%) |
| No | 320 (80.81%) |
| Liver metastases at any time | |
| Yes | 28 (7.07%) |
| No | 368 (92.93%) |
| Lung metastases at any time | |
| Yes | 137 (34.60%) |
| No | 259 (65.40%) |
| ECOG score at second line of treatment start | |
| 0 | 14 (14.00%) |
| 1 | 67 (67.00%) |
| 2 | 16 (16.00%) |
| > = 3 | 3 (3.00%) |
Overall Study Cohort Outcomes
Among all patients with stage IV or recurrent, metastatic KRAS G12C mutant NSCLC with first line systemic therapy recorded after KRAS G12C mutation identification (n = 201), the median first-line rwPFS on systemic therapy for stage IV disease was 9.3 months (95% CI, 7.3–11.8 months) and median OS was 16.8 months (95% CI, 12.7–22.3 months; Figure 1). Treatment data was captured for these 201 patients—in the first-line setting, 89 patients (44%) received platinum-based chemotherapy without a PD-(L)1 inhibitor, 60 patients (30%) were treated with PD-(L)1 inhibitor monotherapy, 36 patients (18%) were treated with platinum based chemotherapy plus a PD-(L)1 inhibitor, and 16 patients (8%) were treated with other regimens. When comparing patients with recurrent, unresectable disease (n = 44) vs. stage IV disease at diagnosis (n = 157) with data on first line systemic therapy, both median first-line OS and rwPFS were similar in patients with recurrent, unresectable disease (median OS: 14.2 months [95% CI, 10.5–26.4 months] vs. 18.7 months [95% CI, 12.4–28.0 months], respectively, and median rwPFS: 10.6 months [95% CI, 7.2–16.5 months] vs. 9.3 months [95% CI, 6.9–12.4 months], respectively).
Figure 1.
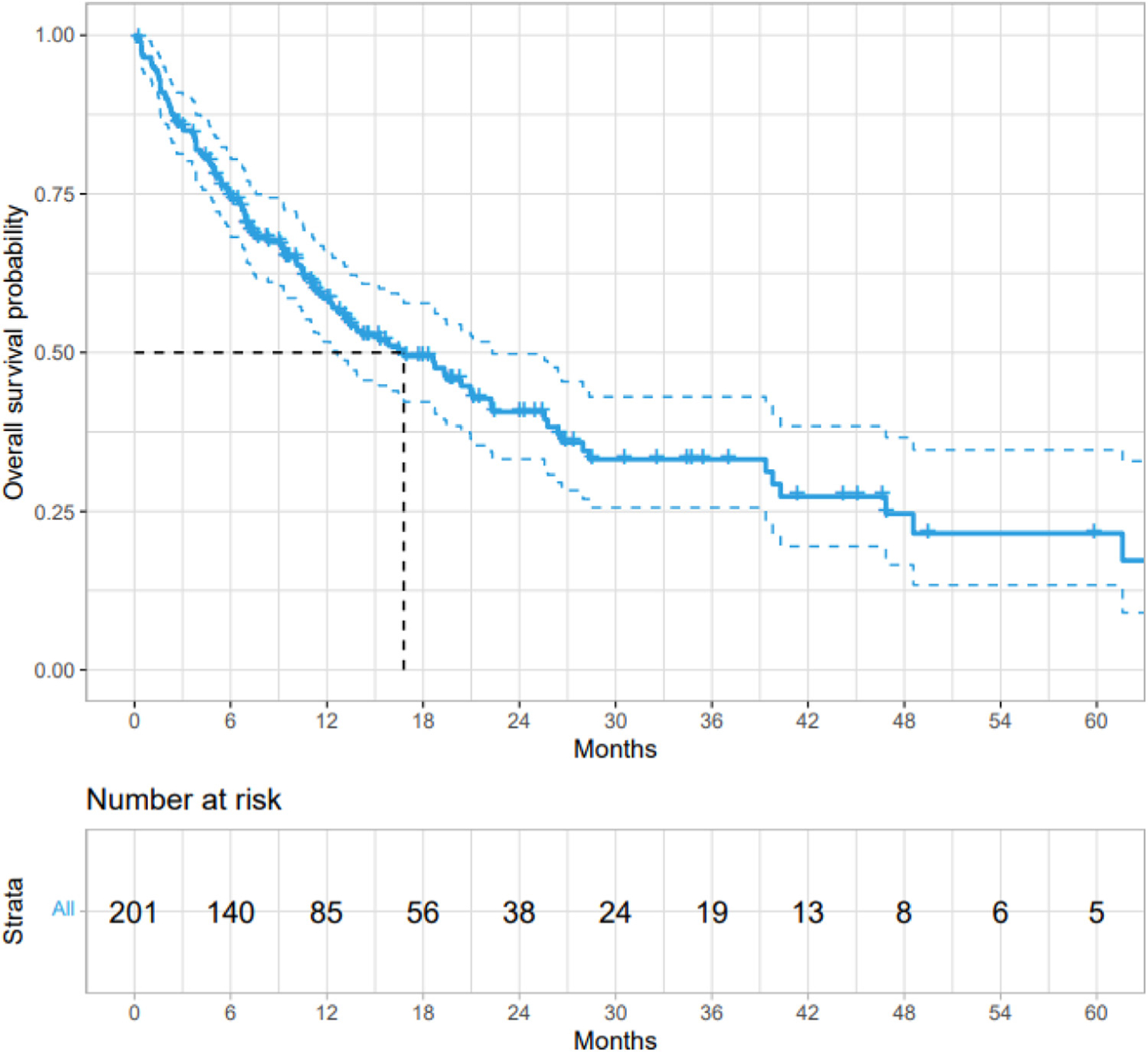
Overall survival on first-line systemic therapy for stage IV or recurrent, metastatic KRAS G12C mutant non–small cell lung cancer (n = 201).
Second-Line and Later Therapy Outcomes
Among patients with documented second-line systemic therapy (n = 123), the second-line median rwPFS was 8.3 months (95% CI, 6.1–11.9 months), and the median rwPFS among 10 patients treated with docetaxel with or without ramucirumab (9 received docetaxel and 1 received docetaxel plus ramucirumab) in the second-line was 4.6 months (95% CI, 1.4-NA). Treatment data was captured for 123 patients—in the second-line setting, 55 patients (45%) were treated with other regimens, 39 patients (31%) were treated with PD-(L)1 inhibitor monotherapy, 18 patients (15%) received platinum based chemotherapy without a PD-(L)1 inhibitor, and 11 patients (9%) were treated with platinum based chemotherapy plus a PD-(L)1 inhibitor. Among patients treated with PD-(L)1 inhibitor monotherapy in the second-line setting the median rwPFS was 9.9 months (95% CI, 3.4-NA), among patients treated with platinum based chemotherapy without a PD-(L)1 inhibitor the rwPFS was 8.9 months (95% CI, 6.6-NA), among patients treated with platinum based chemotherapy with a PD-(L)1 inhibitor the rwPFS was 8.0 months (95% CI, 6.1-NA), and among patients treated with other regimens the median rwPFS was 8.3 months (95% CI, 5.1–15.2 months). When second-line therapy outcomes were analyzed by recurrent, unresectable disease (n = 43) vs. stage IV disease at diagnosis (n = 80), rwPFS was similar (median rwPFS 7.7 months, 95% C,I 6.1–15.4 months in patients with recurrent, unresectable disease vs. 8.3 months, 95% CI, 5.5–16.5 months in patients with stage IV disease).
Comutations and Outcomes
Within the total study population, 49 patients (12%) had a co-occurring STK11 mutation and 3 (1%) had a co-occurring KEAP1 mutation. Among the 12% of patients with a co-occurring KRAS G12C and STK11 mutation, median rwPFS on first-line systemic therapy (n = 23) was 6.0 months (95% CI, 4.7-NA), and median OS was 14.0 months (95% CI, 10.8–35.3 months) (Supplemental Figure 2).
Discussion
This large, multicenter retrospective chart review from 13 academic sites in the United States and Canada of patients with KRAS G12C mutant NSCLC confirms similar retrospective data in this patient cohort, which has noted a first-line rwPFS of 4 to 10 months and median OS of 10 to 15 months.5–9 In the second-line setting where less robust real world data has been reported and prior retrospective reports have been from a single site, community sites (an important contribution to the literature), or international sites, focused on first line outcomes, specific patient subgroups, or the prognostic vs. predictive value of KRAS G12C compared to non-G12C genotypes,5–12 in this academic consortium dataset we observed a relatively short median rwPFS of 4.6 months among patients with KRAS G12C mutant NSCLC treated with docetaxel with or without ramucirumab. Similar to previous reports,5 patients with co-occurring KRAS G12C and STK11 mutations had inferior outcomes with first-line immune checkpoint inhibitor (in this historical dataset, approximately half of patients were treated with immune checkpoint inhibitors in the first line).
Limitations of the study include its retrospective nature, potential immortal time bias in the subgroup OS estimate for brain metastasis at any time, lower than expected frequency of brain metastases compared to other retrospective series in patients with KRAS G12C mutant NSCLC,10,13–15 and that patient numbers among those treated with docetaxel were limited as all patients were treated in academic medical centers and more often enrolled in clinical trials in the relapsed NSCLC setting. Furthermore, standard of care first line therapy changed throughout the treatment interval included in this retrospective series, resulting in just 18% of patients in this cohort receiving first line chemoimmunotherapy, limiting generalizability to the current treatment landscape.
Conclusion
In conclusion, our real-world data suggest a short rwPFS (less than 5 months) among patients with KRAS G12C mutant NSCLC treated with docetaxel containing regimens in the second line, which is congruent with recently reported results from CodeBreak 200 (a randomized trial comparing sotorasib vs. docetaxel in this patient population).16
Supplementary Material
Clinical Practice Points.
Patients with KRAS G12C mutant NSCLC experience a real-world progression-free survival (rwPFS) of approximately 9 months in the first-line and 4 to 5 months in the second-line setting when treated with docetaxel containing regimens.
Patients with KRAS G12C mutant NSCLC with a co-occurring STK11 mutation experienced inferior outcomes when treated with immune checkpoint inhibitors.
Acknowledgment
We thank all participating study sites for collaboration on this project. The authors thank Hil Hsu, PhD and Simon Jones, PhD (Amgen Inc, Thousand Oaks, CA), whose work was funded by Amgen Inc, for study design and methodology in the preparation of this manuscript. WTI was supported by a National Comprehensive Cancer Network Young Investigator award (YIA). This work was funded by Amgen Inc. Except for the role of Amgen personnel as outlined in the acknowledgments, Amgen Inc had no involvement in the study design, collection, analysis and interpretation of data, the writing of this report, or the decision to publish.
Footnotes
Disclosure
WTI reports serving as a consultant for Bristol Myers Squibb, Takeda, Janssen, Genentech, Jazz Pharma, G1 Therapeutics, Mirati, OncLive, Clinical Care Options, Chardan, Outcomes Insights, Cello Health, and Curio Science. AS reports consulting/research funding from Genentech, AstraZeneca, and honoraria/consulting from Amgen. CK has served as a consultant for Novartis, Janssen, Astrazeneca, Sanofi, PierianDx, Diffuse pharmaceuticals, Mirati, and Jazz Pharmaceuticals. RH is a consultant for Targeted Healthcare Communications. JN reports consulting for Aadi Biosciences, Astra Zeneca, Bristol Myers Squibb, Fujirebio, G1 Therapeutics, Genentech, Mindmed, Naveris, Takeda, Western Oncolytics, research support from Genentech and Merck, intellectual property from Cansera, and ownership interests with Cansera, Epic Sciences, Indee Bio, and Quantgene. DRC reports advisory roles / Ad hoc advisory boards/consultations 2022: Appolomics (SRC), AstraZeneca/Daiichi (ILD adjudication committee), Beigene (DSMB) Dizal, EMD Serono, Elevation, Hengrui, (DSMB), Hummingbird, Medtronic, Mersana (ILD adjudication committee), Mirati, Nalo Therapeutics, Onkure, Roche, Takeda; 2021: Abbvie, Amgen, Anheart, Apollomics (SRC), AstraZeneca (SRC/SC), Beigene (DSMC), Bio-Thera (DSMB), Blueprint, Daiichi-Sankyo (ILD adjudication committee), Elevation (SRC), Eli Lilly (DSMB and NCCN), EMD Serono, Helsinn (DSMB), Hengrui (DSMC), Janssen, Kestrel (SAB, Shares), Mersana, Nuvalent (SAB), Puma (NCCN), Ribon, Roche/Genentech, Sanofi, Seattle Genetics, Takeda, Turning Point; 2020: Amgen, Anchiarno (SAB), Apollomics (SRC), AstraZeneca, Bio-Thera (DSMB), BMS, Daiichi-Sankyo (ILD adjudication committee), Eisai, EMD Serono, Elevation (SRC), Eli Lilly, GSK, Helssin, Janssen, Onkure, Mersana, Pfizer, Qilu, Roche, Sanofi, Seattle Genetics, Takeda. MB, SP, CB, VV, MM, TL, PES, AR, KM, NS, XW, and TP report no conflicts of interest.
Data Sharing
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cllc.2023.01.009.
References
- 1.Roberts PJ, Stinchcombe TE. KRAS mutation: should we test for it, and does it matter? J Clin Oncol 2013;31(8):1112–1121. doi: 10.1200/jco.2012.43.0454. [DOI] [PubMed] [Google Scholar]
- 2.Dearden S, Stevens J, Wu YL, Blowers D. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann Oncol 2013;24(9):2371–2376. doi: 10.1093/annonc/mdt205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dogan S, Shen R, Ang DC, et al. Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin Cancer Res 2012;18(22):6169–6177. doi: 10.1158/1078-0432.ccr-11-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skoulidis F, Li BT, Dy GK, et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N Engl J Med 2021;384(25):2371–2381. doi: 10.1056/NEJMoa2103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arbour KC, Rizvi H, Plodkowski AJ, et al. Treatment Outcomes and Clinical Characteristics of Patients with KRAS-G12C-Mutant Non-Small Cell Lung Cancer. Clin Cancer Res 2021;27(8):2209–2215. doi: 10.1158/1078-0432.ccr-20-4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kartolo A, Feilotter H, Hopman W, Fung AS, Robinson A. A single institution study evaluating outcomes of PD-L1 high KRAS-mutant advanced non-small cell lung cancer (NSCLC) patients treated with first line immune checkpoint inhibitors. Cancer Treat Res Commun 2021;27. doi: 10.1016/j.ctarc.2021.100330. [DOI] [PubMed] [Google Scholar]
- 7.Pavan A, Bragadin AB, Calvetti L, et al. Role of next generation sequencing-based liquid biopsy in advanced non-small cell lung cancer patients treated with immune checkpoint inhibitors: impact of STK11, KRAS and TP53 mutations and co-mutations on outcome. Transl Lung Cancer Res 2021;10(1):202–220. doi: 10.21037/tlcr-20-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sebastian M, Eberhardt WEE, Hoffknecht P, et al. KRAS G12C-mutated advanced non-small cell lung cancer: a real-world cohort from the German prospective, observational, nation-wide CRISP Registry (AIO-TRK-0315). Lung Cancer 2021;154:51–61. doi: 10.1016/j.lungcan.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Spira AI, Tu H, Aggarwal S, et al. A retrospective observational study of the natural history of advanced non-small-cell lung cancer in patients with KRAS p.G12C mutated or wild-type disease. Lung Cancer 2021;159:1–9. doi: 10.1016/j.lungcan.2021.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Cui W, Franchini F, Alexander M, et al. Real world outcomes in KRAS G12C mutation positive non-small cell lung cancer. Lung Cancer 2020;146:310–317. doi: 10.1016/j.lungcan.2020.06.030. [DOI] [PubMed] [Google Scholar]
- 11.Illini O, Fabikan H, Hochmair MJ, et al. Characteristics and treatment outcomes in advanced-stage non-small cell lung cancer patients with a KRAS G12C mutation: a real-world study. J Clin Med 2022;11(14):4098. doi: 10.3390/jcm11144098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H, Huang D, Lin G, et al. The prevalence and real-world therapeutic analysis of chinese patients with KRAS-mutant non-small cell lung cancer. Cancer Med 2022;11(19):3581–3592. doi: 10.1002/cam4.4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vassella E, Kashani E, Zens P, et al. Mutational profiles of primary pulmonary adenocarcinoma and paired brain metastases disclose the importance of KRAS mutations. Eur J Cancer 2021;159:227–236. doi: 10.1016/j.ejca.2021.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Wu MY, Zhang EW, Strickland MR, et al. Clinical and imaging features of non-small cell lung cancer with G12C KRAS mutation. Cancers (Basel) 2021;13(14):3572. doi: 10.3390/cancers13143572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tao L, Miao R, Mekhail T, et al. Prognostic value of KRAS mutation subtypes and PD-L1 expression in patients with lung adenocarcinoma. Clin Lung Cancer 2021;22(4):e506–e511. doi: 10.1016/j.cllc.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Johnson ML dLA, Waterhouse D, et al. Sotorasib versus docetaxel for previously treated non-small cell lung cancer with KRAS G12C mutation: CodeBreak 200 phase 3 study. ESMO 2022;2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


