Abstract
Objective
PsA is a chronic disease with heterogeneous clinical manifestations requiring treatment options with long-term efficacy and safety. In this follow-up analysis, the 52-week efficacy and safety of risankizumab 150 mg in patients with active PsA who had previous inadequate response/intolerance to one or more conventional synthetic DMARDs (csDMARD-IR) were evaluated.
Methods
KEEPsAKE 1 is an ongoing, global, phase 3 study with a 24-week, double-blind, placebo-controlled period (period 1) and an open-label extension period (period 2). In period 1, eligible patients were randomized 1:1 to receive subcutaneous risankizumab 150 mg or placebo at weeks 0, 4 and 16. At week 24 (period 2), all continuing patients received open-label risankizumab 150 mg every 12 weeks through week 208.
Results
At week 24, 57.3% of risankizumab-treated patients (n = 483) achieved ≥20% improvement in ACR criteria (ACR20) vs 33.5% of placebo-treated patients (n = 481; P < 0.001). At week 52, 70.0% of patients who were randomized to receive continuous risankizumab therapy and 63.0% of patients who were randomized to receive placebo in period 1 and then receive risankizumab at week 24 achieved ACR20. Similar result trends were observed for other efficacy measures. Risankizumab was well tolerated through 52 weeks of treatment with a consistent safety profile from week 24 through week 52.
Conclusion
In patients with active PsA who were csDMARD-IR, continuous risankizumab treatment demonstrated robust long-term efficacy and was well tolerated through 52 weeks of treatment.
Trial registration
ClinicalTrials.gov, http://clinicaltrials.gov, KEEPsAKE1, NCT03675308.
Keywords: biologic agent, DMARD, IL-23, PsA, risankizumab
Rheumatology key messages.
Risankizumab demonstrated robust and durable efficacy across clinical domains of PsA in patients who were csDMARD-IR.
Risankizumab was generally well tolerated, with a safety profile consistent with psoriasis studies with risankizumab treatment.
The benefits of risankizumab support its use in patients with PsA who were csDMARD-IR.
Introduction
PsA is a chronic, systemic, inflammatory musculoskeletal disease accompanied by psoriatic manifestations that can lead to permanent joint damage, disability [1] and reduced health-related quality of life (HRQoL) [2]. Furthermore, the incidence of PsA is rising in certain populations [3] and is associated with a health inequity compared with the general population. The burden of disease correlates with lower income, increased cost of care and higher risk of developing comorbidities [4].
The clinical manifestations of PsA are multifaceted and can include skin and/or nail psoriasis, arthritis, dactylitis, enthesitis and spondylitis [5]. Because of the heterogeneity in the signs and symptoms of PsA, selecting optimal treatments for individual patients can be challenging [5]. Nonsteroidal anti-inflammatory drugs and local glucocorticoid injections are recommended as initial therapies followed by conventional synthetic DMARD (csDMARD) if initial therapy is ineffective [6]. If treatment goals are still not achieved, therapies targeting TNF, IL-17A, IL-23, IL-12/23, Janus kinase or phosphodiesterase-4 signalling pathways are strongly recommended by the 2021 Group for Research and Assessment of Psoriasis and Psoriatic Arthritis treatment guidelines [7]. Despite the range of available treatment options for PsA, appropriate treatment goals like PsA minimal disease activity (MDA) are only achieved by about one-third of patients [8]; thus, efficacious and well tolerated long-term treatment options are needed to address the range of rheumatologic and dermatologic signs and symptoms of disease for patients who respond inadequately or are intolerant to current standard treatments.
Risankizumab is a humanized immunoglobulin G1 monoclonal antibody that specifically inhibits IL-23 by binding to its p19 subunit and is approved in multiple countries to treat active PsA [9]. KEEPsAKE 1 (NCT03675308) is an ongoing, global, phase 3 multicentre study evaluating the efficacy and safety of risankizumab to treat active PsA in patients who have had inadequate response or intolerance to csDMARD therapy. KEEPsAKE 1 was conducted in parallel with KEEPsAKE 2, which included patients with inadequate response or intolerance to one or two biologic therapies and/or one or more csDMARDs [10]. In the 24-week published results of KEEPsAKE 1, treatment with risankizumab 150 mg at weeks 0, 4 and 16 demonstrated markedly improved signs and symptoms of PsA vs placebo and was well tolerated in patients [11]. Here we report the long-term efficacy, safety and tolerability of risankizumab through 52 weeks of treatment in KEEPsAKE 1.
Methods
A detailed description of the study design and patient population has been previously reported [11]. Briefly, KEEPsAKE 1 is an ongoing, global, phase 3 study that included a 24-week double-blind, placebo-controlled, parallel-group treatment period (period 1) and an open-label extension treatment period from week 24 through week 208 (period 2). In period 1, patients were randomized 1:1 to receive subcutaneous administration of risankizumab 150 mg or placebo at weeks 0, 4 and 16. Period 2 started at week 24, and to maintain blinding of treatment in period 1, patients who were randomized to risankizumab received blinded placebo at week 24, and patients who were randomized to placebo received the first dose of blinded risankizumab at week 24. At week 28, all patients received open-label risankizumab 150 mg every 12 weeks through week 208. Starting at week 36, patients classified as non-responders [those with <20% improvement in tender or swollen joint count on two consecutive visits compared with baseline (a pre-specified protocol withdrawal criterion)], were discontinued from the study drug. This clinical study was conducted in accordance with the operations manual, protocol, International Council for Harmonisation guidelines and applicable guidelines and regulations governing ethical principles and study conduct originating in the Declaration of Helsinki.
Eligible patients were at least 18 years old with a diagnosis of active PsA [symptom onset ≥6 months, meeting the Classification Criteria for Psoriatic Arthritis (CASPAR), five or more tender joints based on 68 joint counts and five or more swollen joints based on 66 joint counts, one or more erosions observed on radiograph and confirmed by central reading or high-sensitivity C-reactive protein level ≥3.0 mg/l, active plaque psoriasis with one or more psoriatic plaques 2 cm or larger in diameter or nail psoriasis]. Patients must have demonstrated an inadequate response, intolerance or contraindication to one or more csDMARDs (defined as csDMARD-IR) (see Supplementary Table S1 for additional eligibility criteria, available at Rheumatology online).
Assessments
Efficacy
Efficacy assessments included the proportion of patients who achieved ≥20% improvement in ACR criteria (ACR20), ACR50 and ACR70. This study also assessed change from baseline in HAQ-Disability Index (HAQ-DI), proportion of patients who achieved ≥90% reduction in Psoriasis Area Severity Index (PASI 90), change from baseline in modified Nail Psoriasis Severity Index (mNAPSI) score [12], change from baseline in Physician Global Assessment of Fingernail Psoriasis (PGA-F) score [13], proportion of patients who achieved resolution of enthesitis [Leeds Enthesitis Index (LEI) = 0], proportion of patients who achieved resolution of dactylitis [Leeds Dactylitis Index (LDI) = 0], proportion of patients who achieved MDA, change from baseline in 36-Item Short Form Health Survey Physical Component Summary score (SF-36 PCS), change from baseline in Functional Assessment of Chronic Illness Therapy-Fatigue Questionnaire score (FACIT-Fatigue), change from baseline in PsA modified Total Sharp Score (PsA-mTSS) [14], proportion of patients with PsA-mTSS ≤0 and proportion of patients with PsA-mTSS ≤0.5. All efficacy outcomes were assessed at weeks 24 and 52.
Safety
Safety was evaluated throughout the study; assessments included monitoring adverse events, performing physical examinations, obtaining vital sign measurements and analysing data from laboratory tests. Safety results, reported for all patients who received any dose of risankizumab 150 mg, are described at week 24 and at the long-term data cut-off date (defined as safety data reported through the 19 April 2021 cut-off date, which includes data through week 52). Laboratory abnormalities were classified by National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 grade.
Statistical analysis
All efficacy analyses were conducted in the full analysis set (all patients who received one dose or more of risankizumab). Continuous efficacy assessments were analysed using mixed-effect model for repeated measures. Up to week 24, observations after patients initiated rescue therapy or concomitant medications for PsA that could have meaningfully impacted continuous efficacy assessments were considered as missing and excluded from the model. After week 24, the mixed-effect model for repeated measures, including as-observed measurements, was used to assess continuous variables. Non-responder imputation incorporating multiple imputation to handle missing data due to COVID-19 (NRI-C) was used to assess binary variables up to week 24 where (i) missing data due to COVID-19 were handled by multiple imputation and (ii) patients with data missing due to reasons other than COVID-19 or after intercurrent events (i.e. initiation of rescue medication or concomitant medications that could meaningfully impact efficacy assessment) were imputed as non-responders. NRI (as observed with imputation), which imputed patients with missing values as non-responders, was used to assess binary variables from week 24 to week 52; as-observed data are also reported. Radiographic endpoints were analysed using an analysis of covariance model incorporating linear extrapolation to impute missing data or data after discontinuation of study drug or initiation of rescue medication. Treatment-emergent adverse events (TEAEs), defined as an adverse event with an onset date on or after the first dose of risankizumab and up to 140 days after the last dose if the patient discontinued the study drug prematurely were summarized using exposure-adjusted event rates [events (E)/100 patient-years (PYs)]. Rates of elevated liver function tests were summarized using exposure-adjusted incidence rates.
Results
Patients
Baseline characteristics and demographics were generally well balanced between groups [11]. Of the 964 patients randomized into period 1 of the study, 940 (97.5%) continued into period 2 and were eligible to receive open-label risankizumab (randomized to receive continuous risankizumab 150 mg, n = 473; placebo to risankizumab 150 mg, n = 467). The most frequent reasons for study discontinuation were <20% improvement in tender/swollen joint count compared with tender/swollen joint count assessed at baseline (a pre-specified protocol withdrawal criterion starting at week 36), withdrawn consent and lack of efficacy (Supplementary Fig. S1, available at Rheumatology online).
Efficacy
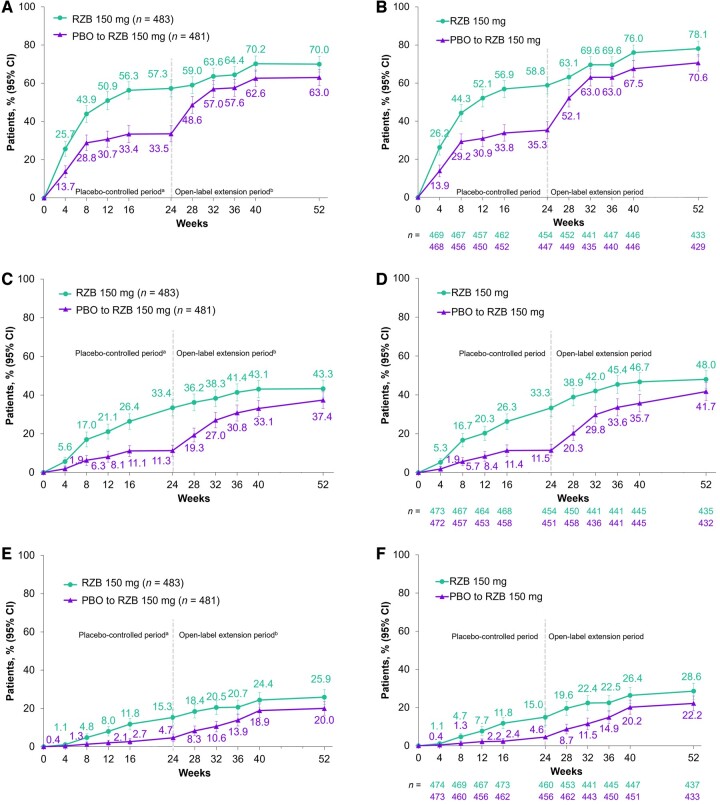
As previously reported [11], at week 24 (period 1), significantly greater proportions of patients receiving risankizumab achieved the primary end point of ACR20 compared with patients receiving placebo (NRI-C, 57.3% vs 33.5%; P < 0.001; Table 1 and Fig. 1A). In patients randomized to receive continuous risankizumab treatment (period 1 and 2), the proportion of patients achieving ACR20 response increased from week 24 (57.3%) to week 52 (70.0%, Fig. 1A). Similarly, for ACR50 and ACR70, there were significant differences between risankizumab and placebo at week 24 (P < 0.001; Table 1). In patients randomized to receive continuous risankizumab treatment, the ACR50 response rate increased from 33.4% at week 24 to 43.3% at week 52, and the ACR70 response rate increased from 15.3% to 25.9% (Fig. 1C and 1E). The observed ACR20/50/70 responses over time are shown in Fig. 1B, D and F.
Table 1.
Efficacy results
| Parameter | Week 24 (Period 1)a |
Week 52 (Period 1 and 2) |
||
|---|---|---|---|---|
| RZB 150 mg | PBO | RZB 150 mg | PBO to RZB 150 mg | |
| n = 483 | n = 481 | n = 483 | n = 481 | |
| ACR20, n (%) (95% CI) | 277 (57.3)***,† | 161 (33.5) | 338 (70.0) | 303 (63.0) |
| (52.9, 61.8) | (29.3, 37.8) | (65.9, 74.1) | (58.7, 67.3) | |
| ACR50, n (%) (95% CI) | 162 (33.4)*** | 54 (11.3) | 209 (43.3) | 180 (37.4) |
| (29.2, 37.7) | (8.4, 14.1) | (38.9, 47.7) | (33.1, 41.7) | |
| ACR70, n (%) (95% CI) | 74 (15.3)*** | 23 (4.7) | 125 (25.9) | 96 (20.0) |
| (12.0, 18.5) | (2.8, 6.7) | (22.0, 29.8) | (16.4, 23.5) | |
| Change in HAQ-DI, mean (95% CI) | −0.31***,† | −0.11 | −0.41 | −0.32 |
| (−0.36, −0.27) | (−0.16, −0.06) | (−0.45, −0.37) | (−0.36, −0.27) | |
| ≥0.35 change in HAQ-DI,bn/n (%) (95% CI) | 208/414 (50.3)*** | 117/419 (27.9) | 238/414 (57.5) | 191/419 (45.6) |
| (45.5, 55.1) | (23.5, 32.2) | (52.7, 62.2) | (40.8, 50.4) | |
| PASI 90,cn/n (%) (95% CI) | 143/273 (52.3)***,† | 27/272 (9.9) | 185/273 (67.8) | 163/272 (59.9) |
| (46.4, 58.3) | (6.4, 13.5) | (62.2, 73.3) | (54.1, 65.8) | |
| Change in mNAPSI,d mean (95% CI) | −9.8***,† | −5.6 | −12.9 | −11.3 |
| (−11.0, −8.6) | (−6.7, −4.4) | (−13.9, −11.9) | (−12.2, −10.3) | |
| Change in PGA-F,d mean (95% CI) | −0.8***,† | −0.4 | −1.2 | −1.1 |
| (−1.0, −0.7) | (−0.5, −0.3) | (−1.3, −1.1) | (−1.2, −1.0) | |
| Resolution of enthesitis,en/n (%) (95% CI) | ||||
| KEEPsAKE 1 | 152/297 (51.2)*** | 108/290 (37.2) | 180/297 (60.6) | 174/290 (60.0) |
| (45.5, 56.9) | (31.7, 42.8) | (55.0, 66.2) | (54.4, 65.6) | |
| Pooledf | 215/444 (48.4)***,† | 156/448 (34.8) | 244/444 (55.0) | 257/448 (57.4) |
| (43.8, 53.1) | (30.4, 39.2) | (50.3, 59.6) | (52.8, 61.9) | |
| Resolution of dactylitis,gn/n (%) (95% CI) | ||||
| KEEPsAKE 1 | 99/148 (66.9)* | 80/147 (54.4) | 116/148 (78.4) | 108/147 (73.5) |
| (59.3, 74.5) | (46.4, 62.5) | (71.7, 85.0) | (66.3, 80.6) | |
| Pooledf | 128/188 (68.1)***,† | 104/204 (51.0) | 143/188 (76.1) | 148/204 (72.5) |
| (61.4, 74.7) | (44.1, 57.8) | (70.0, 82.2) | (66.4, 78.7) | |
| MDA achievement, n (%) (95% CI) | 121/483 (25.0)***,† | 49/481 (10.2) | 183 (37.9) | 132 (27.4) |
| (21.2, 28.9) | (7.5, 12.9) | (33.6, 42.2) | (23.5, 31.4) | |
| Change in SF-36 PCS, mean (95% CI) | 6.5*** | 3.2 | 8.4 | 7.3 |
| (5.8, 7.2) | (2.5, 3.9) | (7.8, 9.1) | (6.7, 8.0) | |
| Change in FACIT-Fatigue, mean (95% CI) | 6.5*** | 3.9 | 7.9 | 6.5 |
| (5.6, 7.3) | (3.1, 4.7) | (7.2, 8.7) | (5.7, 7.3) | |
| Change in PsA-mTSS,h mean (95% CI) | 0.18 | 0.26 | 0.36 | 0.56 |
| (0.05, 0.31) | (0.13, 0.39) | (0.10, 0.63) | (0.30, 0.83) | |
| PsA-mTSS ≤0,hn/n (%) (95% CI) | 402/429 (93.7)* | 384/430 (89.3) | 400/435 (92.0) | 384/430 (89.3) |
| (91.4, 96.0) | (86.4, 92.2) | (89.4, 94.5) | (86.4, 92.2) | |
| PsA-mTSS ≤0.5,hn/n (%) (95% CI) | 409/429 (95.3)** | 391/430 (90.9) | 407/435 (93.6) | 384/430 (89.3) |
| (93.3, 97.3) | (88.2, 93.6) | (91.3, 95.9) | (86.4, 92.2) | |
All changes are least squares mean changes from baseline.
Results based on full analysis set, and NRI-C (week 24) or NRI (as observed with imputation) (week 52) was used for binary endpoints; MMRM was used for continuous endpoints.
Nominal P < 0.001;
nominal P < 0.01;
nominal P < 0.05;
statistically significant under overall type I error control.
Data previously reported, except for the mTSS endpoints [11].
In patients with baseline HAQ-DI ≥0.35.
Among patients with ≥3% body surface area affected by psoriasis.
Among patients with nail psoriasis at baseline (RZB, n = 309; PBO, n = 338).
Defined as Leeds Enthesitis Index = 0 among patients with enthesitis or dactylitis at baseline.
Pooled from KEEPsAKE 1 and KEEPsAKE 2 as a prespecified analysis.
Defined as Leeds Dactylitis Index = 0 among patients with enthesitis or dactylitis at baseline.
Results for PsA-mTSS were recorded from the second reading session and assessed using linear extrapolation. For the PBO to RZB group, all data at week 52 were imputed by linear extrapolation; for patients who switched from PBO to RZB at week 24, the week 24 X-ray was used for extrapolation to impute the data at week 52.
ACR20/50/70: ≥20/50/70% improvement in ACR score; FACIT-Fatigue: Functional Assessment of Chronic Illness Therapy-Fatigue; HAQ-DI: HAQ-Disability Index; MDA: minimal disease activity; MMRM: mixed-effect model for repeated measures; mNAPSI: modified Nail Psoriasis Severity Index; NRI-C: non-responder imputation incorporating multiple imputation to handle missing data due to COVID-19; PASI 90: ≥90% reduction in Psoriasis Area Severity Index; PBO: placebo; PGA-F: Physician Global Assessment of Fingernail Psoriasis; PsA-mTSS: PsA modified Total Sharp Score; RZB: risankizumab; SF-36 PCS: 36 Item Short Form Health Survey Physical Component Summary.
Figure 1.
ACR responses over time. (A) ACR20 (NRI-C/NRI), (B) ACR20 (AO), (C) ACR50 (NRI-C/NRI), (D) ACR50 (AO), (E) ACR70 (NRI-C/NRI) and (F) ACR70 (AO) response rates over the 24-week, double-blind treatment period and open-label extension treatment period from weeks 24 through 52. aBased on full analysis set, NRI-C was used. bBased on full analysis set, NRI (as observed with imputation) was used. Error bars represent the 95% CI. ACR20/50/70: ≥20/50/70% improvement in American College of Rheumatology score; AO: as observed; NRI: non-responder imputation; NRI-C: non-responder imputation incorporating multiple imputation to handle missing data due to COVID-19; PBO: placebo; RZB: risankizumab
Along with increases in ACR response, patients experienced improvements in other signs and symptoms of PsA during risankizumab treatment through week 52. Results for the rates of resolution of enthesitis and resolution of dactylitis among patients with enthesitis or dactylitis at baseline were pooled from the KEEPsAKE 1 and KEEPsAKE 2 studies to align with the prespecified pooled analysis of these endpoints at week 24 [11]. At week 52, in patients who were randomized to receive continuous risankizumab treatment, the proportions of patients with resolution of enthesitis and resolution of dactylitis were 55.0% and 76.1%, respectively, demonstrating maintenance from week 24. Rates for resolution of enthesitis or dactylitis in the KEEPsAKE 1-only population were consistent with the pooled analyses (Table 1).
Differences in radiographic progression, as assessed by change from baseline in PsA-mTSS, among patients treated with risankizumab compared with patients receiving placebo were not significant at week 24 (P = 0.496). At week 24, from the second radiographic reading session, 93.7% of patients receiving risankizumab had no radiographic progression (defined as change from baseline in PsA-mTSS ≤0) vs 89.3% of patients receiving placebo (P = 0.018). Responses were maintained at week 52 in patients receiving continuous risankizumab treatment with 92.0% having no radiographic progression. Similar results were observed for no radiographic progression defined as change in PsA-mTSS ≤0.5 (Table 1).
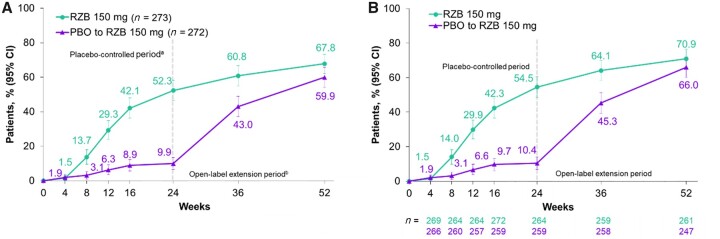
Among patients with psoriasis at baseline (≥3% body surface area affected) who were randomized to receive continuous risankizumab treatment, there was an increase in the percentage of patients achieving PASI 90 response from weeks 24–52, and by week 52, 67.8% of patients achieved PASI 90 (Table 1 and Fig. 2A). The as-observed PASI 90 response rates over time are shown in Fig. 2B.
Figure 2.
PASI 90 response over time. (A) PASI 90 (NRI-C/NRI) and (B) PASI 90 (AO) response rates over the 24-week, double-blind treatment period and the open-label extension treatment period from week 24 through 52. Among patients with ≥3% body surface area affected by psoriasis at baseline. aBased on full analysis set, NRI-C was used. bBased on full analysis set, NRI (as observed with imputation) was used. NRI: non-responder imputation; NRI-C: non-responder imputation incorporating multiple imputation to handle missing data due to COVID-19; PASI 90: ≥90% reduction in Psoriasis Area and Severity Index; PBO: placebo; RZB: risankizumab
For patients with nail psoriasis at baseline, improvements in nail outcomes, as measured by mNAPSI and PGA-F, were also observed from week 24 to week 52 with risankizumab treatment (mixed-effect model for repeated measures, Table 1; as-observed values are shown in Supplementary Table S2, available at Rheumatology online). At week 52, the least squares mean (95% CI) change from baseline was −12.86 (−13.85, −11.87) for mNAPSI and −1.2 (−1.3, −1.1) for PGA-F (Table 1). At week 52, 58.0% (n = 105/181) of patients who were randomized to receive continuous risankizumab treatment achieved a PGA-F score of ‘clear’ or ‘minimal’ with grade 2 or higher improvement (Supplementary Table S2, available at Rheumatology online).
Patients treated with risankizumab also reported additional improvement in physical function from week 24 (Table 1). At week 52, the least squares mean (95% CI) change from baseline in HAQ-DI was −0.41 (−0.45, −0.37), and a clinically meaningful improvement in HAQ-DI (defined as improvement of ≥0.35 among patients with a baseline HAQ-DI ≥0.35) was reported by 57.5% of patients randomized to receive continuous risankizumab treatment, an increase from 50.3% of patients at week 24. Similarly, patients treated with risankizumab also reported numerical improvement in HRQoL and fatigue, as assessed by SF-36 PCS and FACIT-Fatigue. At week 52, the least squares mean (95% CI) change from baseline was 8.43 (7.79, 9.08) for SF-36 PCS and 7.9 (7.2, 8.7) for FACIT-Fatigue.
Among patients randomized to receive continuous risankizumab, the proportion of patients achieving MDA increased from 25.0% at week 24 to 37.9% at week 52 (Table 1).
Improvements in efficacy from week 24 to week 52 were also observed among patients who were initially randomized to receive placebo in period 1 and then received risankizumab in period 2. In this group, the proportion of patients achieving ACR20 increased from 33.5% at week 24 to 63.0% at week 52 (Table 1); the proportions of patients achieving ACR50 and ACR70 also increased from weeks 24–52. The proportion of patients achieving resolution of enthesitis or resolution of dactylitis increased from week 24 through week 52 in both the pooled and KEEPsAKE 1 only analyses (Table 1); numerically greater changes from baseline in LEI and LDI were also observed (Supplementary Table S3, available at Rheumatology online). Improvements from weeks 24–52 were also observed for skin and nail outcomes, as measured by PASI 90, mNAPSI and PGA-F, and for patient-reported outcomes, including HAQ-DI, SF-36 PCS and FACIT-Fatigue (Table 1). By week 52, 27.4% of patients randomized to receive placebo and switch to risankizumab at week 24 had achieved MDA.
Safety
The exposure-adjusted event rate for any TEAEs in patients receiving risankizumab was 177.6 E/100 PYs at week 24 and 143.1 E/100 PYs at the long-term safety cut-off date. TEAEs leading to study drug discontinuation were 2.7 E/100 PYs at week 24 and 2.3 E/100 PYs at the long-term safety cut-off date (Table 2).
Table 2.
Summary of safety during risankizumab treatment
| Events (E/100 PYs) | Week 24 |
Long-terma |
|---|---|---|
| RZB 150 mg | Any RZB 150 mg | |
| n = 483 | n = 946 | |
| PYs = 224.1 | PYs = 958.1 | |
| Any TEAE | 398 (177.6) | 1371 (143.1) |
| Serious TEAE | 15 (6.7) | 71 (7.4) |
| TEAE leading to discontinuation of study drug | 6 (2.7) | 22 (2.3) |
| COVID-19 related TEAE | 1 (0.4) | 61 (6.4) |
| MACE | 0 | 0 |
| Serious infections | 6 (2.7) | 27 (2.8) |
| Opportunistic infections excluding TB and herpes zoster | 0 | 1 (0.1) |
| Active TB | 0 | 0 |
| Herpes zoster | 2 (0.9) | 4 (0.4) |
| Malignant tumors | ||
| NMSC | 0 | 6 (0.6) |
| Other | 0 | 4 (0.4) |
| All deathsb | 1 (0.4) | 2 (0.2) |
Safety reported through data cut-off date (19 April 2021), which includes data though week 52 and all patients who received any RZB 150 mg, including those who started on RZB 150 mg at randomization and who switched from placebo to RZB 150 mg after week 24.
An 81-year-old male patient randomized to RZB on day 1 died of urosepsis on day 96, and a 41-year-old male patient randomized to RZB on day 1 experienced sudden death on day 502.
TEAEs were defined as an adverse event with an onset date that is on or after the first dose of RZB and up to 140 days after the last dose of RZB if patient discontinued study drug prematurely.
E: events; MACE: major adverse cardiovascular events; NMSC: non-melanoma skin cancer; PYs: patient-years; RZB: risankizumab; TB: tuberculosis; TEAE: treatment-emergent adverse events.
KEEPsAKE 1 is being conducted during the COVID-19 pandemic, with period 2 of the study coinciding with the peak of the pandemic. COVID-19 related TEAEs increased from 0.4 E/100 PYs at week 24 to 6.4 E/100 PYs at the long-term cut-off date. The small increases in rates observed from week 24 to the long-term cut-off date included serious TEAEs (6.7 E/100 PYs to 7.4 E/100 PYs) and serious infections (2.7 E/100 PYs to 2.8 E/100 PYs) that were due to COVID-19 related adverse events. Of the 27 serious infections reported in the long-term dataset, 10 (37.0%) were cases of COVID-19, whereas in the week 24 data set, none of the six serious infections reported were related to COVID-19. As of the long-term cut-off date, no events of active tuberculosis, four events (0.4 E/100 PY) of herpes zoster and one event of oropharyngeal candidiasis were reported. No major adverse cardiovascular events were observed. Frequently reported TEAEs in the long-term data set (≥4 E/100 PYs) included COVID-19 (5.1 E/100 PYs), nasopharyngitis (4.8 E/100 PYs), upper respiratory tract infection (4.3 E/100 PYs) and increased alanine aminotransferase (4.0 E/100 PYs). Through the data cut-off date, exposure-adjusted incidence rates (n/100 PYs) of CTCAE grade 3 or higher [>5 times the upper limit of normal (× ULN)] elevations in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were 1.4 and 1.2, respectively, for any patient receiving risankizumab. No CTCAE grade 4 elevations in ALT, AST or bilirubin levels were observed, and there were no elevated liver function test findings that met the criteria for biochemical Hy’s Law (total bilirubin >2 × ULN and ALT or AST ≥3 × ULN; Supplementary Table S4, available at Rheumatology online). Three patients discontinued from the study drug due to transaminase levels that met the protocol-defined withdrawal criteria of ALT or AST >8 × ULN or ALT or AST >5 × ULN for >2 weeks (one patient with medical history of hepatic steatosis, one patient who initiated isoniazid for latent tuberculosis and one patient who initiated fenofibrate for hyperlipoproteinemia); all three conditions were assessed by the investigators as having no reasonable possibility of being related to the study drug.
As of the long-term data cut-off date, two deaths were reported. One newly reported death not related to the study drug was reported after week 24, a 41-year-old male patient randomized to risankizumab on day 1 with relevant medical history of tachycardia, hypertension and fluid retention, experienced serious adverse events of depression (day 85), septicemia (onset day 469 with resolution on day 488) and sudden death on day 502. Additional details surrounding the death are not available.
Discussion
These results through 52 weeks of treatment in the ongoing KEEPsAKE 1 study demonstrate the robust and durable long-term efficacy and safety of risankizumab in patients who were csDMARD-IR. Continuous treatment with risankizumab markedly improved a wide range of signs and symptoms of PsA (including joint, skin and nails) as well as improvement in HRQoL, with efficacy responses maintained through 52 weeks of treatment. Notably, there were improvements observed in these outcomes from week 24 to week 52 in patients who were randomized to receive continuous risankizumab treatment. As expected, patients who switched to risankizumab at week 24 after initial randomization to placebo also experienced improvements in efficacy from weeks 24–52. Significant improvement in joint symptoms, as measured by ACR20/50/70, was observed in patients receiving risankizumab treatment at week 24 with efficacy continuing through 52 weeks of risankizumab treatment. The rapid increase in ACR20 response in patients switching to risankizumab from placebo from week 24 (first risankizumab dose) to week 28 confirms that a single dose of risankizumab 150 mg can lead to improved joint signs and symptoms within 4 weeks.
Enthesitis and dactylitis are associated with increased peripheral and axial joint pain and disease burden [15]. In this study, numerical improvements in the proportion of patients achieving resolution of enthesitis and dactylitis were observed from week 24 to week 52. While comparisons of efficacy between risankizumab and other therapies are limited due to differences in study designs and a lack of head-to-head studies [16, 17], the improvements in enthesitis and dactylitis observed in this study were generally similar to those reported for other therapies targeting TNF-α [18, 19], IL-17 [19] and IL-23 [20] after 52 weeks of treatment.
Long-term risankizumab treatment was associated with limited radiographic progression. At week 52, the mean change in PsA-mTSS from baseline in patients receiving continuous risankizumab treatment was small and similar to that observed at week 24. Furthermore, the proportion of patients with no radiographic progression (defined as PsA-mTSS ≤0) remained stable relative to week 24, with 92.0% having no radiographic progression at week 52. Nail psoriasis, which is associated with impaired dexterity, risk of infection and decreased quality of life [21], also substantially improved with risankizumab treatment with durable efficacy through week 52. Furthermore, risankizumab treatment led to improvements in the SF-36 PCS and FACIT-Fatigue assessments with amelioration in HRQoL continuing through 52 weeks. These findings suggest that risankizumab may help mitigate patients’ disease burden with durable efficacy.
Risankizumab was generally well tolerated through the data cut-off date. The long-term safety profile was stable relative to week 24 and is consistent with safety profiles from studies of patients with psoriasis [22, 23]. No new safety signals were identified, and two deaths observed as of the data cut-off date were assessed to be unrelated to the study drug. Despite the number of COVID-19 related serious infections, the overall serious infection rate remained stable with the rate observed at week 24.
There were no cases of active tuberculosis or inflammatory bowel disease, and rates of herpes zoster and other opportunistic infections were low and stable from week 24. Malignancy rates in the long-term data set were low; non-melanoma skin cancer was the most common malignancy with no other patterns of malignancies observed.
Overall, treatment with risankizumab had a favourable benefit-risk profile. Furthermore, the mechanism of action of risankizumab, which targets IL-23, has been previously demonstrated to be safe and effective in the treatment of PsA and is established in clinical practice [24]. Interestingly, the continued increase in response from week 24 through week 52 observed in this study with continuous risankizumab treatment across various domains including joint symptoms suggests a different clinical dynamic of risankizumab compared with IL-17 and TNF inhibitors [25, 26].
This study has some limitations in that it includes a relatively homogeneous study population, which may limit the generalizability of the results. The switch to open-label risankizumab in both study arms (i.e. no placebo group) after week 24 may bias efficacy results and limit long-term safety interpretations; to mitigate the limitation in interpretation of long-term safety, adverse events are reported as E/100 PYs. In addition, efficacy during the open-label period is biased toward patients responding to therapy, which was mitigated by conducting a non-responder analysis for efficacy. Another limitation is that this study was conducted during the COVID-19 pandemic; however, the conduct of the trial was not markedly impacted by COVID-19 related logistical restrictions and few patient data were missing due to COVID-19.
This study supports the use of risankizumab in patients with active PsA who have inadequate response, intolerance or contraindication to csDMARDs. Overall, treatment with risankizumab improves the diverse clinical signs and symptoms of PsA, with efficacy maintained through 52 weeks. Risankizumab was generally well tolerated, with a long-term safety profile remaining stable relative to week 24. KEEPsAKE 1 remains ongoing and will allow further assessment of long-term efficacy and safety in PsA.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
Acknowledgements
AbbVie Inc. participated in the study design; study research; collection, analysis and interpretation of data; and writing, reviewing and approving this manuscript. All authors had access to the data and participated in the development, review, approval and decision to submit this manuscript for publication. AbbVie and the authors thank all study investigators for their contributions and the patients who participated in this study. AbbVie funded the research for this study and provided writing support for this manuscript. No honoraria or payments were made for authorship. Medical writing assistance, funded by AbbVie, was provided by Jay H. Parekh, PharmD, and Melissa Julyanti, PharmD, of JB Ashtin.
The study is being conducted in accord with International Council for Harmonisation guidelines. The Research Ethics Committees for the Central Denmark Region (Viborg, Denmark) ensured the ethical, scientific and medical appropriateness of the study before it was conducted and approved all relevant documentation. All patients provided written informed consent before enrolment.
Contributor Information
Lars Erik Kristensen, The Parker Institute, Copenhagen University Hospital, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark.
Mauro Keiserman, Rheumatology Section, Pontifical Catholic University, School of Medicine, Porto Alegre, Brazil.
Kim Papp, Probity Medical Research–K Papp Clinical Research, Waterloo, ON, Canada.
Leslie McCasland, Department of Rheumatology, Loyola University Medical Center, Maywood, IL, USA; Department of Veterans Affairs, Hines VA Hospital, Hines, IL, USA.
Douglas White, Rheumatology Department, Waikato Hospital, Hamilton, New Zealand; Waikato Clinical School, University of Auckland, Auckland, New Zealand.
Wenjing Lu, AbbVie Inc., North Chicago, IL, USA.
Ahmed M Soliman, AbbVie Inc., North Chicago, IL, USA.
Ann Eldred, AbbVie Inc., North Chicago, IL, USA.
Lisa Barcomb, AbbVie Inc., North Chicago, IL, USA.
Frank Behrens, Rheumatology & Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP), Fraunhofer Cluster of Excellence for Immune-Mediated Disease (CIMD), Goethe University, Frankfurt, Germany.
Data availability statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual and trial-level data (analysis data sets), as well as other information (e.g. protocols and clinical study reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement. Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Contribution statement
All authors critically reviewed this manuscript and provided final approval for publication. No honoraria or payments were made for authorship. L.E.K., F.B., A.M.S., A.E. and L.B. participated in data interpretation. L.E.K., F.B., M.K., K.P., L.M. and D.W. participated in data acquisition. A.M.S., A.E. and L.B. participated in study concept/design. W.L. participated in statistical analysis.
Funding
This work was supported by AbbVie, Inc., North Chicago, IL, USA.
Disclosure statement: L.E.K. has received honoraria or fees for serving as a speaker or consultant from AbbVie, Amgen, Biogen, Bristol Myers Squibb, Gilead, Janssen, Lilly, Merck, Novartis, Pfizer and UCB. He has received investigator-initiated study grants from AbbVie, Biogen, Janssen, Lilly, Novartis, Pfizer and UCB. M.K. has received honoraria or fees for serving on advisory boards, as a speaker or as a consultant, and has received grants as a principal investigator from AbbVie, Amgen, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Novartis, Pfizer, Roche and UCB. K.P. has received honoraria or fees for serving on advisory boards, as a speaker and as a consultant as well as grants as principal investigator from AbbVie, Amgen, Astellas, Bausch Health (Valeant), Baxalta, Baxter, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Coherus, Dermira, EMD Serono, Forward Pharma, Galderma, Genentech, GlaxoSmithKline, Janssen, Kyowa Kirin, LEO Pharma, Lilly, MedImmune, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Stiefel, Sun Pharma, Takeda and UCB. L.M. has received fees for serving on an advisory board from Lilly. D.W. has received honoraria or fees for serving on advisory boards, as a speaker and as a consultant from AbbVie and Novartis. W.L., A.M.S., A.E. and L.B. are full-time employees of AbbVie, and may hold AbbVie stock or stock options. A.M.S. is listed as an inventor on some AbbVie patents. F.B. has received research grants, honoraria or fees for serving as a consultant or speaker from AbbVie, Amgen, Boehringer Ingelheim, Celgene, Chugai, Galapagos, Genzyme, Gilead, GlaxoSmithKline, Janssen, Lilly, Merck, Novartis, Pfizer, Roche, Sandoz and Sanofi.
References
- 1. Ogdie A, Weiss P.. The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am 2015;41:545–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. FitzGerald O, Ogdie A, Chandran V. et al. Psoriatic arthritis. Nat Rev Dis Primers 2021;7:59. [DOI] [PubMed] [Google Scholar]
- 3. Egeberg A, Kristensen LE, Thyssen JP. et al. Incidence and prevalence of psoriatic arthritis in Denmark: a nationwide register linkage study. Ann Rheum Dis 2017;76:1591–7. [DOI] [PubMed] [Google Scholar]
- 4. Kristensen LE, Jorgensen TS, Christensen R. et al. Societal costs and patients' experience of health inequities before and after diagnosis of psoriatic arthritis: a Danish cohort study. Ann Rheum Dis 2017;76:1495–501. [DOI] [PubMed] [Google Scholar]
- 5. Singh JA, Guyatt G, Ogdie A. et al. Special Article: 2018 American College of Rheumatology/National psoriasis foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol 2019;71:5–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gossec L, Baraliakos X, Kerschbaumer A. et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coates LC, Corp N, van der Windt DA, Soriano ER, Kavanaugh A.. GRAPPA treatment recommendations: an update from the 2020 GRAPPA Annual Meeting. J Rheumatol Suppl 2021;97:65–6. [DOI] [PubMed] [Google Scholar]
- 8. Coates LC, Cook R, Lee KA, Chandran V, Gladman DD.. Frequency, predictors, and prognosis of sustained minimal disease activity in an observational psoriatic arthritis cohort. Arthritis Care Res 2010;62:970–6. [DOI] [PubMed] [Google Scholar]
- 9. Skyrizi (risankizumab-rzaa). Prescribing information. 2022. North Chicago, IL: AbbVie Inc; https://www.rxabbvie.com/pdf/skyrizi_pi.pdf (12 April 2022, date last accessed). [Google Scholar]
- 10. Östör A, Van den Bosch F, Papp K. et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 2 trial. Ann Rheum Dis 2022;81:351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kristensen LE, Keiserman M, Papp K. et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 1 trial. Ann Rheum Dis 2022;81:225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cassell SE, Bieber JD, Rich P. et al. The modified Nail Psoriasis Severity Index: validation of an instrument to assess psoriatic nail involvement in patients with psoriatic arthritis. J Rheumatol 2007;34:123–9. [PubMed] [Google Scholar]
- 13. Hudgens S, Sundaram M, Williams DA.. Evaluation of a novel clinician reported outcome in nail psoriasis [abstract] . Value Health 2016;19:A127. [Google Scholar]
- 14. van der Heijde D, Sharp J, Wassenberg S, Gladman DD.. Psoriatic arthritis imaging: a review of scoring methods. Ann Rheum Dis 2005;64(Suppl 2):ii61–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mease PJ, Karki C, Palmer JB. et al. Clinical characteristics, disease activity, and patient-reported outcomes in psoriatic arthritis patients with dactylitis or enthesitis: results from the corrona psoriatic arthritis/spondyloarthritis registry. Arthritis Care Res 2017;69:1692–9. [DOI] [PubMed] [Google Scholar]
- 16. McInnes IB, Sawyer LM, Markus K. et al. Targeted systemic therapies for psoriatic arthritis: a systematic review and comparative synthesis of short-term articular, dermatological, enthesitis and dactylitis outcomes. RMD Open 2022;8:e002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mourad A, Gniadecki R.. Treatment of dactylitis and enthesitis in psoriatic arthritis with biologic agents: systematic review and metaanalysis. J Rheumatol 2020;47:59–65. [DOI] [PubMed] [Google Scholar]
- 18. Gottlieb AB, Merola JF, Reich K. et al. Efficacy of secukinumab and adalimumab in patients with psoriatic arthritis and concomitant moderate-to-severe plaque psoriasis: results from EXCEED, a randomized, double-blind head-to-head monotherapy study. Br J Dermatol 2021;185:1124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smolen JS, Mease P, Tahir H. et al. Multicentre, randomised, open-label, parallel-group study evaluating the efficacy and safety of ixekizumab versus adalimumab in patients with psoriatic arthritis naïve to biological disease-modifying antirheumatic drug: final results by week 52. Ann Rheum Dis 2020;79:1310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McInnes IB, Rahman P, Gottlieb AB. et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naive patients with active psoriatic arthritis. Arthritis Rheumatol 2022;74:475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baran R. The burden of nail psoriasis: an introduction. Dermatology 2010;221(Suppl 1): 1–5. [DOI] [PubMed] [Google Scholar]
- 22. Reich K, Gooderham M, Thaçi D. et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet 2019;394:576–86. [DOI] [PubMed] [Google Scholar]
- 23. Gordon KB, Strober B, Lebwohl M. et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet 2018;392:650–61. [DOI] [PubMed] [Google Scholar]
- 24. Yang K, Oak ASW, Elewski BE.. Use of IL-23 inhibitors for the treatment of plaque psoriasis and psoriatic arthritis: a comprehensive review. Am J Clin Dermatol 2021;22:173–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smolen JS, Sebba A, Ruderman EM. et al. Efficacy and safety of ixekizumab with or without methotrexate in biologic-naïve patients with psoriatic arthritis: 52-week results from SPIRIT-H2H study. Rheumatol Ther 2020;7:1021–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McInnes IB, Behrens F, Mease PJ. et al. Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3b trial. Lancet 2020;395:1496–505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual and trial-level data (analysis data sets), as well as other information (e.g. protocols and clinical study reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement. Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.