Abstract
Introduction
The PAGANINI study evaluated the efficacy and safety of the selective P2X3 antagonist eliapixant in patients with refractory chronic cough (RCC).
Methods
PAGANINI was a randomized, double-blind, parallel-group, placebo-controlled, multicenter, dose-finding, phase 2b study. Adults with RCC lasting ≥ 12 months and cough severity ≥ 40 mm on a visual analog scale at screening were enrolled. Participants were randomized 1:1:1:1 to twice-daily 25 mg, 75 mg, or 150 mg oral eliapixant or placebo for 12 weeks. The primary endpoint was change from baseline in 24-h cough count after 12 weeks of intervention.
Results
Overall, 310 participants were randomized to twice-daily eliapixant 25 mg (n = 75), 75 mg (n = 78), 150 mg (n = 80), or placebo (n = 77). A statistically significant dose–response signal with eliapixant was detected for the primary endpoint (all dose–response models, adjusted p < 0.1; one-sided). Adverse events (AEs) were reported in 39 (51%) participants with placebo and 43–51 (57–65%) participants receiving eliapixant. The most common AE was dysgeusia, occurring in 1% (n = 1) of the placebo group and 1–16% (n = 1–13) of the eliapixant groups in a dose-related manner. One case of a moderate drug-induced liver injury occurred in a participant receiving 150 mg twice-daily eliapixant.
Conclusion
Eliapixant demonstrated efficacy and a favorable taste tolerability profile in RCC. However, a drug-induced liver injury contributed to intensified liver monitoring in clinical trials with eliapixant and discontinuation of the entire development program in all indications by Bayer AG.
Trial Registration
ClinicalTrials.gov identifier NCT04562155; registered September 18, 2020.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00408-023-00621-x.
Keywords: Chronic cough, Eliapixant, P2X3 receptor antagonist, Phase 2b clinical trial
Introduction
Chronic cough (CC), estimated to affect around 10% of the global adult population [1], is defined as a cough lasting ≥ 8 weeks [2]. CC with unexplained underlying etiology or CC that is unresponsive to conventional treatment are jointly referred to here as refractory CC (RCC) [2]. RCC can have a detrimental impact on patients’ quality of life (QoL) [3] and mental health [4, 5] and results in significant economic burden, with patients experiencing repeated treatment failures and delayed diagnosis [4]. There are no approved drugs for RCC in countries other than Japan [6] and Switzerland [7], resulting in widespread use of off-label treatment options with limited efficacy and a poor safety profile, and non-pharmacologic interventions [4]. There is therefore a large unmet clinical need for efficacious, well-tolerated therapies.
Neuronal hypersensitivity is implicated in the pathogenesis of RCC [8]. Patients with RCC have increased cough reflex sensitivity, which may result from vagal nerve hypersensitivity or changes in the central nervous system projections and central sensitization as presumed underlying mechanisms [8]. P2X3 receptors are thought to play an important role in sensory neural dysregulation associated with RCC [9, 10]. Preclinical studies have shown that P2X2/3 receptors can regulate afferent sensory adenosine triphosphate-mediated signaling in the vagus nerve [9]. Clinical trials of the P2X3 receptor antagonist gefapixant showed efficacy in objective and subjective measures of cough in patients with RCC [11–13]. However, substantial taste-related tolerability issues [11–13], attributed to the block of P2X2/3 receptors on nerves innervating taste buds [14], may limit acceptance of gefapixant by patients.
Eliapixant is a potent P2X3 receptor antagonist with a good tolerability profile in healthy subjects, and high selectivity over the P2X2/3 receptor in vitro, potentially resulting in fewer off-target effects [15–17]. In a phase 2a study, eliapixant significantly reduced cough frequency and severity in patients with RCC, with a lower rate of taste-related side effects than those observed with therapeutic doses of gefapixant [18]. The aim of the phase 2b PAGANINI study was to identify the optimal dose of eliapixant in patients with RCC, to further assess efficacy, and to characterize the safety and tolerability profile of eliapixant over 12 weeks.
Methods
Study Design
PAGANINI (ClinicalTrials.gov NCT04562155) was a randomized, double-blind, parallel-group, placebo-controlled, dose-finding efficacy and safety study conducted at 99 centers in 19 countries (see Supplementary Methods for more details). The study consisted of a 14-day screening period, 12 weeks of randomized treatment, and a 30-day safety follow-up (Supplementary Fig. S1). The study protocol and statistical analysis plan are available on ClinicalTrials.gov.
Eligible participants were centrally randomized 1:1:1:1 by the sponsor using block randomization to receive one of three oral doses of twice-daily eliapixant (25 mg, 75 mg, or 150 mg; Bayer AG, Berlin, Germany) or placebo using Interactive Response Technology (IRT version 2.1; Suvoda, USA), stratified by region. To maintain blinding, tablets containing eliapixant and placebo were identical in size, color, and shape.
Participants
Adults aged ≥ 18 years with RCC lasting ≥ 12 months, with persistent cough for ≥ 8 weeks before screening, and with cough severity ≥ 40 mm measured on a 100 mm visual analog scale (VAS) at screening, were enrolled by the investigators. Full inclusion and exclusion criteria are in the Supplementary Methods.
Procedures
Using an ambulatory cough recording device (VitaloJAK, Vitalograph, Ireland [19]), 24-h cough count monitoring was performed at every visit to Week 12 (see Supplementary Methods for more details). Participants completed the cough severity VAS [20] daily and the Leicester Cough Questionnaire (LCQ) [21] at all visits (see Supplementary Methods for more details). Adverse events (AEs) and other safety outcomes were evaluated throughout the study and at follow-up.
Outcomes
The primary efficacy endpoint was change from baseline in 24-h cough count after 12 weeks of intervention. Secondary efficacy endpoints included: the percentage of participants with ≥ 30% reduction from baseline 24-h cough count after 12 weeks; change from baseline 24-h cough count after 2, 4, and 8 weeks; change from baseline awake cough count per hour after 2, 4, 8, and 12 weeks; change from baseline cough severity after 12 weeks measured by the cough severity VAS; the percentage of participants with ≥ 30-scale unit reduction from baseline after 12 weeks measured by the cough severity VAS [22]; change from baseline cough-related QoL after 12 weeks measured by the LCQ; and the percentage of participants with ≥ 1.3-point increase from baseline after 12 weeks measured by LCQ total score [23].
Treatment-emergent AEs and serious AEs (SAEs) were recorded according to the Medical Dictionary for Regulatory Activities version 24.0. Additional safety assessments are described in the Supplementary Methods. At the study end, participants who spontaneously reported a taste-related AE completed an assessment on taste disturbances.
Statistical Analysis
A multiple comparison procedure modeling (MCP-Mod) approach [24] was used as the prespecified analysis of the primary efficacy endpoint. As PAGANINI was a phase 2b dose-finding study, the MCP-Mod approach was used because it is a well-accepted method for dose finding that efficiently uses the available data better than traditional pairwise comparisons [25, 26]. The MCP-Mod approach enables the estimation of a dose response and the selection of an optimum dose for further phase 3 trials [26].
For the primary endpoint analysis, the raw 24-h cough count was standardized to an average hourly count, then log-transformed as done previously [12, 27]. To detect a dose–response signal, four candidate dose–response models were tested with a single contrast test using the generalized MCP-Mod approach. The null hypothesis, “the response at all doses is equal,” was tested against the alternative, “there is a dose–response relationship.” If at least one of the four individual tests of models was statistically significant (adjusted p of one-sided test ≤ 0.1), a dose–response signal was considered established. The model with the best fit was then used for the estimation of the dose–response curve and the minimum effective dose (MED). For further information on the primary endpoint analysis, see the Supplementary Methods.
Sample size calculations were performed for establishing evidence of a drug effect across the doses. A sample size of 50 participants per dose group was predicted to have at least 85% power to demonstrate a dose–response relationship for the primary efficacy endpoint, using a one-sided test at a type I error rate of α = 0.10 (see the Supplementary Methods for more details).
The secondary endpoint analyses and definitions of the per protocol, full analysis and safety analysis sets are described in the Supplementary Methods.
Statistical evaluation was performed using SAS software version 9.4 or higher (SAS Institute, USA) or ValidR software version 3.5.2 or higher (Mango Solutions, UK). Confirmatory p-values are reported for the analysis of the primary endpoint. The study was not powered for individual pairwise comparisons between dose groups. Analysis of secondary endpoints, sensitivity analyses, and AEs should be interpreted as exploratory.
Results
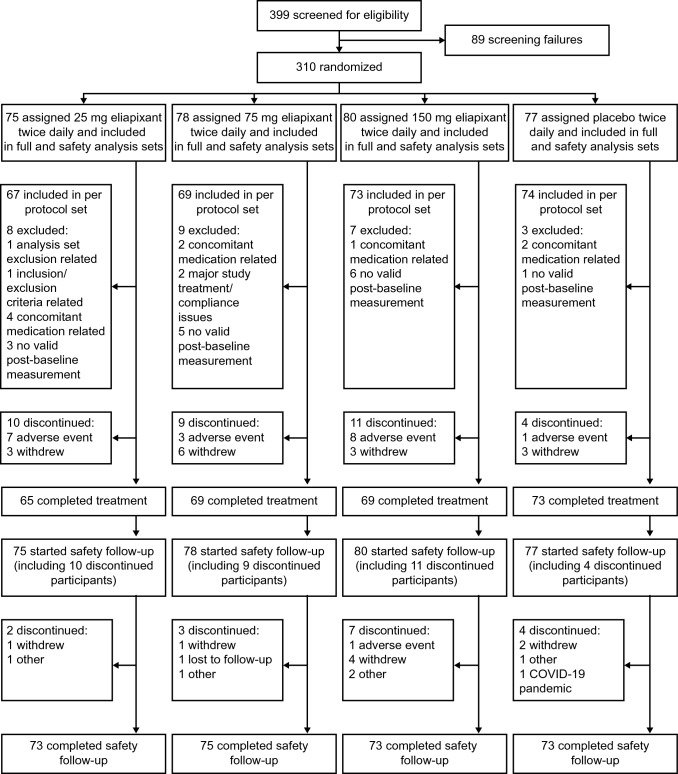
Of 399 participants screened between October 2, 2020 and March 12, 2021, 310 were randomized to eliapixant 25 mg (n = 75), 75 mg (n = 78), 150 mg (n = 80), or placebo (n = 77) (Fig. 1). All randomized participants were included in the full and safety analysis sets. A total of 283 participants were included in the per protocol set (eliapixant 25 mg n = 67, 75 mg n = 69, 150 mg n = 73, placebo n = 74). In total, 276 participants (89%) completed the treatment period.
Fig. 1.
Participant disposition. Includes the 12-week treatment period and the 30-day safety follow-up. If a participant has more than one validity finding that excludes them from an analysis set, all the findings are displayed. All 34 participants (11%) who discontinued the treatment phase of the study entered the safety follow-up. A total of 294 participants (95%) completed the 30-day safety follow-up. COVID-19 corona virus disease 2019
Baseline demographics and clinical characteristics were generally well balanced across the treatment groups (Table 1), although mean 24-h cough count (Table 2) and awake cough count in the eliapixant 150 mg group were slightly lower than in other groups. The baseline awake cough count was higher than the 24-h cough count in all treatment groups. Overall, 76 (27%) participants had a low baseline 24-h cough count of < 10 coughs per hour.
Table 1.
Baseline demographics and clinical characteristics (per protocol set)
| Characteristics | Eliapixant 25 mg twice daily (n = 67) |
Eliapixant 75 mg twice daily (n = 69) |
Eliapixant 150 mg twice daily (n = 73) |
Placebo twice daily (n = 74) |
|---|---|---|---|---|
| Age, years | 61.2 (9.9) | 59.1 (12.1) | 59.3 (11.9) | 56.6 (12.4) |
| ≥ 65 years, n (%) | 24 (36) | 25 (36) | 29 (40) | 26 (35) |
| Female, n (%) | 49 (73) | 56 (81) | 56 (77) | 59 (80) |
| Race, n (%) | ||||
| White | 56 (84) | 60 (87) | 66 (90) | 64 (86) |
| Asian | 10 (15) | 9 (13) | 5 (7) | 10 (14) |
| Other/not reported | 1 (1) | 0 | 2 (3) | 0 |
| Geographic region, n (%) | ||||
| Europe | 35 (52) | 37 (54) | 42 (58) | 40 (54) |
| Japan | 6 (9) | 6 (9) | 5 (7) | 6 (8) |
| Rest of the world | 26 (39) | 26 (38) | 26 (36) | 28 (38) |
| Body mass index, kg/m2 | 27.1 (4.7) | 27.1 (5.5) | 27.9 (5.7) | 27.2 (5.1) |
| Duration of cough, years, n (%) | ||||
| < 10 | 38 (57) | 39 (57) | 46 (63) | 39 (53) |
| ≥ 10 | 29 (43) | 30 (43) | 27 (37) | 35 (47) |
| Tobacco smoking history, n (%) | ||||
| Never | 47 (70) | 51 (74) | 48 (66) | 56 (76) |
| Ex-smoker | 20 (30) | 18 (26) | 25 (34) | 18 (24) |
| Baseline 24-h coughs, per houra | ||||
| < 10, n (%) | 21 (31) | 18 (26) | 20 (27) | 17 (23) |
| ≥ 10, n (%) | 46 (69) | 51 (74) | 53 (73) | 57 (77) |
| ≥ 20, n (%) | 30 (45) | 37 (54) | 31 (42) | 37 (50) |
| ≥ 30, n (%) | 21 (31) | 23 (33) | 15 (21) | 24 (32) |
| Baseline awake coughs, per hour | ||||
| Geometric mean | 23.6 (3.0) | 26.4 (3.0) | 21.2 (2.4) | 24.0 (3.2) |
| < 20, n (%) | 28 (42) | 26 (38) | 30 (41) | 32 (43) |
| ≥ 20, n (%) | 39 (58) | 43 (62) | 43 (59) | 42 (57) |
| Baseline cough severity, VAS 0–100 | ||||
| Arithmetic meanb | 65.5 (14.6) | 67.1 (14.9) | 66.8 (15.9) | 61.5 (18.5) |
| LCQ total score (range 3–21) | ||||
| Arithmetic mean | 12.0 (2.5) | 11.8 (2.8) | 11.2 (2.6) | 11.5 (3.3) |
Data are expressed as mean (SD) unless otherwise stated
LCQ Leicester Cough Questionnaire, SD standard deviation, VAS visual analog scale
aSee Table 2 for baseline geometric mean (SD) 24-h coughs, per hour
bEliapixant 25 mg twice daily n = 62, eliapixant 75 mg twice daily n = 68, eliapixant 150 mg twice daily n = 67, placebo twice daily n = 69
Table 2.
Change from baseline in 24-h cough count after 12 weeks of intervention (per protocol set)
| Eliapixant 25 mg twice daily (n = 67) |
Eliapixant 75 mg twice daily (n = 69) |
Eliapixant 150 mg twice daily (n = 73) |
Placebo twice daily (n = 74) |
|
|---|---|---|---|---|
| Baseline geometric mean (SD) 24-h coughs, per hour | 17.5 (2.9) | 19.2 (2.9) | 15.6 (2.3) | 17.6 (3.1) |
| Week 12 geometric mean (SD) 24-h coughs, per hour | 9.0 (4.0) | 9.1 (3.8) | 8.1 (2.7) | 11.3 (3.1) |
| Relative change of geometric means for 24-h cough at Week 12, % (95% CI) |
− 44 (− 55 to − 29) |
− 53 (− 62 to − 42) |
− 48 (− 57 to − 36) |
− 36 (− 47 to − 23) |
| Change in 24-h cough count at Week 12 relative to placebo, % (95% CI) |
− 12 (− 30 to 11) |
− 27 (− 41 to − 9) |
− 18 (− 33 to < 1) |
CI confidence interval, SD standard deviation
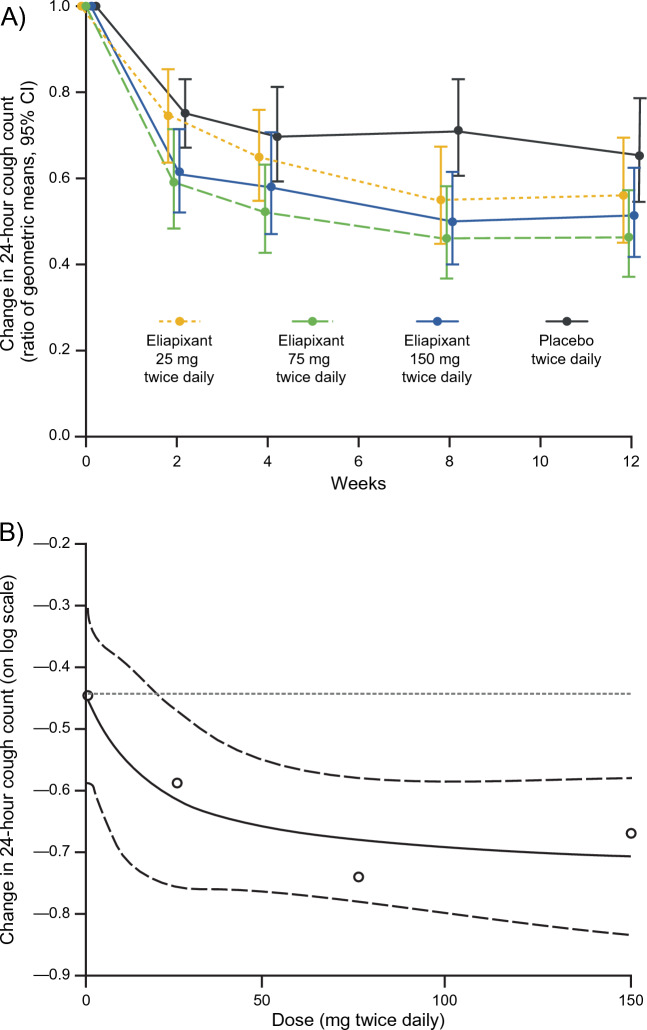
The data for the primary efficacy endpoint, change from baseline in 24-h cough count after 12 weeks of intervention, are shown in Table 2 and Fig. 2A. At Week 12, the 24-h cough count had decreased from baseline in all treatment groups. The largest relative and placebo-adjusted reductions in 24-h cough count from baseline were seen in the 75 mg eliapixant group.
Fig. 2.
(A) Change from baseline in 24-h cough count throughout study period and (B) the estimated dose–response Emax model for the change from baseline to Week 12 in log-transformed 24-h cough count with an 80% CI (per protocol set). In (B), circles indicate the estimated dose response in each dose group adjusted for baseline cough count and geographic region. The dotted horizontal reference line at − 0.44 represents the estimated dose response in the placebo group. The solid line indicates the estimated Emax dose–response model and the dashed lines indicate the 80% CI. CI confidence interval, Emax asymptotic maximum change from placebo effect
For the primary analysis of the primary endpoint, a statistically significant dose–response signal was detected with eliapixant for change from baseline in 24-h cough count at Week 12, with multiplicity-adjusted p-values of < 0.1 for all four candidate models (Supplementary Table S1). As a result of the better model fit, the Emax model was used to derive the MED (Fig. 2B). The MED to achieve a relative change vs. placebo of − 20% (i.e., log[0.8] = − 0.22 on the log-transformed scale (Fig. 2B)) was estimated at ~ 58 mg eliapixant twice daily.
Reductions in 24-h cough count with the two higher doses of eliapixant relative to placebo were observed early at Week 2, with further reductions at Week 4 and Week 8 (Fig. 2A). A ≥ 30% reduction from baseline in 24-h cough count at Week 12 was reported in 34 participants (46%) with placebo and 35–44 participants (52–64%) receiving eliapixant. Compared with placebo, more participants in the 75 mg group reached this responder threshold at Week 12 (mean treatment difference: 18%, 95% confidence interval [CI] 2 to 34, p = 0.03). A smaller treatment difference vs. placebo was observed for the other doses of eliapixant with a mean treatment difference of 6% (95% CI − 10 to 23, p = 0.5) and 8% (95% CI − 9 to 24, p = 0.4) for the 25 mg and 150 mg groups, respectively.
Similar findings to those for 24-h cough count were observed for the change from baseline in awake cough count at all study visits (see Supplementary Results and Supplementary Fig. S2 for more details).
Cough severity was reduced with all doses of eliapixant at Week 12 vs. baseline, with a small numeric reduction vs. placebo (Table 3). More participants in the 75 mg group experienced a ≥ 30-scale unit reduction in cough severity at Week 12 vs. placebo (mean treatment difference of 16%, 95% CI 1 to 31, p = 0.03). A smaller treatment difference vs. placebo was observed for the other doses of eliapixant (Table 3).
Table 3.
Secondary efficacy cough-related endpoints relating to severity and QoL (per protocol set)
| Eliapixant 25 mg twice daily (n = 67) |
Eliapixant 75 mg twice daily (n = 69) |
Eliapixant 150 mg twice daily (n = 73) |
Placebo twice daily (n = 74) |
|
|---|---|---|---|---|
| Cough severitya | ||||
| Mean (SD) change in cough severity from baseline to Week 12 | − 17.7 (23.8) | − 22.7 (23.0) | − 22.9 (24.5) | − 17.0 (21.9) |
| LS mean (SE) change in cough severity from baseline to Week 12b |
− 19.0 (3.1) p < 0.0001 |
− 23.3 (2.9) p < 0.0001 |
− 23.5 (3.0) p < 0.0001 |
− 18.0 (2.7) p < 0.0001 |
| Treatment difference vs. placebo, difference of LS means (SE)b |
− 0.9 (4.0) p = 0.8 |
− 5.3 (3.8) p = 0.2 |
− 5.4 (4.0) p = 0.2 |
|
| Participants with a ≥ 30-scale unit reduction in cough severity from baseline to Week 12, n (%) | 18 (27) | 25 (36) | 20 (27) | 15 (20) |
| Treatment difference vs. placebo, % (SE) |
7 (0.1) p = 0.4 |
16 (0.1) p = 0.03 |
7 (0.1) p = 0.3 |
|
| Cough-related QoLc | ||||
| Mean (SD) change in LCQ total score from baseline to Week 12 | 2.2 (3.4) | 2.5 (3.3) | 2.7 (3.5) | 2.2 (3.1) |
| LS mean (SE) change in LCQ total score from baseline to Week 12b |
2.2 (0.4) p < 0.0001 |
2.5 (0.4) p < 0.0001 |
2.6 (0.4) p < 0.0001 |
2.1 (0.4) p < 0.0001 |
| Treatment difference vs. placebo, difference of LS means (SE)b |
0.1 (0.6) p = 0.9 |
0.4 (0.5) p = 0.5 |
0.5 (0.5) p = 0.4 |
|
| Participants with a ≥ 1.3-point increase in LCQ total score from baseline to Week 12, n (%) | 32 (48) | 42 (61) | 47 (64) | 38 (51) |
| Treatment difference vs. placebo, % (SE) |
− 4 (0.1) p = 0.7 |
10 (0.1) p = 0.3 |
13 (0.1) p = 0.1 |
LCQ Leicester Cough Questionnaire, LS least squares, QoL quality of life, SD standard deviation, SE standard error
aMeasured by the cough severity visual analog scale
bA mixed model repeated measures analysis was applied with baseline value, treatment group, region, visit, and treatment by visit interaction as fixed effects, and participant as a random effect using an unstructured covariance structure
cMeasured by LCQ total score
There was a dose-dependent improvement in LCQ total score after 12 weeks. However, the differences vs. placebo were small (0.1, 95% CI − 1.0 to 1.2, in the 25 mg group; 0.4, 95% CI − 0.7 to 1.4, in the 75 mg group; 0.5, 95% CI − 0.6 to 1.6 in the 150 mg group) (Table 3). The percentage of participants with a ≥ 1.3-point increase in LCQ total score from baseline after 12 weeks was similar between all three doses of eliapixant and placebo.
Sensitivity analyses of the full analysis set confirmed the primary endpoint and secondary endpoint results in the per protocol set (data not shown).
AEs were reported in 39 participants (51%) with placebo and 43–51 participants (57–65%) receiving eliapixant, with most considered mild or moderate (Table 4). The proportion of participants reporting AEs (including those described as severe) was slightly higher in the two higher-dose eliapixant groups than the low-dose eliapixant or placebo groups (Table 4). The most frequently occurring AE was dysgeusia, which occurred in 1 participant (1%) in the placebo group and 1–13 participants (1–16%) in the eliapixant group in a dose-related manner (Table 5). Other AEs relating to taste or smell disorders were similarly more frequent with eliapixant than placebo (Table 5).
Table 4.
Summary of treatment-emergent AEs (safety analysis set)
| Patients, n (%) | Eliapixant 25 mg twice daily (n = 75) |
Eliapixant 75 mg twice daily (n = 78) |
Eliapixant 150 mg twice daily (n = 80) |
Placebo twice daily (n = 77) |
|---|---|---|---|---|
| Any AE | 43 (57) | 51 (65) | 51 (64) | 39 (51) |
| Maximum intensity for any AE | ||||
| Mild | 21 (28) | 31 (40) | 23 (29) | 18 (23) |
| Moderate | 22 (29) | 16 (21) | 25 (31) | 20 (26) |
| Severe | 0 | 3 (4) | 3 (4) | 1 (1) |
| Any study drug-related AE | 9 (12) | 15 (19) | 30 (38) | 9 (12) |
| Maximum intensity for study drug-related AE | ||||
| Mild | 5 (7) | 10 (13) | 16 (20) | 7 (9) |
| Moderate | 4 (5) | 5 (6) | 12 (15) | 2 (3) |
| Severe | 0 | 0 | 2 (3) | 0 |
| AEs leading to study drug discontinuation | 7a (9) | 3b (4) | 8c (10) | 1d (1) |
| Any SAE | 0 | 1 (1) | 2 (3) | 1 (1) |
| Any study drug-related SAE | 0 | 0 | 1 (1) | 0 |
| SAEs leading to study drug discontinuation | 0 | 0 | 1 (1) | 1 (1) |
| AEs with outcome death | 0 | 0 | 0 | 0 |
Treatment-emergent AEs are reported from the start of study intervention administration until 14 days after the last study medication intake
AE adverse event, SAE serious adverse event
an = 1 each for cough, arthralgia, abdominal pain upper, dizziness, diplopia/pain in extremity/headache, musculoskeletal chest pain, or gastroenteritis
bn = 1 each for pancytopenia, asthenia/chest discomfort/hypotension, or palpitations/diarrhea/fatigue/disturbance in attention
cn = 1 each for liver function test abnormal, throat irritations, nausea/chills/fatigue/weight increased, dizziness/dysgeusia/headaches/asthma/hemoptysis, cough, alanine aminotransferase increased/aspartate aminotransferase increased, abdominal pain upper, rash, or arthralgia/pain in extremity
dn = 1 for angina unstable
Table 5.
Most frequently reported treatment-emergent AEs, and AEs related to taste, bleeding, and drug-related hepatic disorders (safety analysis set)
| Patients, n (%) | Eliapixant 25 mg twice daily (n = 75) |
Eliapixant 75 mg twice daily (n = 78) |
Eliapixant 150 mg twice daily (n = 80) |
Placebo twice daily (n = 77) |
|---|---|---|---|---|
| Most frequently reported AEsa | ||||
| Dysgeusia | 1 (1) | 9 (12) | 13 (16) | 1 (1) |
| Headache | 6 (8) | 5 (6) | 6 (8) | 4 (5) |
| Cough | 4 (5) | 7 (9) | 7 (9) | 3 (4) |
| Fatigue | 2 (3) | 6 (8) | 5 (6) | 2 (3) |
| Dry mouth | 1 (1) | 3 (4) | 2 (3) | 4 (5) |
| Dizziness | 2 (3) | 2 (3) | 1 (1) | 5 (6) |
| Nausea | 2 (3) | 2 (3) | 5 (6) | 1 (1) |
| Hypogeusia | 2 (3) | 1 (1) | 4 (5) | 2 (3) |
| Insomnia | 4 (5) | 0 | 1 (1) | 0 |
| Taste-related AEs | ||||
| Dysgeusia | 1 (1) | 9 (12) | 13 (16) | 1 (1) |
| Hypogeusia | 2 (3) | 1 (1) | 4 (5) | 2 (3) |
| Taste disorder | 0 | 2 (3) | 1 (1) | 1 (1) |
| Ageusia | 0 | 0 | 2 (3) | 1 (1) |
| Any AE relating to “taste and smell disorders”b | 3 (4) | 12 (15) | 19 (24) | 5 (6) |
| Any AE relating to “hemorrhages”b | 3 (4) | 2 (3) | 5 (6) | 2 (3) |
| Any AE relating to “drug-related hepatic disorders – comprehensive search”b | 0 | 1 (1) | 3 (4) | 0 |
Treatment-emergent AEs are reported from the start of study intervention administration until 14 days after the last study medication intake
AE adverse event, SAE serious adverse event
aAEs reported in ≥ 5% in any treatment group
bIdentified via standardized Medical Dictionary for Regulatory Activities query
AEs leading to study drug discontinuation were more common with eliapixant than placebo (Table 4). An SAE of abnormal liver tests leading to study drug discontinuation occurred in 1 participant in the 150 mg eliapixant group and was reported as a suspected unexpected serious adverse reaction (SUSAR). No deaths occurred during the study.
Changes in some laboratory safety parameters were reported, including 2 participants receiving eliapixant (75 mg, n = 1; 150 mg, n = 1) who had alanine aminotransferase exceeding the three-fold upper limit of normal, which triggered close liver observation in accordance with the US Food and Drug Administration Guidance for Industry [28] and the study protocol. In the patient receiving eliapixant 150 mg, the SUSAR was considered a moderate drug-induced liver injury (DILI) of hepatocellular origin. The participant prematurely discontinued eliapixant at the 4-week visit because of the liver event, after which the liver enzyme values returned to normal. In the overall population, dose-dependent increases in mean and median values of alkaline phosphatase, fibrinogen, and plasma antithrombin III activity were observed. There were no relevant mean changes in other liver enzymes at any dose of eliapixant during treatment in the overall population. See the Supplementary Results for more details.
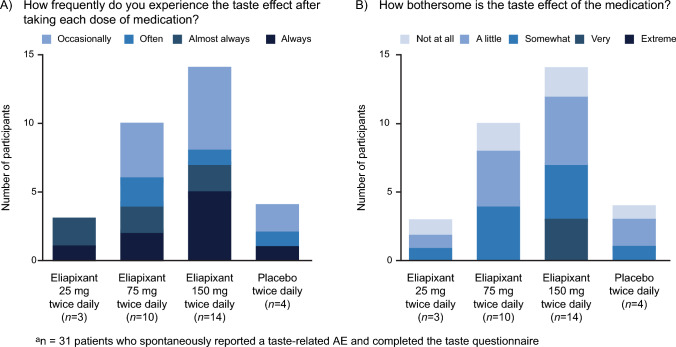
Thirty-one participants who spontaneously reported a taste-related AE during the treatment period completed an end-of-study assessment on taste disturbances (Fig. 3). The frequency and how bothersome the taste disturbances were in the eliapixant groups increased in a dose-related manner (Fig. 3A). No participants described the taste effects as “extremely” bothersome. An answer of “very” bothersome was only recorded in the 150 mg group (Fig. 3B).
Fig. 3.
End-of-study assessment on taste disturbances (safety analysis seta)
Discussion
PAGANINI confirmed data from the phase 2a study suggesting that eliapixant is effective at reducing 24-h cough count in patients with RCC. The detection of a statistically significant dose–response signal with eliapixant was achieved for the primary endpoint of change from baseline in 24-h cough count at Week 12. At Week 12, 24-h cough count was reduced by 27% vs. placebo in the 75 mg group. In an analysis of secondary efficacy endpoints, awake cough count was also reduced by 28% with 75 mg eliapixant vs. placebo at Week 12. Compared with placebo, more participants in the 75 mg eliapixant group reached a ≥ 30% reduction in 24-h cough count and ≥ 30-scale unit reduction in cough severity at Week 12 from baseline; however, cough-related QoL as measured by LCQ total score did not improve.
The phase 2a study of eliapixant demonstrated similar reductions in 24-h cough and awake cough counts to those reported here [18]. However, the improvements in cough severity and cough-related QoL vs. placebo seen in the earlier study [18] were not observed to the same extent. While comparisons between studies should be made with caution, this observation may be explained by the larger placebo response seen in PAGANINI. However, improvements in cough severity and cough-related QoL were reported for the phase 2b gefapixant and sivopixant trials [12, 27], and the phase 3 COUGH-1 and COUGH-2 studies despite large placebo effects [13]. The lack of patient-perceived improvement in cough in this study is therefore difficult to explain. However, it should be noted that PAGANINI was not powered to detect significant differences in patient-reported outcome parameters between treatment groups.
In this study, the efficacy effects in the 150 mg eliapixant group were not greater than those in the 75 mg group, suggesting that a plateau in dose response was reached, as indicated by the estimated dose–response curve. This finding may have also been influenced by the less severe baseline cough characteristics in the 150 mg group. A plateau in dose response for reduction in cough count was also observed with eliapixant in the phase 2a study, although subjective endpoints continued to improve with the highest dose [18]. The plateau in dose response is also supported by data from healthy volunteers [16], whereby the two higher doses of eliapixant had similar trough plasma drug concentrations, and the plasma concentrations predicted to achieve ≥ 80% P2X3 receptor occupancy (the expected threshold for efficacy based on unpublished preclinical studies; data on file, Bayer AG) were reached with both higher doses [16]. Achievement of the primary endpoint and the low MED to achieve 20% improvement over placebo are therefore notable considering the globally heterogeneous study population, the high placebo response, the overall high number of participants experiencing a low baseline 24-h cough count of < 10 coughs per hour, and the lower baseline cough counts and efficacy results in the 150 mg eliapixant group.
The safety and tolerability profiles in PAGANINI are generally consistent with other studies of eliapixant in healthy subjects and the phase 2a study in patients with RCC [16–18]. However, a case of a moderate DILI of hepatocellular origin occurred during treatment with 150 mg eliapixant and contributed to the need for intensified liver monitoring in clinical trials with eliapixant. In a second participant, alanine aminotransferase levels exceeding the three-fold upper limit of normal led to close liver observation, and a dose-dependent increase in mean alkaline phosphatase levels in the overall population was observed during the treatment period. The clinical relevance of increased alkaline phosphatase levels is unclear, as is the origin (liver vs. bone) in the absence of a concurrent increase in the mean values of other liver enzymes. In the phase 2a study of the P2X3 antagonist sivopixant for RCC, a participant receiving sivopixant also experienced a DILI during the trial [29].
Taste-related AEs were reported in 24% of participants in the 150 mg eliapixant group with fewer reports in participants receiving lower doses. One participant discontinued treatment due to dysgeusia as part of a combination of nine AEs. No participants who spontaneously reported a taste-related AE described the effect as “extremely” bothersome. As with the phase 2b study of gefapixant [12], dysgeusia was the most reported AE in PAGANINI. However, taste-related AEs were previously reported in up to 81% of patients with gefapixant in phase 2b [12] compared with up to 24% of participants with eliapixant in this study. In phase 3 trials with gefapixant 45 mg, taste-related AEs were reported by 59% of participants at Week 12 in COUGH-1 and 69% of participants at Week 24 in COUGH-2 [13]. The smaller impact on taste perception with eliapixant may be due to its high selectivity for the P2X3 receptor leading to a low potential for off-target effects mediated by P2X2/3 receptors [16].
Strengths of PAGANINI included that the baseline demographics reflect those seen in the clinical RCC population [30]. Recruitment of participants across 19 countries means the results are likely to reflect the global population of patients with RCC. Limitations of the study include a lack of powered individual pairwise comparisons between dose groups; however, the aim of this study was to establish evidence of a drug effect across the doses to support the dose selection for phase 3 studies [25, 26].
In summary, the PAGANINI study showed that eliapixant was effective at reducing 24-h cough count vs. placebo in patients with RCC. The safety and tolerability profiles in PAGANINI were consistent with other studies of eliapixant in healthy subjects and the phase 2a study in patients with RCC. However, a case of a moderate DILI of hepatocellular origin occurred during treatment with 150 mg eliapixant. This DILI contributed to the need for intensified liver monitoring in clinical trials with eliapixant and the subsequent discontinuation of the entire development program in all indications by Bayer AG.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to thank the investigators and participants for their involvement in the study. Julian Borissoff, MD, PhD (Bayer AG, Berlin, Germany) was the Global Clinical Lead mainly responsible for the study design and conduct. Katrin Roth, PhD (Bayer AG, Berlin, Germany) was the responsible statistician during study planning and setup and assisted with statistical analyses and interpretation of data. Medical writing assistance was provided by Rachael Powis, PhD (Adelphi Communications Ltd, Bollington, UK), funded by Bayer AG (Berlin, Germany) in accordance with Good Publications Practice. Recruitment for this study was supported by the NIHR Manchester Clinical Research Facility, UK.
Author Contributions
KG, UK, RS, PVP, and MW designed the study. AHM, MRS, MV, LMG, and AN collected data. All authors interpreted the data. UK undertook statistical analyses. All authors critically reviewed and revised the manuscript and approved the final version for publication.
Funding
Bayer AG funded the PAGANINI study.
Data Availability
Availability of the data underlying this publication will be determined according to Bayer’s commitment to the European Federation of Pharmaceutical Industries and Associations and Pharmaceutical Research and Manufacturers of America principles for responsible clinical trial data sharing, pertaining to scope, timepoint, and process of data access. Bayer commits to sharing upon request from qualified scientific and medical researchers patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the USA and European Union as necessary for doing legitimate research. This commitment applies to data on new medicines and indications that have been approved by the European Union and US regulatory agencies on or after January 1, 2014. Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymized patient-level data and supporting documents from clinical studies to do further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the study sponsors section of the portal. Data access will be granted to anonymized patient-level data, protocols, and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
Declarations
Conflict of interest
PVD reports remuneration for consultancy from Bayer AG, Bellus Health, Chiesi, Merck, and Shionogi. AHM reports remuneration for consultancy from Bayer AG, Bellus Health, Boehringer Ingelheim, Merck, Pfizer, Procter & Gamble, and Shionogi, lecture fees from AstraZeneca and Boehringer Ingelheim, and grant support from Afferent Pharmaceuticals, Infirst, Merck, and Procter & Gamble. JAS reports research grants and remuneration for consultancy from Algernon, AstraZeneca, Axalbion, Bayer AG, Bellus Health, Boehringer Ingelheim, Chiesi, Merck, Nocion Therapeutics, Shionogi, and Trevi, and non-financial support and royalties from Vitalograph paid to Manchester University NHS Foundation Trust that may be shared with the department in which JAS works. JAS is funded by the Manchester NIHR Biomedical Research Centre and an NIHR Senior Investigator Award. MRS reports research grants from Afferent Pharmaceuticals, Bayer AG, Bellus Health, Merck, NeRRe Therapeutics, and Shionogi, remuneration for lectures from Merck, and has served on advisory committees for Afferent Pharmaceuticals, AstraZeneca, Bayer AG, Bellus Health, Merck, NeRRe Therapeutics, Nocion Therapeutics, and Shionogi and on a Data and Safety Monitoring Board for Nocion Therapeutics. MV reports remuneration for consultancy in litigation relating to acid suppressive therapy, has served on an advisory committee for Bayer AG, Diversatek, Ironwood, ISOThrive, Medtronic, Phathom, and Sanofi, and holds a patent with Diversatek. LG reports a research grant from AstraZeneca, remuneration for lectures from AstraZeneca, Bayer AG, GlaxoSmithKline, MSD, Novartis, and Sanofi, and remuneration for consultancy from AstraZeneca, Bayer AG, Chiesi, GlaxoSmithKline, Merck, MSD, Novartis, and Sanofi. AN reports research grants from Kyorin Pharmaceutical and Novartis and remuneration for lectures from AstraZeneca, GlaxoSmithKline, Kyorin Pharmaceutical, MSD, and Novartis. KG, UK, RS, and MW are employees of Bayer AG. PVP was an employee of Bayer AG at the time of the study and is now an employee of the Janssen Pharmaceutical Companies of Johnson & Johnson. LMG reports research grants from Bayer AG, Bellus Health, Chiesi, Merck, and Shionogi, remuneration for lectures from Bayer AG, Bellus Health, Chiesi, GlaxoSmithKline, Merck, and Shionogi, remuneration for consultancy from Bayer AG, Bellus Health, Chiesi, Merck, NeRRe Therapeutics, Nocion Therapeutics, and Shionogi, and has served on advisory committees for Applied Clinical Intelligence, Bayer AG, Bellus Health, Chiesi, Merck, NeRRe Therapeutics, Nocion Therapeutics, and Shionogi and on a Data and Safety Monitoring Board for Bayer AG.
Ethical Approval
The Institutional Review Board/Independent Ethics Committee at each center approved the protocol. The study was carried out in accordance with Good Clinical Practice guidelines, the Declaration of Helsinki, and the Council for International Organizations of Medical Sciences International Ethical Guidelines.
Consent to Participate
All participants provided written informed consent.
Footnotes
The full list of investigators is provided in the supplementary material.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Song WJ, Chang YS, Faruqi S, Kim JY, Kang MG, Kim S, Jo EJ, Kim MH, Plevkova J, Park HW, Cho SH, Morice AH. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J. 2015;45(5):1479–1481. doi: 10.1183/09031936.00218714. [DOI] [PubMed] [Google Scholar]
- 2.Morice AH, Millqvist E, Bieksiene K, Birring SS, Dicpinigaitis P, Domingo Ribas C, Hilton Boon M, Kantar A, Lai K, McGarvey L, Rigau D, Satia I, Smith J, Song WJ, Tonia T, van den Berg JWK, van Manen MJG, Zacharasiewicz A. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J. 2020;55(1):1901136. doi: 10.1183/13993003.01136-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young EC, Smith JA. Quality of life in patients with chronic cough. Ther Adv Respir Dis. 2010;4(1):49–55. doi: 10.1177/1753465809358249. [DOI] [PubMed] [Google Scholar]
- 4.Morice A, Dicpinigaitis P, McGarvey L, Birring SS. Chronic cough: new insights and future prospects. Eur Respir Rev. 2021;30(162):210127. doi: 10.1183/16000617.0127-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hulme K, Deary V, Dogan S, Parker SM. Psychological profile of individuals presenting with chronic cough. ERJ Open Res. 2017;3(1):00099–02016. doi: 10.1183/23120541.00099-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.KYORIN Pharmaceutical Co. Ltd (2022) MSD K.K. receives manufacturing and marketing approval of LYFNUA® Tablets, world's first selective P2X3 receptor antagonist for the treatment of chronic cough. https://www.kyorin-pharm.co.jp/en/news/2022/001603.shtml. Accessed 14 June 2022
- 7.Swissmedic (2022) Swiss public assessment report: Lyfnua. https://www.swissmedic.ch/dam/swissmedic/en/dokumente/zulassung/swisspar/68065-lyfnua-01-swisspar-20220812.pdf.download.pdf/SwissPAR_Lyfnua.pdf. Accessed 29 Sept 2022
- 8.Mazzone SB, McGarvey L. Mechanisms and rationale for targeted therapies in refractory and unexplained chronic cough. Clin Pharmacol Ther. 2021;109(3):619–636. doi: 10.1002/cpt.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwong K, Kollarik M, Nassenstein C, Ru F, Undem BJ. P2X2 receptors differentiate placodal vs. neural crest C-fiber phenotypes innervating guinea pig lungs and esophagus. Am J Physiol Lung Cell Mol Physiol. 2008;295(5):L858–L865. doi: 10.1152/ajplung.90360.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garceau D, Chauret N. BLU-5937: A selective P2X3 antagonist with potent anti-tussive effect and no taste alteration. Pulm Pharmacol Ther. 2019;56:56–62. doi: 10.1016/j.pupt.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Smith JA, Kitt MM, Butera P, Smith SA, Li Y, Xu ZJ, Holt K, Sen S, Sher MR, Ford AP. Gefapixant in two randomised dose-escalation studies in chronic cough. Eur Respir J. 2020;55(3):1901615. doi: 10.1183/13993003.01615-2019. [DOI] [PubMed] [Google Scholar]
- 12.Smith JA, Kitt MM, Morice AH, Birring SS, McGarvey LP, Sher MR, Li YP, Wu WC, Xu ZJ, Muccino DR, Ford AP, Protocol 012 Investigators Gefapixant, a P2X3 receptor antagonist, for the treatment of refractory or unexplained chronic cough: a randomised, double-blind, controlled, parallel-group, phase 2b trial. Lancet Respir Med. 2020;8(8):775–785. doi: 10.1016/S2213-2600(19)30471-0. [DOI] [PubMed] [Google Scholar]
- 13.McGarvey LP, Birring SS, Morice AH, Dicpinigaitis PV, Pavord ID, Schelfhout J, Nguyen AM, Li Q, Tzontcheva A, Iskold B, Green SA, Rosa C, Muccino DR, Smith JA. Efficacy and safety of gefapixant, a P2X(3) receptor antagonist, in refractory chronic cough and unexplained chronic cough (COUGH-1 and COUGH-2): results from two double-blind, randomised, parallel-group, placebo-controlled, phase 3 trials. Lancet. 2022;399(10328):909–923. doi: 10.1016/S0140-6736(21)02348-5. [DOI] [PubMed] [Google Scholar]
- 14.Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310(5753):1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 15.Davenport AJ, Neagoe I, Bräuer N, Koch M, Rotgeri A, Nagel J, Laux-Biehlmann A, Machet F, Coelho AM, Boyce S, Carty N, Gemkow MJ, Hess SD, Zollner TM, Fischer OM. Eliapixant is a selective P2X3 receptor antagonist for the treatment of disorders associated with hypersensitive nerve fibers. Sci Rep. 2021;11(1):19877. doi: 10.1038/s41598-021-99177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedrich C, Francke K, Gashaw I, Scheerans C, Klein S, Fels L, Smith JA, Hummel T, Morice A. Safety, pharmacodynamics, and pharmacokinetics of P2X3 receptor antagonist eliapixant (BAY 1817080) in healthy subjects: double-blind randomized study. Clin Pharmacokinet. 2022;61(8):1143–1156. doi: 10.1007/s40262-022-01126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein S, Gashaw I, Baumann S, Chang X, Hummel T, Thuß U, Friedrich C. First-in-human study of eliapixant (BAY 1817080), a highly selective P2X3 receptor antagonist: tolerability, safety and pharmacokinetics. Br J Clin Pharmacol. 2022;88(10):4552–4564. doi: 10.1111/bcp.15358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morice A, Smith JA, McGarvey L, Birring SS, Parker SM, Turner A, Hummel T, Gashaw I, Fels L, Klein S, Francke K, Friedrich C. Eliapixant (BAY 1817080), a P2X3 receptor antagonist, in refractory chronic cough: a randomised, placebo-controlled, crossover phase 2a study. Eur Respir J. 2021;58(5):2004240. doi: 10.1183/13993003.04240-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith JA, Holt K, Dockry R, Sen S, Sheppard K, Turner P, Czyzyk P, McGuinness K. Performance of a digital signal processing algorithm for the accurate quantification of cough frequency. Eur Respir J. 2021;58(2):2004271. doi: 10.1183/13993003.04271-2020. [DOI] [PubMed] [Google Scholar]
- 20.Birring SS, Brightling CE, Symon FA, Barlow SG, Wardlaw AJ, Pavord ID. Idiopathic chronic cough: association with organ specific autoimmune disease and bronchoalveolar lymphocytosis. Thorax. 2003;58(12):1066–1070. doi: 10.1136/thorax.58.12.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birring SS, Prudon B, Carr AJ, Singh SJ, Morgan MD, Pavord ID. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ) Thorax. 2003;58(4):339–343. doi: 10.1136/thorax.58.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin Nguyen A, Bacci ED, Vernon M, Birring SS, Rosa C, Muccino D, Schelfhout J. Validation of a visual analog scale for assessing cough severity in patients with chronic cough. Ther Adv Respir Dis. 2021;15:17534666211049743. doi: 10.1177/17534666211049743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raj AA, Pavord ID, Birring SS. Clinical Cough IV: what is the minimal important difference for the Leicester Cough Questionnaire? Handb Exp Pharmacol. 2009;187:311–320. doi: 10.1007/978-3-540-79842-2_16. [DOI] [PubMed] [Google Scholar]
- 24.Bretz F, Pinheiro JC, Branson M. Combining multiple comparisons and modeling techniques in dose-response studies. Biometrics. 2005;61(3):738–748. doi: 10.1111/j.1541-0420.2005.00344.x. [DOI] [PubMed] [Google Scholar]
- 25.European Medicines Agency (EMA) (1994) ICH E4 Dose response information to support drug registration. https://www.ema.europa.eu/en/ich-e4-dose-response-information-support-drug-registration. Accessed 31 Oct 2022
- 26.European Medicines Agency (EMA) (2014) Qualification of MCP Mod as an efficient statistical methodology for model-based design and analysis of Phase II dose finding studies under model uncertainty. https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/qualification-opinion-mcp-mod-efficient-statistical-methodology-model-based-design-analysis-phase-ii_en.pdf. Accessed 14 June 2022
- 27.McGarvey L, Smith JA, Morice A, Birring SS, Chung KF, Dicpinigaitis PV, Niimi A, Benninger MS, Sher M, Matsunaga Y, Miyazaki S, Machida M, Ishihara H, Mahmood A, Gomez JC. A randomized, double-blind, placebo-controlled, parallel-group Phase 2b trial of P2X3 receptor antagonist sivopixant for refractory or unexplained chronic cough. Lung. 2022;201:25–35. doi: 10.1007/s00408-022-00592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Food Drug Administration (FDA) (2009) FDA Guidance for industry. Drug-induced liver injury: premarketing clinical evaluation. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/drug-induced-liver-injury-premarketing-clinical-evaluation. Accessed 11 Nov 2019
- 29.Niimi A, Saito J, Kamei T, Shinkai M, Ishihara H, Machida M, Miyazaki S. Randomised trial of the P2X(3) receptor antagonist sivopixant for refractory chronic cough. Eur Respir J. 2022;59(6):2100725. doi: 10.1183/13993003.00725-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morice AH, Jakes AD, Faruqi S, Birring SS, McGarvey L, Canning B, Smith JA, Parker SM, Chung KF, Lai K, Pavord ID, van den Berg J, Song WJ, Millqvist E, Farrell MJ, Mazzone SB, Dicpinigaitis P, Chronic Cough Registry A worldwide survey of chronic cough: a manifestation of enhanced somatosensory response. Eur Respir J. 2014;44(5):1149–1155. doi: 10.1183/09031936.00217813. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Availability of the data underlying this publication will be determined according to Bayer’s commitment to the European Federation of Pharmaceutical Industries and Associations and Pharmaceutical Research and Manufacturers of America principles for responsible clinical trial data sharing, pertaining to scope, timepoint, and process of data access. Bayer commits to sharing upon request from qualified scientific and medical researchers patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the USA and European Union as necessary for doing legitimate research. This commitment applies to data on new medicines and indications that have been approved by the European Union and US regulatory agencies on or after January 1, 2014. Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymized patient-level data and supporting documents from clinical studies to do further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the study sponsors section of the portal. Data access will be granted to anonymized patient-level data, protocols, and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.