Abstract
Telomere shortening, a marker of cellular aging, has been linked to hospitalization and the severity of COVID-19. In this systematic review and meta-analysis, the mean difference in telomere length between non-severe and severe COVID-19 individuals was pooled to determine the association between short telomeres and COVID-19 severity. Relevant studies were retrieved through searches conducted in PubMed-Medline, Scopus, EMBASE, Medrxiv, Biorxiv, EuroPMC, and SSRN databases up to November 2022. Selected studies were systematically reviewed and assessed for risk of bias using AXIS tool. The standardized mean difference in telomere length between non-severe and severe COVID-19 was pooled using random-effects model. A total of thirteen studies were included in the review, out of which seven (1332 patients with the severe COVID-19 disease and 6321 patients with non-severe COVID-19) were eligible for meta-analysis. The estimated pooled mean difference in Leukocyte telomere length between severe COVID-19 and non-severe COVID-19 was 0.39 (95% CI − 0.02 to 0.81, I2 = 93.5%) with substantial heterogeneity. Our findings do not provide clear evidence for association of shorter telomere length and severe COVID-19 disease. More extensive studies measuring absolute telomere length with age and gender adjustments are needed to draw definitive conclusions on the potential causal association between telomere shortening and COVID-19 severity.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11262-023-02010-1.
Keywords: SARS-COV-2, Telomere shortening, Covid-19, Severity, Adverse outcome
Introduction
COVID-19, caused by SARS-Cov-2, has posed a significant public health threat since its emergence. Individual’s clinical presentations of COVID-19 range from a minor illness to a severe infection resulting in death, the major cause for concern [1]. Elderly individuals with co-morbidities, in particular, are at significant risk of developing a severe illness [2]. Several risk factors and biomarkers that contribute to COVID-19 severity have been investigated to understand why some individuals have more severe diseases than others [3]. Previous studies have shown a possible link between leukocyte telomere length (LTL) and the risk of developing severe disease among covid-19 patients [4]. Telomere length (TL) has long been linked to cellular aging and is considered to be the cell’s biological clock [5]. Telomeres are repeat sequences of short nucleotides located at the ends of linear chromosomes. Telomere shortening is a phenomenon in which a small segment of telomeric sequence is lost each time a cell divides due to the end replication problem, which protects the genetic information [5]. Shorter telomere length has been linked to cardiometabolic outcomes like stroke, myocardial infarction, and type 2 diabetes, according to meta-analyses [6–8]. LTL has recently been proposed as a marker of replicative capacity and repairability, which may influence an individual’s response to SARS-CoV-2 infection within the hematopoietic system, irrespective of age [9]. Shorter LTL has been linked to hospitalization and COVID-19 severity in a few case–control studies, but most of the studies have small sample sizes, which might not detect true associations [10–12]. Few bidrectional mendelian randomization studies shown contradicting results [13–15]. Therefore, we aim to do a systematic review and meta-analysis to determine the association between LTL and COVID-19 severity.
Methods
The present systematic review was conducted adhering to the preferred reporting items of systematic reviews and meta-analysis (PRISMA) 2020 guidelines [16], and the protocol was registered at PROSPERO (PROSPERO ID: CRD42022311400). Observational studies that evaluated the association of TL or telomerase activity with COVID-19 severity were systematically searched using key terms from inception to November 24, 2022, in PubMed-Medline, Scopus, EMBASE, Medrxiv, Biorxiv, EuroPMC, and SSRN databases. The search terms were constructed based on the PEO framework (i.e., Problem COVID-19, Exposure telomere shortening and Outcome-Severe COVID-19 disease). Additional keywords identified during the search were also included in the systematic search. A sensitivity and precision maximizing strategy were adopted to identify the relevant studies. The detailed search terms and strategies are reported in Suppl Table 1, 2. The last search was performed on November 24, 2022.
Studies that assessed the LTL or telomerase activity in COVID-19 individuals and studied its association with the severity of COVID-19 illness were considered for this systematic review. Interventional studies and studies that did not have relevant information for the systematic review were excluded.
Study screening and selection
The authors (BSB and AL) individually assessed the titles and abstracts of the papers listed from the electronic database search for their potential inclusion. Authors (BSB and AL) independently reviewed the full text of publications identified during screening. On mutual agreement, the authors compiled the final list of studies that met the inclusion and exclusion criteria for inclusion in the review.
Data extraction and analysis
Relevant details required to meet the proposed objectives were extracted from the studies using the data extraction form created in Microsoft Excel 2016. Author names, study titles, publication year, and contact information of the corresponding author, characteristics of the study population (age, gender, etc.), disease-related information (COVID-19 severity, hospitalization duration, etc.), and telomere-related information (method of telomere measurement, LTL, etc.) were recorded in the data extraction sheet. Data on central tendency (mean/median) and dispersion (standard deviation (SD)/standard error (SE)/interquartile range/95 percent confidence interval (CI)) were extracted independently from the included studies (HM and AL). The data were used for further analysis after being verified for consistency. For studies reporting outcome variables in units other than the conventional/standard units, the outcome parameters were converted to uniform units using standard conversion factors. All values were converted to mean and SD by using the formula proposed by Hozo et al. before performing the meta-analysis [17].
Studies reporting LTL for severe and non-severe groups were included in the meta-analysis. Mean and SD of the LTL were pooled for the non-severe COVID-19 and severe COVID-19 groups and standardized mean difference (SMD) was estimated. As the included studies have measured and reported LTL in different ways, we have used SMD (using Cohen’s method) as the effect measure in our meta-analysis [18]. COVID-19 involving respiratory dysfunction, radiographic lung abnormalities, intensive care unit (ICU) admission, and mechanical ventilation was considered to be “severe” [19, 20]. Hospitalization due to COVID-19 is also considered to be “severe” if the comparator group included non-hospitalized COVID-19. For studies reporting more than one severity group, groups were categorized into severe and non-severe, based on the above definition of severity, following which grand mean and SD were calculated using the following formula [21].
Decomposition of means and standard deviation
- For each group
-
oΣx = mean * n;
-
pΣx2 = SD2(n − 1) + ((Σx)2/n)
-
o
- The values are then added together
-
otn = sum of all (n)
-
ptx = sum of all Σx
-
qtxx = sum of all Σx2
-
o
- The combined calculations are
-
oCombined n = tn
-
pCombined mean = tx/tn
-
qCombined SD = sqrt((txx-tx2/tn)/(tn − 1))
-
o
Visual assessment of forest plots, the Cochran-Q test, and I-squared (I2) statistics were used to assess heterogeneity among included studies. The I2 value greater than 25% or Cochrane-Q less than 0.1 indicated the presence of heterogeneity between the included studies. A random-effect model with DerSimonian and Laird was applied [22]. The source of heterogeneity was further investigated by sensitivity analysis. Publication bias could not be assessed due to insufficient studies [23].
Data were recorded using a Microsoft excel sheet and analyzed using Stata version 16 (2019) [24]. Two-sided p < 0.05 was considered statistically significant except for the heterogeneity test, wherein p < 0.10 was considered significant [25].
Risk of bias assessment
The AXIS instrument, which consists of 20 components with three responses: Yes, No, and Do not know, was used to assess the risk of bias [26]. The quality of included studies was appraised separately by two authors (AL and HM), and disagreements were addressed by consensus.
Results
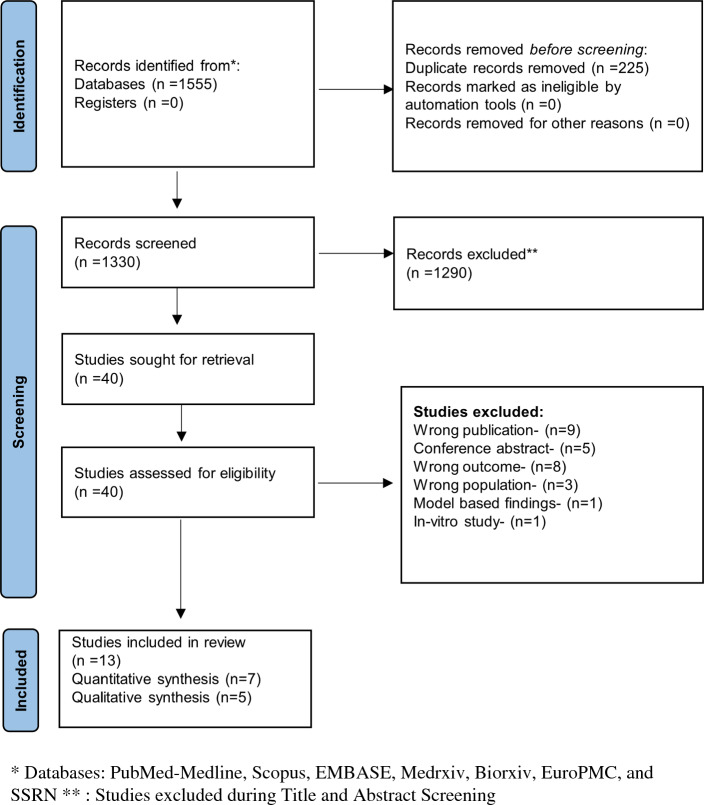
A total of 1596 studies were retrieved through a systematic literature search. The titles and abstracts of 1371 studies were reviewed after duplicates were removed. Forty of those studies were deemed relevant for full-text retrieval. Thirteen articles fulfilled the eligibility requirements during full-text screening and were included in the study, whereas 27 studies were excluded (Fig. 1). Out of the 13 included studies for the systematic review [10–15, 27–33], only seven studies that reported mean Telomere length were eligible for quantitative synthesis [10–12, 29, 30, 32, 33]. The characteristics of included studies and the reasons for the exclusion of individual studies are given in Table 1 and Suppl Table 3, respectively. Though we had performed the search in pre-print databases, none of the articles retrieved from the pre-print databases met the inclusion criteria. Thus, all our included studies were peer-reviewed.
Fig. 1.
PRISMA flow chart
Table 1.
Characteristics of included studies
| S. No | Study | Study groups (sample size) | Telomere measurement | Telomere length (Mean ± SD) | Statistical measure and analysis | Variables | Short telomere cut-off | Conclusion |
|---|---|---|---|---|---|---|---|---|
| 1 | Sanchez-Vazquez et al. [10] |
COVID-19 1. Mild (7) 2. Moderate (35) 3. Severe (39) 4. Acute (5) |
q-PCR (relative ratio) TRF HT-Q-FISH |
13.81 ± 1.66 13.97 ± 1.96 12.33 ± 2.82 13.64 ± 0.69 (Data were extracted from Fig. 6A of the original study and the mean ± SD was calculated) |
r − 0.145 (Correlation) | Short telomeres and severity score | < 3 kb | Shorter telomeres are associated with increased severity in COVID-19 patients |
| 2 | Dos Santos et al. [11] |
1. Symptomatic COVID-19 negative (12) 2. COVID-19 positive no hospitalization (15) 3. COVID-19 respiratory dysfunction (17) 4. COVID-19 ICU mechanical ventilation (9) |
q-PCR (relative ratio) |
94.36 ± 51.10 70.80 ± 50.16 35.88 ± 9.624 11.05 ± 3.134 (Data were obtained from the author through private correspondence via email) |
Ref 0.043 0.002 (Ref: no hospitalization) |
Short telomeres and COVID-19respiratory dysfunction (moderate) Short telomeres (< median LTL) and COVID-19 ICUmechanical ventilation (severe) |
Median LTL (38.8 AU) | Shorter LTL is significantly associated with severe COVID-19 infection |
| 3 | Froidure et al. [12] |
Hospitalized COVID-19 patient 1. TL < 10 percentile (28) 2. TL > 10 percentile (42) |
Flow-FISH | – |
OR 3.24 (1.21–8.55) Ref: TL > 10% |
Short telomeres vs. critical disease (ICU/Death) | TL < 10% | Short telomeres are associated with increased risk for ICU admission or mortality, regardless of age in COVID-19 patients |
| 4 | Wang et al. [33] |
1. COVID-19 with hospitalization (5861) 2. COVID-19 without hospitalization (914) |
q-PCR (relative ratio) |
− 0.14 ± 0.97 − 0.03 ± 1.00 (Data were obtained from Table 1 of the original study |
Composite: 1.17 (1.05–1.30) Component: 1.17 (hospitalization) 1.32 (critical care) 1.36 (respiratory support) 1.36 (Death) Ref: (No hospitalization) |
LTL (age adjusted) (per 1 SD shorter) and adverse outcomes | (per 1 SD shorter) | Shorter LTL is independently associated with severe COVID-19 and hospitalization |
| 5 | Alessia Mongelli et al. [31] |
1. COVID-19 free (144) 2. Post-COVID-19 (117) |
q-PCR (absolute) |
10.67 ± 11.69 3.03 ± 2.39 (Data were obtained from Table II of the original study) |
– | – | – | TL shortening is associated with significant acceleration of biological age, mainly in the younger COVID-19 individuals |
| 6 | McGroder et al. [30] |
COVID-19 survivors (4 months post-COVID-19) 1. Normal CT (31) 2. Non-fibrotic (13) 3. Fibrotic (32) |
q-PCR-relative ratio |
1.4738 (0.1798) 1.3748 (0.2164) 1.3714 (0.2812) (Data were obtained from the author through private correspondence via email) |
1.5 (1.06–1.72) Ref: Normal CT (Multivariable logistic regression) |
Age-adjusted Telomere length (per 10% decrease in TL) and fibrotic-like patterns | (per 10% decrease in TL) | Short telomeres are associated with fibrotic Interstitial Lung Abnormalities in COVID-19 survivors |
| 7 | Franzen et al. [29] |
1. COVID-19 with ARDS (12) 2. COVID-19 without ARDS (5) |
Flow-FISH |
5.83 ± 1.80 6.24 ± 1.88 (Data were extracted from Fig. 6A of the original study and the mean ± SD was calculated) |
– | – | – | Shortened telomere length or accelerated epigenetic age is not associated with severe COVID-19 |
| 8 | Benetos et al. [27] |
1. COVID-19 (17) 2. Non-COVID-19 (21) |
TESLA (absolute) SB |
3.42 ± 0.33 3.52 ± 0.39 6.52 ± 0.57 6.64 ± 0.80 (Data were obtained from Supplementary Table 1 of the original study) |
– | – | – | Short telomeres are associated with lower lymphocyte count in COVID-19 older patients |
| 9 | Retuerto et al. [32] |
1. Healthy control group (169) 2. COVID-19 hospitalized cohort (251) WHO OS 3. Hospitalized no oxygen therapy WHO OS 4. Oxygen by mask or nasal prongs WHO OS 5. Non-invasive ventilation or high-flow oxygen WHO OS 6. Intubation and mechanical ventilation WHO OS 7. Ventilation and additional organ support WHO OS 8. Death |
q-PCR-absolute length |
7.1536 ± 0.4719 (non-severe COVID-19-Combined mean ± SD of WHO OS 3 & 4) 4.8677 ± 1.2554 (Severe COVID-19—Combined mean ± SD of WHO OS 5, 6, 7, 8) (Data were extracted from Fig. 2B of the original study and converted to mean ± SD) |
r = − 0.03, p = 0.72 (correlation analysis) p = 0.6 (ANOVA) |
Duration of oxygen therapy (a measure of covid-19 severity) vs age-adjusted TL Either age-adjusted or absolute (Kb) TL was similarly decreased in all the groups by COVID-19 severity scores |
Shorter TL is not associated with COVID-19 clinical outcomes nor with persistent post-COVID-19 manifestations | |
| 10 | Cao et al. [28] |
1. Healthy individuals (232) 2. Non-severe COVID-19 (194) 3. Severe COVID-19 (213) |
DNA methylation-based estimator |
4.8676 ± 1.2554 3.7288 ± 1.092 (Data were extracted from Fig. 1f of the original study and the mean ± SD was calculated) |
p < 0.05 (Distribution of telomere attrition in health, non-severe COVID-19 and severe COVID-19) | DNAm TL attrition and severe COVID-19 patients |
Accelerated epigenetic aging is associated with the risk of SARS-CoV-2 infection and developing severe COVID-19 Severe COVID-19 patients had significant DNAm TL attrition acceleration compared with non-severe COVID-19 patients |
|
| 11 | Jiang et al. [14] |
1. Symptomatic Hospitalized COVID-19 patients (9,986) 2. Population controls (1,877,672) (European-ancestry participants) |
q-PCR-relative ratio | NR | OR 0.85 (0.70 to 1.03) | OR of COVID-19 outcomes per-standard deviation (SD) increase in genetically predicted LTL (IVW meta-analysis) | Per SD increase | Genetic evidence does not support shorter LTL as a causal risk factor for COVID-19 susceptibility or severity |
| 12 | Xu et al. [15] |
1. COVID-19 patients with severe symptoms (5,101) 2. Control including individuals without severe COVID-19/without COVID-19 (1,383,241) |
NR | NR |
OR 0.97 (0.66 to 1.42) OR − 0.01 (− 0.02 to − 0.001) |
Odds of severe COVID-19 with short TL Odds of telomere shortening due to severe COVID-19 (IVW meta-analysis) |
NR |
No causal relationship between telomere length and COVID-19 severity Severe COVID-19 could lead to accelerated telomere wear and shorter telomere lengths |
| 13 | Huang et al. [13] |
1. Critically ill COVID-19 (1,388,342) 2. LTL (4,721,74) |
NR | NR | OR 1.00 (0.79–1.28) | Odds of critically ill COVID-19 with LTL (IVW meta-analysis) | NR | LTL was not causally related to critically ill COVID‐19 |
ARDS acute respiratory distress syndrome, AU arbitrary unit, FISH fluorescence in situ hybridization, HT-Q-FISH high-throughput quantitative fluorescence in situ hybridization, ICU intensive care unit, IVW inverse-variance weighted, LTL leukocyte telomere length, NR not reported, OR odds ratio, q-PCR quantitative polymerase chain reaction, sTL shorter telomere length, SD standard deviation, SB southern blotting, TL telomere length, TRF telomere restriction fragment, TeSLA telomere shortest length assay, kb kilobasepairs, Ref reference, WHO OS World Health Organization ordinal scale
Seven studies included for meta-analysis consisted of 6321 participants with a mean age of 53.07 years in the non-severe COVID-19 group and 1332 participants with a mean age of 59.34 in the severe COVID-19 group. The sample size of individual studies ranged from 17 to 6775. Of the thirteen studies, three studies were conducted in 2020 [12, 30, 33], probably during the first wave of the pandemic, five studies were conducted in 2021 [10, 11, 27, 29, 31], and five studies were conducted in 2022 [13–15, 28, 32]. None of the studies stated the COVID-19 strain. In all the studies, peripheral venous blood samples were used. Telomere length was measured using the q-PCR method in seven studies [10, 11, 14, 29–33], TESLA [27] and southern blotting (SB) [27] in one study, Flow-FISH method [10, 12] in two studies, and DNA methylation-based estimation in one study [28]. Of the thirteen studies included in this systematic review, seven studies [10–12, 29, 30, 32, 33] measured LTL in COVID-19 individuals with varying severity, two [27, 31] studies measured LTL in COVID-19 vs. non-COVID-19 individuals with no mention of severity and three studies [14, 28, 32] studied LTL and COVID-19 outcomes association using two-sample bidirectional mendelian randomization.
Absolute LTL was reported in three studies [10, 27, 31], and the relative ratio was reported in five studies [10–12, 14, 30, 33]. None of the studies reported telomerase activity. Out of the eleven studies which studied the association between telomere shortening and COVID-19 severity, seven studies [11–15, 30, 33] reported the odds of getting a severe COVID-19 disease with short telomeres and two studies reported a correlation between short telomeres and COVID-19 severity [10, 32]. The cut-off for short telomeres varied across studies including LTL < 3 kb, median LTL, LTL < 10 percentile, per 1 SD shorter, per 10% decrease in LTL, and per SD increase; hence, meta-analysis of the odds ratio could not be performed. Of the thirteen studies included for systematic review, nine studies concluded that short telomeres were associated with a higher risk of COVID-19 severity/susceptibility [10–12, 27, 28, 30–33] whereas four studies concluded that telomere shortening is not associated with the severity of COVID-19 [13–15, 29].
Meta-analysis
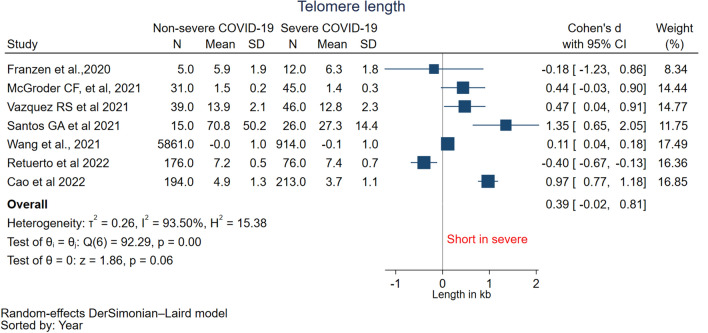
The meta-analysis pooled the mean LTL from 1332 patients with severe COVID-19 disease and 6321 patients with non-severe COVID-19 disease from seven studies [10, 11, 28–30, 32, 33] to evaluate the relationship between LTL and COVID-19 severity. The estimated pooled standardized mean difference in LTL was 0.39 (95% CI − 0.02 to 0.81, I2 = 93.5%) with high heterogeneity suggesting no significant difference in telomere length between non-severe and severe COVID-19 (Fig. 2). The standardized mean difference was also pooled using a fixed-effect model which showed similar results (0.40 (− 0.05 to 0.84)) (Supp Fig. 1). Each study’s influence on the pooled mean difference was assessed using sensitivity analysis by omitting one study at a time (Suppl Table 4). Omitting the study Franzen et al. [29] influenced the pooled mean difference (0.49 (95% CI 0.02 to 0.97, I2 = 84.61%) which was significant between the severe and non-severe COVID-19. Subgroup analysis based on age-adjusted LTL revealed that only age-unadjusted LTL subgroup showed significant difference (0.89 (0.48 to 1.29)) whereas age-adjusted LTL subgroup showed no significant difference in the mean LTL (0.00 (− 0.36 to 0.36) (Supp Fig. 2).
Fig. 2.
Forest plot of pooled mean difference of telomere length between non-severe COVID-19 and severe COVID-19
Risk of bias assessment
The current synthesis appraised the methodological quality of the eight cross-sectional studies included in the systematic review using the AXIS tool. The response was “Yes” in all the studies for 3 out of 20 questions related to statistical significance, description of statistical method, and description of analysis in the form, indicating a low risk of bias for these questions. The response was “No” or “Do not know,” signifying moderate-serious bias in all the studies for sample size justification and non-response bias questions. For all the other questions, most of the studies showed a low risk of bias (Suppl Table 5).
Discussion
The current systematic review and meta-analysis were undertaken to summarize the evidence on the association between telomere shortening and COVID-19 severity. The mean difference in LTL between individuals with severe COVID-19 and individuals without severe COVID-19 was pooled; the results indicate that there was no significant difference in LTL between the two groups. However, sensitivity analysis performed by omitting Franzen et al. [29] indicates a significantly shorter LTL in severe COVID-19. A qualitative analysis of studies that examined the odds of having severe COVID-19 disease with short telomeres revealed conflicting results. Hence, the current evidence is insufficient for decisive understanding regarding the causal relationship between telomere shortening and COVID-19 severity.
Most of the studies that reported a link between short telomeres and COVID-19 severity measured LTL after SARS-COV-2 infection, making it difficult to conclude whether telomere shortening preceded or was caused by COVID-19 [10–12, 29, 30]. In light of this, Wang et al. findings suggest that LTL is associated with a higher risk of poor COVID-19 outcomes regardless of age [33], where LTL was measured several years before the onset of COVID-19. The results of this study show reverse causality is much less likely and suggests that SARS-CoV-2 does not cause telomere shortening. On contrary, three bidirectional mendelian randomization studies report that LTL is not causally related to critical COVID-19 and vice versa [13–15].
As pre-existing chronic diseases such as hypertension, type 2 diabetes, atherosclerosis, and cancer are known to increase the risk of severe COVID-19 infection [34], it stands to reason that telomere shortening could have occurred prior to COVID-19 infection as a result of the pre-existing illnesses. Short telomeres have been associated with a number of disorders, including hypertension, type 2 diabetes, stroke [6, 7], lung cancer [35], and overall survival in patients with colorectal cancer [36], and the majority of these conditions are also linked to unfavorable outcomes in COVID-19. In the general population, short telomeres have been linked to an increased risk of all-cause mortality [37] and an even higher risk of disease-specific mortality. Age is another confounding factor, which is linked to both telomere shortening and severe COVID-19 illness. According to our observations from age-adjusted and age-unadjusted LTL subgroup analysis, there is no apparent variation in telomere length between COVID-19 severe and non-severe patients in the age-adjusted subgroup.
Although few studies show a link between telomere shortening and COVID-19 severity irrespective of age, the exact mechanisms that link telomere shortening to the severity of COVID-19 are unclear. TL-dependent T-cell lymphopenia, commonly witnessed in COVID-19, is the mechanism put forward by many authors, which is believed to be the connecting link between telomere shortening and COVID-19 severity [11, 33, 38]. Among the studies included in this systematic review, only one study reported significantly reduced lymphocyte count in COVID-19 compared to non-COVID-19 individuals [27]. In Froidure et al. study, lymphocyte count in COVID-19 individuals with short telomeres (TL < 10th percentile) is not reported to be significantly different from COVID-19 individuals with long telomeres (TL > 10th percentile) [12]. Other studies have not reported any data on lymphocyte count. Therefore, the proposed TL-dependent T-cell lymphopenia mechanism is still considered a hypothesis and needs validation. All the studies have measured Telomere in leukocytes as LTL has been used as a proxy for TL in leukocyte lineages and other somatic cells [9, 33]. Further studies measuring TL in isolated T-cells and investigating lymphocyte count may provide some evidence to support the proposed mechanism. Animal studies show that telomerase activation effectively treats diseases associated with aging and telomere damage, such as pulmonary fibrosis, by reversing the process of telomere shortening [39], opening up a new avenue for research in COVID-19 treatment. Apart from telomere shortening, several other risk factors such as Kynurenine, genetic factors, etc., for severe COVID-19 have been proposed [40–42], and their possible relationships to telomere length need to be investigated. There are certain limitations in our systematic review, primarily due to lack of primary data or the heterogeneity of available data from current literature. First, the method used to measure telomeres varied across studies, with q-PCR being the most commonly used and others including Flow-FISH, Southern blotting, and so on. Second, some studies reported LTL in absolute numbers, whereas others reported relative ratios. Third, each study adopted a different threshold or cutoff value for short telomeres, adding heterogeneity. Wang et al. [33] was the most heterogeneous of the included studies as it reported log-transformed and z-standardized mean LTL. Fourth, not all the studies reported age- and gender-adjusted LTL; hence, confounding effect of age and gender on telomere shortening or COVID-19 severity could not be ruled out. Telomere shortening could also be influenced by pre-existing illnesses; however, this was not demonstrated in our meta-analysis because none of the included studies provided information on co-morbidities.Another lacuna observed in most of the included studies is the small sample size. Furthermore, the definition of COVID-19 severity was not standard across all the studies which is also seen to be limitation.
Conclusion
Our systematic review found conflicting results on the association between shorter telomere length and COVID-19 severity. According to the findings of our meta-analysis, there is no proof that telomere shortening causes severe COVID-19 illness. However, additional substantial high-quality studies examining absolute telomere length that are controlled for age, gender, and co-morbidities are needed in order to draw firm conclusions in this regard.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are thankful to Santos GA et al. 2021 and McGroder CF et al. 2021 for providing us with the data on mean telomere length that greatly helped our research.
Author contributions
HM contributed to conceptualization, data curation, formal analysis, and original draft. LA contributed to data curation, formal analysis, and review & editing. BBS contributed to conceptualization, data curation, formal analysis, inputs on original draft Investigation, methodology, software, and review & editing.
Funding
This study received no funding. However, the health technology resource center is supported by Department of Health Research, New Delhi.
Data availability
All data used for the analysis were obtained from secondary sources that are publicly available. The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Declarations
Conflict of interest
None.
Ethical approval
This article does not involve human participants or animals.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Madhumitha Haridoss and Lavanya Ayyasamy have contributed equally to this work.
References
- 1.Thevarajan I, Buising KL, Cowie BC. Clinical presentation and management of COVID-19. Med J Aust. 2020;213(3):134–139. doi: 10.5694/mja2.50698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perrotta F, et al. COVID-19 and the elderly: insights into pathogenesis and clinical decision-making. Aging Clin Exp Res. 2020;32(8):1599–1608. doi: 10.1007/s40520-020-01631-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballow M, Haga CL. Why do some people develop serious COVID-19 disease after infection, while others only exhibit mild symptoms? J Allergy Clin Immunol Pract. 2021;9(4):1442–1448. doi: 10.1016/j.jaip.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aviv A. Telomeres and COVID-19. FASEB J. 2020;34(6):7247–7252. doi: 10.1096/fj.202001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88(2):557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 6.D'Mello MJ, et al. Association between shortened leukocyte telomere length and cardiometabolic outcomes: systematic review and meta-analysis. Circ Cardiovasc Genet. 2015;8:82–90. doi: 10.1161/CIRCGENETICS.113.000485. [DOI] [PubMed] [Google Scholar]
- 7.Tellechea ML, Pirola CJ. The impact of hypertension on leukocyte telomere length: a systematic review and meta-analysis of human studies. J Hum Hypertens. 2017;31(2):99–105. doi: 10.1038/jhh.2016.45. [DOI] [PubMed] [Google Scholar]
- 8.Haycock PC, et al. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aviv A, Shay JW. Reflections on telomere dynamics and ageing-related diseases in humans. Philos Trans R Soc Lond B Biol Sci. 2018 doi: 10.1098/rstb.2016.0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez-Vazquez R, et al. Shorter telomere lengths in patients with severe COVID-19 disease. Aging (Albany NY) 2021;13(1):1–15. doi: 10.18632/aging.202463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dos Santos GA, et al. Shorter leukocyte telomere length is associated with severity of COVID-19 infection. Biochem Biophys Rep. 2021;27:101056. doi: 10.1016/j.bbrep.2021.101056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Froidure A, et al. Short telomeres increase the risk of severe COVID-19. Aging (Albany NY) 2020;12(20):19911–19922. doi: 10.18632/aging.104097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang D, et al. Association between COVID-19 and telomere length: a bidirectional Mendelian randomization study. J Med Virol. 2022;94:5354. doi: 10.1002/jmv.28008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang L, et al. Telomere length and COVID-19 outcomes: a two-sample bidirectional mendelian randomization study. Front Genet. 2022 doi: 10.3389/fgene.2022.805903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu W, et al. Causal association of epigenetic aging and COVID-19 severity and susceptibility: a bidirectional Mendelian randomization study. Front Med. 2022 doi: 10.3389/fmed.2022.989950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):1–10. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen J. Statistical power analysis for the behavioral sciences. Abingdon: Routledge; 2013. [Google Scholar]
- 19.WHO . Clinical management of COVID-19: interim guidance, 27 May 2020. Geneva: World Health Organization; 2020. [Google Scholar]
- 20.Taskforce NCCE (2022) Caring for people with COVID‐19: living guidelines.
- 21.Altman DG, Machin D, Bryant TN, Gardner MJ. Statistics with confidence: confidence intervals and statistical guidelines. London: BMJ Books; 2000. [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Egger M, et al. Bias in meta-analysis detected by a simple. Graphical Test BMJ. 1997;315:629. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.StataCorp . Stata statistical software: release 16. College Station: StataCorp LLC; 2019. [Google Scholar]
- 25.Deeks JJ, et al. Cochrane handbook for systematic reviews of interventions. New Jersey: Wiley; 2019. Analysing data and undertaking meta-analyses; pp. 241–284. [Google Scholar]
- 26.Downes MJ, et al. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS) BMJ Open. 2016;6(12):e011458. doi: 10.1136/bmjopen-2016-011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benetos A, et al. The nexus between telomere length and lymphocyte count in seniors hospitalized with COVID-19. J Gerontol A Biol Sci Med Sci. 2021;76(8):e97–e101. doi: 10.1093/gerona/glab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao X, et al. Accelerated biological aging in COVID-19 patients. Nat Commun. 2022 doi: 10.1038/s41467-022-29801-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franzen JAO, et al. Epigenetic clocks are not accelerated in COVID-19 patients. Int J Mol Sci. 2021 doi: 10.3390/ijms22179306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGroder CF, et al. Pulmonary fibrosis 4 months after COVID-19 is associated with severity of illness and blood leucocyte telomere length. Thorax. 2021;76(12):1242–1245. doi: 10.1136/thoraxjnl-2021-217031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mongelli A, et al. Evidence for biological age acceleration and telomere shortening in COVID-19 survivors. Int J Mol Sci. 2021 doi: 10.3390/ijms22116151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Retuerto M, et al. Shorter telomere length is associated with COVID-19 hospitalization and with persistence of radiographic lung abnormalities. Immun Ageing. 2022 doi: 10.1186/s12979-022-00294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Q, et al. Shorter leukocyte telomere length is associated with adverse COVID-19 outcomes: a cohort study in UK Biobank. EBioMedicine. 2021 doi: 10.1016/j.ebiom.2021.103485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khoury E, et al. Differences in outcomes and factors associated with mortality among patients with SARS-CoV-2 infection and cancer compared with those without cancer: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(5):e2210880. doi: 10.1001/jamanetworkopen.2022.10880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, et al. The association of telomere length in peripheral blood cells with cancer risk: a systematic review and meta-analysis of prospective studies. Cancer Epidemiol Biomarkers Prev. 2017;26(9):1381–1390. doi: 10.1158/1055-9965.EPI-16-0968. [DOI] [PubMed] [Google Scholar]
- 36.Jia H, Wang Z. Telomere length as a prognostic factor for overall survival in colorectal cancer patients. Cell Physiol Biochem. 2016 doi: 10.1159/000438614. [DOI] [PubMed] [Google Scholar]
- 37.Wang Q, et al. Telomere length and all-cause mortality: a meta-analysis. Ageing Res Rev. 2018 doi: 10.1016/j.arr.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Aviv A. Short telomeres and severe COVID-19: the connection conundrum. EBioMedicine. 2021 doi: 10.1016/j.ebiom.2021.103513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Jesus BB, et al. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol Med. 2012 doi: 10.1002/emmm.201200245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willette AA, et al. Using machine learning to predict COVID-19 infection and severity risk among 4510 aged adults: a UK Biobank cohort study. Sci Rep. 2022;12(1):7736. doi: 10.1038/s41598-022-07307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mangge H, et al. Increased kynurenine indicates a fatal course of COVID-19. Antioxidants (Basel) 2021 doi: 10.3390/antiox10121960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guin D, et al. Human genetic factors associated with pneumonia risk, a cue for COVID-19 susceptibility. Infect Genet Evol. 2022;102:105299. doi: 10.1016/j.meegid.2022.105299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used for the analysis were obtained from secondary sources that are publicly available. The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.