Abstract
Extracellular vesicles (EVs) are a group of nanoscale membrane-bound organelles including exosomes, microvesicles (MVs), membrane particles, and apoptotic bodies, which are released from almost all eukaryotic cells. Owing to their ingredients, EVs can be employed as biomarkers for human diseases. Interestingly, EVs show favorable features as candidates for targeted drug delivery and thus, they are suggested as ideal drug carriers as well as good vaccines for various human diseases including cancer. Among various drugs loaded in EVs for targeted drug delivery, immune checkpoint inhibitors (ICIs), including antibodies against programmed cell death-1 (PD-1), programmed death-ligand 1 (PD-L1), and cytotoxic-T-lymphocyte-associated protein 4 (CTLA-4), have attracted an increasing attention for cancer researchers and clinicians. Animal and clinical studies have shown combination of EVs and immunotherapy antibodies to improve the efficacy and reduce possible side effects in systemic administration of ICIs. In this review, we discuss the EVs and their significance in drug delivery with a focus on cancer immunotherapy agents.
Graphical Abstract
Keywords: Extracellular vesicle (EV), Exosome, Drug delivery, Immunotherapy, Programmed cell death-1 (PD-1), Programmed death-ligand 1 (PD-L1)
Introduction
Cancers are responsible for the leading cause of mortality around the world, which also affects quality of life of millions of people [1]. The burden of cancer is increasing annually where according to the GLOBOCAN cancer statistics 2020, more than 19 million new cases of cancer and 10 million related deaths were recorded for 2020 [2], with both showing a rise compared to those for 2018 (18.1 million newly diagnosed cases and 9.6 million deaths) [3]. Since the discovery of cancer pathogenesis basics, the main goal of oncologists and cancer researchers was to find efficient treatments, which exclusively target and eliminate the cancer cells without posing damage to healthy tissues. Anticancer therapies still remain mainly dependent on surgical approaches, which along with associated therapies including chemotherapy, radiotherapy, and cryotherapy constitute the main strategies and pillars of human cancer treatment [4]. Those treatments are considerably aggressive, non-specific, and destructive to healthy tissues. Cancer immunotherapy, instead, shows less aggressiveness and damaging impact on healthy tissues aiming at activation of host immune defenses against cancer cells and thus introduced as the forth modality of cancer therapy [5]. This novelty in anticancer therapies was based on the fact that a majority of tumor cells express some antigens on their surface, which can induce immune rejection actions. This anticancer strategy has shown practical results in rejection of tumors alone or in combination with other approaches [6, 7], such as chemotherapy [8, 9], radiotherapy [10, 11], interferons [12], nanoparticles [13], or using more than one ICI together [14]. As a revolutionizing anticancer strategy, immunotherapy has strongly improved outcomes among cancer patients [15].
Extracellular vesicles (EVs) are a group of submicron membrane-bound organelles, which differ in origin, ingredient, production process, size, and shape; however, they are all composed of a lipid bilayer membrane and released from almost all living cells [16]. EVs carry various biologic macromolecule ingredients including DNA, RNA, proteins, and lipids, which are released into the extracellular environment, transfer cargo to recipient cells, and thus play important roles in cell-to-cell communication [17]. This is due to high and heterogeneous protein content so that they are called “vectorial signalosomes” [18, 19]. The involvement of EVs in intercellular communication is extensively documented among all kingdoms [20]. EVs can affect the phenotype and features of target cells through modification of gene expression in various routes including de novo translation, post-translational modification, and impacting signaling pathways [21–23]. Additionally, this process can help cells to eliminate toxic or redundant unnecessary compounds, such as chemotherapeutic drugs [24], components of infectious organisms, and intracellular components [25, 26]. EVs are shed from the majority of cells including platelets and megakaryocytes and accordingly can be found in circulation as well as biological fluids like cerebrospinal fluid (CSF), amniotic fluid, urine, saliva, bile, breast milk, and blood [27–30]. Functionally, EVs are suggested as essential players in various biologic processes, and are potentially involved in the pathogenesis as well as being considered biomarkers for a wide variety of human diseases, such as autoimmune disorders, neurodegenerative diseases, diabetes, cardiometabolic diseases, infection, and cancer [28, 31–33]. The potential for transporting pharmacologic compounds has made EVs as promising vehicles for drug delivery with potentials in treatment of various human diseases like inflammatory conditions, septic shock, renal injury, and malignancies, such as prostate, colon, and breast cancers [34–41]. The aim of this review is to discuss the potentials of EVs in delivery of immunotherapies for cancer treatment.
Cancer immunotherapy: advances and challenges
Generally, tumor immunotherapy is considered an alternative cancer treatment modality defined as an approach to modify or stimulate host immune responses against tumor cells [42, 43]. Compared to other therapeutic modalities being employed to treat early tumors, the primary aim of immunotherapy is to prevent tumor metastasis and improve the quality of life among affected patients [42]. Historically, it was first documented in 1891 when a bone sarcoma surgeon, William B. Coley, found the efficacy of streptococcal toxins (Coley toxins) in shrinking inoperable malignant tumors particularly bone and soft tissue sarcomas [44, 45]. These efforts, although did not achieve considerable success due to unacceptability by clinicians as well as development of radiotherapy and chemotherapy for cancer therapy, were the first experiences of cancer immunotherapy by the “father of immunotherapy” [45]. Later, during 1970–1980s researchers found that activation of lymphocytes by lectins or interleukin (IL)-2 gives them the capability of targeting cancer cells [46–48]. Those findings in later years motivated clinical trials for evaluation of the efficacy of cytokines (e.g., IL-2 and interferon-α; IFN-α) in cancer therapy, which then confirmed for clinical use [49–52]. Rather than cytokines, cancer immunotherapy is conducted through several other compounds like vaccines, stimulated effector cells, and antibodies [42]. Cancer vaccines (e.g., HER2/neu [53], Canvaxin [54], M-VAX [55], and Oncophage [56]) are composed of protein/polypeptides, whole cell, and viral vector vaccines that induce immunosurveillance against a specific antigen on cancer cells and develop active immunity against tumors and thus called active immunotherapy. Promising results are reported for stimulation of anti-tumor responses particularly from dendritic cells (DCs) pulsed with anti-CD44 immunoglobulin G (IgG) opsonized tumor cells [57]; however, on the viewpoint of clinical applicability, those therapeutic strategies are extremely costly and not available for treating a wide range of patients that makes their clinical application restricted to small settings [42]. Among cancer immunotherapy modalities, immune checkpoint inhibitors or blockers (ICIs, ICBs) have seen the most advances being considered leading approaches in tumor immunotherapy [58]. This approach targets immune checkpoints that their interaction with corresponding receptors compromises the anti-tumor function of T cells [59]. In fact, tumors exploit that system, including immune checkpoints and their ligands that are reportedly upregulated in the tumor microenvironment, to escape immune responses, and ICIs are developed to target those receptors or their ligands to reverse the immunocompromising effect of tumors [17]. Among the targets of ICI compounds, the programmed cell death 1 (PD-1) (also known as cluster of differentiation 279 or CD279), the cytotoxic-T-lymphocyte-associated protein 4 (CTLA-4; CD152), and T cell immunoglobulin mucin-3 (TIM-3) are the best-known examples entered clinical practice, while the therapeutic strategy for the former is through targeting the interaction between PD-1 receptor and its ligand (PD-L1) [58, 60, 61]. PD-1/PD-L1 and CTLA-4 regulate the function and activation of T cells, and hindering those actions is the underlying mechanism for several ICIs, which have shown unprecedented advances in cancer immunotherapy during the past decades in combating various malignancies [17]. Accordingly, several antibodies targeting these molecules received approval by the US Food and Drug Administration (FDA). These include nivolumab, pembrolizumab, and cemiplimab as approved inhibitors of PD-1 receptor, and atezolizumab, avelumab, and durvalumab received approval for anti-PD-L1 immunotherapy, as well as ipilimumab for targeting CTLA-4 [17, 58]. Administration of ICIs significantly improved clinical outcomes among patients with various cancer types (an average response of 20–40% for over 80% of refractory Hodgkin’s lymphoma) [62, 63]. Those metrics are unprecedented for ICIs; however, the rate and durability of response to ICI therapy are heterogeneous and still remain low for a majority of cases [64, 65]. For instance, although melanoma patients show the highest response to ICIs compared with other cancers, a majority of those patients receiving immunotherapy (by 70%) do not respond to anti-PD-1 therapy, while 20–30% of responders also experience tumor relapse and progression [66, 67]. The underlying mechanisms responsible for either primary (also called intrinsic) or acquired resistance to ICIs among cancer patients are the subject to a considerable number of studies, suggesting complex identity of resistance and involvement of various aspects, including tumor-intrinsic factors, such as genetic alterations, metabolism, inflammation, and neovascularization, tumor local microenvironment factors, and host-related factors [64, 65, 68–72]. Possible causes include low or no expression of PD-L1, tumor mutational burden, and epigenetic properties, disturbances in critical signaling pathways, and gut bacterial species in hosts [58]. In this regard, extracellular vesicles have emerged as role players in contribution to chemoresistance and as carriers of medications in cancer therapy.
Extracellular vesicles: classification, functions in tumor immunity, and potentials in cancer immunotherapy
First evidence of vesicle discovery was reported by Erwin Chargaff and Randolph West upon study of thromboplastic protein in 1946 [73]. They reported the impact of centrifuge-isolated cell-free component of blood on clotting time [73]. Twenty years later, Peter Wolf using electron microscopy revealed the identity of subcellular fraction contained platelet-derived vesicles [74]. Those subcellular particles in literature were initially referred as several terms like microvesicles, ectosomes, and shedding vesicles [75–77]. To date, we know that both prokaryotes and eukaryotes, including almost all human cells, release vesicles to the outer environment [78–80]. This process in bacteria helps intercellular communication and coordination of their social activities, together contributing to act as multicellular organisms, while it also plays role in multicellular organisms to function as an ecosystem [81]. Packaging biologic cargo in vesicles, which is selective, provides protection against degradation, while it also suggests a highly efficient storage and delivery approach targeting specific recipients based on surface receptors [26, 82]. EVs are known to play a role in various substantial biological processes like intercellular communication, cell survival, angiogenesis, coagulation, and inflammation, as well as functioning in various organs, such as the immune system, cardiovascular system, kidneys, reproduction, central nervous system, and musculoskeletal system [26, 83]. Although still in their infancy, EV-based therapeutics (drug delivery, cancer vaccines, and antigen presentation) are suggested as promising therapeutics as well as diagnostic tools for an increasing number of human disorders, particularly various malignancies.
Classification and functions of extracellular vesicles
Although the classification of EVs has subjected to changes and constant updates, on the viewpoint of biogenesis they are divided into two main classes, namely ectosomes and exosomes. Ectosomes are larger compared to the second group, have an average diameter up to 1000 nm, and include microvesicles, microparticles, and large vesicles, which are developed by cell membrane budding in the outward direction [84, 85]. Conversely, exosomes originate from endosomes [85]. Besides, based on phenotype and physical features, EVs are classified into four types: exosomes, microvesicles (MVs), membrane particles, and apoptotic vesicles (bodies), with either which differs in features like biogenesis, cargo composition, and function [86, 87]. All those classes are engaged in intercellular communication, but apoptotic vesicles which are taken by phagocytic cells post release to the extracellular environment [88]. Currently, there is no specific biomarker for characterization of subclasses of EVs. Additionally, isolation techniques are not able to purify a specific subclass and mainly yield a mixture [30]. However, isolation of EVs can be conducted by size exclusion in affinity chromatography and antibody differentiation or using ligands based on suspected surface biomarkers [30, 89–91]. Due to considerable overlap between the sizes of smaller subgroups of EVs, which is routinely applied for classification of identified vesicles, the broad term “extracellular vesicle” (EV in brief) is generally used to cover all types of cell-derived vesicles [83]. EVs are enriched in proteins of the tetraspanin family (e.g., CD63, CD9, CD81) and lipids, such as phosphatidylserine, ganglioside, and cholesterol [92, 93]. Fundamental technical deficiencies have caused considerable challenges in progress of our knowledge about the functions of EVs in vivo [94].
Exosomes are the most studied cup-shaped class of EVs with a diameter of 40–160 nm (average: 100 nm), which are originated from endosomes and released from most of eukaryotic cells [86]. Exosomes are generated by double invagination of cell membrane, sequential storage in early and late endosomes, and then formation of multivesicular bodies (MVBs) [84]. MVBs contain intraluminal vesicles (ILVs), which are released to the extracellular environment as exosomes through fusion to the plasma membrane and then exocytosis [84]. The generated exosomes show heterogenicities in their source, size, and content as well as function [84]. Exosomes are mainly characterized using transmission electron microscopy (TEM) and nanoparticle tracking analysis (NTA) as well as flow cytometry (FACS) and resistive pulse sensing (RPS) [26, 95]. To isolate and purify exosomes, several methods based on size and density screening are used, which include ultracentrifugation, density-gradient centrifugation, ultrafiltration, immune-affinity capture, and precipitation [82, 95]. The composition of exosomes, like all EVs, is dependent on the originating cells, so they may contain various ingredients including DNA, microRNA (miRNA), circular RNA (circRNA), protein, and lipid, as well as cellular metabolites and cell membrane proteins [30, 84, 86, 96]. Although concise roles of exosomes are unknown and being investigated in numerous studies, they are postulated to assist cell homeostasis [84] and importantly are suggested to play roles in various human diseases like development and progression of tumors [97–99]. Exosomes can be derived from normal or tumor cells (tumor-derived exosomes; TEXs), in which they functionally alter the phenotype and reprogram recipient cells in order to assist several major hallmarks of cancer, including enhancing proliferation, epithelial-mesenchymal transition (EMT), metastasis, angiogenesis, affecting the tumor microenvironment (TME), and suppression of immune responses [100–102]. These effects together contribute to orchestrating several critical stages of cancer development and metastatic progression [87, 103]. TEXs are found in body fluids and accordingly suggested with potentials in diagnosis as well as therapeutic targets for cancer therapy [102].
Among ectosomes, MVs are larger classes of EVs with an average size of 100–1000 nm in diameter, which are generated by direct budding from plasma membrane. Apoptotic vesicles are even larger compared to other subgroups and considered the largest subcellular structures with 1000–5000 nm diameter originated from those cells undergoing programmed cell death [83]. Similar to exosomes, MVs and membrane particles show cup-shaped morphologies in TEM studies; however, the latter subgroup of ectosomes are only released from epithelial cells (and thus can be found in biological fluids contacting the epithelium layer), while MVs and apoptotic vesicles are released from almost all cells [86]. Additionally, composition analyses have also shown differences among those subgroups of ectosomes. Uptake of MVs by recipient cells is conducted via membrane fusion [104], while this process is through endolytic events for other EVs [105]. Apoptotic vesicles are enriched in histones and DNA, while membrane particles mainly have prominin-1 (CD133) in their composition [86]. Membrane particles have a size range similar to exosomes, but their densities are lower on sucrose gradients. Although tumor-derived EVs are known to assist development and progression of malignancies through affecting signaling pathways, non-malignant-derived EVs can also contribute to tumor development [106].
The impact of tumor-derived extracellular vesicles on tumor immune microenvironment
Exosomes are reported in physiological conditions to play role in antigen presentation to T cells, activation of immune responses via the major histocompatibility complex (MHC)–restricted pathways, initial T cell priming, and mature cell differentiation as well as creation of effector functions [95, 107, 108]. Studies show that EVs are directly involved in the tumor immune invasion through various mechanisms including stimulating the death of T lymphocytes [109]. This phenomenon is repeatedly reported when tumor-derived EVs expressing immune-related ligands, such as Fas-ligand (CD95L), on their outer layer induce apoptosis of T cells upon encountering ligand-positive cells [110–112]. Owing to a broad content of cargos transferred among cancer cells, EVs are known to be involved in various malignant features of tumor cells including pro-proliferative and metastasis-enhancing functions, inducing angiogenesis, conferring chemoresistance, and reprogramming tumor metabolism [87]. Emerging evidence unveils the substantial roles of EVs in establishment and maintenance or modulation of TME [113]. These include sustained cell growth, evading anti-proliferative restrictions, resistance against cell death, getting genomic instabilities, and fundamental alterations in the stromal cell lineages, with all causing the development of functionally remodeled TME [87]. Additionally, EV-mediated information exchange within TME may affect intrusion, metastasis, and several other biological behaviors of tumors [114].
Tumor-derived EVs in intercellular communication
Within the TME, immune cells including B and T lymphocytes, macrophages, and DCs interact with cancer and stromal cells following infiltrating the tumor tissue [115]. Accordingly, tumor cells can target immune cells via EV-mediated delivery of stimulatory or suppressing bioactive molecules together contributing to regulation of proliferation, expanding the number and activity of immune cells [116–119]. Tumor-derived EVs provide a communication net between cancer cells with the immune system and affect several types of immune cells, such as DCs, CD8+ T cells, tumor-associated macrophages (TAMs), natural killer (NK) cells, and regulatory T cells (Tregs) in addition to fibroblasts, platelets, and epithelial cells [106].
Tumor-derived EVs impact the malignant phenotype of cancer cells as well as immune cells in TME
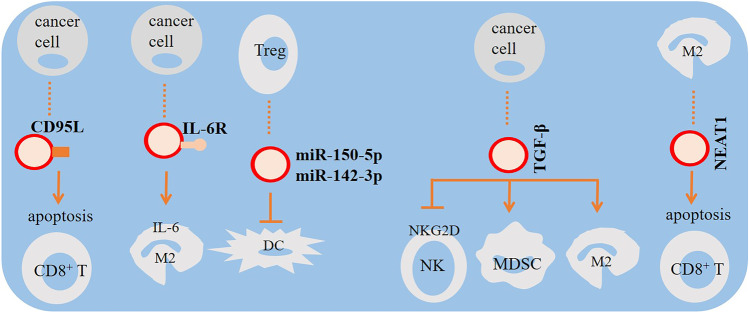
Tumor-derived MVs inhibit the proliferation, modulate the functions, and induce apoptosis of CD8+ T cells, suggesting that those MVs induced immune suppression contributing to tumor escape [120]. Chow et al. demonstrated that breast cancer–derived exosomes induce activation of nuclear factor-κB (NF-κB) in macrophages which led to the production of pro-inflammatory cytokines like IL-6, tumor necrosis factor (TNF), granulocyte colony-stimulating factor (GCSF), and CCL2 [121]. The impact of miRNA-loaded tumor-derived exosomes on the malignant phenotype of targeted immune cells is clearly shown in a study which demonstrated that oral squamous cell carcinoma (OSCC) cells exhibited more aggressive phenotype upon treatment with cancer-associated fibroblasts (CAFs)–derived exosomes [122]. Those effects were revealed to be exerted through downregulation of exosomal miR-34a-5p and activation of the AKT/GSK-3β/β-catenin/Snail signaling pathway [122]. These findings indicate that tumor-derived exosomes may stimulate pro-inflammatory mechanisms of distant macrophages [121]. EVs are also found to negatively impact the cytotoxic effect of NK cells, for instance, by ectopic expression of the soluble form of the ligand of NKp30 (BAG6) contributing to invading the immune system in animal models [123]. Similarly, the interaction between EVs derived from Tregs and DCs is reported to suppress the function of DCs contributing to development of an immunosuppressive TME via exosomal transfer of miR-150-5p and miR-142-3p [124]. Besides, exosomes secreted from tumor cells under hypoxic conditions of TME contain transforming growth factor-β (TGF-β) to reduce surface expression of NKG2D for further suppression of NK cell activity [125]. Tumor-derived exosomes containing TGF-β also increase macrophage polarization toward pro-tumor M2 phenotype [126] and augment activity of myeloid-derived suppressor cells (MDSCs) [127] for further strengthening the immunosuppressive power of tumor [128, 129]. M2 macrophages secreted exosomal NEAT1 to promote CD8+ T cell apoptosis through inducing PD-L1 expression (130). Moreover, EVs can enhance the efficiency of antigen presentation by DCs through alkalinizing their phagosomal compartment as well as transfer of DNA fragments, like GAPDH gene to induce their maturation [131–134]. Taken together, cancer cell–derived EVs can negatively affect the functions of immune cells, modulate the regulatory arms, and induce antigen-specific tolerance contributing to immune invasion [109]. It should be noted that unlike a majority of experiments which found EVs with anti-apoptotic functions, a study reported that NK cell–derived exosomes exert cytotoxic effects on melanoma cells (Fig. 1) [135].
Fig. 1.
The impact of tumor-derived extracellular vesicles (EVs) on immune ecosystem. Tumor-derived EVs expressing Fas-ligand (CD95L) induce CD8+ T cell apoptosis. CD8+ T cell apoptosis is also induced by pro-tumor macrophage type 2 (M2)–derived exosomes. Tumor cells also release exosomes to promote M2 macrophage polarization and increase the activity of myeloid-derived suppressor cells (MDSCs) and M2 macrophages, hampering the function of natural killer (NK) cells. This is mediated through IL-6 and transforming growth factor-β (TGF-β). Dampening dendritic cell (DC) maturation by regulatory T cell (Treg)–derived exosomes is also important for boosting the immunosuppressive profile of cancer
The impact of tumor-derived extracellular vesicles on cancer immunotherapy outcomes
Tumor-derived EVs and particularly TEXs have the potential of transferring immunosuppressive molecules, such as PD-L1, TGF-β, Fas-ligand (FasL), and TNF-related apoptosis-inducing ligand (TRAIL), which can act as mediators of tumor invasion [136–139]. PD-L1 is found on the outer layer of TEXs isolated from plasma samples of patients with various types of malignancies. EVs expressing PD-L1 are associated with disease progression and accelerating tumor progression [140–142]. The TEX-loaded ligand is shown to promote carcinogenesis in murine oral squamous cell carcinoma [143], while it has also revealed immunosuppressive activity at least in two distinct studies, which evaluated its roles in melanoma and prostate cancer patients [144, 145]. Notably, Poggio et al. found that specifically secreted exosomal PD-L1 suppressed T cell activation in vitro and also inhibited the activity of T cells assessed in draining lymph node [144]. Remarkably, tumor cells lacking the exosomal PD-L1 suppressed the growth of wild-type tumor, while exogenous expression of PD-L1 rescued immune suppression and tumor growth [144]. The same impact of exosomal PD-L1 on activity of CD8+ T cells and tumor growth was already shown in another study, where it caused suppression of systemic anti-tumor activity [145]. Also, among patients diagnosed with advanced melanoma who received anti-PD-1 therapy (pembrolizumab), responders demonstrated aberrant exosomal PD-L1 mostly after 6 weeks of therapy compared to non-responders [145]. Those findings suggested TEX PD-L1 as a biomarker for immune activation after starting treatment and prediction of response to anti-PD-1 therapy [145, 146].
Additionally, EVs are suggested to affect the outcomes of chimeric antigen receptor (CAR)-T cell therapy, which is engineering of T cells against a specific cancer antigen as an anticancer therapeutic strategy [147–149]. CAR-T cell–derived EVs have the novel potential in immunotherapy of solid tumors [149], which is discussed below. Particularly, tumor-derived EVs are found to affect the efficacy of CAR-T cell therapy to target CD171 [150]. Study shows that exposure of CAR-T cells to neuroblastoma cell–derived EVs negatively affected tumor cytotoxicity of CAR-T cells [150]. Notably, chronic lymphocytic leukemia (CLL)–derived EVs are able to impair the function of CART cells through inducing their exhaustion [151].
Extracellular vesicles as drug carriers
Known applications of EVs include anticancer therapy, vaccines, immunomodulatory treatments, and drug delivery [152]. After more than two decades of extensive studies on EVs, they are being focused as engineered carriers of cancer therapy in addition to their diagnostic values [153]. Synthetic drug delivery methods, including nanocarrier-based systems, are developed to improve the pharmacokinetic and pharmacodynamic properties, and to enhance the efficacy and minimize the toxicity of drug compounds compared with natural delivery systems [154–156].
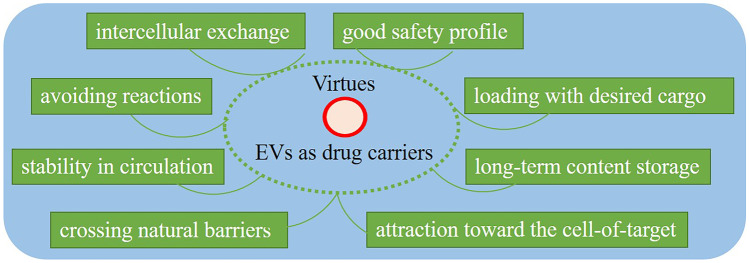
Among those synthetic carriers, liposomes, as the bilayer lipid structures, are the best-known commercially available with the most extensive potential applications in drug delivery. Studies revealed the significance of EVs as “cell-free therapies” due to several unique physical and functional properties, which makes them advantageous for scale up production over cell therapies [20, 153]. These include endogenous cellular sorting and packaging, intrinsic targeting properties, potential of crossing natural barriers due to their small size enabling them to pass through the extracellular matrix (ECM), enhanced biocompatibility, evading phagocytosis (through the surface molecule CD47) [157] and extending half-life of pharmacological cargo, lack of immunogenicity and avoiding reactions unlike viral vectors and synthetic carriers [158], stability in circulation, good safety profile due to endogenous origin, and unique possibilities for loading with desired exogenous cargos as well as the ability of intercellular exchange, which together make them ideal carriers of bioactive compounds [156, 159–162].
Notably, EVs can fuse with plasma membrane and release their drug content directly into the cytoplasm and accordingly protect loaded phrenological from lysosomal degradation [163]. The EV-originating cells are engineered to develop various platforms for loading therapeutic cargoes and conjugating targeting moieties (Fig. 2) [159]. Accordingly, various isolated EVs, including those derived from stem cells, allogenic and autologous EVs, and drug-loaded EVs are being investigated or have shown efficacy and safety as therapeutic carriers in preclinical and clinical studies [20].
Fig. 2.
Virtues related to the application of extracellular vesicles (EVs) in cancer therapy
The EV-based therapeutics have demonstrated promising indices for various human disorders, such as infections, Alzheimer’s disease, immune disorders, heart ischemic conditions, various types of cancers, a few suggested for coronavirus disease (COVID-19), and also for tissue regeneration and stem cell therapy [20, 164–166]. Results of animal studies support hopeful achievements suggesting EVs as potent drug delivery vehicles for various human diseases [159]. A majority of preclinical experiments have focused over cancer therapy [160]. Owing to their nanoscale sizes, EVs can be employed for passive targeting of cancers through the enhanced permeation and retention (EPR) impact [167].
For the first time in 2013, Mizrak et al. evaluated the impact of EV-based drug delivery to cancers [168]. They generated HEK-293 cell–derived MVs expressing the suicide gene cytosine deaminase fused to uracil phosphoribosyltransferase (UPRT) and incubated isolated EVs with schwannomas in animal model. Results demonstrated that weekly intra-tumoral injection of MVs resulted in significant regression of tumors upon treatment with prodrug 5-fluorocytosine (5-FC) [168]. The same results were then achieved in another study by systemic delivery of epidermal growth factor receptor (EGFR)–targeted exosomes loaded with the anti-tumor miRNA let-7a to EGFR-positive breast tumors in mice models [169]. Moreover, EVs are successfully engineered to deliver small interfering RNAs (siRNAs) to cancer cells for targeting specific oncogenes, such as TGF-β [170]. Interestingly, scientists developed EVs loaded with chemotherapeutic agents, such as doxorubicin [171, 172], cisplatin [173], paclitaxel [38, 174], gemcitabine [175], and methotrexate [176], as well as curcumin [34], aimed at improving the therapeutic anticancer efficacy and minimizing the side effects of chemotherapy through targeted delivery, among them some have entered clinical trials [160]. Additionally, some EV-loaded proteins or peptides are also exploited with anticancer therapeutic potential in several studies [35, 177–179].
Extracellular vesicles as carriers of anti-checkpoint antibodies
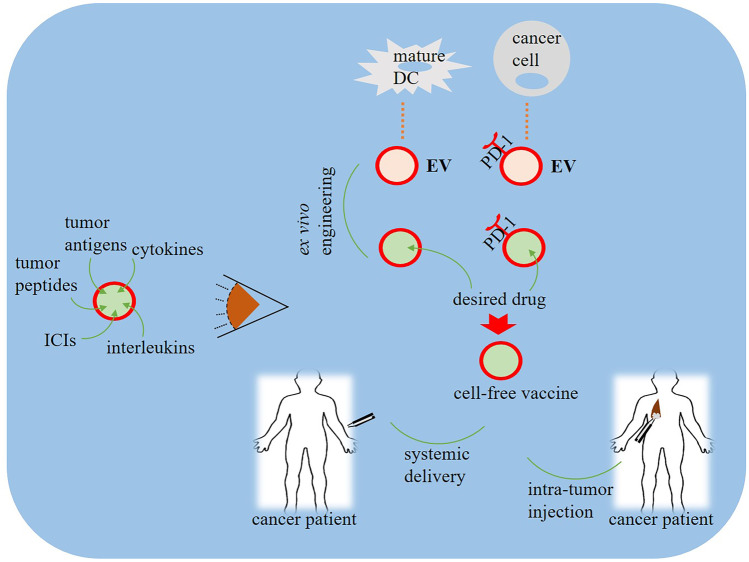
The potential of EVs in cancer immunotherapy is particularly due to their role in the crosstalk between cancer cells and immune cells [153]. Thanks to transmembrane and membrane-anchored proteins, EVs can enhance endocytosis and accordingly increase the intracellular delivery of anticancer therapeutic cargos [180]. Although a majority focused on the roles of EVs in the development of resistance to monoclonal antibodies used for cancer immunotherapy. Theoretically, owing to the drug carrier potential of EVs, they can be employed for targeted delivery of immunotherapeutic agents, aiming at improving the efficacy and to reduce the possible side effects upon systemic delivery. The immunotherapeutic potentials of EVs were first reported in the late 1990s when Zitvogel et al. found that DC-derived exosomes induced specific cytotoxic activity of T lymphocytes in vitro as well as suppressed tumor growth in vivo [181]. Currently, although no EV-based anticancer medicine is approved for clinical use, there are ongoing strategies focusing on application of natural/engineered EVs to enhance anticancer immune responses or to overcome immunosuppressive activities [179, 182]. EVs expressing antibodies against checkpoint proteins are so suggested as potential cancer immunotherapies examined along with gene and chemotherapies for various cancers [183, 184]. Accordingly, EVs are used for loading with clinical monoclonal antibodies and targeted delivery of immunotherapy agents over cancer cells. Several agents used as cancer immunotherapies are loaded into EVs to improve their targeting efficacy against tumor tissues. These include ICIs, tumor antigens, tumor peptides, cytokines, interleukins, cancer vaccines, and two classes of major histocompatibility complex (MHC-I and MHC-II) [185]. Studies revealed that exosomes derived from DCs, as the most potent cells in presentation of antigens, as well as cancer cells are suggested as potential cell-free vaccines for cancer immunotherapy [186, 187]. Among cancer immunotherapy agents, ICIs are tried in various studies aiming to improve EV-based therapeutics. Zhang et al., for instance, developed engineered cellular nanovesicles (NVs) expressing PD-1 receptor on their outer surface to target the PD-1/PD-L1 immune inhibitory axis [188]. PD-1 NVs showed enhanced anticancer activity, and long-time presence in melanoma cells, while being able to bind to PD-1. Exosomal PD-L1 acts as a substantial role player in immunosuppression and response to pembrolizumab immunotherapy (Fig. 3) [145]. Chen et al. showed that EVs expressing a high-affinity variant human PD-1 protein (havPD-1) inhibited the proliferation and induced apoptosis of cancer cells [189]. Additionally, havPD-1 EVs blocked T cell suppression by the PD-L1 mechanism. Interestingly, treatment with havPD-1 EVs showed profound anticancer activity in xenograft models and also, therapeutic effects of havPD-1 EVs are comparable with anti-PD-1 antibodies, suggesting the potency of EVs as nanodrug carriers for combined chemotherapy-immunotherapy [189]. Antigen-loaded EVs are employed to improve anti-PD-1/PD-L1 immunotherapy responses [190], exerting an effect similar to that for metformin-loaded microparticles on anti-PD-1 antibody responses [191]. Trastuzumab emtansine (T-DM1) is an antibody–drug conjugate composed of trastuzumab as a humanized immunoglobulin G1 (IgG1) monoclonal antibody against the extracellular domain of HER2, and DM1, a microtubule polymerization inhibitor [192]. The human epidermal growth factor receptor 2 (HER2) gene is reportedly found to show high expression in a considerable proportion of breast cancer tissues as well as in ovarian and gastric cancer patients and is associated with poorer prognosis among those patients. This is the reason for the development of therapeutic strategies using monoclonal antibodies to target this gene [193, 194]. T-DM1 has been a subject to various trials being suggested as a developing therapy for HER2-positive breast cancer patients [195–198]. Transmission electron microscopy and flow cytometry analysis revealed that T-DM1 bound to type-A exosomes isolated from SKBR-3 and SNU-216 HER2-positive cancer cells and then used for targeting other cancer cells. Treatment of HER2-positive cancer cells with T-DM1-containing exosomes contributed to reduction in cell viability, and induction of apoptosis through activation of caspases [199].
Fig. 3.
Protocols for application of extracellular vesicles (EVs) in cancer immunotherapy. EVs are collected from mature anti-tumor immune cells, such as dendritic cells (DCs), or from tumor cells and modified ex vivo with desired agents including tumor antigens, tumor peptides, cytokines, interleukins, and immune checkpoint inhibitor (ICI) antibodies to form cell-free vaccines. Such modified vesicles are then administered to cancer patients via either systemic delivery or intra-tumor injection. EVs collected from tumor cells express tumor-derived antigens and checkpoints, such as programmed death-1 (PD-1), so modification of the content of such vesicles with desired agents will exert desired anti-tumor effects due to more attraction toward the target tumor area
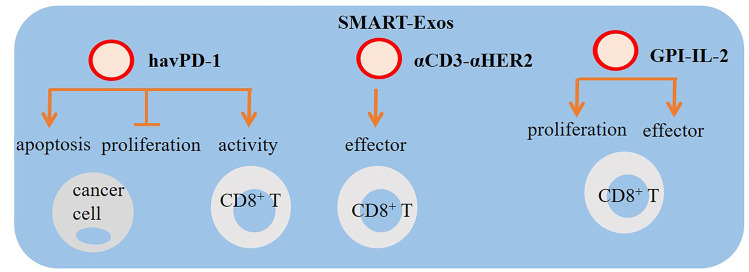
Moreover, genetically modified EVs expressing two different monoclonal antibodies, defined with a distinguishing term called synthetic multivalent antibodies retargeted exosome (SMART-Exos), are tested in cancer cells to target both T cells and cancer cells, which their application resulted in enhanced anticancer responses [200, 201]. In the former study, Shi et al. engineered SMART-Exos (αCD3-αHER2 SMART-Exos) by genetic display to express anti-human CD3 antibody and another antibody against the human HER2 for dual targeting of T cells and HER2-positive breast cancer cells [200]. Thus, those exosomes simultaneously recognized T cells and cancer cells to redirect immune effector cells conferring controlled anticancer immunity. Interestingly, SMART-Exos were demonstrated to successfully activate and direct human T cells to HER2-positive cells, while also induced cytotoxic effect on those cancer cells with high potency and specificity. Moreover, pharmacokinetic evaluation of αCD3-αHER2 SMART-Exos revealed good stability, efficacy, and toxicity (by measuring creatinine levels for kidney function and alanine aminotransferase (ALT) biomarker for liver damages) in xenograft mice models of hepatocellular carcinoma (HCC) (Fig. 4) [200]. Similarly, SMART-Exos expressing dual monoclonal antibodies against CD3 for T cells and EGFR for targeting cancer cells were found to bind to both T cells and EGFR-positive triple negative breast cancer (TNBC) cells [201]. In vitro toxicity investigation demonstrated that SMART-Exos caused a strong and specific cytotoxicity against TNBC cells expressing EGFR. Those anticancer effects of SMART-Exos were confirmed in xenograft animal study [201]. These brilliant findings suggest exosomes with promising therapeutic potential in cancer immunotherapy. To bring EVs into clinical practice, several clinical trials are being conducted to evaluate the impact of EVs in immunotherapy or in combination with other anticancer therapies [108]. A recruiting study (NCT03985696), for instance, entitled “Exosomes and Immunotherapy in Non-Hodgkin B-cell Lymphomas” aims at investigating the significance of exosomes in response to immunotherapies by measuring exosomal levels of CD20 and PDL-1 isolated from diffuse large B cell lymphomas (DLBCL) cells. Researchers conducting NCT04427475 are trying to explore the potential application of exosomal PD-L1 in predicting response to pablolizumab and nafulizumab in patients with non-small cell lung cancer (NSCLC). In two other distinct trials (NCT02890849 and NCT02869685), the significance of PD-L1 levels in plasma exosomes is explored in monitoring clinical responses to radiotherapy/immunotherapy.
Fig. 4.
Extracellular vesicle (EV)–based methods in cancer immunotherapy. High-affinity variant human PD-1 protein (havPD-1), synthetic multivalent antibodies retargeted exosome (SMART-Exos), and glycosyl-phosphatidylinositol (GPI)-anchored IL-2 are examples of EV-based approaches for targeting tumors. EVs expressing havPD-1 can be used as a method for inhibiting the proliferation, inducing apoptosis of cancer cells, and reinvigorating T cell effector function. Therapeutic effects of havPD-1 EVs are comparable with anti-PD-1 antibodies, suggesting the potency of EVs as nanodrug carriers for combined chemotherapy-immunotherapy. SMART-Exos is tested in cancer cells to target both T cells and cancer cells aiming to boost anticancer responses. Expressing GPI-IL-2 on tumor-derived exosomes and their further representation on dendritic cells (DCs) induces proliferation of T lymphocytes and augments the efficiency of antigen-specific responses in cytotoxic T cells
Interleukin-loaded extracellular vesicles in cancer immunotherapy
Cytokines are proteins, which play various roles with immunomodulatory functions and importantly as intermediates of interactions between immune cells to develop a synchronized and specific response against antigens [202, 203]. They are particularly involved in various oncogenic mechanisms as well as innate and adoptive anticancer immunity [204–206], while tumor-promoting cytokines contribute to enhanced stemness and oncogenic potential [204]. Among cytokines, six interleukin families, namely IL-1, IL-2, IL-6, IL-8, IL-10, and IL-17, are described [207], which are known to mediate immune-inflammatory cell interactions with potentials in promotion of growth, differentiation, and activation of immune cells [208]. Accordingly, interleukins play substantial roles in regulating the functions of immune cells as well as inflammation and fever [208]. Chronic inflammation is recognized as a driver of carcinogenesis in various human cancers [209, 210], and thus, it is not surprising to know that interleukins have been employed for a long time in cancer therapies established as the first successful immunotherapies [205]. Theoretically, TME can be targeted via delivery of inflammatory interleukins or through removing suppressive factors [137]. Recombinant interferon alpha-2b (IFNα2b) was the first cytokine, which showed anti-proliferative impact and received approval for therapeutic application in patients with hairy cell leukemia [211]. Additionally, pro-inflammatory cytokine IL-2 at high doses was the first interleukin, which was approved by FDA in 1990s for use in patients with metastatic melanoma and renal cancer [212]. Subsequently, several interleukins are suggested for anticancer immunotherapy, while an explosion has happened in the number of clinical and preclinical studies investigating the potentials of whole cytokines in cancer therapy [206]. To this aim, targeting immunomodulatory interleukins could be used as a potential anticancer therapy [210, 213]. Collectively, EV-mediated interleukins are suggested with diagnostic potentials, while loading in exosomes is particularly found with promising therapeutic potentials for anticancer purposes [214]. Encapsulation of interleukins in EVs has been suggested as an effective candidate for delivery of interleukins [215]. EVs isolated from genetically engineered MC38 colorectal cancer (CRC) cells overexpressing IL-12 and/or small heterogeneous RNA (shRNA) against TGF-β1 are shown to induce growth inhibition and sustained anti-tumor effect, while those TEXs isolated from unmodified cells did not show this effect [137]. Zhang et al. expressed glycosyl-phosphatidylinositol (GPI)-anchored IL-2 on TEXs and found that GPI-IL-2 expressing DCs induced proliferation of T lymphocytes and enhanced the efficiency of antigen-specific responses in cytotoxic T cells [216]. Moreover, conjugation of EVs with the IL-4 receptor binding peptide (IL4RPep-1) is reported to improve targeting efficiency of EV drug delivery to the site of thyroid cancer in vivo [217]. Also, imatinib-loaded exosomes with fused fragments of IL-3 are shown to target the chronic myeloid leukemia (CML) cells and inhibited cell growth both in vitro and in vivo [218]. Accordingly, the fusion of EVs with interleukins may be considered a therapeutic drug delivery approach against cancer [214].
Antigen-loaded extracellular vesicles in cancer immunotherapy
Given the fact that EVs can contain both immune-stimulatory molecules and tumor antigens, the extraction of those EVs from antigen presenting cells (APCs) may help in overcoming against intra-tumoral heterogeneity [219]. Accordingly, various tumor-associated antigens (e.g., OVA) can be loaded into EVs to induce the anticancer immune responses and antineoplastic activities [220]. Application of antigen-loaded EVs is suggested as another strategy for cancer immunotherapy [221]. Transfer of those antigens via EVs may facilitate activation of NK cells and antigen-specific responses of T cells contributing to restricting the growth of tumor cells [222]. It has shown promising results in improving anti-PD-1/PD-L1 therapy in an ICI-refractory cancer model [190]. Interestingly, antigen-loaded EVs are nominated as cancer vaccines to induce appropriate antigen-specific anticancer responses. This potential of EVs has been a subject to a number of clinical trials (e.g., see NCT01159288 on www.clinicaltrials.gov).
Extracellular vesicles in adoptive T cell therapy
Passive immunization through injection of T lymphocytes is now referred as adoptive T cell therapy that is mainly conducted for cancer patients via transfusion of autologous or allogeneic T cells [223]. CAR-T cell therapy is the process of adoptive T cell transfer with cytotoxic activity against cancer cells, which is being considered an emerging therapeutic approach for a wide variety of human malignancies [224, 225]. CAR-T cells are reported to release EVs and based on their biological functions, T cells–derived exosomes that contain CAR are suggested to represent therapeutic potentials as direct attackers in cancer immunotherapy [226]. Reciprocally, TEXs can impact the function and efficacy of CAR-T cell therapy [150, 227, 228]. CAR-T EVs may be exploited to overcome CAR-T cell therapy failure [229]. Among a heterogeneous population of exosomes isolated from CAR-T cell medium, those contain CAR are shown to express high levels of cytotoxic molecules, which gives them potential to attack cancer cells with potent anti-tumor activity and improved safety in xenograft models compared with CAR-T cell therapy [226]. Moreover, CAR-containing EVs have further advantages over CAR-T cell therapy, which include crossing the blood barrier, higher efficiency against solid tumors, lack of neurotoxicity, and CRS, as well as resistance to immunosuppression mediated by tumoral PD-L1 since those EVs do not contain PD-1 [230].
Conclusions, limitations, and future perspectives
EVs, particularly exosomes, have shown promising achievements in diagnosis and therapy. Being able to carry various bioactive molecules along with the potential to cross biological barriers, good biocompatibility, avoid immunogenicity, stability, and good safety profile have made EVs as ideal candidates for targeted drug delivery in addition to biomarker and vaccine applications. Considerable advances are made in study of EVs during the recent decade; however, the field is still in the infancy of development and clinical translation of EVs requires improved methods for large-scale production with high yields and isolation and purification [231–233]. Although EVs are promising biologic drug delivery machinery, large-scale engineered generation of EVs requires special consideration and strategies [234, 235]; meanwhile, their clinical applications are limited mainly due to lack of appropriate scalable isolation methods in addition to requirement for efficient drug loading technologies [160]. Successful translation of EVs requires several factors including cost-effective production methods, highly sensitive isolation, and characterization technologies as well as drug loading methods with wide application [20]. Some studies have shown the feasibility of producing EVs according to the Good Manufacturing Practice (GMP) standards on small scales. However, large-scale production remains challenging and production of EVs for therapeutic goals seems far from reality due to technical deficiencies [236]. Furthermore, cytokine release syndrome (CRS) is a major safety concern for application of EVs in cancer immunotherapy [237]. Notably, clinical-grade EVs are produced for anticancer therapies in large scales under standards [238, 239] highlighting hopes for rapid translation of EV-based therapeutics into clinical practice [189]. Overall, antigen loading efficacy needs optimization for achieving enhanced immunotherapy [95]. Additionally, safety of produced EVs to avoid CRS and removal of pathogens along with GMP standard requirements are other obstacles to overcome for bringing EVs into clinical settings [237, 240, 241].
Acknowledgements
This work is supported by Kurdistan University of Medical Sciences (Ethical Code: IR.MUK.REC.1401.433).
Author contribution
Collection and revision of information: SN and KM; conceptualization: KM; writing, original draft preparation, review, and editing: JM and KM. The authors have read and agreed to publish the manuscript.
Availability of data and material
Not applicable.
Declarations
Ethics approval
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Highlights
∙ EVs play essential roles in intercellular communication.
∙ EVs are appropriate vehicles for targeted drug delivery.
∙ EVs may affect responses to ICIs.
∙ Loading desired agents in EVs improves immunotherapy efficacy.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127(16):3029–3030. doi: 10.1002/cncr.33587. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed]
- 3.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed]
- 4.Baba AI, Câtoi C. Comparative oncology: Publishing House of the Romanian Academy Bucharest; 2007. [PubMed]
- 5.Kirkwood JM, Butterfield LH, Tarhini AA, Zarour H, Kalinski P, Ferrone S. Immunotherapy of cancer in 2012. CA: A Cancer J Clin. 2012;62(5):309–35. [DOI] [PMC free article] [PubMed]
- 6.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 7.Page DB, Bear H, Prabhakaran S, Gatti-Mays ME, Thomas A, Cobain E, et al. Two may be better than one: PD-1/PD-L1 blockade combination approaches in metastatic breast cancer. npj Breast Cancer. 2019;5(1):34. [DOI] [PMC free article] [PubMed]
- 8.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 9.Page D, Pucilowska J, Bennetts L, Kim I, Sanchez K, Martel M, et al. Abstract P2–09–03: updated efficacy of first or second-line pembrolizumab (pembro) plus capecitabine (cape) in metastatic triple negative breast cancer (mTNBC) and correlations with baseline lymphocyte and naïve CD4+ T-cell count. Cancer Research. 2019;79(4_Supplement):P2–09–3-P2--3.
- 10.McArthur HL, Barker CA, Gucalp A, Lebron-Zapata L, Wen YH, Phung A, et al. A single-arm, phase II study assessing the efficacy of pembrolizumab (pembro) plus radiotherapy (RT) in metastatic triple negative breast cancer (mTNBC). Am Soc Clin Oncol. 2018.
- 11.Luke JJ, Lemons JM, Karrison TG, Pitroda SP, Melotek JM, Zha Y, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol. 2018;36(16):1611. doi: 10.1200/JCO.2017.76.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loncharich MF, Anderson CW. Interferon inhibition for lupus with anifrolumab: critical appraisal of the evidence leading to FDA approval. Wiley Online Library; 2022. [DOI] [PMC free article] [PubMed]
- 13.Emami F, Banstola A, Vatanara A, Lee S, Kim JO, Jeong J-H, et al. Doxorubicin and anti-PD-L1 antibody conjugated gold nanoparticles for colorectal cancer photochemotherapy. Mol Pharm. 2019;16(3):1184–1199. doi: 10.1021/acs.molpharmaceut.8b01157. [DOI] [PubMed] [Google Scholar]
- 14.Perets R, Bar J, Rasco DW, Ahn MJ, Yoh K, Kim DW, et al. Safety and efficacy of quavonlimab, a novel anti-CTLA-4 antibody (MK-1308), in combination with pembrolizumab in first-line advanced non-small-cell lung cancer. Ann Oncol. 2021;32(3):395–403. doi: 10.1016/j.annonc.2020.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Najafi S, Majidpoor J, Mortezaee K. The impact of microbiota on PD-1/PD-L1 inhibitor therapy outcomes: a focus on solid tumors. Life Sci. 2022;310:121138. doi: 10.1016/j.lfs.2022.121138. [DOI] [PubMed] [Google Scholar]
- 16.Asemani Y, Najafi S, Ezzatifar F, Zolbanin NM, Jafari R. Recent highlights in the immunomodulatory aspects of Treg cell-derived extracellular vesicles: special emphasis on autoimmune diseases and transplantation. Cell Biosci. 2022;12(1):67. doi: 10.1186/s13578-022-00808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahangar Davoodi N, Najafi S, Naderi Ghale-Noie Z, Piranviseh A, Mollazadeh S, Ahmadi Asouri S, et al. Role of non-coding RNAs and exosomal non-coding RNAs in retinoblastoma progression. Front Cell Dev Biol. 2022;10. [DOI] [PMC free article] [PubMed]
- 18.Najafi S. Circular RNAs as emerging players in cervical cancer tumorigenesis; a review to roles and biomarker potentials. Int J Biol Macromol. 2022;206:939–953. doi: 10.1016/j.ijbiomac.2022.03.103. [DOI] [PubMed] [Google Scholar]
- 19.Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins. RNA and lipids Nucleic acids research. 2012;40(D1):D1241–D1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16(7):748–759. doi: 10.1038/s41565-021-00931-2. [DOI] [PubMed] [Google Scholar]
- 21.Van Niel G, d’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 22.Maas SL, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27(3):172–188. doi: 10.1016/j.tcb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulcahy LA, Pink RC, Carter DRF. Routes and mechanisms of extracellular vesicle uptake. Journal of extracellular vesicles. 2014;3(1):24641. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S, Naerdemann W, et al. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol Cancer Ther. 2005;4(10):1595–1604. doi: 10.1158/1535-7163.MCT-05-0102. [DOI] [PubMed] [Google Scholar]
- 25.Zwaal R, Comfurius P, Bevers E. Surface exposure of phosphatidylserine in pathological cells. Cellular and Molecular Life Sciences CMLS. 2005;62(9):971–988. doi: 10.1007/s00018-005-4527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuana Y, Sturk A, Nieuwland R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013;27(1):31–39. doi: 10.1016/j.blre.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Van der Pol E, Böing A, Gool E, Nieuwland R. Recent developments in the nomenclature, presence, isolation, detection and clinical impact of extracellular vesicles. J Thromb Haemost. 2016;14(1):48–56. doi: 10.1111/jth.13190. [DOI] [PubMed] [Google Scholar]
- 28.Shah R, Patel T, Freedman JE. Circulating extracellular vesicles in human disease. N Engl J Med. 2018;379(10):958–966. doi: 10.1056/NEJMra1704286. [DOI] [PubMed] [Google Scholar]
- 29.Lässer C, Seyed Alikhani V, Ekström K, Eldh M, Torregrosa Paredes P, Bossios A, et al. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med. 2011;9(1):1–8. doi: 10.1186/1479-5876-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yáñez-Mó M, Siljander PR-M, Andreu Z, Bedina Zavec A, Borràs FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4(1):27066. [DOI] [PMC free article] [PubMed]
- 31.Koniusz S, Andrzejewska A, Muraca M, Srivastava AK, Janowski M, Lukomska B. Extracellular vesicles in physiology, pathology, and therapy of the immune and central nervous system, with focus on extracellular vesicles derived from mesenchymal stem cells as therapeutic tools. Front Cell Neurosci. 2016;10. [DOI] [PMC free article] [PubMed]
- 32.Lu M, DiBernardo E, Parks E, Fox H, Zheng S-Y, Wayne E. The role of extracellular vesicles in the pathogenesis and treatment of autoimmune disorders. Front Immunol. 2021;12. [DOI] [PMC free article] [PubMed]
- 33.Anderson HC, Mulhall D, Garimella R. Role of extracellular membrane vesicles in the pathogenesis of various diseases, including cancer, renal diseases, atherosclerosis, and arthritis. Lab Invest. 2010;90(11):1549–1557. doi: 10.1038/labinvest.2010.152. [DOI] [PubMed] [Google Scholar]
- 34.Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18(9):1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wan Y, Wang L, Zhu C, Zheng Q, Wang G, Tong J, et al. Aptamer-conjugated extracellular nanovesicles for targeted drug delivery preparation of cancer-targeted extracellular nanovesicles. Can Res. 2018;78(3):798–808. doi: 10.1158/0008-5472.CAN-17-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang RH, Hu C-MJ, Luk BT, Gao W, Copp JA, Tai Y, et al. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano letters. 2014;14(4):2181–8. [DOI] [PMC free article] [PubMed]
- 37.Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AM, et al. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. 2012;56(2):293–304. doi: 10.1016/j.ymeth.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Saari H, Lázaro-Ibáñez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M. Microvesicle-and exosome-mediated drug delivery enhances the cytotoxicity of paclitaxel in autologous prostate cancer cells. J Control Release. 2015;220:727–737. doi: 10.1016/j.jconrel.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 39.O’Brien K, Lowry MC, Corcoran C, Martinez VG, Daly M, Rani S, et al. miR-134 in extracellular vesicles reduces triple-negative breast cancer aggression and increases drug sensitivity. Oncotarget. 2015;6(32):32774. doi: 10.18632/oncotarget.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012;3:359. doi: 10.3389/fphys.2012.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Najafi M, Majidpoor J, Toolee H, Mortezaee K. The current knowledge concerning solid cancer and therapy. J Biochem Mol Toxicol. 2021;35(11):e22900. doi: 10.1002/jbt.22900. [DOI] [PubMed] [Google Scholar]
- 42.Schuster M, Nechansky A, Kircheis R. Cancer immunotherapy. Biotechnol J. 2006;1(2):138–147. doi: 10.1002/biot.200500044. [DOI] [PubMed] [Google Scholar]
- 43.Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366(26):2517–2519. doi: 10.1056/NEJMe1205943. [DOI] [PubMed] [Google Scholar]
- 44.Nauts HC, Fowler GA, Bogatko FH. A review of the influence of bacterial infection and of bacterial products (Coley’s toxins) on malignant tumors in man; a critical analysis of 30 inoperable cases treated by Coley’s mixed toxins, in which diagnosis was confirmed by microscopic examination selected for special study. Acta Med Scand Suppl. 1953;276:1–103. [PubMed] [Google Scholar]
- 45.McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J. 2006;26:154–8. [PMC free article] [PubMed]
- 46.Grimm EA, Mazumder A, Zhang H, Rosenberg S. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982;155(6):1823–41. [DOI] [PMC free article] [PubMed]
- 47.Strausser J, Mazumder A, Grimm E, Lotze M, Rosenberg S. Lysis of human solid tumors by autologous cells sensitized in vitro to alloantigens. J Immunol. 1981;127(1):266–271. doi: 10.4049/jimmunol.127.1.266. [DOI] [PubMed] [Google Scholar]
- 48.Mazumder A, Grimm EA, Zhang HZ, Rosenberg SA. Lysis of fresh human solid tumors by autologous lymphocytes activated in vitro with lectins. Can Res. 1982;42(3):913–918. [PubMed] [Google Scholar]
- 49.Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6:S11–S14. [PubMed] [Google Scholar]
- 50.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clinical Oncol. 1999;17(7):2105-. [DOI] [PubMed]
- 51.Dutcher JP, Creekmore S, Weiss GR, Margolin K, Markowitz AB, Roper M, et al. A phase II study of interleukin-2 and lymphokine-activated killer cells in patients with metastatic malignant melanoma. J Clin Oncol. 1989;7(4):477–485. doi: 10.1200/JCO.1989.7.4.477. [DOI] [PubMed] [Google Scholar]
- 52.Krown SE, Burk MW, Kirkwood JM, Kerr D, Morton DL, Oettgen H. Human leukocyte(alpha) interferon in metastatic malignant melanoma: the American Cancer Society phase II trial. Cancer Treat Rep. 1984;68(5):723–726. [PubMed] [Google Scholar]
- 53.Renard V, Sonderbye L, Ebbehøj K, Rasmussen PB, Gregorius K, Gottschalk T, et al. HER-2 DNA and protein vaccines containing potent Th cell epitopes induce distinct protective and therapeutic antitumor responses in HER-2 transgenic mice. J Immunol. 2003;171(3):1588–1595. doi: 10.4049/jimmunol.171.3.1588. [DOI] [PubMed] [Google Scholar]
- 54.Motl SE. Technology evaluation: Canvaxin, John Wayne Cancer Institute/CancerVax. Curr Opin Mol Ther. 2004;6(1):104–111. [PubMed] [Google Scholar]
- 55.Berd D. M-Vax: an autologous, hapten-modified vaccine for human cancer. Expert Rev Vaccines. 2004;3(5):521–527. doi: 10.1586/14760584.3.5.521. [DOI] [PubMed] [Google Scholar]
- 56.Wood C, Escudier B, Gorelov S, Krajka K, Lacombe L, Fossa S, et al. A multicenter randomized study of adjuvant heat-shock protein peptide-complex 96 (HSPPC-96) vaccine in patients with high-risk of recurrence after nephrectomy for renal cell carcinoma (RCC)-a preliminary report. J Clin Oncol. 2004;22(14_suppl):2618-.
- 57.Pilon-Thomas S, Verhaegen M, Kuhn L, Riker A, Mulé JJ. Induction of anti-tumor immunity by vaccination with dendritic cells pulsed with anti-CD44 IgG opsonized tumor cells. Cancer Immunol Immunother. 2006;55(10):1238–1246. doi: 10.1007/s00262-005-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–249. doi: 10.1146/annurev-pathol-042020-042741. [DOI] [PubMed] [Google Scholar]
- 59.Naimi A, Mohammed RN, Raji A, Chupradit S, Yumashev AV, Suksatan W, et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Communication and Signaling. 2022;20(1):44. doi: 10.1186/s12964-022-00854-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mortezaee K. B7x in cancer immunity and immunotherapy. Int Immunopharmacol. 2023;118:110133. doi: 10.1016/j.intimp.2023.110133. [DOI] [PubMed] [Google Scholar]
- 61.Rouzbahani E, Majidpoor J, Najafi S, Mortezaee K. Cancer stem cells in immunoregulation and bypassing anti-checkpoint therapy. Biomed Pharmacother. 2022;156:113906. doi: 10.1016/j.biopha.2022.113906. [DOI] [PubMed] [Google Scholar]
- 62.Nayak L, Iwamoto FM, LaCasce A, Mukundan S, Roemer MGM, Chapuy B, et al. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood. 2017;129(23):3071–3073. doi: 10.1182/blood-2017-01-764209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bai R, Chen N, Li L, Du N, Bai L, Lv Z, et al. Mechanisms of cancer resistance to immunotherapy. Front Oncol. 2020;10:1290. doi: 10.3389/fonc.2020.01290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pitt Jonathan M, Vétizou M, Daillère R, Roberti María P, Yamazaki T, Routy B, et al. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity. 2016;44(6):1255–1269. doi: 10.1016/j.immuni.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 66.Ott PA, Bang YJ, Piha-Paul SA, Razak ARA, Bennouna J, Soria JC, et al. T-cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol. 2019;37(4):318–327. doi: 10.1200/JCO.2018.78.2276. [DOI] [PubMed] [Google Scholar]
- 67.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 68.Wang S, Xie K, Liu T. Cancer immunotherapies: from efficacy to resistance mechanisms – not only checkpoint matters. Front Immunol. 2021;12. [DOI] [PMC free article] [PubMed]
- 69.Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer. 2018;118(1):9–16. doi: 10.1038/bjc.2017.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mortezaee K, Majidpoor J, Najafi S. VISTA immune regulatory effects in bypassing cancer immunotherapy: updated. Life Sciences. 2022:121083. [DOI] [PubMed]
- 71.Mortezaee K. HHLA2 immune-regulatory roles in cancer. Biomed Pharmacother. 2023;162:114639. doi: 10.1016/j.biopha.2023.114639. [DOI] [PubMed] [Google Scholar]
- 72.Mortezaee K, Majidpoor J, Najafi S, Tasa D. Bypassing anti-PD-(L)1 therapy: mechanisms and management strategies. Biomed Pharmacother. 2023;158:114150. doi: 10.1016/j.biopha.2022.114150. [DOI] [PubMed] [Google Scholar]
- 73.Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166(1):189–197. doi: 10.1016/S0021-9258(17)34997-9. [DOI] [PubMed] [Google Scholar]
- 74.Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13(3):269–288. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 75.Holme PA, Solum NO, Brosstad F, Røger M, Abdelnoor M. Demonstration of platelet-derived microvesicles in blood from patients with activated coagulation and fibrinolysis using a filtration technique and western blotting. Thromb Haemost. 1994;72(11):666–671. [PubMed] [Google Scholar]
- 76.Hess C, Sadallah S, Hefti A, Landmann R, Schifferli J-A. Ectosomes released by human neutrophils are specialized functional units. J Immunol. 1999;163(8):4564–4573. doi: 10.4049/jimmunol.163.8.4564. [DOI] [PubMed] [Google Scholar]
- 77.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19(2):43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 78.Lee EY, Choi DY, Kim DK, Kim JW, Park JO, Kim S, et al. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics. 2009;9(24):5425–5436. doi: 10.1002/pmic.200900338. [DOI] [PubMed] [Google Scholar]
- 79.György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68(16):2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beveridge TJ. Structures of gram-negative cell walls and their derived membrane vesicles. J Bacteriol. 1999;181(16):4725–4733. doi: 10.1128/JB.181.16.4725-4733.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21(1):319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 82.Mortezaee K, Majidpoor J, Fathi F. Extracellular vesicle isolation, purification and evaluation in cancer diagnosis. Expert Rev Mol Med. 2022:1–44. [DOI] [PubMed]
- 83.Yates AG, Pink RC, Erdbrügger U, Siljander PR-M, Dellar ER, Pantazi P, et al. In sickness and in health: the functional role of extracellular vesicles in physiology and pathology in vivo. J Extracell Vesicles. 2022;11(1):e12151. [DOI] [PMC free article] [PubMed]
- 84.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. [DOI] [PMC free article] [PubMed]
- 85.Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25(6):364–372. doi: 10.1016/j.tcb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 86.van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64(3):676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- 87.Han L, Lam EWF, Sun Y. Extracellular vesicles in the tumor microenvironment: old stories, but new tales. Mol Cancer. 2019;18(1):59. doi: 10.1186/s12943-019-0980-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Budnik V, Ruiz-Cañada C, Wendler F. Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci. 2016;17(3):160–172. doi: 10.1038/nrn.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mathivanan S, Lim JW, Tauro BJ, Ji H, Moritz RL, Simpson RJ. Proteomics analysis of A33 immunoaffinity-purified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature. Mol Cell Proteomics. 2010;9(2):197–208. doi: 10.1074/mcp.M900152-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110(1):13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 91.Atai NA, Balaj L, van Veen H, Breakefield XO, Jarzyna PA, Van Noorden CJ, et al. Heparin blocks transfer of extracellular vesicles between donor and recipient cells. J Neurooncol. 2013;115(3):343–351. doi: 10.1007/s11060-013-1235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jankovičová J, Sečová P, Michalková K, Antalíková J. Tetraspanins, more than markers of extracellular vesicles in reproduction. Int J Mol Sci. 2020;21(20):7568. doi: 10.3390/ijms21207568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen S, Datta-Chaudhuri A, Deme P, Dickens A, Dastgheyb R, Bhargava P, et al. Lipidomic characterization of extracellular vesicles in human serum. Journal of circulating biomarkers. 2019;8:1849454419879848. doi: 10.1177/1849454419879848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ramirez MI, Amorim MG, Gadelha C, Milic I, Welsh JA, Freitas VM, et al. Technical challenges of working with extracellular vesicles. Nanoscale. 2018;10(3):881–906. doi: 10.1039/C7NR08360B. [DOI] [PubMed] [Google Scholar]
- 95.Xie F, Zhou X, Fang M, Li H, Su P, Tu Y, et al. Extracellular vesicles in cancer immune microenvironment and cancer immunotherapy. Advanced Science. 2019;6(24):1901779. doi: 10.1002/advs.201901779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Najafi S. The emerging roles and potential applications of circular RNAs in ovarian cancer: a comprehensive review. J Cancer Res Clin Oncol. 2022. [DOI] [PMC free article] [PubMed]
- 97.Brinton LT, Sloane HS, Kester M, Kelly KA. Formation and role of exosomes in cancer. Cell Mol Life Sci. 2015;72(4):659–671. doi: 10.1007/s00018-014-1764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Steinbichler TB, Dudás J, Riechelmann H, Skvortsova I-I. The role of exosomes in cancer metastasis. Semin Cancer Biol. 2017;44:170–181. doi: 10.1016/j.semcancer.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 99.Tickner JA, Urquhart AJ, Stephenson S-A, Richard DJ, O’Byrne KJ. Functions and therapeutic roles of exosomes in cancer. Front Oncol. 2014;4. [DOI] [PMC free article] [PubMed]
- 100.Whiteside TL. Tumor-derived exosomes and their role in cancer progression. Adv Clin Chem. 2016;74:103–141. doi: 10.1016/bs.acc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kok VC, Yu CC. Cancer-derived exosomes: their role in cancer biology and biomarker development. Int J Nanomedicine. 2020;15:8019–8036. doi: 10.2147/IJN.S272378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu K, Zhang C, Du T, Gabriel ANA, Wang X, Li X, et al. Progress of exosomes in the diagnosis and treatment of lung cancer. Biomed Pharmacother. 2021;134:111111. doi: 10.1016/j.biopha.2020.111111. [DOI] [PubMed] [Google Scholar]
- 103.Mortezaee K. Organ tropism in solid tumor metastasis: an updated review. Future Oncol. 2021;17(15):1943–1961. doi: 10.2217/fon-2020-1103. [DOI] [PubMed] [Google Scholar]
- 104.Del Conde I, Shrimpton CN, Thiagarajan P, López JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106(5):1604–1611. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 105.Svensson KJ, Christianson HC, Wittrup A, Bourseau-Guilmain E, Lindqvist E, Svensson LM, et al. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J Biol Chem. 2013;288(24):17713–17724. doi: 10.1074/jbc.M112.445403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tao S-C, Guo S-C. Role of extracellular vesicles in tumour microenvironment. Cell Communication and Signaling. 2020;18(1):163. doi: 10.1186/s12964-020-00643-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maia J, Caja S, Strano Moraes MC, Couto N, Costa-Silva B. Exosome-based cell-cell communication in the tumor microenvironment. Frontiers in cell and developmental biology. 2018;6:18. doi: 10.3389/fcell.2018.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Srivastava A, Rathore S, Munshi A, Ramesh R. Extracellular vesicles in oncology: from immune suppression to immunotherapy. AAPS J. 2021;23(2):30. doi: 10.1208/s12248-021-00554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Webber J, Yeung V, Clayton A, editors. Extracellular vesicles as modulators of the cancer microenvironment. Semin Cell Dev Biol. 2015:Elsevier. [DOI] [PubMed]
- 110.Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med. 2002;195(10):1303–1316. doi: 10.1084/jem.20011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huber V, Fais S, Iero M, Lugini L, Canese P, Squarcina P, et al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology. 2005;128(7):1796–1804. doi: 10.1053/j.gastro.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 112.Taylor DD, Gerçel-Taylor Ç, Lyons KS, Stanson J, Whiteside TL. T-cell apoptosis and suppression of T-cell receptor/CD3-ζ by Fas ligand-containing membrane vesicles shed from ovarian tumors. Clin Cancer Res. 2003;9(14):5113–5119. [PubMed] [Google Scholar]
- 113.Mathew M, Zade M, Mezghani N, Patel R, Wang Y, Momen-Heravi F. Extracellular vesicles as biomarkers in cancer immunotherapy. Cancers. 2020;12(10):2825. doi: 10.3390/cancers12102825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Le MT, Hamar P, Guo C, Basar E, Perdigão-Henriques R, Balaj L, et al. miR-200–containing extracellular vesicles promote breast cancer cell metastasis. J Clin Investig. 2014;124(12):5109–5128. doi: 10.1172/JCI75695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li X, Yang Y, Huang Q, Deng Y, Guo F, Wang G, et al. Crosstalk between the tumor microenvironment and cancer cells: a promising predictive biomarker for immune checkpoint inhibitors. Front Cell Dev Biol. 2021;9:738373. doi: 10.3389/fcell.2021.738373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 117.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Whiteside TL. Exosomes and tumor-mediated immune suppression. J Clin Investig. 2016;126(4):1216–1223. doi: 10.1172/JCI81136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, Whiteside TL. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes1. J Immunol. 2009;183(6):3720–3730. doi: 10.4049/jimmunol.0900970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chow A, Zhou W, Liu L, Fong MY, Champer J, Van Haute D, et al. Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-κB. Sci Rep. 2014;4(1):5750. doi: 10.1038/srep05750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Cunha G, Hein P, et al. Carcinoma-associated fibroblasts stimulate tumor progression of initiated human epithelium. Breast Cancer Res. 2000;2(1):1-. [DOI] [PMC free article] [PubMed]
- 123.Reiners KS, Topolar D, Henke A, Simhadri VR, Kessler J, Sauer M, et al. Soluble ligands for NK cell receptors promote evasion of chronic lymphocytic leukemia cells from NK cell anti-tumor activity. Blood. 2013;121(18):3658–3665. doi: 10.1182/blood-2013-01-476606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tung SL, Boardman DA, Sen M, Letizia M, Peng Q, Cianci N, et al. Regulatory T cell-derived extracellular vesicles modify dendritic cell function. Sci Rep. 2018;8(1):1–12. doi: 10.1038/s41598-018-24531-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Berchem G, Noman MZ, Bosseler M, Paggetti J, Baconnais S, Le Cam E, et al. Hypoxic tumor-derived microvesicles negatively regulate NK cell function by a mechanism involving TGF-β and miR23a transfer. Oncoimmunology. 2016;5(4):e1062968. doi: 10.1080/2162402X.2015.1062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Baig MS, Roy A, Rajpoot S, Liu D, Savai R, Banerjee S, et al. Tumor-derived exosomes in the regulation of macrophage polarization. Inflamm Res. 2020;69:435–451. doi: 10.1007/s00011-020-01318-0. [DOI] [PubMed] [Google Scholar]
- 127.Tian X, Shen H, Li Z, Wang T, Wang S. Tumor-derived exosomes, myeloid-derived suppressor cells, and tumor microenvironment. J Hematol Oncol. 2019;12:1–18. doi: 10.1186/s13045-019-0772-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mortezaee K. Myeloid-derived suppressor cells in cancer immunotherapy-clinical perspectives. Life Sci. 2021;277:119627. doi: 10.1016/j.lfs.2021.119627. [DOI] [PubMed] [Google Scholar]
- 129.Mortezaee K. Normalization in tumor ecosystem: opportunities and challenges. Cell Biol Int. 2021;45(10):2017–2030. doi: 10.1002/cbin.11655. [DOI] [PubMed] [Google Scholar]
- 130.Yin L, Wang Y. Extracellular vesicles derived from M2-polarized tumor-associated macrophages promote immune escape in ovarian cancer through NEAT1/miR-101–3p/ZEB1/PD-L1 axis. Cancer Immunol Immunother. 2022:1–16. [DOI] [PMC free article] [PubMed]
- 131.Dionisi M, De Archangelis C, Battisti F, Rahimi Koshkaki H, Belleudi F, Zizzari IG, et al. Tumor-derived microvesicles enhance cross-processing ability of clinical grade dendritic cells. Front Immunol. 2018;9:2481. doi: 10.3389/fimmu.2018.02481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ma J, Wei K, Zhang H, Tang K, Li F, Zhang T, et al. Mechanisms by which dendritic cells present tumor microparticle antigens to CD8(+) T cells. Cancer Immunol Res. 2018;6(9):1057–1068. doi: 10.1158/2326-6066.CIR-17-0716. [DOI] [PubMed] [Google Scholar]
- 133.Kitai Y, Kawasaki T, Sueyoshi T, Kobiyama K, Ishii KJ, Zou J, et al. DNA-containing exosomes derived from cancer cells treated with topotecan activate a STING-dependent pathway and reinforce antitumor immunity. J Immunol. 2017;198(4):1649–1659. doi: 10.4049/jimmunol.1601694. [DOI] [PubMed] [Google Scholar]
- 134.Zhang H, Tang K, Zhang Y, Ma R, Ma J, Li Y, et al. Cell-free tumor microparticle vaccines stimulate dendritic cells via cGAS/STING signaling tumor cell–derived microparticles as vaccine. Cancer Immunol Res. 2015;3(2):196–205. doi: 10.1158/2326-6066.CIR-14-0177. [DOI] [PubMed] [Google Scholar]
- 135.Zhu L, Kalimuthu S, Gangadaran P, Oh JM, Lee HW, Baek SH, et al. Exosomes derived from natural killer cells exert therapeutic effect in melanoma. Theranostics. 2017;7(10):2732. doi: 10.7150/thno.18752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Morrissey SM, Yan J. Exosomal PD-L1: roles in tumor progression and immunotherapy. Trends in Cancer. 2020;6(7):550–558. doi: 10.1016/j.trecan.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rossowska J, Anger N, Wegierek K, Szczygieł A, Mierzejewska J, Milczarek M, et al. Antitumor potential of extracellular vesicles released by genetically modified murine colon carcinoma cells with overexpression of interleukin-12 and shRNA for TGF-β1. Front Immunol. 2019;10. [DOI] [PMC free article] [PubMed]
- 138.Jo EB, Lee YS, Lee H, Park JB, Park H, Choi Y-L, et al. Combination therapy with c-met inhibitor and TRAIL enhances apoptosis in dedifferentiated liposarcoma patient-derived cells. BMC Cancer. 2019;19(1):496. doi: 10.1186/s12885-019-5713-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Seo N, Akiyoshi K, Shiku H. Exosome-mediated regulation of tumor immunology. Cancer Sci. 2018;109(10):2998–3004. doi: 10.1111/cas.13735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Theodoraki M-N, Yerneni SS, Hoffmann TK, Gooding WE, Whiteside TL. Clinical significance of PD-L1+ exosomes in plasma of head and neck cancer patients. Clin Cancer Res. 2018;24(4):896–905. doi: 10.1158/1078-0432.CCR-17-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Theodoraki M-N, Yerneni SS, Hoffmann TK, Gooding WE, Whiteside TL. Clinical significance of PD-L1+ exosomes in plasma of head and neck cancer patients PD-L1+ exosomes in plasma of HNC patients. Clin Cancer Res. 2018;24(4):896–905. doi: 10.1158/1078-0432.CCR-17-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Razzo BM, Ludwig N, Hong CS, Sharma P, Fabian KP, Fecek RJ, et al. Tumor-derived exosomes promote carcinogenesis of murine oral squamous cell carcinoma. Carcinogenesis. 2020;41(5):625–633. doi: 10.1093/carcin/bgz124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Razzo BM, Ludwig N, Hong C-S, Sharma P, Fabian KP, Fecek RJ, et al. Tumor-derived exosomes promote carcinogenesis of murine oral squamous cell carcinoma. Carcinogenesis. 2019;41(5):625–633. doi: 10.1093/carcin/bgz124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Poggio M, Hu T, Pai C-C, Chu B, Belair CD, Chang A, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177(2):414–27.e13. doi: 10.1016/j.cell.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Daassi D, Mahoney KM, Freeman GJ. The importance of exosomal PDL1 in tumour immune evasion. Nat Rev Immunol. 2020;20(4):209–215. doi: 10.1038/s41577-019-0264-y. [DOI] [PubMed] [Google Scholar]
- 147.Tang XJ, Sun XY, Huang KM, Zhang L, Yang ZS, Zou DD, et al. Therapeutic potential of CAR-T cell-derived exosomes: a cell-free modality for targeted cancer therapy. Oncotarget. 2015;6(42):44179–44190. doi: 10.18632/oncotarget.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jogalekar MP, Rajendran RL, Khan F, Dmello C, Gangadaran P, Ahn BC. CAR T-cell-based gene therapy for cancers: new perspectives, challenges, and clinical developments. Front Immunol. 2022;13:925985. doi: 10.3389/fimmu.2022.925985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Aharon A, Horn G, Bar-Lev TH, Zagagi Yohay E, Waks T, Levin M, et al. Extracellular vesicles derived from chimeric antigen receptor-T cells: a potential therapy for cancer. Hum Gene Ther. 2021;32(19–20):1224–1241. doi: 10.1089/hum.2021.192. [DOI] [PubMed] [Google Scholar]
- 150.Ali S, Toews K, Schwiebert S, Klaus A, Winkler A, Grunewald L, et al. Tumor-derived extracellular vesicles impair CD171-specific CD4+ CAR T cell efficacy. Front Immunol. 2020;11. [DOI] [PMC free article] [PubMed]
- 151.Cox MJ, Lucien F, Sakemura R, Boysen JC, Kim Y, Horvei P, et al. Leukemic extracellular vesicles induce chimeric antigen receptor T cell dysfunction in chronic lymphocytic leukemia. Mol Ther. 2021;29(4):1529–1540. doi: 10.1016/j.ymthe.2020.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lener T, Gimona M, Aigner L, Börger V, Buzas E, Camussi G, et al. Applying extracellular vesicles based therapeutics in clinical trials – an ISEV position paper. Journal of Extracellular Vesicles. 2015;4(1):30087. doi: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Marar C, Starich B, Wirtz D. Extracellular vesicles in immunomodulation and tumor progression. Nat Immunol. 2021;22(5):560–570. doi: 10.1038/s41590-021-00899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Svenson S. Clinical translation of nanomedicines. Curr Opin Solid State Mater Sci. 2012;16(6):287–294. doi: 10.1016/j.cossms.2012.10.001. [DOI] [Google Scholar]
- 155.Çağdaş M, Sezer AD, Bucak S. Liposomes as potential drug carrier systems for drug delivery. Application of nanotechnology in drug delivery. 2014;1:1–50. [Google Scholar]
- 156.van der Meel R, Fens MH, Vader P, Van Solinge WW, Eniola-Adefeso O, Schiffelers RM. Extracellular vesicles as drug delivery systems: lessons from the liposome field. J Control Release. 2014;195:72–85. doi: 10.1016/j.jconrel.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 157.Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Van den Boorn JG, Schlee M, Coch C, Hartmann G. SiRNA delivery with exosome nanoparticles. Nat Biotechnol. 2011;29(4):325–326. doi: 10.1038/nbt.1830. [DOI] [PubMed] [Google Scholar]
- 159.Elsharkasy OM, Nordin JZ, Hagey DW, de Jong OG, Schiffelers RM, Andaloussi SEL, et al. Extracellular vesicles as drug delivery systems: why and how? Adv Drug Deliv Rev. 2020;159:332–343. doi: 10.1016/j.addr.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 160.Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106:148–156. doi: 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 161.Wiklander OPB, Brennan M, Lötvall J, Breakefield XO, El Andaloussi S. Advances in therapeutic applications of extracellular vesicles. Science translational medicine. 2019;11(492). [DOI] [PMC free article] [PubMed]
- 162.Tang TT, Lv LL, Lan HY, Liu BC. Extracellular vesicles: opportunities and challenges for the treatment of renal diseases. Front Physiol. 2019;10:226. doi: 10.3389/fphys.2019.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharmaceutica Sinica B. 2016;6(4):287–296. doi: 10.1016/j.apsb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wiklander OPB, Brennan MÁ, Lötvall J, Breakefield XO, EL Andaloussi S. Advances in therapeutic applications of extracellular vesicles. Science translational medicine. 2019;11(492):eaav8521. [DOI] [PMC free article] [PubMed]
- 165.György B, Hung ME, Breakefield XO, Leonard JN. Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmacol Toxicol. 2015;55:439–464. doi: 10.1146/annurev-pharmtox-010814-124630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fais S, O’Driscoll L, Borras FE, Buzas E, Camussi G, Cappello F, et al. Evidence-based clinical use of nanoscale extracellular vesicles in nanomedicine. 2016. [DOI] [PubMed]
- 167.Maeda H. Macromolecular therapeutics in cancer treatment: the EPR effect and beyond. J Control Release. 2012;164(2):138–144. doi: 10.1016/j.jconrel.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 168.Mizrak A, Bolukbasi MF, Ozdener GB, Brenner GJ, Madlener S, Erkan EP, et al. Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Mol Ther. 2013;21(1):101–108. doi: 10.1038/mt.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ohno S-i, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21(1):185–91. [DOI] [PMC free article] [PubMed]
- 170.Zhang Y, Li L, Yu J, Zhu D, Zhang Y, Li X, et al. Microvesicle-mediated delivery of transforming growth factor β1 siRNA for the suppression of tumor growth in mice. Biomaterials. 2014;35(14):4390–4400. doi: 10.1016/j.biomaterials.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 171.Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35(7):2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 172.Kuerban K, Gao X, Zhang H, Liu J, Dong M, Wu L, et al. Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced anti-tumor efficacy in non-small-cell lung cancer. Acta Pharmaceutica Sinica B. 2020;10(8):1534–1548. doi: 10.1016/j.apsb.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ma J, Zhang Y, Tang K, Zhang H, Yin X, Li Y, et al. Reversing drug resistance of soft tumor-repopulating cells by tumor cell-derived chemotherapeutic microparticles. Cell Res. 2016;26(6):713–727. doi: 10.1038/cr.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine: Nanotech, Bio Med. 2016;12(3):655–64. [DOI] [PMC free article] [PubMed]
- 175.Li Y-J, Wu J-Y, Wang J-M, Hu X-B, Cai J-X, Xiang D-X. Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer. Acta Biomater. 2020;101:519–530. doi: 10.1016/j.actbio.2019.10.022. [DOI] [PubMed] [Google Scholar]
- 176.Tang K, Zhang Y, Zhang H, Xu P, Liu J, Ma J, et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat Commun. 2012;3(1):1–11. doi: 10.1038/ncomms2282. [DOI] [PubMed] [Google Scholar]
- 177.Zhao C, Busch DJ, Vershel CP, Stachowiak JC. Multifunctional transmembrane protein ligands for cell-specific targeting of plasma membrane-derived vesicles. Small. 2016;12(28):3837–3848. doi: 10.1002/smll.201600493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang D, Lee H, Zhu Z, Minhas JK, Jin Y. Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2017;312(1):L110–L121. doi: 10.1152/ajplung.00423.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Koh E, Lee EJ, Nam G-H, Hong Y, Cho E, Yang Y, et al. Exosome-SIRPα, a CD47 blockade increases cancer cell phagocytosis. Biomaterials. 2017;121:121–129. doi: 10.1016/j.biomaterials.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 180.Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017;38(6):754–763. doi: 10.1038/aps.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell derived exosomes. Nat Med. 1998;4(5):594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 182.Ochyl LJ, Bazzill JD, Park C, Xu Y, Kuai R, Moon JJ. PEGylated tumor cell membrane vesicles as a new vaccine platform for cancer immunotherapy. Biomaterials. 2018;182:157–166. doi: 10.1016/j.biomaterials.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Srivastava A, Babu A, Filant J, Moxley KM, Ruskin R, Dhanasekaran D, et al. Exploitation of exosomes as nanocarriers for gene-, chemo-, and immune-therapy of cancer. J Biomed Nanotechnol. 2016;12(6):1159–1173. doi: 10.1166/jbn.2016.2205. [DOI] [PubMed] [Google Scholar]
- 184.Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. OncoImmunology. 2016;5(4):e1071008. doi: 10.1080/2162402X.2015.1071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Giacobino C, Canta M, Fornaguera C, Borrós S, Cauda V. Extracellular vesicles and their current role in cancer immunotherapy. Cancers. 2021;13(9):2280. doi: 10.3390/cancers13092280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nikfarjam S, Rezaie J, Kashanchi F, Jafari R. Dexosomes as a cell-free vaccine for cancer immunotherapy. J Exp Clin Cancer Res. 2020;39(1):1–20. doi: 10.1186/s13046-020-01781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Naseri M, Bozorgmehr M, Zöller M, Ranaei Pirmardan E, Madjd Z. Tumor-derived exosomes: the next generation of promising cell-free vaccines in cancer immunotherapy. Oncoimmunology. 2020;9(1):1779991. doi: 10.1080/2162402X.2020.1779991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang X, Wang C, Wang J, Hu Q, Langworthy B, Ye Y, et al. PD-1 blockade cellular vesicles for cancer immunotherapy. Adv Mater. 2018;30(22):1707112. doi: 10.1002/adma.201707112. [DOI] [PubMed] [Google Scholar]
- 189.Chen Y, Wang L, Zheng M, Zhu C, Wang G, Xia Y, et al. Engineered extracellular vesicles for concurrent anti-PDL1 immunotherapy and chemotherapy. Bioact Mater. 2022;9:251–265. doi: 10.1016/j.bioactmat.2021.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Veerman RE, Akpinar GG, Offens A, Steiner L, Larssen P, Lundqvist A, et al. Antigen-loaded extracellular vesicles induce responsiveness to anti–PD-1 and anti–PD-L1 treatment in a checkpoint refractory melanoma model. Cancer Immunol Res. 2023:OF1-OF11. [DOI] [PMC free article] [PubMed]
- 191.Wei Z, Zhang X, Yong T, Bie N, Zhan G, Li X, et al. Boosting anti-PD-1 therapy with metformin-loaded macrophage-derived microparticles. Nat Commun. 2021;12(1):440. doi: 10.1038/s41467-020-20723-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Girish S, Gupta M, Wang B, Lu D, Krop IE, Vogel CL, et al. Clinical pharmacology of trastuzumab emtansine (T-DM1): an antibody–drug conjugate in development for the treatment of HER2-positive cancer. Cancer Chemother Pharmacol. 2012;69(5):1229–1240. doi: 10.1007/s00280-011-1817-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Figueroa-Magalhães MC, Jelovac D, Connolly RM, Wolff AC. Treatment of HER2-positive breast cancer. The Breast. 2014;23(2):128–136. doi: 10.1016/j.breast.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tai W, Mahato R, Cheng K. The role of HER2 in cancer therapy and targeted drug delivery. J Control Release. 2010;146(3):264–275. doi: 10.1016/j.jconrel.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Krop IE, Beeram M, Modi S, Jones SF, Holden SN, Yu W, et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol. 2010;28(16):2698–2704. doi: 10.1200/JCO.2009.26.2071. [DOI] [PubMed] [Google Scholar]
- 196.Burris HA, Rugo HS, Vukelja SJ, Vogel CL, Borson RA, Limentani S, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)–positive breast cancer after prior HER2-directed therapy. J Clin Oncol. 2011;29(4):398–405. doi: 10.1200/JCO.2010.29.5865. [DOI] [PubMed] [Google Scholar]
- 197.Girish S, Gupta M, Wang B, Lu D, Krop IE, Vogel CL, et al. Clinical pharmacology of trastuzumab emtansine (T-DM1): an antibody-drug conjugate in development for the treatment of HER2-positive cancer. Cancer Chemother Pharmacol. 2012;69(5):1229–1240. doi: 10.1007/s00280-011-1817-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody–cytotoxic drug conjugate. Can Res. 2008;68(22):9280–9290. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 199.Barok M, Puhka M, Vereb G, Szollosi J, Isola J, Joensuu H. Cancer-derived exosomes from HER2-positive cancer cells carry trastuzumab-emtansine into cancer cells leading to growth inhibition and caspase activation. BMC Cancer. 2018;18(1):1–12. doi: 10.1186/s12885-018-4418-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shi X, Cheng Q, Hou T, Han M, Smbatyan G, Lang JE, et al. Genetically engineered cell-derived nanoparticles for targeted breast cancer immunotherapy. Mol Ther. 2020;28(2):536–547. doi: 10.1016/j.ymthe.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cheng Q, Shi X, Han M, Smbatyan G, Lenz H-J, Zhang Y. Reprogramming exosomes as nanoscale controllers of cellular immunity. J Am Chem Soc. 2018;140(48):16413–16417. doi: 10.1021/jacs.8b10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yoshimoto T, Morishima N, Okumura M, Chiba Y, Xu M, Mizuguchi J. Interleukins and cancer immunotherapy. Immunotherapy. 2009;1(5):825–844. doi: 10.2217/imt.09.46. [DOI] [PubMed] [Google Scholar]
- 203.Akdis M, Burgler S, Crameri R, Eiwegger T, Fujita H, Gomez E, et al. Interleukins, from 1 to 37, and interferon-γ: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2011;127(3):701–21.e1–70. [DOI] [PubMed]
- 204.Anestakis D, Petanidis S, Kalyvas S, Nday CM, Tsave O, Kioseoglou E, et al. Mechanisms and applications of interleukins in cancer immunotherapy. Int J Mol Sci. 2015;16(1):1691–1710. doi: 10.3390/ijms16011691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Briukhovetska D, Dörr J, Endres S, Libby P, Dinarello CA, Kobold S. Interleukins in cancer: from biology to therapy. Nat Rev Cancer. 2021;21(8):481–499. doi: 10.1038/s41568-021-00363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Holder PG, Lim SA, Huang CS, Sharma P, Dagdas YS, Bulutoglu B, et al. Engineering interferons and interleukins for cancer immunotherapy. Adv Drug Deliv Rev. 2022;182:114112. doi: 10.1016/j.addr.2022.114112. [DOI] [PubMed] [Google Scholar]
- 207.Li J, Huang L, Zhao H, Yan Y, Lu J. The role of interleukins in colorectal cancer. Int J Biol Sci. 2020;16(13):2323–2339. doi: 10.7150/ijbs.46651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mizel SB. The interleukins1. FASEB J. 1989;3(12):2379–2388. doi: 10.1096/fasebj.3.12.2676681. [DOI] [PubMed] [Google Scholar]
- 209.Bruni D, Angell HK, Galon J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer. 2020;20(11):662–680. doi: 10.1038/s41568-020-0285-7. [DOI] [PubMed] [Google Scholar]
- 210.Zhang J, Veeramachaneni N. Targeting interleukin-1β and inflammation in lung cancer. Biomarker Research. 2022;10(1):5. doi: 10.1186/s40364-021-00341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Pfeffer LM, Dinarello CA, Herberman RB, Williams BR, Borden EC, Bordens R, et al. Biological properties of recombinant α-interferons: 40th anniversary of the discovery of interferons. Can Res. 1998;58(12):2489–2499. [PubMed] [Google Scholar]
- 212.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192(12):5451–5458. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhou Y, Husman T, Cen X, Tsao T, Brown J, Bajpai A, et al. Interleukin 15 in cell-based cancer immunotherapy. Int J Mol Sci [Internet]. 2022;23(13). [DOI] [PMC free article] [PubMed]
- 214.Souza AG, Colli LM. Extracellular vesicles and interleukins: novel frontiers in diagnostic and therapeutic for cancer. Front Immunol. 2022;13. [DOI] [PMC free article] [PubMed]
- 215.Tang TT, Wang B, Wu M, Li ZL, Feng Y, Cao JY, et al. Extracellular vesicle-encapsulated IL-10 as novel nanotherapeutics against ischemic AKI. Sci Adv. 2020;6(33):eaaz0748. [DOI] [PMC free article] [PubMed]
- 216.Zhang J, Zhang Y, Luo C, Xia Y, Chen H, Wu X. Glycosyl-phosphatidylinositol-anchored interleukin-2 expressed on tumor-derived exosomes induces antitumor immune response in vitro. Tumori. 2010;96(3):452–459. doi: 10.1177/030089161009600313. [DOI] [PubMed] [Google Scholar]
- 217.Gangadaran P, Gunassekaran GR, Rajendran RL, Oh JM, Vadevoo SM, Lee HW, et al. Interleukin-4 receptor targeting peptide decorated extracellular vesicles as a platform for in vivo drug delivery to thyroid cancer. Biomedicines [Internet]. 2022;10(8). [DOI] [PMC free article] [PubMed]
- 218.Bellavia D, Raimondo S, Calabrese G, Forte S, Cristaldi M, Patinella A, et al. Interleukin 3- receptor targeted exosomes inhibit in vitro and in vivo chronic myelogenous leukemia cell growth. Theranostics. 2017;7(5):1333–1345. doi: 10.7150/thno.17092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Vitale I, Shema E, Loi S, Galluzzi L. Intratumoral heterogeneity in cancer progression and response to immunotherapy. Nat Med. 2021;27(2):212–224. doi: 10.1038/s41591-021-01233-9. [DOI] [PubMed] [Google Scholar]
- 220.Qazi KR, Gehrmann U, Domange Jordö E, Karlsson MCI, Gabrielsson S. Antigen-loaded exosomes alone induce Th1-type memory through a B-cell-dependent mechanism. Blood. 2009;113(12):2673–2683. doi: 10.1182/blood-2008-04-153536. [DOI] [PubMed] [Google Scholar]
- 221.Morishita M, Takahashi Y, Matsumoto A, Nishikawa M, Takakura Y. Exosome-based tumor antigens–adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomaterials. 2016;111:55–65. doi: 10.1016/j.biomaterials.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 222.Tian H, Li W. Dendritic cell-derived exosomes for cancer immunotherapy: hope and challenges. Annals of Translational Medicine. 2017;5(10):221. doi: 10.21037/atm.2017.02.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Investig. 2007;117(6):1466–1476. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348(6230):62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.June CH, Riddell SR, Schumacher TN. Adoptive cellular therapy: a race to the finish line. Sci Transl Med. 2015;7(280):280ps7-ps7. [DOI] [PubMed]
- 226.Fu W, Lei C, Liu S, Cui Y, Wang C, Qian K, et al. CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity. Nat Commun. 2019;10(1):4355. doi: 10.1038/s41467-019-12321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Cox MJ, Lucien F, Sakemura R, Boysen JC, Kim Y, Horvei P, et al. Leukemic extracellular vesicles induce chimeric antigen receptor T cell dysfunction in chronic lymphocytic leukemia. Mol Ther. 2021;29(4):1529–1540. doi: 10.1016/j.ymthe.2020.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhu X, Hu H, Xiao Y, Li Q, Zhong Z, Yang J, et al. Tumor-derived extracellular vesicles induce invalid cytokine release and exhaustion of CD19 CAR-T Cells. Cancer Lett. 2022;536:215668. doi: 10.1016/j.canlet.2022.215668. [DOI] [PubMed] [Google Scholar]
- 229.Extracellular vesicles derived from chimeric antigen receptor-T cells a potential therapy for cancer. Hum Gene Ther. 2021;32(19–20):1224–1241. doi: 10.1089/hum.2021.192. [DOI] [PubMed] [Google Scholar]
- 230.Calvo V, Izquierdo M. T lymphocyte and CAR-T cell-derived extracellular vesicles and their applications in cancer therapy. Cells. 2022;11(5):790. doi: 10.3390/cells11050790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Jong AY, Wu C-H, Li J, Sun J, Fabbri M, Wayne AS, et al. Large-scale isolation and cytotoxicity of extracellular vesicles derived from activated human natural killer cells. Journal of Extracellular Vesicles. 2017;6(1):1294368. doi: 10.1080/20013078.2017.1294368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zhao Z, McGill J, Gamero-Kubota P, He M. Microfluidic on-demand engineering of exosomes towards cancer immunotherapy. Lab Chip. 2019;19(10):1877–1886. doi: 10.1039/C8LC01279B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Watson DC, Bayik D, Srivatsan A, Bergamaschi C, Valentin A, Niu G, et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials. 2016;105:195–205. doi: 10.1016/j.biomaterials.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ng CY, Kee LT, Al-Masawa ME, Lee QH, Subramaniam T, Kok D, et al. Scalable production of extracellular vesicles and its therapeutic values: a review. Int J Mol Sci. 2022;23(14). [DOI] [PMC free article] [PubMed]
- 235.Hahm J, Kim J, Park J. Strategies to enhance extracellular vesicle production. Tissue Engineering and Regenerative Medicine. 2021;18(4):513–524. doi: 10.1007/s13770-021-00364-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.de Jong OG, Kooijmans SAA, Murphy DE, Jiang L, Evers MJW, Sluijter JPG, et al. Drug delivery with extracellular vesicles: from imagination to innovation. Acc Chem Res. 2019;52(7):1761–1770. doi: 10.1021/acs.accounts.9b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):1–14. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Mendt M, Kamerkar S, Sugimoto H, McAndrews KM, Wu C-C, Gagea M, et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI insight. 2018;3(8). [DOI] [PMC free article] [PubMed]
- 239.Lamparski HG, Metha-Damani A, Yao J-Y, Patel S, Hsu D-H, Ruegg C, et al. Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods. 2002;270(2):211–226. doi: 10.1016/S0022-1759(02)00330-7. [DOI] [PubMed] [Google Scholar]
- 240.Rohde E, Pachler K, Gimona M. Manufacturing and characterization of extracellular vesicles from umbilical cord–derived mesenchymal stromal cells for clinical testing. Cytotherapy. 2019;21(6):581–592. doi: 10.1016/j.jcyt.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 241.Burnouf T, Agrahari V, Agrahari V. Extracellular vesicles as nanomedicine: hopes and hurdles in clinical translation. Int J Nanomedicine. 2019;14:8847–8859. doi: 10.2147/IJN.S225453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.