Abstract
Myocardial infarction is associated with increased risk for vascular dementia. In both myocardial infarction and vascular dementia, there is evidence that elevated inflammatory biomarkers are associated with worsened clinical outcomes. Myocardial infarction leads to a systemic inflammatory response, which may contribute to recruitment or activation of myeloid cells, including monocytes, microglia, and perivascular macrophages, within the central nervous system. However, our understanding of the causative roles for these cells linking cardiac injury to the development and progression of dementia is incomplete. Herein, we provide an overview of inflammatory cellular and molecular links between myocardial infarction and vascular dementia and discuss strategies to resolve inflammation after myocardial infarction to limit neurovascular injury.
2. Introduction
Myocardial infarction (MI) is a leading cause of morbidity and mortality worldwide [1]. While advances in treatment, such as percutaneous intervention with thrombolytics, has improved acute survival after MI, this has led to a growing and aging population of patients that are susceptible to post-MI sequalae, including heart failure (HF), cancer [2], and dementia [3]. Similar to HF, the prevalence of dementia is increasing worldwide [4]. The two most common causes of cognitive impairment include Alzheimer’s disease (AD) and vascular dementia (VaD). Clinical observations have shown that MI is associated with a higher risk for VaD, but not AD or other dementias [3]. This association can be explained in part by the shared risk factors that underlie both MI and VaD, including obesity, arteriosclerosis, diabetes, metabolic syndrome, hypertension, and advanced age [5][6]. Despite these similarities, little is known about the precise underlying mechanisms linking these diseases.
VaD is generally defined as dementia that is associated with clinical evidence of stroke, vascular brain injury, or cerebrovascular disease [7]. VaD is a heterogeneous disease encompassing different subtypes of cognitive disorders associated with cerebrovascular disease and its manifestations, including small or large cerebral vessel dementia, multi-infarct dementia, hypoperfusive dementia, hemorrhagic dementia, and subcortical ischemic dementia [8]. Given its broad definition, VaD can coexist with multiple neurodegenerative disorders, including the plaques and tangles of AD, and an ongoing challenge is determining whether cognitive decline in a dementia patient is a consequence solely of VaD (pure) or a combination of VaD with other neurodegenerative diseases (mixed) [7]. Cardiovascular risk factors, including hypertension, diabetes, and atherosclerosis, increase the risk for VaD as well as AD [7][9], further complicating clinical diagnoses. Despite the associations between cardiovascular disease and AD, cognitive deterioration in AD is generally accepted to arise from the diffuse amyloid beta deposits or plaques and Tau neurofibrillary tangles that lead to “damage from within the brain” [10]. In contrast, stronger contributions of cardiovascular disease have been reported for VaD compared to AD [3][11][12], suggesting that the brains of VaD patients may “reside in a bad neighborhood,” where extracerebral comorbidities lead to progressive damage to the brain vasculature. While cardiovascular disease and its lifestyle risk factors increase the risk for VaD, a major adverse cardiovascular event, such as MI, may precipitate VaD or accelerate progression from mild cognitive impairment to severe VaD.
Although the exact molecular mechanisms linking MI to VaD are unknown, it has been proposed that systemic inflammation leading to impaired cerebral blood flow, oxidative stress, and endothelial dysfunction within the central nervous system (CNS) links ischemic cardiac injury to white matter injury and adverse brain function. MI elicits a robust systemic inflammatory response that includes production of cytokines and chemokines and mobilization of myeloid cells, including monocytes and neutrophils, from the spleen and bone marrow into the peripheral circulation. Heightened levels of systemic inflammation are associated with increased risk for cognitive impairment [13] and higher levels of proinflammatory cytokines, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α, have been observed in both plasma [14] and cerebrospinal fluid (CSF) [15] of VaD patients compared to healthy age-matched controls without dementia. In the sections that follow, we review clinical and preclinical evidence for inflammatory links between MI and VaD.
2. Clinical links between MI and VaD
Some of the earliest clinical evidence in humans linking myocardial infarction to increased risk for dementia comes from the Bronx Aging Study [16]. In this study, 488 subjects, including 137 men and 285 women, were evaluated for changes in cognitive function using the Blessed Test of Information, Memory, and Concentration over a period of two to seven years. While the investigators controlled for baseline demographic, psychosocial, and medical history, women were found to be over three times more likely to develop dementia when compared to men and this risk increased five-fold when comparing women with a history of MI to those without MI. The higher prevalence of dementia in women compared to men may be explained in part by the stronger association of cardiovascular risk factors, including hypertension, smoking, and diabetes, with MI in women [17]. Other factors that may increase MI-induced dementia risk in women compared to men include sex-related differences in hormones, longevity, systemic inflammation, and microvascular dysfunction [18].
In a larger cohort of 4,971 subjects aged 55 to 94 years old, the single-center, population-based Rotterdam Study found a similar association between prior history of MI and worsened cognitive performance [19]. Since the study subjects had a large age range, it confirmed earlier reports finding an association between increasing age or lower education and cognitive impairment [20], and found that the prior history of MI was associated with worse cognitive performance independent of the effects of age or education [19]. Patients with unrecognized MI, which is a MI that is asymptomatic during the acute phase but eventually detected by electrocardiography, were also found to be at increased risk for VaD [21]. Subsequent analyses revealed that subjects with a history of MI were more likely to have white matter lesions, which appear as hyperintense signals on T2-weighted magnetic resonance imaging (MRI) images and are indicative of small vessel vascular brain disease, neurodegeneration, or inflammation [22]. Additional imaging studies using transcranial doppler found cortical microembolisms in 17% of patients within 72 hours after MI [23], and post-mortem analyses of the brains of MI patients revealed an increase in the prevalence of cortical microinfarcts compared to controls [24], demonstrating that MI triggers neuropathological correlates of VaD.
More recently and largest study to date, the nationwide Danish population-cohort study compared 314,911 patients with MI to 1,573,193 individuals from the general population without an MI diagnosis and matched for sex, birth and calendar year and found that MI was associated with higher risk of VaD, which was further increased in patients who had a stroke or developed severe HF during the first year after MI [3]. Interestingly, MI was not associated with all-cause dementia, AD, or other dementia subtypes, indicating that the pathological links were specific for MI and VaD. While individuals who experience an MI are likely to have a higher burden of cardiovascular disease, which would increase the risk for VaD, a longitudinal study of 7,888 subjects over a 12-year period, found that despite the higher burden of vascular risk factors, accelerated cognitive decline was observed only after MI, but not before [25]. To control for a higher burden of vascular risk factors at baseline, the Improve Cardiovascular Outcomes in high-risk older patieNts (ICON1) study assessed cognitive decline in 298 older patients with non-ST-elevation acute coronary syndrome [26]. Despite the high prevalence of shared risk factors for MI and VaD among all the study participants, including hypertension, diabetes mellitus, hyperlipidemia, and history of smoking, recurrent MI was independently associated with cognitive decline at the one-year follow-up assessment. Taken together, while shared risk factors may predispose to both MI and VaD, these studies suggest that MI may independently trigger pathophysiological pathways that result in VaD.
Additional studies have found strong associations between MI and mild cognitive impairment (MCI), which can progress to VaD [27]. In a large cohort of 6,455 cognitively intact, postmenopausal women aged 65 to 79 years old and followed for 8.4 years, the Women’s Health Initiative Memory Study found that a history of MI doubled the risk for MCI or probable dementia compared to women without MI [28]. Similarly, in a large cohort of 2,963 men and women aged 65 to 84 years old and followed 3.5 years, the Italian Longitudinal Study on Aging found that coronary artery disease, which included a history of MI or angina pectoris, was associated with increased risk for progression of MCI to dementia [29]. The study also found that among those that progressed to dementia, 33% progressed to VaD [29]. Finally, in 1,701 participants with a mean age of 76 years old followed over a period of 20 years, the Atherosclerosis Risk in Communities study found that a history of MI was associated with increased prevalence of intracranial atherosclerotic stenosis in individuals with MCI [30]. Intracranial atherosclerotic stenosis was reported in 53% of VaD patients during a retrospective review of 103 dementia patients in a Singapore dementia clinic [31], suggesting this may play a role in VaD pathogenesis. Intracranial atherosclerotic stenosis can lead to progressive cerebral damage through spontaneous cerebral emboli, which have been observed in greater frequency in VaD patients compared to age and sex matched controls [32]. Together, these studies suggest that MI may accelerate progression from MCI to VaD.
Other adverse cardiac events, including sudden cardiac arrest [33], coronary-artery bypass surgery [34], or congenital heart disease [35], have also been associated with cognitive impairment and increased risk for dementia. However, the pathophysiological links between these other adverse cardiac events and cognitive decline are likely distinct from those between MI and VaD. For example, cardiac arrest leads to global CNS hypoxia-ischemia followed by reperfusion injury after return of spontaneous circulation [36]. In contrast, there is little evidence to support a role for acute ischemic injury in the CNS after MI. A case-controlled study of 50 patients with varying severity of HF and 50 healthy control subjects matched for age, gender, and intelligence found that while history of MI was associated with increased severity of cognitive impairment, the extent of left ventricular ejection fraction (LVEF), or the amount of blood pumped out of the left ventricle after each contraction, was not associated with severity of cognitive impairment [37]. A separate study of 82 patients with history of MI that divided patients based on LVEFs above or below 40%, also found no relationship between LVEF level and cognitive impairment measured three months after MI [38]. Likewise, cerebral oxygen levels were only modestly reduced during the first 24 hours after cardiac injury before returning to baseline levels by one week in a murine model of permanent occlusion MI [39]. Thus, the mechanisms that accelerate VaD after MI are likely specific to MI.
3. Inflammatory links between MI and VaD
MI causes the release of inflammatory cytokines and chemokines into the circulation, which may initiate the neuropathological pathways that contribute to the development of VaD. After MI, recognition of damaged cardiac tissue by Toll-like receptors (TLR) expressed on myeloid cells, including monocytes, neutrophils, and macrophages, leads to production of proinflammatory cytokines, including IL-1β [40], IL-6 [41], and TNF-α [42]. Release of these cytokines into circulation initiates the inflammatory phase, which includes mobilization of immune cells from the spleen and bone marrow, endothelial cell activation, and infiltration of immune cells into the infarcted myocardium [43]. During the inflammatory phase, the levels of proinflammatory cytokine release determines the extent of systolic dysfunction and risk for HF progression [41]. It also contributes to the initiation of the reparative and proliferative phases, which are characterized by clearance of dead cardiac tissue, production of specialized proresolving mediators (SPM), inflammation resolution, and mature scar formation. Failure to resolve inflammation during the latter phases after MI leads to persistence of inflammatory cytokines within the circulation, sustained hepatic release of C-reactive protein (CRP), a biomarker for inflammation, and increased risk for secondary adverse events [42].
Similar to MI, higher levels of proinflammatory cytokines have been observed during VaD. In a study comparing plasma levels of IL-1β, IL-6, and TNF-α among 182 subjects with VaD, AD, or age-matched controls without dementia and adjusted for confounding variables, VaD patients were found to have higher levels of IL-1β, IL-6, and TNF-α compared to controls [14]. While AD patients also exhibited higher levels of IL-1β and TNF-α compared to controls, the increase in IL-6 was specific to VaD patients. The heightened inflammation systemically during VaD also appears to manifest locally within the CNS, as IL-6 levels were found to be significantly elevated in the CSF of VaD patients when compared to patients with AD or cerebrovascular disease without dementia [15]. TNF-α levels have also been found to be increased in CSF of VaD patients compared to healthy controls and this was positively correlated with the levels of sulfatide, a marker of white matter degradation [44]. Another hallmark of vascular pathophysiology is oxidative stress-induced expression of adhesion molecules, including vascular cell adhesion molecule (VCAM)-1, on cerebrovascular endothelial cells. In VaD patients, plasma levels of soluble VCAM-1 were increased compared to non-demented older controls [45], indicative of a state of endothelial dysfunction. Higher levels of soluble VCAM-1 were also found in AD patients compared to controls [45] and separately, no association was found between high plasma levels of soluble VCAM-1 and dementia risk [46]. This suggests that endothelial dysfunction is a consequence of a heightened inflammatory state and manifests in a variety of neurodegenerative disorders. Taken together, heightened systemic inflammation may reflect not only peripheral disease but also neuropathological mechanisms related to VaD.
Increased levels of inflammatory cytokines in the plasma of VaD patients raises the question of whether inflammation is involved in the pathogenic pathways leading to VaD. To address this question, four large population-based cohort studies examined the prospective associations between CRP or IL-6 to dementia and its major subtypes, AD and VaD. In the Honolulu-Asia Aging Study (HAAS), blood samples were collected from a cohort of 8,006 Japanese American men from 1968–1970 who were subsequently reexamined 25 years later for dementia [47]. In a random subsample of 1,050 cases from HAAS, high serum levels of CRP at midlife were found to significantly increase the risk for both AD and VaD 25 years later by approximately three-fold, which was independent of cardiovascular risk factors and disease [47]. In contrast to AD, the risk for VaD increased with increasing concentrations of CRP, suggesting the association between CRP and dementia may be stronger for VaD than AD [47]. While the HAAS was only conducted in men, a case-cohort study within the Rotterdam Study examined 915 men and women who were free of dementia at baseline plasma collection and subsequently followed for incident dementia. This study also found an association between high levels of CRP as well as IL-6 and α1-antichymotrypsin with increased risk for VaD [46]. Similarly, the Conselice Study of Brain Aging, which followed incident dementia over a four year period in a dementia-free Italian elderly cohort comprised of both men and women, found a combination of high levels of both CRP and IL-6 in plasma at intake was associated with increased risk of VaD, independent of socio-demographic confounders and traditional risk factors [48]. Interestingly, this association was specific for VaD as neither inflammatory marker in this study was associated with changes in risk for AD [48]. While neither the Rotterdam Study nor Conselice Study performed sex-related analyses, a population-based, longitudinal cohort study of 305 men and women aged 90 years and older found that when analyses were performed separately for men and women, higher CRP levels were associated with significantly higher risk for all-cause dementia in women only [49];. Together, these studies provide evidence that inflammation precedes the onset of clinical symptoms and is involved in VaD pathogenesis.
While there is substantial evidence that inflammation increases VaD risk and disease progression, few studies have explored the relationship specifically between MI-induced inflammation and VaD. In a small prospective study of 78 adults with pre-existing cardiovascular disease that performed neuropsychological assessments 1 year after measurement of plasma CRP levels, high CRP levels were found to be associated with cognitive decline but no significant differences were found among individuals with history of MI compared to groups with other types of cardiovascular disease [50]. In contrast, a separate study of 536 patients with a history of MI at least 6 months to no longer than 5 years before enrollment and/or stable angina that were evaluated for cognitive performance 20 years after measurement of plasma CRP levels, found that patients with higher CRP levels at entry to the study were associated with poorer cognitive performance overall [51]. Higher peak levels of CRP in the acute phase after MI are predictive of progression to HF [52], suggesting that the extent of inflammation after MI is an important determinant in dementia risk. This underscores the importance of obtaining measurements of inflammatory mediators closer to the time of MI as these will likely be more informative in predicting the links between MI and VaD. Additional information on inflammatory cell numbers and activation status within the periphery and in the CNS will also aid our understanding of the relationship between MI-induced inflammation and VaD.
In contrast to the limited human studies, mounting experimental evidence from rodent models of MI supports a link between MI-induced neuroinflammation and VaD-like disease. In mice and rats, MI is surgically-induced by temporarily or permanently ligating the left ascending coronary artery to achieve reperfused or nonreperfused MI, respectively [53]. Advantages of this model include the ability to study the kinetics of neuroinflammation after MI and direct contributions of MI-induced neuroinflammation to cognitive impairment through conditional knockouts or CNS-targeted interventions. It also enables studies of post-MI neuroinflammation independent or in combination with other cardiovascular risk factors, such as aging, hypertension, obesity, and diabetes. Similar to VaD patients, inflammatory cytokines, including IL-1β, IL-6, and TNF-α, are increased in the brains of rats after acute MI [54] and remain elevated into the progression to HF four to eight weeks after MI [55]. Furthermore, behavioral studies have revealed that rats and mice exhibit multiple deficits in cognitive function after MI that recapitulates many of the features of human VaD. In rats with HF eight weeks after MI, depression-like behavior was increased and spatial learning and memory were impaired as measured using the forced swim test [56] and Morris water maze [57], respectively. Likewise, in mice with HF eight to twelve weeks after MI, anxiety-like behavior was increased and short-term recognition and memory was impaired as measured using the elevated plus maze [58] and non-spatial novel object recognition test [59], respectively. Treatment of HF mice with an anti-TNF-α biologic six weeks after MI improved short-term recognition and memory [59], linking neuroinflammation to cognitive impairment after experimental MI.
While rodent models are useful for studying the kinetics and cellular responses linking MI-induced neuroinflammation to cognitive decline, there are important limitations. For example, the increased maturation rate in mice compared to humans makes it difficult to extrapolate the time frame of cognitive decline in mice to humans after MI. Laboratory mice and humans have average lifespans of two and 80 years, respectively, so it has been estimated that 9.125 mouse days are equal to one human year [60]. Therefore, the increased cognitive decline observed eight to twelve weeks after MI in mice translates to six to nine years after MI in humans. This time frame is consistent with many of the human studies described above. Another important caveat to the murine studies of experimental MI is that many studies utilized mature animals aged three to six months old, which is the human equivalent of 20 to 30 years. This suggests that MI-induced neuroinflammation increases risk for cognitive impairment independent of age. However, innate and adaptive immune function changes with age [61], so this may affect the extent of cognitive decline after MI in older patients. Finally, few studies have examined whether the sex differences in cognitive impairment observed after MI in humans are reproduced in rodent models. One study using 10 week old male and female rats found that male rats were more susceptible to MI-induced cognitive impairment [62]. This is in contrast to the increased risk of cognitive decline reported in older women after MI [16]. The cognitive protection observed in mature female rats may have been due in part to an active estrous cycle, as menopause is associated with elevated risk for dementia [63]. Thus, additional studies focused on sex differences and using aged rodents are needed to more fully recapitulate the human disease.
4. Immune cell activation in the periphery and CNS after MI
CNS resident microglia and perivascular macrophages are capable of producing the inflammatory cytokines that increase VaD risk and disease progression. However, compared to other neurodegenerative conditions in humans, such as AD, far less to little is known, about their role in neuroinflammation after MI. Evidence of neuroinflammation after acute MI in humans comes from a study of three patients that underwent positron emission tomography (PET) within four to six days after MI [64]. Using an imaging agent that targeted the mitochondrial translocator protein (TSPO), which is expressed in activated microglia and macrophages, increased signal was observed in the temporal and frontobasal cortex, hypothalamus, and cerebellum in MI patients compared to healthy controls. This paralleled changes in the heart, where a higher TSPO signal was also observed in the infarct region compared to remote tissue, demonstrating increased myeloid cell activation in both the heart and brain after MI. Increased activation of microglia and accumulation of macrophages has also been observed in post-mortem histopathological analyses of VaD patients [65], with macrophages prevalent in multiple white matter regions and positioned between fibrotic, hypertrophied blood vessels and white matter injury [66]. This raises the possibility that activation of CNS resident immune cells leads to neuroinflammation and disruption of the blood brain barrier after MI.
The most abundant immune cell in the CNS are microglia, which exert homeostatic functions critical for CNS maintenance and undergo proliferative, morphological, and functional changes in response to CNS injury. Microglial activation was first observed in the hypothalamic paraventricular nucleus (PVN) two to five weeks after MI in rats [67]. Following MI, microglia acquired an activated morphology defined by increased immunostaining for CD11b, enlarged soma, and reduced but considerably thicker and shorter processes. While CD11b is not specific to microglia and is a common myeloid cell marker, activated microglia increase expression of CD11b and other surface markers, including CD68, CX3CR1, Iba-1, and MHCII [68]. Subsequent kinetic analyses revealed that microglial activation in the hypothalamic PVN did not happen within the first 24 hours after MI but rather it occurred gradually and was sustained for weeks after MI [69]. Interestingly, microglial activation occurred without changes in the total number of microglia. While these initial studies only examined microglial activation in the hypothalamic PVN, additional studies have found that MI leads to activated microglia in brain regions involved in cardiovascular regulation, including the rostral ventrolateral medulla (RVLM), nucleus tractus solitarius (NLS), and periaqueductal grey (PAG) [70]. Microglial activation after MI has also been confirmed using TSPO imaging agents. In mice, TSPO signal was elevated in the brain one week after MI and observed throughout the cortex [64]. This indicates that MI-induced microglial activation spreads beyond the limited regions identified through morphological assessments. Importantly, localized skeletal muscle inflammation induced by intramuscular injection of lipopolysaccharide (LPS) into the hindlimb did not increase TSPO signal in the brain, demonstrating that the MI-induced neuroinflammation was specific to myocardial injury and not a generalized response to muscle injury.
Although it is clear that microglia are activated after MI, less is known about the functional consequences of microglial activation after MI. Upon recognition of endogenous ligands released from damaged or dead tissue, TLR4 activation on microglia leads to downstream NF-κB signaling and proinflammatory cytokine production. After MI in rats, TLR4 expression and NF-κB signaling were increased in microglia within the hypothalamic PVN [71]. In line with this finding, microglia isolated from the mouse hippocampus four weeks after MI displayed increased production of proinflammatory mediators, including IL-6, TNF-α, interferon-γ, and inducible nitric oxide synthase [72]. In contrast, gene expression profiling of microglia four days after MI in mice, revealed few changes in the microglial transcriptome compared to steady-state microglia [73]. The lack of transcriptional changes in microglia in the acute phase after MI are consistent with the delayed kinetics observed for microglial activation after MI. Interestingly, steady-state microglia also exhibited the lowest baseline inflammatory phenotype compared to tissue-resident macrophages from the lung, heart, kidney, or liver [73], suggesting that even subtle changes in microglial inflammatory function after MI may have profound effects on cognition.
In addition to microglia, perivascular macrophages are another population of CNS tissue-resident macrophage, which are found in the perivascular space and surround cerebral arterioles and venules. Given their close association to blood vessels, perivascular macrophages have crucial roles in the maintenance and permeability of cerebral vasculature during steady-state and disease [74]. MI and VaD risk factors, such as hypertension, promote reactive oxygen species (ROS) generation by perivascular macrophages leading to vascular oxidative stress and neurovascular dysfunction [75]. In hypertensive mice, pharmacological depletion of perivascular macrophages attenuated ROS production [75] and preserved cognitive function [76], linking perivascular macrophage ROS production to cognitive impairment. Increased levels of ROS are also observed within the brains of mice after MI [72], identifying a potential role for perivascular macrophages in neurovascular and cognitive dysfunction after MI. Interestingly, scavenging of ROS within the CNS through intracerebroventricular delivery of the ROS scavenger, superoxide dismutase, decreased cardiomyocyte apoptosis and improved LVEF after MI [77]. Along with cognitive dysfunction, this implicates MI-induced neuroinflammation in a deleterious feedback loop that deteriorates cardiac function.
Besides neurovascular maintenance, perivascular macrophages have also been implicated in neurohormonal and metabolic changes after MI. Following MI, perivascular macrophages increased expression of cyclooxygenase 2 (COX-2), which generates the hormone-like prostaglandin E2 (PGE2) [78]. This led to elevated levels of PGE2 in the CSF, followed by activation of neurons in the hypothalamic PVN, and culminated with increased sympathetic drive as measured by elevations in blood pressure, heart rate, and renal sympathetic nerve activity. Separately, PGE2 has been shown to act in an autocrine manner and signal through its receptor, EP2, on macrophages during aging [79]. This suppressed macrophage metabolism, as aged macrophages primarily relied on glycolysis for their energetic needs, and led to maladaptive, proinflammatory responses. After MI, glycolytic metabolism was increased in the brains of rats within the first ten days as measured by increased uptake of the radiolabeled glucose analog, 18F-fluorodeoxyglucose (18F-FDG), on PET [57]. However, by 60 days after MI, 18F-FDG uptake in the brain was significantly reduced demonstrating a gradual suppression of glycolytic metabolism after MI [57]. This implies that MI-induced changes in perivascular macrophage metabolism may precede neuroinflammation and cognitive decline.
Increased recruitment of monocytes and neutrophils to the CNS may also contribute to neuroinflammation after MI. Monocytes and neutrophils originate in the bone marrow and spleen and are released into circulation after MI to traffic to sites of tissue injury. In mice, monocyte and neutrophil abundance was increased in the brain within the first day after MI and remained elevated for at least one week compared to non-infarcted controls [73]. While it is unknown if monocytes and neutrophils accumulate in the human brain after MI, higher plasma levels of CD14, a co-receptor for bacterial LPS expressed on monocytes, was associated with higher risk for VaD [80], implying that MI-induced changes in myeloid cell recruitment to the CNS may precede neuroinflammation and cognitive decline. In line with these findings, the percentage of circulating inflammatory CD14++CD16+ intermediate monocytes was found to be positively associated with both the abundance of white matter lesions and disease progression over a nine year period in VaD patients [81]. Although many studies have focused on monocyte and neutrophil recruitment from bone marrow reservoirs within the tibia and femur, recent studies have identified reservoirs for these cells in the CNS adjacent skull and vertebral bone marrow. Skull bone marrow was found to contain direct vascular channels to the brain parenchyma facilitating monocyte and neutrophil migration during both homeostasis and after CNS injury [82][83]. Interestingly, skull bone marrow-derived monocytes were transcriptionally-distinct and less inflammatory compared to blood-borne monocytes [83]. Engraftment of bone marrow-derived macrophages in the brains of mice following lethal irradiation were also found to remain transcriptionally distinct from host microglia and exhibit a gene signature similar to that observed in disease associated microglia [84]. This raises the possibility that local or peripheral sourcing of myeloid cells may lead to MI-induced neuroprotection or neuropathology, respectively. Recruitment of blood-borne monocytes to the injured brain has been shown to rely on the interaction between CC chemokine receptor 2 (CCR2) expressed on monocytes and its ligand, CC motif ligand 2 (CCL2) [85]. Genetic deletion or pharmacological inhibition of CCR2 blocked CNS monocyte infiltration and preserved cognitive function in mouse models of acute brain injury [85][86][87]. In humans, higher levels of blood Ccr2 transcript levels were associated with worsened cognitive decline over a nine-year period, suggesting that blood-borne monocytes may have a neuropathogenic role during VaD [88]. Similar protective and pathogenic roles have been ascribed to cardiac resident macrophages and blood-borne monocytes, respectively, in the infarcted heart [89][90]. Together, this necessitates a better understanding of myeloid cell sourcing during MI-induced neuroinflammation.
In VaD patients, blood-borne monocytes exhibit an increased production of inflammatory cytokines after stimulation [81], which is characteristic of a trained immunity phenotype. Trained immunity results from stimuli-induced epigenetic and metabolic reprogramming of monocytes leading to enhanced inflammatory cytokine production following re-stimulation. Evidence for trained immunity after MI was recently observed in mice, where MI-induced epigenetic reprogramming of bone marrow-derived Ly6Chi monocytes led to an immunosuppressive phenotype that accelerated tumor growth in a mouse model of breast cancer [91]. The longlasting effects of trained immunity occur in part through reprogramming hematopoietic stem and progenitor cells in the bone marrow towards myelopoiesis, the process by which progenitor cells differentiate into mature myeloid cells [92]. Elevated levels of granulocyte-macrophage colony-stimulating factor (GM-CSF), a central mediator of myelopoiesis, have been found in both cerebrospinal fluid and serum of VaD patients compared to healthy controls [93], indicating that trained immunity after MI may accelerate VaD.
Since cognitive impairment develops in the subsequent weeks after MI, as opposed to the initial inflammatory phase [57], this implies that cellular crosstalk between immune cells and CNS parenchymal cells is involved in the onset of cognitive decline. Numerous studies have observed neuronal activation after MI, which may contribute to cognitive dysfunction. Neuronal activation as measured by increased expression of the activation markers, Fos and Fos related antigens, was observed in association with activated microglia after MI in regions involved in sympathetic nerve activity, including the hypothalamic PVN, RVLM, NTS, and PAG [70]. There is considerable interest in understanding how MI-induced neuroinflammation leads to neuronal activation in these regions as increased sympathetic drive may facilitate fatal arrhythmias during the acute phase after MI [94], while sustained activation contributes to the pathogenesis of HF [95]. Activated neurons within the hypothalamic PVN were also found to express IL-1β and TNF-α [96], suggesting that neurons may not only be passive bystanders during MI-induced neuroinflammation. In contrast, neuronal production of brain-derived neurotrophic factor (BDNF) after MI [97], led to increased circulating levels of BDNF where it mediated cardioprotective responses by increasing cardiomyocyte survival. Likewise and in contrast to its putative role in fatal arrhythmias [94], the neuropeptide oxytocin was found to attenuate neuroinflammation through inhibition of TLR4-stimulated proinflammatory cytokine production by microglia [98], demonstrating that not all neuronal function is likely to be deleterious after MI.
The increased presence of white matter lesions after MI also suggests the involvement of oligodendrocytes, specialized glial cells responsible for generating and maintaining the multilayer myelin sheath that surrounds axons of the CNS [99]. The myelin sheath supports axonal signal transduction and acts as a conduit to provide essential metabolic support from the oligodendrocyte to the axon [100]. Loss of oligodendrocytes leads to myelin and neuronal degeneration and is associated with behavioral changes and cognitive decline [101]. Indirect evidence for impaired function or loss of oligodendrocytes after MI come from gross histological and imaging studies. Post-mortem examinations of the brain nine days after recurrent MI revealed pallor of myelin staining and focal ballooning of myelin sheaths [102], pathological changes associated with oligodendrocytes. Since several myelin proteins exhibit a relatively long half-life, changes in behavior and cognitive function are often delayed following loss of oligodendrocytes. This is exemplified by a case of a 68-year-old woman with recurrent MI [103]. While MRI imaging 10 days after her second MI revealed subtle changes and she was discharged with no neurological deficits, in the weeks that followed, she progressively developed behavioral changes and cognitive impairment. A follow-up T1-weighted MRI four months after MI revealed abnormal hypointense regions of white matter, indicative of myelin loss. In line with these findings, little neuronal damage was detected within ten days after nonreperfused MI in rats, but by 60 days after MI, neuronal degeneration as measured by changes in Nissl staining was widespread throughout the frontal cortex and hippocampus [57]. Taken together, these findings necessitate further investigations defining the crosstalk among immune, parenchymal, and stromal cells in the CNS, to delineate their roles in cardiovascular and cognitive function after MI.
5. Targeting inflammation after MI to prevent VaD
The associations among MI, VaD, and inflammation has fueled therapeutic efforts targeting inflammation to slow or halt disease progression. Some of the first trials targeting inflammation in lowering dementia risk focused on the use of non-steroidal anti-inflammatory drugs (NSAID), which are used for pain relief in a variety of inflammatory conditions. While results have varied, NSAIDs have been shown to be ineffective in preventing VaD. A prospective, population-based cohort study of NSAID usage as a preventative or modifier of cognitive decline in 6,989 subjects revealed that NSAID usage did not affect risk for VaD [104]. In contrast, a case control study using electronic medical records of 31,083 patients matched on age, gender, and index date found that NSAID or glucocorticoid therapy increased the risk for VaD [105]. Similarly, NSAID usage is contraindicated after MI as it increases the risk of bleeding and excess thrombotic events [106]. The lack of efficacy of NSAIDs may reflect their broad immunosuppressive effects and necessitate strategies that target specific inflammatory cytokines or pathways to inhibit neuropathological pathways while preserving key neuroprotective immune function.
Recent evidence that supports specific targeting of inflammatory mediators after MI to limit secondary adverse events comes from two randomized, double-blind, placebo-controlled trials. In the Canakinumab Antiinflammatory Thrombosis Outcomes Study, 10,061 patients with a history of MI were treated with canakinumab, a monoclonal antibody targeting IL-1β, or placebo. In patients treated with canakinumab, IL-1β blockade led to a significant reduction in recurrent adverse cardiovascular events [107]. The greatest benefits were conferred to patients who had exhibited the largest reductions in systemic inflammation as measured by decreases in CRP levels [108]. Separately, in the Colchicine Cardiovascular Outcomes Trial, treatment of 4,475 patients within 30 days after MI with placebo or colchicine, an anti-inflammatory medication used to treat gout and also known to inhibit IL-1β production [109], also led to a significantly lower risk for secondary adverse cardiovascular events [110]. While it is unclear whether inhibition of IL-1β would reduce risk for VaD in MI patients, administration of colchicine to patients with inflammatory disorders, including gout [111] and Familial Mediterranean fever [112], reduced risk of dementia and improved cognitive performance. This suggests that targeted inhibition of inflammatory pathways holds promise in the prevention and treatment of VaD.
As an alternative to targeting inflammatory mediators, strategies that promote inflammation resolution after MI may also reduce risk of VaD or slow cognitive decline. Inflammation resolution after MI is an active process coordinated in part through the production of SPMs, which includes lipoxins, resolvins, and maresins [113]. SPMs activate specific G-protein-coupled receptors on immune cells to reduce inflammatory activation and promote tissue repair through clearance of dead cells. Elevated plasma SPM levels at the time of MI have been shown to lower risk for secondary adverse events [114]. Similarly, elevated plasma levels of lipoxins [115] and resolvins [116] within seven days after a stroke were associated with better cognitive outcomes, suggesting that SPMs may be a viable therapeutic target to attenuate MI-induced neurovascular injury. Since resolvins and maresins are derived from polyunsaturated fatty acids (PUFA), including eicosapentaenoic acid and docosahexaenoic acid, interventional studies have tested whether dietary supplementation with PUFAs confers protection after MI. In the GISSI-Prevenzione trial, which started administering PUFAs or placebo to 11,324 patients within three months after MI, supplementation with PUFAs was found to significantly reduce the risk for death, secondary MI, and stroke compared to controls [117]. Likewise, a small trial of 20 elderly VaD patients randomly assigned to PUFA or placebo treatment, observed a statistically significant, albeit transient, improvement in cognitive function [118]. In contrast, the Alpha Omega Trial, which administered PUFAs or placebo to 4,837 patients who had a MI up to 10 years before study intake, found no effect for PUFA supplementation on reducing adverse cardiovascular events [119] or improvement in cognitive function [120]. These opposing results demonstrate that the timing of therapeutic intervention is critical in changing the trajectory of disease progression after MI.
Since MI and VaD share vascular risk factors, combined approaches that target both inflammation as well as the underlying comorbidities may confer the greatest cognitive protection. For example, type 2 diabetes mellitus (T2DM) is associated with worsened outcomes after MI [121] and increased risk for VaD [122]. During T2DM, acute and chronic hyperglycemia increases oxidative stress [123], which leads to vascular dysfunction and inflammation. In T2DM patients with a history of MI, use of glucose lowering drugs, including metformin [124], dipeptidyl peptidase 4 (DPP4) inhibitors [125], and sodium glucose lowering transport 2 receptor (SGLT2) inhibitors [126], reduced major adverse cardiovascular events and promoted long-term survival. While dementia risk is higher in T2DM patients both with and without pharmacotherapy [127], metformin, DPP4 inhibitors, and SGLT2 inhibitors have been found to attenuate the absolute increase in dementia risk [128]. This reduction may be specific for VaD, as DPP4 inhibitors were found to decrease the risk of VaD but not AD in T2DM patients [129]. Interestingly, a small retrospective study of 60 elderly T2DM patients with pre-existing mild cognitive impairment, observed that administration of both metformin and DPP4 inhibitors led to preservation of cognitive function over the course of 6 months compared to metformin treatment alone [130]. This suggests that it is better late than never to target underlying comorbidities that elevate risk for VaD after MI.
Finally, as inflammation increases VaD risk years in advance of clinical onset, primary prevention through lifestyle modifications is critical in limiting inflammation to reduce VaD risk. The Lancet Commission on dementia prevention, intervention, and care estimated that 35% of dementia cases are attributable to a combination of modifiable lifestyle risk factors [131]. Examples of modifiable lifestyle risk factors associated with increased risk for dementia or cognitive decline include fragmented or short sleep duration [132] and physical inactivity [133]. While the mechanisms linking lifestyle risk factors to VaD are likely complex, trained immunity through epigenetic reprogramming of innate immune cells and their progenitor cells within the bone marrow of mice is directly affected by fragmented sleep [134], physical inactivity [135], or high-fat diet [92]. This leads to increased production and circulation of inflammatory monocytes and culminates in systemic inflammation. Lifestyle interventions such as undisturbed sleep, exercise, and healthy diet, limits these pathological changes and importantly, maintains or improves cognitive function in at-risk individuals [136]. For example, in a large cohort of 1,260 at-risk individuals aged 60 to 77 years old, the double-blind randomized controlled Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability found that 2 years of multidomain intervention (diet, exercise, cognitive training, vascular risk monitoring) maintained or improved cognitive function compared to the control group, which only received general health advice [136]. Additional protection may occur through increased production of anti-inflammatory mediators. For example, strength exercise training for 28 weeks in older women with cognitive decline increased anti-inflammatory IL-10 and reduced the circulating numbers of proinflammatory monocytes compared to controls [137]. This translated to an increase in cognitive performance, indicating that immunomodulatory lifestyle interventions, even in the elderly, provide an additional strategy to reduce the risk for VaD after MI.
7. Concluding Remarks and Future Perspectives
Evidence from clinical and preclinical studies continues to resolve inflammatory links between MI and VaD (Figure 1). While there is a wealth of knowledge on inflammatory responses after MI, we still lack a clear understanding on how MI triggers the neuroinflammation that accelerates VaD. It is still unclear whether neuroinflammation arises directly from microglia and perivascular macrophages recognizing danger signals released by the infarcted heart or indirectly through systemic elevations of inflammatory cytokines and myeloid cells. Activation of microglia and neurons in regions that regulate cardiovascular function raises the question on whether MI-induced CNS activation initiates a deleterious loop that perpetually worsens cardiac and cognitive function. Besides myeloid cells, MI also leads to activation of dendritic cells and adaptive immune cells, including T and B cells [138], but it is unclear whether these cells also accumulate in the brains of VaD patients and contribute to cognitive decline. Since animal models have advanced our understanding of the kinetics and immune populations underlying neuroinflammation after MI, additional studies using conditional or temporal knockouts to deplete specific cell populations or proteins holds promise in teasing apart contributions of CNS resident or recruited cells in disease pathogenesis. Furthermore, application of cutting-edge tools, including single-cell RNA sequencing, will enable investigation into the crosstalk among immune, parenchymal, and stromal cells in the CNS to delineate their roles in cognitive decline after MI. In humans, imaging and cellular analyses during the acute phases after MI will clarify the relationships between MI and neuroinflammation and determine whether the findings in animal models translate to human disease. A better understanding of the influence of cardiovascular disease risk factors and sex differences on MI-induced neuroinflammation are also needed to inform therapeutic strategies. VaD is a heterogenous disease so therapeutic strategies targeting neuroinflammation after MI will need to account for multiple comorbidities and likely combine pharmacotherapies with lifestyle modifications to confer the greatest preservation of cognitive function after MI. Ultimately, animal and human studies will continue to advance our understanding of the inflammatory links between MI and VaD leading to novel therapeutic strategies and culminating in beneficial clinical outcomes in humans.
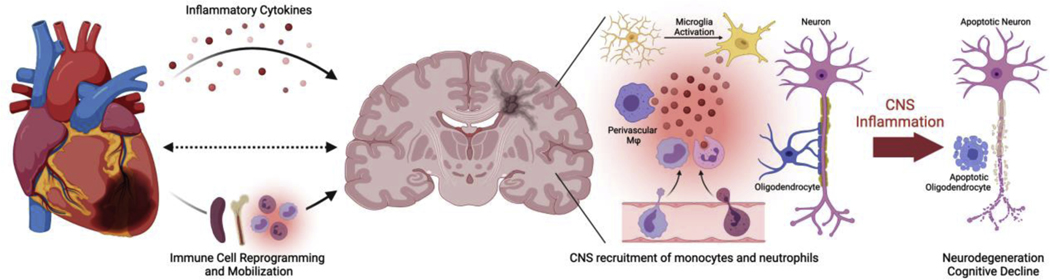
Figure 1. Proposed inflammatory links between MI and VaD.
Following MI, levels of inflammatory cytokines are increased in the peripheral blood. In the spleen and bone marrow, myeloid cells, such as neutrophils and monocytes, undergo epigenetic reprogramming and are released into the circulation. Within the CNS, MI leads to activation and proinflammatory cytokine production by microglia and perivascular macrophages. Combined increase of both local and systemic inflammation leads to disruption in the blood brain barrier and CNS infiltration of neutrophils and monocytes from the periphery. Sustained inflammation and disruption of the CNS vasculature leads to progressive loss of oligodendrocytes and neurons. This results in clinical VaD, characterized by white matter lesions and cognitive deterioration.
ACKNOWLEDGMENT
This work was supported by the National Institutes of Health (grants RF1AG072080 to MEF and BP). The figure was created with Biorender.com.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA, Samad Z, Satou GM, Schroeder EB, Shah SH, Shay CM, Stokes A, VanWagner LB, Wang N-Y, Tsao CW, null null, Heart Disease and Stroke Statistics—2021 Update, Circulation. 143 (2021) e254–e743. 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- [2].Tal Hasin, Yariv Gerber, Susan A Weston., Ruoxiang Jiang, Killian Jill M., Manemann Sheila M., Cerhan James R., Roger Véronique L., Heart Failure After Myocardial Infarction Is Associated With Increased Risk of Cancer, Journal of the American College of Cardiology. 68 (2016) 265–271. 10.1016/j.jacc.2016.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sundbøll J, Horváth-Puhó E, Adelborg K, Schmidt M, Pedersen L, Bøtker HE, Henderson VW, Toft Sørensen H, Higher Risk of Vascular Dementia in Myocardial Infarction Survivors, Circulation. 137 (2018) 567–577. 10.1161/CIRCULATIONAHA.117.029127. [DOI] [PubMed] [Google Scholar]
- [4].Wolters FJ, Ikram M, Epidemiology of Dementia: The Burden on Society, the Challenges for Research, in: Perneczky R (Ed.), Biomarkers for Alzheimer’s Disease Drug Development, Springer New York, New York, NY, 2018: pp. 3–14. 10.1007/978-1-4939-7704-8_1. [DOI] [PubMed] [Google Scholar]
- [5].Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L, Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study, The Lancet. 364 (2004) 937–952. 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- [6].Gorelick PB, Risk Factors for Vascular Dementia and Alzheimer Disease, Stroke. 35 (2004) 2620–2622. 10.1161/01.STR.0000143318.70292.47. [DOI] [PubMed] [Google Scholar]
- [7].Gorelick PB, Scuteri A, Black SE, DeCarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S, Vascular Contributions to Cognitive Impairment and Dementia, Stroke. 42 (2011) 2672–2713. 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kalaria RN, The pathology and pathophysiology of vascular dementia, Neuropharmacology. 134 (2018) 226–239. 10.1016/j.neuropharm.2017.12.030. [DOI] [PubMed] [Google Scholar]
- [9].Newman AB, Fitzpatrick AL, Lopez O, Jackson S, Lyketsos C, Jagust W, Ives D, DeKosky ST, Kuller LH, Dementia and Alzheimer’s Disease Incidence in Relationship to Cardiovascular Disease in the Cardiovascular Health Study Cohort, Journal of the American Geriatrics Society. 53 (2005) 1101–1107. 10.1111/j.1532-5415.2005.53360.x. [DOI] [PubMed] [Google Scholar]
- [10].Trejo-Lopez JA, Yachnis AT, Prokop S, Neuropathology of Alzheimer’s Disease, Neurotherapeutics. (2021). 10.1007/s13311-021-01146-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Raffaitin C, Gin H, Empana J-P, Helmer C, Berr C, Tzourio C, Portet F, Dartigues J-F, Alpérovitch A, Barberger-Gateau P, Metabolic Syndrome and Risk for Incident Alzheimer’s Disease or Vascular Dementia: The Three-City Study, Diabetes Care. 32 (2009) 169–174. 10.2337/dc08-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Javanshiri K, Waldö ML, Friberg N, Sjövall F, Wickerström K, Haglund M, Englund E, Atherosclerosis, Hypertension, and Diabetes in Alzheimer’s Disease, Vascular Dementia, and Mixed Dementia: Prevalence and Presentation, Journal of Alzheimer’s Disease. 65 (2018) 1247–1258. 10.3233/JAD-180644. [DOI] [PubMed] [Google Scholar]
- [13].Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Tylavsky FA, Newman AB, The Metabolic Syndrome, Inflammation, and Risk of Cognitive Decline, JAMA. 292 (2004) 2237–2242. 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- [14].Zuliani G, Ranzini M, Guerra G, Rossi L, Munari MR, Zurlo A, Volpato S, Atti AR, Blè A, Fellin R, Plasma cytokines profile in older subjects with late onset Alzheimer’s disease or vascular dementia, Journal of Psychiatric Research. 41 (2007) 686–693. 10.1016/j.jpsychires.2006.02.008. [DOI] [PubMed] [Google Scholar]
- [15].Wada-Isoe K, Wakutani Y, Urakami K, Nakashima K, Elevated interleukin-6 levels in cerebrospinal fluid of vascular dementia patients, Acta Neurologica Scandinavica. 110 (2004) 124–127. 10.1111/j.1600-0404.2004.00286.x. [DOI] [PubMed] [Google Scholar]
- [16].Aronson MK, Ooi WL, Morgenstern H, Hafner A, Masur D, Crystal H, Frishman WH, Fisher D, Katzman R, Women, myocardial infarction, and dementia in the very old, Neurology. 40 (1990) 1102. 10.1212/WNL.40.7.1102. [DOI] [PubMed] [Google Scholar]
- [17].Millett ERC, Peters SAE, Woodward M, Sex differences in risk factors for myocardial infarction: cohort study of UK Biobank participants, BMJ. 363 (2018). 10.1136/bmj.k4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Volgman AS, Merz CNB, Aggarwal NT, Bittner V, Bunch TJ, Gorelick PB, Maki P, Patel HN, Poppas A, Ruskin J, Russo AM, Waldstein SR, Wenger NK, Yaffe K, Pepine CJ, Sex Differences in Cardiovascular Disease and Cognitive Impairment: Another Health Disparity for Women?, Journal of the American Heart Association. 8 (2019) e013154. 10.1161/JAHA.119.013154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Breteler MMB, Claus JJ, Grobbee DE, Hofman A, Cardiovascular disease and distribution of cognitive function in elderly people: the Rotterdam study, BMJ. 308 (1994) 1604. 10.1136/bmj.308.6944.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Deary IJ, Corley J, Gow AJ, Harris SE, Houlihan LM, Marioni RE, Penke L, Rafnsson SB, Starr JM, Age-associated cognitive decline, British Medical Bulletin. 92 (2009) 135–152. 10.1093/bmb/ldp033. [DOI] [PubMed] [Google Scholar]
- [21].Ikram MA, van Oijen M, de Jong FJ, Kors JA, Koudstaal PJ, Hofman A, Witteman JCM, Breteler MMB, Unrecognized Myocardial Infarction in Relation to Risk of Dementia and Cerebral Small Vessel Disease, Stroke. 39 (2008) 1421–1426. 10.1161/STROKEAHA.107.501106. [DOI] [PubMed] [Google Scholar]
- [22].Bots ML, van Swieten JC, Cerebral white matter lesions and atherosclerosis in the Rotterdam Study., Lancet. 341 (1993) 1232. [DOI] [PubMed] [Google Scholar]
- [23].Nadareishvili ZG, Choudary Z, Joyner C, Brodie D, Norris JW, Cerebral Microembolism in Acute Myocardial Infarction, Stroke. 30 (1999) 2679–2682. 10.1161/01.STR.30.12.2679. [DOI] [PubMed] [Google Scholar]
- [24].Richardson Kathryn, Stephan Blossom C.M., Ince Paul G., Brayne Carol, Matthews Fiona E. and Margaret M Esiri on behalf of the MRC Cognitive Function and Ageing Neuropathology Study Group, The Neuropathology of Vascular Disease in the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS), Current Alzheimer Research. 9 (2012) 687–696. 10.2174/156720512801322654. [DOI] [PubMed] [Google Scholar]
- [25].Wuxiang Xie, Fanfan Zheng, Li Yan, Baoliang Zhong, Cognitive Decline Before and After Incident Coronary Events, Journal of the American College of Cardiology. 73 (2019) 3041–3050. 10.1016/j.jacc.2019.04.019. [DOI] [PubMed] [Google Scholar]
- [26].Gu SZ, Beska B, Chan D, Neely D, Batty JA, Adams‐Hall J, Mossop H, Qiu W, Kunadian V, Cognitive Decline in Older Patients With Non‐ST Elevation Acute Coronary Syndrome, Journal of the American Heart Association. 8 (2019) e011218. 10.1161/JAHA.118.011218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Meguro K, Akanuma K, Meguro M, Kasai M, Ishii H, Yamaguchi S, Prognosis of Vascular Mild Cognitive Impairment Includes Vascular Dementia Onset and Death by Cardiovascular Disease: Reanalysis From the Osaki-Tajiri Project, Journal of Stroke and Cerebrovascular Diseases. 21 (2012) 607–611. 10.1016/j.jstrokecerebrovasdis.2011.01.009. [DOI] [PubMed] [Google Scholar]
- [28].Haring B, Leng X, Robinson J, Johnson KC, Jackson RD, Beyth R, Wactawski-Wende J, von Ballmoos MW, Goveas JS, Kuller LH, Wassertheil‐Smoller S, Cardiovascular Disease and Cognitive Decline in Postmenopausal Women: Results From the Women’s Health Initiative Memory Study, Journal of the American Heart Association. 2 (n.d.) e000369. 10.1161/JAHA.113.000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Solfrizzi V, Panza F, Colacicco AM, D’Introno A, Capurso C, Torres F, Grigoletto F, Maggi S, Del Parigi A, Reiman EM, Caselli RJ, Scafato E, Farchi G, Capurso A, Vascular risk factors, incidence of MCI, and rates of progression to dementia, Neurology. 63 (2004) 1882. 10.1212/01.WNL.0000144281.38555.E3. [DOI] [PubMed] [Google Scholar]
- [30].Suri MFK, Zhou J, Qiao Y, Chu H, Qureshi AI, Mosley T, Gottesman RF, Wruck L, Sharrett AR, Alonso A, Wasserman BA, Cognitive impairment and intracranial atherosclerotic stenosis in general population, Neurology. 90 (2018) e1240. 10.1212/WNL.0000000000005250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].De Silva DA, Ancalan M, Doshi K, Chang H-M, Wong M-C, Chen C, Intracranial large artery disease in Alzheimer’s disease and vascular dementia among ethnic Asians, European Journal of Neurology. 16 (2009) 643–645. 10.1111/j.1468-1331.2009.02551.x. [DOI] [PubMed] [Google Scholar]
- [32].Purandare N, Burns A, Daly KJ, Hardicre J, Morris J, Macfarlane G, McCollum C, Cerebral emboli as a potential cause of Alzheimer’s disease and vascular dementia: casecontrol study, BMJ. 332 (2006) 1119. 10.1136/bmj.38814.696493.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Buanes EA, Gramstad A, Søvig KK, Hufthammer KO, Flaatten H, Husby T, Langørgen J, Heltne J-K, Cognitive function and health-related quality of life four years after cardiac arrest, Resuscitation. 89 (2015) 13–18. 10.1016/j.resuscitation.2014.12.021. [DOI] [PubMed] [Google Scholar]
- [34].Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, Mark DB, Reves JG, Blumenthal JA, Longitudinal Assessment of Neurocognitive Function after Coronary-Artery Bypass Surgery, N Engl J Med. 344 (2001) 395–402. 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- [35].Bagge CN, Henderson VW, Laursen HB, Adelborg K, Olsen M, Madsen NL, Risk of Dementia in Adults With Congenital Heart Disease, Circulation. 137 (2018) 1912–1920. 10.1161/CIRCULATIONAHA.117.029686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Elmer J, Callaway CW, The Brain after Cardiac Arrest, Semin Neurol. 37 (2017) 19–24. 10.1055/s-0036-1597833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sauvé MJ, Lewis WR, Blankenbiller M, Rickabaugh B, Pressler SJ, Cognitive Impairments in Chronic Heart Failure: A Case Controlled Study, Journal of Cardiac Failure. 15 (2009) 1–10. 10.1016/j.cardfail.2008.08.007. [DOI] [PubMed] [Google Scholar]
- [38].Dikić A, Radmilo L, Živanović Ž, Keković G, Sekulić S, Kovačić Z, Radmilo R, Cognitive impairment and depression after acute myocardial infarction: associations with ejection fraction and demographic characteristics, Acta Neurologica Belgica. (2020). 10.1007/s13760-020-01440-0. [DOI] [PubMed] [Google Scholar]
- [39].David H, Ughetto A, Gaudard P, Plawecki M, Paiyabhroma N, Zub E, Colson P, Richard S, Marchi N, Sicard P, Experimental Myocardial Infarction Elicits Time-Dependent Patterns of Vascular Hypoxia in Peripheral Organs and in the Brain, Frontiers in Cardiovascular Medicine. 7 (2021) 406. 10.3389/fcvm.2020.615507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Guillen I, Blanes M, Gomez-Lechon MJ, Castell JV, Cytokine signaling during myocardial infarction: sequential appearance of IL-1 beta and IL-6, American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 269 (1995) R229–R235. 10.1152/ajpregu.1995.269.2.R229. [DOI] [PubMed] [Google Scholar]
- [41].Gabriel AS, Martinsson A, Wretlind B, Ahnve S, IL-6 levels in acute and post myocardial infarction: their relation to CRP levels, infarction size, left ventricular systolic function, and heart failure, European Journal of Internal Medicine. 15 (2004) 523–528. 10.1016/j.ejim.2004.07.013. [DOI] [PubMed] [Google Scholar]
- [42].Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E, Elevation of Tumor Necrosis Factor-α and Increased Risk of Recurrent Coronary Events After Myocardial Infarction, Circulation. 101 (2000) 2149–2153. 10.1161/01.CIR.101.18.2149. [DOI] [PubMed] [Google Scholar]
- [43].Prabhu SD, Frangogiannis NG, The Biological Basis for Cardiac Repair After Myocardial Infarction, Circulation Research. 119 (2016) 91–112. 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tarkowski E, Tullberg M, Fredman P, Wikkelsö C, Correlation between Intrathecal Sulfatide and TNF-α Levels in Patients with Vascular Dementia, Dementia and Geriatric Cognitive Disorders. 15 (2003) 207–211. 10.1159/000068780. [DOI] [PubMed] [Google Scholar]
- [45].Zuliani G, Cavalieri M, Galvani M, Passaro A, Munari MR, Bosi C, Zurlo A, Fellin R, Markers of endothelial dysfunction in older subjects with late onset Alzheimer’s disease or vascular dementia, Journal of the Neurological Sciences. 272 (2008) 164–170. 10.1016/j.jns.2008.05.020. [DOI] [PubMed] [Google Scholar]
- [46].Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, Stijnen T, Hofman A, Witteman JCM, Breteler MMB, Inflammatory Proteins in Plasma and the Risk of Dementia: The Rotterdam Study, Archives of Neurology. 61 (2004) 668–672. 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- [47].Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ, Early inflammation and dementia: A 25-year follow-up of the Honolulu-Asia aging study, Annals of Neurology. 52 (2002) 168–174. 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- [48].Ravaglia G, Forti P, Maioli F, Chiappelli M, Montesi F, Tumini E, Mariani E, Licastro F, Patterson C, Blood inflammatory markers and risk of dementia: The Conselice Study of Brain Aging, Neurobiology of Aging. 28 (2007) 1810–1820. 10.1016/j.neurobiolaging.2006.08.012. [DOI] [PubMed] [Google Scholar]
- [49].Kravitz BA, Corrada MM, Kawas CH, Elevated C-reactive protein levels are associated with prevalent dementia in the oldest-old, Alzheimer’s & Dementia. 5 (2009) 318–323. 10.1016/j.jalz.2009.04.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hoth KF, Haley AP, Gunstad J, Paul RH, Poppas A, Jefferson AL, Tate DF, Ono M, Jerskey BA, Cohen RA, Elevated C-Reactive Protein Is Related to Cognitive Decline in Older Adults with Cardiovascular Disease, Journal of the American Geriatrics Society. 56 (2008) 1898–1903. 10.1111/j.1532-5415.2008.01930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Weinstein G, Lutski M, Goldbourt U, Tanne D, C-reactive protein is related to future cognitive impairment and decline in elderly individuals with cardiovascular disease, Archives of Gerontology and Geriatrics. 69 (2017) 31–37. 10.1016/j.archger.2016.11.002. [DOI] [PubMed] [Google Scholar]
- [52].Stumpf C, Sheriff A, Zimmermann S, Schaefauer L, Schlundt C, Raaz D, Garlichs CD, Achenbach S, C-reactive protein levels predict systolic heart failure and outcome in patients with first ST-elevation myocardial infarction treated with coronary angioplasty, Arch Med Sci. 13 (2017) 1086–1093. 10.5114/aoms.2017.69327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lindsey ML, de Castro Brás LE, DeLeon-Pennell KY, Frangogiannis NG, Halade GV, O’Meara CC, Spinale FG, Kassiri Z, Kirk JA, Kleinbongard P, Ripplinger CM, Brunt KR, Reperfused vs. nonreperfused myocardial infarction: when to use which model, American Journal of Physiology-Heart and Circulatory Physiology. 321 (2021) H208–H213. 10.1152/ajpheart.00234.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Francis J, Chu Y, Johnson AK, Weiss RM, Felder RB, Acute myocardial infarction induces hypothalamic cytokine synthesis, American Journal of Physiology-Heart and Circulatory Physiology. 286 (2004) H2264–H2271. 10.1152/ajpheart.01072.2003. [DOI] [PubMed] [Google Scholar]
- [55].Sun N, Mei Y, Hu Z, Xing W, Lv K, Hu N, Zhang T, Wang D, Ghrelin attenuates depressive-like behavior, heart failure, and neuroinflammation in postmyocardial infarction rat model, European Journal of Pharmacology. 901 (2021) 174096. 10.1016/j.ejphar.2021.174096. [DOI] [PubMed] [Google Scholar]
- [56].Wang H-W, Ahmad M, Jadayel R, Najjar F, Lagace D, Leenen FHH, Inhibition of inflammation by minocycline improves heart failure and depression-like behaviour in rats after myocardial infarction, PLOS ONE. 14 (2019) e0217437. 10.1371/journal.pone.0217437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yang T, Lu Z, Wang L, Zhao Y, Nie B, Xu Q, Han X, Li T, Zhao J, Cheng W, Wang B, Wu A, Zhu H, Meng W, Shang H, Zhao M, Dynamic Changes in Brain Glucose Metabolism and Neuronal Structure in Rats with Heart Failure, Neuroscience. 424 (2020) 34–44. 10.1016/j.neuroscience.2019.10.008. [DOI] [PubMed] [Google Scholar]
- [58].Hong X, Bu L, Wang Y, Xu J, Wu J, Huang Y, Liu J, Suo H, Yang L, Shi Y, Lou Y, Sun Z, Zhu G, Behnisch T, Yu M, Jia J, Hai W, Meng H, Liang S, Huang F, Zou Y, Ge J, Increases in the Risk of Cognitive Impairment and Alterations of Cerebral β-amyloid Metabolism in Mouse Model of Heart Failure, PLOS ONE. 8 (2013) e63829. 10.1371/journal.pone.0063829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Meissner A, Visanji NP, Momen MA, Feng R, Francis BM, Bolz S, Hazrati L, Tumor Necrosis Factor‐α Underlies Loss of Cortical Dendritic Spine Density in a Mouse Model of Congestive Heart Failure, Journal of the American Heart Association. 4 (n.d.) e001920. 10.1161/JAHA.115.001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Dutta S, Sengupta P, Men and mice: Relating their ages, Life Sciences. 152 (2016) 244–248. 10.1016/j.lfs.2015.10.025. [DOI] [PubMed] [Google Scholar]
- [61].Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S, Innate immunity in aging: impact on macrophage function, Aging Cell. 3 (2004) 161–167. 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- [62].Gouweleeuw L, Hovens IB, Liu H, Naudé PJW, Schoemaker RG, Differences in the association between behavior and neutrophil gelatinase-associated lipocalin in male and female rats after coronary artery ligation, Physiology & Behavior. 163 (2016) 7–16. 10.1016/j.physbeh.2016.04.040. [DOI] [PubMed] [Google Scholar]
- [63].Gilsanz P, Lee C, Corrada MM, Kawas CH, Quesenberry CP, Whitmer RA, Reproductive period and risk of dementia in a diverse cohort of health care members, Neurology. 92 (2019) e2005. 10.1212/WNL.0000000000007326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Thackeray JT, Hupe HC, Wang Y, Bankstahl JP, Berding G, Ross TL, Bauersachs J, Wollert KC, Bengel FM, Myocardial Inflammation Predicts Remodeling and Neuroinflammation After Myocardial Infarction, Journal of the American College of Cardiology. 71 (2018) 263–275. 10.1016/j.jacc.2017.11.024. [DOI] [PubMed] [Google Scholar]
- [65].Llorens F, Hermann P, Villar-Piqué A, Diaz-Lucena D, Nägga K, Hansson O, Santana I, Schmitz M, Schmidt C, Varges D, Goebel S, Dumurgier J, Zetterberg H, Blennow K, Paquet C, Baldeiras I, Ferrer I, Zerr I, Cerebrospinal fluid lipocalin 2 as a novel biomarker for the differential diagnosis of vascular dementia, Nature Communications. 11 (2020) 619. 10.1038/s41467-020-14373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Rosenberg GA, Sullivan N, Esiri MM, White Matter Damage Is Associated With Matrix Metalloproteinases in Vascular Dementia, Stroke. 32 (2001) 1162–1168. 10.1161/01.STR.32.5.1162. [DOI] [PubMed] [Google Scholar]
- [67].Rana I, Stebbing M, Kompa A, Kelly DJ, Krum H, Badoer E, Microglia activation in the hypothalamic PVN following myocardial infarction, Brain Research. 1326 (2010) 96–104. 10.1016/j.brainres.2010.02.028. [DOI] [PubMed] [Google Scholar]
- [68].Greter M, Lelios I, Croxford AL, Microglia Versus Myeloid Cell Nomenclature during Brain Inflammation, Frontiers in Immunology. 6 (2015). 10.3389/fimmu.2015.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Dworak M, Stebbing M, Kompa AR, Rana I, Krum H, Badoer E, Sustained activation of microglia in the hypothalamic PVN following myocardial infarction, Autonomic Neuroscience. 169 (2012) 70–76. 10.1016/j.autneu.2012.04.004. [DOI] [PubMed] [Google Scholar]
- [70].Dworak M, Stebbing M, Kompa AR, Rana I, Krum H, Badoer E, Attenuation of microglial and neuronal activation in the brain by ICV minocycline following myocardial infarction, Autonomic Neuroscience. 185 (2014) 43–50. 10.1016/j.autneu.2014.03.007. [DOI] [PubMed] [Google Scholar]
- [71].Wang Y, Hu H, Yin J, Shi Y, Tan J, Zheng L, Wang C, Li X, Xue M, Liu J, Wang Y, Li Y, Li X, Liu F, Liu Q, Yan S, TLR4 participates in sympathetic hyperactivity Post-MI in the PVN by regulating NF-κB pathway and ROS production, Redox Biology. 24 (2019) 101186. 10.1016/j.redox.2019.101186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Zhang W, Luo P, Myocardial Infarction Predisposes Neurodegenerative Diseases, Journal of Alzheimer’s Disease. 74 (2020) 579–587. 10.3233/JAD-191225. [DOI] [PubMed] [Google Scholar]
- [73].Hoyer FF, Naxerova K, Schloss MJ, Hulsmans M, Nair AV, Dutta P, Calcagno DM, Herisson F, Anzai A, Sun Y, Wojtkiewicz G, Rohde D, Frodermann V, Vandoorne K, Courties G, Iwamoto Y, Garris CS, Williams DL, Breton S, Brown D, Whalen M, Libby P, Pittet MJ, King KR, Weissleder R, Swirski FK, Nahrendorf M, Tissue-Specific Macrophage Responses to Remote Injury Impact the Outcome of Subsequent Local Immune Challenge, Immunity. 51 (2019) 899–914.e7. 10.1016/j.immuni.2019.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lapenna A, De Palma M, Lewis CE, Perivascular macrophages in health and disease, Nature Reviews Immunology. 18 (2018) 689–702. 10.1038/s41577-018-0056-9. [DOI] [PubMed] [Google Scholar]
- [75].Faraco G, Sugiyama Y, Lane D, Garcia-Bonilla L, Chang H, Santisteban MM, Racchumi G, Murphy M, Van Rooijen N, Anrather J, Iadecola C, Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension, J Clin Invest. 126 (2016) 4674–4689. 10.1172/JCI86950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kerkhofs D, van Hagen BT, Milanova IV, Schell KJ, van Essen H, Wijnands E, Goossens P, Blankesteijn WM, Unger T, Prickaerts J, Biessen EA, van Oostenbrugge RJ, Foulquier S, Pharmacological depletion of microglia and perivascular macrophages prevents Vascular Cognitive Impairment in Ang II-induced hypertension, Theranostics. 10 (2020) 9512–9527. 10.7150/thno.44394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lindley TE, Infanger DW, Rishniw M, Zhou Y, Doobay MF, Sharma RV, Davisson RL, Scavenging superoxide selectively in mouse forebrain is associated with improved cardiac function and survival following myocardial infarction, American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 296 (2009) R1–R8. 10.1152/ajpregu.00078.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Yu Y, Zhang Z-H, Wei S-G, Serrats J, Weiss RM, Felder RB, Brain Perivascular Macrophages and the Sympathetic Response to Inflammation in Rats After Myocardial Infarction, Hypertension. 55 (2010) 652–659. 10.1161/HYPERTENSIONAHA.109.142836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Minhas PS, Latif-Hernandez A, McReynolds MR, Durairaj AS, Wang Q, Rubin A, Joshi AU, He JQ, Gauba E, Liu L, Wang C, Linde M, Sugiura Y, Moon PK, Majeti R, Suematsu M, Mochly-Rosen D, Weissman IL, Longo FM, Rabinowitz JD, Andreasson KI, Restoring metabolism of myeloid cells reverses cognitive decline in ageing, Nature. 590 (2021) 122–128. 10.1038/s41586-020-03160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Pase MP, Himali JJ, Beiser AS, DeCarli C, McGrath ER, Satizabal CL, Aparicio HJ, Adams HHH, Reiner AP, Longstreth WT, Fornage M, Tracy RP, Lopez O, Psaty BM, Levy D, Seshadri S, Bis JC, Association of CD14 with incident dementia and markers of brain aging and injury, Neurology. 94 (2020) e254. 10.1212/WNL.0000000000008682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Noz Marlies P, ter Telgte Annemieke, Wiegertjes Kim, Joosten Leo AB, Netea Mihai G, de Leeuw Frank-Erik, Riksen Niels P., Trained Immunity Characteristics Are Associated With Progressive Cerebral Small Vessel Disease, Stroke. 49 (2018) 2910–2917. 10.1161/STROKEAHA.118.023192. [DOI] [PubMed] [Google Scholar]
- [82].Herisson F, Frodermann V, Courties G, Rohde D, Sun Y, Vandoorne K, Wojtkiewicz GR, Masson GS, Vinegoni C, Kim J, Kim D-E, Weissleder R, Swirski FK, Moskowitz MA, Nahrendorf M, Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration, Nature Neuroscience. 21 (2018) 1209–1217. 10.1038/s41593-018-0213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Andrea Cugurra, Tornike Mamuladze, Justin Rustenhoven, Taitea Dykstra, Giorgi Beroshvili, Greenberg Zev J, Baker Wendy, Zach Papadopoulos, Antoine Drieu, Susan Blackburn, Mitsuhiro Kanamori, Simone Brioschi, Jasmin Herz, Schuettpelz Laura G., Marco Colonna, Igor Smirnov, Jonathan Kipnis, Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma, Science. 373 (2021) eabf7844. 10.1126/science.abf7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Shemer A, Grozovski J, Tay TL, Tao J, Volaski A, Süß P, Ardura-Fabregat A, Gross-Vered M, Kim J-S, David E, Chappell-Maor L, Thielecke L, Glass CK, Cornils K, Prinz M, Jung S, Engrafted parenchymal brain macrophages differ from microglia in transcriptome, chromatin landscape and response to challenge, Nature Communications. 9 (2018) 5206. 10.1038/s41467-018-07548-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV, Absence of the Chemokine Receptor CCR2 Protects Against Cerebral Ischemia/Reperfusion Injury in Mice, Stroke. 38 (2007) 1345–1353. 10.1161/01.STR.0000259709.16654.8f. [DOI] [PubMed] [Google Scholar]
- [86].Morganti JM, Riparip L-K, Chou A, Liu S, Gupta N, Rosi S, Age exacerbates the CCR2/5-mediated neuroinflammatory response to traumatic brain injury, Journal of Neuroinflammation. 13 (2016) 80. 10.1186/s12974-016-0547-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hsieh CL, Niemi EC, Wang SH, Lee CC, Bingham D, Zhang J, Cozen ML, Charo I, Huang EJ, Liu J, Nakamura MC, CCR2 Deficiency Impairs Macrophage Infiltration and Improves Cognitive Function after Traumatic Brain Injury, Journal of Neurotrauma. 31 (2014) 1677–1688. 10.1089/neu.2013.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Harries LW, Bradley-Smith RM, Llewellyn DJ, Pilling LC, Fellows A, Henley W, Hernandez D, Guralnik JM, Bandinelli S, Singleton A, Ferrucci L, Melzer D, Leukocyte CCR2 Expression Is Associated with Mini-Mental State Examination Score in Older Adults, Rejuvenation Research. 15 (2012) 395–404. 10.1089/rej.2011.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Dick SA, Macklin JA, Nejat S, Momen A, Clemente-Casares X, Althagafi MG, Chen J, Kantores C, Hosseinzadeh S, Aronoff L, Wong A, Zaman R, Barbu I, Besla R, Lavine KJ, Razani B, Ginhoux F, Husain M, Cybulsky MI, Robbins CS, Epelman S, Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction, Nature Immunology. 20 (2019) 29–39. 10.1038/s41590-018-0272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, Mann DL, Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart, Proc Natl Acad Sci USA. 111 (2014) 16029. 10.1073/pnas.1406508111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Koelwyn GJ, Newman AAC, Afonso MS, van Solingen C, Corr EM, Brown EJ, Albers KB, Yamaguchi N, Narke D, Schlegel M, Sharma M, Shanley LC, Barrett TJ, Rahman K, Mezzano V, Fisher EA, Park DS, Newman JD, Quail DF, Nelson ER, Caan BJ, Jones LW, Moore KJ, Myocardial infarction accelerates breast cancer via innate immune reprogramming, Nature Medicine. 26 (2020) 1452–1458. 10.1038/s41591-020-0964-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Christ A, Günther P, Lauterbach MAR, Duewell P, Biswas D, Pelka K, Scholz CJ, Oosting M, Haendler K, Baßler K, Klee K, Schulte-Schrepping J, Ulas T, Moorlag SJCFM, Kumar V, Park MH, Joosten LAB, Groh LA, Riksen NP, Espevik T, Schlitzer A, Li Y, Fitzgerald ML, Netea MG, Schultze JL, Latz E, Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming, Cell. 172 (2018) 162–175.e14. 10.1016/j.cell.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Tarkowski E, Wallin A, Regland B, Blennow K, Tarkowski A, Local and systemic GM-CSF increase in Alzheimer’s disease and vascular dementia, Acta Neurologica Scandinavica. 103 (2001) 166–174. 10.1034/j.1600-0404.2001.103003166.x. [DOI] [PubMed] [Google Scholar]
- [94].Roy RK, Augustine RA, Brown CH, Schwenke DO, Activation of oxytocin neurons in the paraventricular nucleus drives cardiac sympathetic nerve activation following myocardial infarction in rats, Communications Biology. 1 (2018) 160. 10.1038/s42003-018-0169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J, The Sympathetic Nervous System in Heart Failure: Physiology, Pathophysiology, and Clinical Implications, Journal of the American College of Cardiology. 54 (2009) 1747–1762. 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- [96].Kang Y-M, Zhang Z-H, Johnson RF, Yu Y, Beltz T, Johnson AK, Weiss RM, Felder RB, Novel Effect of Mineralocorticoid Receptor Antagonism to Reduce Proinflammatory Cytokines and Hypothalamic Activation in Rats With Ischemia-Induced Heart Failure, Circulation Research. 99 (2006) 758–766. 10.1161/01.RES.0000244092.95152.86. [DOI] [PubMed] [Google Scholar]
- [97].Okada S, Yokoyama M, Toko H, Tateno K, Moriya J, Shimizu I, Nojima A, Ito T, Yoshida Y, Kobayashi Y, Katagiri H, Minamino T, Komuro I, Brain-Derived Neurotrophic Factor Protects Against Cardiac Dysfunction After Myocardial Infarction via a Central Nervous System–Mediated Pathway, Arteriosclerosis, Thrombosis, and Vascular Biology. 32 (2012) 1902–1909. 10.1161/ATVBAHA.112.248930. [DOI] [PubMed] [Google Scholar]
- [98].Yuan L, Liu S, Bai X, Gao Y, Liu G, Wang X, Liu D, Li T, Hao A, Wang Z, Oxytocin inhibits lipopolysaccharide-induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide-treated mice, Journal of Neuroinflammation. 13 (2016) 77. 10.1186/s12974-016-0541-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Clayton BLL, Popko B, Endoplasmic reticulum stress and the unfolded protein response in disorders of myelinating glia, Brain Research. 1648 (2016) 594–602. 10.1016/j.brainres.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Simons M, Nave K-A, Oligodendrocytes: Myelination and Axonal Support, Cold Spring Harbor Perspectives in Biology. 8 (2016). 10.1101/cshperspect.a020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Wang F, Ren S-Y, Chen J-F, Liu K, Li R-X, Li Z-F, Hu B, Niu J-Q, Xiao L, Chan JR, Mei F, Myelin degeneration and diminished myelin renewal contribute to age-related deficits in memory, Nature Neuroscience. 23 (2020) 481–486. 10.1038/s41593-020-0588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Ginsberg MD, Hedley-Whyte ET, Richardson EP Jr, Hypoxic-Ischemic Leukoencephalopathy in Man, Archives of Neurology. 33 (1976) 5–14. 10.1001/archneur.1976.00500010007002. [DOI] [PubMed] [Google Scholar]
- [103].Jayakrishnan R, Rashid N, Cheenath RT, Verghese J, Grinker’s myelinopathy: The rarely reported consequence of hypoxic brain injury, Radiology Case Reports. 16 (2021) 3311–3314. 10.1016/j.radcr.2021.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].in ‘t Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, Breteler MMB, Stricker BHC, Nonsteroidal Antiinflammatory Drugs and the Risk of Alzheimer’s Disease, N Engl J Med. 345 (2001) 1515–1521. 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- [105].Dregan A, Chowienczyk P, Armstrong D, Patterns of anti-inflammatory drug use and risk of dementia: a matched case–control study, European Journal of Neurology. 22 (2015) 1421–1428. 10.1111/ene.12774. [DOI] [PubMed] [Google Scholar]
- [106].Schjerning Olsen A-M, Gislason GH, McGettigan P, Fosbøl E, Sørensen R, Hansen ML, Køber L, Torp-Pedersen C, Lamberts M, Association of NSAID Use With Risk of Bleeding and Cardiovascular Events in Patients Receiving Antithrombotic Therapy After Myocardial Infarction, JAMA. 313 (2015) 805–814. 10.1001/jama.2015.0809. [DOI] [PubMed] [Google Scholar]
- [107].Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease, N Engl J Med. 377 (2017) 1119–1131. 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- [108].Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ, Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ, Kastelein J, Koenig W, Genest J, Lorenzatti A, Varigos J, Siostrzonek P, Sinnaeve P, Fonseca F, Nicolau J, Gotcheva N, Yong H, Urina-Triana M, Milicic D, Cifkova R, Vettus R, Anker SD, Manolis AJ, Wyss F, Forster T, Sigurdsson A, Pais P, Fucili A, Ogawa H, Shimokawa H, Veze I, Petrauskiene B, Salvador L, Cornel JH, Klemsdal TO, Medina F, Budaj A, Vida-Simiti L, Kobalava Z, Otasevic P, Pella D, Lainscak M, Seung K-B, Commerford P, Dellborg M, Donath M, Hwang J-J, Kultursay H, Flather M, Ballantyne C, Bilazarian S, Chan W, East C, Forgosh L, Harris B, Ligueros M, Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial, The Lancet. 391 (2018) 319–328. 10.1016/S0140-6736(17)32814-3. [DOI] [PubMed] [Google Scholar]
- [109].Robertson S, Martínez GJ, Payet CA, Barraclough JY, Celermajer DS, Bursill C, Patel S, Colchicine therapy in acute coronary syndrome patients acts on caspase-1 to suppress NLRP3 inflammasome monocyte activation, Clinical Science. 130 (2016) 1237–1246. 10.1042/CS20160090. [DOI] [PubMed] [Google Scholar]
- [110].Tardif J-C, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, Berry C, López-Sendón J, Ostadal P, Koenig W, Angoulvant D, Grégoire JC, Lavoie M-A, Dubé M-P, Rhainds D, Provencher M, Blondeau L, Orfanos A, L’Allier PL, Guertin M-C, Roubille F, Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction, N Engl J Med. 381 (2019) 2497–2505. 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- [111].Min KH, Kang SO, Oh SJ, Han JM, Lee KE, Association Between Gout and Dementia in the Elderly: A Nationwide Population-Based Cohort Study, The American Journal of Geriatric Psychiatry. (2021). 10.1016/j.jagp.2021.01.016. [DOI] [PubMed] [Google Scholar]
- [112].Leibovitz A, Lidar M, Baumoehl Y, Livneh A, Segal R, Colchicine therapy and the cognitive status of elderly patients with familial Mediterranean fever., The Israel Medical Association Journal : IMAJ. 8 7 (2006) 469–72. [PubMed] [Google Scholar]
- [113].Fredman G, Spite M, Specialized pro-resolving mediators in cardiovascular diseases, Molecular Aspects of Medicine. 58 (2017) 65–71. 10.1016/j.mam.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Iolanda Lázaro, Ferran Rueda, Cediel Germán Ortega Emilio, Cosme García-García, Aleix Sala-Vila, Antoni Bayés-Genís, Circulating Omega-3 Fatty Acids and Incident Adverse Events in Patients With Acute Myocardial Infarction, Journal of the American College of Cardiology. 76 (2020) 2089–2097. 10.1016/j.jacc.2020.08.073. [DOI] [PubMed] [Google Scholar]
- [115].Wang X, Miao Z, Xu X, Schultzberg M, Zhao Y, Reduced Levels of Plasma Lipoxin A4 Are Associated with Post-Stroke Cognitive Impairment, Journal of Alzheimer’s Disease. 79 (2021) 607–613. 10.3233/JAD-201050. [DOI] [PubMed] [Google Scholar]
- [116].Kotlęga D, Peda B, Drozd A, Zembroń-Łacny A, Stachowska E, Gramacki J, Szczuko M, Prostaglandin E2, 9S-, 13S-HODE and resolvin D1 are strongly associated with the post-stroke cognitive impairment, Prostaglandins & Other Lipid Mediators. 156 (2021) 106576. 10.1016/j.prostaglandins.2021.106576. [DOI] [PubMed] [Google Scholar]
- [117].Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial, The Lancet. 354 (1999) 447–455. 10.1016/S0140-6736(99)07072-5. [DOI] [PubMed] [Google Scholar]
- [118].Terano T, Fujishiro S, Ban T, Yamamoto K, Tanaka T, Noguchi Y, Tamura Y, Yazawa K, Hirayama T, Docosahexaenoic acid supplementation improves the moderately severe dementia from thrombotic cerebrovascular diseases, Lipids. 34 (1999) S345–S346. 10.1007/BF02562338. [DOI] [PubMed] [Google Scholar]
- [119].Kromhout D, Giltay EJ, Geleijnse JM, n–3 Fatty Acids and Cardiovascular Events after Myocardial Infarction, N Engl J Med. 363 (2010) 2015–2026. 10.1056/NEJMoa1003603. [DOI] [PubMed] [Google Scholar]
- [120].Geleijnse JM, Giltay EJ, Kromhout D, Effects of n-3 fatty acids on cognitive decline: A randomized, double-blind, placebo-controlled trial in stable myocardial infarction patients, Alzheimer’s & Dementia. 8 (2012) 278–287. 10.1016/j.jalz.2011.06.002. [DOI] [PubMed] [Google Scholar]
- [121].Stevens RJ, Coleman RL, Adler AI, Stratton IM, Matthews DR, Holman RR, Risk Factors for Myocardial Infarction Case Fatality and Stroke Case Fatality in Type 2 Diabetes, Diabetes Care. 27 (2004) 201. 10.2337/diacare.27.1.201. [DOI] [PubMed] [Google Scholar]
- [122].Ahtiluoto S, Polvikoski T, Peltonen M, Solomon A, Tuomilehto J, Winblad B, Sulkava R, Kivipelto M, Diabetes, Alzheimer disease, and vascular dementia, Neurology. 75 (2010) 1195. 10.1212/WNL.0b013e3181f4d7f8. [DOI] [PubMed] [Google Scholar]
- [123].Chang C-M, Hsieh C-J, Huang J-C, Huang I-C, Acute and chronic fluctuations in blood glucose levels can increase oxidative stress in type 2 diabetes mellitus, Acta Diabetologica. 49 (2012) 171–177. 10.1007/s00592-012-0398-x. [DOI] [PubMed] [Google Scholar]
- [124].Ritsinger V, Lagerqvist B, Lundman P, Hagström E, Norhammar A, Diabetes, metformin and glucose lowering therapies after myocardial infarction: Insights from the SWEDEHEART registry, Diabetes and Vascular Disease Research. 17 (2020) 1479164120973676. 10.1177/1479164120973676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Wang M-T, Lin S-C, Tang P-L, Hung W-T, Cheng C-C, Yang J-S, Chang H-T, Liu C-P, Mar G-Y, Huang W-C, The impact of DPP-4 inhibitors on long-term survival among diabetic patients after first acute myocardial infarction, Cardiovascular Diabetology. 16 (2017) 89. 10.1186/s12933-017-0572-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Furtado RHM, Bonaca MP, Raz I, Zelniker TA, Mosenzon O, Cahn A, Kuder J, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Nicolau JC, Gause-Nilsson IAM, Fredriksson M, Langkilde AM, Sabatine MS, Wiviott SD, Dapagliflozin and Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus and Previous Myocardial Infarction, Circulation. 139 (2019) 2516–2527. 10.1161/CIRCULATIONAHA.119.039996. [DOI] [PubMed] [Google Scholar]
- [127].Hsu C-C, Wahlqvist ML, Lee M-S, Tsai H-N, Incidence of Dementia is Increased in Type 2 Diabetes and Reduced by the Use of Sulfonylureas and Metformin, Journal of Alzheimer’s Disease. 24 (2011) 485–493. 10.3233/JAD-2011-101524. [DOI] [PubMed] [Google Scholar]
- [128].Wium-Andersen IK, Osler M, Jørgensen MB, Rungby J, Wium-Andersen MK, Antidiabetic medication and risk of dementia in patients with type 2 diabetes: a nested case–control study, European Journal of Endocrinology. 181 (2019) 499–507. 10.1530/EJE-19-0259. [DOI] [PubMed] [Google Scholar]
- [129].Chen K-C, Chung C-H, Lu C-H, Tzeng N-S, Lee C-H, Su S-C, Kuo F-C, Liu J-S, Hsieh C-H, Chien W-C, Association between the Use of Dipeptidyl Peptidase 4 Inhibitors and the Risk of Dementia among Patients with Type 2 Diabetes in Taiwan, Journal of Clinical Medicine. 9 (2020). 10.3390/jcm9030660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Borzì AM, Condorelli G, Biondi A, Basile F, Vicari ESD, Buscemi C, Luca S, Vacante M, Effects of vildagliptin, a DPP-4 inhibitor, in elderly diabetic patients with mild cognitive impairment, Archives of Gerontology and Geriatrics. 84 (2019) 103896. 10.1016/j.archger.2019.06.001. [DOI] [PubMed] [Google Scholar]
- [131].Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, Cooper C, Fox N, Gitlin LN, Howard R, Kales HC, Larson EB, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N, Dementia prevention, intervention, and care, The Lancet. 390 (2017) 2673–2734. 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- [132].Sabia S, Fayosse A, Dumurgier J, van Hees VT, Paquet C, Sommerlad A, Kivimäki M, Dugravot A, Singh-Manoux A, Association of sleep duration in middle and old age with incidence of dementia, Nature Communications. 12 (2021) 2289. 10.1038/s41467-021-22354-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Verdelho A, Madureira S, Ferro JM, Baezner H, Blahak C, Poggesi A, Hennerici M, Pantoni L, Fazekas F, Scheltens P, Waldemar G, Wallin A, Erkinjuntti T, Inzitari D, Physical Activity Prevents Progression for Cognitive Impairment and Vascular Dementia, Stroke. 43 (2012) 3331–3335. 10.1161/STROKEAHA.112.661793. [DOI] [PubMed] [Google Scholar]
- [134].McAlpine CS, Kiss MG, Rattik S, He S, Vassalli A, Valet C, Anzai A, Chan CT, Mindur JE, Kahles F, Poller WC, Frodermann V, Fenn AM, Gregory AF, Halle L, Iwamoto Y, Hoyer FF, Binder CJ, Libby P, Tafti M, Scammell TE, Nahrendorf M, Swirski FK, Sleep modulates haematopoiesis and protects against atherosclerosis, Nature. 566 (2019) 383–387. 10.1038/s41586-019-0948-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Frodermann V, Rohde D, Courties G, Severe N, Schloss MJ, Amatullah H, McAlpine CS, Cremer S, Hoyer FF, Ji F, van Koeverden ID, Herisson F, Honold L, Masson GS, Zhang S, Grune J, Iwamoto Y, Schmidt SP, Wojtkiewicz GR, Lee I-H, Gustafsson K, Pasterkamp G, de Jager SCA, Sadreyev RI, MacFadyen J, Libby P, Ridker P, Scadden DT, Naxerova K, Jeffrey KL, Swirski FK, Nahrendorf M, Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells, Nature Medicine. 25 (2019) 1761–1771. 10.1038/s41591-019-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, Bäckman L, Hänninen T, Jula A, Laatikainen T, Lindström J, Mangialasche F, Paajanen T, Pajala S, Peltonen M, Rauramaa R, Stigsdotter-Neely A, Strandberg T, Tuomilehto J, Soininen H, Kivipelto M, A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial, The Lancet. 385 (2015) 2255–2263. 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- [137].Chupel MU, Direito F, Furtado GE, Minuzzi LG, Pedrosa FM, Colado JC, Ferreira JP, Filaire E, Teixeira AM, Strength Training Decreases Inflammation and Increases Cognition and Physical Fitness in Older Women with Cognitive Impairment, Frontiers in Physiology. 8 (2017) 377. 10.3389/fphys.2017.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Hofmann U, Frantz S, Role of Lymphocytes in Myocardial Injury, Healing, and Remodeling After Myocardial Infarction, Circulation Research. 116 (2015) 354–367. 10.1161/CIRCRESAHA.116.304072. [DOI] [PubMed] [Google Scholar]