Abstract
Objectives:
Experimental models have demonstrated a link between exposure to perfluoroalkyl substances (PFAS) and decreased fertility and fecundability; however, human studies are scarce. We assessed the associations between preconception plasma PFAS concentrations and fertility outcomes in women.
Methods:
In a case-control study nested within the population-based Singapore Preconception Study of Long-Term Maternal and Child Outcomes (S-PRESTO), we measured PFAS in plasma collected in 2015–2017 from 382 women of reproductive age trying to conceive. Using Cox proportional hazards regression (fecundability ratios [FRs]) and logistic regression (odds ratios [ORs]) models, we assessed the associations of individual PFAS with time-to-pregnancy (TTP), and the likelihoods of clinical pregnancy and live birth, respectively, over one year of follow-up, adjusting for analytical batch, age, education, ethnicity, and parity. We used Bayesian weighted quantile sum (BWQS) regression to assess the associations of the PFAS mixture with fertility outcomes.
Results:
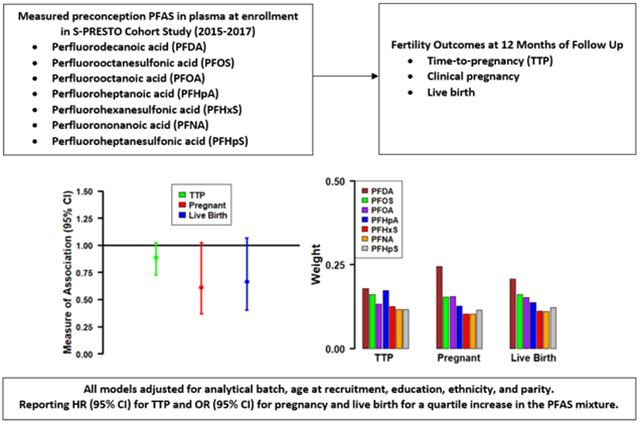
We found a 5–10% reduction in fecundability per quartile increase of exposure to individual PFAS (FRs [95% CIs] for clinical pregnancy=0.90 [0.82, 0.98] for PFDA; 0.88 [0.79, 0.99] for PFOS; 0.95 [0.86, 1.06] for PFOA; 0.92 [0.84, 1.00] for PFHpA). We observed similar decreased odds of clinical pregnancy (ORs [95% CIs]=0.74 [0.56, 0.98] for PFDA; 0.76 [0.53, 1.09] for PFOS; 0.83 [0.59, 1.17] for PFOA; 0.92 [0.70, 1.22] for PFHpA) and live birth per quartile increases of individual PFAS and the PFAS mixture (ORs [95% CIs]=0.61 [0.37, 1.02] for clinical pregnancy, and 0.66 [0.40, 1.07] for live birth). Within the PFAS mixture, PFDA followed by PFOS, PFOA, and PFHpA were the biggest contributors to these associations. We found no evidence of association for PFHxS, PFNA, and PFHpS and the fertility outcomes examined.
Conclusions:
Higher PFAS exposures may be associated with decreased fertility in women. The potential impact of ubiquitous PFAS exposures on infertility mechanisms require further investigation.
Keywords: PFAS, Endocrine disrupting chemicals, Fecundability, Pregnancy outcomes, Women’s health
Graphical Abstract
1. Introduction
Infertility is the inability of an individual who is actively attempting conception to become pregnant within 12 months or longer of initiating unprotected sexual intercourse on a regular basis (Vander Borght and Wyns, 2018). Infertility is an important public health problem due to its adverse effects on affected individuals’ mental health (Fallahzadeh et al., 2019; Yusuf, 2016). Approximately 8–12% of couples experience infertility worldwide (Vander Borght and Wyns, 2018). The use of assisted reproductive technology (ART) among women living in Singapore is currently on the rise (Huang et al., 2021a). Furthermore, the prevalence of primary infertility among women who desired to get pregnant in South Asia was nearly 2.5% in 2010, which exceeded that of many other parts of the world (Mascarenhas et al., 2012). Additionally, Asian women are more likely to delay the usage of infertility treatments and have lower success rates after fertility treatments compared to white women (Vu et al., 2021). Emerging evidence suggests that environmental chemical exposures may affect fertility in women (Luo et al., 2022; Messerlian et al., 2016; Thomsen et al., 2017; Tranfo et al., 2012). However, few studies have examined this association to date, and no previous study included women residing in Singapore.
Perfluoroalkyl substances (PFAS) comprise a class of ubiquitous and persistent chemicals that are commonly found in drinking water (Domingo and Nadal, 2019; Sunderland et al., 2019) and a wide variety of consumer products such as waterproof clothing (van der Veen et al., 2020; van der Veen et al., 2022), furniture (Schildroth et al., 2022), non-stick cookware (Herzke et al., 2012), baking paper (Kotthoff et al., 2015), and food packaging (Curtzwiler et al., 2021; Ramírez Carnero et al., 2021; Susmann et al., 2019). PFAS are detected in the blood of >99% of populations tested in the United States (Kato et al., 2011), Singapore (Sum et al., 2022), and other regions due to common daily exposure and the long half-lives (in years) in human tissues of many PFAS (Olsen et al., 2007).
Exposure to PFAS may result in infertility in women via endocrine disruption (Rickard et al., 2022) and through increasing the risk for common reproductive conditions such as endometriosis (Miller et al., 2017; Mohammed Rasheed and Hamid, 2020; Singh et al., 2016; Wang et al., 2017) and polycystic ovary syndrome (González, 2012; Tarkun et al., 2006; Velez et al., 2021; Wang et al., 2019). In particular, higher levels of PFAS exposure could lead to alterations in estradiol and progesterone levels in women, resulting in impairment of the reproductive system (Barrett et al., 2015; Chaparro-Ortega et al., 2018) due to the important role that they play in the menstrual cycle (Reed and Carr, 2000).
A recent meta-analysis of previous epidemiology studies conducted mainly in non-Asian populations found evidence that exposures to PFOS and PFOA may decrease fertility in women but noted large heterogeneity in effect estimates across studies and limited data on other PFAS (Wang et al., 2023). Notably, previous studies have not assessed the potential impact of PFAS exposure as a mixture. This is an important limitation as populations are exposed simultaneously to multiple PFAS, rather than a single PFAS compound. Therefore, we assessed the extent to which preconception plasma PFAS concentrations and PFAS mixture exposures were associated with fertility outcomes in a population-based cohort of women from Singapore who were of reproductive age and attempting to conceive naturally within a one-year period of follow-up. Consistent with experimental evidence, we hypothesized that higher plasma PFAS concentrations will be associated with decreased fecundability and reduced likelihoods of clinical pregnancy and live birth in reproductive age women.
2. Materials and Methods
2.1. Study Design and Population
The present study included women who were enrolled in the Singapore Preconception Study of Long-Term Maternal and Child Outcomes (S-PRESTO) (Loo et al., 2021). S-PRESTO is a population-based prospective cohort study designed to assess the effects of maternal preconception health on adverse health outcomes in women and their offspring (Loo et al., 2021). A total of 1,032 women aged from 18 to 45 years who were actively trying to conceive were enrolled in the origin cohort between February 2015 and October 2017, and followed-up for at least 1 year. In total, each participant attended three preconception visits within the first few months, and then was followed up by phone calls at 6, 9, and 12 months to track pregnancy status. Recruitment occurred from the community and at KK Women’s and Children’s Hospital, which is Singapore’s largest public maternity hospital. Women who had been actively trying to conceive for more than 18 months before recruitment, who were currently pregnant, were using oral or implanted contraception, or with an intrauterine contraceptive device in situ in the past 1 month or who were undergoing fertility treatment or had a diagnosis of type 1 or type 2 diabetes, or were on systemic steroids, anticonvulsants, HIV or Hepatitis B or C medications in the past 1 month were excluded from the S-PRESTO study (Loo et al., 2021). Only Chinese, Malay, Indian, or a mixture of these ethnicities were recruited.
We conducted a case-control study nested within the S-PRESTO cohort to evaluate the association between PFAS exposure and fertility. The present analysis included a sub-study of 382 women with PFAS concentrations measured in preconception blood samples, including 332 women who achieved clinical pregnancy and a random subset of 50 women from among those who failed to conceive within one year of follow up. The 332 women who became clinically pregnant consisted of all women who conceived in the S-PRESTO cohort who had a blood sample available for PFAS analysis, and the 50 women who did not conceive (i.e. controls) comprised a random subset of the remaining such S-PRESTO participants. All participants provided a signed informed consent. Ethical approvals of the study protocols were obtained by the SingHealth Centralised Institutional Review Board (reference 2014/692/D) and the Institutional Review Board at Icahn School of Medicine at Mount Sinai.
2.2. Assessment of PFAS exposures
A total of 15 PFAS were measured in one preconception plasma sample collected from each woman at study enrollment from 2015–2017. Samples were drawn, fractionated, and stored immediately frozen at −80°C until laboratory analyses. All PFAS analyses occurred between December 4, 2020 and January 16, 2021 and were performed by targeted LC-MS/MS (Coggan et al., 2019; Kato et al., 2018) at the Frank R. Lautenberg Environmental Health Sciences Laboratory at Icahn School of Medicine at Mount Sinai, NY, USA. The samples were analyzed in six batches. Measured PFAS included perfluorodecanoic acid (PFDA), linear and branched perfluorooctanesulfonic acid (PFOS), linear perfluorooctanoic acid (PFOA), perfluoroheptanoic acid (PFHpA), perfluorohexanesulfonic acid (PFHxS), perfluorononanoic acid (PFNA), perfluoroheptanesulfonic acid (PFHpS), perfluorobutanesulfonic acid (PFBS), N-methylperfluorooctanesulfonamido acetic acid (NMeFOSAA), N-ethyl perfluorooctanesulfonamidoacetic acid (NEtFOSAA), 6:2 fluorotelomer sulfonate (6:2 FTS), 6:2 polyfluoroalkyl phosphate ester (6:2 PAP), 6:2 fluorotelomer phosphate diester (6:2 diPAP), perfluorooctanesulfonamide (PFOSA), and perfluorodecanesulfonic acid (PFDS).
We followed the analytical method of the Centers for Disease Control and Prevention (CDC) for PFAS in plasma (Kato et al., 2018) with minor modifications (Coggan et al., 2019; Reagen et al., 2008). Quantification of PFAS was based on isotope-dilution liquid chromatography with tandem mass spectrometry method. In brief, 13C stable isotope labeled internal standards were added to each sample, followed by solid-phase extraction with an Oasis WAX mixed-mode polymeric reversed-phase 96-well plate (30 mg sorbent per well, 30 μm particle size; Waters Corporation, Milford, MA). Low-volume sample aliquoting (0.2 ml) and clean-up procedures were automated using a liquid handler (epMotion 5075vtc; Eppendorf, Hauppauge, NY). The LC-MS/MS (Agilent 1290 Infinity II UHPLC coupled with 6470A triple quadrupole MS, Agilent Technologies, Wilmington, DE) was operated in electrospray negative mode for ionization and multiple reaction monitoring (MRM) for quantification. Chromatographic separation was achieved on an InfinityLab Poroshell 120 EC-C18, 1.9 μm, 100 × 2.1 mm analytical column with 5 × 2.1 mm guard cartridge (Agilent Technologies, Wilmington, DE). Instrument contribution to background PFAS was removed by Eclipse Plus C18, 3.5 μm, 50 × 4.6 mm delay column (Agilent Technologies, Wilmington, DE). Mobile phase A was 5mM ammonium acetate in LC-MS grade water, and mobile phase B was 5mM ammonium acetate in LC-MS grade methanol and water (95:5 v/v). Quality controls (QCs) included in each batch were procedural and instrumental blanks, matrix spikes in the lower, middle and upper range of assay validation, NIST standard reference material (SRM 1957: Organic Contaminants in Non-Fortified Human Serum and SRM 1958: Organic Contaminants in Fortified Human Serum), and archived proficiency testing material. Batchwise relative standard deviations (RSDs) of QCs during analysis of the study specimens were <20% for target analytes in reference or fortified material, except for analytes at or below the limit of quantification (LOQ) (LOQ=3xLOD) where RSDs up to 30% were accepted. Intra-batch precision (CV) was below 10% and inter-batch precision was below 20% for QC analytes above the LOQ. Recoveries in batch QCs were between 70% and 130%. The Lautenberg Laboratory is part of the Human Health Exposure Analysis Resource (HHEAR), a continuation of Children’s Health Exposure Analysis Resource (CHEAR), which has participated and is qualified in proficiency testing programs for PFAS in human serum conducted by CTQ-AMAP (https://www.inspq.qc.ca/en/ctq/eqas/amap/description) (CTQ, 2021) and G-EQUAS (http://www.g-equas.de/) (Göen et al., 2012).
Out of 15 PFAS measured, seven had concentrations above the limit of detection (LOD) for at least 33% of women and were subsequently included in statistical analyses. These seven PFAS (LOD) included total PFOS (0.20 ng/ml for linear and branched), PFOA (0.50 ng/ml), PFHpA (0.20 ng/ml), PFDA (0.50 ng/ml), PFHxS (0.10 ng/ml), PFNA (0.50 ng/ml), and PFHpS (0.20 ng/ml). We excluded the remaining eight PFAS from subsequent statistical analyses due to low detection rates. We substituted PFAS concentrations below the LOD to be equal to half of the LOD in our analyses, as has been done previously (Vélez et al., 2015). We calculated total PFOS as the sum of linear and branched PFOS.
2.3. Assessment of Fertility Outcomes
Pregnancy screening procedures conducted in the S-PRESTO cohort have previously been detailed elsewhere (Loy et al., 2018). Briefly, participants were screened for pregnancy at each preconception visit using a urinary pregnancy test kit (Biotron Diagnostics, USA) (Loy et al., 2018). Women were provided with pregnancy test kits to use in between preconception visits and were reminded to perform home urine pregnancy tests, if their menstrual periods were late for 3–4 days, or 2 weeks after unprotected intercourse, and to contact the research staff if they test positive. The pregnancy test kits were sensitive to 25 mIU/ml of human chorionic gonadotropin (hCG) (Loy et al., 2018). In the event of a positive pregnancy test, participants underwent a viability ultrasound scan after 6 weeks’ amenorrhea to confirm a clinical pregnancy. In the absence of any update within 6, 9, and 12 months of recruitment, S-PRESTO research staff conducted a follow-up call to determine the woman’s pregnancy status over the past year. All women were followed for at least 1 year while attempting to conceive.
We assessed TTP, clinical pregnancy, and live birth as the study outcomes in our analyses. We assessed TTP based on the number of observed menstrual cycles required to achieve pregnancy over 12 months of follow-up, as detailed previously (Wise et al., 2020). In order to account for left truncation, we based our risk sets on observed cycles at risk. We used the date of the last menstrual period (LMP) as the starting date and the date of conception or the date of the last follow-up call as the ending date for women who conceived and those who did not conceive, respectively. We used these starting and ending dates to calculate the observation time interval taken to achieve a clinical pregnancy for each participant. Finally, we calculated TTP in menstrual cycles using the following formula:
For those who achieved a clinical pregnancy, one more cycle was added (Loy et al., 2018) under the assumption that the actual conception occurred 0.5–1.0 menstrual cycles after the LMP (Huang et al., 2021b). In the analyses of TTP, we excluded three participants with missing data on the start and end dates of the menstrual cycle and an additional three participants with missing data on the TTP measurement in days. In addition to TTP, we also assessed clinical pregnancy and live birth as dichotomous outcomes (0: no, 1: yes) denoting whether the woman conceived by the end of the 12 month follow-up period, and whether they delivered. Delivery information was abstracted from clinical records by trained study staff using standardized case report forms.
2.4. Additional covariates
We collected data on sociodemographic characteristics for each participant based on interviews with the women conducted by trained study staff at the enrollment visit. These data included participants’ ethnicity, education, parity, and smoking status. Women’s age at recruitment was calculated based on recorded date of birth. Only a few women were of mixed ethnicity and were reclassified as Chinese, if of partial Chinese descent (n=6), or Malay, if of mixed Malay-Indian ethnicity (n=1) to handle small number categories, as these ethnic groups are the most largely represented in our study population.
2.5. Statistical Analysis
We reported descriptive statistics to summarize the demographic composition of the study population and compare it to the overall cohort. We also compared demographic characteristics between clinical pregnancy or live birth subgroups using Wilcoxon rank sum tests for continuous variables and chi-squared tests or Fisher’s exact test for categorical variables. We computed Spearman pairwise correlations between the individual PFAS concentrations under study.
We analyzed TTP using Cox proportional hazards regression models to estimate fecundability ratios (FR, 95% CIs) interpreted as the risk of being clinically pregnant per quartile increase in PFAS exposures. Results did not considerably differ using accelerated failure time (AFT) models with a discrete time Weibull distribution or a Poisson regression model to assess TTP, as sensitivity analyses. The proportional hazards assumption was met in our analyses (p > 0.05 for the Schoenfeld test for all seven PFAS). We used logistic regression models to estimate the odds ratios (OR, 95% CIs) for being clinically pregnant within a year of follow up, or delivering a live birth per quartile increase in PFAS exposures. Additionally, Bayesian weighted quantile sum-Cox proportional hazards (BWQS-PH) regression models were used to assess the combined effects of the multiple PFAS with TTP to calculate a FR. A Bayesian weighted quantile sum (BWQS) model (Colicino et al., 2020) estimates the combined effects of multiple exposures on an outcome, and a Cox proportional hazards model (Cox, 1972) estimates the hazard rate of an event given that a participant has not yet experienced the event at a given point in time. Therefore, a BWQS-PH model estimates the combined effects of multiple exposures on the hazard rate of a survival outcome. Our code for fitting the BWQS-PH models has been made publicly available (https://github.com/DaniaValvi/S-PRESTO_Fertility) (Yao, 2023). Moreover, we used BWQS to calculate the ORs for clinical pregnancy and live birth. In the BWQS models, we set the thinning parameter to one, and we used two Markov chains with 10,000 iterations each. We selected confounders included in the statistical models based on a priori knowledge (Kingsley et al., 2018; Liang et al., 2021; Sadecki et al., 2022) and results of our bivariate analyses evaluating PFAS relationships with covariates, and adjusted all models for analytical batch, age at recruitment, education, ethnicity, and parity. No missing data were present in these covariates. Effect estimates (95% CIs) from all analyses are expressed per quartile increase in PFAS exposures to facilitate comparison of the magnitude of effects across statistical methods. We checked linearity between the seven PFAS and clinical pregnancy by adding orthogonal polynomials of degree one and two into our models and confirming that the quadratic terms were not statistically significant for any of the seven PFAS.
We used inverse probability weighting (IPW) in all models to account for the different selection probabilities for women’s inclusion in the present nested case-control design for PFAS assessment due to pregnancy status, loss to follow up, and blood sample availability. Weights were calculated for each participant in the entire origin cohort from a logistic regression model of the probability of inclusion in the PFAS laboratory analyses based on baseline predictors (age at recruitment, education, ethnicity, and parity). We present all estimates and 95% confidence intervals from the weighted statistical models.
Sensitivity analyses included: (1) repeating all analyses using PFDA, PFHpA, and PFHpS as binary variables (instead of in quartiles), dichotomized at the LOD value because of larger numbers of women having concentrations below the LOD for these three PFAS; (2) the exclusion of women with irregular menstrual cycles (n=24) in our mixture analyses; (3) the exclusion of the few participants of mixed-race (n=7) from the statistical models, and (4) analyses adjusting for fruit intake and fast food intake.
We interpreted results emphasizing on effect magnitude (estimates and confidence intervals) and consistent association patterns without solely relying on statistical significance. We performed all statistical analyses in R (version 4.2.1). We used the rstan (Stan Development Team, 2020) and BWQS (Colicino et al., 2020) packages for our mixture analyses.
3. Results
Median (IQR) age at recruitment was 30.5 (28.1, 32.8) years (Table 1). Median (IQR) BMI at preconception was 22.0 (20.1, 25.2) kg/m2. The majority of women (77.2%) were Chinese, attained a university level education (67.0%), were nulliparous (64.4%) and had never smoked (91.1%). Only three (0.8%) women had a diagnosis of polycystic ovary syndrome. PFOS and PFOA had the highest concentrations on average compared to other PFAS with a median (IQR) of 2.49 (1.83, 3.35) ng/ml and 1.76 (1.28, 2.28) ng/ml, respectively. The distribution of all 15 PFAS measured is displayed in Supplementary Table 1, and the pairwise correlations between PFAS included in our analyses are shown in Supplementary Figure 1. Spearman correlation coefficients between PFAS ranged from 0.03 (PFHxS and PFHpA as well as PFHpS and PFDA) to 0.74 (PFOS and PFHpS). Average TTP (median (IQR)) was 5 (2, 10) menstrual cycles (Kaplan-Meier curve shown in Supplementary Figure 2). Women who conceived over the study period, compared to those who did not conceive, were more likely to be of Chinese ethnicity, had a higher education, were less likely to be nulliparous and had lower plasma concentrations of certain PFAS (Table 1). We observed similar differences in covariates distributions between women who delivered a live birth vs. those who did not (Supplementary Table 2). Women included compared to those excluded from the present analyses were of younger age (median: 30.5 vs 31.5 years) more likely to have attained university level education (67.0% vs 58.3%) and be Chinese (77.2% vs 71.1%) and did not differ in parity or smoking history (Supplementary Table 3). We did not observe any substantial differences in sociodemographic characteristics when comparing women included to those excluded from the current analyses by clinical pregnancy status (Supplementary Table 4).
Table 1.
Population Characteristics Overall and by Pregnancy Status
| Overall (N=382) |
Conceived (N=332) |
Did Not Conceive (N=50) |
P-Valueb | |
|---|---|---|---|---|
| Median (IQR) or N (%) a | Median (IQR) or N (%) a | Median (IQR) or N (%) a | ||
| Age at Recruitment (years) | 30.5 (28.1, 32.8) | 30.4 (28.1, 32.6) | 32.0 (28.2, 33.8) | 0.09 |
| BMI at Preconception (kg/m2)c | 22.0 (20.1, 25.2) | 21.9 (20.2, 25.0) | 23.4 (19.6, 25.7) | 0.52 |
| Highest Education Completed at Preconception | <0.001 | |||
| Primary/Secondary/Post-Secondary | 126 (33.0%) | 98 (29.5%) | 28 (56.0%) | |
| University | 256 (67.0%) | 234 (70.5%) | 22 (44.0%) | |
| Ethnicity | 0.12 | |||
| Chinese | 295 (77.2%) | 262 (78.9%) | 33 (66.0%) | |
| Indian | 29 (7.6%) | 23 (6.9%) | 6 (12.0%) | |
| Malay | 58 (15.2%) | 47 (14.2%) | 11 (22.0%) | |
| History of Parity | 0.05 | |||
| 0 | 246 (64.4%) | 207 (62.3%) | 39 (78.0%) | |
| >0 | 136 (35.6%) | 125 (37.7%) | 11 (22.0%) | |
| Smoking Status | 0.46 | |||
| Never Smoked | 348 (91.1%) | 304 (91.6%) | 44 (88.0%) | |
| Former Smoker | 20 (5.2%) | 17 (5.1%) | 3 (6.0%) | |
| Current Smoker | 14 (3.7%) | 11 (3.3%) | 3 (6.0%) | |
| Polycystic Ovary Syndrome | >0.99 | |||
| No | 379 (99.2%) | 329 (99.1%) | 50 (100.0%) | |
| Yes | 3 (0.8%) | 3 (0.9%) | 0 (0.0%) | |
| PFDA (ng/ml) d | 0.25 (0.25, 0.63) | 0.25 (0.25, 0.62) | 0.25 (0.25, 0.74) | 0.25 |
| PFOS (ng/ml) d , e | 2.49 (1.83, 3.35) | 2.43 (1.82, 3.28) | 3.00 (1.94, 3.95) | 0.01 |
| PFOA (ng/ml) d | 1.76 (1.28, 2.28) | 1.74 (1.24, 2.23) | 1.99 (1.52, 2.54) | 0.02 |
| PFHpA (ng/ml) d | 0.10 (0.10, 0.45) | 0.10 (0.10, 0.44) | 0.10 (0.10, 0.49) | 0.29 |
| PFHxS (ng/ml) | 0.58 (0.44, 0.79) | 0.57 (0.43, 0.78) | 0.64 (0.45, 0.97) | 0.12 |
| PFNA (ng/ml) d | 0.64 (0.25, 1.01) | 0.62 (0.25, 1.01) | 0.68 (0.25, 0.98) | 0.84 |
| PFHpS (ng/ml) d | 0.10 (0.10, 0.22) | 0.10 (0.10, 0.22) | 0.21 (0.10, 0.24) | 0.02 |
| TTP (menstrual cycles) | 5 (2, 10) | 4 (2, 8) | 14 (13, 16) | <0.001 |
For continuous variables, we are reporting median (IQR). For categorical variables, we are reporting N (%).
Comparison of covariate distributions between groups of women who conceived and did not conceive using Wilcoxon rank sum tests for continuous variables and chi-squared tests for categorical variables. For categorical variables with counts less than five, we used Fisher’s exact test instead.
BMI at preconception is missing for one participant who conceived.
PFAS concentrations below the LOD were substituted by half of the LOD.
We are reporting total PFOS in this table.
In individual PFAS analyses (Table 2), we observed decreased fecundability (FR [95% CI]) per quartile increase in preconception plasma concentrations of PFDA (0.90 [0.82, 0.98]), PFOS (0.88 [0.79, 0.99]), PFOA (0.95 [0.86, 1.06]), and PFHpA (0.92 [0.84, 1.00]). Moreover, we observed reduced likelihood of clinical pregnancy (OR [95%CI]) per quartile increase in PFDA (0.74 [0.56, 0.98]) and PFOS (0.76 [0.53, 1.09]). Associations between individual PFAS and live birth were in the same direction (ORs < 1) as for clinical pregnancy but did not reach the statistical significance level (Table 2). In PFAS mixture analyses, we observed a consistent pattern of decreased fecundability (FR [95% CI]=0.89 [0.73, 1.02]), and reduced likelihoods for clinical pregnancy (OR [95% CI]=0.61 [0.37, 1.02]) and live birth (OR [95% CI]=0.66 [0.40, 1.07]) per quartile increase in the PFAS mixture (Table 2). PFDA followed by PFOS, PFOA, and PFHpA were the biggest contributors to the PFAS mixture associations (Figure 1). We did not observe evidence of an association with the fertility outcomes in any of our analyses for PFHxS, PFNA, and PFHpS.
Table 2.
Associations of PFAS Concentrations at Preconception with Fertility Outcomesa
| TTP FR (95% CI) |
Clinical Pregnancy OR (95% CI) |
Live Birth OR (95% CI) |
|
|---|---|---|---|
| PFDA (ng/ml) | 0.90 (0.82, 0.98) | 0.74 (0.56, 0.98) | 0.78 (0.60, 1.03) |
| PFOS (ng/ml) | 0.88 (0.79, 0.99) | 0.76 (0.53, 1.09) | 0.78 (0.54, 1.11) |
| PFOA (ng/ml) | 0.95 (0.86, 1.06) | 0.83 (0.59, 1.17) | 0.86 (0.62, 1.20) |
| PFHpA (ng/ml) | 0.92 (0.84, 1.00) | 0.92 (0.70, 1.22) | 0.91 (0.69, 1.21) |
| PFHxS (ng/ml) | 0.95 (0.85, 1.07) | 0.98 (0.70, 1.37) | 0.97 (0.70, 1.35) |
| PFNA (ng/ml) | 0.98 (0.90, 1.08) | 0.98 (0.75, 1.30) | 0.99 (0.75, 1.31) |
| PFHpS (ng/ml) | 0.97 (0.89, 1.06) | 0.89 (0.67, 1.19) | 0.90 (0.67, 1.20) |
| PFAS Mixture b | 0.89 (0.73, 1.02) | 0.61 (0.37, 1.02) | 0.66 (0.40, 1.07) |
Effect estimates (95% CI) per quartile increase in PFAS from Cox proportional hazards regression (FRs) and logistic regression (ORs) models.
Effect estimates per quartile increase in the PFAS mixture from BWQS regression.
All estimates shown are adjusted for batch, age at recruitment, education, ethnicity, and parity.
Figure 1.
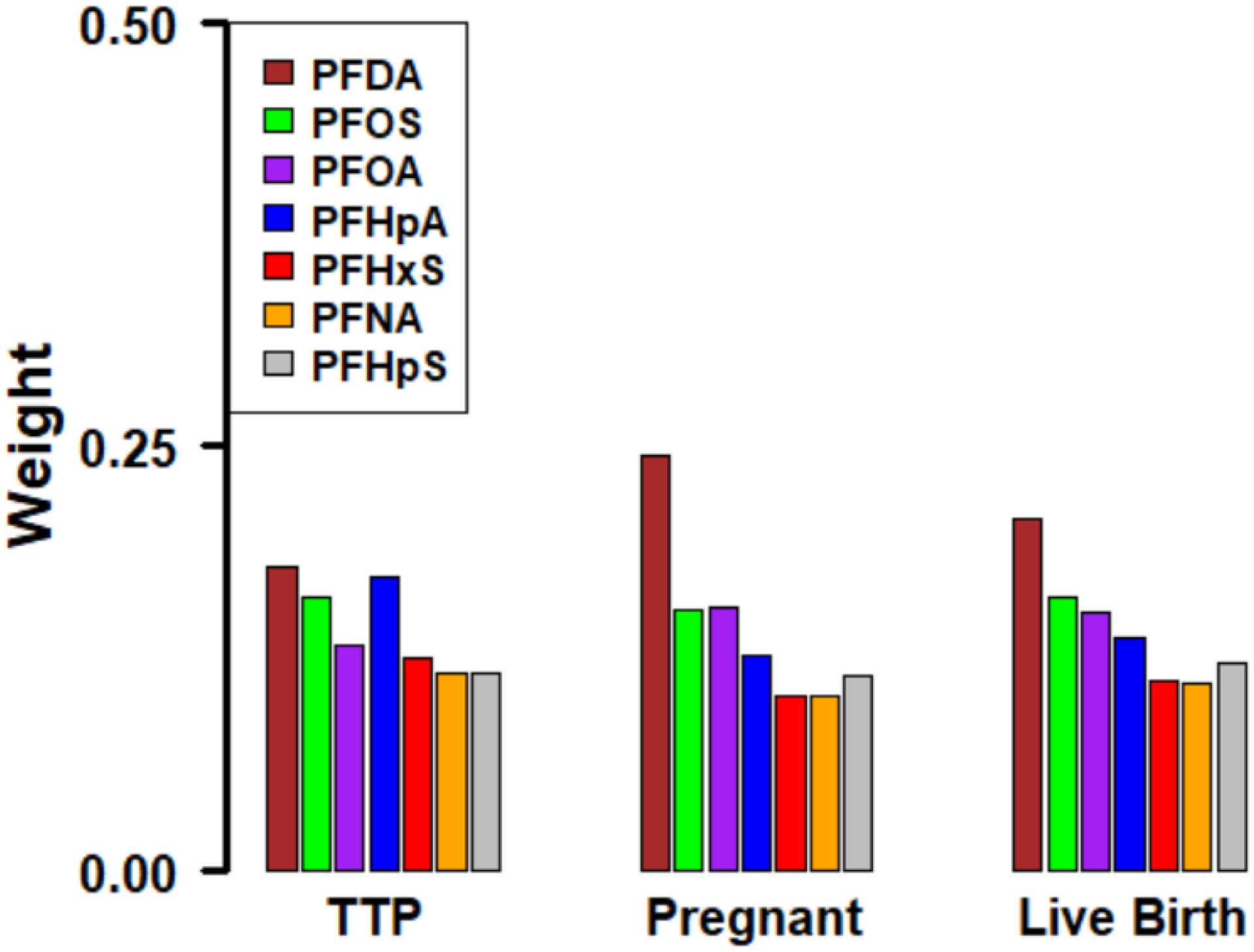
Contributions of Each Individual PFAS in the PFAS Mixture Associations from the BWQS Regression Analysis a
aThe weights shown in the figure sum to one.
In sensitivity analyses analyzing PFDA, PFHpA, and PFHpS as dichotomous variables, effect estimates for all fertility outcomes, though non-significant, were in the same direction and of stronger magnitude (Supplementary Table 5), as the effect estimates obtained from the main analyses (Table 2). Our results for TTP also did not considerably change in our AFT models or our Poisson regression models (Supplementary Table 6). Our results also remained similar after excluding the 24 participants with irregular menstrual cycles from our mixture analyses (Supplementary Table 7) and after adjusting for fruit intake and fast food intake (Supplementary Table 8). Moreover, results from all analyses remained the same after exclusion of the seven participants of mixed-race (data not shown).
4. Discussion
In this population-based study of women from Singapore who were attempting pregnancy over a one-year follow-up period, we found that higher exposure to PFAS, individually and as a mixture, is associated with reduced probability for clinical pregnancy and live birth. More specifically, we found suggestive evidence of a 5–10% reduction on average in fecundability per quartile increase of PFDA, PFOS, PFOA, and PFHpA, and a 30–40% reduction on average in the likelihood of clinical pregnancy and live birth per quartile increase in the PFAS mixture. We found no clear evidence for an association between exposures to PFHxS, PFNA, and PFHpS and the fertility outcomes examined. Our results support the notion that exposure to PFAS may disrupt reproductive function in women, as suggested in previous female animal models (Cao et al., 2020; Feng et al., 2015), and underscore the need for further investigation to elucidate the potential impact of ubiquitous PFAS exposures on women’s fertility and underlying biological mechanisms.
Our results add to a growing body of literature implicating PFAS as a potential risk factor of infertility in women (Wang et al., 2023). For example, a recent study conducted in the Shanghai Birth Cohort found associations between higher 6:2 diPAP concentrations in women and reduced fecundability and greater odds of infertility, defined as a TTP greater than 12 menstrual cycles (Luo et al., 2022). In our study, 6:2 diPAP concentrations were below the LOD for almost all women and therefore we did not examine this association. This previous study also did not observe an association between PFDA concentrations and fecundability or infertility, whereas we observed an association in the present study. This discrepancy could be explained in part by differences in the PFAS mixtures and exposure ranges between the two studies. Our findings are consistent with prior work conducted in the Danish National Birth Cohort (Fei et al., 2009) which showed associations between higher PFOS and PFOA concentrations and infertility, defined as a TTP greater than 12 months or the usage of infertility treatment. A previous pregnancy cohort study conducted in Canada (Vélez et al., 2015) further showed an association of higher PFOA exposure with TTP, in line to our findings. Similarly, a previous study conducted in Norway (Whitworth et al., 2012) showed associations with PFOS and PFOA exposure in relation to subfecundity, which is consistent with our findings. However, the study conducted in Canada also found that PFHxS was associated with infertility, for which we observed no clear association in our study, and no association for PFOS (Vélez et al., 2015). The discrepant results between our study and this previous study may in part be attributed to the retrospective collection of self-reported TTP data from pregnant women, as well as differences in the PFHxS concentrations between the two cohorts, because PFHxS concentrations were double as high in the previous study (median: 1 ng/ml vs 0.58 ng/ml in our study). Additionally, our null findings for certain PFAS (PFHxS and PFNA) are in line with another prior study conducted in a Danish cohort (Vestergaard et al., 2012).
In our analyses, we observed associations with infertility outcomes for both long-chain PFAS (PFOS, PFOA, PFDA) as well as the short-chain PFHpA. PFHpA has a shorter biological half-life in humans (estimated in days to few months) compared to other long-chain PFAS (half-lives estimated in years) (Xu et al., 2020). Our finding about a potential association between PFHpA and women’s infertility is of particular importance because long-chain PFAS are being replaced by short-chain PFAS that are considered as ‘safer’ alternatives in recent years (Sunderland et al., 2019). This finding motivates future research into the effects of emerging, short-chained PFAS on infertility that have not been previously studied (Wang et al., 2023).
Previous animal studies focused on long-chain (Feng et al., 2015) and short-chain (Cao et al., 2020) PFAS suggest reproductive hormone disruption as a potential mechanism for explaining the PFAS effects on female fertility. In particular, female mice who are chronically exposed to PFOS have lower concentrations of luteinizing hormone (LH), follicle stimulating hormone (FSH), and gonadotropin releasing hormone (GnRH) (Feng et al., 2015), which play a role in the process of ovulation (Holesh et al., 2022). Our results with respect to PFOS support the notion that this could be a potential mechanism in humans. In PFAS mixture analyses, we found PFDA followed by other PFAS to be the main driver of the associations with fertility outcomes. Biological mechanisms underlying the potential effects of PFDA and PFAS as a mixture on female fertility require further elucidation in experimental models. In addition, future epidemiology studies integrating female reproductive hormones and omic biomarkers can help to elucidate potential mechanisms underlying the PFAS associations with infertility in humans.
PFAS exposure in the present study is lower in comparison to a previous preconception cohort study in Shanghai, China, with median PFOS and PFOA concentrations above 10 ng/ml (Zhou et al., 2017), which considerably exceeds the median concentrations measured in the present study of women in Singapore. Preconception blood samples used to measure the PFAS were collected from 2013–2015 in this previous study, compared to 2015–2017 in the present study. Concentrations for PFAS examined are also generally lower in our cohort than those observed in delivery samples collected in 2009–2011 from an older Singaporean cohort (Sum et al., 2022). In particular, PFBS was detected in only 1% of preconception samples in our study but 100% of cord and maternal peripheral blood at delivery in the previous cohort. This may reflect in part declines in environmental PFAS exposures over the preceding 4–5 years differential exposures during pregnancy, and/or potential effects of pregnancy dynamics. Other studies assessing the relationship between PFAS and infertility in women in Canada and Norway have reported higher median PFOS concentrations than that of the present study, but their reported median PFOA concentrations have been more comparable (Vélez et al., 2015; Whitworth et al., 2012). Therefore, findings from the present study suggest that PFAS exposures at preconception may contribute to infertility even at the lower PFAS exposure ranges detected in the S-PRESTO cohort study compared to other previous populations.
The present study has important strengths. These include the prospective design that ensured that PFAS exposures preceded the outcomes under study and allowed us to evaluate observed fertility outcomes within one year of follow up. Other strengths are the multiethnic composition of the S-PRESTO cohort consisting of Chinese, Indian, and Malay ancestries which is generally representative of Singapore’s citizen and permanent resident population (Singapore, 2020), the measurement of a wide list of PFAS, and the use of exposure mixture methods to assess the joint effect of the PFAS mixture on the fertility outcomes. Furthermore, we assessed a cohort of women who were residing in Singapore population, which addresses a notable gap in the literature in this area because no prior studies have assessed the effects of PFAS on infertility in women who live there. Further research in other Asian populations is needed, as PFAS concentrations in water are above the recommended levels in many countries, such as China, Japan, and South Korea (Baluyot et al., 2021). Additionally, replacement chemicals, such as perfluoroalkyl carboxylic acids and perfluorooctane sulfonamide substances, have been found to be relatively high in Singaporean water catchments (Chen et al., 2017), and their effects are otherwise poorly studied.
The present study has a few limitations that are worth noting. One limitation is the fact that we relied on observed pregnancy outcomes and could not further assess associations with reproductive hormones as a potential mechanism as hormone measures are not available in this cohort. However, efforts are underway in this and other deeply-phenotyped cohorts to investigate potential underlying mechanisms. Additionally, our PFAS assessment was targeted to PFAS that have been detected at high concentrations in other populations (Chang et al., 2021; Christensen et al., 2019) and other Singaporean pregnancy cohorts (Sum et al., 2022), but PFAS consist of a particularly large class of chemicals and many emerging PFAS were not studied. Another limitation is that only a subset of women from the parent cohort study were selected for the PFAS assessment which may increase chance of selection bias; to address this limitation we used IPW in statistical analyses to provide generalizable results for the parent cohort study. Notably, we also calculated TTP using self-reported data on the minimum and maximum menstrual cycle lengths for each woman, which could potentially be subject to recall bias. Lastly, we were unable to account for male PFAS exposure and male infertility history in our analyses. Future studies in other populations are needed to elucidate the potential impacts of PFAS mixtures on fertility in women, considering also other emerging PFAS, and underlying mechanisms.
4.1. Conclusions
In conclusion, we found suggestive evidence that higher plasma PFAS concentrations measured at preconception (individual PFAS and as a mixture) are associated with a reduced likelihood of clinical pregnancy over a year of follow-up and live birth in women from Singapore. We observed stronger associations for PFAS as a mixture, suggesting a reduction in the likelihood of clinical pregnancy and live birth per quartile PFAS mixture increment, with PFDA, PFOS, PFOA, and PFHpA being the biggest contributors to these associations. The present study makes a valuable contribution to the literature because it is a prospective population-based preconception study of Asian women aiming to conceive naturally in Singapore, which may be more susceptible to infertility, and considered potential PFAS mixture effects. Future investigation is essential to better understand the potential effects and modifiable mechanisms of PFAS on female infertility and inform potential public health or clinical interventions.
Supplementary Material
Highlights.
We investigated associations between PFAS concentrations and fertility outcomes in a cohort of women residing in Singapore.
Study findings show that higher PFAS exposures are associated with decreased fertility in women.
PFDA followed by PFOS, PFOA, and PFHpA were the biggest contributors to the PFAS mixture associations with fertility outcomes.
Acknowledgements
We are grateful to all S-PRESTO cohort participants and study staff for their efforts. The S-PRESTO study group includes: Airu Chia, Anna Magdalena Fogel, Anne Eng Neo Goh, Anne Hin Yee Chu, Anne Rifkin-Graboi, Anqi Qiu, Bee Wah Lee, Bernard Su Min Chern, Bobby Kyungbeom Cheon, Candida Vaz, Christiani Jeyakumar Henry, Ciaran Gerard Forde, Claudia Chi, Dawn Xin Ping Koh, Desiree Y. Phua, Doris Ngiuk Lan Loh, Elaine Phaik Ling Quah, Elizabeth Huiwen Tham, Evelyn Chung Ning Law, Evelyn Xiu Ling Loo, Fabian Kok Peng Yap, Faidon Magkos, Falk Müller-Riemenschneider, George Seow Heong Yeo, Hannah Ee Juen Yong, Helen Yu Chen, Heng Hao Tan, Hong Pan, Hugo P S van Bever, Hui Min Tan, Ives Yubin Lim, Izzuddin Bin Mohd Aris, Jeannie Tay, Jerry Kok Yen Chan, Jia Xu, Joanne Su-Yin Yoong, Johan Gunnar Eriksson, Jonathan Tze Liang Choo, Jonathan Y. Bernard, Jonathan Yinhao Huang, Jun Shi Lai, Karen Mei Ling Tan, Keith M. Godfrey, Kenneth Yung Chiang Kwek, Keri McCrickerd, Kok Hian Tan, Kok Wee Chong, Kothandaraman Narasimhan, Kuan Jin Lee, Li Chen, Lieng Hsi Ling, Ling-Wei Chen, Lourdes Mary Daniel, Lynette Pei-Chi Shek, Marielle V. Fortier, Mary Foong-Fong Chong, Mei Chien Chua, Melvin Khee-Shing Leow, Michael J. Meaney, Michelle Zhi Ling Kee, Min Gong, Mya Thway Tint, Navin Michael, Neerja Karnani, Ngee Lek, Oon Hoe Teoh, Peter David Gluckman, Priti Mishra, Queenie Ling Jun Li, Sambasivam Sendhil Velan, See Ling Loy, Seng Bin Ang, Sharon Ng, Shiao-Yng Chan, Shirong Cai, Shu-E Soh, Si Hui Goh, Sok Bee Lim, Stella Tsotsi, Stephen Chin-Ying Hsu , Sue-Anne Ee Shiow Toh, Suresh Anand Sadananthan, Teng Hong Tan, Tong Wei Yew, Varsha Gupta, Victor Samuel Rajadurai, Wee Meng Han, Wei Wei Pang, Wen Lun Yuan, Yanan Zhu, Yap Seng Chong, Yin Bun Cheung, Yiong Huak Chan, Yung Seng Lee, Zai Ru Cheng. Data are available through reasonable request to the S-PRESTO Executive Committee. Details on how to apply as well as summaries of available data can be found at: https://gustodatavault.sg/policies. Further questions can be direction to jonathan_huang@sics.a-star.edu.sg.
Funding:
This work is supported by the National Research Foundation (NRF) under the Open Fund-Large Collaborative Grant (grant number MOH-000504) administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC) and the Agency for Science, Technology and Research (A*STAR); and the US National Institute of Environmental Health Sciences-NIEHS (grant numbers U2CES026561, P30ES023515).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interests
The authors do not have any competing interests to declare.
References
- Baluyot JC, Reyes EM, Velarde MC. Per- and polyfluoroalkyl substances (PFAS) as contaminants of emerging concern in Asia’s freshwater resources. Environ Res 2021; 197: 111122. [DOI] [PubMed] [Google Scholar]
- Barrett ES, Chen C, Thurston SW, Haug LS, Sabaredzovic A, Fjeldheim FN, et al. Perfluoroalkyl substances and ovarian hormone concentrations in naturally cycling women. Fertil Steril 2015; 103: 1261–70.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao XY, Liu J, Zhang YJ, Wang Y, Xiong JW, Wu J, et al. Exposure of adult mice to perfluorobutanesulfonate impacts ovarian functions through hypothyroxinemia leading to down-regulation of Akt-mTOR signaling. Chemosphere 2020; 244: 125497. [DOI] [PubMed] [Google Scholar]
- Chang CJ, Ryan PB, Smarr MM, Kannan K, Panuwet P, Dunlop AL, et al. Serum per- and polyfluoroalkyl substance (PFAS) concentrations and predictors of exposure among pregnant African American women in the Atlanta area, Georgia. Environ Res 2021; 198: 110445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaparro-Ortega A, Betancourt M, Rosas P, Vázquez-Cuevas FG, Chavira R, Bonilla E, et al. Endocrine disruptor effect of perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) on porcine ovarian cell steroidogenesis. Toxicol In Vitro 2018; 46: 86–93. [DOI] [PubMed] [Google Scholar]
- Chen H, Reinhard M, Nguyen TV, You L, He Y, Gin KY. Characterization of occurrence, sources and sinks of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in a tropical urban catchment. Environ Pollut 2017; 227: 397–405. [DOI] [PubMed] [Google Scholar]
- Christensen KY, Raymond M, Meiman J. Perfluoroalkyl substances and metabolic syndrome. Int J Hyg Environ Health 2019; 222: 147–153. [DOI] [PubMed] [Google Scholar]
- Coggan TL, Anumol T, Pyke J, Shimeta J, Clarke BO. A single analytical method for the determination of 53 legacy and emerging per- and polyfluoroalkyl substances (PFAS) in aqueous matrices. Anal Bioanal Chem 2019; 411: 3507–3520. [DOI] [PubMed] [Google Scholar]
- Colicino E, Pedretti NF, Busgang SA, Gennings C. Per- and poly-fluoroalkyl substances and bone mineral density: Results from the Bayesian weighted quantile sum regression. Environ Epidemiol 2020; 4: e092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DR. Regression models and life- tables. Journal of the Royal Statistical Society: Series B (Methodological) 1972; 34: 187–202. [Google Scholar]
- CTQ. AMAP: AMAP Ring Test for Persistent Organic Pollutants in Human Serum. The Centre de toxicologie du Québec, Quebec, Canada, 2021. [Google Scholar]
- Curtzwiler GW, Silva P, Hall A, Ivey A, Vorst K. Significance of Perfluoroalkyl Substances (PFAS) in Food Packaging. Integr Environ Assess Manag 2021; 17: 7–12. [DOI] [PubMed] [Google Scholar]
- Domingo JL, Nadal M. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature. Environ Res 2019; 177: 108648. [DOI] [PubMed] [Google Scholar]
- Fallahzadeh H, Zareei Mahmood Abadi H, Momayyezi M, Malaki Moghadam H, Keyghobadi N. The comparison of depression and anxiety between fertile and infertile couples: A meta-analysis study. Int J Reprod Biomed 2019; 17: 153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Lipworth L, Olsen J. Maternal levels of perfluorinated chemicals and subfecundity. Hum Reprod 2009; 24: 1200–5. [DOI] [PubMed] [Google Scholar]
- Feng X, Wang X, Cao X, Xia Y, Zhou R, Chen L. Chronic Exposure of Female Mice to an Environmental Level of Perfluorooctane Sulfonate Suppresses Estrogen Synthesis Through Reduced Histone H3K14 Acetylation of the StAR Promoter Leading to Deficits in Follicular Development and Ovulation. Toxicol Sci 2015; 148: 368–79. [DOI] [PubMed] [Google Scholar]
- Göen T, Schaller KH, Drexler H. External quality assessment of human biomonitoring in the range of environmental exposure levels. Int J Hyg Environ Health 2012; 215: 229–32. [DOI] [PubMed] [Google Scholar]
- González F Inflammation in Polycystic Ovary Syndrome: underpinning of insulin resistance and ovarian dysfunction. Steroids 2012; 77: 300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzke D, Olsson E, Posner S. Perfluoroalkyl and polyfluoroalkyl substances (PFASs) in consumer products in Norway - a pilot study. Chemosphere 2012; 88: 980–7. [DOI] [PubMed] [Google Scholar]
- Holesh JE, Bass AN, Lord M. Physiology, Ovulation. StatPearls. StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC., Treasure Island (FL), 2022. [PubMed] [Google Scholar]
- Huang JY, Cai S, Huang Z, Tint MT, Yuan WL, Aris IM, et al. Analyses of child cardiometabolic phenotype following assisted reproductive technologies using a pragmatic trial emulation approach. Nat Commun 2021a; 12: 5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Loy SL, Chen WQ, Eriksson JG, Chong YS, Huang Z, et al. Retinal microvasculature and time to pregnancy in a multi-ethnic pre-conception cohort in Singapore. Hum Reprod 2021b; 36: 2935–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Kalathil AA, Patel AM, Ye X, Calafat AM. Per- and polyfluoroalkyl substances and fluorinated alternatives in urine and serum by on-line solid phase extraction-liquid chromatography-tandem mass spectrometry. Chemosphere 2018; 209: 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999–2008. Environ Sci Technol 2011; 45: 8037–45. [DOI] [PubMed] [Google Scholar]
- Kingsley SL, Eliot MN, Kelsey KT, Calafat AM, Ehrlich S, Lanphear BP, et al. Variability and predictors of serum perfluoroalkyl substance concentrations during pregnancy and early childhood. Environ Res 2018; 165: 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotthoff M, Müller J, Jürling H, Schlummer M, Fiedler D. Perfluoroalkyl and polyfluoroalkyl substances in consumer products. Environ Sci Pollut Res Int 2015; 22: 14546–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Chen Y, Wang Q, Chen H, Cui C, Xu X, et al. Prevalence and associated factors of infertility among 20–49 year old women in Henan Province, China. Reprod Health 2021; 18: 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo EXL, Soh SE, Loy SL, Ng S, Tint MT, Chan SY, et al. Cohort profile: Singapore Preconception Study of Long-Term Maternal and Child Outcomes (S-PRESTO). Eur J Epidemiol 2021; 36: 129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy SL, Cheung YB, Soh SE, Ng S, Tint MT, Aris IM, et al. Female adiposity and time-to-pregnancy: a multiethnic prospective cohort. Hum Reprod 2018; 33: 2141–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo K, Liu X, Zhou W, Nian M, Qiu W, Yang Y, et al. Preconception exposure to perfluoroalkyl and polyfluoroalkyl substances and couple fecundity: A couple-based exploration. Environ Int 2022; 170: 107567. [DOI] [PubMed] [Google Scholar]
- Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med 2012; 9: e1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian C, Souter I, Gaskins AJ, Williams PL, Ford JB, Chiu YH, et al. Urinary phthalate metabolites and ovarian reserve among women seeking infertility care. Hum Reprod 2016; 31: 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JE, Ahn SH, Monsanto SP, Khalaj K, Koti M, Tayade C. Implications of immune dysfunction on endometriosis associated infertility. Oncotarget 2017; 8: 7138–7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed Rasheed HA, Hamid P. Inflammation to Infertility: Panoramic View on Endometriosis. Cureus 2020; 12: e11516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. Half-life of serum elimination of perfluorooctanesulfonate,perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 2007; 115: 1298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez Carnero A, Lestido-Cardama A, Vazquez Loureiro P, Barbosa-Pereira L, Rodríguez Bernaldo de Quirós A, Sendón R. Presence of Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) in Food Contact Materials (FCM) and Its Migration to Food. Foods 2021; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagen WK, Ellefson ME, Kannan K, Giesy JP. Comparison of extraction and quantification methods of perfluorinated compounds in human plasma, serum, and whole blood. Anal Chim Acta 2008; 628: 214–21. [DOI] [PubMed] [Google Scholar]
- Reed BG, Carr BR. The Normal Menstrual Cycle and the Control of Ovulation. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, et al. , editors. https://europepmc.org/article/nbk/nbk279054 [PubMed] [Google Scholar]
- Rickard BP, Rizvi I, Fenton SE. Per- and poly-fluoroalkyl substances (PFAS) and female reproductive outcomes: PFAS elimination, endocrine-mediated effects, and disease. Toxicology 2022; 465: 153031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadecki E, Weaver A, Zhao Y, Stewart EA, Ainsworth AJ. Fertility trends and comparisons in a historical cohort of US women with primary infertility. Reprod Health 2022; 19: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildroth S, Rodgers KM, Strynar M, McCord J, Poma G, Covaci A, et al. Per-and polyfluoroalkyl substances (PFAS) and persistent chemical mixtures in dust from U.S. colleges. Environ Res 2022; 206: 112530. [DOI] [PubMed] [Google Scholar]
- Singapore. Census 2020. In: Statistics, editor, 2020. [Google Scholar]
- Singh AK, Dutta M, Chattopadhyay R, Chakravarty B, Chaudhury K. Intrafollicular interleukin-8, interleukin-12, and adrenomedullin are the promising prognostic markers of oocyte and embryo quality in women with endometriosis. J Assist Reprod Genet 2016; 33: 1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan Development Team. RStan: the R interface to Stan, 2020.
- Sum KK, Tint MT, Aguilera R, Dickens BSL, Choo S, Ang LT, et al. The socioeconomic landscape of the exposome during pregnancy. Environ Int 2022; 163: 107205. [DOI] [PubMed] [Google Scholar]
- Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol 2019; 29: 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susmann HP, Schaider LA, Rodgers KM, Rudel RA. Dietary Habits Related to Food Packaging and Population Exposure to PFASs. Environ Health Perspect 2019; 127: 107003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkun I, Cetinarslan B, Türemen E, Cantürk Z, Biyikli M. Association between Circulating Tumor Necrosis Factor-Alpha, Interleukin-6, and Insulin Resistance in Normal-Weight Women with Polycystic Ovary Syndrome. Metab Syndr Relat Disord 2006; 4: 122–8. [DOI] [PubMed] [Google Scholar]
- Thomsen AM, Riis AH, Olsen J, Jönsson BA, Lindh CH, Hjollund NH, et al. Female exposure to phthalates and time to pregnancy: a first pregnancy planner study. Hum Reprod 2017; 32: 232–238. [DOI] [PubMed] [Google Scholar]
- Tranfo G, Caporossi L, Paci E, Aragona C, Romanzi D, De Carolis C, et al. Urinary phthalate monoesters concentration in couples with infertility problems. Toxicol Lett 2012; 213: 15–20. [DOI] [PubMed] [Google Scholar]
- van der Veen I, Hanning AC, Stare A, Leonards PEG, de Boer J, Weiss JM. The effect of weathering on per- and polyfluoroalkyl substances (PFASs) from durable water repellent (DWR) clothing. Chemosphere 2020; 249: 126100. [DOI] [PubMed] [Google Scholar]
- van der Veen I, Schellenberger S, Hanning AC, Stare A, de Boer J, Weiss JM, et al. Fate of Per- and Polyfluoroalkyl Substances from Durable Water-Repellent Clothing during Use. Environ Sci Technol 2022; 56: 5886–5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Borght M, Wyns C. Fertility and infertility: Definition and epidemiology. Clin Biochem 2018; 62: 2–10. [DOI] [PubMed] [Google Scholar]
- Velez LM, Seldin M, Motta AB. Inflammation and reproductive function in women with polycystic ovary syndrome†. Biol Reprod 2021; 104: 1205–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vélez MP, Arbuckle TE, Fraser WD. Maternal exposure to perfluorinated chemicals and reduced fecundity: the MIREC study. Hum Reprod 2015; 30: 701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard S, Nielsen F, Andersson AM, Hjøllund NH, Grandjean P, Andersen HR, et al. Association between perfluorinated compounds and time to pregnancy in a prospective cohort of Danish couples attempting to conceive. Hum Reprod 2012; 27: 873–80. [DOI] [PubMed] [Google Scholar]
- Vu M, Nguyen A, Alur-Gupta S. Asian Americans and Infertility: Genetic Susceptibilities, Sociocultural Stigma and Access to Care. F&S Reports 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Zhang R, Jin F, Lou H, Mao Y, Zhu W, et al. Perfluoroalkyl substances and endometriosis-related infertility in Chinese women. Environ Int 2017; 102: 207–212. [DOI] [PubMed] [Google Scholar]
- Wang W, Hong X, Zhao F, Wu J, Wang B. The effects of perfluoroalkyl and polyfluoroalkyl substances on female fertility: A systematic review and meta-analysis. Environ Res 2023; 216: 114718. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhou W, Wu S, Liang F, Li Y, Zhang J, et al. Perfluoroalkyl substances exposure and risk of polycystic ovarian syndrome related infertility in Chinese women. Environ Pollut 2019; 247: 824–831. [DOI] [PubMed] [Google Scholar]
- Whitworth KW, Haug LS, Baird DD, Becher G, Hoppin JA, Skjaerven R, et al. Perfluorinated compounds and subfecundity in pregnant women. Epidemiology 2012; 23: 257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LA, Willis SK, Mikkelsen EM, Wesselink AK, Sørensen HT, Rothman KJ, et al. The Association between Seafood Intake and Fecundability: Analysis from Two Prospective Studies. Nutrients 2020; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Fletcher T, Pineda D, Lindh CH, Nilsson C, Glynn A, et al. Serum Half-Lives for Short- and Long-Chain Perfluoroalkyl Acids after Ceasing Exposure from Drinking Water Contaminated by Firefighting Foam. Environ Health Perspect 2020; 128: 77004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M S-PRESTO_Fertility, 2023. https://github.com/DaniaValvi/S-PRESTO_Fertility.
- Yusuf L. Depression, anxiety and stress among female patients of infertility; A case control study. Pak J Med Sci 2016; 32: 1340–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Zhang L, Tong C, Fang F, Zhao S, Tian Y, et al. Plasma Perfluoroalkyl and Polyfluoroalkyl Substances Concentration and Menstrual Cycle Characteristics in Preconception Women. Environ Health Perspect 2017; 125: 067012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


