This systematic review is the final update of a living systematic review on the use of masks for prevention of SARS-CoV-2 infection in health care and community settings. The authors summarize changes from the last update and the cumulation of randomized and observational evidence on use of masks for the prevention of COVID-19.
Abstract
Background:
Optimal use of masks for preventing COVID-19 is unclear.
Purpose:
To update an evidence synthesis on N95, surgical, and cloth mask effectiveness in community and health care settings for preventing SARS-CoV-2 infection.
Data Sources:
MEDLINE, EMBASE, medRxiv (3 June 2022 to 2 January 2023), and reference lists.
Study Selection:
Randomized trials of interventions to increase mask use and risk for SARS-CoV-2 infection and observational studies of mask use that controlled for potential confounders.
Data Extraction:
Two investigators sequentially abstracted study data and rated quality.
Data Synthesis:
Three randomized trials and 21 observational studies were included. In community settings, mask use may be associated with a small reduced risk for SARS-CoV-2 infection versus no mask use, on the basis of 2 randomized trials and 7 observational studies. In routine patient care settings, surgical masks and N95 respirators may be associated with similar risk for SARS-CoV-2 infection, on the basis of 1 new randomized trial with some imprecision and 4 observational studies. Evidence from observational studies was insufficient to evaluate other mask comparisons due to methodological limitations and inconsistency.
Limitation:
Few randomized trials, studies had methodological limitations and some imprecision, suboptimal adherence and pragmatic aspects of randomized trials potentially attenuated benefits, very limited evidence on harms, uncertain applicability to Omicron variant predominant era, meta-analysis not done due to heterogeneity, unable to formally assess for publication bias, and restricted to English-language articles.
Conclusion:
Updated evidence suggests that masks may be associated with a small reduction in risk for SARS-CoV-2 infection in community settings. Surgical masks and N95 respirators may be associated with similar infection risk in routine patient care settings, but a beneficial effect of N95 respirators cannot be ruled out.
Primary Funding Source:
None.
Preventive measures, including use of respiratory protective devices (“masks”), are recommended to reduce risk for COVID-19, the disease caused by SARS-CoV-2 infection. Several types of masks (N95 respirators, surgical masks, and cloth masks) are available, with variability in filtration efficacy, fluid resistance, and fit (1). Factors affecting SARS-CoV-2 transmission and potentially affecting mask effectiveness include viral transmission levels, circulating variants, degree of immunity, behaviors (for example, use of other personal protective equipment and infection control measures), and exposures in different settings (for example, home, community, or workplace [including health care settings]). In June 2020, we published the first version of a living, rapid review on masks and risk for SARS-CoV-2 and other respiratory infections (2). We found insufficient evidence to determine effects of masks on SARS-CoV-2 infection, on the basis of 2 observational studies. Observational evidence suggested an association between mask use versus nonuse and reduced SARS-CoV-1 infection risk in community settings and an association between N95s versus surgical masks and reduced SARS-CoV-1 infection risk in health care settings, but the studies had methodological limitations. For influenza or influenza-like illness, randomized controlled trials (RCTs) indicated probably no difference between surgical versus no mask in community settings, although adherence was low, and probably similar effects of N95 and surgical masks in health care settings.
We subsequently published 8 updates (last search completed 2 June 2022 [3]), with low to moderate strength evidence for an association between mask use and decreased risk for SARS-CoV-2 infection in community settings based on 2 RCTs and 10 observational studies. The RCTs differed from observational studies by evaluating interventions aimed at increasing mask use, rather than outcomes associated with actual (self-reported) mask use. Evidence on N95 versus surgical masks in health care settings and risk for SARS-CoV-2 infection remained insufficient on the basis of observational studies with methodological limitations and inconsistency.
While conducting update 8, we were aware of a completed RCT of N95 versus surgical masks and planned a final update after its publication (4). The purpose of this update is to incorporate this RCT and other new studies. This update focuses on SARS-CoV-2 infection, given increased evidence availability and uncertain applicability of non–SARS-CoV-2 infections. Due to a change in conclusions, this report met criteria for a major update (5).
Methods
The key questions for the initial review were developed with input from staff at the American College of Physicians and the Agency for Healthcare Research and Quality (AHRQ). The protocol was posted on the AHRQ Effective Health Care Program website (6). From the original protocol, we removed key questions on prevention of influenza or influenza-like illness, SARS-CoV-1, and Middle East respiratory syndrome coronavirus. To focus on higher-quality evidence, we previously (7) modified the protocol to exclude observational studies that did not control for confounders, studies relying solely on self-report for SARS-CoV-2 infection diagnosis, and non–peer-reviewed studies, unless data were collected after February 2021 (Delta and Omicron variant predominant period). We also decreased the frequency of updates and replaced rapid with standard systematic review methods, including dual abstract review, formal critical appraisal of observational studies, and dual critical appraisal and data abstraction. Supplement Table 1 describes the revised inclusion criteria and protocol modifications. This final update was triggered by the publication of a new RCT comparing masks types in health care settings (4). Although no further updates are planned, additional updates may be warranted by the publication of new RCTs that could affect review findings. The key questions were:
Key question 1: What is the effectiveness and comparative effectiveness of respirators (N95 or equivalent), face masks (surgical), and cloth masks in addition to standard precautions in community and health care (high- or non–high-risk) settings for prevention of SARS-CoV-2 infection?
Key question 2: What is the evidence for extended or reuse of N95 respirators for prevention of SARS-CoV-2 infection?
Data Sources and Searches
Searches for this update were done from 3 June 2022 to 2 January 2023 on PubMed, MEDLINE, and Elsevier EMBASE using the same search strategies (Supplement Table 2) as the original review. We also searched medRxiv and reviewed reference lists of relevant articles.
Study Selection
We selected studies using the criteria described in Supplement Table 1. The population was health care workers (HCWs) and persons in the community. Interventions were N95 (or equivalent) filtering facepiece respirators, surgical masks, and cloth masks. We included RCTs, cohort studies, and case–control studies on mask use versus no mask use, different mask types, consistency of mask use, and reuse or extended versus standard mask use. Outcomes were SARS-CoV-2 infection, on the basis of laboratory testing or meeting clinical criteria for COVID-19, and harms. We applied revised eligibility criteria to previously included studies. We excluded ecological studies and studies on mask policies without information on individual mask use (reviewed elsewhere [8]) and restricted inclusion to English-language articles.
One investigator reviewed each citation for potential full-text review and reviewed each full-text article for inclusion. A second investigator verified exclusion decisions at both the citation and full-text level; disagreements were resolved through consensus.
Quality Assessment
Study quality was assessed using criteria adapted from the U.S. Preventive Services Task Force (9).
Data Synthesis and Analysis
Meta-analysis was not done due to study design variability; methodological limitations; and differences in study populations, comparisons, outcome definitions, and settings. For cluster randomized trials, we reported risk estimates adjusted for cluster effects when available (10). For observational studies, we reported adjusted risk estimates except when mask use was not included in the model selection process, in which case we reported the univariate risk estimate and noted exclusion from the multivariate model. We synthesized evidence separately for community and health care settings and separately for RCTs and observational studies. For mask use versus nonuse, we evaluated evidence for any or unspecified mask use and for specific mask types. We examined how findings differed when poor-quality studies or studies of SARS-CoV-2 infection based solely on seropositivity were excluded. We did not formally assess for publication bias using graphical or statistical methods due to few RCTs and methodological limitations and heterogeneity in the observational studies (11). We updated a previously developed evidence map showing the strength of evidence and effect direction for each mask comparison and setting. The strength of evidence was classified as high, moderate, low, or insufficient on the basis of study design, quality, inconsistency, indirectness, and imprecision (12). This is our final planned update.
Role of the Funding Source
Funding for the initial review and 2 updates was from AHRQ; after update 2, no additional funding was received. AHRQ had no role in subsequent updates and was not involved in the decision to submit this article for publication.
Results
Literature searches for the initial review and updates identified a total of 9694 citations, including 1498 for this update (Figure 1). Three RCTs (Table) (4, 13, 14) and 21 observational studies (in 22 publications [Supplement Table 3]) (15–36) were included in this update (Figure 1). The prior update (3) included 2 RCTs (both done in community settings) (13, 14) and 24 observational studies (15, 16, 20, 23–29, 31–44). Eight prior observational studies did not meet revised inclusion criteria because they relied on self-reported SARS-CoV-2 infection (2 studies) (40, 42), did not control for confounders (5 studies) (37–39, 41, 43), or evaluated mask policies (1 study) (44), leaving 15 prior observational studies (8 in community settings [16, 24–26, 29, 33, 34, 36] and 7 in health care settings [15, 20, 23, 27, 28, 31, 32, 35]). For this update, we added 1 new RCT of N95 versus surgical masks in health care settings (4) and 6 new observational studies (2 in community settings [17, 22] and 4 in health care settings [18, 19, 21, 30]).
Figure 1. Literature flow diagram.
ILI = influenza-like illness; MERS-CoV = Middle East respiratory syndrome coronavirus; VRI = viral respiratory illness.
* Includes 37 studies of SARS-CoV-1, MERS-CoV, influenza, ILI, or VRI.
† Not addressed in this update.
Table.
Study Characteristics of RCTs of Mask Use
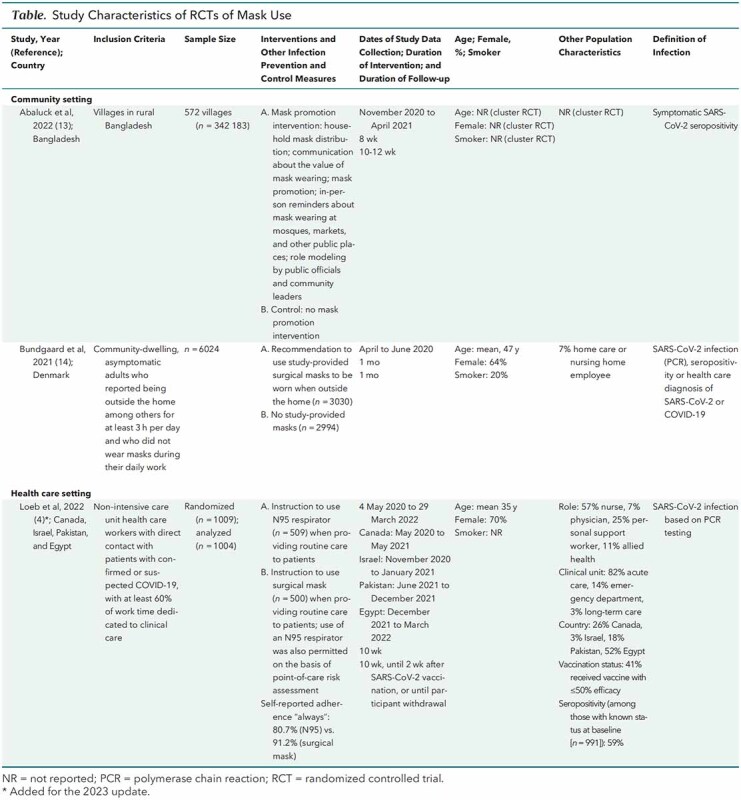
All RCTs were open label because blinding to mask use or type was not possible. One RCT (13, 14) had incomplete outcomes assessment and differential recruitment, and 1 RCT (4) reported several protocol changes (Supplement Table 4). Methodological shortcomings in the observational studies included unclear or low participation rate, potential recall bias, failure to report attrition or missing data, and potential residual confounding due to incomplete assessment of SARS-CoV-2 exposures and infection control measures (Supplement Table 5). Key question 1 study results are summarized in Supplement Table 6. No study evaluated extended or reuse of N95 respirators (key question 2).
Community Settings
We identified no new studies of mask use versus nonuse. In previous updates, 2 RCTs (13, 14) and 10 observational studies (16, 25, 26, 29, 33, 34, 36, 40, 42, 43) provided low to moderate strength evidence for mask use (any or unspecified type) versus nonuse and decreased SARS-CoV-2 infection risk in community settings. One good-quality Danish RCT (n = 6024) found a mask use recommendation associated with a small, nonstatistically significant reduction in SARS-CoV-2 infection risk (on the basis of antibody testing, polymerase chain reaction [PCR], or hospital diagnosis) at 1 month (1.8% vs. 2.1%; odds ratio [OR], 0.82 [95% CI, 0.54 to 1.23]) (14). There were no differences in mask effects based on age (≤48 vs. >48 years), sex, or daily time outside the home (≤4.5 vs. >4.5 hours). The RCT was not designed to assess masks as source control. In addition, mask adherence was suboptimal (46% as recommended, 47% predominantly as recommended), and high implementation of other infection control measures could have attenuated benefits. A large (n > 340 000), fair-quality cluster randomized trial done in Bangladesh found villages randomly assigned to a community-level intervention to promote mask use associated with decreased risk for symptomatic SARS-CoV-2 seroprevalence (0.68% vs. 0.76%; adjusted prevalence ratio, 0.90 [CI, 0.82 to 0.995]) and COVID-19 symptoms prevalence (7.63% vs. 8.60%; adjusted prevalence ratio, 0.88 [CI, 0.83 to 0.93]) versus control villages at 10 to 12 weeks (13). Mask use was 42% in intervention and 13% in control villages. Effects were slightly greater for surgical masks (adjusted prevalence ratio, 0.89 [CI, 0.78 to 0.997]) than cloth masks (adjusted prevalence ratio, 0.94 [CI, 0.78 to 1.10]), but CIs overlapped. Benefits of masks increased with older age in surgical mask villages (adjusted prevalence ratio, 0.65 [CI, 0.45 to 0.84] for persons ≥60 years and 0.97 [CI, 0.83 to 1.10] for persons <40 years) but not in cloth mask villages. Methodological limitations included failure to perform serologic testing in 60% of symptomatic patients (proportion similar in intervention and control villages) and slightly higher recruitment in intervention than control villages.
Of 10 prior observational studies, 9 found mask use (any or unspecified type) associated with decreased SARS-CoV-2 infection versus nonuse (adjusted risk estimates ranged from 0.04 to 0.86). The 10th study reported an imprecise estimate not favoring mask use (OR, 2.3 [CI, 0.67 to 8.25]) (43). That study and 2 others (40, 42) did not meet revised inclusion criteria. In 7 remaining studies, adjusted risk estimates ranged from 0.10 to 0.60 (16, 25, 26, 29, 33, 34, 36). Four studies used a case–control design, 2 were cross-sectional, and 1 was a retrospective cohort. In 4 studies, participants had community (25, 33, 34) or household (36) contact with COVID-19 cases; 1 study evaluated persons returning from high-prevalence countries (29), and 2 studies selected patients who had laboratory testing (16, 26). Mask use comparisons (for example, mask use vs. no mask use, mask use when outdoors vs. no mask, or mask use when exposed to index case vs. no mask) and SARS-CoV-2 infection definitions (PCR [5 studies]; undefined laboratory testing [1 study]; or a combination of clinical, epidemiologic, and laboratory criteria [1 study]) varied (Supplement Table 3). One case–control study of persons exposed in 3 large COVID-19 clusters was rated poor quality because of high potential for recall bias but reported results consistent with the other studies (25).
The strength of evidence remained low for reduced risk for SARS-CoV-2 infection with surgical masks versus no mask on the basis of 2 prior RCTs (adjusted prevalence ratio, 0.89 [CI, 0.78 to 0.997] [13] and OR, 0.82 [CI, 0.52 to 1.23] [14]) and 2 observational studies (16, 25) and insufficient for N95 respirators versus no mask (16) or cloth mask versus no mask (13, 16, 25) (Figure 2; Supplement Table 7). There were no new studies and insufficient evidence for surgical versus cloth masks (13, 16) and N95 versus surgical masks (16).
Figure 2. Masks for prevention of SARS-CoV-2 evidence map.
RCT = randomized controlled trial.
* N95 or equivalent/similar respirators (for example, P2, FFP2, FFP3).
† New evidence added for this update.
In previous updates, 4 observational studies evaluated more versus less consistent mask use and SARS-CoV-2 infection in community settings (16, 24, 25, 36). Although they found more consistent mask use associated with decreased SARS-CoV-2 infection risk, SARS-CoV-2 infection was based only on seropositivity in 1 study (25), and the studies had methodological limitations, providing insufficient evidence (Figure 2 and Supplement Table 7). Two new, fair-quality observational studies evaluated consistency of mask use (Supplement Table 3) (17, 22). Although 1 cross-sectional study (n = 1337; Brazil) found more consistent mask use associated with reduced SARS-CoV-2 seropositivity risk (adjusted OR, 0.30 [CI, 0.11 to 0.81]) (22), another large (n = 10 250; Germany) new cohort study did not find an association between more consistent use and decreased risk for PCR-confirmed SARS-CoV-2 infection (adjusted hazard ratio [HR], 1.27 [CI, 0.63 to 2.27]) (17). The evidence for consistency of mask use remained insufficient (Figure 2 and Supplement Table 7). No included study of masks in community settings evaluated harms.
Health Care Settings
Prior updates identified no RCTs on masks and SARS-CoV-2 infection in health care settings. Five previously included observational studies provided insufficient evidence on N95 versus surgical masks due to methodological limitations and inconsistency (risk estimates ranged from 0.60 to 7.1) (27, 31, 35, 37, 41). Three previously included studies did not adjust for confounders and were excluded from this update (35, 37, 41). The 2 remaining cohort studies were inconsistent. A study of Swiss HCWs (n = 3259) found “mostly” FFP2 respirator use associated with decreased SARS-CoV-2 seropositivity risk versus “mostly” surgical mask use after adjustment for various exposures (adjusted HR, 0.80 [CI, 0.64 to 1.00]) (27). The other study (n = 963) found FFP2 respirator use in Italian HCWs associated with a marked increased risk for PCR-confirmed SARS-CoV-2 infection, but it only adjusted for sex (adjusted OR, 7.1 [CI, 3.6 to 13.9]) (31).
One new RCT (4) and 2 new observational studies (18, 19) compared N95 versus surgical masks in health care settings. The RCT (n = 1009) was done in Canada, Israel, Pakistan, and Egypt and compared instruction to use N95 versus surgical masks in HCWs providing routine patient care (4). Surgical masks were noninferior to N95s for risk for PCR-confirmed SARS-CoV-2 infection (HR, 1.14 [CI, 0.77 to 1.69]) on the basis of a prespecified noninferiority margin of up to a doubling of risk. The trial was open label and pragmatic (for example, patients randomly assigned to surgical masks could use N95s if indicated, on the basis of point-of-care risk assessment). Self-reported adherence “all the time” was reported by 81% in the N95 group and 91% in the surgical mask group; adherence was also high in a monitored participant subset. The protocol had several modifications to expand enrollment criteria, decrease follow-up duration, account for variable duration of follow-up, and account for COVID-19 vaccination uptake; these did not seem to have biased findings. A post hoc analysis found that HRs ranged from 0.95 in Egypt to 2.83 in Canada; however, country-specific estimates were very imprecise other than for Egypt, which accounted for 74% of cases (HR, 0.95 [CI, 0.60 to 1.50]). N95 masks were associated with a nonstatistically significant increased risk for mask-related adverse events (13.6% vs. 10.8%; risk ratio, 1.25 [CI, 0.87 to 1.79]), primarily due to increased discomfort and headaches. There were few withdrawals due to adverse events (3 with N95s and 1 with surgical masks).
Two new fair-quality case–control studies (2607 and 2045 cases) from France and Canada each found N95 respirators associated with decreased risk for SARS-CoV-2 versus surgical masks, although estimates were imprecise (adjusted OR, 0.85 [CI, 0.55 to 1.29] [18] and adjusted OR, 0.6 [CI, 0.3 to 1.1] during nonaerosol generating procedures and adjusted OR [CI, 0.2 to 2.0] during aerosol generating procedures, in postvaccination period [19]). On the basis of the new RCT, the strength of evidence was changed from insufficient to low for similar effects of N95 and surgical masks when providing routine patient care in health care settings (Figure 2 and Supplement Table 7).
Prior updates found insufficient evidence to determine effects of mask use versus nonuse (5 studies [20, 31, 35, 39, 41]) or more versus less consistent mask use in health care settings (4 studies [15, 23, 28, 38]) on SARS-CoV-2 infection risk. Excluding 3 studies (38, 39, 41) not meeting revised inclusion criteria left 3 prior studies of mask use versus nonuse with inconsistent results (20, 31, 35) and 3 studies of more versus less consistent mask use in which SARS-CoV-2 infection was based on seropositivity only (15) or the estimate was very imprecise (23, 28).
A new cross-sectional study (n = 2952; Italy) found any mask use by occupational medicine unit workers associated with decreased risk for PCR-confirmed SARS-CoV-2 infection versus nonuse (adjusted OR, 0.63 [CI, 0.45 to 0.87]); findings were similar but more imprecise for FFP2 or FFP3 use versus nonuse (adjusted OR, 0.48 [CI, 0.21 to 1.09]) (21). Two new observational studies compared more versus less consistent mask use in health care settings. A case–control study (2046 cases; Canada) found always masking at work associated with a nonstatistically significant decreased risk for PCR-confirmed SARS-CoV-2 infection versus not always masking (adjusted OR, 0.6 [CI, 0.3 to 1.4]) (19), and a cohort study (n = 129; Brazil) found mask use all of the time associated with a statistically significant reduction in risk for SARS-CoV-2 seropositivity versus mask use some of the time (adjusted OR, 0.18 [CI, 0.04 to 0.85]) (30). The evidence for mask use versus nonuse and more versus less consistent mask use remained insufficient (Figure 2 and Supplement Table 7).
Discussion
This update summarizes the evidence on the effectiveness and comparative effectiveness of masks for preventing SARS-CoV-2 infection. Figure 2 is an updated evidence map showing the strength of evidence for key mask comparisons by setting (community or health care) (see also Supplement Table 7). The original review, done near the start of the COVID-19 pandemic, included only 2 observational studies on SARS-CoV-2 infection that provided insufficient evidence (2). Although evidence on other respiratory illnesses was more robust, applicability to SARS-CoV-2 was uncertain. An expanded evidence base enabled this update to focus on SARS-CoV-2 infections.
As in prior updates, our main finding for community settings was low to moderate strength evidence that mask use (any or unspecified type) may be associated with a small reduction in risk for SARS-CoV-2 infection versus no masks. Although 2 previously included RCTs each found interventions to increase mask use associated with a small reduction in risk for SARS-CoV-2 infection, interventions (a recommendation to wear masks versus a community-level mask promotion intervention) and settings (transmission rates, use of other infection control measures, socioeconomic status, and other factors) differed, and adherence was suboptimal (13, 14). In addition, 1 RCT reported imprecise estimates and did not assess masks as source control, and high uptake of other infection control measures could have attenuated benefits (14). Methodological limitations in the other RCT included differential recruitment and incomplete outcomes assessment (13). The risk reduction was around 10% to 18%, which may be important on a population level, especially when considering cumulative effects over time. Observational studies of masks versus no masks consistently found masks associated with decreased risk for SARS-CoV-2 infection but had methodological limitations and some imprecision. The evidence on surgical versus cloth masks or more versus less consistent mask use remained insufficient.
In health care settings, a new RCT found that effects of instruction to use surgical masks were noninferior to instruction to use N95 respirators for routine patient care (4). However, noninferiority was defined as less than a doubling of risk, with the CI consistent with up to a 70% increase in risk. Due to a single trial with imprecision, the strength of evidence was low. In addition, the RCT could have reported attenuated benefits of N95 respirators in the health care setting due to infections acquired in the community or home (45). Although results were similar when patients were stratified according to presence of nonwork exposures, the analysis was post hoc and based on self-reported and known exposures (4). The trial was done in 4 countries (Egypt, Canada, Pakistan, and Israel) that varied with regard to COVID-19 seroprevalence, vaccination status, Omicron predominance, personal protective equipment use, and other factors. A post hoc stratified analysis indicated potential heterogeneity by country, but estimates from all countries were imprecise except for Egypt, which accounted for nearly 75% of the SARS-CoV-2 infections. Results are likely most applicable to settings similar to Egypt (for example, Omicron-predominant, high baseline COVID-19 seroprevalence) and do not apply to situations in which routine N95 use is recommended (for example, around aerosol-generating procedures) (4). Observational studies on N95 versus surgical masks had methodological limitations and inconsistency, and evidence on other mask comparisons in health care settings remained insufficient.
Evidence on harms remained very limited. One new RCT found N95s associated with a nonstatistically significant increased risk for bothersome mask-related harms versus surgical mask, with no serious events (4). Prior RCTs did not report harms, and observational studies were not designed to evaluate harms. As detailed in the original review (2), reporting of harms in RCTs of masks and other respiratory viruses was suboptimal but did not indicate serious harms. There remains no evidence on extended or reuse of N95 respirators and risk for SARS-CoV-2 infection.
This update differs from other systematic reviews of masks (46–51) by focusing on SARS-CoV-2 infection and (due to our living review approach) being more up to date, including a key new RCT (4). For example, a recently updated systematic review concluded that wearing masks in the community probably makes little or no difference versus no masks in preventing acute respiratory viral infection but combined earlier trials of influenza-like illness or influenza with trials of SARS-CoV-2 infection, despite differences in viral circulation levels and transmission potentially affecting mask effectiveness (46). Findings in the review for N95 versus surgical masks were based only on RCTs of influenza or influenza-like illness, as the new RCT was identified too late to be included in analyses (4). In addition to focusing on SARS-CoV-2, we prospectively implemented additional protocol changes to focus on higher-quality and more relevant evidence. Application of updated and more stringent eligibility criteria resulted in exclusion of some previously included studies, which did not affect findings.
Despite focusing on higher-quality studies, the evidence base continues to have important limitations. Randomized controlled trials were few and had some imprecision and methodological shortcomings. In addition, RCTs evaluated interventions to promote or encourage mask use and were designed pragmatically, improving applicability but potentially attenuating estimated effects due to suboptimal adherence and crossover. For example, the RCT of N95 versus surgical masks permitted HCWs randomly assigned to surgical masks to use N95s (crossover) in situations perceived to be at high risk for transmission, which could have diminished the relative benefits of N95s (4). Only 1 RCT was designed to include the effects of masks to prevent SARS-CoV-2 infection acquisition and as source control (13). Observational studies were based on actual mask use but remained highly susceptible to recall bias due to reliance on self-report and confounding. Although studies of masks focused on use in specific (for example, health care, home, or community) settings, exposures may occur in multiple settings, complicating interpretation of findings (45). Studies of cloth masks often provided few details about material, number of layers, and fit (52), and some studies evaluated masks with limited or uncertain generalizability. For example, 1 RCT used specially designed washable surgical masks and cloth masks made of higher filtration materials than in commonly available commercial masks (14). In some studies, SARS-CoV-2 infection was based solely on antibody testing, which is not recommended for diagnosis of acute infection (53). Little evidence is available from the Omicron-predominant era.
The review process had limitations. We did not attempt meta-analysis owing to study methodological shortcomings and heterogeneity in study designs, comparisons, and outcomes and did not formally assess for publication bias due to heterogeneity and few studies for most comparisons. We restricted inclusion to English-language articles and excluded ecological studies and studies on mask policies that did not provide information on individual mask use, which may provide complementary information (8).
Additional research would further clarify the comparative effectiveness of masks for prevention of SARS-CoV-2 infection. Future studies should have adequate statistical power for primary as well as stratified analyses. Assessing masks as source control represent a challenge, requiring evaluation of SARS-CoV-2 infections in communities of masked and unmasked persons. Studies should use appropriate methods for diagnosing SARS-CoV-2 infection, describe key mask characteristics, evaluate adherence, and assess harms as well as benefits. Although well-conducted observational studies could supplement RCTs, susceptibility to recall bias and residual confounding represent an important limitation that would require intensive, prospective measurement of behaviors and exposures by external observers to overcome.
In conclusion, updated evidence suggests that masks may be associated with a small reduction in risk for SARS-CoV-2 infection in community settings. Surgical masks and N95 respirators may be associated with similar risk for infection in health care settings, but a beneficial effect of N95 respirators cannot be ruled out.
Supplementary Material
Footnotes
This article was published at Annals.org on 16 May 2023.
References
- 1. World Health Organization. Mask use in the context of COVID-19. Accessed at www.who.int/publications/i/item/advice-on-the-use-of-masks-in-the-community-during-home-care-and-in-healthcare-settings-in-the-context-of-the-novel-coronavirus-(2019-ncov)-outbreak on 22 February 2023.
- 2. Chou R, Dana T, Jungbauer R, et al. Masks for prevention of respiratory virus infections, including SARS-CoV-2, in health care and community settings. A living rapid review. Ann Intern Med. 2020;173:542-555. [PMID: ] doi: 10.7326/M20-3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chou R, Dana T, Jungbauer R. Update alert 8: masks for prevention of respiratory virus infections, including SARS-CoV-2, in health care and community settings [Letter]. Ann Intern Med. 2022;175:W108-W109. [PMID: ] doi: 10.7326/L22-0272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Loeb M, Bartholomew A, Hashmi M, et al. Medical masks versus N95 respirators for preventing COVID-19 among health care workers. A randomized trial. Ann Intern Med. 2022;175:1629-1638. [PMID: ] doi: 10.7326/M22-1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Laine C, Taichman DB, Guallar E, et al. Keeping up with emerging evidence in (almost) real time. Ann Intern Med. 2020;173:153-154. [PMID: ] doi: 10.7326/M20-2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Effective Health Care Program. Masks for prevention of COVID-19 in healthcare and community settings. Accessed at https://effectivehealthcare.ahrq.gov/products/masks-covid/protocol on 22 February 2023.
- 7. Chou R, Dana T, Jungbauer R. Update alert 7: masks for prevention of respiratory virus infections, including SARS-CoV-2, in health care and community settings [Letter]. Ann Intern Med. 2022;175:W58-W59. [PMID: ] doi: 10.7326/L21-0783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ford N, Holmer HK, Chou R, et al. Mask use in community settings in the context of COVID-19: a systematic review of ecological data. EClinicalMedicine. 2021;38:101024. [PMID: ] doi: 10.1016/j.eclinm.2021.101024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. U.S. Preventive Services Task Force. Criteria for assessing internal validity of individual studies. Accessed at www.uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes/procedure-manual/procedure-manual-appendix-vi-criteria-assessing-internal-validity-individual-studies on 22 February 2023.
- 10. Campbell MK, Grimshaw JM, Elbourne DR. Intracluster correlation coefficients in cluster randomized trials: empirical insights into how should they be reported. BMC Med Res Methodol. 2004;4:9. [PMID: ] doi: 10.1186/1471-2288-4-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. [PMID: ] doi: 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 12. Berkman ND, Lohr KN, Ansari MT, et al. Grading the strength of a body of evidence when assessing health care interventions: an EPC update. J Clin Epidemiol. 2015;68:1312-1324. [PMID: ] doi: 10.1016/j.jclinepi.2014.11.023 [DOI] [PubMed] [Google Scholar]
- 13. Abaluck J, Kwong LH, Styczynski A, et al. Impact of community masking on COVID-19: a cluster-randomized trial in Bangladesh. Science. 2022;375:eabi9069. [PMID: ] doi: 10.1126/science.abi9069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bundgaard H, Bundgaard JS, Raaschou-Pedersen DET, et al. Effectiveness of adding a mask recommendation to other public health measures to prevent SARS-CoV-2 infection in Danish mask wearers. A randomized controlled trial. Ann Intern Med. 2021;174:335-343. [PMID: ] doi: 10.7326/M20-6817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Akinbami LJ, Vuong N, Petersen LR, et al. SARS-CoV-2 seroprevalence among healthcare, first response, and public safety personnel, Detroit metropolitan area, Michigan, USA, May-June 2020. Emerg Infect Dis. 2020;26:2863-2871. [PMID: ] doi: 10.3201/eid2612.203764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andrejko KL, Pry JM, Myers JF, et al; California COVID-19 Case-Control Study Team. Effectiveness of face mask or respirator use in indoor public settings for prevention of SARS-CoV-2 infection - California, February-December 2021. MMWR Morb Mortal Wkly Rep. 2022;71:212-216. [PMID: ] doi: 10.15585/mmwr.mm7106e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baumkötter R, Yilmaz S, Zahn D, et al. Protective behavior and SARS-CoV-2 infection risk in the population - results from the Gutenberg COVID-19 study. BMC Public Health. 2022;22:1993. [PMID: ] doi: 10.1186/s12889-022-14310-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Belan M, Charmet T, Schaeffer L, et al. SARS-CoV-2 exposures of healthcare workers from primary care, long-term care facilities and hospitals: a nationwide matched case-control study. Clin Microbiol Infect. 2022;28:1471-1476. [PMID: ] doi: 10.1016/j.cmi.2022.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carazo S, Villeneuve J, Laliberté D, et al. Risk and protective factors for severe acute respiratory coronavirus virus 2 (SARS-CoV-2) infection among healthcare workers: a test-negative case-control study in Québec, Canada. Infect Control Hosp Epidemiol. 2022:1-10. [PMID] [PMID: ] doi: 10.1017/ice.2022.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chatterjee P, Anand T, Singh KJ, et al. Healthcare workers & SARS-CoV-2 infection in India: a case-control investigation in the time of COVID-19. Indian J Med Res. 2020;151:459-467. [PMID: ] doi: 10.4103/ijmr.IJMR_2234_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Collatuzzo G, Mansour I, Ciocan C, et al. Effectiveness of prevention of SARS-CoV-2 transmission among unvaccinated Italian healthcare workers. Med Lav. 2022;113:e2022050. [PMID: ] doi: 10.23749/mdl.v113i6.13577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. da Silva Torres MK, Lopes FT, de Lima ACR, et al. Changes in the seroprevalence and risk factors between the first and second waves of COVID-19 in a metropolis in the Brazilian Amazon. Front Cell Infect Microbiol. 2022;12:932563. [PMID: ] doi: 10.3389/fcimb.2022.932563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Davido B, Gautier S, Riom I, et al; Garches COVID-19 Collaborative Group. The first wave of COVID-19 in hospital staff members of a tertiary care hospital in the greater Paris area: a surveillance and risk factors study. Int J Infect Dis. 2021;105:172-179. [PMID: ] doi: 10.1016/j.ijid.2021.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Doernberg SB, Holubar M, Jain V, et al; CHART Study Consortium. Incidence and prevalence of coronavirus disease 2019 within a healthcare worker cohort during the first year of the severe acute respiratory syndrome coronavirus 2 pandemic. Clin Infect Dis. 2022;75:1573-1584. [PMID: ] doi: 10.1093/cid/ciac210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Doung-Ngern P, Suphanchaimat R, Panjangampatthana A, et al. Case-control study of use of personal protective measures and risk for SARS-CoV 2 infection, Thailand. Emerg Infect Dis. 2020;26:2607-2616. [PMID: ] doi: 10.3201/eid2611.203003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gonçalves MR, Dos Reis RCP, Tólio RP, et al. Social distancing, mask use, and transmission of severe acute respiratory syndrome coronavirus 2, Brazil, April-June 2020. Emerg Infect Dis. 2021;27:2135-2143. [PMID: ] doi: 10.3201/eid2708.204757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haller S, Güsewell S, Egger T, et al. Impact of respirator versus surgical masks on SARS-CoV-2 acquisition in healthcare workers: a prospective multicentre cohort. Antimicrob Resist Infect Control. 2022;11:27. [PMID: ] doi: 10.1186/s13756-022-01070-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Howard-Anderson JR, Adams C, Dube WC, et al. Occupational risk factors for severe acute respiratory coronavirus virus 2 (SARS-CoV-2) infection among healthcare personnel: a 6-month prospective analysis of the COVID-19 Prevention in Emory Healthcare Personnel (COPE) Study. Infect Control Hosp Epidemiol. 2022;43:1664-1671. [PMID: ] doi: 10.1017/ice.2021.518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lio CF, Cheong HH, Lei CI, et al. Effectiveness of personal protective health behaviour against COVID-19. BMC Public Health. 2021;21:827. [PMID: ] doi: 10.1186/s12889-021-10680-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Madureira R, Ferreira SA, Marion MAL, et al. Seroprevalence of SARS-CoV-2 in emergency department healthcare workers at Sírio-Libanês hospital, Brazil. Health Secur. 2022;20:359-367. [PMID: ] doi: 10.1089/hs.2022.0045 [DOI] [PubMed] [Google Scholar]
- 31. Piapan L, De Michieli P, Ronchese F, et al. COVID-19 outbreak in healthcare workers in hospitals in Trieste, North-east Italy. J Hosp Infect. 2020;106:626-628. [PMID: ] doi: 10.1016/j.jhin.2020.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Piapan L, De Michieli P, Ronchese F, et al. COVID-19 outbreaks in hospital workers during the first COVID-19 wave. Occup Med (Lond). 2022;72:110-117. [PMID: ] doi: 10.1093/occmed/kqab161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rebmann T, Loux TM, Arnold LD, et al. SARS-CoV-2 transmission to masked and unmasked close contacts of university students with COVID-19 - St. Louis, Missouri, January-May 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1245-1248. [PMID: ] doi: 10.15585/mmwr.mm7036a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sugimura M, Chimed-Ochir O, Yumiya Y, et al. The association between wearing a mask and COVID-19. Int J Environ Res Public Health. 2021;18. [PMID: ] doi: 10.3390/ijerph18179131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Venugopal U, Jilani N, Rabah S, et al. SARS-CoV-2 seroprevalence among health care workers in a New York City hospital: a cross-sectional analysis during the COVID-19 pandemic. Int J Infect Dis. 2021;102:63-69. [PMID: ] doi: 10.1016/j.ijid.2020.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang Y, Tian H, Zhang L, et al. Reduction of secondary transmission of SARS-CoV-2 in households by face mask use, disinfection and social distancing: a cohort study in Beijing, China. BMJ Glob Health. 2020;5. [PMID: ] doi: 10.1136/bmjgh-2020-002794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fletcher JJ, Feucht EC, Hahn PY, et al. Healthcare-acquired coronavirus disease 2019 (COVID-19) is less symptomatic than community-acquired disease among healthcare workers. Infect Control Hosp Epidemiol. 2022;43:490-496. [PMID: ] doi: 10.1017/ice.2021.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Heinzerling A, Stuckey MJ, Scheuer T, et al. Transmission of COVID-19 to health care personnel during exposures to a hospitalized patient - Solano County, California, February 2020. MMWR Morb Mortal Wkly Rep. 2020;69:472-476. [PMID: ] doi: 10.15585/mmwr.mm6915e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Khalil MM, Alam MM, Arefin MK, et al. Role of personal protective measures in prevention of COVID-19 spread among physicians in Bangladesh: a multicenter cross-sectional comparative study. SN Compr Clin Med. 2020;2:1733-1739. [PMID: ] doi: 10.1007/s42399-020-00471-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sharif N, Alzahrani KJ, Ahmed SN, et al. Protective measures are associated with the reduction of transmission of COVID-19 in Bangladesh: a nationwide cross-sectional study. PLoS One. 2021;16:e0260287. [PMID: ] doi: 10.1371/journal.pone.0260287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sims MD, Maine GN, Childers KL, et al. Coronavirus disease 2019 (COVID-19) seropositivity and asymptomatic rates in healthcare workers are associated with job function and masking. Clin Infect Dis. 2021;73:S154-S162. [PMID: ] doi: 10.1093/cid/ciaa1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tjaden AH, Gibbs M, Runyon M, et al; COVID-19 Community Research Partnership Study Group. Association between self-reported masking behavior and SARS-CoV-2 infection wanes from pre-Delta to Omicron-predominant periods - North Carolina COVID-19 Community Research Partnership (NC-CCRP). Am J Infect Control. 2023;51:261-267. [PMID: ] doi: 10.1016/j.ajic.2022.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van den Broek-Altenburg EM, Atherly AJ, Diehl SA, et al. Jobs, housing, and mask wearing: cross-sectional study of risk factors for COVID-19. JMIR Public Health Surveill. 2021;7:e24320. [PMID: ] doi: 10.2196/24320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang X, Pan Z, Cheng Z. Association between 2019-nCoV transmission and N95 respirator use [Letter]. J Hosp Infect. 2020;105:104-105. [PMID: ] doi: 10.1016/j.jhin.2020.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chou R, Dana T, Buckley DI, et al. Update alert 10: epidemiology of and risk factors for coronavirus infection in health care workers [Letter]. Ann Intern Med. 2022;175:W8-W9. [PMID: ] doi: 10.7326/M21-4294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jefferson T, Dooley L, Ferroni E, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev. 2023;1:CD006207. [PMID: ] doi: 10.1002/14651858.CD006207.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bartoszko JJ, Farooqi MAM, Alhazzani W, et al. Medical masks vs N95 respirators for preventing COVID-19 in healthcare workers: a systematic review and meta-analysis of randomized trials. Influenza Other Respir Viruses. 2020;14:365-373. [PMID: ] doi: 10.1111/irv.12745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li Y, Liang M, Gao L, et al. Face masks to prevent transmission of COVID-19: a systematic review and meta-analysis. Am J Infect Control. 2021;49:900-906. [PMID: ] doi: 10.1016/j.ajic.2020.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chu DK, Akl EA, Duda S, et al; COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973-1987. [PMID: ] doi: 10.1016/S0140-6736(20)31142-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Long Y, Hu T, Liu L, et al. Effectiveness of N95 respirators versus surgical masks against influenza: a systematic review and meta-analysis. J Evid Based Med. 2020;13:93-101. [PMID: ] doi: 10.1111/jebm.12381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. MacIntyre CR, Chughtai AA. A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patients. Int J Nurs Stud. 2020;108:103629 [PMID: ] doi: 10.1016/j.ijnurstu.2020.103629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Clapp PW, Sickbert-Bennett EE, Samet JM, et al; US Centers for Disease Control and Prevention Epicenters Program. Evaluation of cloth masks and modified procedure masks as personal protective equipment for the public during the COVID-19 pandemic. JAMA Intern Med. 2021;181:463-469. [PMID: ] doi: 10.1001/jamainternmed.2020.8168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Centers for Disease Control and Prevention. Interim guidelines for COVID-19 antibody testing in clinical and public health settings. Accessed at www.cdc.gov/coronavirus/2019-ncov/hcp/testing/antibody-tests-guidelines.html on 22 February 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




