Abstract
MCL, Mincle and Dectin‐2 are C‐type lectin receptors expressed by subsets of myeloid cells, and their genes cluster together in the APLEC/Dectin‐2 gene complex. We have previously shown that MCL and Mincle form a heterodimer in the rat, and others have shown that MCL and Dectin‐2 form a heterodimer in the mouse. In the rat, Dectin‐2 is a pseudogene, but here, we examine the association of the three receptors in human. In co‐transfection experiments analyzed with flow cytometry and immunoprecipitation, we here show that human MCL and Mincle form a disulphide‐linked heterodimer that associates with the signalling adaptor molecule FcεRIγ, in accordance with our previous findings in the rat. In contrast to previous findings in the rat, data in this paper indicate a direct association of MCL with FcεRIγ, as previously shown for mouse MCL. We were unable to demonstrate the formation of a heterodimer between human MCL and Dectin‐2. Thus, despite similarities, there may be important differences in the conformation of these receptors between rat, mouse and human, and this may have functional consequences.
Keywords: cell activation, cell surface molecules, cells, dendritic cells, molecules, monocytes/macrophages, neutrophils, processes
Abbreviations
- APLEC
Antigen‐presenting lectin‐like complex
- Dectin‐2
Dendritic cell‐associated C‐type lectin‐2
- MCL
Macrophage C‐type lectin
- Mincle
Macrophage inducible C‐type lectin
1. INTRODUCTION
Macrophage inducible C‐type lectin (Mincle) and macrophage C‐type lectin (MCL, also called CLEC4D or CLECSF8) are receptors expressed on cells of myeloid origin. They belong to the C‐type lectin‐like domain superfamily, and their genes lie in the gene complex APLEC (antigen‐presenting lectin‐like complex) on human chromosome 12 together with dendritic cell‐associated C‐type lectin‐2 (Dectin‐2), dendritic cell immunoreceptor (DCIR) and dendritic cell lectin (DLEC, also called BDCA‐2). 1
The first evidence that APLEC receptors may function as pathogen recognition receptors came with the observation that Dectin‐2 was able to recognize a wide range of fungi. 2 , 3 Mouse Mincle was also shown to selectively recognize some fungal species, in particular Malassezia 4 and Fonsecaea 5 , 6 species. In addition, mouse Mincle has been shown to recognize mycobacteria of the M tuberculosis complex, by binding to mycobacterial cord factor, trehalose 6,6'‐dimycolate (TDM). 7 , 8 Mice deficient for Mincle have been shown to be more susceptible to infection with M bovis, 9 suggesting that Mincle has an important in vivo role in the immune response to mycobacteria. Mouse MCL has also been shown to bind to TDM, 10 although we were unable to demonstrate recognition of mycobacteria by rat MCL. 11 Delineation of the ligand recognized by MCL is made more complex by its reported association with Mincle 12 and Dectin‐2. 13 In addition to these exogenous ligands, mouse Mincle has also been reported to recognize the endogenous ligands spliceosome‐associated protein 130 (SAP130) 14 and β‐glucosylceramide 15 released during cell death.
The majority of activating C‐type lectin receptors (CLRs) signal via associated adaptor proteins. Mouse and rat Mincle have been shown to associate with FcεRIγ. 14 , 16 Likewise, mouse Dectin‐2 has been shown to associate with FcεRIγ. 3 MCL carries no known signalling motifs in its cytoplasmic domain, and lacks the charged residue in the transmembrane domain thought to be important for association with signalling adaptor proteins. Nevertheless, mouse MCL was reported to associate with FcεRIγ, 10 although others failed to show this. 17 In the rat, we showed that there was no direct association between MCL and FcεRIγ, but we could show an indirect association via Mincle. 11 Thus, there are reported differences in the biology of rat and mouse Mincle and MCL. Furthermore, Dectin‐2 is a pseudogene in all rat strains examined, thus ruling out the existence of MCL/Dectin‐2 heterodimers in the rat.
We have here examined the relationship between these three receptors in the human, and demonstrate differences in the formation of complexes between human MCL and Mincle or Dectin‐2 compared to previously published rodent data.
2. MATERIALS AND METHODS
2.1. Cells, expression constructs, and transfection
293T cells were obtained from ATCC. Cell lines were grown in cRPMI (RPMI 1640 supplemented with 5% heat‐inactivated FBS, 50 µM 2‐mercaptoethanol, and 1x antibiotic‐antimycotic (ThermoFisher)).
Full‐length open‐reading frames of human Mincle (NM014358, bases 109‐766), DCIR (NM016184, bases 263‐974), or Dectin‐2 (NM001007033, bases 151‐778) were cloned in to the EMCV‐SRα vector followed by a C‐terminal (extracellular) FLAG epitope tag. Human MCL (NM080387, bases 231‐876) was cloned into the same vector, but with a C‐terminal Myc‐tag. Human FcεRI‐γ was cloned into the pDISPLAY vector (Thermo Fisher) with a IGκ leader sequence, an N‐terminal HA tag and full‐length FcεRI‐γ (NM004106, bases 82‐288). 293T cells were transiently transfected using polyethylenimine (PEI) ‘Max’ (m.w. 40,000, Polysciences). 18 Briefly, 1x105 293T cells were plated per well in a 24‐well plate, and left overnight to adhere. 1 μg of total vector DNA was added to 60 μl of PBS, and mixed with 60 μl PEI (43 μg/ml in water). After incubation at room temperature for 30 minutes, 100 μl of this mix was added to each well. In initial experiments (shown in Figures 1 and 2), 400 ng of each receptor DNA (or empty vector) and 200 ng of FcεRI‐γ DNA (or empty vector) were used. For the titration experiments, 450 ng of each receptor DNA were used together with up to 100 ng of FcεRI‐γ DNA and empty vector up to a total of 1 μg.
FIGURE 1.
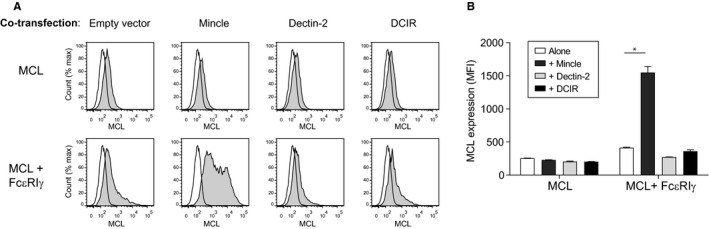
Human MCL requires Mincle and FcεRIγ for full surface expression. Flow cytometry analysis of expression of MCL on the surface of 293T cells transfected with MCL alone, or together with Mincle, Dectin‐2 or DCIR, either with or without FcεRIγ. Data are displayed as histograms A, and summarized as geometric mean fluorescent intensity (MFI) in B. Filled histograms show receptor‐specific staining, and open histograms show isotype control antibody binding. Data from one experiment are shown, representative of at least three independent experiments. Statistical significance was determined using one‐way ANOVA, with Dunnett correction for multiple comparisons.* P = .012
FIGURE 2.
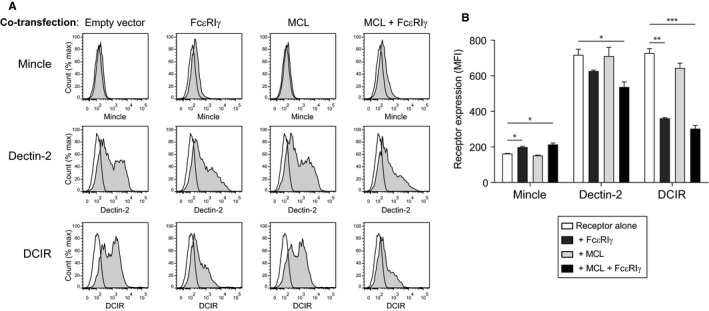
Expression of Mincle, Dectin‐2 or DCIR on the surface of 293T cells. 293T cells were transfected with receptor alone, or co‐transfected with MCL, FcεRIγ or MCL and FcεRIγ. Data are displayed as histograms A, and summarized as geometric mean fluorescent intensity in B. Filled histograms show receptor‐specific staining, and open histograms show isotype control antibody binding. Data from one experiment are shown, representative of at least three independent experiments. Statistical significance was determined using one‐way ANOVA, with Dunnett correction for multiple comparisons.* P < .05, ** P < .01, *** P < .001
2.2. Antibodies and flow cytometry
Antibodies used in the studies described herein were anti‐hMincle (AT16E3, Acris Antibodies); anti‐hMCL (clone #413512), anti‐hDCIR (clone #216110) and anti‐hDectin‐2 (clone #545925) (all R&D Systems); anti‐FLAG (M2, Sigma‐Aldrich); and anti‐HA (16B12, Covance).
For flow cytometry studies, data were acquired using a FACSCanto II (BD Biosciences) and analyzed using FlowJo software (Tree Star).
For the titration experiments with FcεRIγ vector, cells were transfected in triplicate for each experiment, and data shown are pooled from two independent experiments, normalized to geometric mean fluorescence intensity (MFI) of the individual receptors transfected alone without FcεRIγ vector (arbitrarily set to 100). Statistical analysis was performed using GraphPad Prism software. For analysis of receptor induction by FcεRIγ, statistical significance of varying vector amount was determined using one‐way ANOVA, with Dunnett correction for multiple comparisons. For comparison of receptor expression between groups, statistical significance was determined using multiple t‐tests with Holm‐Sidak correction for multiple comparisons.
2.3. Immunoprecipitation and Western blotting
Transfected 293T cells were lysed in digitonin lysis buffer 20 mM Tris‐HCl, pH 7.8, 1% digitonin (Calbiochem), 0.12% Triton ×‐100, 150 mM NaCl, 1 mM MgSO4, and 0.01% sodium azide)). Lysates were immunoprecipitated with Protein G Dynabeads (Invitrogen), separated by SDS‐PAGE, and detected by ECL as detailed previously. 11 For immunoprecipitation, anti‐hMCL was used. For Western blotting, a biotinylated anti‐FLAG mAb (M2, Sigma‐Aldrich) was used as the primary antibody, followed by streptavidin‐HRP (Jackson ImmunoReseach Laboratories) or anti‐hMCL followed by a HRP‐conjugated goat anti‐mouse secondary antibody (Jackson ImmunoReseach Laboratories). Comparison of specific FLAG‐detected band densities was performed using ImageJ software. 19
3. RESULTS
3.1. MCL expression is induced by Mincle, but not Dectin‐2
We have previously demonstrated that rat Mincle and MCL form a disulphide‐linked heterodimer that associates with FcεRIγ, 11 and this was later confirmed to be also true for the mouse. 20 , 21 MCL has also been reported to associate with another receptor encoded by the same receptor gene complex, Dectin‐2. 13 We therefore transfected 293T cells with combinations of the human receptors either with or without FcεRIγ, to see if there were combinations that led to increased surface expression, analyzed by flow cytometry. Co‐transfection with Mincle alone did not lead to increased surface expression of MCL. The same was true for co‐transfections with Dectin‐2 or DCIR constructs, respectively. However, surface expression of MCL could be induced by co‐transfection with FcεRIγ alone, and was further augmented in triple‐transfection experiments with Mincle in addition to FcεRIγ and MCL (Figure 1).
In contrast, Dectin‐2 transfection did not lead to increased MCL expression in either the presence or the absence of FcεRIγ. Control co‐transfection with DCIR did not affect MCL expression in either the presence or the absence of FcεRIγ (Figure 1).
3.2. Mincle and Dectin‐2 expression are not induced by MCL
Looking at surface expression of Mince and Dectin‐2, the expression of Mincle was not enhanced by co‐transfection with MCL in either the presence or the absence of FcεRIγ (Figure 2). The same was true for Dectin‐2. While co‐transfection with FcεRIγ led to a slight increase in Mincle expression on the cell surface, it surprisingly led to a decrease in Dectin‐2 expression. Remarkably, it also led to a decrease in DCIR expression on the cell surface, despite the fact that DCIR is not known to associate with FcεRIγ (Figure 2). MCL.
Although FcεRIγ‐vector was a minor component of the transfections (200 ng of a total 1 µg), we wondered whether it could be having non‐specific toxic effects on receptor expression. We therefore titrated the amount of FcεRIγ‐vector DNA used in the transfections. When the amount of FcεRIγ‐vector DNA in the transfection exceeded about 2.5% of the total DNA, the expression of DCIR began to decrease (Figure 3D).
FIGURE 3.
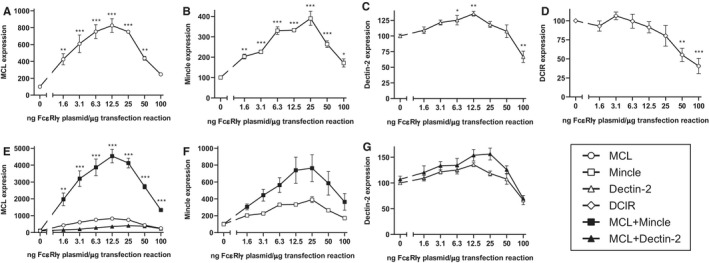
FcεRIγ has a dose‐dependent effect on receptor expression on the cell surface. Effect of varying amounts of FcεRIγ DNA on expression of MCL A, Mincle B, Dectin‐2 C, or DCIR D, following transfection of 293T cells. MFI–geometric mean fluorescence intensity. Significant differences from receptor alone are indicated. E, Effect of varying amounts of FcεRIγ DNA on expression of MCL transfected alone or together with Mincle or Dectin‐2. Significant increases over transfection with MCL together with an identical amount of FcεRIγ are indicated. F, Effect of varying amounts of FcεRIγ on expression of Mincle transfected alone or together with MCL. G, Effect of varying amounts of FcεRIγ on expression of Dectin‐2 transfected alone or together with MCL. Cells were transfected in triplicate for each experiment, and data shown are pooled from two independent experiments, normalized to MFI of the individual receptors transfected alone without FcεRIγ (arbitrarily set to 100). Statistical significance of varying FcεRIγ vector amount A‐D, was determined using one way ANOVA, with Dunnett correction for multiple comparisons. For comparison of receptor expression between groups E‐G, statistical significance was determined using multiple t‐tests with Holm–Sidak correction for multiple comparisons. *P < .05, **P < .01, ***P < .001
The titration of FcεRIγ‐vector DNA also had profound effects on the induction of receptor expression. Again, the receptor induction was optimal when FcεRIγ‐vector DNA constituted approximately 1%‐2% of the total transfected DNA. At this concentration, the induction of MCL (Figure 3A) and Mincle (Figure 3B), as well as Dectin‐2 (Figure 3C) by co‐transfection with FcεRIγ was apparent, confirming that all three receptors associate with this signalling adaptor chain.
The titration also revealed optimal induction of MCL expression in the presence of Mincle, and confirmed that Dectin‐2 did not induce expression of MCL regardless of the amount of FcεRIγ‐vector DNA used (Figure 3E).
Despite this optimization, MCL could not be demonstrated to significantly induce expression of either Mincle (Figure 3F) or Dectin‐2 (Figure 3G), although a clear trend was apparent for Mincle.
3.3. Mincle can be co‐immunoprecipitated with MCL
To confirm the association of human Mincle with MCL, we co‐transfected 293T cells with MCL and FcεRIγ, together with FLAG tagged Mincle, Dectin‐2 or DCIR. Immunoprecipitation of MCL followed by Western blotting with anti‐FLAG antibody showed co‐precipitation of a specific MCL‐associated band only in Mincle co‐transfections. This band migrated with a molecular weight of approximately 28 kDa on a reducing gel, consistent with Mincle in monomeric form (Figure 4A). Given the reported association of Dectin‐2 and MCL in the mouse (18), the failure to co‐precipitate human Dectin‐2 with MCL was surprising. Upon extensive exposure, a faint band could be detected in the Dectin‐2 lane, but at this stage, a faint band could also be detected in the DCIR lane (Figure 4B). These bands appeared to be in relative proportion to the bands in whole cell lysate (Figure 4C), and probably represented non‐specific background binding. In contrast, Mincle was substantially enriched in MCL immunoprecipitates, confirming specific association between these two receptors. Comparison of the specific FLAG‐detected band densities in the whole cell lysate and following immunoprecipitation confirmed that Mincle was substantially enriched by immunoprecipitation with anti‐MCL antibody, while DCIR and Dectin‐2 were not (Figure 4D).
FIGURE 4.
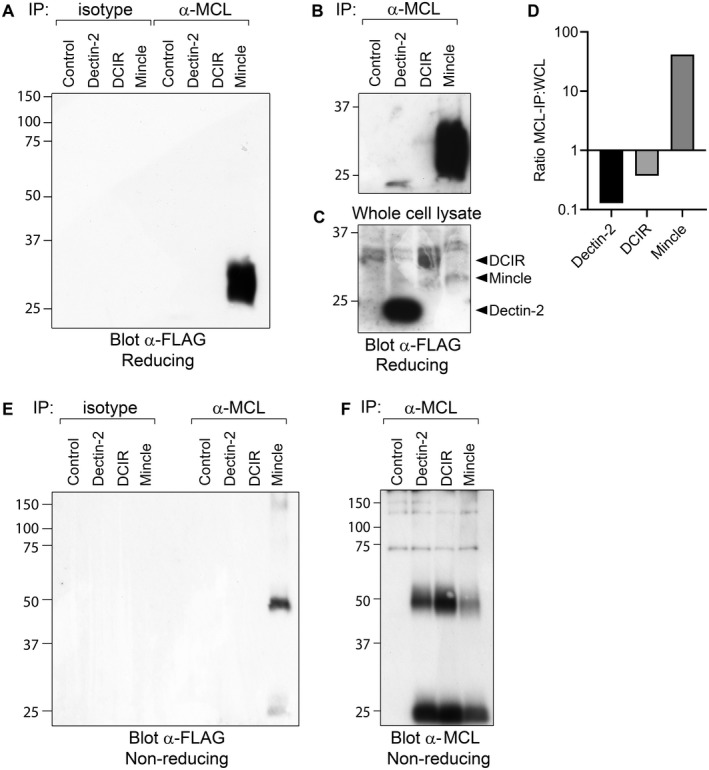
Co‐immunoprecipitation of Mincle with MCL. 293T cells transfected with MCL and optimal amounts of FcεRIγ, together with FLAG‐Dectin‐2, FLAG‐DCIR or FLAG‐Mincle were lysed, immunoprecipitated with anti‐MCL or isotype control antibody, and separated on 10% SDS‐PAGE gels. A, Western blot analysis of immunoprecipitates under reducing conditions with anti‐FLAG antibody. A longer exposure time of the same blot is shown B. C, Whole cell lysates blotted with anti‐FLAG antibody. ImageJ was used to compare band intensities, and the ratio of specific band density in immunoprecipitation versus whole cell lysate is shown D. Immunoprecipitates were also separated under non‐reducing conditions, blotted, and detected with anti‐FLAG E or anti‐MCL F, antibodies
Separation of the immunoprecipitates on a non‐reducing gel revealed a band with a molecular weight of just over 50 kDa, consistent with a disulphide‐linked Mincle‐MCL heterodimer (Figure 4E). A control blot showed that significant amounts of MCL could be detected in each immunoprecipitate, although somewhat less MCL was detected in Mincle‐transfected cells (Figure 4F).
4. DISCUSSION
We demonstrate here that human Mincle and MCL form a disulphide‐linked heterodimer that forms a heteromeric complex together with FcεRIγ, as we have previously shown for the rat receptors. In contrast to the rat, both MCL and Mincle appeared to associate with FcεRIγ, suggesting that the stoichiometry of the human receptor complex may differ from that of the rat.
Rat MCL expresses readily on the cell surface after transfection, and the expression level is unaffected by co‐transfection with Mincle or FcεRIγ. 11 This is true regardless of the amount of FcεRIγ vector DNA used in the transfection. In contrast, mouse and human MCL were not expressed at significant levels on the cell surface when transfected alone into non‐myeloid cell lines. 17 Here, we were also only able to detect low surface expression of human MCL when MCL was transfected alone, but expression could be induced 8‐fold on the cell surface by co‐transfection with optimal amounts of FcεRIγ. In the presence of Mincle, the optimal amount of FcεRIγ lead to a 45‐fold induction of MCL. Although Miyake and colleagues were able to demonstrate association of mouse MCL with FcεRIγ, 10 Graham and colleagues were unable to show this. 17 The difference may be explained by differing amounts of FcεRIγ expressed in these two studies, as with higher amounts of FcεRIγ, we were unable to see substantial induction of human MCL expression. This requirement for optimal amounts of FcεRIγ may have broader implications, because in separate studies, we also saw similar toxic effects with the adaptor proteins CD3ζ and DAP12 in co‐transfections with other receptors (unpublished results). The mechanism mediating this inhibition of receptor expression by higher expression of adaptor protein is unclear, but it appears to be a general effect on the transcription/translation machinery; expression of EGFP (cytosolic) by transfection with an EGFP expression vector was also inhibited by co‐transfection with higher amounts of FcεRIγ vector, ruling out endoplasmic reticulum/Golgi complex‐mediated effects (unpublished results).
Zhu and colleagues found that MCL associates with Dectin‐2. 13 This article looked at both human and mouse Dectin‐2. It was not always clear which species was used in which experiment, making interpretation difficult. Yamasaki and colleagues also reported unpublished observations of human MCL associating with Dectin‐2 in co‐precipitation experiments 21 ; however, Dectin‐2 expression levels were not different in wildtype and MCL‐deficient mice, suggesting that MCL does not affect Dectin‐2 expression in mice. 21 Here, we were unable to show a direct association of human MCL with Dectin‐2. Dectin‐2, in contrast to Mincle, did not induce expression of MCL on the cell surface (Figure 3E). Neither could MCL induce Dectin‐2 expression on the cell surface (Figure 3G). A Dectin‐2 band was observed in MCL‐immunoprecipitations from 293T cells co‐transfected with the two receptors, but only upon extensive exposure of the blot, probably a result of background binding due to the more efficient expression of Dectin‐2 in these cells. Dectin‐2 was not enriched in immunoprecipitations when compared to the amount of the receptor in whole cell lysate. Indeed, Dectin‐2 enrichment was slightly lower than enrichment of DCIR, an inhibitory receptor that is unlikely to have a functional association with MCL. In contrast, Mincle was substantially enriched in MCL immunoprecipitations, confirming the robust association of these two receptors in the human. The reason for the background binding is not clear, as the magnetic beads used for the immunoprecipitation have low non‐specific binding, and were extensively washed. It could conceivably be due to co‐precipitation in undissociated membrane microdomains, or to non‐specific association via exposed hydrophobic transmembrane domains.
Although we were unable to demonstrate a direct association of human MCL with Dectin‐2, we cannot rule out that an additional component is required for an MCL‐Dectin‐2 receptor complex that was absent from our system, but is present in macrophages –however, our data does not support a direct association between MCL and Dectin‐2. Like MCL and Mincle, our data suggest that also human Dectin‐2 associates with FcεRIγ, although induction of Dectin‐2 expression by FcεRIγ was less pronounced than the induction of Mincle and MCL (Figure 3A‐C). However, expression of Dectin‐2 was less dependent upon association with FcεRIγ, and Dectin‐2 expressed efficiently alone (Figure 2).
In the rat strains studied so far, Dectin‐2 is a pseudogene, 1 suggesting that an MCL‐Dectin‐2 complex was not evolutionarily necessary for the rat. The loss of Dectin‐2 seems strange given the broad recognition of fungi by this receptor, but there may be a degree of redundancy among fungi‐binding pattern recognition receptors. This redundancy is not mediated by Mincle, however, because rat Mincle, just like mouse and human Mincle, recognizes a limited subset of fungi, notably Malassezia (unpublished results).
What is the role of MCL in the human receptor complex? In the rat, this role seemed fairly clear – MCL was required for significant Mincle expression on the cell surface, so we presumed that its role was a structural one. 11 Although human Mincle could be expressed at reasonable levels on the cell surface in the absence of MCL, MCL did appear to enhance Mincle expression, although this effect did not reach statistical significance. At the same time, and in contrast to the rat, human Mincle considerably enhanced MCL expression. It has recently been shown that human MCL SNPs are associated with an increased susceptibility to tuberculosis. 22 Although one explanation for this would be a direct recognition of mycobacteria by human MCL, this was not shown, and we have been unable to show such recognition (unpublished results). Future studies will need to address this important question.
This work highlights differences between human and rodent receptor complexes that have implications for the choice of animal models for mapping function of the human receptors.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
VB, ALP, PCS, SF, ED and MD contributed to study design, analysis of data and writing/revision of the manuscript. VB, ALP, PCS and MD collected data.
ACKNOWLEDGMENTS
We would like to thank Wendi Jensen for her technical assistance. This work was supported by funding from the University of Oslo, and the Anders Jahre Fund.
Blankson V, Lobato‐Pascual A, Saether PC, Fossum S, Dissen E, Daws MR. Human macrophage C‐type lectin forms a heteromeric receptor complex with Mincle but not Dectin‐2. Scand J Immunol. 2022;95:e13149. doi: 10.1111/sji.13149
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Flornes LM, Bryceson YT, Spurkland A, Lorentzen JC, Dissen E , Fossum S. Identification of lectin‐like receptors expressed by antigen presenting cells and neutrophils and their mapping to a novel gene complex. Immunogenetics. 2004;56:506‐517. [DOI] [PubMed] [Google Scholar]
- 2. McGreal EP, Rosas M, Brown GD, et al. The carbohydrate‐recognition domain of Dectin‐2 is a C‐type lectin with specificity for high mannose. Glycobiology. 2006;16(5):422‐430 [DOI] [PubMed] [Google Scholar]
- 3. Sato K, Yang XL, Yudate T, et al. Dectin‐2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J Biol Chem. 2006;281:38854‐38866. [DOI] [PubMed] [Google Scholar]
- 4. Yamasaki S, Matsumoto M, Takeuchi O, et al. C‐type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc Natl Acad Sci USA. 2009;106:1897‐1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sousa MG, Reid DM, Schweighoffer E, et al. Restoration of pattern recognition receptor costimulation to treat chromoblastomycosis, a chronic fungal infection of the skin. Cell Host Microbe. 2011;9:436‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wevers BA, Kaptein TM, Zijlstra‐Willems EM, et al. Fungal engagement of the C‐type lectin mincle suppresses dectin‐1‐induced antifungal immunity. Cell Host Microbe. 2014;15(4):494‐505. [DOI] [PubMed] [Google Scholar]
- 7. Matsunaga I, Moody DB. Mincle is a long sought receptor for mycobacterial cord factor. J Exp Med. 2009;206(13):2865‐2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ishikawa E, Ishikawa T, Morita YS, et al. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C‐type lectin Mincle. J Exp Med. 2009;206(13):2879‐2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Behler F, Steinwede K, Balboa L, et al. Role of Mincle in alveolar macrophage‐dependent innate immunity against mycobacterial infections in mice. J Immunol. 2012;189(6):3121‐3129. [DOI] [PubMed] [Google Scholar]
- 10. Miyake Y, Toyonaga K, Mori D, et al. C‐type lectin MCL is an FcRgamma‐coupled receptor that mediates the adjuvanticity of mycobacterial cord factor. Immunity. 2013;38:1050‐1062. [DOI] [PubMed] [Google Scholar]
- 11. Lobato‐Pascual A, Saether PC, Dahle MK, et al. Rat macrophage C‐type lectin is an activating receptor expressed by phagocytic cells. PLoS One. 2013;8:e57406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lobato‐Pascual A, Saether PC, Fossum S, Dissen E, Daws MR. Mincle, the receptor for mycobacterial cord factor, forms a functional receptor complex with MCL and FcepsilonRI‐gamma. Eur J Immunol. 2013;43:3167‐3174. [DOI] [PubMed] [Google Scholar]
- 13. Zhu LL, Zhao XQ, Jiang C, et al. C‐type lectin receptors Dectin‐3 and Dectin‐2 form a heterodimeric pattern‐recognition receptor for host defense against fungal infection. Immunity. 2013;22(39):324‐334. [DOI] [PubMed] [Google Scholar]
- 14. Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Mincle is an ITAM‐coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9(10):1179‐1188. [DOI] [PubMed] [Google Scholar]
- 15. Nagata M, Izumi Y, Ishikawa E, et al. Intracellular metabolite beta‐glucosylceramide is an endogenous Mincle ligand possessing immunostimulatory activity. Proc Natl Acad Sci USA. 2017;18(114):E3285‐E3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lobato‐Pascual A, Saether PC, Fossum S, Dissen E, Daws MR. Mincle, the receptor for mycobacterial cord factor, forms a functional receptor complex with MCL and FcepsilonRI‐gamma. Eur J Immunol. 2013;43:3167‐3174. [DOI] [PubMed] [Google Scholar]
- 17. Graham LM, Gupta V, Schafer G, et al. The C‐type lectin receptor CLECSF8 (CLEC4D) is expressed by myeloid cells and triggers cellular activation through Syk kinase. J Biol Chem. 2012;27(287):25964‐25974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huh SH, Do HJ, Lim HY, et al. Optimization of 25 kDa linear polyethylenimine for efficient gene delivery. Biologicals. 2007;35(3):165‐171. [DOI] [PubMed] [Google Scholar]
- 19. Schindelin J, Arganda‐Carreras I, Frise E, et al. Fiji: an open‐source platform for biological‐image analysis. Nat Methods. 2012;28(9):676‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kerscher B, Wilson GJ, Reid DM, et al. Mycobacterial receptor, Clec4d (CLECSF8, MCL), is coregulated with Mincle and upregulated on mouse myeloid cells following microbial challenge. Eur J Immunol. 2016;46:381‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miyake Y, Masatsugu OH, Yamasaki S. C‐type lectin receptor MCL facilitates mincle expression and signaling through complex formation. J Immunol. 2015;1(194):5366‐5374. [DOI] [PubMed] [Google Scholar]
- 22. Wilson GJ, Marakalala MJ, Hoving JC, et al. The C‐type lectin receptor CLECSF8/CLEC4D is a key component of anti‐mycobacterial immunity. Cell Host Microbe. 2015;11(17):252‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.


