Abstract
Objective
Scalp seborrheic dermatitis (SD) is a chronic, relapsing, and inflammatory scalp disease. Studies indicate a global bacterial and fungal microbiota shift of scalp SD, as compared to healthy scalp. Ketoconazole and selenium disulfide (SeS2) improve clinical signs and symptoms in both scalp dandruff and SD.
Aim
The main objective of this study was to investigate the changes in the scalp microbiota diversity and counts in subjects with scalp SD during a two‐phase treatment period.
Material and methods
The scalp microbiota and clinical efficacy were investigated in 68 subjects with mild‐to‐moderate scalp SD after an initial one‐month treatment with 2% ketoconazole, and after a 2‐month maintenance phase, either with a 1% SeS2‐based shampoo or its vehicle.
Results
Thirty one subjects in the active and 37 subjects in the vehicle group participated. Ketoconazole provided an improvement of clinical symptoms (adherent (−1.75 p < 0.05), non‐adherent (−1.5, p < 0.05)) flakes and erythema (scores 1.67–0.93, p < 0.001), in an increased fungal diversity and in a significant (p < 0.005) decrease of Malassezia spp. SeS2 provided an additional clinical improvement (−0.8; p = 0.0002 and −0.7; p = 0.0081 for adherent and non‐adherent flakes, respectively, at Day 84) compared to the vehicle associated with a low Malassezia spp. count and an additional significant (p < 0.001) decrease of the Staphylococcus spp. level.
Conclusion
Selenium disulfide provides an additional benefit on the scalp microbiota and in clinical symptoms of SD and dandruff after treatment with ketoconazole. The results confirm the role of Staphylococcus spp. in scalp SD and open possible perspectives for preventing relapses.
Keywords: ketoconazole, scalp microbiota, seborrheic dermatitis, selenium disulfide
1. INTRODUCTION
Seborrheic dermatitis (SD) is a chronic and relapsing inflammatory skin condition of sebum‐rich areas such as the scalp. It is characterized by erythema, mild‐to‐moderate scaling resulting in greasy and flaky scalp, and is sometimes associated with pruritus. 1 In the adult population, its prevalence is up to 5%, with a higher prevalence in immunocompromised patients and in patients with neurologic diseases. 2 , 3 When only mild scaling without visible inflammation is observed, SD is called dandruff (D). The prevalence of dandruff in the general population has been estimated at 15%–20%. 1 Various environmental, intrinsic and host immune factors may contribute to the development of SD, leading to an alteration of the sebaceous gland activity and sebum composition, epidermal barrier function, and skin surface fungal colonization, which ultimately leads to inflammation. Among these factors, lipophilic Malassezia yeasts may play a key role.
At a species level, M. restricta and M. globosa are the most frequent species associated with a healthy scalp. 4 , 5 Specific strains of M. restricta have been associated with lesional skin of SD patients at the genotypic level. 6 In addition, Malassezia spp. is able to metabolize and oxidize sebum‐derived lipids such as triglycerides, squalene, and fatty acids into inflammatory compounds and to produce indole derivatives (malassezin, indolocarbazole), while an activity against aryl hydrocarbon receptors may impact skin inflammation. 7 , 8 Malassezia species, especially M. restricta, are able to induce cytotoxicity to keratinocytes in vitro, suggesting an active role in the accelerated flake formation. 9
Moreover, bacterial skin microbiota changes may also be involved in the pathogenesis of SD. 10 , 11 , 12 The healthy scalp microbiota is characterized by a low bacterial diversity, as compared to the other body sites, and is dominated by Cutibacterium acnes (formerly Propionibacterium acnes), Staphylococcus epidermidis, and yeasts, especially Malassezia spp. 13 , 14 In D/SD scalp, bacterial changes correspond to a higher diversity. 15 , 16 This corresponds to a disequilibrium between the 2 dominant bacteria Staphylococcus (higher) and Cutibacterium (lower). 17 , 18 , 19 , 20 These modifications are correlated to severity of scaling and, to an extent, to the forehead of SD patients. 16 Additional microbial markers such as Aspergillus and Pseudomonas have been also proposed. 21 Similar to skin diseases related to a microbiome imbalance such as atopic dermatitis and acne vulgaris, deviation from the healthy scalp microbiome may trigger an inflammatory response in scalp SD. 1
Current SD treatments consist of topical applications of antifungals and anti‐inflammatory agents. 22 Ketoconazole is a fungistatic agent, which limits Malassezia restricta, M. globosa, and M. furfur growth, without inhibiting S. epidermidis or S. aureus. 23 In vivo, the clinical efficacy of ketoconazole used at 1%–2% resulted in a reduction in Malassezia spp. loads supporting the contribution of these fungi in the physiopathology of SD. 24 , 25 When ketoconazole treatment is stopped, SD symptoms relapse. 24 , 26
Selenium disulfide shampoo (SeS2) is another effective means in the treatment of dandruff, a milder form of seborrheic dermatitis. 27 SeS2 has antifungal properties against Malassezia furfur and also inhibits Staphylococcus epidermidis growth in vitro. 23 , 28 Past reports on the treatment of scalp SD/D have focused on Malassezia spp and counts, while bacterial microbiota changes have only been poorly described. 29 , 30 Moreover, the SeS2‐based shampoo contains salicylic acid, known for its keratolytic activity, allowing to reduce flakes on the scalp. 22 , 31 We performed an 8‐week maintenance study to investigate the clinical benefit and effect of SeS2 on the scalp microbiota, compared to its vehicle after a 4‐week initial treatment with ketoconazole. The present study provides insights into the effect of ketoconazole and SeS2 on the scalp microbiota using next‐generation sequencing and qPCR quantification of the 3 major microbial markers of SD/D scalp: Staphylococcus, Cutibacterium, and Malassezia. 18 We also hypothesized that using a treatment that acts on the bacterial microbiota of the scalp may provide additional benefits for scalp SD symptoms.
2. METHODOLOGY
This was a randomized comparative, double‐blind, parallel group study. The study was approved by an Institutional Review Board (CPP Sud‐Ouest & Outremer III) and the French Agency for Security of Health Products (ANSM) (ID‐RCB number: 2017‐A00148‐45). The study was registered in the clinical trial database under the number NCT04057950 (www.clinicaltrials.gov). All subjects provided written informed consent prior to enrollment in the study.
2.1. Subject selection and study design
Seventy‐seven (77) subjects, aged between 18 and 65 years, were recruited in the outpatient dermatology clinic of a public hospital (51 subjects) and at one investigational site (26 subjects), both located in Paris. Suitable subjects had to have mild‐to‐moderate SD on the scalp with high flake severity corresponding to an adherent flake score of ≥2.5 (ranging 0–5) and total (adherent + non‐adherent) flake score of ≥4 (ranging from 0 to 10). 32
The study was organized in two phases: a treatment phase (D0 to D28) during which all subjects received a 2% ketoconazole foaming gel (6 g monodose sachets, Ketoderm®, Janssen‐Cilag™) to be applied twice a week for 4 weeks. During the maintenance phase (D28 to D84), subjects were randomized in 2 groups. One group (active group, n = 35) received a SeS2‐1% salicylic acid shampoo (Dercos® DS anti Dandruff, Vichy Laboratoires) and the 2nd group (vehicle group, n = 42) received the vehicle; products were to be applied for 8 weeks three times a week for 8 weeks. Subjects were asked to wash their hair with the shampoo or its vehicle and to rinse the hair immediately after washing and to re‐apply the shampoo or its vehicle and to leave it on the scalp for 2 min prior to rinsing and drying of the hair.
2.2. Clinical evaluations
Clinical assessments were performed at D0, 14, 21, 28, 56, 70, and 84. The severity of scaling was assessed according to a 0–5 grading flake for adherent flake (0: no flakes, 5: flakes in very large quantity or in thick layers on the scalp) and a 0–5 grading scale for non‐adherent flakes as described previously. A 0–5 grading scale (from 0: none to 5: severe) was used for scalp erythema and for irritation, and a 1–5 grading scale (1: very dry to 5: very oily) was used for greasiness. Clinical grading was performed on 8 different sites symmetrically distributed on the left and right side (4 sites each) of the upper part of the scalp. Gradings were summed up and averaged, yielding an overall clinical grading of the scalp status. In addition, subjects self‐assessed the flake severity of their scalp and the intensity of scalp pruritus through a 0–9 grading scale (0: absent; 9: intensely perceived) at 3 time points (at day 0, 28, and 84).
2.3. Scalp microbiota analysis
The detailed microbiota analysis protocols are described in earlier reports. 15 , 17 Briefly, 2 areas of the scalp were sampled per subject at every time point using 2 separate sterile cotton swabs for QPCR quantification and NGS analysis, respectively. Subjects were asked not to wash their scalps for the 2 days prior to the sampling procedure. Samples from the scalp area (vertex or crown) with a squamous score >2.5 were obtained at baseline (D0), at the end of the treatment phase (D28), and during the cosmetic shampoo phase at 2 visits (D56, D84). To ensure the repositioning of the sampling on the same scalp location during follow‐up, the hair from the sampling area was cut at 5 mm from the scalp surface every 2 weeks. To limit the variability during sampling, the same technician sampled the subject during the study. Sampling was conducted as previously described with minor modifications. 17 A sterile cotton swab soaked in a solution containing collection solution (0.15 M NaCl and 0.1% Tween 20) was rubbed onto the scalp surface (among the hair) with a zig‐zag pattern, to cover a total surface of 4 cm2 in a non‐overlapping manner. At the end of the procedure, the head of each swab was cut from the handle and placed into a tube containing 5 ml of collection buffer. These wet swabs were stored at 4°C and processed for DNA isolation within 24 h followed by QPCR analysis. In parallel to scalp samples, sterile cotton swabs were cut from the handle and placed in the collection buffer and further processed using an identical procedure, as negative controls. For NGS, sampling was conducted as described above, except that swabs were kept dry (dry swabs) at the end of the sampling in an Eppendorf sterile tube and stored at −80°C until DNA extraction and NGS analysis. Similarly, a few sterile cotton swabs were kept as negative controls.
For NGS, 16S and ITS amplicon libraries were prepared using specific bacterial 16S rRNA (V1‐27S and V3‐535R) and specific fungal ITS1 (ITS‐18SF and ITS‐5.8S1R) hypervariable regions and sequenced on an Illumina Miseq system for 300 pb paired‐end sequencing at the Genomics Center, CHU de Québec‐Université Laval Research Center, Canada. Sequence processing and bioinformatics are described in SI Materials and Methods. qPCR was performed on all the samples using primers and TaqMan MGB probes as previously reported. 17
2.4. Statistics
Descriptive statistics were carried out on raw data at D0, D28, and D84 and other times (D14, D21, and D70) for clinical assessments.
To show the ketoconazole treatment effect, a time effect analysis was performed on the whole study population using the linear mixed model with each study parameter as dependent variable; time as fixed and repeated factor: overall time effect and comparisons Dx versus D0 with adjustment for multiple comparison according to the Bonferroni procedure; subject as random variable, unstructured model as covariance matrix, checking of residuals distribution normality assumption using normal QQ plot. The measurements homogeneity between the active and vehicle groups at D0 and D28 was tested using unpaired t‐test or Mann‐Whitney test if the distribution normality assumption was rejected.
For the maintenance phase, a time effect analysis was performed using the linear mixed model according to the same design as described above, but here, the analysis was split by product and each Dx was compared to D28 with adjustment for multiple comparison according to the Bonferroni procedure if required. The comparison between the 2 investigational products was performed using ANCOVA with difference Dx‐D28 for each study parameter as dependent variable; fixed factors: center, treatment, and interaction center—treatment (as there was no significant interaction between center and treatment for any of the study criteria, the interaction was removed from model); covariate: measurements at D28.
A gap compared to residuals distribution normality was found for raw data of QPCR (Staphylococcus, Cutibacterium, Malassezia), a log10 transformation of data, was therefore applied to obtain normally distributed residuals for each QPCR variable.
For sequencing data analysis, R software was used (vegan: Community Ecology Package. R package version 2.4–5. 33 Pairwise analysis of Similarities (ANOSIM 15 ) was applied to assess differences based on treatment and time point. ANOSIM global R value ranges from 1 to −1 (R ~ 0 indicates the same level of variation within and between groups). ANOSIM test was performed using α = 0.05 for statistical significance. Alpha diversity (Shannon) was analyzed following the same method as for clinical parameters (linear mixed model, see above). Benjamini‐Hochberg procedure was used for multiple comparisons adjustment.
3. RESULTS
3.1. Patient demographic and baseline data
From the 77 recruited subjects, 68 subjects completed the study: 31 subjects (21 women, 10 men, mean age 40 ± 2 years) in the active group and 37 subjects (29 women, 8 men, aged 42 years ± 2) in the vehicle group. Nine (9) subjects were either lost to follow‐up or had major protocol deviations.
3.2. Clinical efficacy on flake, seborrhea, and erythema
During treatment with ketoconazole, a significant (p < 0.05) reduction in the scaling of the scalp was observed, as measured by adherent flakes (average decrease: −1.75) and not adherent flakes (average decrease: −1.5) at D28 compared to D0 (Figure 1A). During the maintenance phase, a significant additional reduction in flake scores was observed in the active group, compared to the vehicle group at D70 (average decrease: −0.9; p = 0.0019 and average decrease: −0.8; p = 0.0011 for adherent and non‐adherent flakes, respectively) and D84 (average decrease: −0.8; p = 0.0002 and average decrease: −0.7; p = 0.0081 for adherent and non‐adherent flakes, respectively).
FIGURE 1.
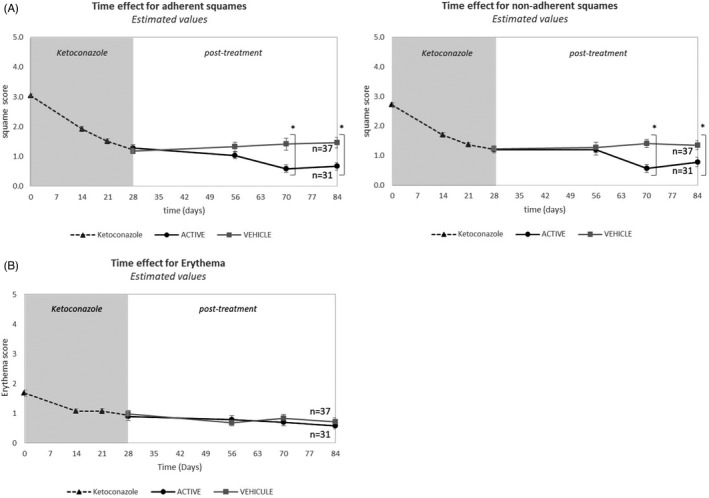
Changes in the scores of adherent and non‐adherent flakes from D0 to D84, during the two phases of the study. Scores, established on 8 different scalp regions, are expressed as mean ± SD). (Phase 1, ketoconazole treatment: Large dot line; Phase 2, vehicle treatment: small dot line and active treatment: full line)
Scalp seborrhea did not change significantly during the entire duration of the study, (data not shown).
Erythema significantly decreased (p < 0.001) between D0 and D28 (average scores 1.67–0.93) and continued to decrease during the maintenance phase. However, the decrease was not significant in the active group (from 0.88 to 0.57; Figure 1B). Irritation scores showed a similar trend.
Self‐assessments paralleled the clinical assessments: subjects perceived a strong decrease in flakes and pruritus at the end of the first phase (D28), as compared to the baseline (average scores from 6.47 to 2.72, p < 0.0001 for the flakes and 5.47 to 1.84, p < 0.0001 for pruritus) and an additional significant decrease of both flakes and pruritus in the active group at D84 compared to D28 (average scores from 2.77 to 1.12, p < 0.0001 for the squamous status and from 2.16 to 0.81, p < 0.0004 for pruritus). Conversely, at D84 compared to D28, there was no significant additional decrease with the vehicle for flakes (average scores from 2.78 to 2.61, p = 0.8845) and for pruritus (from 1.57 to 1.89, p = 0.3496).
3.3. Scalp microbiota changes during ketoconazole and SeS2 treatment
The bacterial and fungal microbiota of 68 subjects was analyzed for the treatment and maintenance phase. At the genus level, Cutibacterium spp. and Staphylococcus spp. were the 2 most abundant bacterial genera in scalp SD subjects (>75% of the reads at all‐time points, Figure 2A). Malassezia genus comprised the majority of fungi in almost all of the samples (>80% of the reads in scalp SD samples at D0; Figure 2B). Taxonomic assignment of Malassezia taxa at species level allowed to identify Malassezia restricta, M. globosa, M. sympodialis, and M. slooffiae, M. restricta being the most abundant species in the scalp SD samples (>70%; Figure S1). Differences between the fungal and bacterial profiles, after ketoconazole treatment, showed a decrease of the relative abundance of Malassezia genus in the 2 study groups compared to D0. In the maintenance phase, Staphylococcus abundance had decreased at D56 and D84 only in the active group, compared to D28. To further characterize the scalp microbiota, comparisons between the initial and maintenance phase, bacterial and fungal diversity analyses were made.
FIGURE 2.
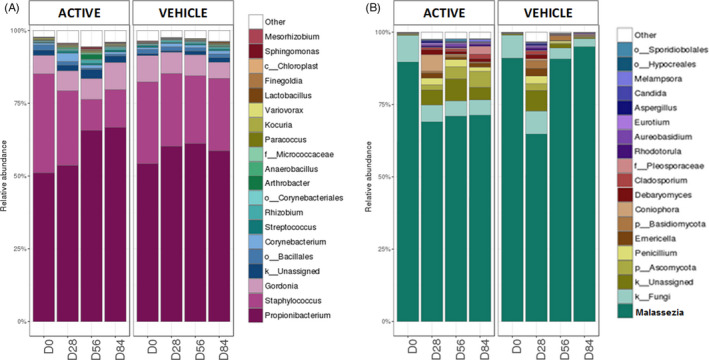
The top 20 most represented bacterial (A) and fungal (B) genera over time for the active and the vehicle group
Changes in the alpha diversity (Shannon index, Figure 3A) of the bacterial microbiota during ketoconazole treatment were minor (<+0.10), while the beta diversity (ANOSIM comparison, Figure 4A,B) did not show any difference between D0 and D28.
FIGURE 3.
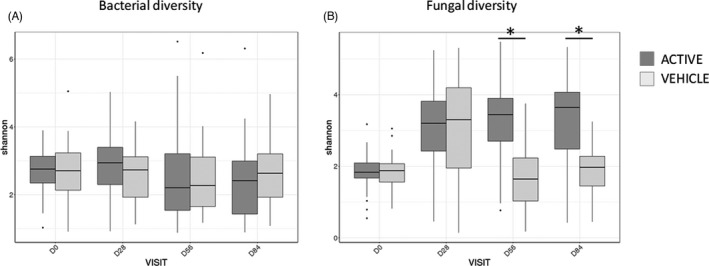
Alpha diversity: variations of the Shannon index for bacterial (A) and fungal (B) microbiota over time, for the active and vehicle group (*: significant p‐value adjusted)
FIGURE 4.
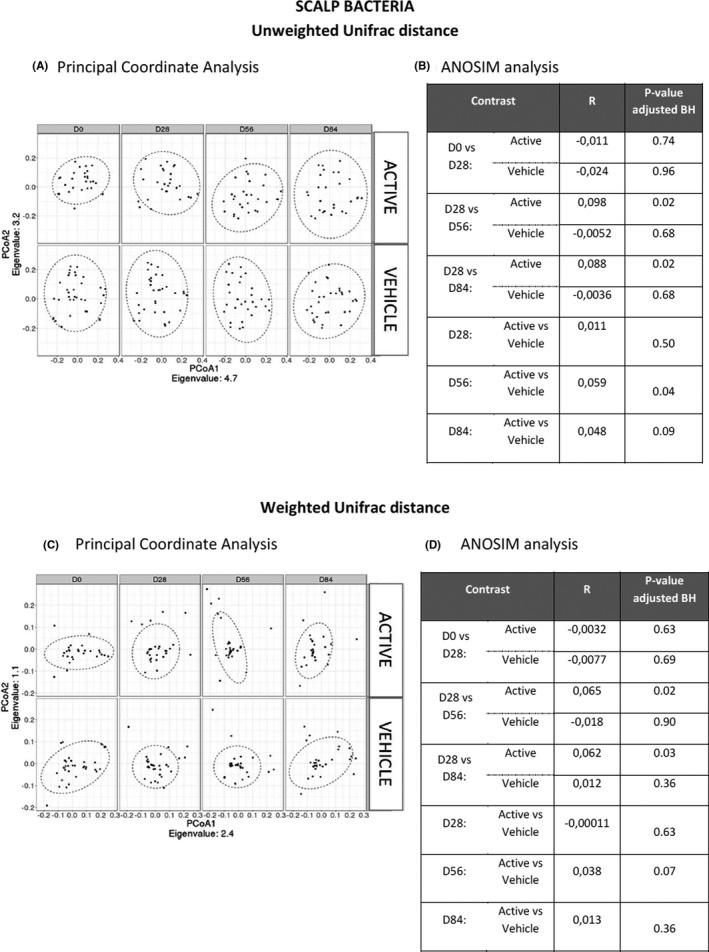
Beta diversity for Unifrac distances. The variation of the Unifrac distances (describing the variation of the beta diversity for the bacterial species) is presented as PCoA (A,C), along with the comparison table between time points and groups using ANOSIM analysis (B,D). Two sets of indexes were investigated: the weighted and unweighted unifrac distances which give information focused on the relative abundance and species presence/absence respectively. BH: Benjamini‐Hochberg procedure was used for multiple comparisons adjustment
During the maintenance period, a weak decrease of the Shannon index (Figure 3A) was measured at D56 (p < 0.05) and D84 (p < 0.05) compared to D28 in the active group, while no significant difference was observed with the vehicle. When comparing the beta diversities, significant differences were observed in the active group at D56 (R = 0.098; p < 0.05) and D84 (R = 0.088; p < 0.05) compared to D28, for unweighted unifrac distances (Figure 4C,D). The 2 groups were significantly different for the beta diversity (weighted and unweighted unifrac distance) at D56 (R = 0.059; p < 0.05) according to the ANOSIM test. Only the active shampoo was associated with a decrease in the bacterial diversity and significant modifications of the bacterial community composition.
Ketoconazole treatment led to a significant increase in the alpha diversity of the fungal microbiota (median: +1.26 and +1.47 in the active and vehicle group, respectively; Figure 3B) and to a significant difference (ANOSIM test: R = 0.37; p < 0.005 and R = 0.51; p < 0.005 in the active and vehicle group, respectively; Figure 5A,B).
FIGURE 5.
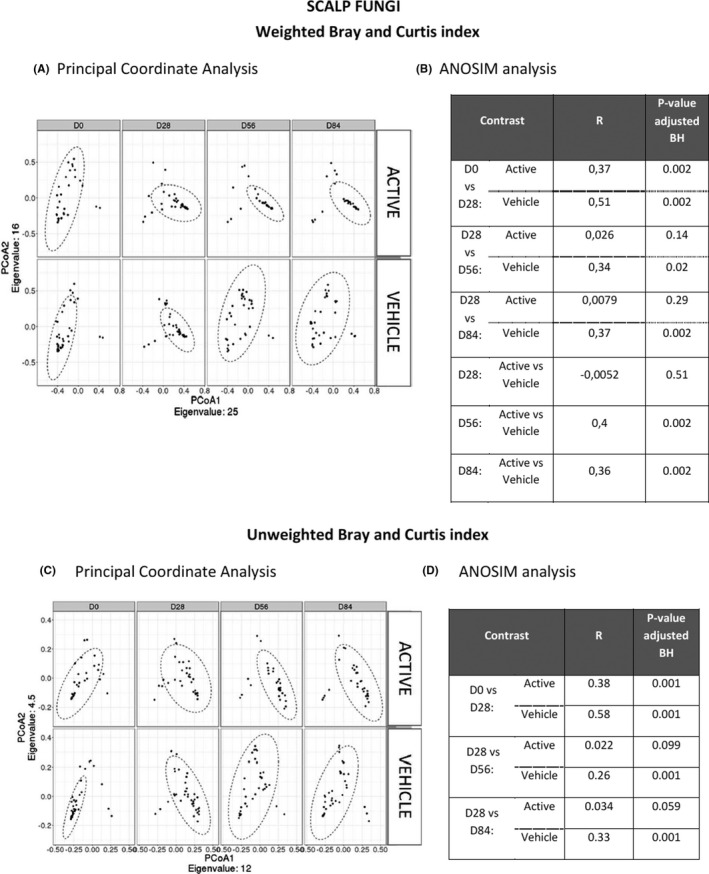
Beta diversity using the Bray‐Curtis index. The variation of the Bray‐Curtis index (describing the variation of the beta diversity for the fungal species) is presented as PCoA (A,C), along with the comparison table between time points and groups using ANOSIM analysis (B,D). Two sets of indexes were investigated: Bray‐Curtis weighted and Bray‐Curtis unweighted indexes which give information focused on the relative abundance and species presence/absence respectively. BH: Benjamini‐Hochberg procedure was used for multiple comparisons adjustment
The Shannon index remained stable at D56 and D84 compared to D28 in the active group (Figure 3B), while a significant decrease was observed at D56 and D84 with a value close to D0 (before treatment) in the vehicle group. Moreover, the fungal alpha diversity at D56 and D84 was significantly lower in the active group compared to the vehicle group (p < 0.05). Similar trends were observed using the ANOSIM comparison (Figure 5C,D).
3.4. SeS2 shampoo efficacy is associated with an additional decrease in Staphylococcus spp.
A significantly lower amount of Malassezia spp. (from 5.059 to 2.791 log10(cells/cm2) (median value), p < 0.001) and Staphylococcus spp. (from 5.036 to 4.566 log10(cells/cm2) (median value), p < 0.01) was observed at D28, after a treatment phase with ketoconazole compared to D0 with no effect on the Cutibacterium spp. load (Figure 6).
FIGURE 6.
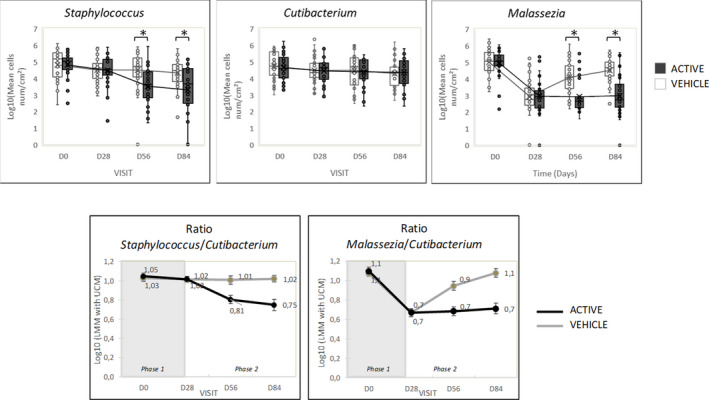
Quantitative changes in Malassezia; Cutibacterium and Staphylococcus counts (log10(cells /cm2) during initial treatment with ketoconazole and during maintenance with either active or vehicle shampoo and changes in the ratios between microbial species Malassezia/Cutibacterium and Cutibacterium/Staphylococcus
During the maintenance phase, in the active group, the Malassezia load remained low at 2.668 and 2.736 log10(cells/cm2) (median value) at D56 and D84, while the Staphylococcus spp. load continued to decrease significantly at D56 and D84 (from 4.566 to 3.640 and 3.658 log10(cells/cm2) (median value), p < 0.001) compared to D28. Conversely, in the vehicle group, Malassezia spp. increased significantly at D56 and D84, as compared to D28 (from 2.734 to 4.090 and 4.673 log10 (cells/cm2; median value), p < 0.001) although flake scores were low. Staphylococcus spp. and Cutibacterium spp. counts remained unchanged in the vehicle group.
The Malassezia/Cutibacterium spp. ratio was significantly lower (p = 0.005) after ketoconazole treatment (mean ratio = 0.7) as compared to D0 (mean ratio = 1.1) (Figure 6) and remained low in the active group during maintenance; while in the vehicle group, the ratio had increased at D84, returning to almost baseline values. The Staphylococcus/Cutibacterium ratio decreased significantly (p < 0.05) solely with active shampoo at D84 compared to D28 (mean ratio from 1.02 to 0.75; Figure 6).
4. DISCUSSION
Both ketoconazole and SeS2 are effective against dandruff and scalp SD. 27 However, little is yet known regarding their impact on the scalp microbiome in vivo.
The present characterization of the bacterial and fungal microbiome of subjects with SD scalp shows that ketoconazole significantly decreases the Malassezia load and enriches the fungal diversity, including genera such as Penicillium, Debaryomyces, Cladosporium, Rhodotolura, Aspergillus, and Candida, which are all common scalp colonizers. 16 , 17 Moreover, ketoconazole does not impact on the bacterial diversity.
Maintenance with SeS2 maintained a very low level of Malassezia spp. counts and a high fungal diversity. In addition, the bacterial composition was slightly modified (beta diversity), consisting mainly of a significant decrease of the Staphylococcus spp. load close to that of reported healthy scalp levels. 17 , 19 Not surprisingly, this was associated with an additional clinical improvement of the flake severity, probably due to the keratolytic agent salicylic acid contained in the shampoo. Staphylococcus spp. is considered to be strongly correlated with the severity of D/SD. 21 , 34 The present findings confirm that Staphylococcus spp. is a microbial marker for D/SD improvement.
Staphylococcus spp., presumably S. epidermidis, according to previous reports on European scalps, exhibits dual roles on the skin as either colonizer or as pathogen. 17 , 35 It participates in the reinforcement of tight junctions (TJ) and in the decrease of inflammation. 36 Recent reports suggest that the overabundance of S. epidermidis observed in atopic patients may play a role in the process of damaging the skin barrier by expressing a particular cysteine protease able to degrade the tight junction protein desmoglelin‐1. 37 Our data may allow to support the second hypothesis, because 2 weeks after the decrease in Staphylococcus spp. counts, a decrease of the flake severity was observed. However, we cannot exclude the presence of other Staphylococcus species, such as S. aureus, that have been associated with other inflammatory scalp diseases, such as folliculitis decalvans and scalp psoriasis. 38 , 39 , 40 , 41 , 42 , 43 Moreover, Bäsler et al. showed that chronic exposure to an over‐colonization of S. aureus can induce a decrease of TJ. 44 Therefore, a clear definition at a taxonomic level of Staphylococcus species associated to scalp SD may help to develop optimal management strategies of scalp SD.
Dysbiosis has been associated with various chronic inflammatory diseases; disorders such as atopic dermatitis and psoriasis with a loss of bacterial diversity and increase in Staphylococcus spp. have been reported in comparison with healthy subjects. 45 , 46 In scalp D/SD, existing data suggest a different phenomenon, as the microbial diversity is particularly low, compared to other body sites and a loss of balance between the three species Malassezia, Staphylococcus, and Cutibacterium can be observed. 10 , 14 , 17 , 20 Decreasing Malassezia spp. counts is beneficial for scalp with SD. Our study shows that suppressing, at least partially, Staphylococcus spp., leads to an additional improvement. Furthermore, studies of Staphylococcus spp. species in a more extensive taxonomical analysis and functional characterization of the bacterial‐scalp interactions might generate novel hypotheses and open new perspectives regarding the pathogenesis of scalp D/SD. Moreover, a maintenance regimen with an active shampoo once a week could be of interest in the context of a chronic condition.
In addition to these results regarding the impact of ketoconazole and SeS2 on the scalp microbiome, the present study confirms the clinical efficacy of ketoconazole on the management of scalp SD symptoms, while a maintenance treatment using SeS2 shampoo may provide an additional, significant improvement of SD symptoms. 24 , 25 , 26
In 2019, Suchonwanit et al. 47 provided new insights about biophysical characteristics in subjects with scalp SD. The authors showed that transepidermal water loss, stratum corneum hydration, skin surface pH, and skin surface lipids were impacted in subjects with scalp SD. Thus, in addition to the disturbed skin microbiota which we observed in our study, these disturbed biophysical characteristics play a role in the skin barrier disruption and skin inflammation and suggesting that rebalancing the scalp microbiota will help to restore scalp homeostasis.
Further research in a larger population may be necessary to confirm our observations and those made by Suchonwanit et al through a clinical study assessing, biophysical, clinical, and microbial features. 47
5. CONCLUSIONS
The present study confirms the additional benefit of SeS2 shampoo in the maintenance setting on the scalp, microbiota, and clinical symptoms in SD and dandruff after an initial treatment with ketoconazole. The results also highlight the role of Staphylococcus spp. in the management of scalp SD and open possible perspectives for preventing relapses.
CONFLICT OF INTERESTS
PM, CC, MT, AO, CM and AG are employees of L'Oréal Research and Innovation. DK is an employee of Vichy Laboratoires, AB was an employee of Vichy Laboratoires at the time the study was conducted. PR received honoraria from L’Oréal to conduct the study.
AUTHOR CONTRIBUTIONS
Philippe Massiot, Cécile Clavaud, Marie Thomas, Alban Ott, Audrey Guéniche, Ségolène Panhard, Benoît Muller and Céline Michelin participated in the conduct of the study and analysis of study results. Pascal Reygagne served as the investigator. Anne Bouloc and Delphine Kerob served as medical experts. All authors participated in writing the manuscript and all approved its content.
ETHICAL APPROVAL
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received.
Supporting information
Figure S1
SI Materials and Methods
ACKNOWLEDGMENTS
The authors acknowledge the editorial assistance of Karl Patrick Göritz, SMWS Scientific Writing Services, France.
Massiot P, Clavaud C, Thomas M, et al. Continuous clinical improvement of mild‐to‐moderate seborrheic dermatitis and rebalancing of the scalp microbiome using a selenium disulfide–based shampoo after an initial treatment with ketoconazole. J Cosmet Dermatol. 2022;21:2215–2225. 10.1111/jocd.14362
Funding information
This study was funded by Vichy Laboratoires, Levallois, France and supported by L’Oréal Research and Innovation.
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Wikramanayake TC, Borda LJ, Miteva M, Paus R. Seborrheic dermatitis‐looking beyond Malassezia. Exp Dermatol. 2019;28(9):991‐1001. [DOI] [PubMed] [Google Scholar]
- 2. Gaitanis G, Magiatis P, Hantschke M, Bassukas ID, Velegraki A. The Malassezia genus in skin and systemic diseases. Clin Microbiol Rev. 2012;25(1):106‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hay RJ. Malassezia, dandruff and seborrhoeic dermatitis: an overview. Br J Dermatol. 2011;165(Suppl 2):2‐8. [DOI] [PubMed] [Google Scholar]
- 4. Grice EA, Dawson TL Jr. Host‐microbe interactions: Malassezia and human skin. Curr Opin Microbiol. 2017;40:81‐87. [DOI] [PubMed] [Google Scholar]
- 5. Theelen B, Cafarchia C, Gaitanis G, Bassukas ID, Boekhout T, Dawson TL Jr. Malassezia ecology, pathophysiology, and treatment. Med Mycol. 2018;56(suppl_1):S10‐S25. [DOI] [PubMed] [Google Scholar]
- 6. Tajima M, Sugita T, Nishikawa A, Tsuboi R. Molecular analysis of Malassezia microflora in seborrheic dermatitis patients: comparison with other diseases and healthy subjects. J Invest Dermatol. 2008;128(2):345‐351. [DOI] [PubMed] [Google Scholar]
- 7. Magiatis P, Pappas P, Gaitanis G, et al. Malassezia yeasts produce a collection of exceptionally potent activators of the Ah (dioxin) receptor detected in diseased human skin. J Invest Dermatol. 2013;133(8):2023‐2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jourdain R, Moga A, Vingler P, et al. Exploration of scalp surface lipids reveals squalene peroxide as a potential actor in dandruff condition. Arch Dermatol Res. 2016;308(3):153‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Donnarumma G, Perfetto B, Paoletti I, et al. Analysis of the response of human keratinocytes to Malassezia globosa and restricta strains. Arch Dermatol Res. 2014;306(8):763‐768. [DOI] [PubMed] [Google Scholar]
- 10. Park T, Kim HJ, Myeong NR, et al. Collapse of human scalp microbiome network in dandruff and seborrhoeic dermatitis. Exp Dermatol. 2017;26(9):835‐838. [DOI] [PubMed] [Google Scholar]
- 11. Soares RC, Zani MB, Arruda AC, Arruda LH, Paulino LC. Malassezia intra‐specific diversity and potentially new species in the skin microbiota from Brazilian healthy subjects and seborrheic dermatitis patients. PLoS One. 2015;10(2):e0117921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanaka A, Cho O, Saito C, Saito M, Tsuboi R, Sugita T. Comprehensive pyrosequencing analysis of the bacterial microbiota of the skin of patients with seborrheic dermatitis. Microbiol Immunol. 2016;60(8):521‐526. [DOI] [PubMed] [Google Scholar]
- 13. Perez Perez GI, Gao Z, Jourdain R, et al. Body site is a more determinant factor than human population diversity in the healthy skin microbiome. PLoS One. 2016;11(4):e0151990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shibagaki N, Suda W, Clavaud C, et al. Aging‐related changes in the diversity of women's skin microbiomes associated with oral bacteria. Sci Rep. 2017;7(1):10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saxena R, Mittal P, Clavaud C, et al. Comparison of healthy and dandruff scalp microbiome reveals the role of commensals in scalp health. Front Cell Infect Microbiol. 2018;8:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soares RC, Camargo‐Penna PH, de Moraes VC, et al. Dysbiotic bacterial and fungal communities not restricted to clinically affected skin sites in dandruff. Front Cell Infect Microbiol. 2016;6:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clavaud C, Jourdain R, Bar‐Hen A, et al. Dandruff is associated with disequilibrium in the proportion of the major bacterial and fungal populations colonizing the scalp. PLoS One. 2013;8(3):e58203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grimshaw SG, Smith AM, Arnold DS, Xu E, Hoptroff M, Murphy B. The diversity and abundance of fungi and bacteria on the healthy and dandruff affected human scalp. PLoS One. 2019;14(12):e0225796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang L, Clavaud C, Bar‐Hen A, et al. Characterization of the major bacterial‐fungal populations colonizing dandruff scalps in Shanghai, China, shows microbial disequilibrium. Exp Dermatol. 2015;24(5):398‐400. [DOI] [PubMed] [Google Scholar]
- 20. Xu Z, Wang Z, Yuan C, et al. Dandruff is associated with the conjoined interactions between host and microorganisms. Sci Rep. 2016;6:24877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin Q, Panchamukhi A, Li P, et al. Malassezia and Staphylococcus dominate scalp microbiome for seborrheic dermatitis. Bioprocess Biosyst Eng. 2021;44(5):965‐975. [DOI] [PubMed] [Google Scholar]
- 22. Borda LJ, Perper M, Keri JE. Treatment of seborrheic dermatitis: a comprehensive review. J Dermatolog Treat. 2019;30(2):158‐169. [DOI] [PubMed] [Google Scholar]
- 23. Leong C, Schmid B, Buttafuoco A, Glatz M, Bosshard PP. In vitro efficacy of antifungal agents alone and in shampoo formulation against dandruff‐associated Malassezia spp. and Staphylococcus spp. Int J Cosmet Sci. 2019;41(3):221‐227. [DOI] [PubMed] [Google Scholar]
- 24. Pierard GE, Arrese JE, Pierard‐Franchimont C, De doncker P. Prolonged effects of antidandruff shampoos ‐ time to recurrence of Malassezia ovalis colonization of skin. Int J Cosmet Sci. 1997;19(3):111‐117. [DOI] [PubMed] [Google Scholar]
- 25. Pierard‐Franchimont C, Pierard GE, Arrese JE, De Doncker P. Effect of ketoconazole 1% and 2% shampoos on severe dandruff and seborrhoeic dermatitis: clinical, squamometric and mycological assessments. Dermatology. 2001;202(2):171‐176. [DOI] [PubMed] [Google Scholar]
- 26. Ortonne JP, Nikkels AF, Reich K, et al. Efficacious and safe management of moderate to severe scalp seborrhoeic dermatitis using clobetasol propionate shampoo 0.05% combined with ketoconazole shampoo 2%: a randomized, controlled study. Br J Dermatol. 2011;165(1):171‐176. [DOI] [PubMed] [Google Scholar]
- 27. Danby FW, Maddin WS, Margesson LJ, Rosenthal DA. A randomized, double‐blind, placebo‐controlled trial of ketoconazole 2% shampoo versus selenium sulfide 2.5% shampoo in the treatment of moderate to severe dandruff. J Am Acad Dermatol. 1993;29(6):1008‐1012. [DOI] [PubMed] [Google Scholar]
- 28. Schmidt A, Rühl‐Hörster B. In vitro susceptibility of Malassezia furfur. Arzneimittelforschung. 1996;46(4):442‐444. [PubMed] [Google Scholar]
- 29. Kamamoto CSL, Nishikaku AS, Gompertz OF, Melo AS, Hassun KM, Bagatin E. Cutaneous fungal microbiome: Malassezia yeasts in seborrheic dermatitis scalp in a randomized, comparative and therapeutic trial. Dermatoendocrinol. 2017;9(1):e1361573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zani MB, Soares RC, Arruda AC, de Arruda LH, Paulino LC. Ketoconazole does not decrease fungal amount in patients with seborrhoeic dermatitis. Br J Dermatol. 2016;175(2):417‐421. [DOI] [PubMed] [Google Scholar]
- 31. Melhorn S. Use of salicylic acid oils on the scalp. Hautarzt. 2017;68(3):248‐249. [DOI] [PubMed] [Google Scholar]
- 32. Reygagne P, Bastien P, Couavoux MP, et al. The positive benefit of Lactobacillus paracasei NCC2461 ST11 in healthy volunteers with moderate to severe dandruff. Benef Microbes. 2017;8(5):671‐680. [DOI] [PubMed] [Google Scholar]
- 33. Oksanen JB, Blanchet FG, Friendly M, et al. vegan. Community Ecology Package. R package version 24‐5; 2017.
- 34. Tamer F, Yuksel ME, Sarifakioglu E, Karabag Y. Staphylococcus aureus is the most common bacterial agent of the skin flora of patients with seborrheic dermatitis. Dermatol Pract Concept. 2018;8(2):80‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brown MM, Horswill AR. Staphylococcus epidermidis‐Skin friend or foe? PLoS Pathog. 2020;16(11):e1009026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Belkaid Y, Segre JA. Dialogue between skin microbiota and immunity. Science. 2014;346(6212):954‐959. [DOI] [PubMed] [Google Scholar]
- 37. Cau L, Williams MR, Butcher AM, et al. Staphylococcus epidermidis protease EcpA can be a deleterious component of the skin microbiome in atopic dermatitis. J Allergy Clin Immunol. 2021;147(3):955‐966.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Amaya M, Tajima M, Okubo Y, Sugita T, Nishikawa A, Tsuboi R. Molecular analysis of Malassezia microflora in the lesional skin of psoriasis patients. J Dermatol. 2007;34(9):619‐624. [DOI] [PubMed] [Google Scholar]
- 39. Chang HW, Yan D, Singh R, et al. Alteration of the cutaneous microbiome in psoriasis and potential role in Th17 polarization. Microbiome. 2018;6(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gomez‐Moyano E, Crespo‐Erchiga V, Martinez‐Pilar L, et al. Do Malassezia species play a role in exacerbation of scalp psoriasis? J Mycol Med. 2014;24(2):87‐92. [DOI] [PubMed] [Google Scholar]
- 41. Prohic A. Identification of Malassezia species isolated from scalp skin of patients with psoriasis and healthy subjects. Acta Dermatovenerol Croat. 2003;11(1):10‐16. [PubMed] [Google Scholar]
- 42. Matard B, Donay JL, Resche‐Rigon M, et al. Folliculitis decalvans is characterized by a persistent, abnormal subepidermal microbiota. Exp Dermatol. 2020;29(3):295‐298. [DOI] [PubMed] [Google Scholar]
- 43. Matard B, Cavelier‐Balloy B, Reygagne P. Epidermal psoriasiform hyperplasia, an unrecognized sign of folliculitis decalvans: a histological study of 26 patients. J Cutan Pathol. 2017;44(4):352‐357. [DOI] [PubMed] [Google Scholar]
- 44. Bäsler K, Galliano MF, Bergmann S, et al. Biphasic influence of Staphylococcus aureus on human epidermal tight junctions. Ann N Y Acad Sci. 2017;1405(1):53‐70. [DOI] [PubMed] [Google Scholar]
- 45. Alekseyenko AV, Perez‐Perez GI, De Souza A, et al. Community differentiation of the cutaneous microbiota in psoriasis. Microbiome. 2013;1(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kong HH, Oh J, Deming C, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22(5):850‐859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Suchonwanit P, Triyangkulsri K, Ploydaeng M, Leerunyakul K. Assessing biophysical and physiological profiles of scalp seborrheic dermatitis in the thai population. Biomed Res Int. 2019;2019:5128376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
SI Materials and Methods
Data Availability Statement
Data available on request from the authors.


