OBJECTIVES:
This narrative review article seeks to highlight the effects of citrate on physiology during massive transfusion of the bleeding patient.
DATA SOURCES:
A limited library of curated articles was created using search terms including “citrate intoxication,” “citrate massive transfusion,” “citrate pharmacokinetics,” “hypocalcemia of trauma,” “citrate phosphate dextrose,” and “hypocalcemia in massive transfusion.” Review articles, as well as prospective and retrospective studies were selected based on their relevance for inclusion in this review.
STUDY SELECTION:
Given the limited number of relevant studies, studies were reviewed and included if they were written in English. This is not a systematic review nor a meta-analysis.
DATA EXTRACTION AND SYNTHESIS:
As this is not a meta-analysis, new statistical analyses were not performed. Relevant data were summarized in the body of the text.
CONCLUSIONS:
The physiologic effects of citrate independent of hypocalcemia are poorly understood. While a healthy individual can rapidly clear the citrate in a unit of blood (either through the citric acid cycle or direct excretion in urine), the physiology of hemorrhagic shock can lead to decreased clearance and prolonged circulation of citrate. The so-called “Diamond of Death” of bleeding—coagulopathy, acidemia, hypothermia, and hypocalcemia—has a dynamic interaction with citrate that can lead to a death spiral. Hypothermia and acidemia both decrease citrate clearance while circulating citrate decreases thrombin generation and platelet function, leading to ionized hypocalcemia, coagulopathy, and need for further transfusion resulting in a new citrate load. Whole blood transfusion typically requires lower volumes of transfused product than component therapy alone, resulting in a lower citrate burden. Efforts should be made to limit the amount of citrate infused into a patient in hemorrhagic shock while simultaneously addressing the induced hypocalcemia.
Keywords: citrate phosphate dextrose, coagulopathy of trauma, hypocalcemia of trauma, blood transfusion
KEY POINTS
Question: While much of the focus in recent years has been on addressing hypocalcemia during massive transfusion, the extent to which citrate plays a separate and independent role in the physiology of the massively bleeding patient remains unclear.
Findings: Although some studies in 50–70 years ago found risk with transfusion of large amounts of citrate, it has largely not been investigated in the modern era, despite significant changes to resuscitation practices including the emphasis on transfusion of plasma and platelets with 1:1:1 transfusion, which contain a large citrate load.
Meaning: The focus on correcting hypocalcemia in massive transfusion is important, however, may be missing the mark as the pathophysiology of hypocalcemia in severe bleeding is poorly understood, and the under-appreciated risk of transfusing large citrate loads may be greater than just causing hypocalcemia.
Transfusion practices for resuscitation of the exsanguinating patient have shifted away from crystalloid resuscitation and toward damage control resuscitation with whole blood (WB) or balanced components in a 1:1:1 fashion (1–4), with an emphasis on early administration of platelets and fresh frozen plasma (FFP) (5). With this focus on early administration of blood products, massive transfusion protocols (MTPs, commonly defined as transfusion of at least 10 U of blood in a 24-hr period) have become widely implemented (5). The Prevalence of hypocalcemia in the massively transfused population has become a well-described phenomenon (6–12).
This is likely even more pronounced when using component therapy; compared with WB resuscitation, solely using component therapy typically results in transfusion of larger volumes of blood product (2, 13–16). More transfused products means more transfused citrate, which chelates free serum calcium and can worsen hypocalcemia. Retrospective studies have documented how hypocalcemia in massive transfusion is a risk factor for coagulopathy, death, additional transfusion requirements, increased ICU length of stay, ventilator days, need for emergent surgery, and even discharge destination (8, 11, 17, 18).
Some progress has been made; there is now an emphasis on measurement of ionized calcium (iCa) or total calcium/iCa ratio rather than total calcium alone (approximately 50% of circulating calcium biologically available) (19). Additionally, there now are guidelines for calcium supplementation during ongoing resuscitation from the Joint Trauma System Clinical Practice Guidelines (20). The lack of prospective randomized controlled trials investigating calcium supplementation in massive transfusion represents a critical gap in the implementation of MTPs, but a deeper understanding of calcium homeostasis and the pharmacodynamics of citrate during massive transfusion is necessary in order to address the root issues (21, 22).
Much of the literature agrees that citrate, which is an additive used to prevent spontaneous coagulation during banking, is at least partially responsible for the hypocalcemia noted in massively transfused patients (7, 9, 11, 18, 23, 24). Although a significant percentage of trauma patients present with hypocalcemia prior to transfusion of blood products (10, 25), there is no shortage of studies demonstrating citrated blood components inducing hypocalcemia in nontrauma patients and animals (26–29). Kahn et al (29) took intraoperative measurements of nontrauma patients receiving transfusions and found that the rate (peak rates were approximately 33 mL/kg/hr) and volume (patients included received at least 2,500 mL and more than half received > 5,000 mL) of transfusion significantly decreased iCa levels during and after transfusion, increased circulating citrate levels during transfusion, and significantly prolonged the corrected Q-T intervals, although no hemodynamic instability was noted.
Yet, relatively little attention in recent years has been paid to the deleterious effects of circulating citrate independent of its effect on serum calcium levels. This narrative review will describe citrate’s impact on physiology in the massively bleeding patient.
METHODS
PubMed was the primary source of information for our curated library. We began by searching for “citrate intoxication during massive transfusion” and began reviewing articles written in English. Search terms were expanded to include terms such as “citrate pharmacokinetics,” “hypocalcemia of trauma,” “citrate phosphate dextrose,” and “hypocalcemia in massive transfusion.” Studies broadly fit into two categories: case reports or small prospective studies done by infusing citrate into live animal and human models (typically written between 1945 and 1980) and retrospective cohort studies of large massive transfusion databases (typically written after 2000). Included articles were limited to those indexed in PubMed or Ovid, and with the exception of some unpublished data from our group, no unpublished studies were included in this review. The last day that PubMed was searched was April 13, 2023.
Background
Citrate is the deprotonated form of citric acid and typically exists in the body in equilibrium between citrate–3 + H+ ↔ citrate–2 (30). Citrate is a substrate in the citric acid cycle (CAC) where it is ultimately metabolized to bicarbonate and adenosine triphosphate (ATP) (30, 31). Citrate, with its three carboxyl groups, has a dissociation constant of ~3.1, 4.7, and 6.4, is an important buffer (30, 32). Given its importance in the CAC, the majority of citrate metabolism takes place in the liver, as well as in skeletal muscle and the kidneys (31, 32). Citrate is also excreted directly in urine without being metabolized (30).
In blood banking, citrate is used for its ability to prevent coagulation by chelating calcium, dropping the iCa to zero (31). Calcium plays an important role in several steps of coagulation including platelet signaling and generation of activated forms of factors II, VII, IX, X, and protein C and S.
The first nondirect transfusion of citrated blood took place in March of 1914 (33). Over the following decades, iterations of citrate-based preservatives were produced. In the 1950s and 1960s, Gibson et al (34–37) developed citrate phosphate dextrose (CPD) and citrate phosphate dextrose adenine (CPD-A), which improved on the tonicity and pH of storage solutions and allowed for storage for up to a month. CPD and CPD-A remain the backbone of anticoagulant solutions for blood banking in the United States, and the standards for approving new storage solutions have not significantly changed since they were developed (15).
As blood transfusions became more common, reports of “citrate intoxication” began appearing in the literature (38, 39). While healthy people can clear transfused citrate within minutes, Wexler et al (40) demonstrated in 1949 that newborns undergoing exchange transfusion for erythroblastosis fetalis had significantly increased serum concentrations of citrate during transfusion compared with healthy newborns receiving lower blood volumes. The elevated serum citrate levels were associated with tetany, and the one newborn who died within 24 hours of exchange transfusion demonstrated an inability to remove citrate from circulation. A postmortem revealed extensive liver damage in that newborn. Physicians took note, but dismissed the findings as a fringe case, with Yendt (39) stating in 1957 that the rate of 5–10 mg/kg/min of citrate was “unlikely to occur in adults.”
While that may have been the case in 1957, some quick math tells us that does not necessarily hold true today. The literature often states that a unit of packed RBCs (pRBCs) has 3,000 mg of citrate, although this is likely an overestimate (18, 23, 41). FFP and platelet concentrate (PC) have higher concentrations of citrate than pRBCs, as they have more plasma than packed red cells (18). Consider the following scenario where a 250 mL unit of FFP containing 1,000 mg of citrate is run through a rapid infuser over 30 seconds:
The dose of 25 mg/kg/min is 2.5× higher than what Yendt (39) considered to be a dangerous dose. Yendt (39) noted that when the rate of transfusion was increased to 500 mL in 15 minutes in adults, all patients in the study with liver disease and half without had a rise of serum citrate to levels he stated would be, “apt to result in significant reduction in the amount of ionized [emphasis his] calcium in the extracellular fluids (39).” As the liver is responsible for metabolizing the majority of citrate, it is intuitive that decreased liver function could lead to increased levels of circulating citrate (42). Kramer et al (43) measured peak citrate levels and citrate clearance of cirrhotic and noncirrhotic critically ill (but nonbleeding) ICU patients receiving a continuous infusion of sodium citrate. They found that while noncirrhotic patients had normal clearance and peak citrate levels, cirrhotic patients had significantly higher peak citrate concentrations (1.6 vs 1.01 mmol/L; p = 0.007), decreased citrate clearance (340 vs 710 mL/min; p = 0.002), and a prolonged citrate half-life (69 vs 36 min; p = 0.001). Furthermore, metabolic changes were similar between the two groups, although the cirrhotic group took longer to change pH and bicarbonate.
Thanapongsatorn et al (27) similarly found reduced rates of citrate clearance in patients with liver failure, while Zhang et al (44) found no difference in citrate clearance between healthy volunteers and patients with acute kidney injury, suggesting that liver function is the key determinant of citrate clearance.
Critically ill patients can be exposed to large volumes of citrate through another modality: continuous renal replacement therapy (CRRT). CRRT relies on anticoagulation to prevent coagulation in the circuit; the Kidney Disease Improving Global Outcomes organization currently recommends regional citrate anticoagulation (RCA) over heparin (44). While multiple studies and meta-analyses have found RCA to be safe, it has been associated with acid-base disturbances (both metabolic acidosis and alkalosis), increased total calcium/iCa ratio, hypocalcemia, increased levels of lactate during CRRT, and bleeding events during and after CRRT (44, 45). In a meta-analysis, Zhang et al (44) demonstrated no significant difference between pH, serum lactate, and total calcium/iCa between patients with and without liver failure on CRRT. These acid-base disturbances and decrease in iCa are well described in the renal replacement literature, so raised awareness may prevent worse outcomes, while in massively bleeding patients (trauma, massive gastrointestinal bleeds, postpartum hemorrhage), the citrate content of the resuscitative fluids is often an afterthought if it is considered at all.
In the 1960s and 1970s, resuscitation practices for the hemorrhaging patient moved away from resuscitation with blood products toward massive amounts of crystalloid (46). Several landmark studies in the 21st century including a study by Holcomb et al (1) have shifted resuscitation back toward balanced resuscitation with components in a 1:1:1 ratio or WB, reintroducing citrate into resuscitation (47).
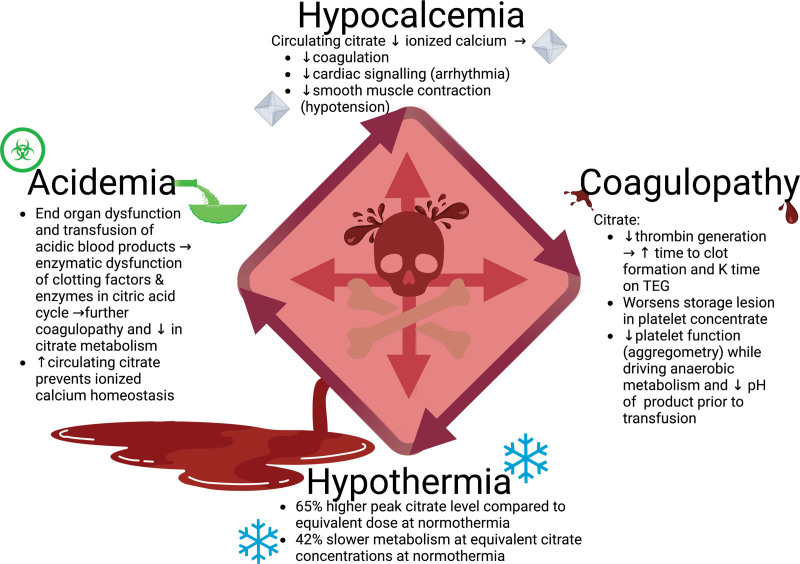
The Diamond of Death
In 2020, Ditzel et al (23) published a review of the literature around transfusion-induced hypocalcemia. They suggested updating the “lethal triad” of coagulopathy, hypothermia, and acidemia to include hypocalcemia; what the authors referred to as the “Diamond of Death.” They noted that hypocalcemia during the resuscitation of a trauma patient was common and associated with negative patient outcomes. While hypocalcemia plays role in the patient’s pathophysiology, citrate also likely has detrimental effects independent of the level of hypocalcemia (Fig. 1).
Figure 1.
Citrate’s interaction with the diamond of death. Figure created with biorender.com. TEG = thromboelastography.
Coagulopathy
Citrate has been demonstrated to affect clot formation independent of calcium level. Mann et al (48) performed a series of experiments where freshly collected donor blood was anticoagulated with either sodium citrate, corn trypsin inhibitor (CTI) which blocks the contact pathway “without” chelating calcium, or a combination of CTI and citrate. The CTI-citrate samples were divided into two subgroups where they were either preincubated with calcium to reverse the effect of citrate before reversing the CTI, while the other subgroup had both citrate and CTI reversed at the same time. To account for the effect of recalcification of samples with an excess of citrate (adding the same amount of calcium to a citrated sample would result in a lower iCa than a sample with less citrate), the authors performed titration studies to ensure that the iCa after recalcification was similar in all experimental groups.
They found that even when CTI-citrate samples were preincubated with calcium before reversing the effect of CTI, the samples exposed to citrate produced significantly less thrombin (with a longer lag time before thrombin generation) even when ensuring the same level of iCa. Aggregation studies revealed reduced aggregation in the presence of citrate. Thromboelastography analysis demonstrated a prolonged K-time (measure of kinetics, which is dependent on the amplification and thrombin burst phase of coagulation) in samples exposed to citrate, even if preincubated with calcium.
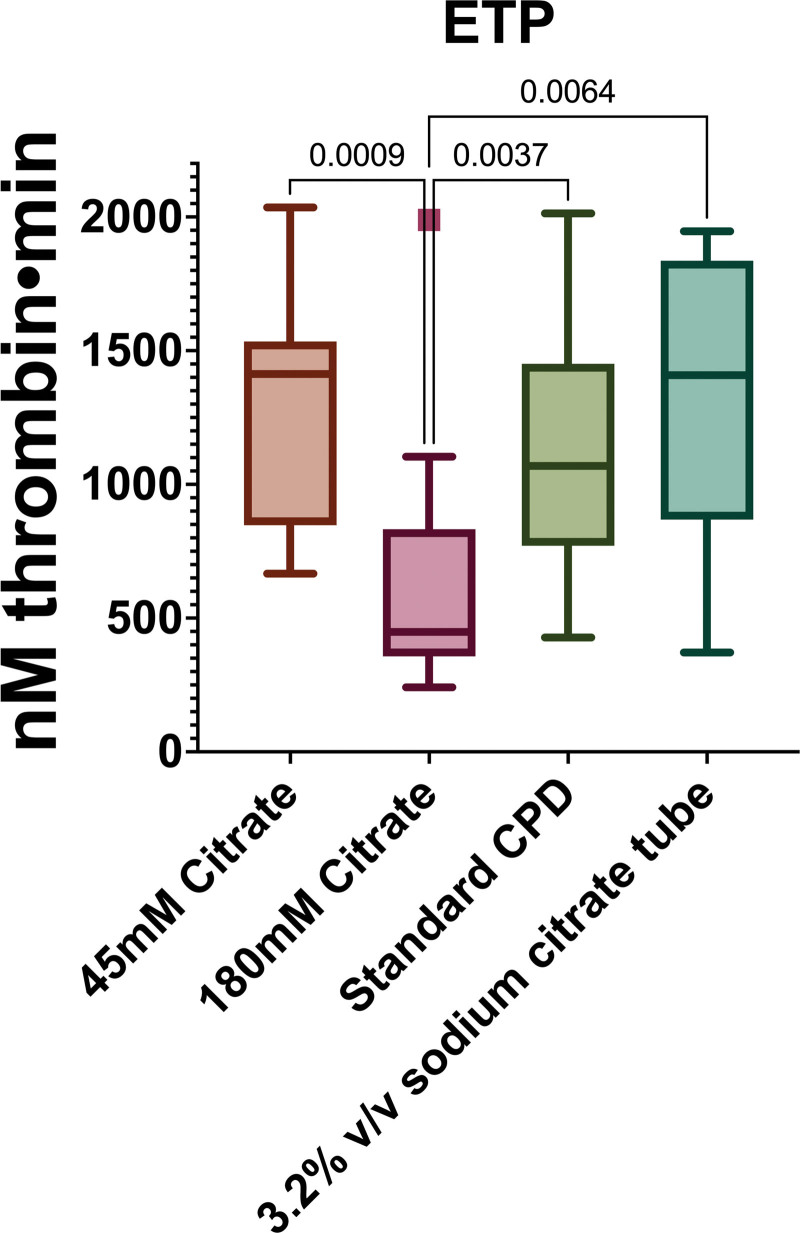
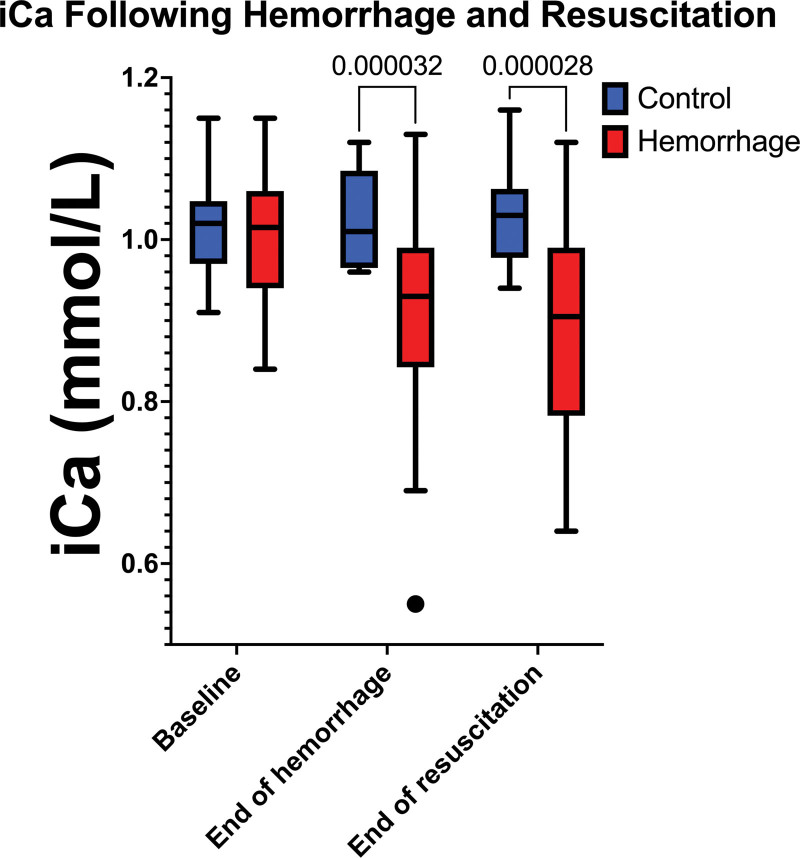
Unpublished work from our group shows a similar effect of citrate on clot formation. A homemade formulation of CPD with half as much stock citrate (45 mM vs the usual 90 mM, with the pH titrated to match the control) had higher peak thrombin generation and higher estimated thrombin potential as measured by calibrated automated thrombography (Fig. 2). Similarly, CPD with twice as much citrate failed to meaningfully generate thrombin or polymerize fibrin, suggesting that increasing levels of circulating citrate can attenuate thrombin generation, leading to coagulopathy. Additionally, in a controlled rat hemorrhage model where blood was withdrawn from an arterial catheter into a citrate tube and was autotransfused back into the rat over 3 hours, the transfused rats had a significantly lower iCa compared with sham rats with no hemorrhage or transfusion (Fig. 3).
Figure 2.
Unpublished internal data on the effect of different amounts of citrate on thrombin generation as measured by calibrated automated thrombography. Standard citrate phosphate dextrose (CPD) has a citrate concentration of 90 mM. Endogenous thrombin potential (ETP) is the area under the curve of the thrombin generation. While the assay is calibrated for 3.2% citrate volume/volume, the change in citrate in the samples can be taken to represent a change in a person’s ability to metabolize citrate, possibly resulting in a buildup of citrate.
Figure 3.
The ionized calcium (iCa) of male Sprague-Dawley rats before hemorrhage, at the end of hemorrhage, and at the end of resuscitation. The control rats had an incision and venipuncture, but no hemorrhage or resuscitation. The hemorrhage rats had their blood collected into citrated tubes which was then autotransfused back into them for resuscitation. n = 12 for control group and 40 for the hemorrhage group, Supplemental Figure 1 (http://links.lww.com/CCX/B200) shows endogenous thrombin potential as well as lag time, peak thrombin generation, time to peak, and velocity.
Several studies have examined the effect of citrate in the storage solution for PC. PC is either derived from apheresis or via centrifugation of WB into its components. PC has traditionally been stored in plasma, although several generations of platelet additive solution (PAS) have been developed to create an optimal storage environment (49).
Getz et al (50) demonstrated that addition of citrate to PAS resulted in a dose-dependent increase in glucose utilization, formation of reactive oxygen species, lactate formation, and expression of P-selectin (surface marker of activation). They went on to demonstrate that platelets stored in citrate-free PAS had improvements in all these metrics and had at least a 10% improvement in aggregation response. Isola et al (51) removed citrate from PAS and again demonstrated decreased lactate generation (and a corresponding improvement in pH of the PC), as well as reducing apoptosis and P-selectin expression while improving aggregation responses to multiple agonists.
The type of product being transfused can impact coagulopathy as well. Hemorrhage resuscitation moved away from WB transfusion to component therapy to allow for easier storage and transportation to remote locations like Vietnam during the war; however, it appears to have the unintended consequence of leading to worse outcomes for massively transfused patients (13–15). A unit of fresh WB compared with an equivalent amount of WB reconstituted from components has a higher hematocrit, higher platelet count, and better platelet and coagulant activity, which leads to less product being transfused and significantly improved outcomes (15). Byerly et al (18) found that pRBC transfusion was an independent risk factor for severe hypocalcemia in trauma patients and was associated with worse outcomes. This is relevant to current resuscitation practices of balanced 1:1:1 resuscitation; FFP and PC deliver significantly higher loads of citrate relative to historical practices of resuscitation with crystalloid and pRBCs, worsening the risk for hypocalcemia.
While the benefits of WB are multifactorial, the decreased volume of transfused product means a lower volume of citrate being introduced; it should be noted this effect is entirely theoretical and has not been adequately studied in a prospective manner. Additionally, given the limited availability of WB (either cold-stored or fresh) at most civilian centers, most studies of resuscitation with WB versus component are retrospective and the WB group typically gets a combination of WB and components versus components alone. Furthermore, there are differences between cold-stored WB and freshly collected WB. Fresh WB can be transfused as quickly as 30 minutes after activation of the emergency blood drive (15), meaning the components will not have been impacted by storage lesion, which has been demonstrated to be worsened by citrate.
Acidemia
Anticoagulant solutions such as CPD are all acidic; stock CPD solution has a pH between 5.0 and 6.0 prior to mixing with blood. Similarly, all blood products have a pH below physiologic prior to transfusion (52–54). Once intracellular, citrate inhibits glycolysis and stimulates fatty acid synthesis, potentially limiting cells from mobilizing stores of glucose (32). Additionally, many of the enzymes needed for metabolism of citrate in the CAC do not function well at subphysiologic pH.
Studies of massively transfused patients have noted that acidemia (typically defined as pH < 7.35) is an independent risk factor for mortality (9, 55, 56). However, this acidemia is a bit paradoxical, as much of the physiology of shock might lead one to expect a metabolic alkalosis (9, 32). As blood volume and renal perfusion pressure drop, kidneys reabsorb sodium and bicarbonate—the so-called “contraction alkalosis.” As citrate is transfused, it is metabolized to bicarbonate, using up 3 H+ ions in the process—again, which would lead to an expected alkalosis.
However, as anaerobic metabolism struggles to keep up with ATP demand, ion pumps such as H+/K+ and Na-K-ATP fail (57), leading to trapped ions, intracellular edema, and cell death (58, 59). As blood is transfused, lysed red cells can lead to a transfusion-related hyperkalemia. Serum potassium will shift into cells, and the resulting intracellular to extracellular potassium shift and corresponding H+ efflux to maintain normal serum potassium concentration would cause an acidosis (32). As shock leads to a type A hyperlactatemia, excess lactate ions may bind calcium and worsen hypocalcemia (22).
This may help explain the observed paradox of acidemia and hypocalcemia. Typically, as pH drops and the amount of free H+ in the serum increases, the H+ ions displace calcium bound to negatively charged serum proteins and increase the iCa (31), yet the sickest hemorrhagic patients are often acidemic and hypocalcemic due to failures of ion transport and increased lactate production in the anaerobic state. The acidemic patient may be unable to metabolize citrate as efficiently, leading to prolonged circulation of the molecule and subsequent worsening of hypocalcemia (32, 55).
While citrate may not significantly contribute directly to acidemia, a vicious cycle of acidemia results in prolonged circulation of citrate which prevents the body from normalizing iCa levels either through dissociation from serum proteins or mobilization from bone, leading to worse coagulopathy, shock physiology, and further acidemia.
Hypothermia
Hypothermia can have profound consequences on human physiology, including the coagulation cascade and worsening a bleeding patient’s coagulopathy (55, 60). Hypothermia has been demonstrated to reduce citrate metabolism: Ludbrook and Wynn (38) performed a series of experiments in dogs and humans to quantify rates of citrate metabolism and excretion. They infused acid citrate dextrose (ACD) at a constant rate and measured serum and urine levels of citrate until they reached a steady state. They were then able to extrapolate the rate of metabolism. They then took the same subjects and subjected them to hypothermia (given the difficulty of performing this in humans, the humans were given a bolus of ACD while the dogs were subjected to a constant infusion as was done at normothermia). They reported that at the same rate of infusion in the dogs, the peak citrate concentration was 65% higher in the presence of hypothermia, and at equivalent citrate levels, metabolism was 42% slower. The human subjects demonstrated similar changes in their serum citrate concentration.
CONCLUSIONS
Many institutions recognize the risk of hypocalcemia with citrated transfusions, although addressing the hypocalcemia alone rather than the buildup of citrate may be inadequate. The retrospective study by Chanthima et al (61) found that while greater than 83% of included patients had hypocalcemia at first measurement, there was no difference in survival between those who did and did not get calcium. However, it may be that patients are simply not getting enough calcium, as clinicians may be underestimating the persistence of citrate in a patient in hemorrhagic shock. In the study by Chanthima et al (61), only 51% of included patients received any calcium, and greater than 85% had hypocalcemia at the end of 3 hours of resuscitation, suggesting that even those who did get calcium did not get enough.
Short-term improvement in the care of the massively transfused patient should involve guided replacement of calcium, but the long-term solution to the deleterious effects of massive transfusion must involve addressing the root problem. This review is limited by a lack of large-scale randomized data; extrapolating from retrospective databases or small prospective studies in controlled conditions cannot fully describe the true burden of citrate buildup during massive transfusion. Blood products anticoagulated with citrate are more acidic than blood, contain an excess of a molecule that intentionally prevents coagulation (and has the unintended effect of attenuating platelet function and thrombin generation), and in our sickest patients potentially leads to a vicious cycle of worsening coagulopathy, acidemia, and hypocalcemia which leads to more product transfusion and worsening of those parameters. Efforts should be made to explore alternative anticoagulants that limit the ill effects of citrate in stored blood products.
ACKNOWLEDGMENTS
We would like to thank the support of CE Wade and the Center for Translational Injury Research.
Supplementary Material
Footnotes
Drs. Cox and Gill received sponsored research funding from Coagulex and Equity/Royalty (interest via UTHealth). UTHealth has an institutional conflicts of interest for holding equity in Coagulex (to Dr. Gill). Dr. Olson is supported by National Institute of Neurological Disorders and Stroke R21NS116302. Dr. Schriner was supported by a T32 fellowship from the National Institute of General Medical Sciences of the National Institutes of Health under award number 2T32GM008792. The remaining authors have disclosed that they do not have any potential conflicts of interest.
The views expressed in this article are those of the author(s) and do not reflect the official policy or position of the U.S. Army Medical Department, Department of the Army, Department of Defense, or the U.S. Government.
Drs. Schriner, Cotton, and Gill conceived of the review and all authors had input in drafting the article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
REFERENCES
- 1.Holcomb JB, Tilley BC, Baraniuk S, et al. ; PROPPR Study Group: Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: The PROPPR randomized clinical trial. JAMA 2015; 313:471–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brill JB, Tang B, Hatton G, et al. : Impact of incorporating whole blood into hemorrhagic shock resuscitation: Analysis of 1,377 consecutive trauma patients receiving emergency-release uncrossmatched blood products. J Am Coll Surg 2022; 234:408–418 [DOI] [PubMed] [Google Scholar]
- 3.Jones AR, Frazier SK: Increased mortality in adult patients with trauma transfused with blood components compared with whole blood. J Trauma Nurs 2014; 21:22–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braverman MA, Smith AA, Ciaraglia AV, et al. ; San Antonio Whole Blood Consortium: The regional whole blood program in San Antonio, TX: A 3-year update on prehospital and in-hospital transfusion practices for traumatic and non-traumatic hemorrhage. Transfusion 2022; 62:S80–S89 [DOI] [PubMed] [Google Scholar]
- 5.Meneses E, Boneva D, McKenney M, et al. : Massive transfusion protocol in adult trauma population. Am J Emerg Med 2020; 38:2661–2666 [DOI] [PubMed] [Google Scholar]
- 6.Escandon MA, Tapia AD, Fisher AD, et al. : An analysis of the incidence of hypocalcemia in wartime trauma casualties. Med J (Ft Sam Houst Tex) 2022:17–21 [PubMed] [Google Scholar]
- 7.Wray JP, Bridwell RE, Schauer SG, et al. : The diamond of death: Hypocalcemia in trauma and resuscitation. Am J Emerg Med 2021; 41:104–109 [DOI] [PubMed] [Google Scholar]
- 8.Kronstedt S, Roberts N, Ditzel R, et al. : Hypocalcemia as a predictor of mortality and transfusion. A scoping review of hypocalcemia in trauma and hemostatic resuscitation. Transfusion 2022; 62:S158–S166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasudeva M, Mathew JK, Groombridge C, et al. : Hypocalcemia in trauma patients: A systematic review. J Trauma Acute Care Surg 2021; 90:396–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Webster S, Todd S, Redhead J, et al. : Ionised calcium levels in major trauma patients who received blood in the emergency department. Emerg Med J 2016; 33:569–572 [DOI] [PubMed] [Google Scholar]
- 11.MacKay EJ, Stubna MD, Holena DN, et al. : Abnormal calcium levels during trauma resuscitation are associated with increased mortality, increased blood product use, and greater hospital resource consumption: A pilot investigation. Anesth Analg 2017; 125:895–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douville NJ, Davis R, Jewell E, et al. : Volume of packed red blood cells and fresh frozen plasma is associated with intraoperative hypocalcaemia during large volume intraoperative transfusion. Transfus Med 2021; 31:447–458 [DOI] [PubMed] [Google Scholar]
- 13.Cap AP, Beckett A, Benov A, et al. : Whole blood transfusion. Mil Med 2018; 183(Suppl_2):44–51 [DOI] [PubMed] [Google Scholar]
- 14.Shea SM, Staudt AM, Thomas KA, et al. : The use of low-titer group O whole blood is independently associated with improved survival compared to component therapy in adults with severe traumatic hemorrhage. Transfusion 2020; 60(Suppl 3):S2–S9 [DOI] [PubMed] [Google Scholar]
- 15.Spinella PC: Warm fresh whole blood transfusion for severe hemorrhage: U.S. military and potential civilian applications. Crit Care Med 2008; 36(7 Suppl):S340–S345 [DOI] [PubMed] [Google Scholar]
- 16.Salamea-Molina JC, Himmler AN, Valencia-Angel LI, et al. : Whole blood for blood loss: Hemostatic resuscitation in damage control. Colomb Med (Cali) 2020; 51:e4044511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magnotti LJ, Bradburn EH, Webb DL, et al. : Admission ionized calcium levels predict the need for multiple transfusions: A prospective study of 591 critically ill trauma patients. J Trauma 2011; 70:391–395; discussion 395–397 [DOI] [PubMed] [Google Scholar]
- 18.Byerly S, Inaba K, Biswas S, et al. : Transfusion-related hypocalcemia after trauma. World J Surg 2020; 44:3743–3750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baird GS: Ionized calcium. Clin Chim Acta 2011; 412:696–701 [DOI] [PubMed] [Google Scholar]
- 20.Cap AP, Gurney J, Spinella PC, et al. : Joint Trauma System Clinical Practice Guideline Damage Control Resuscitation. 2019. Available at: https://jts.amedd.army.mil/assets/docs/cpgs/Damage_Control_Resuscitation_12_Jul_2019_ID18.pdf. Accessed January 18, 2023
- 21.Hall C, Nagengast AK, Knapp C, et al. : Massive transfusions and severe hypocalcemia: An opportunity for monitoring and supplementation guidelines. Transfusion 2021; 61:S188–S194 [DOI] [PubMed] [Google Scholar]
- 22.DeBot M, Sauaia A, Schaid T, et al. : Trauma-induced hypocalcemia. Transfusion 2022; 62:S274–S280 [DOI] [PubMed] [Google Scholar]
- 23.Ditzel RM, Jr, Anderson JL, Eisenhart WJ, et al. : A review of transfusion- and trauma-induced hypocalcemia: Is it time to change the lethal triad to the lethal diamond? J Trauma Acute Care Surg 2020; 88:434–439 [DOI] [PubMed] [Google Scholar]
- 24.Vasudeva M, Mathew JK, Fitzgerald MC, et al. : Hypocalcaemia and traumatic coagulopathy: An observational analysis. Vox Sang 2020; 115:189–195 [DOI] [PubMed] [Google Scholar]
- 25.Conner JR, Benavides LC, Shackelford SA, et al. : Hypocalcemia in military casualties from point of injury to surgical teams in Afghanistan. Mil Med 2021; 186(Suppl 1):300–304 [DOI] [PubMed] [Google Scholar]
- 26.Bunker JP, Bendixen HH, Murphy AJ: Hemodynamic effects of intravenously administered sodium citrate. N Engl J Med 1962; 266:372–377 [DOI] [PubMed] [Google Scholar]
- 27.Thanapongsatorn P, Chaijamorn W, Sirivongrangson P, et al. : Citrate pharmacokinetics in critically ill liver failure patients receiving CRRT. Sci Rep 2022; 12:1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayes JK, Bremer RA, Wong KC, et al. : Continuous monitoring of serum ionized calcium in the dog during sodium citrate infusion using an extracorporeal blood shunt. Can Anaesth Soc J 1980; 27:458–463 [DOI] [PubMed] [Google Scholar]
- 29.Kahn RC, Jascott D, Carlon GC, et al. : Massive blood replacement: Correlation of ionized calcium, citrate, and hydrogen ion concentration. Anesth Analg 1979; 58:274–278 [DOI] [PubMed] [Google Scholar]
- 30.Weiner JW, IV: Renal Acidification Mechanisms. In: Brenner & Rector’s The Kidney. Eleventh Edition. Yu A. (Eds). Philadelphia, PA, Elsevier, 2020,. pp 262–263 [Google Scholar]
- 31.Monchi M: Citrate pathophysiology and metabolism. Transfus Apher Sci 2017; 56:28–30 [DOI] [PubMed] [Google Scholar]
- 32.Dzik WH, Kirkley SA: Citrate toxicity during massive blood transfusion. Transfus Med Rev 1988; 2:76–94 [DOI] [PubMed] [Google Scholar]
- 33.Van Hee R: The development of blood transfusion: The role of Albert Hustin and the influence of World War I. Acta Chir Belg 2015; 115:247–255 [DOI] [PubMed] [Google Scholar]
- 34.Gibson JG, 2nd: Citrate-phosphate-dextrose: An improved anticoagulant preservative solution for human blood. Vox Sang 1967; 13:106–107 [PubMed] [Google Scholar]
- 35.Gibson JG, 2nd, Gregory CB, Button LN: Citrate-phosphate-dextrose solution for preservation of human blood: A further report. Transfusion 1961; 1:280–287 [DOI] [PubMed] [Google Scholar]
- 36.Gibson JG, 2nd, Kevy S, Pennell RC: Citrate-phosphate-dextrose: An improved anticoagulant preservative solution for human blood. Bibl Haematol 1968; 29:758–763 [DOI] [PubMed] [Google Scholar]
- 37.Gibson JG, 2nd, Rees SB, Mc MT, et al. : A cltrate-phosphatedextrose solution for the preservation of human blood. Am J Clin Pathol 1957; 28:569–578 [DOI] [PubMed] [Google Scholar]
- 38.Ludbrook J, Wynn V: Citrate intoxication; a clinical and experimental study. Br Med J 1958; 2:523–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yendt ER: Citrate intoxication. Can Med Assoc J 1957; 76:141–144 [PMC free article] [PubMed] [Google Scholar]
- 40.Wexler IB, Pincus JB, Natelson S, et al. : The fate of citrate in erythroblastotic infants treated with exchange transfusion. J Clin Invest 1949; 28:474–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li K, Xu Y: Citrate metabolism in blood transfusions and its relationship due to metabolic alkalosis and respiratory acidosis. Int J Clin Exp Med 2015; 8:6578–6584 [PMC free article] [PubMed] [Google Scholar]
- 42.Chung HS, Cho SJ, Park CS: Effects of liver function on ionized hypocalcaemia following rapid blood transfusion. J Int Med Res 2012; 40:572–582 [DOI] [PubMed] [Google Scholar]
- 43.Kramer L, Bauer E, Joukhadar C, et al. : Citrate pharmacokinetics and metabolism in cirrhotic and noncirrhotic critically ill patients. Crit Care Med 2003; 31:2450–2455 [DOI] [PubMed] [Google Scholar]
- 44.Zhang W, Bai M, Yu Y, et al. : Safety and efficacy of regional citrate anticoagulation for continuous renal replacement therapy in liver failure patients: A systematic review and meta-analysis. Crit Care 2019; 23:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bianchi NA, Altarelli M, Eckert P, et al. : Complications of regional citrate anticoagulation for continuous renal replacement therapy: An observational study. Blood Purif 2020; 49:567–575 [DOI] [PubMed] [Google Scholar]
- 46.Thompson P, Strandenes G: The History of Fluid Resuscitation for Bleeding. In: Damage Control Resusucitation. Spinella P. (Ed). Springer, Cham, 2019, pp 3–29 [Google Scholar]
- 47.Shoemaker WC: Comparison of the relative effectiveness of whole blood transfusions and various types of fluid therapy in resuscitation. Crit Care Med 1976; 4: 71–78 [DOI] [PubMed] [Google Scholar]
- 48.Mann KG, Whelihan MF, Butenas S, et al. : Citrate anticoagulation and the dynamics of thrombin generation. J Thromb Haemost 2007; 5:2055–2061 [DOI] [PubMed] [Google Scholar]
- 49.Azuma H, Hirayama J, Akino M, et al. : Platelet additive solution - electrolytes. Transfus Apher Sci 2011; 44:277–281 [DOI] [PubMed] [Google Scholar]
- 50.Getz TM, Turgeon A, Wagner SJ: Sodium citrate contributes to the platelet storage lesion. Transfusion 2019; 59:2103–2112 [DOI] [PubMed] [Google Scholar]
- 51.Isola H, Ravanat C, Rudwill F, et al. : Removal of citrate from PAS-III additive solution improves functional and biochemical characteristics of buffy-coat platelet concentrates stored for 7 days, with or without INTERCEPT pathogen reduction. Transfusion 2021; 61:919–930 [DOI] [PubMed] [Google Scholar]
- 52.Bailey DN, Bove JR: Chemical and hematological changes in stored CPD blood. Transfusion 1975; 15:244–249 [DOI] [PubMed] [Google Scholar]
- 53.Gaudry PL, Duffy C, Joseph D: The pH and hydrogen ion content of stored A.C.D. blood. Anaesth Intensive Care 1974; 2:247–250 [DOI] [PubMed] [Google Scholar]
- 54.Gaudry PL, Duffy C, Joseph D: The pH and titratable acidity of stored CPD blood. Anaesth Intensive Care 1980; 8:353–355 [DOI] [PubMed] [Google Scholar]
- 55.Sihler KC, Napolitano LM: Complications of massive transfusion. Chest 2010; 137:209–220 [DOI] [PubMed] [Google Scholar]
- 56.Vivien B, Langeron O, Morell E, et al. : Early hypocalcemia in severe trauma. Crit Care Med 2005; 33:1946–1952 [DOI] [PubMed] [Google Scholar]
- 57.Sayeed MM, Senior RM, Chaudry IH, et al. : Active sodium-potassium transport and ATP levels in lung and liver during shock. Surg Forum 1974; 25:5–7 [PubMed] [Google Scholar]
- 58.Chaudry IH, Sayeed MM, Baue AE: Effect of hemorrhagic shock on tissue adenine nucleotides in conscious rats. Can J Physiol Pharmacol 1974; 52:131–137 [DOI] [PubMed] [Google Scholar]
- 59.Hagberg S, Haljamae H, Rockert H: Shock reactions in skeletal muscle. 3. The electrolyte content of tissue fluid and blood plasma before and after induced hemorrhagic shock. Ann Surg 1968; 168:243–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reynolds BR, Forsythe RM, Harbrecht BG, et al. ; Inflammation and Host Response to Injury Investigators: Hypothermia in massive transfusion: Have we been paying enough attention to it? J Trauma Acute Care Surg 2012; 73:486–491 [PubMed] [Google Scholar]
- 61.Chanthima P, Yuwapattanawong K, Thamjamrassri T, et al. : Association between ionized calcium concentrations during hemostatic transfusion and calcium treatment with mortality in major trauma. Anesth Analg 2021; 132:1684–1691 [DOI] [PubMed] [Google Scholar]