INTRODUCTION
Cardiovascular disease remains the most common cause of death among patients with diabetes (1–3). Two recently approved classes of medications for Type 2 diabetes mellitus (T2DM) reduce the risk of cardiovascular events. Glucagon-like peptide-1 agonists (GLP1RA) reduce death from cardiovascular causes, nonfatal myocardial infarction, and nonfatal stroke in patients T2DM(5–10). Sodium-glucose co-transporter 2 inhibitors (SGLT2i) reduce heart failure events by 27% to 39% and decrease the frequency of worsening renal disease by 39% compared to placebo in patients with T2DM. The 2018 American Diabetes Association Standard of Medical Care Consensus incorporated new ASCVD recommendation the use of GLP1RA and/or SGLT2I as adjunctive therapy to metformin for patients with T2DM and with high risk for cardiovascular disease(11).
Despite this evidence, uptake has generally been sub-optimal (12–13). There are critical questions regarding how therapy uptake has varied based on medical and sociodemographic characteristics. Prior analyses evaluated treatment with GLP1RA and SGLT2i among patients with T2DM treated by endocrinologists and cardiologists between 2015 and 2019 (14–15). They found overall lower likelihood of treatment among high-risk patients with heart failure (HF) and among historically disadvantaged racial and ethnic groups. However, several important questions remain. First, most patients with T2DM are primarily managed by primary care. It is unclear how treatment rates compare among these patients compared with patients treated by endocrinology and whether there is substantial variation in treatment rates within specialties. Second, it is unknown whether racial and ethnic disparities exist within clinician practices or if disparities are primarily related to who treats a given patient. Finally, the use of these therapies may have shifted after they were incorporated into clinical guidelines in 2018.
This study evaluated the use of GLP1RA and SGLT2i therapy among patients with T2DM in 2018–2019 using a commercial insurance database. We analyzed the association between medical and sociodemographic characteristics with the likelihood of GLP1RA/SGLT2i therapy to identify disparities and areas of risk-treatment paradox. We evaluated whether identified disparities persisted after adjusting for differences in clinician practices.
RESEARCH DESIGN AND METHODS
Data
We used the Optum de-identified Clinformatics® DataMart a database comprising administrative health claims for members of commercial and Medicare Advantage plans across all 50 states. The DataMart included medical and pharmacy claims, enrollment information, inpatient data, and clinician characteristics. The database includes approximately 87 million unique individuals from between 2003 and 2020. Data access requests are to be sent to Optum; statistical code will be made available upon request.
Study Population
We selected patients with diabetes aged 18 years or older with a clinic visit to a primary care clinician or endocrinologist between January 1, 2018 through January 1, 2019. We identified patients with T2DM based on at least one clinician diagnosis (using International Classification of Diseases [ICD]-Version 9 or Version 10 codes listed in Supplement Table 1) on an evaluation and management (E&M) claim or an inpatient claim preceding their clinic visit. To ensure the cohort had medically treated diabetes, we required at least one diabetes medication filled in the prior year: biguanides (i.e., Metformin), DPP4, sulfonylureas, thiazolidinediones, insulin, GLP1RA, or SGLT2i. Additionally, we required at least one medication filled in the subsequent 180 days. Finally, to limit missing data, we excluded patients without continuous insurance enrollment for 1 year before the index date and 180 days post-index date.
We linked each patient to a single clinician. For patients with endocrinology and primary care visits, we selected the endocrinologist. For patients with visits with multiple primary care clinicians or endocrinology clinicians, we selected the clinician with the most visits. If there was a tie in number of visits, we selected the clinician with the last encounter. We used the last clinic visit between January 1, 2018 through January 1, 2019 with the selected clinician as the index date.
Study Variables
We evaluated demographic characteristics, including age, sex, and race/ethnicity. Race and ethnicity are defined in mutually exclusive categories in the database: Asian, Black, Hispanic (any race), and Non-Hispanic White. We also analyzed sociodemographic data, which included level of educational attainment (less than 12th grade, high school diploma, less than Bachelor Degree, Bachelor Degree Plus, and unknown), and household income level (<$40,000, $40,000–49,000, $50,000–59,000, $60,000–74,000, $75,000–99,000 and >$100,000).
We evaluated medical history based on diagnoses within the 1 year prior to the index clinic visit. We included a wide array of medical comorbidities (codes in Supplement Table 1). In addition to diagnoses, we also identified certain conditions based on procedures using Current Procedural Terminology (CPT) or ICD-Procedure Coding System (ICD-PCS) or admissions based on the Medicare Severity-Diagnosis Related Group (MS-DRG). For example, we characterized patients as having IHD if they underwent coronary revascularization or had an acute myocardial infarction admission. We identified patients on dialysis based on either dialysis diagnosis codes or procedures codes.
We separated patients with IHD and HF into higher and lower risk groups. For IHD, we stratified patients into three groups: those with revascularization or IHD-related admissions within the prior 12 months, those with IHD diagnoses without IHD-related hospitalization or revascularization, and those without IHD. For HF, we stratified patients into those with a heart failure hospitalization within the prior 12 months, those with a HF diagnosis without a recent HF hospitalization, and those without HF.
We estimated diabetes severity using the adjusted Diabetes Complications Severity Index (aDCSI), which estimates the burden of diabetes-related complications using claims diagnoses. It has been shown to be predict mortality and hospitalization among patients with diabetes(17). We categorized aDCSI: 0, 1–2, 3–4, and ≥5).
We identified medication fills using prescription data. We captured both medications filled in the 180 days following the index date and the 12 months before the index date. Our primary metric was a medication fill of either GLP1RA or SGLT2i within 180 days of the index clinic visit as the primary outcome. As a secondary outcome, we evaluated GLP1RA and SGLT2i therapy individually.
Statistical Analysis
We calculated summary statistics of our baseline patient cohort with mean and standard deviation for normally distributed statistics, median and interquartile range for statistics with skewed distributions, and frequencies for count variables. We compared patient characteristics - age, sex, race/ethnicity, income, education level, and medical comorbidities – across those who received SGLT2i or GLP1RA versus those who did not using effect size estimates - Cramer’s V for categorical variables and Cohen’s d for continuous variables. We assumed a difference of over 0.1 was considered a meaningful difference and a difference exceeding 0.5 was a large difference (18).
We conducted multivariable logistic regression to examine factors associated with SGLT2i/GLP1RA therapy within 180 days of the index clinic visit with nested models. For all models, we adjusted for patient age, sex, census division, whether they were evaluated by an endocrinologist during the study period, and if they received insulin therapy. In each model, we clustered standard errors by the assigned clinician.
We first evaluated the association between high-risk medical characteristics (CKD, HF, and IHD) with GLP1RA/SGLT2i therapy with adjustment for other medical comorbidities. We then ran three alternate models to evaluate these associations. First, we added adjustment for sociodemographic characteristics (sex, race/ethnicity, and household income). Second, we used a mixed effects model with a random intercept for clinician to evaluate the within-practice effects of high-risk medical characteristics. Finally, we excluded patients with severe, life-threatening comorbidities (cancer, dementia, and end-stage renal disease). We then evaluated the association between these high-risk characteristics with each therapy individually. Finally, we evaluated the association between the aDCSI and GLP1RA/SGLT2i therapy without adjusting for other medical comorbidities given the substantial multicollinearity between diabetes complications and other comorbidities.
Second, we evaluated the association between sociodemographic characteristics with GLP1RA/SGLT2i therapy. First, we evaluated the association between age and sex with GLP1RA/SGLT2i therapy while adjusting for medical comorbidities. We then sequentially evaluated the association between our outcome and race/ethnicity, income, and education. In each model, we adjusted for sex and age but not other medical comorbidities given these diagnoses may be related to the quality of diabetes care and the documentation of these diagnoses may vary across sociodemographic characteristics. We performed two sensitivity analyses of the race/ethnicity analysis. First, we adjusted for medical comorbidities. Second, we adjusted for individual clinician practices using a mixed effects model with a random intercept for the treating clinician. By adjusting for differences between clinician practices, the model measured the within-clinician associations between race/ethnicity and GLP1RA/SGLT2i treatment; in other words, this model estimated whether patients of different races had a different likelihood of receiving GLP1RA/SGLT2i from the same clinician. Finally, we modeled interactions between the presence of cardiovascular disease (IHD or HF), income, and race/ethnicity; we plotted the adjusted probabilities of GLP1RA/SGLT2i treatment as a function of these three variables.
The final set of analyses evaluated clinician variation in treatment rates after adjustment for patient characteristics. We calculated the median odds ratio from the mixed effects model with a random intercept for treating clinician. Conceptually, the median odds ratio represents the difference in odds of a patient receiving therapy when treated by two randomly selected clinicians. It captures clinician-level variance, not between-individual variance within a clinician’s practice, and is always ≥ 1. We ran separate models for endocrinologists and primary care clinicians.
Analyses were conducted in STATA. A p-value of <0.05 was considered statistically significant. Data access for this project was provided by the Stanford Center for Population Health Sciences Data Core. This study was approved by the Stanford Institutional Review Board.
RESULTS
We analyzed a final cohort of 793,525 patients with a diagnosis of T2DM and receipt of a diabetes medication (see Supplement Figure 1). Table 1 displays the patient characteristics. There were 182,726 (26.48%) patients with prior insulin use. A large proportion (360,140 [44.3%]) had no diabetes-related complications (aDCSI of 0) and only 125,843 (15.9%) were seen by an endocrinologist.
Table 1.
Patient Characteristics
| Total | No GLP1RA or SGLT2i | GLP1RA or SGLT2i | SMD | |
|---|---|---|---|---|
| N=793,525 | N=667,889 | N=125,636 | ||
| Age, years | 67.1 (12.5) | 68.1 (12.4) | 61.4 (11.3) | 0.55 |
| Census Division | 0.06 | |||
| East North Central | 103,009 (13.0%) | 87,070 (13.0%) | 15,939 (12.7%) | |
| East South Central | 30,658 (3.9%) | 24,468 (3.7%) | 6,190 (4.9%) | |
| Middle Atlantic | 57,789 (7.3%) | 49,992 (7.5%) | 7,797 (6.2%) | |
| Mountain | 67,135 (8.5%) | 57,713 (8.6%) | 9,422 (7.5%) | |
| New England | 23,726 (3.0%) | 20,861 (3.1%) | 2,865 (2.3%) | |
| Pacific | 90,405 (11.4%) | 78,744 (11.8%) | 11,661 (9.3%) | |
| South Atlantic | 241,212 (30.4%) | 202,609 (30.3%) | 38,603 (30.7%) | |
| Unknown | 3,081 (0.4%) | 2,693 (0.4%) | 388 (0.3%) | |
| West North Central | 44,649 (5.6%) | 36,219 (5.4%) | 8,430 (6.7%) | |
| West South Central | 131,861 (16.6%) | 107,520 (16.1%) | 24,341 (19.4%) | |
| Race/Ethnicity | 0.03 | |||
| Asian | 35,489 (4.5%) | 30,944 (4.6%) | 4,545 (3.6%) | |
| Black | 127,068 (16.0%) | 108,945 (16.3%) | 18,123 (14.4%) | |
| Hispanic | 133,884 (16.9%) | 114,040 (17.1%) | 19,844 (15.8%) | |
| Missing | 23,914 (3.0%) | 19,831 (3.0%) | 4,083 (3.2%) | |
| Non-Hispanic White | 473,170 (59.6%) | 394,129 (59.0%) | 79,041 (62.9%) | |
| Endocrinology Visit | 109,821 (13.8%) | 78,260 (11.7%) | 31,561 (25.1%) | 0.38 |
| aDSCI Score | 0.07 | |||
| 0 | 360,140 (45.4%) | 295,586 (44.3%) | 64,554 (51.4%) | |
| 1 | 99,331 (12.5%) | 82,054 (12.3%) | 17,277 (13.8%) | |
| 2 | 139,038 (17.5%) | 118,841 (17.8%) | 20,197 (16.1%) | |
| 3–4 | 115,330 (14.5%) | 100,387 (15.0%) | 14,943 (11.9%) | |
| 5+ | 79,686 (10.0%) | 71,021 (10.6%) | 8,665 (6.9%) | |
| Medication Fills in Last 90 days | ||||
| Number of Diabetes Therapies | 1.4 (0.9) | 1.2 (0.8) | 2.1 (1.1) | 0.60 |
| Insulin | 185,151 (23.3%) | 147,698 (22.1%) | 37,453 (29.8%) | 0.18 |
| Meglitinide | 5,584 (0.7%) | 4,513 (0.7%) | 1,071 (0.9%) | 0.02 |
| Metformin | 440,253 (55.5%) | 372,012 (55.7%) | 68,241 (54.3%) | −0.03 |
| Thiazolidine | 43,425 (5.5%) | 34,512 (5.2%) | 8,913 (7.1%) | 0.08 |
| DPP4 Inhibitors | 92,548 (11.7%) | 72,387 (10.8%) | 20,161 (16.0%) | 0.16 |
| Sulfonylureas | 194,954 (24.6%) | 164,193 (24.6%) | 30,761 (24.5%) | 0.00 |
| GLP1RA | 57,581 (7.3%) | 4,607 (0.7%) | 52,974 (42.2%) | 1.54 |
| SGLT2i | 53,730 (6.8%) | 3,979 (0.6%) | 49,751 (39.6%) | 1.50 |
| Comorbidities | ||||
| Alcohol Use Disorder | 15,348 (1.9%) | 13,307 (2.0%) | 2,041 (1.6%) | −0.03 |
| Cardiac Arrhythmias | 78,880 (9.9%) | 70,246 (10.5%) | 8,634 (6.9%) | −0.12 |
| Bipolar Disorder | 121,717 (15.3%) | 101,442 (15.2%) | 20,275 (16.1%) | 0.02 |
| Cancer | 69,668 (8.8%) | 61,346 (9.2%) | 8,322 (6.6%) | −0.09 |
| Cerebrovascular Disease | 57,828 (7.3%) | 51,280 (7.7%) | 6,548 (5.2%) | −0.10 |
| Chronic Kidney Disease | 218,574 (27.5%) | 189,774 (28.4%) | 28,800 (22.9%) | −0.12 |
| End-stage Renal Disease | 4,113 (0.5%) | 3,967 (0.6%) | 146 (0.1%) | −0.07 |
| Chronic Liver Disease | 31,614 (4.0%) | 25,334 (3.8%) | 6,280 (5.0%) | 0.06 |
| Chronic Obstructive Pulmonary Disease | 90,630 (11.4%) | 80,071 (12.0%) | 10,559 (8.4%) | −0.11 |
| Dementia | 23,352 (2.9%) | 21,954 (3.3%) | 1,398 (1.1%) | −0.13 |
| Heart Failure | ||||
| No recent admission | 85,467 (10.8%) | 75,204 (11.3%) | 10,263 (8.2%) | −0.10 |
| With admission | 10,574 (1.3%) | 9,729 (1.5%) | 845 (0.7%) | −0.07 |
| Hyperlipidemia | 589,444 (74.3%) | 490,712 (73.5%) | 98,732 (78.6%) | 0.09 |
| Hypertension | 145,102 (18.3%) | 127,910 (19.2%) | 17,192 (13.7%) | −0.14 |
| Ischemic Heart Disease | ||||
| No IHD Admission or Revascularization | 113,522 (14.3%) | 97,826 (14.6%) | 15,696 (12.5%) | −0.06 |
| With Admission or Revascularization | 63,587 (8.0%) | 54,911 (8.2%) | 8,676 (6.9%) | −0.05 |
| Neurologic Disorder | 110,449 (13.9%) | 95,176 (14.3%) | 15,273 (12.2%) | −0.06 |
| Obesity | 280,602 (35.4%) | 222,231 (33.3%) | 58,371 (46.5%) | 0.26 |
| Peripheral Arterial Disease | 196,327 (24.7%) | 169,196 (25.3%) | 27,131 (21.6%) | −0.08 |
| Psychotic Disorder | 7,365 (0.9%) | 6,397 (1.0%) | 968 (0.8%) | −0.02 |
| Thyroid Disorder | 151,266 (19.1%) | 127,113 (19.0%) | 24,153 (19.2%) | 0.01 |
| Educational Attainment | 0.02 | |||
| Less than High School | 8,400 (1.1%) | 7,231 (1.1%) | 1,169 (0.9%) | |
| High School | 302,668 (38.1%) | 257,546 (38.6%) | 45,122 (35.9%) | |
| College Degree | 401,548 (50.6%) | 336,452 (50.4%) | 65,096 (51.8%) | |
| Graduate School | 78,282 (9.9%) | 64,427 (9.6%) | 13,855 (11.0%) | |
| Unknown | 2,627 (0.3%) | 2,233 (0.3%) | 394 (0.3%) | |
| Household Income, Thousand $ | 0.07 | |||
| <40 | 229,148 (28.9%) | 198,385 (29.7%) | 30,763 (24.5%) | |
| ≥$40 and <50 | 62,259 (7.8%) | 53,684 (8.0%) | 8,575 (6.8%) | |
| ≥$50 and <60 | 66,250 (8.3%) | 56,979 (8.5%) | 9,271 (7.4%) | |
| ≥60 and <75 | 84,417 (10.6%) | 71,600 (10.7%) | 12,817 (10.2%) | |
| ≥75 and <100 | 106,889 (13.5%) | 88,810 (13.3%) | 18,079 (14.4%) | |
| ≥$100 | 144,405 (18.2%) | 114,384 (17.1%) | 30,021 (23.9%) | |
| Unknown | 100,157 (12.6%) | 84,047 (12.6%) | 16,110 (12.8%) | |
Of the total cohort, 125,636 patients (15.8%) received either GLP1RA or SGLT2i therapy during the study period (9.5% received a GLP1RA and 8.6% an SGLT2i). The average age for those who did and did not receive either an SGLT2i or GLP1RA during the study period was 61.4 years and 68.1 years, respectively. Among the 109,821 patients seen by an endocrinologist, 28.7% (31,561) received GLP1RA/SGLT2i therapy compared with 13.8% among those who did not see an endocrinologist. Among patients with a history of HF or IHD, 11.6% (11,108 of 96,041) and 13.8% (24,372 of 177,709) patients received a GLP1RA/SGLT2i, respectively, compared with 16.7% (125,636 of 578,997) among patients without either condition. Among the 279,006 patients treated with more than 1 diabetes medication in the 90 days preceding the index visit, GLP1RA/SGLT2i therapy was used by 32.6% of patients.
Association between Comorbidities and SGLT2i or GLP1RA Prescription
We subsequently performed analyses evaluating the association between medical characteristics and GLP1RA/SGLT2i therapy (Table 2). After adjusting for age and other comorbidities, patients with IHD had slightly higher odds of receiving SGLT2i/GLP1RA therapy compared with those without IHD. Patients with a recent IHD-related admission or revascularization had an adjusted odds ratio [AOR] of 1.05 (95% CI: 1.02–1.07) while other patients with IHD had an aOR of 1.07 (95% CI: 1.05–1.09) compared with patients without an IHD-related admission or diagnosis. Patients with HF with and without a recent HF admission were less likely to receive GLP1RA/SGLT2i (aOR: 0.62 [95% CI: 0.57–0.67] and 0.93 [95% CI 0.90–0.95], respectively) compared with those without HF.
Table 2.
Association Between Medical Characteristics and GLP1RA/SGLT2i Treatment
| Model A1 (n=793,525) |
Model B: Model A + Sociodemographic Characteristics1 (n=793,525) |
Model C: Model A + Clinician Practice Adjustment1 (n=793,525) |
Model D: Model A Excluding Cancer, Dementia, and ESRD1 (n=699,982) |
|
|---|---|---|---|---|
| Chronic Kidney Disease | 1.04 (1.02–1.06) | 1.05 (1.03–1.07) | 1.05 (1.03–1.07) | 1.04 (1.02–1.06) |
| Heart failure | ||||
| With Recent Admission | 0.62 (0.57–0.67) | 0.63 (0.58–0.68) | 0.62 (0.57–0.67) | 0.60 (0.55–0.65) |
| Without Recent Admission | 0.93 (0.90–0.95) | 0.94 (0.91–0.96) | 0.94 (0.91–0.96) | 0.92 (0.90–0.95) |
| Ischemic Heart Disease | ||||
| Admission or Revascularization | 1.05 (1.02–1.07) | 1.04 (1.01–1.07) | 1.05 (1.02–1.08) | 1.06 (1.03–1.09) |
| Without Admission or Revascularization | 1.07 (1.05–1.09) | 1.07 (1.04–1.09) | 1.07 (1.05–1.10) | 1.07 (1.04–1.09) |
Adjusted for patient age, sex, census division, insulin treatment, medical comorbidities, and whether the patient was treated by endocrinology.
For IHD and HF, the likelihood of treatment was similar when evaluating each therapy individually. IHD was associated with higher likelihood of GLP1RA therapy (aOR: 1.03; 95% CI: 1.01–1.05) and SGLT2i therapy (aOR: 1.10; 95% CI: 1.07–1.12). HF was associated with lower likelihood of GLP1RA therapy (aOR: 0.88; 95% CI: 0.86–0.91) and SGLT2i therapy (aOR: 0.72; 95% CI: 0.70–0.75).
The association between CKD and treatment differed between GLP1RA and SGLT2i therapy. Patients with CKD, without evidence of end-stage renal disease, had a slightly higher odds of GLP1RA/SGLT2i therapy (aOR: 1.04 [95% CI: 1.02–1.06]. CKD was associated with higher odds of GLP1RA treatment (aOR: 1.21; 95% CI: 1.19–1.24), but lower odds of SGLT2i treatment (aOR: 0.74; 95% CI: 0.72–0.76).
In a separate model, we evaluated the association between the number of diabetes-related complications and the odds of GLP1RA/SGLT2i therapy. Patients with the highest burden of diabetes-related complications (aDCSI ≥5) had lower odds (aOR: 0.81; 95% CI: 0.77–0.82) of receiving GLP1RA/SGLT2i therapy compared with patients without diabetes-related complications (aDCSI of 0) (Supplement Table 2). This finding was consistent after excluding patients with cancer, dementia, and end-stage renal disease (aOR: 0.85; 95% CI: 0.83–0.88 for aDCSI of ≥5 compared with aDCSI of 0).
Sociodemographic Characteristics
The association between sociodemographic characteristics and GLP1RA/SGLT2i therapy is shown in Table 3. Older patients were less likely to receive GLP1RA/SGLT2i therapy during the study period (aOR: 0.33 [95% CI 0.32 – 0.66] for patients aged 75–85; aOR: 0.14 [95% CI 0.13 – 0.15] for patients aged ≥ 85 years compared with patients ≤ 45 years) after adjustment for medical comorbidities.
Table 3.
Association Between Sociodemographic Characteristics and GLP1RA/SGLT2i Therapy
| Model A: Race/Ethnicity1 (n=793,525) |
Model B: Income1 (n=793,525) |
Model C: Education1 (n=793,525) |
Model D: Model A + Medical Comorbidities1 (n=793,525) | Model E: Model A + Clinician Practice Adjustment1 (n=793,525) |
|
|---|---|---|---|---|---|
| Sex, Women (Ref: Men) | 0.95 (0.94–0.97) | 0.97 (0.96–0.99) | 0.95 (0.93–0.96) | 0.95 (0.94–0.96) | 0.95 (0.94–0.96) |
| Age (Ref: <45 years old) | |||||
| ≥45 to <55 years | 1.60 (1.55–1.65) | 1.59 (1.54–1.64) | 1.61 (1.56–1.66) | 1.50 (1.46–1.55) | 1.64 (1.60–1.69) |
| ≥55 to <65 years | 1.28 (1.24–1.32) | 1.28 (1.24–1.32) | 1.30 (1.26–1.34) | 1.22 (1.19–1.26) | 1.31 (1.28–1.35) |
| ≥65 to <75 years | 0.65 (0.63–0.67) | 0.66 (0.64–0.68) | 0.66 (0.64–0.68) | 0.64 (0.62–0.66) | 0.65 (0.63–0.67) |
| ≥75 to <85 years | 0.31 (0.30–0.32) | 0.32 (0.31–0.33) | 0.32 (0.31–0.33) | 0.33 (0.32–0.34) | 0.31 (0.30–0.32) |
| ≥85 years | 0.12 (0.11–0.13) | 0.13 (0.12–0.14) | 0.12 (0.12–0.13) | 0.14 (0.13–0.15) | 0.12 (0.11–0.13) |
| Race/Ethnicity (Ref: non-Hispanic White) | |||||
| Asian | 0.80 (0.77–0.84) | --- | --- | 0.83 (0.80–0.87) | 0.78 (0.75–0.81) |
| Black | 0.80 (0.79–0.82) | --- | --- | 0.82 (0.80–0.84) | 0.80 (0.78–0.81) |
| Hispanic | 0.88 (0.86–0.90) | --- | --- | 0.87 (0.84–0.89) | 0.88 (0.86–0.90) |
| Missing | 0.97 (0.93–1.00) | --- | --- | 0.97 (0.93–1.01) | 0.97 (0.94–1.01) |
| Household Income, thousand $ (Ref: ≥100) | |||||
| ≥75 to <100 | --- | 0.91 (0.89–0.93) | --- | --- | --- |
| ≥60 to <75 | --- | 0.86 (0.83–0.88) | --- | --- | --- |
| ≥50K to <60K | --- | 0.80 (0.78–0.82) | --- | --- | --- |
| ≥40 to <50 | --- | 0.77 (0.75–0.79) | --- | --- | --- |
| <40 | --- | 0.75 (0.73–0.76) | --- | --- | --- |
| Missing | --- | 0.77 (0.75–0.79) | --- | --- | --- |
| Educational Attainment (Ref: Graduate Degree) | |||||
| Less than High School | --- | --- | 0.85 (0.78–0.92) | --- | --- |
| High School | --- | --- | 0.88 (0.85–0.90) | --- | --- |
| College | --- | --- | 0.97 (0.95–1.00) | --- | --- |
| Missing | --- | --- | 0.87 (0.77–0.97) | --- | --- |
Adjusted for patient age, sex, census division, insulin treatment, and whether the patient was treated by endocrinology.
We also found disparities across sex and race/ethnicity (Table 3). Women had lower odds of GLP1RA/SGLT2i treatment (aOR: 0.94, 95% CI 0.93 – 0.95). This was similar after adjusting for patient income, education, or medical comorbidities (Table 3). Asian, Black, and Hispanic patients also had lower odds of GLP1RA/SGLT2i therapy compared with non-Hispanic White patients (Asian aOR: 0.80 [95% CI 0.77 – 0.84]; Black: aOR: 0.80 [95% CI 0.79–0.82]; Hispanic: 0.88 [95% CI: 0.86 – 0.90]).
The disparities were similar within clinician practices. After adjusting for between-clinician differences, the associations between sex and race/ethnicity with GLP1RA/SGLT2i therapy remained relatively unchanged. Within a clinician’s practice, both women (aOR: 0.95, 95% CI: 0.94–0.95) and Black patients (aOR 0.80, 95% CI: 0.78–0.81) had lower odds of GLP1RA/SGLT2i therapy (Table 3).
In terms of household income, we found a significant dose-dependent association between income and the odds of GLP1RA/SGLT2i therapy. Compared with patients with income <$40,000, the aOR of SGLT2 or GLP1 prescription during the study period was 1.18 (95% CI: 1.15–1.21) for patients with a household income ≥$75,000 and <$100,000 and 1.30 (95% CI 1.27–1.33, p<0.01) for patients with a household income ≥$100,000. Educational attainment was also associated with likelihood of GLP1RA/SGLT2i therapy. Patients with a high school-level education had lower odds (aOR: 0.85; 95% CI: 0.78–0.82).
We analyzed the interaction between cardiovascular disease (ischemic heart disease or heart failure), race/ethnicity, and household income by calculating the adjusted probability of GLP1RA/SGLT2i therapy based on these three variables (Figure 1). There was a significant interaction between low household income and Black race (OR 0.90; 95% CI: 0.82–0.98 compared with non-Hispanic White), indicating low-income Black patients had lower GLP1RA/SGLT2i therapy rates than low-income, non-Hispanic White patients.
Figure 1.
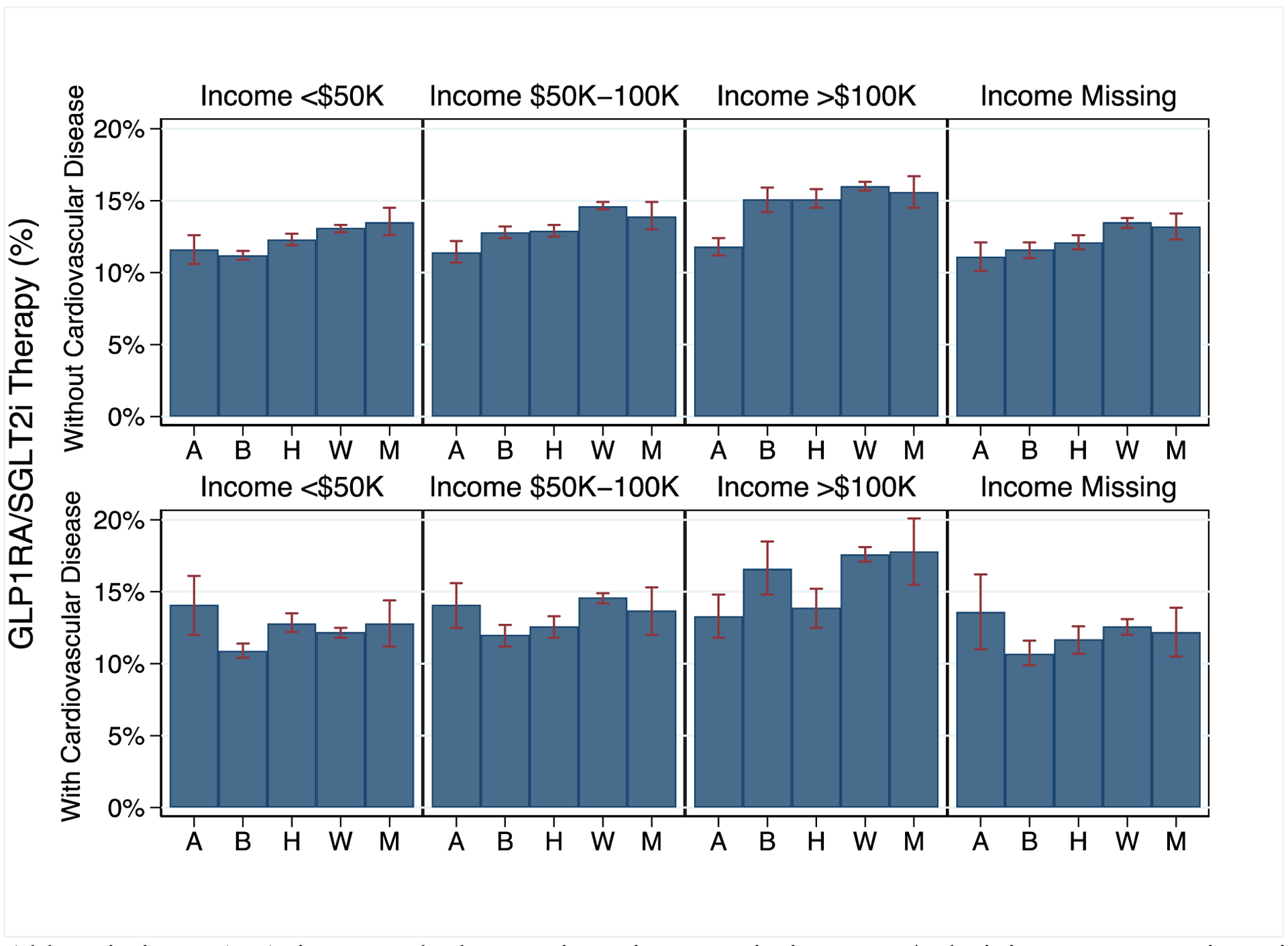
Adjusted Probability of GLP1RA/SGLT2i Therapy Based on Cardiovascular Disease, Race/Ethnicity, and Income
Abbreviations: A: Asian, B: Black, H: Hispanic. M: Missing Race/Ethnicity, W: Non-Hispanic White.
Clinician variation
The odds of SGLT2i or GLP1RA therapy were higher for those treated by an endocrinologist (AOR 2.11 [95% CI 2.04–2.17]) after adjustment for medical comorbidities. There were 1,718 endocrinology and 7,078 primary care clinician groups with more than 20 patients with GLP1RA/SGLT2i therapy. For endocrinologists, the adjusted probability of GLP1RA/SGLT2i therapy ranged from 5.6%-67.0% (median: 25.1%; IQR 19.8%-31.3%) (Figure 2a). The median odds ratio of GLP1RA/SGLT2i therapy was 1.70 (95% CI: 1.65–1.75), indicating there was a 70% difference in the odds of GLP1RA/SGLT2i therapy for a given patient between two randomly selected endocrinologists. For primary care clinicians, the adjusted probability of GLP1RA/SGLT2i therapy ranged from 1.9%-51.7% (median: 8.6%; IQR 6.0%-12.8%) (Figure 2b). The median odds ratio was 2.04 (95% CI: 2.00–2.09). These results were similar after adjusting for sociodemographic characteristics (Supplement Table 3).
Figure 2.
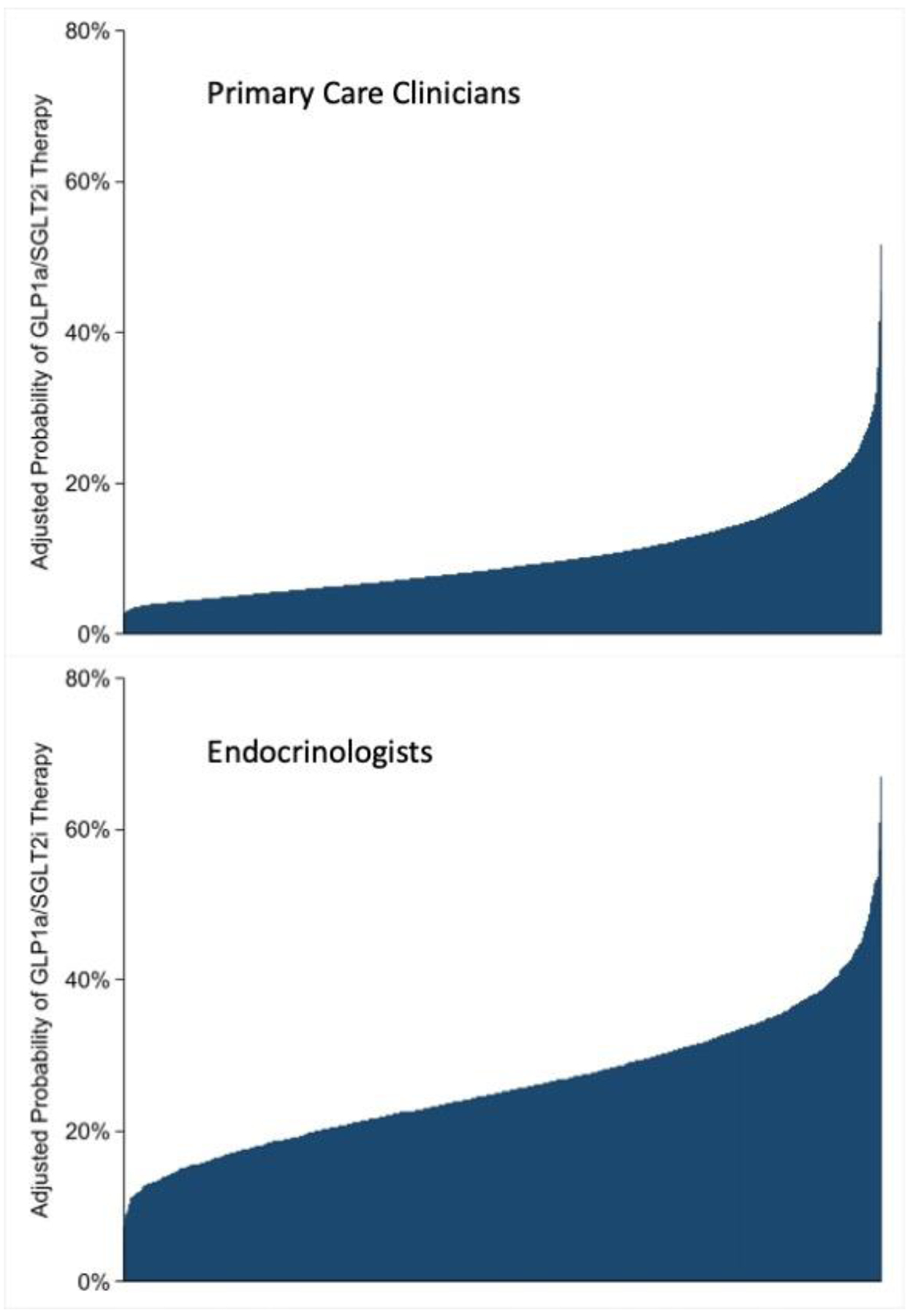
Variation in Adjusted GLP1RA/SGLT2i Therapy Rates Across Primary Care Clinicians and Endocrinologists
DISCUSSION
Among a commercially insured cohort of patients with T2DM in 2018–2019, only 16% received GLP1RA/SGLT2i therapy. We discovered important patient-specific disparities in treatment. Asian, Black, and Hispanic patients, older patients and those with lower income had lower odds of GLP1RA/SGLT2i therapy, which persisted despite adjustment for medical comorbidities or clinician practice. We also found evidence of a striking risk-treatment paradox. Patients with HF and those with higher rate of diabetes-related complications were less likely to receive GLP1RA/SGLT2i therapy than patients without these diagnoses. These results identify a number of opportunities for improving our implementation of these novel therapies to reduce cardiovascular morbidity.
Clinical guidelines have endorsed the use of GLP1RA and SGLT2i therapy as integral components of the care for diabetes (11,20) In 2019, the American Diabetes Association (ADA) released updated guidelines in support of cardioprotective GLP1RA or SGLT2i when metformin monotherapy was no longer sufficient for patients with T2DM(19). Additionally, position statements published by the ADA which came out just before our study cohort in November of 2017 recommended empagliflozin and liraglutide for patients with existing atherosclerotic cardiovascular disease (21). Despite the robust evidence for the cardiovascular and renal benefits of these therapies in multiple guidelines and position statements, we found a significant variability in their uptake even across endocrinologists. There might be several explanations for the sluggish adoption among individual providers. Familiarity with these medications, knowing which patients would benefit, and the patient’s risk profile likely all factor into clinicians’ decisions to initiate GLP1RA or SGLT2i (13,20). Clinicians and patients may also be reluctant to change therapies when clinical status is stable. This inertia often overlooks previous data demonstrating the high persistent risk of clinical events among patients with glycemic control, especially among those with existing cardiovascular disease(22). Clinicians may also have discomfort in recommending diabetes therapies for the cardioprotective benefits as opposed to glycemic control. This emphasizes the need to overcome the perception of GLP1RA/SGLT2i as glucose-lowering, diabetes medications. Instead, these therapies should be rebranded as preventive therapies for the cardiovascular and renal diseases they prevent.
The high cost of GLP1RA/SGLT2i therapies presents unique barriers related to patient affordability and prior authorization barriers(23,24). This is consistent with the lower odds of GLP1RA/SGLT2i therapy among those with low household income. The unaffordability of clinically effective therapies is a critical factor in driving disparities. Reducing out-of-pocket costs can have important effects on improving adherence and reducing disparities(24). These findings emphasize the need for reform in pharmaceutical cost-sharing(22,38). Patient affordability is especially critical for cost-effective therapies in which the high costs are justified based on their clinical benefit (25,26), which has been the case for SGLT2i among high-risk groups with CKD or HF. In addition to the actual unaffordability of high-cost medication, implicit bias related to cost may contribute to disparities. Clinicians are often unaware of out-of-pocket costs and may presume a drug is unaffordable despite patient access programs such as the Low-Income Subsidy for Medicare patients(27,29). Additionally, patients and clinicians are often unaware of program eligibility, with over 1/3 of eligible patients unenrolled(28).
In addition to sociodemographic disparities, we found an important risk-treatment paradox. The highest-risk patients were not substantially more likely to receive GLP1RA/SGLT2i therapy. In fact, patients with HF or with more diabetes-related complications were less likely to receive these therapies. However, these high-risk patients would derive the highest absolute benefit with treatment. We also demonstrated that the risk-treatment paradox persisted within high-risk subgroups. Patients with recent HF hospitalizations had lower odds of GLP1RA/SGLT2i therapy than patients with HF without a recent HF admission. Patients with a recent IHD-related admissions/revascularization did not have higher odds of GLP1RA/SGLT2i therapy. The risk-treatment paradox has been established across multiple disease states, including statin prescription(30), percutaneous coronary intervention(31) and in marginalized groups such as elderly patients(33) and women(34). While there may be concerns regarding side effects in patients with more comorbidities, there are now extensive safety data across broad populations. Similar guideline-directed research has been done in other domains, such as evolution of formulary coverage and utilization strategies in prescription of DOAC or PCSK9 therapies (35, 36).
Our analysis builds on prior studies that demonstrated the low uptake of GLP1RA/SGLT2i therapy among patients with T2DM(14,15). Eberly and colleagues also found low rates of GLP1RA and SGLT2i therapy among commercially insured individuals in the Optum database between 2015–2019 with lower treatment rates among Black patients and those with lower average neighborhood income. While we used a similar cohort, there are several important differences and unique findings in our analysis. First, we included patients with diabetes managed by primary care. We found patients managed by primary care without endocrinology had substantially lower likelihood of GLP1RA/SGLT2i therapy, even after adjusting for comorbidities and sociodemographic characteristics. Second, we restricted our cohort to the period after the diabetes treatment guidelines explicitly recommended GLP1RA/SGLT2i, yet we found treatment rates for GLP1RA/SGLT2i remained low, including among those with cardiovascular or renal disease. Third, we demonstrated patient-level income and education were both associated with the likelihood of GLP1RA/SGLT2i therapy.
Our study also builds on prior work by evaluating GLP1RA/SGLT2i treatment rates within clinician practices and comparing rates across practices. Treatment disparities may be related to differences in treatment practices among clinicians who disproportionately treat a given group (between-clinician differences) or differences in how individual clinicians treat patients of different groups (within-clinician differences) or both. Understanding the structure of disparities is critical. For example, if disparities are driven by between-clinician differences, the primary factor is the general quality of care of the clinicians treating a given group rather than differential treatment by a given clinician. We found disparities in GLP1RA/SGLT2i remained unchanged after adjusting for between-clinician differences. This illustrates that race- and ethnicity-based disparities were secondary to differential treatment within clinician practices. Understanding the causes of these disparities is beyond the scope of what we can determine from this analysis, but ethnic and racial discrimination and structural racism may be important contributors.
We found a substantial range in treatment rates across clinicians. There was a 70% difference in the odds of receiving a GLP1RA/SGLT2i between two randomly selected endocrinologists after accounting for other patient characteristics, including income. This is consistent with an earlier analysis that demonstrated profound between-practice variation in prescription of second-generation diabetic medications(37). This variation strengthens the argument that higher treatments rates are obtainable. Quality improvement efforts should focus on increasing the use of GLP1RA/SGLT2i, particularly among high-risk populations that are most likely to benefit.
Careful implementation of guidelines also has a powerful effect on adoption. There are multiple potential strategies to improving adoption of SGLT2i and GLP1RA. First, the benefit of starting these therapies should be disseminated across medical specialties. Diabetic patients at high risk of adverse cardiovascular and renal outcomes are often cared for by a range of specialists, including cardiologists and nephrologists. Educating clinicians across specialists about the benefit of these therapies may improve therapy uptake. Second, quality improvement registries of diabetes patients may promote uptake of these therapies via audit and feedback strategies (39). Third, inclusion of these medications on hospital formularies would facilitate inpatient initiation and increase the likelihood that patients’ providers will continue them outpatient. Finally, reduction in patient cost-sharing to improve affordability is critical. The Inflation Reduction Act of 2022 will cap Medicare Part D drug cost-sharing to $2,000 annually starting in 2025 (40).
Our study has important limitations. First, this study used claims data which limits clinical granularity. Second, Optum SES includes only patients with Medicare Advantage or commercial insurance. Therapy rates may be even lower among patients without insurance or with Medicaid. Finally, we did not directly link the prescription claims with a given clinician and instead assumed the endocrinologist or primary care clinician treating the patient were responsible.
In conclusion, we found sub-optimal adoption of GLP1RA/SGLT2i therapy among patients with medically treated T2DM. Overall therapy rates were low. Individual clinician practices were less likely to treat Asian, Black, and Hispanic patients with GLP1RA/SGLT2i than their non-Hispanic, White patients. Patients with heart failure and more diabetes-related complications had lower treatment rates despite having a greater absolute likelihood of benefit with treatment. Overall, shifting the narrative around these medications from one of a “glucocentric” view to a cardio-renal lens is paramount to not only improving uptake of these protective medications into practice but ensuring accessibility to patients who would derive the most benefit. Rebranding GLP1RA/SGLT2i therapies in this way will further promote their use among patients at high risk of adverse cardiovascular and renal outcomes. These findings underscore the urgency of identifying strategies to improve adoption of T2DM therapies that have cardiovascular and renal benefits, especially among high-risk and historically disadvantaged groups
Supplementary Material
Author Disclosures:
E.C.V. reports no disclosures
M.B. reports no disclosures
J.C. reports no disclosures
D.J.M. reports no disclosures
F.R. reports consulting fees from Novo Nordisk, consulting fees and equity in HealthPals and reports advisory boards for Novartis and Amgen
A.T.S. reports National Heart, Lung, and Blood Institute K23 award supporting work by A.T.S
References
- 1.National Diabetes Statistics Report. US Department of Health and Human Services, Centers for Disease Control and Prevention. 2020.
- 2.Gregg EW, Cheng YJ, Srinivasan M, Lin J, Geiss LS, Albright AL, Imperatore G. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet. 2018: 391; 2430–2440 [DOI] [PubMed] [Google Scholar]
- 3.Emerging Risk Factors Collaboration, Sarwar N, Gao P, Kondapally Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CDA, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010; 375(9733): 2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leon BM & Maddox TM. Diabetes and Cardiovascular Disease: Epidemiology, Biological Mechanisms, Treatment Recommendations and Future Research. World Journal of Diabetes. 2015; 6(13): 1246–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bethel MA, Patel RA, Merrill P et al. Cardiovascular outcomes with glucagon-like peptide 1 receptor agonists with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinology. 2018; 6(2): 105–113 [DOI] [PubMed] [Google Scholar]
- 6.Marso SP, Daniels GH, Brown-Frandsen K et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. NEJM. 2016; 375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marso SP, Bain SC, Consoli A et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. NEJM. 2016; 375: 1834–1844 [DOI] [PubMed] [Google Scholar]
- 8.Husain M, Bikenfeld AL, Donsmark M et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. NEJM. 2019; 381: 841–851 [DOI] [PubMed] [Google Scholar]
- 9.Packer M, Anker SD, Butler J et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. NEJM. 2020; 383: 1413–1424 [DOI] [PubMed] [Google Scholar]
- 10.McMurray JJ, Solomon SD, Inzucchi SE et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. 2019; 381: 1995–2008 [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association, Standards of Medical Care in Diabetes – 2018. Diabetes Care. 2018; 41(1): S1–S156.29222369 [Google Scholar]
- 12.Vaduganathan M, Sathiyakumar V, Singh A et al. Prescriber Patterns of SGLT2i After Expansions of US Food and Drug Administration Labeling. JACC. 2018; 72(25): 3370–337 [DOI] [PubMed] [Google Scholar]
- 13.Garjon F Adoption of New Drugs by Physicians: a survival analysis. BMC Health Services Research. 2012; 12(56). 10.1186/1472-6963-12-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eberly LA, Yang L, Eneanya ND et al. Association of Race/Ethnicity, Gender, and Socioeconomic Status with Sodium-Glucose Cotransporter 2 Inhibitor Use Among Patients with Diabetes in the US. JAMA Network Open. 2021; 4(4): e216139. doi: 10.1001/jamanetworkopen.2021.6139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eberly LA, Yang L, Essien UR et al. Racial, Ethnic, and Socioeconomic Inequities in Glucagon-Like Peptide-1 Receptor Agonist Use Among Patients with Diabetes in the US. JAMA Network Open. 2021; 2(21): e214182. doi: 10.1001/jamahealthforum.2021.4182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu A, Yue Y, Desai RP & Argulian E. Racial and Ethnic Differences in Antihypertensive Medication Use and Blood Pressure Control Among US Adults with Hypertension. 2017;10:e003166. [DOI] [PubMed] [Google Scholar]
- 17.Young BA, Lin E, von Korff MV et al. Diabetes Complications Severity Index and Risk of Mortality, Hospitalization and Healthcare utilization. American Journal of Managed Care. 2008; 14(1): 15–23. [PMC free article] [PubMed] [Google Scholar]
- 18.Lakens D Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Frontiers in Psychology. 2013; 4:863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, D’Alessio DA, Davies MJ. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD). Diabetes Care. 2020; 43:487–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCoy IE, Han J, Montez-Rath ME, Chertow GM, Rhee J. Patient and Provider Characteristics Associated with Sodium-Glucose Cotransporter 2 Inhibitor Prescription in Patients with Diabetes and Proteinuric Chronic Kidney Disease. Clinical Diabetes. 2020; 38(3): 240–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Diabetes Association, Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes – 2018. Diabetes Care. 2018; 41(Supplement_1): S73–S85 [DOI] [PubMed] [Google Scholar]
- 22.McCoy RG, Dykoff HJ, Sangaralingham L, Ross JS, Karaca-Mandic P, Montori VM, Shah ND. Adoption of New Glucose-Lowering Medications in the US - The Case of SGLT2 Inhibitors: Nationwide Cohort Study. Diabetes Technology &Therapeutics. 2019; 21(12): 702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao Y, Peterson E, Pagidipati N. Barriers to Prescribing Glucose-Lowering Therapies with Cardiometabolic Benefits. American Heart Journal. 2020; 24: 47–53 [DOI] [PubMed] [Google Scholar]
- 24.Choudhry NK, Avorn J, Glynn RJ et al. Full Coverage for Preventive Medications after Myocardial Infarction. NEJM. 2011; 365: 2088–2097 [DOI] [PubMed] [Google Scholar]
- 25.Parizo JT, Goldhaber-Fiebert JD, Salomon JA, Khush KK, Spertus JA, Heidenreich PA, Sandhu AT. Cost-Effectiveness of Dapagliflozin for Treatment of Patients with Heart Failure With Reduced Ejection Fraction. JAMA Cardiology. 2021; 6(8): 926–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tisdale RL, Cusick MM, Aluri KZ, Handley TJ, Cummings Joyner AK, Salomon JA, Chertow GM, Goldhaber-Fiebert JD, Owens DK. Cost-Effectiveness of Dapagliflozin for Non-Diabetes Chronic Kidney Disease. Journal of General Internal Medicine. 2022; doi: 10.1007/s11606-021-07311-5. Online ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Low Income Subsidy for Medicare Prescription Drug Coverage. Centers for Medicare & Medicaid Services. Updated December 1, 2021. Accessed March 2, 2022. https://www.cms.gov/Medicare/Eligibility-and-Enrollment/LowIncSubMedicarePresCov [Google Scholar]
- 28.Society of General Internal Medicine. The Relationship Between Take-up of Prescription Drug Subsidies and Medicaid Among Low-Income Medicare Beneficiaries. Journal of General Internal Medicine. 2020; 36(9): 2873–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandhu AT & Heidenreich PA. The Affordability of Guideline-Directed Medical Therapy: Cost Sharing is a Critical Barrier to Therapy Adoption. Circulation. 2021: 143:1073–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ko DT, Mamdani M, Alter DA. Lipid-Lowering Therapy With Statins in High-Risk Elderly Patients: The Treatment-Risk Paradox. JAMA. 2004; 291(15): 1864–1870 [DOI] [PubMed] [Google Scholar]
- 31.Krumholz HM Radford MJ Wang Y Chen J Heiat A Marciniak TA National use and effectiveness of β-blockers for the treatment of elderly patients after acute myocardial infarction: National Cooperative Cardiovascular Project. JAMA 1998;280623–629 [DOI] [PubMed] [Google Scholar]
- 32.McAlister FA Taylor L Teo KK et al. Clinical Quality Improvement Network (CQIN) Investigators, The treatment and prevention of coronary heart disease in Canada: do older patients receive efficacious therapies? J Am Geriatr Soc 1999;47811–818 [DOI] [PubMed] [Google Scholar]
- 33.Vittinghoff E Shlipak MG Varosy PD et al. Heart and Estrogen/progestin Replacement Study Research Group, Risk factors and secondary prevention in women with heart disease: the Heart and Estrogen/progestin Replacement Study. Ann Intern Med 2003;13881–89 [DOI] [PubMed] [Google Scholar]
- 34.Psotka MA, Fiuzat M, Solomon SD, Chauhan C, Felker GM, Butler J, Teerlink JR, Sinha S, O’Connor CM, Konstam MA. Challenges and Potential Improvements to patient Access to Pharmaceuticals: Examples from Cardiology. Circulation. 2020; 142: 790–798 [DOI] [PubMed] [Google Scholar]
- 35.Hess GP, Natarajan P, Faridi KF, Fievitz A, Valsdottir L, Yeh RW. Proprotein convertase subtilisin/kexin type 9 inhibitor therapy: payer approvals and rejections, and patient characteristics for successful prescribing. Circulation. 2017; 136:2210–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dayoub EJ, Ross JS, Shah ND, Dhruva SS. Evolution of Medicare formulary coverage changes for antithrombotic therapies after guideline updates. Circulation. 2019; 140:1227–1230. [DOI] [PubMed] [Google Scholar]
- 37.Gilstrap LG, Blair RA, Huskamp HA et al. Assessment of Second Generation Diabetes Medication Initiation Among Medicare Enrollees From 2007–2015. JAMA Network Open. 2020; 3(5): e205411. doi: 10.1001/jamanetworkopen.2020.5411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomson S, Schang L, Chernew ME. Value-Based Cost Sharing In the United States and Elsewhere Can Increase Patient’s Use of High-Value Goods and Services. Health Affairs. 2013; 32(4): 704–712 [DOI] [PubMed] [Google Scholar]
- 39.Fan W, Song Y, Inzucchi SE et al. Composite Cardiovascular Risk Factor target Achievement and its Predictors in US Adults with Diabetes: The Diabetes Collaborative Registry. Diabetes, Obesity and Metabolism. 2019; 21: 1121–1127 [DOI] [PubMed] [Google Scholar]
- 40.Inflation Reduction Act, H.R. 5376, 117th Cong (2022). https://www.congress.gov/bill/117th-congress/house-bill/5376/text
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


