Abstract
There is a chronic shortage of donor lungs for pulmonary transplantation due, in part, to low lung utilization rates in the United States. We performed a retrospective cohort study using data from the Scientific Registry of Transplant Recipients database (2006–2019) and developed the Lung Donor (LUNDON) acceptability score. A total of 83219 brain-dead donors were included and were randomly divided into derivation (n=58314, 70%) and validation (n=24905, 30%) cohorts. The overall lung acceptance was 27.3% (n=22767). Donor factors associated with the lung acceptance were age, maximum creatinine, PaO2/FiO2 ratio, mechanism of death by asphyxiation or drowning, history of cigarette use (≥20 pack-years), history of myocardial infarction, chest x-ray appearance, bloodstream infection, and the occurrence of cardiac arrest after brain death. The prediction model had high discriminatory power (C statistic=0.891, 95% CI=0.886–0.895) in the validation cohort. We developed a web-based, user-friendly tool (available at https://sites.wustl.edu/lundon) that provides the predicted probability of donor lung acceptance. LUNDON score was associated with recipient survival in patients with high lung allocation scores. In conclusion, the multivariable LUNDON score uses readily available donor characteristics to reliably predict lung acceptability. Widespread adoption of this model may standardize lung donor evaluation and improve lung utilization rates.
Keywords: Lung transplant, donor selection
Introduction
Lung transplantation is the only feasible option to prolong life and improve function in many patients with end-stage lung disease. Despite significant advances in transplant outcomes over the last several decades, there remains a chronic shortage of available donor organs1, due in part to low lung utilization rates (number of lung donors/total number of potential organ donors). For example, while liver and kidney utilization rates exceed 80–90% in brain-dead donors, lung utilization rates remain around 25%2. As a consequence, every year nearly 15% of patients on the lung waitlist either die or become too sick for transplantation2. Therefore, developing objective methods to increase lung utilization is essential to address this chronic donor shortage.
A contributing factor to low lung utilization rates is the absence of well-accepted criteria for determining the quality of donor lungs. Conventionally, lung donors have been evaluated using the International Society for Heart and Lung Transplantation (ISHLT) donor guidelines. ISHLT guidelines empirically define an ideal “standard criteria donor” as age <55, limited smoking history, normal chest x-ray, normal bronchoscopy, lack of infection, and ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) greater than 300 to 3503, those who do not meet these standards are referred to as an “extended criteria donor.” These guidelines are variably followed in real-world practice, however, with more than half of accepted lung allografts meeting extended criteria4. Furthermore, recipients of extended criteria donor lungs have outcomes that are comparable to standard criteria lungs, raising concerns about the utility of the extended versus standard criteria donor definition altogether5,6. Leveraging higher rates of lung acceptance among these non-ideal – but usable – donors is essential in order to increase the number of available lungs7.
There is a critical need for a contemporary, well-calibrated predictive model with high discrimination to assess potential lung donors. While prior studies have proposed objective models, these efforts have been significantly hampered by small sample sizes, methodologic challenges, and lack of validation7–13. Additionally, most donor acceptability models to date have either included recipient characteristics or modelled recipient outcomes, both of which confound the independent evaluation of lungs. Indeed, such models ignore the 75% of the donor pool that is never accepted for lung transplant. Developing an acceptability of donor lung score based strictly on donor criteria should therefore standardize and enhance the lung evaluation process, while potentially improving lung acceptance rates.
To address this gap in knowledge, and to inform current practices in lung transplantation, we performed a retrospective cohort study using data from the Scientific Registry of Transplant Recipients (SRTR) database to develop a lung donor acceptability score incorporating universally collected, clinically relevant donor variables.
Methods
Data Source and Study Population
This study was performed using the Scientific Registry of Transplant Recipients (SRTR) database, which collates data from the United Network for Organ Sharing (UNOS) and Organ Procurement and Transplantation Network (OPTN) database along with several other sources14. We used the SRTR standard analysis donor files, which contain demographic and clinical information on all deceased solid-organ donors. Inclusion criteria for this study were any individuals who donated at least 1 organ (kidney, liver, pancreas, intestine, lung, and/or heart) in the United States between 2006 (post-lung allocation score [LAS] era) and 2019 (pre-COVID-19 pandemic). Exclusion criteria were donation after circulatory death (DCD), age <16 years old, and those donors who did not have a chest x-ray performed (Figure 1). Donor lungs undergoing ex vivo lung perfusion (EVLP) were also unavailable in the dataset. We randomly split the cohort in a 70:30 fashion to develop our derivation and validation cohorts, respectively.
Figure 1.
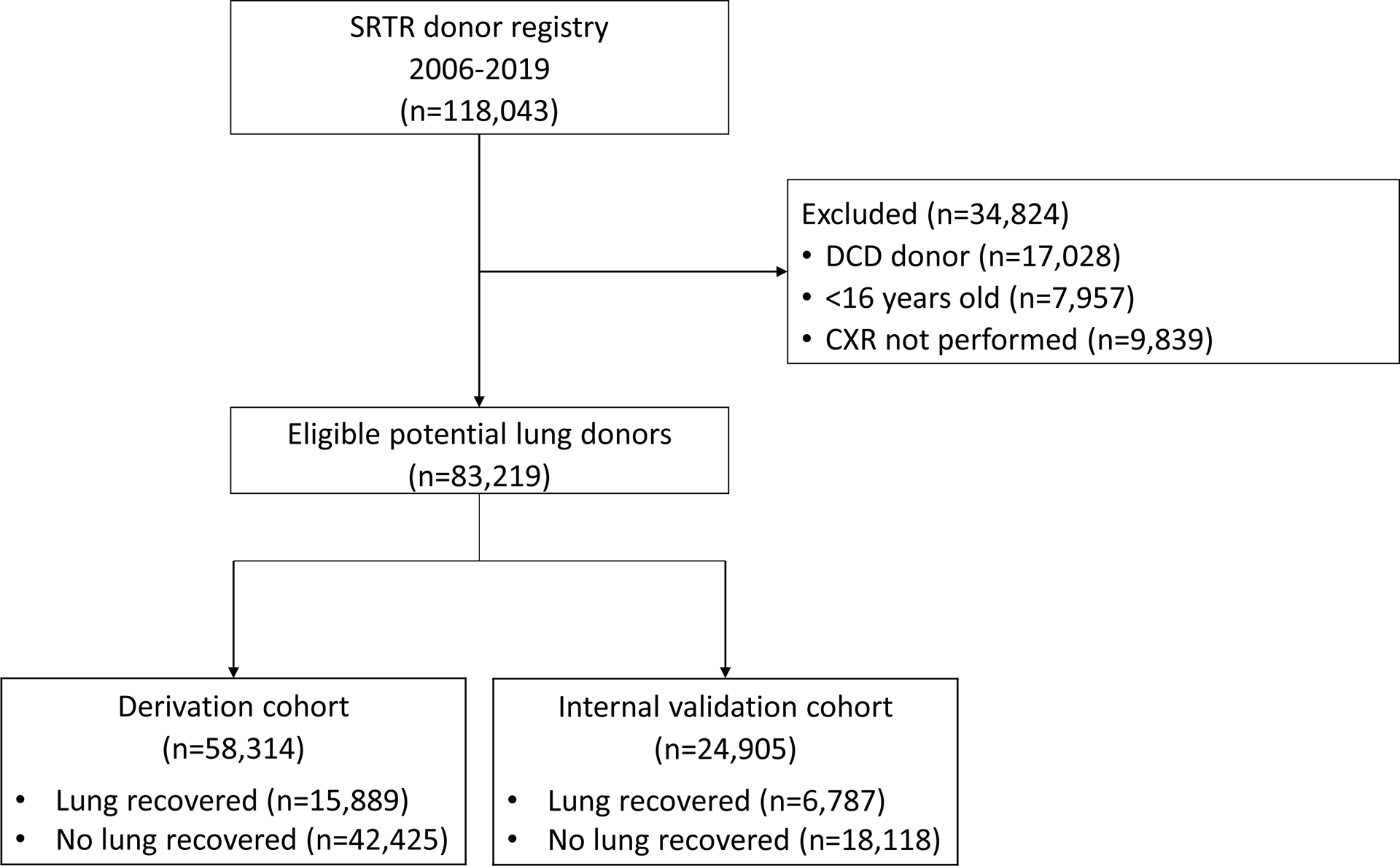
Formation of the derivation and internal validation cohorts
Consort diagram displaying exclusion criteria for the study cohort, which was randomly split (70:30) into derivation and internal validation cohorts.
The study was performed according to the TRIPOD guidelines for developing risk prediction models15. The Washington University in St. Louis Human Research Protection Office deemed the project to be non-human subject research and therefore IRB exempt.
Study Outcomes
We defined lung acceptance, our outcome of interest, as procurement of at least 1 lung from a donor. We did not discriminate between whether a single or double lung allograft was procured. We also did not consider discard rates (i.e., a lung being discarded after procurement) as we thought that this likely reflected logistical and recipient-related factors, which do not reflect the inherent acceptability of the donor lungs, and therefore would bias our analyses. Recipient covariates and outcomes were not considered in the model, as such models would exclude the 75% of lung allografts that are not used for transplant2.
Candidate Predictor Variables
The SRTR database contains roughly 250 donor variables, including demographic information, laboratory values, and imaging characteristics14. A team of subject matter experts with representation from thoracic surgery, lung transplant pulmonary medicine, and donor critical care reviewed each of these variables and decided a priori, based on clinical expertise and contemporary literature, to consider a subset of these variables in our prediction model (eTable1 in supplement). Some variables were excluded outright based on practicality and statistical precedent of contemporary predictive modeling. For example, donor race was removed since inclusion of race as a variable in risk prediction modeling has been widely criticized as propagating racial and racist disparities in health16–18. Additionally, bronchoscopy findings were excluded since bronchoscopy is often a late-stage factor in the evaluation of potential donor lungs, with many rejected donors never receiving a bronchoscopy. Similarly, organ procurement organization (OPO) center location was removed. Even though transplant activities vary greatly by center19,20, inclusion of this variable (which is based on historical data) could further propagate suboptimal transplant activity in poorly-performing centers. Finally, infectious serologies were removed as use of positive donor organs is being increasingly considered and is likely to expand21,22.
To further assess the accuracy of our model and to compare it to historical practice standards, we also evaluated donor acceptability based on the ISHLT standard donor criteria. For this analysis, we defined standard criteria as age <55 years old, PaO2/FiO2 ratio >300, less than 20 pack-year smoking history, and normal chest x-ray findings. We compared the sensitivity, specificity, positive predictive value, and negative predictive value of this binary model to our developed model (using various score cutoffs). Choosing a theoretical “cutoff” was necessary for this comparison since our developed score is a continuous score while the ISHLT criteria produce a binary “score.”
Recipient Outcomes
The association of LUNDON score and recipient outcomes was also assessed. Adult patients (≥18 years) who underwent lung transplantation (2006–2018) were abstracted from the SRTR lung transplant recipient database. Patients who received multi-organ transplantation or had prior transplantation of any type were excluded. Additionally, patients with missing LAS or LUNDON scores (i.e., donor after circulatory death, < 16 years, or missing chest X-ray) were excluded.
Statistical Analysis
Missing data were present for less than 2% of all candidate variables that we included in the final model. Missing values were imputed using single stochastic imputation using the Fully Conditional Specification (FCS) using the PROC MI function in SAS (SAS Institute, Cary, NC). For continuous variables, the linearity of the relationship between the candidate predictor variable and outcome was assessed using a restricted cubic spline function and the likelihood ratio test for non-linearity (lgtphcurv9 macro in SAS23). For variables that violated this relationship, transformations were performed to achieve a linear relationship between the transformed continuous predictor variable and the outcome24.
To develop our model for predicting lung acceptability, we constructed multivariable logistic regression models utilizing the derivation cohort. We used stepwise backward selection with a significance value of 0.15 to determine variables that could be removed from the model. Following development of this initial model, we then fit several simplified models by sequentially removing predictor variables (based on clinical judgement from multiple providers) to derive a more parsimonious model25. Each of the simplified models was compared with the original multivariable model to verify that discrimination and calibration were preserved despite removing these variables.
To evaluate the performance of the prediction model, we first examined calibration by measuring the agreement between predicted vs. observed outcome probabilities by decile and assessing the calibration slope and intercept26. We also assessed the discriminatory accuracy of the prediction model by using c-statistics.
To create a more clinically relevant risk score, we first developed an integer-based score using previously described methods27. This score was constructed so that higher values represented a higher probability of lung acceptance, and vice versa. We also hypothesized that the score may be more clinically useful when given as a predicted probability (0–100) as opposed to an integer (ie, “based on historical data, what is the probability that these lungs would have been accepted for transplantation?”). Therefore, we created an easy-to-use, web-based tool (i.e., the “LUNDON” acceptability score) with the assistance of our institutional IT support group (https://sites.wustl.edu/lundon).
To assess recipient outcomes, Kaplan-Meier survival analysis was performed for five-year graft survival across the three LUNDON score groups. Recipient survival was assessed based on donor LUNDON score (≤40 low, 40–60 intermediate, >60 high) in both the overall cohort and in three subgroups stratified by recipient LAS (≤50, 50–70, >70). Graft survival was a composite outcome of graft failure or patient death, whichever occurred first. Two-tailed Pvalues of less than 0.05 were considered statistically significant. Analyses were performed using SAS Studios 3.81 (SAS Institute, Cary, NC).
Results
A total of 83219 donors were included in the study (Figure 1), of whom 58314 (70.1%) and 24905 (29.9%) were in the derivation and validation cohorts, respectively. The overall likelihood of lung acceptance in the combined cohorts was 27.3% (n=22767). The mean (SD) donor age was 43.0 (15.9) years. A majority of donors were male (58.7%, n=48869) and of white race (64.7%, n=53865). The most common mechanisms of death were blunt trauma (20.5%, n=17034) and penetrating gunshot or stab wound (10.0%, n=8302). Significant smoking history (>20 pack-years) was reported for 24.8% (n=20654) of donors. The mean (SD) PaO2/FiO2 ratio was 302.2 (142.7). Additional demographic and clinical factors are shown in Table 1.
Table 1.
Donor characteristics in the derivation and validation cohorts.
| Derivation Cohort (n=58314) | Validation Cohort (n=24905) | Total (n=83219) | ||||
|---|---|---|---|---|---|---|
| Donor Age (mean, SD) | 43.0 | 15.9 | 43.0 | 15.9 | 43.0 | 15.9 |
| Gender (n, %) | ||||||
| Female | 24015 | 41.18 | 10335 | 41.50 | 34350 | 41.28 |
| Male | 34299 | 58.82 | 14570 | 58.50 | 48869 | 58.72 |
| Race (n, %) | ||||||
| Black or African American | 10246 | 17.57 | 4428 | 17.78 | 14674 | 17.63 |
| Hispanic/Latino | 8266 | 14.17 | 3449 | 13.85 | 11715 | 14.08 |
| Unknown or Other | 2080 | 3.57 | 885 | 3.55 | 2965 | 3.56 |
| White | 37722 | 64.69 | 16143 | 64.82 | 53865 | 64.73 |
| BMI (mean, SD) | 28.1 | 6.7 | 28.1 | 6.8 | 28.1 | 6.8 |
| Mechanism of Death (n, %) | ||||||
| Drowning | 229 | 0.39 | 85 | 0.34 | 314 | 0.38 |
| Drug Overdose | 4753 | 8.15 | 2127 | 8.54 | 6880 | 8.27 |
| Asphyxiation | 1896 | 3.25 | 851 | 3.42 | 2747 | 3.30 |
| GSW or Stab Wound | 5883 | 10.09 | 2419 | 9.71 | 8302 | 9.98 |
| Blunt Trauma | 11927 | 20.45 | 5107 | 20.51 | 17034 | 20.47 |
| Other | 33626 | 57.66 | 14316 | 57.48 | 47942 | 57.61 |
| Cardiac Arrest after Death (n, %) | 4067 | 6.97 | 1753 | 7.04 | 5820 | 6.99 |
| Smoking History (>20 pack-years) (n, %) | ||||||
| No | 42817 | 73.42 | 18278 | 73.39 | 61095 | 73.41 |
| Unknown | 1018 | 1.75 | 452 | 1.81 | 1470 | 1.77 |
| Yes | 14479 | 24.83 | 6175 | 24.79 | 20654 | 24.82 |
| CDC High-risk Criteria (n, %) | ||||||
| No | 47926 | 82.19 | 20501 | 82.32 | 68427 | 82.23 |
| Unknown | 95 | 0.16 | 37 | 0.15 | 132 | 0.16 |
| Yes | 10293 | 17.65 | 4367 | 17.53 | 14660 | 17.62 |
| History of MI (n, %) | ||||||
| No | 55103 | 94.49 | 23557 | 94.59 | 78660 | 94.52 |
| Unknown | 839 | 1.44 | 351 | 1.41 | 1190 | 1.43 |
| Yes | 2372 | 4.07 | 997 | 4.00 | 3369 | 4.05 |
| Infection (n, %) | ||||||
| Bloodstream infection | 5550 | 9.52 | 2342 | 9.40 | 7892 | 9.48 |
| Pneumonia | 31001 | 53.16 | 13206 | 53.03 | 44207 | 53.12 |
| UTI | 7914 | 13.57 | 3384 | 13.59 | 11298 | 13.58 |
| Chest X-ray (n, %) | ||||||
| Abnormal | 43886 | 75.26 | 18662 | 74.93 | 62548 | 75.16 |
| Normal | 14428 | 24.74 | 6243 | 25.07 | 20671 | 24.84 |
| PaO2/FiO2 ratio (mean, SD) | 302.3 | 142.8 | 302.8 | 143.0 | 302.4 | 142.9 |
| Hematocrit (mean, SD) | 30.3 | 5.7 | 30.4 | 5.8 | 30.3 | 5.8 |
| Maximum Creatinine (mean, SD) | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 |
GSW=gunshot wound, UTI=urinary tract infection, MI=myocardial infarction, PaO2/FiO2=ratio of arterial partial pressure of oxygen to fraction of inspired oxygen
The results of the final multivariable model are shown in Table 2. Donor factors associated with the acceptance of at least 1 donor lung were age, maximum creatinine, PaO2/FiO2 ratio, mechanism of death by asphyxiation or drowning (no vs. yes, odds ratio [OR] 1.349, 95% CI 1.195–1.522), smoking history (>20 pack-years) (no vs. yes, OR 2.695, 95% CI 2.516–2.886), history of myocardial infarction (no vs. yes, OR 1.443, 95% CI 1.237–1.684), chest x-ray appearance (normal vs. abnormal, OR 2.023, 95% CI 1.921–2.130), bloodstream infection (no vs. yes, OR 1.306, 95% CI 1.200–1.421), and the occurrence of cardiac arrest (since neurological event that led to declaration of brain death) (no vs. yes, OR 1.281, 95% CI 1.162–1.413).
Table 2.
Final multivariable analysis of factors associated with donor lung acceptability in derivation cohort
| Parameter | β | Odds Ratio | 95% Confidence Interval | P-value | |
|---|---|---|---|---|---|
| Age (transformed) a | −0.00082 | -- | -- | -- | <.0001 |
| Maximum creatinine (transformed) a | 0.7133 | -- | -- | -- | <.0001 |
| PaO2/FiO2 ratio (transformed) a,b | 0.2501 | -- | -- | -- | <.0001 |
| Mechanism of death by asphyxiation/drowning (no vs.yes) | 0.2991 | 1.349 | 1.195 | 1.522 | <.0001 |
| Smoking history (>20 pack-years) (no vs. yes) | 0.9912 | 2.695 | 2.516 | 2.886 | <.0001 |
| Smoking history (>20 pack-years) (unknown vs. yes) | 0.6949 | 2.004 | 1.622 | 2.475 | <.0001 |
| History of MI (no vs. yes) | 0.3669 | 1.443 | 1.237 | 1.684 | <.0001 |
| History of MI (no vs. unknown) | −0.0393 | 0.961 | 0.718 | 1.288 | 0.7919 |
| Bloodstream infection (no vs. yes) | 0.2669 | 1.306 | 1.200 | 1.421 | <.0001 |
| Chest x-ray (normal vs. abnormal) c | 0.7046 | 2.023 | 1.921 | 2.130 | <.0001 |
| Cardiac Arrest (no vs. yes) d | 0.2478 | 1.281 | 1.162 | 1.413 | <.0001 |
Model intercept = −6.0705
Transformed age variable calculated as (age-20)2, transformed maximum creatinine variable calculated as (creatinine)−1/4, transformed PaO2/FiO2 ratio variable calculated as (PaO2/FiO2-200)1/2, with values less than 200 equal to 0.
To be determined within 2 hours prior to potential lung offer
To be determined within 3 hours prior to potential lung offer
Since neurological event that led to declaration of brain death
MI=myocardial infarction, PaO2/FiO2= ratio of arterial partial pressure of oxygen to fraction of inspired oxygen
We next assessed the performance of our nine-variable model in the derivation and validation cohort. In the derivation cohort, the model C statistic was 0.888 (95% CI 0.8850.890). The calibration slope and intercept were 1.000 (95% CI 0.972–1.028) and 0.000 (95% CI −0.011–0.011), respectively. In the validation cohort, the model maintained high discrimination with a C statistic of 0.891 (95% CI 0.886–0.895) and excellent calibration (calibration intercept = −0.002, 95% CI −0.019–0.014, calibration slope = 1.007, 95% CI 0.965–1.050, eFigure1–2).
Based on the multivariable model, we developed an integer-based score to predict the probability of donor lung acceptability. This integer-based score had a maximum value of 30, with higher scores representing more acceptable lungs. As shown in Figure 2, the predicted probability of donor lung acceptance ranged from 0.5% for donors with a score of 0 points to a maximum of 82.3% for donors with a score of 30 points. To enhance the dissemination and implementation of our prediction model28, however, we also developed the online LUNg DONor (“LUNDON”) acceptability score. This easy-to-use, web-based calculator (https://sites.wustl.edu/lundon) outputs the predicted probability of donor acceptance, with higher percentages representing more acceptable lungs (eFigure3). An example of how the online scoring tool could be used is shown in the Box.
Figure 2.
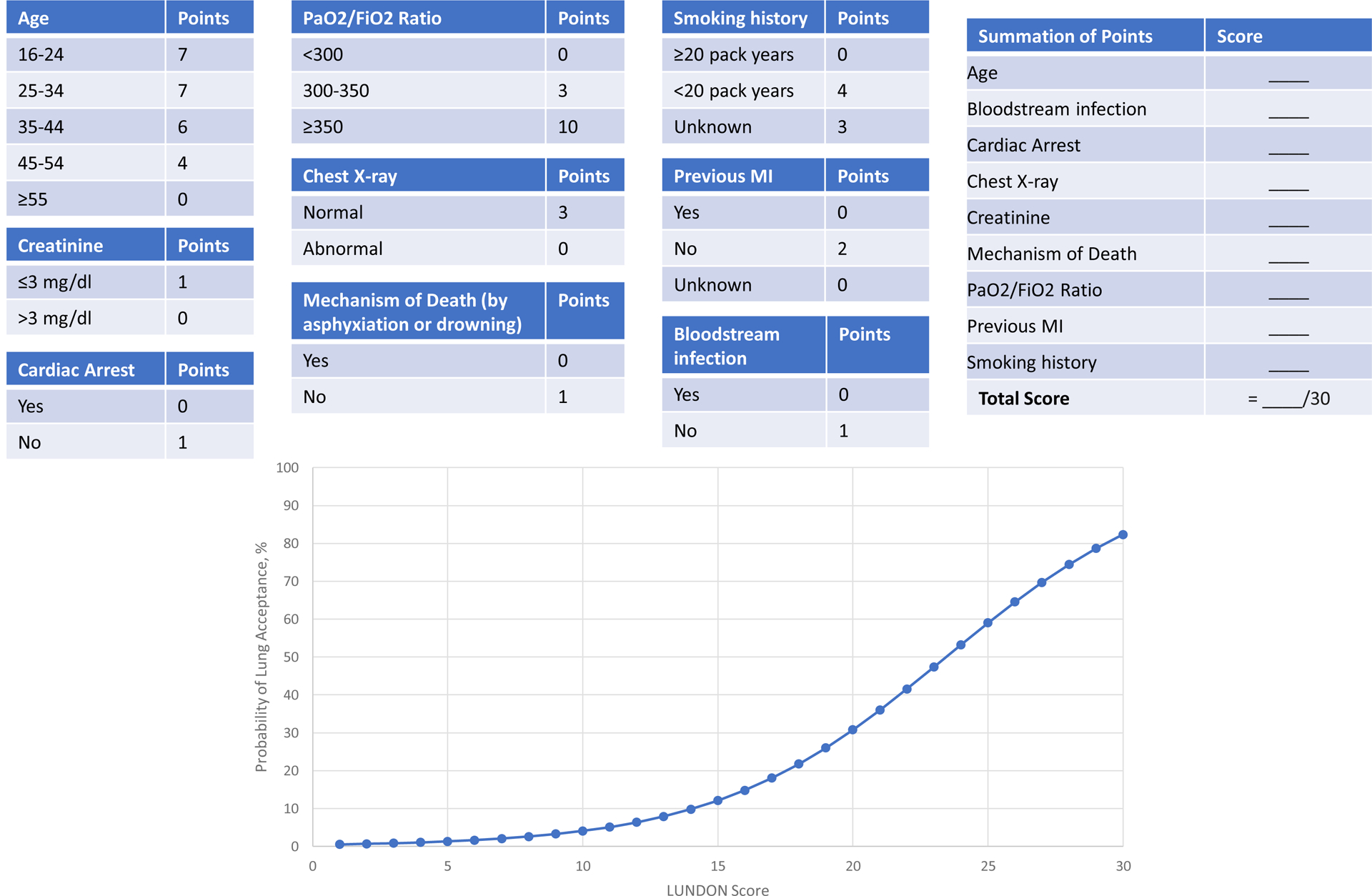
Integer-based score for predicting donor lung acceptability
Integer-based score for the acceptability of donor lungs. Higher scores represent donor lungs with higher probability of acceptance.
Box. Potential examples of LUNDON score interpretation.
| Online LUNDON Score | Integer Score | Interpretation |
|---|---|---|
| <10% | <15 | Low-acceptability donor lungs
|
| 10–60% | 15–25 | Moderate-acceptability donor lungs
|
| >60% | >25 | High-acceptability donor lungs
|
We next compared our LUNDON score to the ISHLT standard criteria to assess each method’s accuracy of identifying acceptable donor lungs. According to the ISHLT guidelines for lung donation, 11.7% of donors met standard criteria. The sensitivity and positive predictive value of the ISHLT guidelines for predicting lung acceptability in the validation cohort were 31.8% (95% CI 30.7–32.9%) and 74.0% (95% CI 72.4–75.6%), respectively (Table 3). Using an arbitrary LUNDON score cutoff of 25% (ie, 25% probability that lungs would be accepted), the model had a higher sensitivity (87.5%, 95% CI 86.7–88.2%) and slightly lower positive predictive value (57.5%, 95% CI 56.5–58.5%) for predicting lung acceptability. Using a higher LUNDON score cutoff of 60% (ie, 60% probability that lungs would be accepted), the model maintained a higher sensitivity (50.6%, 95% CI 49.4–51.8%) and similar positive predictive value (74.4%, 95% CI 73.1–75.6%).
Table 3.
Comparing current guidelines with the LUNDON model
| Statistic | ISHLT standard criteria | LUNDON score >25%a | LUNDON score >60%b |
|---|---|---|---|
| Sensitivity (%, 95% CI) | 31.8 (30.7–32.9) | 87.5 (86.7–88.2) | 50.6 (49.4–51.8) |
| Specificity (%, 95% CI) | 95.8 (95.5–96.1) | 75.8 (75.2–76.4) | 93.5 (93.1–93.8) |
| Positive predictive value (%, 95% CI) | 74.0 (72.4–75.6) | 57.5 (56.5–58.5) | 74.4 (73.1–75.6) |
| Negative predictive value (%, 95% CI) | 79.0 (78.4–79.5) | 94.2 (93.8–94.5) | 83.5 (83.0–84.0) |
Corresponds to integer score of approximately 20
Corresponds to integer score of approximately 25
ISHLT= International Society for Heart and Lung Transplantation
A total of 21,321 patients were included in the recipient cohort. Patient baseline characteristics are shown in eTable 2. The median LUNDON score was 61 (IQR 41–76), and median LAS was 41 (IQR 35–52). There were 5,153 (24.2%) patients in the low LUNDON score group (≤40), 5,104 (23.9%) in the intermediate group (40–60), and 11,064 (51.9%) in the high LUNDON score (>60) groups respectively. Low and intermediate LUNDON score donors were increasingly utilized in the past decade (Figure 3), with the median LUNDON score decreasing from 65 (IQR 45–80) in 2006–2008 to 58 (IQR 38–74) in 2017–2018.
Figure 3.
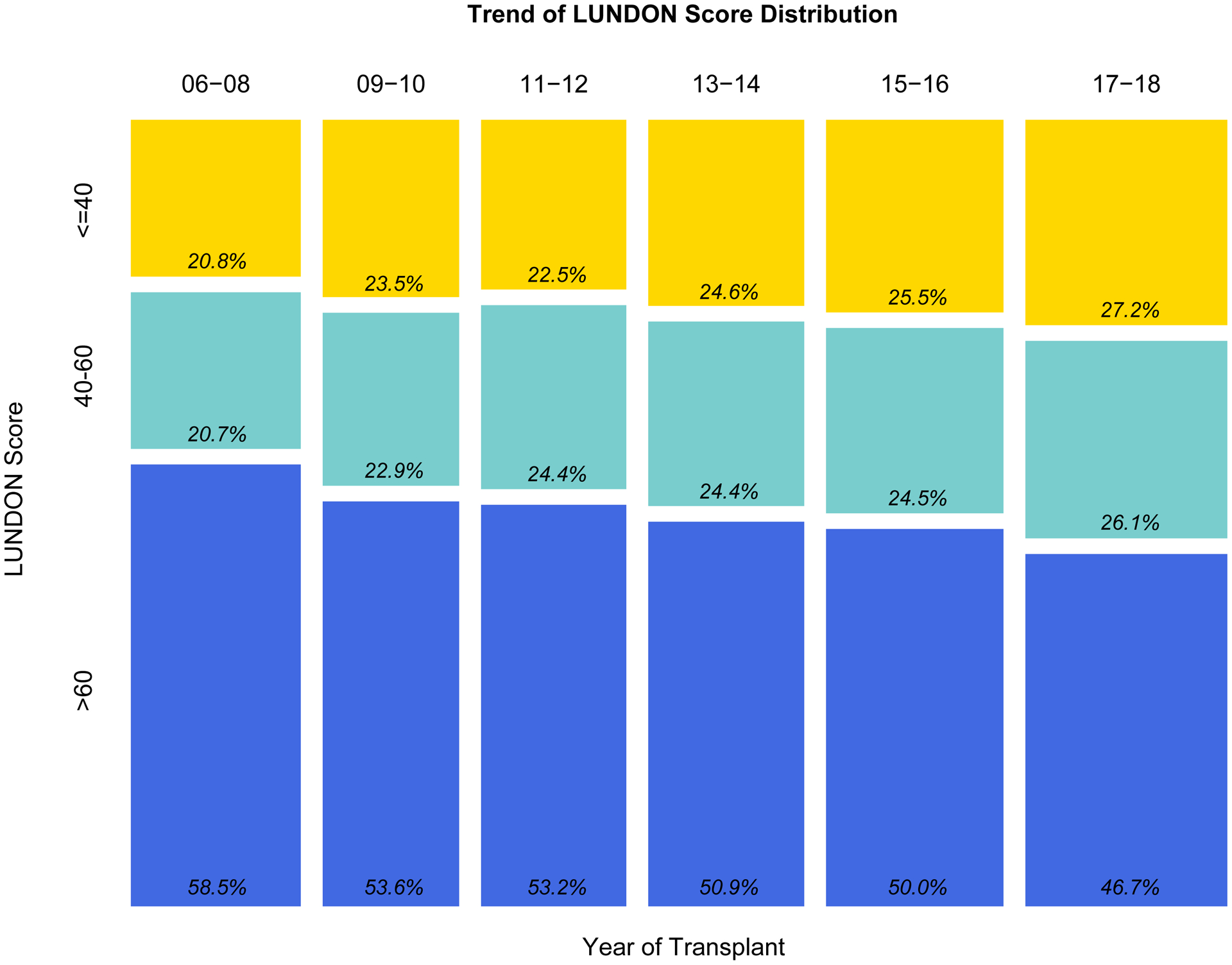
Proportion of low, intermediate, and high LUNDON score from 2006 to 2018.
Relative changes in LUNDON scores (grouped as low, intermediate, high scores) based on year of transplantation.
Unadjusted five-year graft survival rates in the overall cohort were 53.4% (95% CI 51.7%−55.1%) for low, 55.3% (95% CI 53.7%−57.0%) for intermediate, and 56.2% (95% CI 55.1%−57.3%) for high LUNDON score patients (p=0.027, Figure 4). In subgroup analysis, unadjusted Kaplan-Meier curves showed no significant differences in five-year graft survival across the three LUNDON score groups in recipients with LAS ≤50 (n=15,473, p=0.37, eFigure 4A) and recipients with LAS 50–70 (n=2,924, p=0.24, eFigure 4B). However, in the LAS >70 cohort (n=2,924), significantly lower five-year graft survival was noted in patients receiving low LUNDON score donor lungs (p=0.031, eFigure 4C). Five-year graft survival rates in the LAS >70 cohort were 46.9% (95% CI 42.5%−51.8%), 54.3% (95% CI 49.8%−59.1%), and 54.0% (95% CI 51.0%−57.1%) for low, intermediate, and high LUNDON score donors, respectively.
Figure 4.
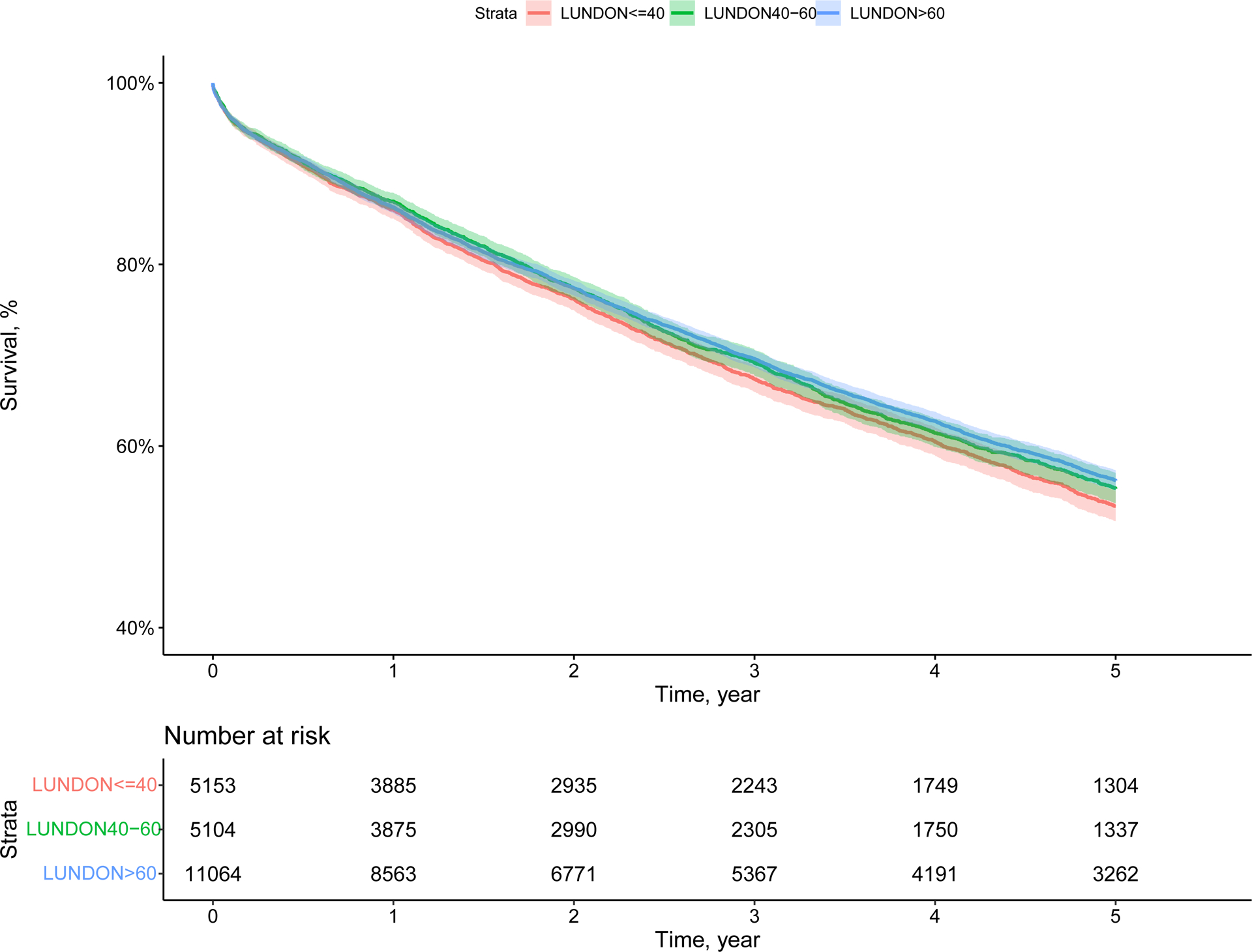
Kaplan-Meier curves for five-year graft survival in the overall cohort stratified by LUNDON score.
Overall survival among recipients stratified by donor LUNDON score (p=0.027).
Discussion
In this study, we describe the development of the LUNDON score, a measure of the acceptability of donor lungs based on objective donor characteristics. Leveraging large-scale data from the SRTR database, we created a nine-variable, parsimonious model for predicting donor lung acceptability that had high discrimination and calibration. The purpose of this model is to promote standardization of the lung donor evaluation process beyond what is currently available using the ISHLT criteria. This tool can fill a critical gap in the field of lung transplantation and may contribute to improved lung utilization rates in the United States.
Several other groups have attempted to quantify the acceptability of donor lungs through scoring algorithms. The most well-known of these was developed by Oto and colleagues, in which they identified five donor factors associated with lung acceptability (age, smoking history, chest x-ray, secretions, and PaO2/FiO2 ratio)13. However, this score was derived using a modest cohort of 87 patients at a single institution in Australia, limiting its widespread applicability. Smits and colleagues developed a similar score, the Eurotransplant Lung Donor Score (ELDS), which adds bronchoscopy findings to this predictive model. Several other models have also been recently described, including a 5-variable (pulmonary infection, diabetes, PaO2/FiO2 ratio, smoking history, and age) Zurich Donor Score29–31. However, these models are limited in that several include recipient characteristics and others arbitrarily include donor variables based largely on stringent ISHLT criteria. Importantly, donor evaluation models are not widely used in clinical practice in the United States. Our model, in contrast, provides a robust, systematic assessment of donor factors influencing lung donor selection in the modern transplant era. We balanced our variable selection process with clinical utility, to develop a model with very good performance characteristics using variables that are readily available for all potential lung donors.
As expected, our model inputs had some overlap with previous scores (age, smoking history, chest x-ray, and PaO2/FiO2 ratio), however, we also identified several novel variables that predict lung donor acceptance (maximum creatinine, mechanism of death, history of MI, bloodstream infection, and occurrence of cardiac arrest). A distinguishing feature of our model is that it evaluates only donor variables and outcomes. Other groups have evaluated donor lung quality, developing models that include both recipient characteristics and recipient outcomes8,12. While important, such models only include data from donors accepted for transplant and do not consider the remaining 75% of potential lung donors. These models may inform appropriate matching of donors and recipients based on risk factors but are unlikely to improve overall lung utilization rates. Admittedly, any lung donor offer is evaluated in the context of recipient characteristics, including age, diagnosis, acuity, height, degree of allosensitization, etc. However, an objective, data-driven donor scoring system can provide lung transplant clinicians anchoring information and an important baseline which can be assimilated into the decision-making process about individual donor offers.
Despite career-long training in Bayesian decision-making, medical professionals commonly rely on heuristics to estimate disease probabilities and treatment-related outcomes32. Furthermore, such heuristics may lead to significant under- or overestimation of actual evidence-based probability or risk33. “Anchoring and adjustment” is one such heuristic that influences how individuals intuitively assess probabilities. According to the anchoring and adjustment heuristic, decision makers employ a certain starting point (“anchor”) and adjust their estimates based on objective or subjective criteria34. However, if these anchors are based on erroneous or inaccurate cognitive biases that a clinician carries (like from prior experience), the resulting clinical decision-making will be similarly biased, a phenomenon called anchoring-and-adjustment bias35. Such decision-making processes are exemplified in the field of cognitive psychology which defines 2 types of reasoning: type I (intuitive or heuristic reasoning) versus type II (analytical or systematic reasoning)36. Donor lung utilization decisions are often determined by type I reasoning, reliant upon the quick, anchored, “gut-feelings” of transplant clinicians as opposed to more evidence-based type II reasoning. The bias associated with such reasoning can be mitigated with standardized lung donor evaluation processes, such as the LUNDON score.
The transplant community has experience with risk prediction scores. For example, the Kidney Donor Profile Index (KDPI) plays a major role in donor allocation for renal transplant37. Widespread implementation of this algorithm in the renal transplant community has resulted in increased utilization of kidneys from marginal donors38. Our LUNDON score, while similar to KDPI in purpose (i.e., increasing organ utilization rates), does not consider recipient outcomes. Therefore, this score will allow for standardization of the lung utilization process even in the 75% of donors who are not used for lung transplant. Seamless adoption of this score by transplant centers and OPOs should be possible given the widespread use of other scores by these organizations.
Low lung utilization rates contribute to the chronic shortage of donor lung allografts for transplant2. A possible cause of this is the continued reliance of the transplant community on the ISHLT standard criteria to assess potential donors. These criteria, when evaluated at face value, produce a binary result for every donor (standard or extended) which is inadequate for assessing potential donors, particularly with prior studies showing that a majority of accepted lung offers come from extended criteria donors4. In other words, anchoring on these overly stringent criteria have led to low lung acceptance rates over the last several decades, particularly in marginal but potentially usable donors. Providing an objective evidence-based score may mitigate anchoring bias associated with these ISHLT criteria and thus improve lung utilization rates.
There are several potential applications of this model, which require further (external) validation and study (Box). In particular, the modifiable factors in the score (e.g., creatinine, PaO2/FiO2 ratio, bloodstream infection, chest x-ray appearance) may be attractive targets for donor optimization. For example, donors with low-to-moderate acceptability scores may benefit from more robust optimization by OPOs and transplant centers, perhaps even at specializeddonor-care facilities39. Lung protective management or prone ventilation could augment lung utilization in these otherwise marginal donors39,40. Similarly, EVLP could be highly beneficial in donors with marginal scores. Further research on such situations is required. For donors with high scores, expedited pulmonary transplant workup may be able to commence more efficiently while specific recipient characteristics such as height, degree of allosensitization, or other factors that limit the recipient access to organs are considered in the decision-making process. This model also carries potentially significant policy implications. For example, OPTN policies state that OPOs are individually responsible for defining “acceptable” donor organs, which allows for significantly difference criteria between sites20,41. Widespread adoption of this LUNDON score in the United States (which is comprised of 57 OPOs) could standardize the current system for defining acceptable donor lungs. If so, this could have several positive implications including reducing disparities in donor selection, reducing administrative burden associated with making offers, and standardizing lung acceptance rates as a quality metric to assess performance across different OPOs and transplant centers42. Further research into this is needed.
Another implicit finding of this study is the importance of the surgical procurement team in evaluating appropriate donors. When possible, in-person (or “in situ”) evaluation by surgical procurement specialists is ideal, especially for moderate- and high-scoring donors43.
A critical finding of our study is that even low scoring (i.e., less acceptable) donor lungs appear to be safe for most lung transplant recipients, with similar survival rates regardless of LUNDON score. This study adds to a growing body of literature suggesting that “non-ideal” donor lungs can be used safely in most scenarios5,6. Notably, additional research is warranted to determine if high scoring (i.e., most acceptable) lungs should be reserved for patients with high LAS. Regardless, these recipient findings extend the practical applications of the LUNDON score beyond a general overall assessment of the acceptability of donor lungs, by providing valuable information regarding the prognosis of donor lungs after transplantation, thereby informing donor organ selection in potential recipients with varying acuity.
This study has several strengths. First, it leverages the large-scale SRTR dataset, which includes information on all transplant donors in the United States. Second, it uses robust methodology to consider a large number of clinically relevant potential predictor variables and how these contribute to donor lung acceptability. Third, the analysis was performed using data from the post-LAS era, which reflects modern transplant practices. Conversely, this study has some limitations. First, the study uses retrospective data to predict the acceptability of donor lungs. Consequently, it will be important to evaluate this score over time as transplant practices change, especially since the goal of this score is to optimize lung utilization. Second, this score excludes some potentially relevant donor factors that are not consistently available in the SRTR database (like length of intubation and bronchoscopy results). Clinicians will have to use this score in the context of other important clinical variables (both donor- and recipient-specific) to decide lung acceptability on a case-by-case basis. Third, this score was developed using primarily North American donors and therefore may not reflect practices in other countries. Fourth, this score does not consider DCD donors, due to the vastly dynamic factors that affect DCD lung utilization. Finally, this model does not consider recent advances in donor optimization, most notably EVLP44.
Conclusions
Using a large, multicenter cohort of brain-dead organ donors in the United States, we systematically identified key donor variables that were highly associated with donor lung utilization. We used these variables to create the LUNDON score, an objective and novel measure of donor lung acceptability. This contemporary predictive model that objectively assesses potential lung donors can fill an important gap in the field of lung transplantation and potentially improve lung utilization rates.
Supplementary Material
Acknowledgements
Brendan T. Heiden has funding through a cardiothoracic surgery NIH 5T32HL007776-25 grant. Varun Puri, Su-Hsin Chang, and Yan Yan have funding through a NIH 1R01HL146856-01A1 grant. The authors would like to recognize Christian Oncken, Ken Wamsley, and the Washington University Department of Surgery IT staff for help in developing the website.
Source of Funding:
Funded in part by NIH 5T32HL007776-25 (BTH), NIH 1R01HL146856-01A1 (VP, S-HC, YY)
Abbreviation List
- BMI
body mass index
- DCD
donation after circulatory death
- EVLP
ex vivo lung perfusion
- ISHLT
International Society for Heart and Lung Transplantation
- LAS
lung allocation score
- OPO
organ procurement organization
- OPTN
Organ Procurement and Transplantation Network
- PaO2/FiO2
ratio of arterial partial pressure of oxygen to fraction of inspired oxygen
- SRTR
Scientific Registry of Transplant Recipients
- UNOS
United Network for Organ Sharing
Footnotes
Meeting Presentations: None
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability
The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). Data requests should be directed to SRTR (https://www.srtr.org/). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
References
- 1.Valapour M, Skeans MA, Heubner BM, et al. OPTN/SRTR 2012 Annual Data Report: Lung. Am J Transplant. 2014,14:139–165. [DOI] [PubMed] [Google Scholar]
- 2.Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2017 Annual Data Report: Lung. Am J Transplant. 2019,19:404–484. [DOI] [PubMed] [Google Scholar]
- 3.Copeland H, Hayanga JWA, Neyrinck A, et al. Donor heart and lung procurement: A consensus statement. J Hear Lung Transplant. 2020,39:501–517. [DOI] [PubMed] [Google Scholar]
- 4.Reyes KG, Mason DP, Thuita L, et al. Guidelines for Donor Lung Selection: Time for Revision? Ann Thorac Surg. 2010,89:1756–1765. [DOI] [PubMed] [Google Scholar]
- 5.Sundaresan S, Semenkovich J, Ochoa L, et al. Successful outcome of lung transplantation is not compromised by the use of marginal donor lungs. J Thorac Cardiovasc Surg. 1995,109:1075–1080. [DOI] [PubMed] [Google Scholar]
- 6.Choi AY, Jawitz OK, Raman V, et al. Predictors of Older Donor Lung Use: Are We Too Good at Saying No? Ann Thorac Surg. 2020,110:1683–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smits JM, Van Der Bij W, Van Raemdonck D, et al. Defining an extended criteria donor lung: An empirical approach based on the Eurotransplant experience. Transpl Int. 2011,24:393–400. [DOI] [PubMed] [Google Scholar]
- 8.Loor G, Radosevich DM, Kelly RF, et al. The University of Minnesota Donor Lung Quality Index: A Consensus-Based Scoring Application Improves Donor Lung Use. Ann Thorac Surg. 2016,102:1156–1165. [DOI] [PubMed] [Google Scholar]
- 9.Grimm JC, Valero V, Magruder JT, et al. A novel risk score that incorporates recipient and donor variables to predict 1-year mortality in the current era of lung transplantation. J Hear Lung Transplant. 2015,34:1449–1454. [DOI] [PubMed] [Google Scholar]
- 10.Huppmann P, Neurohr C, Leuschner S, et al. The Munich-LTX-Score: predictor for survival after lung transplantation. Clin Transplant. 2012,26:173–183. [DOI] [PubMed] [Google Scholar]
- 11.Mulligan MJ, Sanchez PG, Evans CF, et al. The use of extended criteria donors decreases one-year survival in high-risk lung recipients: A review of the United Network of Organ Sharing Database. J Thorac Cardiovasc Surg. 2016,152:891–898.e2. [DOI] [PubMed] [Google Scholar]
- 12.Whited WM, Trivedi JR, van Berkel VH, et al. Objective Donor Scoring System for Lung Transplantation. Ann Thorac Surg. 2019,107:425–429. [DOI] [PubMed] [Google Scholar]
- 13.Oto T, Levvey BJ, Whitford H, et al. Feasibility and Utility of a Lung Donor Score: Correlation With Early Post-Transplant Outcomes. Ann Thorac Surg. 2007,83:257–263. [DOI] [PubMed] [Google Scholar]
- 14.The SRTR Database Available from: https://www.srtr.org/about-the-data/the-srtrdatabase/. Accessed May 3, 2021.
- 15.Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ.,350. Epub ahead of print January 7, 2015. DOI: 10.1136/bmj.g7594. [DOI] [PubMed] [Google Scholar]
- 16.Vyas DA, Eisenstein LG, Jones DS. Hidden in Plain Sight — Reconsidering the Use of Race Correction in Clinical Algorithms. N Engl J Med. 2020,383:874–882. [DOI] [PubMed] [Google Scholar]
- 17.Waters EA, Colditz GA, Davis KL. Essentialism and Exclusion: Racism in Cancer Risk Prediction Models. J Natl Cancer Inst.. Epub ahead of print April 2021. DOI: 10.1093/jnci/djab074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waters EA, Taber JM, McQueen A, et al. Translating Cancer Risk Prediction Models into Personalized Cancer Risk Assessment Tools: Stumbling Blocks and Strategies for Success. Cancer Epidemiol biomarkers Prev a Publ Am Assoc Cancer Res cosponsored by Am Soc Prev Oncol. 2020,29:2389–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thabut G, Christie JD, Kremers WK, et al. Survival differences following lung transplantation among US transplant centers. JAMA - J Am Med Assoc. 2010,304:53–60. [DOI] [PubMed] [Google Scholar]
- 20.Halpern SE, McConnell A, Peskoe SB, et al. A three-tier system for evaluation of organ procurement organizations’ willingness to pursue and utilize nonideal donor lungs. Am J Transplant. 2021,21:1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cypel M, Feld JJ, Galasso M, et al. Prevention of viral transmission during lung transplantation with hepatitis C-viraemic donors: an open-label, single-centre, pilot trial. Lancet Respir Med. 2020,8:192–201. [DOI] [PubMed] [Google Scholar]
- 22.Tullius SG, Rabb H. Improving the Supply and Quality of Deceased-Donor Organs for Transplantation. N Engl J Med. 2018,378:1920–1929. [DOI] [PubMed] [Google Scholar]
- 23.%lgtphcurv9 | Donna Spiegelman | Harvard T.H. Chan School of Public Health Available from: https://www.hsph.harvard.edu/donna-spiegelman/software/lgtphcurv9/. Accessed May 3, 2021.
- 24.Sultan AA, West J, Grainge MJ, et al. Development and validation of risk prediction model for venous thromboembolism in postpartum women: Multinational cohort study. BMJ.,355. Epub ahead of print December 5, 2016. DOI: 10.1136/bmj.i6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altman DG, Vergouwe Y, Royston P, et al. Prognosis and prognostic research: Validating a prognostic model. BMJ. 2009,338:1432–1435. [DOI] [PubMed] [Google Scholar]
- 26.SAS Macros Available from: https://ncook.bwh.harvard.edu/sas-macros.html. Accessed June 10, 2021.
- 27.Sullivan LM, Massaro JM, D’Agostino RB. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004,23:1631–1660. [DOI] [PubMed] [Google Scholar]
- 28.Heiden BT, Tetteh E, Robbins KJ, et al. Dissemination and Implementation Science in Cardiothoracic Surgery: A Review and Case Study. Ann Thorac Surg.,0. Epub ahead of print September 2021. DOI: 10.1016/J.ATHORACSUR.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehrsam JP, Held U, Opitz I, et al. A new lung donor score to predict short and long-term survival in lung transplantation. J Thorac Dis. 2020,12:5485–5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reul RM, Loor G, Garcha PS, et al. Allograft discard risk index for lung transplantation. J Hear lung Transplant Off Publ Int Soc Hear Transplant. 2021,40:1658–1667. [DOI] [PubMed] [Google Scholar]
- 31.Schwarz S, Rahimi N, Kifjak D, et al. Comparison of donor scores in bilateral lung transplantation-A large single-center analysis. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2021,21:2132–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phang SH, Ravani P, Schaefer J, et al. Internal Medicine residents use heuristics to estimate disease probability. Can Med Educ J. 2015,6:e71. [PMC free article] [PubMed] [Google Scholar]
- 33.S H, SH P, JP S, et al. Estimation of post-test probabilities by residents: Bayesian reasoning versus heuristics? Adv Health Sci Educ Theory Pract. 2014,19:393–402. [DOI] [PubMed] [Google Scholar]
- 34.Hughes TM, Dossett LA, Hawley ST, et al. Recognizing Heuristics and Bias in Clinical Decision-making. Ann Surg. 2020,271:813–814. [DOI] [PubMed] [Google Scholar]
- 35.Senay I, Kaphingst KA. Anchoring-and-Adjustment Bias in Communication of Disease Risk. Med Decis Making. 2009,29:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ralston S, Penman ID, Strachan MWJ, et al. , eds. Davidson’s principles and practice of medicine. 23rd Edition. Elsevier, 2016. [Google Scholar]
- 37.AB M, J L, LM F, et al. A Risk Index for Living Donor Kidney Transplantation. Am J Transplant. 2016,16:2077–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foutz J, Carrico RJ, Sisaithong K, et al. One Year of COIIN Outcomes: Did Cohorts A and B Maintain Graft Survival? [Abstract]. 2020 Am Transpl Congr. Available from: https://atcmeetingabstracts.com/abstract/one-year-of-coiin-outcomes-did-cohorts-a-and-bmaintain-graft-survival/. 2020. Accessed August 24, 2021. [Google Scholar]
- 39.Chang SH, Kreisel D, Marklin GF, et al. Lung Focused Resuscitation at a Specialized Donor Care Facility Improves Lung Procurement Rates. Ann Thorac Surg. 2018,105:1531–1536. [DOI] [PubMed] [Google Scholar]
- 40.Marklin GF, O’Sullivan C, Dhar R. Ventilation in the prone position improves oxygenation and results in more lungs being transplanted from organ donors with hypoxemia and atelectasis. J Hear Lung Transplant. 2021,40:120–127. [DOI] [PubMed] [Google Scholar]
- 41.Organ Procurement and Transplantation Network (OPTN) Policies Available from: https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf. Accessed June 11, 2021.
- 42.Andreoni KA. Now is the time for the Organ Procurement and Transplantation Network to change regulatory policy to effectively increase transplantation in the United States, Carpe Diem. Am J Transplant. 2020,20:2026–2029. [DOI] [PubMed] [Google Scholar]
- 43.Martens A, Neyrinck A, Van Raemdonck D. Accepting donor lungs for transplant: let Lisa and Bob finish the job! European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2016,50:832–833. [DOI] [PubMed] [Google Scholar]
- 44.Divithotawela C, Cypel M, Martinu T, et al. Long-term Outcomes of Lung Transplant with Ex Vivo Lung Perfusion. JAMA Surg. 2019,154:1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). Data requests should be directed to SRTR (https://www.srtr.org/). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.


