Abstract
OBJECTIVE
To identify a postpartum lipidomic signature associated with gestational diabetes mellitus (GDM) and investigate the role of the identified lipids in the progression to type 2 diabetes (T2D).
RESEARCH DESIGN AND METHODS
This prospective cohort study enrolled 1,409 women at 24–72 h after delivery of a singleton baby and followed them prospectively at the Boston Medical Center. The lipidome was profiled by liquid chromatography-tandem mass spectrometry. Diagnoses of GDM and incident T2D were extracted from medical records and verified using plasma glucose levels.
RESULTS
Mean (SD) age of study women at baseline was 28.5 (6.6) years. A total of 219 (16.4%) women developed incident diabetes over a median follow-up of 11.8 (interquartile range 8.2–14.8) years. We identified 33 postpartum lipid species associated with GDM, including 16 inverse associations (primarily cholesterol esters and phosphatidylcholine plasmalogens), and 17 positive associations (primarily diacyglycerols and triacyglycerols). Of these, four were associated with risk of incident T2D and mediated ∼12% of the progression from GDM to T2D. The identified lipid species modestly improved the predictive performance for incident T2D above classical risk factors when the entire follow-up period was considered.
CONCLUSIONS
GDM was associated with a wide range of lipid metabolic alterations at early postpartum, among which some lipid species were also associated with incident T2D and mediated the progression from GDM to T2D. The improvements attained by including lipid species in the prediction of T2D provides new insights regarding the early detection and prevention of progression to T2D.
Graphical Abstract
Introduction
The prevalence of type 2 diabetes (T2D) has more than doubled globally alongside the epidemic of obesity, making it a leading cause of morbidity in the U.S. and worldwide (1). In parallel, the prevalence of gestational diabetes mellitus (GDM), a frequent complication of pregnancy, has been rising worldwide over the past several decades (2) and has become a public health concern because of its profoundly adverse implications for the short- and long-term health of mother and child (3,4). A meta-analysis showed that women with a history of GDM were 10 times more likely to develop T2D than those with a normoglycemic pregnancy (4). The cumulative incidence of T2D was shown to increase greatly in the first 5 years after a GDM pregnancy (5), suggesting a critical need to identify high-risk individuals and intervene at an early stage.
Another prominent metabolic change during pregnancy is lipid metabolism owing to adaptations to meet the requirements of fetal growth (6). In women with GDM, lipid homeostasis further deteriorates, accompanied by abnormal glucose tolerance (7). Studies have documented lipidomic signatures associated with GDM in early and midpregnancy (8). One study showed that elevated lipid levels during pregnancy decline slowly at postpartum (9). However, it is unknown whether GDM-related lipidomic signatures at early postpartum persist into later postpartum or whether these lipidomic profiles can inform the prediction of T2D in later life.
Using the Boston Birth Cohort (BBC), a racially and ethnically diverse birth cohort with a long follow-up period, we aimed to identify an early postpartum lipidomic signature associated with GDM and to examine whether the lipidomic profile varied between women who had GDM versus those with preexisting diabetes in the index pregnancy. We also prospectively investigated whether the identified GDM-related lipidomic signature can facilitate the early prediction of T2D years later. Finally, we explored the potential mediation effect of the identified lipid species in the progression from GDM to T2D.
Research Design and Methods
Study Participants
The ongoing BBC enrolled 8,623 racially and ethnically diverse mother-infant pairs from 1998 to 2019 at Boston Medical Center (BMC). Detailed information on enrollment has been described previously (10). Briefly, on recruitment (at baseline, within 24–72 h after delivery), eligible mothers completed a questionnaire by in-person interview to collect sociodemographic, diet, environmental, and health information and medical history, as well as underwent a blood draw. A subset of mother-child pairs who continued their medical care at BMC and consented to participate in the postnatal follow-up study have been followed prospectively up to a maximum of 21 years. As of 2020, 3,394 mother-child pairs have been followed (10). Of those followed mothers, 1,409 had plasma lipidome quantified at baseline and are the focus of this study. The number of included study participants and the attrition is shown in Supplementary Fig. 1. The baseline characteristics of the mothers included in this study were comparable with those of the total enrolled and the total followed mothers in the BBC (Supplementary Table 1). The study protocol was approved by the institutional review boards of BMC and Johns Hopkins University Bloomberg School of Public Health. All study participants gave written informed consent.
Plasma Lipidomic Profiling
The plasma lipidome was profiled using high-throughput liquid chromatography-tandem mass spectrometry at the Broad Institute. A description of the plasma lipidomic profiling is provided in the Supplementary Material. This study included a total of 209 lipid species attributable to 17 different lipid classes/subclasses, including 12 cholesterol esters (CEs), 4 ceramides, 11 diacylglycerols (DAGs), 9 lysophosphatidylcholines (LPCs), 8 lysophosphatidylethanolamines (LPEs), 3 monoacylglycerols, 24 phosphatidylcholines (PCs), 15 phosphatidylcholine plasmalogens (PC-Ps), 14 phosphatidylethanolamines, 12 phosphatidylethanolamine plasmalogens, 3 phosphatidylinositols (PIs), 1 phosphatidylserine, 9 sphingomyelins (SMs), 1 sphingolipid, 79 triacylglycerols (TAGs), 2 sterol lipids, and 2 other lipids (Supplementary Table 2).
Definition of GDM, Preexisting Diabetes, and Incident T2D
ICD-9-CM and ICD-10-CM diagnosis codes, history of antidiabetic medication prescription, as well as laboratory testing of plasma glucose profile were abstracted from the electric medical record. The definition and description of the adjudication of GDM, preexisting diabetes, and incident T2D are provided in the Supplementary Material. We computed follow-up time from the date of delivery to the date of incident T2D diagnosis, last visit, or the end of follow-up, whichever came first. Since there was a small number (n = 23) of women with follow-up length >20 years, we grouped these women to 20 years.
Perinatal Covariates
Data on race and ethnicity, educational attainment, smoking status during pregnancy, medical and reproductive history, and prepregnancy weight and height were self-reported via an in-person questionnaire. For this study, we grouped self-identified race and ethnicity into non-Hispanic Black versus other (including non-Hispanic White, Asian, Pacific Islander, mixed race, and other race). We grouped educational attainment into high school and below versus college and above. We classified smoking status during pregnancy into nonsmoker versus smoker (former or continuous smoker). We dichotomized parity into primiparous versus multiparous. We calculated prepregnancy BMI as weight in kilograms divided by height in meters squared and defined overweight or obesity (OWO) as BMI ≥25 kg/m2 (11). We extracted caesarean delivery, gestational age at birth, pregnancy complications, family history of diabetes, postpartum obesity, and metabolic syndrome from the electronic medical record. Hypertensive disorders during pregnancy included chronic/gestational hypertension; preeclampsia; eclampsia; or hemolysis, elevated liver enzymes, and low platelets syndrome (12).
Statistical Analysis
To reduce batch effects and remove the effect of potential outliers, we normalized the lipidome data using inverse normal transformation. We calculated Pearson correlation coefficients among lipid species.
We used linear regression models to identify lipid species associated with GDM or preexisting diabetes after adjusting for age at delivery, race and ethnicity, educational attainment, smoking status during pregnancy, parity, prepregnancy BMI, and hypertensive disorders during pregnancy. We fit Cox proportional hazards regression models to examine the association between the identified lipid species and incident T2D, adjusting for the same covariates as well as family history of diabetes. We used multiplicative interaction terms and stratified models to examine effect modification of associations by OWO.
We also explored the joint effects of lipid species by calculating a predictive risk score among women who developed GDM in the index pregnancy. To avoid overfitting and account for the intercorrelation of lipid species, we used a penalized regression approach for lipid species selection. We generated a predictive risk score by computing the weighted sum of covariates from the 10-fold cross-validation Lasso prediction models. Descriptions of the penalized Cox proportional hazards regression model and predictive risk score calculation are provided in the Supplementary Material.
We further assessed the ability of lipid species to predict incident T2D using the receiver operating characteristic (ROC) area under the curve (AUC). We calculated the AUC using ROC analysis for two models: 1) base model, which was based on classical diabetes risk factors, and 2) full model, which was a combination of classical risk factors and identified lipid species. To capture the time-varying accuracy of the prediction models, we calculated the time-dependent ROC curve using a cumulative cases/dynamic controls approach to predict cumulative (prevalent) cases over a fixed future time interval using the R package survivalROC (13) and plotted time-dependent AUC against time to explore the discriminative performance of predictive models across the entire follow-up period.
To examine the potential mediation effect of identified lipid species on the progression from GDM to T2D, we performed a mediation analysis for multiple mediators simultaneously using the R package mma (14), which allows correlations among the mediators. We included postpartum obesity and metabolic syndrome as indicators of insulin resistance in the mediation model. Given that breastfeeding may play a role in the progression to T2D (15), we also included breastfeeding status in the model.
We performed all analyses using RStudio version 2021.09.0.351 (Posit Software, Boston, MA), and all statistical tests were two-sided. We calculated the false discovery rate using the Benjamini-Hochberg method to correct for multiple testing, and a corrected P < 0.05 was considered significant.
Results
Baseline Characteristics of the Study Population
Mean (SD) age of the study women at baseline was 28.5 (6.6) years. During the median follow-up of 11.8 years (interquartile range 8.2–14.8 years), 219 (16.4%) women developed incident diabetes (216 T2D, 2 type 1 diabetes, and 1 other specific diabetes). In the predictive analysis, we focused on incident T2D. As such, we excluded 3 women who developed type 1 diabetes or other specific diabetes during follow-up, 73 who had preexisting diabetes, and 13 who were lost to follow-up. Finally, the predictive analysis included 1,320 women (Supplementary Fig. 1). The characteristics of the study participants are summarized according to incident T2D status among the women included in the predictive analysis (Table 1) or diabetes status during pregnancy among the total study population (Supplementary Table 3). Compared with those who did not develop T2D, women with incident T2D were typically older, had a higher prepregnancy BMI, and were most likely to have GDM during the index pregnancy and obesity and metabolic syndrome at follow-up.
Table 1.
Baseline and follow-up characteristics of study participants who were included in the predictive analysis (n = 1,320)
| Characteristic | Nondiabetic (n = 1,104) | Incident T2D (n = 216) | P |
|---|---|---|---|
| Baseline | |||
| Age at delivery (years) | 27.93 (6.50) | 30.22 (6.85) | <0.001 |
| Race and ethnicity | |||
| Non-Hispanic Black | 700 (63.4) | 150 (69.4) | 0.106 |
| Other race | 404 (36.6) | 66 (30.6) | |
| Educational attainment | |||
| High school and below | 721 (65.3) | 151 (69.9) | 0.22 |
| College and above | 383 (34.7) | 65 (30.1) | |
| Parity | |||
| Primiparous | 497 (45.0) | 85 (39.4) | 0.145 |
| Multiparous | 607 (55.0) | 131 (60.6) | |
| Smoking status during pregnancy | |||
| Nonsmoker | 921 (83.4) | 175 (81.0) | 0.446 |
| Smoker | 183 (16.6) | 41 (19.0) | |
| BMI (kg/m2) | 25.90 (5.86) | 29.66 (7.73) | <0.001 |
| OWO prepregnancy | 532 (48.2) | 150 (69.4) | <0.001 |
| Glucose tolerance status in pregnancy | |||
| Normal glucose tolerance | 1,000 (90.6) | 161 (74.5) | <0.001 |
| GDM | 104 (9.4) | 55 (25.5) | |
| Family history of diabetes | 29 (2.6) | 11 (5.1) | 0.086 |
| Follow-up | |||
| Breastfeeding status | 0.009 | ||
| Formula exclusively | 252 (22.8) | 69 (31.9) | |
| Mixed feeding | 768 (69.6) | 128 (59.3) | |
| Breastfeed exclusively | 84 (7.6) | 19 (8.8) | |
| Obesity | 560 (50.7) | 168 (77.8) | <0.001 |
| Metabolic syndrome | 36 (3.3) | 27 (12.5) | <0.001 |
Data are n (%) or mean (SD).
Identification of GDM-Related Lipid Species
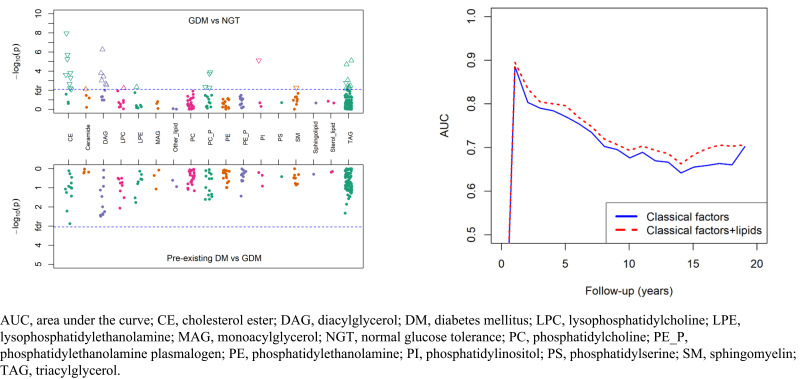
GDM was significantly associated with 33 lipid species after correction for multiple testing (Supplementary Table 4 and Supplementary Fig. 2A). Among those lipids, 16 were inversely associated with GDM, including CEs, PC-Ps, PIs, and SMs, while the other 17 lipid species were positively associated with GDM, mostly TAGs and DAGs. These associations were independent of age at delivery, race and ethnicity, education, parity, smoking during pregnancy, prepregnancy BMI, and hypertensive disorders during pregnancy. After further adjustment for breastfeeding status, the results did not change substantially (Supplementary Fig. 2B). Correlation analysis of the 33 lipid species showed that most lipid species were correlated (Supplementary Fig. 3).
Comparison of Lipidomic Profile
Comparisons of postpartum lipidomic profiles between women who developed GDM during the index pregnancy and those who had preexisting diabetes are presented in Supplementary Fig. 4. Although the levels of 26 lipid species in women with preexisting diabetes appeared different from those in women who had GDM in the index pregnancy, none passed the false discovery rate threshold of 0.05.
GDM-Associated Lipid Species and Incident T2D in the Total Study Population
Fourteen of 33 postpartum lipid species associated with GDM were also associated with the development of T2D after delivery, but only four remained statistically significant after multiple testing correction (Table 2). Of those, three lipid species [PI(36:2), PC(P-36:2), and SM(14:0)] that were negatively associated with GDM also showed an inverse association with the risk of incident T2D; adjusted hazard ratios (HRs) ranged from 0.79 to 0.82 per 1-SD increment in lipid species. One lipid species [TAG(56:5)] that was positively associated with GDM also showed a positive association with the risk of incident T2D, with an adjusted HR of 1.22 per 1-SD increment. In addition, there were 10 lipid species associated with incident T2D at a nominal significance (raw P < 0.05). The directions of the associations between these lipid species and incident T2D were almost always the same as that between GDM and the lipid species (Supplementary Table 5). When the 33 lipids were summed into classes/subclasses, three (PC-P, PI, and SM) of the nine classes/subclasses were significantly associated with incident T2D after correcting for multiple testing (Supplementary Fig. 5 and Supplementary Table 6).
Table 2.
Associations of individual lipid species with GDM or the risk of incident T2D
| GDM* | Incident T2D† | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lipid species | β | SE | P | FDR P | HR | 95% CI | P | FDR P | Class or subclass |
| PI(36:2) | −0.38 | 0.08 | 7.5E-06 | 2.7E-04 | 0.79 | 0.69–0.91 | 8.7E-04 | 2.9E-02 | PI |
| SM(14:0) | −0.23 | 0.08 | 5.3E-03 | 4.1E-02 | 0.82 | 0.72–0.94 | 3.8E-03 | 4.1E-02 | SM |
| TAG(56:5) | 0.23 | 0.08 | 5.2E-03 | 4.1E-02 | 1.22 | 1.06–1.41 | 4.5E-03 | 4.1E-02 | TAG |
| PC(P-36:2) | −0.31 | 0.08 | 1.9E-04 | 3.6E-03 | 0.82 | 0.72–0.94 | 5.0E-03 | 4.1E-02 | PC-P |
| TAG(54:10) | −0.27 | 0.09 | 1.7E-03 | 2.0E-02 | 0.84 | 0.74–0.96 | 1.3E-02 | 6.6E-02 | TAG |
| TAG(56:8) | 0.26 | 0.08 | 8.4E-04 | 1.2E-02 | 1.21 | 1.04–1.40 | 1.3E-02 | 6.6E-02 | TAG |
| CE(16:1) | −0.37 | 0.08 | 2.0E-06 | 1.4E-04 | 0.83 | 0.72–0.97 | 1.5E-02 | 6.6E-02 | CE |
| TAG(56:7) | 0.35 | 0.08 | 2.0E-05 | 5.9E-04 | 1.19 | 1.03–1.38 | 1.6E-02 | 6.6E-02 | TAG |
| PC(P-36:4) | −0.24 | 0.09 | 5.3E-03 | 4.1E-02 | 0.86 | 0.75–0.98 | 2.4E-02 | 8.2E-02 | PC-P |
| PC(P-36:1) | −0.32 | 0.08 | 1.3E-04 | 3.4E-03 | 0.86 | 0.75–0.98 | 2.5E-02 | 8.2E-02 | PC-P |
| CE(14:0) | −0.45 | 0.08 | 1.1E-08 | 2.3E-06 | 0.85 | 0.73–0.99 | 3.2E-02 | 9.6E-02 | CE |
| PC(P-34:1)-A | −0.24 | 0.09 | 4.4E-03 | 4.0E-02 | 0.87 | 0.76–0.99 | 3.8E-02 | 1.0E-01 | PC-P |
| CE(18:0) | −0.22 | 0.08 | 7.8E-03 | 5.0E-02 | 0.87 | 0.76–0.99 | 4.1E-02 | 1.0E-01 | CE |
| CE(18:1) | −0.31 | 0.08 | 2.4E-04 | 4.3E-03 | 0.87 | 0.75–1.00 | 4.8E-02 | 1.1E-01 | CE |
Lipid species were inverse normally transformed. FDR, false discovery rate.
Difference of postpartum lipid species between women who had GDM in the index pregnancy and those with a nondiabetic pregnancy (reference group) was estimated from linear regression models adjusted for age at delivery, race and ethnicity, educational attainment, smoking status during pregnancy, parity, prepregnancy BMI, and hypertensive disorder during pregnancy.
HRs for incident T2D per 1-SD increase in each lipid species were estimated from Cox proportional hazards regression models adjusted for age at delivery, race and ethnicity, educational attainment, smoking status during pregnancy, parity, prepregnancy BMI, hypertensive disorder during pregnancy, and family history of diabetes.
Prediction of Incident T2D in the Total Study Population
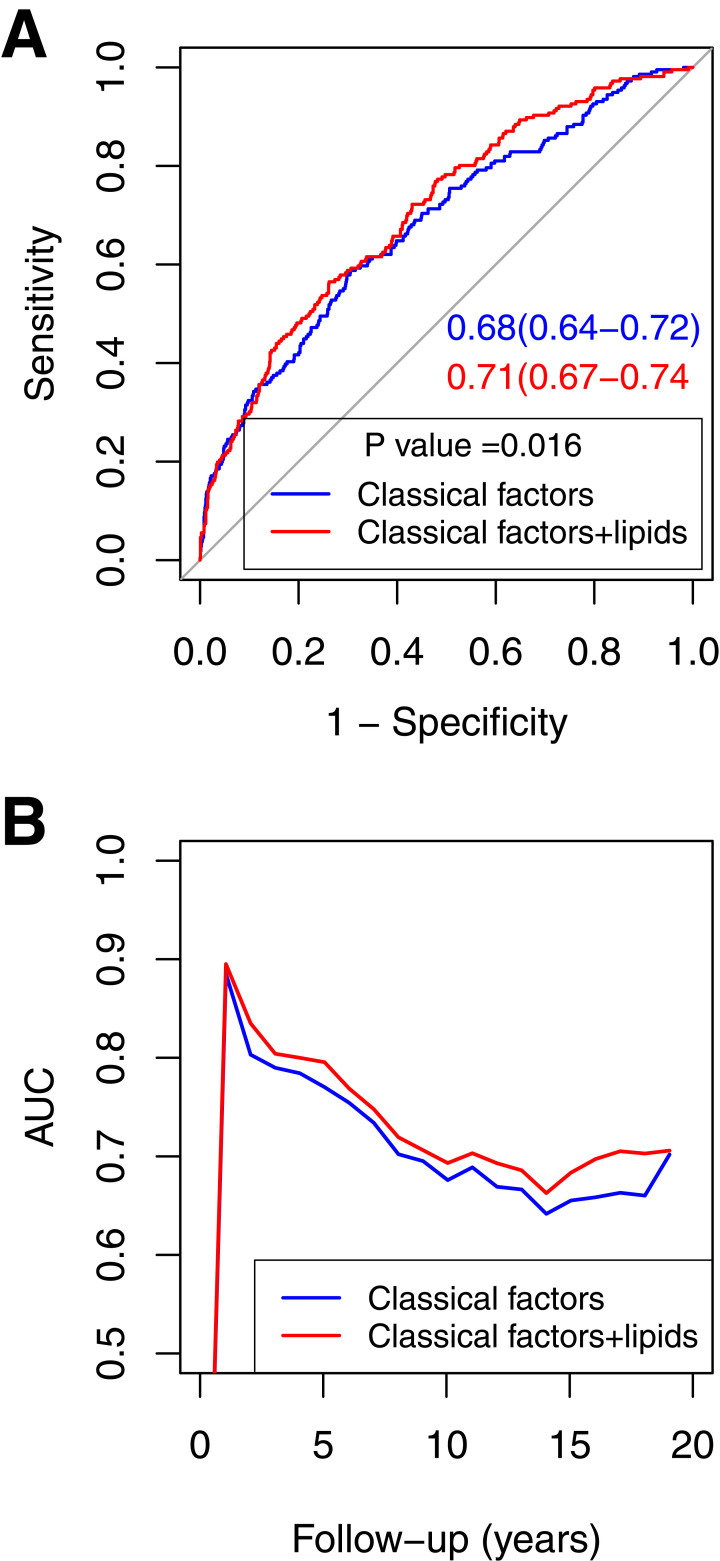
We constructed two predictive models. The base model was composed of six classical risk factors, including age at delivery, race and ethnicity, smoking during pregnancy, prepregnancy BMI, GDM history, and family history of diabetes. The full model consisted of the six classical risk factors as well as four incident T2D-associated lipid species [PC(P-36:2), PI(36:2), SM(14:0), and TAG(56:5)]. To assess the discriminatory ability of the established models, we conducted ROC curve analyses. Adding four lipid species to the base model significantly increased the AUC from 0.68 (95% CI 0.64–0.72) to 0.71 (95% CI 0.67–0.74; P = 0.016) (Fig. 1A). The likelihood ratio test for the model comparison was also statistically significant (P = 0.0049). The time-dependent ROC curve showed that the full model consistently performed better than the base model over the 20 years of follow-up (Fig. 1B).
Figure 1.
ROC curves and time-dependent AUCs based on the base model and full model across 20 years of follow-up. The ROC curves (A) and time-dependent AUCs (B) were generated from the total predictive analysis sample (n = 1,320). The base model included six classical factors (age at delivery, race and ethnicity, smoking during pregnancy, prepregnancy BMI, GDM history, and family history of diabetes). The full model included a combination of the six classical risk factors and four lipid species [PC(P-36:2), PI(36:2), SM(14:0), and TAG(56.5)] that were associated with incident T2D.
Prediction of Progression to T2D Among Women Who Had GDM in the Index Pregnancy
Three lipid species were individually associated with incident T2D among women who developed GDM in the index pregnancy, but none of the lipids remained statistically significant after correction for multiple testing (Supplementary Table 7). To examine the joint effect of these lipid species, we calculated a predictive risk score using lipid species selected by the Lasso regression model in combination with five of the classical risk factors (age at delivery, race and ethnicity, smoking during pregnancy, prepregnancy BMI, and family history of diabetes) that were included in the base model. The HRs of progression to T2D per a 1-SD increase in the predictive risk score were 1.45 (95% CI 1.25–1.68) in the base model and 1.72 (95% CI 1.32–2.23) in the full model at 20 years follow-up (Table 3). When targeting incident T2D for the first 5, 10, and 15 years, separately, the predictive risk scores from the full model were always associated with a higher HR compared with those from the base models. The time-dependent ROC curve showed a better performance for the full model across the first 15 years of follow-up compared with the base model (Supplementary Fig. 6B). However, when the estimates were obtained without considering survival time, the full model did not show an improved predictive ability (Supplementary Fig. 6A).
Table 3.
Predictive risk score and incident T2D among women who had GDM in the index pregnancy
| Length of follow-up (years) | Total n | Cases,* n (%) | Base model | Full model | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |||
| 5 | 134 | 30 (22.3) | 1.41 (1.19–1.67) | 5.0E-05 | 2.09 (1.49–2.95) | 2.4E-05 |
| 10 | 148 | 44 (29.7) | 1.44 (1.23–1.68) | 6.5E-06 | 1.65 (1.25–2.19) | 4.5E-04 |
| 15 | 157 | 53 (33.8) | 1.44 (1.24–1.68) | 2.5E-06 | 1.69 (1.30–2.20) | 8.4E-05 |
| 20 | 159 | 55 (34.6) | 1.45 (1.25–1.68) | 1.6E-06 | 1.72 (1.32–2.23) | 5.6E-05 |
The predictive risk scores were calculated as the sum of regression coefficients of covariates from the predictive models built by the Lasso regression model. The base model included age at delivery, race and ethnicity, smoking during pregnancy, prepregnancy BMI, and family history of diabetes. The full model included LPE(22:6), PC(PA-34:1), PC(P-36:1), PC(P-36:4), PI(36:2), Cer(d18:1/16:0), SM(14:0), CE(16:1), CE(18:0), CE(18:1), CE(20:3), CE (22:4), DAG(34:1), DAG(36:2), DAG(36:3), DAG(36:4), TAG(54:4), TAG(56:5), TAG(56:7), TAG(56:9), and TAG(56:10) in addition to all covariates in the base model. HRs were for incident T2D per 1-SD increase in predictive risk score.
Cases represented accumulated incident T2D.
Mediation Effect of Lipid Species on the Progression From GDM to T2D
As shown in Supplementary Table 8, the total effect and direct effect of GDM on progression to T2D were 1.30 (95% CI 0.94–1.68) and 0.87 (95% CI 0.54–1.23), respectively. Four lipid species [PC(P-36:2), PI(36:2), SM(14:0), and TAG(C56:5)] jointly explained the progression by 12% as a mediator. Postpartum obesity, metabolic syndrome, and breastfeeding mediated the progression by 3.7%, 2.0%, and 1.1%, respectively.
Sensitivity Analysis
When we additionally adjusted for gestational age at birth, caesarean delivery, and vitamin intake in the third trimester in the models for associations between GDM and lipid species, we obtained a similar magnitude of associations for most lipid species (Supplementary Table 9). To reduce the potential influence of women who had GDM in the index pregnancy and progressed to T2D at the time of sampling, we conducted sensitivity analyses after excluding those who had GDM during pregnancy and developed T2D in the first year postpartum. The results did not change substantially (Supplementary Fig. 2C and Supplementary Fig. 4, bottom). In addition, the associations between GDM and lipid species remained essentially unchanged when we restricted the analysis to women with prepregnancy OWO (Supplementary Table 10). We did not observe any significant effect modification for OWO. We further compared the lipidomic profile among women who developed T2D during follow-up according to the tempo of T2D development. Women who developed T2D in the first 5 years postpartum had a tendency of lower levels of PC-Ps and CEs but higher levels of TAGs than those with T2D that occurred >5 years after delivery (Supplementary Table 11).
Conclusions
We observed several key findings in this large, prospective cohort study. First, we identified a GDM-associated postpartum lipidomic signature measured within 24–72 h after delivery, a period with intense metabolic changes. Second, we documented a similarity in the postpartum lipidomic profile between women who developed GDM in the index pregnancy and those with preexisting diabetes. Third, we demonstrated that four GDM-related lipid species were associated with incident T2D during a median of 12 years of follow-up and may mediate ∼12% of the progression from GDM to T2D. Finally, we constructed a predictive model by incorporating lipid species with classical diabetes risk factors, which modestly improved the discriminatory ability for predicting future risk of T2D among the total study population.
Our study identified a GDM-associated early postpartum lipidomic signature, which was characterized by higher levels of DAGs, a group of TAGs, LPCs, LPEs, and ceramides but lower levels of CEs, PC-Ps, PIs, and SMs. Our findings are consistent with a previous study reporting that women with previous GDM had lower plasma levels of PIs, but higher levels of LPCs than control participants at 22 months after pregnancy (16). Another study also documented an adverse lipid trajectory defined by traditional lipidemia measures (i.e., elevated TAG, total cholesterol, and LDL cholesterol, but lower levels of HDL cholesterol) from prepregnancy to 2 years postpartum in women with a recent GDM pregnancy (17). In addition, a similar lipidomic pattern measured in the first or second trimester has been associated with GDM (8). In the general population, similar lipidomic patterns were also associated with insulin resistance and T2D (18,19). Taken together, our data suggest that women who developed GDM during pregnancy had an unfavorable lipidomic profile at early postpartum.
The positive associations of DAGs and a group of TAGs with T2D risk is clearly shown in the literature (18,20–22). In line with these studies, we found that women who had GDM in the index pregnancy had higher levels of DAGs and a group of TAGs at early postpartum than those with a nondiabetic pregnancy. A study demonstrated that DAG is responsible for lipid-induced insulin resistance by activating protein kinase C (23). DAG can be derived from de novo synthesis, breakdown of phospholipids, and intermediates of TAG metabolism (23). It is noteworthy that the rise of intermediate lipids (e.g., DAGs) may also result from the fragmentation process of mass spectrometry. In this context, it should not generally be considered a biomarker on its own.
We did not find a consistent pattern of associations with TAGs by number of carbon atoms or double bonds. Still, we observed higher postpartum plasma levels of molecular species of TAGs containing arachidonic acid (AA) (C20:4), an essential long-chain polyunsaturated fatty acid in women who had GDM in the index pregnancy compared with women with a nondiabetic pregnancy. In agreement with our findings, several studies have reported an increased esterified AA content in phospholipids in patients with diabetes (24,25). The underlying mechanism remains elusive. One potential explanation may be the increased activity of fatty acid desaturase under hyperinsulinemia (26) along with a lower amount of elongation of AA to fatty acid 22:4n-6 (27). During gestation, maternal circulating lipid levels increase to provide enough metabolic substrates for fetal growth (6). Although the elevated lipid levels due to pregnancy decline slowly after delivery (28), it is unclear whether fetal body size can inform the maternal lipid profile at early postpartum.
In line with previous studies (18), our study found that women who developed GDM during the index pregnancy had a lower CE level at early postpartum compared with nondiabetic pregnancies. Given an increased transfer of CE from HDL toward triglyceride-rich lipoproteins and impaired cellular cholesterol efflux to plasma (29), an impairment of cholesterol esterification and CE metabolism may underlie this phenomenon. Furthermore, we observed significantly lower levels of PC-Ps in women after a GDM pregnancy. Our findings align with previous studies that showed a negative association of PC-P with insulin resistance (30). As plasmalogens may prevent lipoprotein oxidation as serum antioxidants (31), our data suggest a low-grade lipid peroxidation in early postpartum after a GDM pregnancy. We also revealed an elevated ceramide level in women who developed GDM in the index pregnancy. Given that ceramide dysregulation may lead to impairment of β-cell function (32), our findings suggest that these women may also have underlying β-cell dysfunction, which is in accordance with the literature (33).
On the other hand, we observed a similar postpartum lipidomic profile between women who developed GDM in the index pregnancy and those with preexisting diabetes, suggesting a shared common pathophysiological feature underlying both diabetic conditions. Although most women with GDM experience restored glucose homeostasis after delivery, they tend to continue having inadequate insulin secretion (34) and to be insulin resistant (35), suggesting that GDM might represent a prediabetic condition. In a similar setting, a study reported a comparable lipidomic profile between prediabetes and T2D (20). Taken together, in terms of these metabolic alterations in women after a GDM pregnancy, GDM may mirror the earlier stages of T2D (36).
From the clinical perspective, an important question is who among women with a GDM history are most likely to progress to T2D? To answer this question, we developed a predictive risk score using lipid species selected from a Lasso model coupled with classical risk factors. The predictive risk score was strongly associated with the progression to T2D after a GDM pregnancy. Our results were consistent with previous studies, which showed that these classes of lipid species measured at 6–9 weeks postpartum were associated with progression to T2D (21,22). Moreover, another study showed that decreased phospholipid trajectories from baseline (6–9 weeks postpartum after a GDM pregnancy) to 2 years of follow-up contributed to T2D progression (37). However, while the predictive risk score tended to improve the discriminatory ability provided by classical risk factors across the first 15 years of the follow-up period, it did not show improved predictive power when survival time was not taken into account. The potential reason could be due to a relatively small number of women included in this analysis.
Our study also revealed that the progression from GDM to T2D may be partially (∼12%) explained by the lipidomic profile measured at early postpartum. The underlying mechanism remains unclear. A few studies documented a role of lipidomic dysregulation in β-cell dysfunction (32) and insulin resistance (23), suggesting dyslipidemia as an independent predictor of incident T2D (38). On the other hand, studies have shown that dyslipidemia may be driven by insulin resistance in T2D (33). Nevertheless, our findings provide new insights into the potential role of lipidomic metabolism in progression to T2D that warrant further investigation.
Strengths and Limitations
Our study has several strengths, including the prospective design and long follow-up period. Our lipidomic platform covered a wide variety of lipid species. Moreover, we used new analytical methods to examine the mediation effect by simultaneously accounting for multiple mediators/confounders and to calculate the AUC by capturing the time-varying accuracy of prediction models. Finally, our prospective birth cohort study design avoids the limitation of reverse causality.
We also acknowledge several limitations. First, lipidomic profiling was conducted at only one time point. As such, we could not capture the changes of the lipidomic profile over time. Second, since glucose tolerance was not assessed in women with a GDM pregnancy at the time of sampling, we could not exclude those who had progressed to T2D before sampling from this group. However, as the development of T2D at early postpartum after a GDM pregnancy is rare (∼2%) (39), we do not think that the inclusion of a small number of women with T2D in this group distorted the large effect estimates obtained by our study. Third, the prevalence of a family history of diabetes in our study population was much lower than reported in the general literature (8,40), which may be attributable to missing documentation. Fourth, the AUC for classical risk factors in our study was slightly lower than that reported in the literature (40). The reason may be partly due to not including plasma glucose variables in the model. Finally, given that our study population was drawn from predominantly U.S. urban Black, Indigenous, and people of color living in low-income communities, caution should be used when generalizing our findings to other populations.
In summary, we identified a panel of 33 plasma lipid species at early postpartum associated with GDM. We also demonstrated that these lipid species modestly improve prediction of T2D beyond classical risk factors. These lipid biomarkers may have a role in identifying women at high risk for T2D who should undertake postpartum lifestyle intervention.
Article Information
Acknowledgments. The authors thank the study participants; the nursing staff of Labor and Delivery, BMC; and the field team for contributions to the BBC. Linda Rosen and the clinical data warehouse assisted in obtaining relevant clinical information; Ms. Rosen was compensated for her time.
Funding. The clinical data warehouse service is supported by Boston University’s Clinical and Translational Institute and the National Institutes of Health Clinical and Translational Science Award (U54-TR001012). The BBC (the parent study) is supported in part by the National Institutes of Health (Eunice Kennedy Shriver National Institute of Child Health and Human Development grants 2R01HD041702, R01HD086013, and R01HD098232, and National Institute of Environmental Health Sciences grants R01ES031272, R01ES031521, and U01ES034983), and Health Resources and Services Administration of the U.S. Department of Health and Human Services (grant UT7MC45949).
The contents of the article are solely the responsibility of the authors and do not necessarily represent the official views of the supporting agencies.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. G.W. and X.W. designed and conceptualized the study and drafted and revised the manuscript. J.P.B., T.R.B., X.H., C.P., and X.W. interpreted the results and reviewed and revised the manuscript. C.P. contributed to the acquisition of data. All authors contributed to the discussion, reviewed and edited the manuscript, and approved the final manuscript. G.W. and X.W. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.22289449.
References
- 1. Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus--present and future perspectives. Nat Rev Endocrinol 2011;8:228–236 [DOI] [PubMed] [Google Scholar]
- 2. Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care 2007;30(Suppl. 2):S141–S146 [DOI] [PubMed] [Google Scholar]
- 3. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007;30(Suppl. 2):S251–S260 [DOI] [PubMed] [Google Scholar]
- 4. Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ 2020;369:m1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002;25:1862–1868 [DOI] [PubMed] [Google Scholar]
- 6. Darmady JM, Postle AD. Lipid metabolism in pregnancy. Br J Obstet Gynaecol 1982;89:211–215 [DOI] [PubMed] [Google Scholar]
- 7. Herrera E, Ortega-Senovilla H. Disturbances in lipid metabolism in diabetic pregnancy - are these the cause of the problem? Best Pract Res Clin Endocrinol Metab 2010;24:515–525 [DOI] [PubMed] [Google Scholar]
- 8. Rahman ML, Feng YA, Fiehn O, et al. Plasma lipidomics profile in pregnancy and gestational diabetes risk: a prospective study in a multiracial/ethnic cohort. BMJ Open Diabetes Res Care 2021;9:e001551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Montes A, Walden CE, Knopp RH, Cheung M, Chapman MB, Albers JJ. Physiologic and supraphysiologic increases in lipoprotein lipids and apoproteins in late pregnancy and postpartum. Possible markers for the diagnosis of “prelipemia”. Arteriosclerosis 1984;4:407–417 [DOI] [PubMed] [Google Scholar]
- 10. Pearson C, Bartell T, Wang G, et al. Boston Birth Cohort Profile: Rationale and Study Design. Precis Nutr 2022;1:e00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention . Defining adult overweight and obesity. Accessed 23 November 2020. Available from https://www.cdc.gov/obesity/adult/defining.html
- 12. Wang G, Divall S, Radovick S, et al. Preterm birth and random plasma insulin levels at birth and in early childhood. JAMA 2014;311:587–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 2000;56:337–344 [DOI] [PubMed] [Google Scholar]
- 14. Yu Q, Scribner RA, Leonardi C, et al. Exploring racial disparity in obesity: a mediation analysis considering geo-coded environmental factors. Spat Spatio-Temporal Epidemiol 2017;21:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Z, Lai M, Piro AL, et al. Intensive lactation among women with recent gestational diabetes significantly alters the early postpartum circulating lipid profile: the SWIFT study. BMC Med 2021;19:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anderson SG, Dunn WB, Banerjee M, et al. Evidence that multiple defects in lipid regulation occur before hyperglycemia during the prodrome of type-2 diabetes. PLoS One 2014;9:e103217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Retnakaran R, Shah BR. Impact of pregnancy on the trajectories of cardiovascular risk factors in women with and without gestational diabetes. Diabetes Obes Metab 2021;23:2364–2373 [DOI] [PubMed] [Google Scholar]
- 18. Razquin C, Toledo E, Clish CB, et al. Plasma lipidomic profiling and risk of type 2 diabetes in the PREDIMED trial. Diabetes Care 2018;41:2617–2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rhee EP, Cheng S, Larson MG, et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest 2011;121:1402–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meikle PJ, Wong G, Barlow CK, et al. Plasma lipid profiling shows similar associations with prediabetes and type 2 diabetes. PLoS One 2013;8:e74341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khan SR, Mohan H, Liu Y, et al. The discovery of novel predictive biomarkers and early-stage pathophysiology for the transition from gestational diabetes to type 2 diabetes. Diabetologia 2019;62:687–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lai M, Al Rijjal D, Röst HL, Dai FF, Gunderson EP, Wheeler MB. Underlying dyslipidemia postpartum in women with a recent GDM pregnancy who develop type 2 diabetes. eLife 2020;9:e59153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Timmers S, Schrauwen P, de Vogel J. Muscular diacylglycerol metabolism and insulin resistance. Physiol Behav 2008;94:242–251 [DOI] [PubMed] [Google Scholar]
- 24. Pelikánová T, Kohout M, Válek J, Base J, Stefka Z. Fatty acid composition of serum lipids and erythrocyte membranes in type 2 (non-insulin-dependent) diabetic men. Metabolism 1991;40:175–180 [DOI] [PubMed] [Google Scholar]
- 25. Takahashi R, Morita I, Saito Y, Ito H, Murota S. Increased arachidonic acid incorporation into platelet phospholipids in type 2 (non-insulin-dependent) diabetes. Diabetologia 1984;26:134–137 [DOI] [PubMed] [Google Scholar]
- 26. Shin CS, Lee MK, Park KS, et al. Insulin restores fatty acid composition earlier in liver microsomes than erythrocyte membranes in streptozotocin-induced diabetic rats. Diabetes Res Clin Pract 1995;29:93–98 [DOI] [PubMed] [Google Scholar]
- 27. Wijendran V, Bendel RB, Couch SC, et al. Maternal plasma phospholipid polyunsaturated fatty acids in pregnancy with and without gestational diabetes mellitus: relations with maternal factors. Am J Clin Nutr 1999;70:53–61 [DOI] [PubMed] [Google Scholar]
- 28. Mankuta D, Elami-Suzin M, Elhayani A, Vinker S. Lipid profile in consecutive pregnancies. Lipids Health Dis 2010;9:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Borggreve SE, De Vries R, Dullaart RP. Alterations in high-density lipoprotein metabolism and reverse cholesterol transport in insulin resistance and type 2 diabetes mellitus: role of lipolytic enzymes, lecithin:cholesterol acyltransferase and lipid transfer proteins. Eur J Clin Invest 2003;33:1051–1069 [DOI] [PubMed] [Google Scholar]
- 30. Floegel A, Stefan N, Yu Z, et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 2013;62:639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wallner S, Schmitz G. Plasmalogens the neglected regulatory and scavenging lipid species. Chem Phys Lipids 2011;164:573–589 [DOI] [PubMed] [Google Scholar]
- 32. Boslem E, Meikle PJ, Biden TJ. Roles of ceramide and sphingolipids in pancreatic β-cell function and dysfunction. Islets 2012;4:177–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fu J, Retnakaran R. The life course perspective of gestational diabetes: an opportunity for the prevention of diabetes and heart disease in women. EClinicalMedicine 2022;45:101294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kautzky-Willer A, Prager R, Waldhausl W, et al. Pronounced insulin resistance and inadequate beta-cell secretion characterize lean gestational diabetes during and after pregnancy. Diabetes Care 1997;20:1717–1723 [DOI] [PubMed] [Google Scholar]
- 35. Homko C, Sivan E, Chen X, Reece EA, Boden G. Insulin secretion during and after pregnancy in patients with gestational diabetes mellitus. J Clin Endocrinol Metab 2001;86:568–573 [DOI] [PubMed] [Google Scholar]
- 36. Buchanan TA, Xiang A, Kjos SL, Watanabe R. What is gestational diabetes? Diabetes Care 2007;30(Suppl. 2):S105–S111 [DOI] [PubMed] [Google Scholar]
- 37. Lai M, Liu Y, Ronnett GV, et al. Amino acid and lipid metabolism in post-gestational diabetes and progression to type 2 diabetes: A metabolic profiling study. PLoS Med 2020;17:e1003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. D’Agostino RB Jr, Hamman RF, Karter AJ, Mykkanen L, Wagenknecht LE; Insulin Resistance Atherosclerosis Study Investigators . Cardiovascular disease risk factors predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes Care 2004;27:2234–2240 [DOI] [PubMed] [Google Scholar]
- 39. Werner EF, Has P, Tarabulsi G, Lee J, Satin A. Early postpartum glucose testing in women with gestational diabetes mellitus. Am J Perinatol 2016;33:966–971 [DOI] [PubMed] [Google Scholar]
- 40. Sun L, Liang L, Gao X, et al. Early prediction of developing type 2 diabetes by plasma acylcarnitines: a population-based study. Diabetes Care 2016;39:1563–1570 [DOI] [PubMed] [Google Scholar]