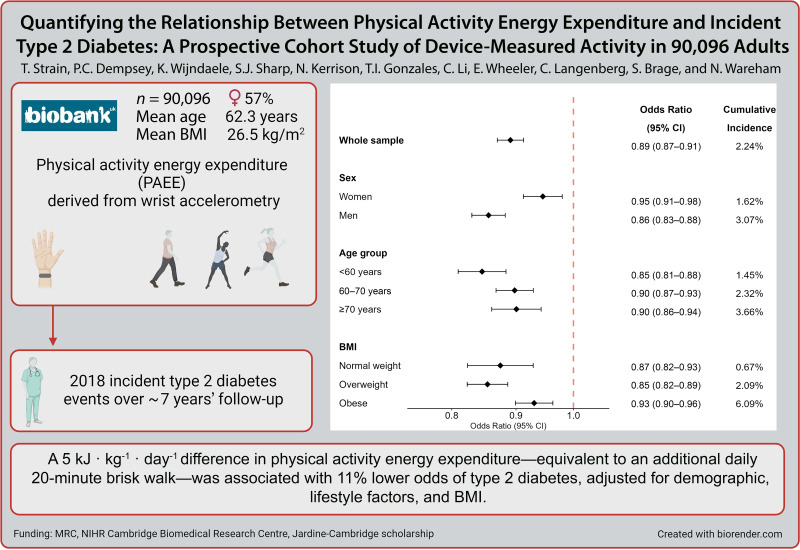
Abstract
OBJECTIVE
To investigate the association between accelerometer-derived physical activity energy expenditure (PAEE) and incident type 2 diabetes (T2D) in a cohort of middle-aged adults and within subgroups.
RESEARCH DESIGN AND METHODS
Data were from 90,096 UK Biobank participants without prevalent diabetes (mean 62 years of age; 57% women) who wore a wrist accelerometer for 7 days. PAEE was derived from wrist acceleration using a population-specific method validated against doubly labeled water. Logistic regressions were used to assess associations between PAEE, its underlying intensity, and incident T2D, ascertained using hospital episode and mortality data up to November 2020. Models were progressively adjusted for demographic, lifestyle factors, and BMI.
RESULTS
The association between PAEE and T2D was approximately linear (n = 2,018 events). We observed 19% (95% CI 17–21) lower odds of T2D per 5 kJ · kg−1 · day−1 in PAEE without adjustment for BMI and 11% (9–13) with BMI adjustment. The association was stronger in men than women and weaker in those with obesity and higher genetic susceptibility to obesity. There was no evidence of effect modification by genetic susceptibility to T2D or insulin resistance. For a given level of PAEE, odds of T2D were lower among those engaging in more moderate-to-vigorous activity.
CONCLUSIONS
There was a strong linear relationship between PAEE and incident T2D. A difference in PAEE equivalent to an additional daily 20-min brisk walk was associated with 19% lower odds of T2D. The association was broadly similar across population subgroups, supporting physical activity for diabetes prevention in the whole population.
Graphical Abstract
Introduction
There is a well-established inverse association between self-reported physical activity and incident type 2 diabetes (T2D) in observational studies (1–5), which is supported by evidence of prevention in randomized controlled trials (6–8). However, quantification of the association between habitual physical activity energy expenditure (PAEE) has proven to be challenging because of the intrinsic limitations in translating self-reported participation in particular activities into accurate estimates of PAEE. For example, the recall and social desirability biases inherent to self-report methods may differ by weight status (9). Thus, there are remaining uncertainties about the dose response relationship between physical activity and incident T2D. These uncertainties impact on public health messaging as it remains unclear how much benefit would be obtained from small changes in population-level PAEE.
The importance of using PAEE to investigate dose-response relationships is that it allows public health recommendations to be framed in terms of the benefits of physical activity of any type, potentially informing more specific or targeted prevention strategies. The best method for estimating PAEE is using stable isotopes to assess total energy expenditure, from which a measure of resting energy expenditure is subtracted (10,11). However, applying this technique at sufficiently large scale to enable the study of disease incidence in the general population remains prohibitively expensive. The use of wearables such as accelerometers to measure physical activity offers a viable alternative to objectively quantify dose-response associations with health outcomes (12–15), complementing previous studies using self-report of behaviors (1,16–18). To date, few studies have investigated the association between accelerometer-measured physical activity and incident T2D (19–22), and none of these have parameterized PAEE using methods validated against gold-standard stable isotope measurements. In addition, previous studies had smaller sample sizes, limiting the investigation of effect modification by population stratification or exploration of volume-intensity interactions.
The aims of this study were to investigate the association between accelerometer-derived PAEE and incident T2D in a large (n = 90,096) cohort of middle-aged adults without known diabetes at baseline. We also examined whether associations differ in subgroups defined by a range of demographic and health-related characteristics. Finally, we investigated whether different intensity profiles are associated with incident T2D.
Research Design and Methods
Study Population
The UK Biobank is a prospective study of over half a million adults aged 40–69 years living in Great Britain when recruited in 2006 to 2010, as explained in detail elsewhere (23). Briefly, participants completed a touchscreen questionnaire and undertook nurse interview and anthropometric assessment at a designated interview center. A subsample (n = 103,670) was invited to wear a wrist accelerometer 5 years after initial recruitment (24). Some participants (n = 8,697) undertook one or two additional assessment center visits in the interim (see Supplementary Fig. 1 for an overview).
Accelerometry Measurement and Processing
Accelerometry subsample participants were requested to wear a triaxial accelerometer (AX3; Axivity, Newcastle upon Tyne, U.K.) on their dominant wrist continuously for 7 days. Raw acceleration was collected at 100 Hz resolution, calibrated to local gravity (25), and low-pass filtered at 20 Hz to eliminate machine noise. Movement-related acceleration was calculated as vector magnitude minus gravitational acceleration in 5-s epochs and summarized into proportions of daily time spent at different movement intensity levels for each participant (26). Nonwear time (awake or sleep) was identified as extended periods of nonmovement and imputed using the average of similar time-of-day vector magnitude and intensity distribution data points with 1-min granularity on different days of the measurement. We excluded those with inadequate data for calibration (n = 11), those who had insufficient wear time (no wear data in each 1-h period of the 24-h cycle from the whole period of wear or <72 h of total wear; n = 6,985), and those with an average acceleration >100 milligravities (mg) (n = 13), as explained elsewhere (24).
As in our previous work (12), we used a population-specific equation (13) to convert time spent in each movement intensity category into PAEE in kilojoules per kilogram per day. This was derived in a separate validation study through regression to PAEE measured by individually calibrated combined heart rate and trunk acceleration in 1,695 U.K. adults; the resulting wrist acceleration-based estimate of PAEE was subsequently validated against total PAEE, measured by the gold-standard stable isotope method, and resting indirect calorimetry in 97 adults (Supplementary Fig. 2) (14). In addition, we derived the fraction of PAEE from moderate-to-vigorous physical activity (%MVPA; any activity with a movement intensity >125 mg [equivalent to 3 METs from combined sensing]), expressed as a percentage to enable the study of joint activity volume and intensity associations (12). A scatter plot between PAEE and %MVPA is shown in Supplementary Fig. 3. We excluded one individual who was a clear outlier (PAEE >150 kJ · kg−1 · day−1 and %MVPA >80%). We also derived time spent in MVPA (hours per day). Season of wear was parameterized as two sine functions.
Diabetes Ascertainment
Participants were considered to have prevalent diabetes (any type) if they self-reported any diabetes other than gestational diabetes, or self-reported diabetes medication at recruitment (insulin, sulfonylureas, glitazones, meglitinides, or acarbose), or had a hospital episode statistics (HES) event with ICD-10 codes E10–E14 prior to accelerometry (n = 3,619). We excluded those with prevalent diabetes of any type and those for whom no prevalent diabetes status could be inferred (n = 5). Compared with the method developed by Eastwood et al. (27), we counted a further 524 individuals as prevalent cases, primarily because we used self-reported (via touchscreen interview) diabetes diagnosis as evidence. Eastwood et al. (27) identified 10 individuals as prevalent cases that our algorithm did not; we excluded these in order to be conservative. Incident T2D was ascertained through HES and mortality records with ICD code E11 without E10 or E14 without E10–E13 (27). HES records were available until 30 November 2020 in England, 28 February 2018 in Wales, and 31 October 2020 in Scotland. Death records were available until 30 November 2020. We used the method from Eastwood et al. (27) to infer diagnosis date by taking the midpoint between the last record without diabetes and the date of the first record with diabetes.
Potential Confounders
Data were obtained at initial recruitment through a touchscreen questionnaire and anthropometric assessment. Blood samples were also collected at this point. For participants who took part in further in-person assessments prior to accelerometry (n = 9,171), we used the data from the time point closest to the accelerometry measurement. Exceptions were sex and Townsend index of deprivation (based on postcode) that were only obtained at baseline and ethnicity (assumed not to have changed) and family medical history in which a condition was counted even if it was at any of the measurement points. We have previously shown that the majority of covariates are stable over this period with the exceptions of employment status and medication use, in which there were trends toward unemployment and greater medication use at later visits (12).
We considered the following variables to be potential confounders with plausible associations to both exposure and outcome: sex (men/women), age (in years), ethnicity (White/non-White), Townsend index of deprivation, highest educational level achieved (degree or above/any other qualification/no qualification), employment status (unemployed/in paid or self-employment), smoking status (never/previous/current), alcohol consumption (never/less than twice a week/at least three times a week), fruit and vegetable intake (a score from 0–4 taking into account questions on cooked and raw vegetables and fresh and dried fruit consumption), parental history of diabetes (yes/no), and sleep duration (<7/7–8/>8 h) (Supplementary Fig. 4). We considered BMI to be a potential confounder but also a potential mediator of the association between physical activity and T2D given the plausible bidirectional associations between obesity and activity (28); in sensitivity analyses, we also considered abdominal obesity using waist circumference.
Those missing data in any potential confounder were excluded from the main analysis (n = 2,940). Multiple imputation using chained equations was used to impute these missing data for a sensitivity analysis. All potential confounders, PAEE, and incident T2D were included in the imputation model.
Potential Effect Modifiers
The following variables were investigated as potential effect modifiers: sex, age, ethnicity, BMI status, prevalent cardiovascular disease (CVD) status, prevalent cancer status, and tertiles of cardiorespiratory fitness, grip strength, genetic predisposition scores for T2D, insulin resistance, and BMI.
The association was not estimated in those with BMI <18.5 kg/m2 as the sample size was too small. Prevalent CVD was determined using both self-reported data and HES records up to accelerometry. CVD was classified as ICD-9 410–414 or 430–439, ICD-10 I20–25 or I60–69, or self-reported angina, chest pain, leg pain while walking normally, heart attack, or stroke. Prevalent cancer was determined using both self-reported data and HES records (ICD-9 140–199, 201–208, 209.1–209.3, 209.7–209.9, and 235–239 and ICD-10 C0–99). Cardiorespiratory fitness was estimated from resting heart rate measures taken during blood pressure measurements at the initial recruitment visit. Grip strength was measured at the same time point; we averaged the values from both hands. Age- and sex-specific tertiles were derived. Genotyping was performed using the UK BiLEVE and UK Biobank Axiom arrays and initial quality control performed by the UK Biobank (29). We used the v3 release of the genetic data, imputed to the full set of Haplotype Reference Consortium reference panel (30) and the merged UK10K and 1000 Genomes Phase III reference panels (31). Approximately 93 million directly genotyped and imputed autosomal genetic markers were available after quality control. From these, we derived genetic risk scores for T2D using 424 single nucleotide polymorphisms (SNPs) (32), insulin resistance using 53 SNPs (33) and BMI using 97 SNPs (34), weighted by their relative effect size extracted from the reference genome-wide association studies. Participants were excluded from the specific analysis if they had a missing value of the stratification variable.
Statistical Analysis
As likely date of T2D diagnosis was inferred rather than measured, our primary analysis used logistic regression. Cubic splines with four evenly spaced knots were used to examine the shape of the dose-response relationship between PAEE and incident T2D. We fit three models progressively adjusting for covariates. Model 0 adjusted for age, sex, and season of accelerometry wear. Season of wear is not a confounder (not associated with the outcome) but explains considerable variance in the exposure and is included to improve the precision of estimates (35). Model 1 additionally adjusted for all other demographic and lifestyle variables and parental history of diabetes; model 2 additionally adjusted for BMI, which may be considered to partially be on the causal pathway between PA and T2D. All continuous covariates except BMI met the linearity assumption as assessed visually by fractional polynomials. The shape of the BMI association was best modeled by including both a linear and a log-transformed term, determined using likelihood ratio tests. The reference value was 25 kJ · kg−1 · day−1, approximately the fifth percentile of the PAEE distribution in the sample.
We estimated the linear association between PAEE (per 5 kJ · kg−1 · day−1) and incident T2D using the same three levels of adjustment. We also estimated the association between PAEE and T2D within subgroups of the potential effect modifiers. P values for interaction were reported based on a likelihood ratio test comparing models with and without an interaction term. Genetic risk score analyses were additionally adjusted for the UK Biobank genotyping arrays and 10 genetic principal components but did not adjust for ethnicity as analyses were restricted to those of White European ancestry (the population in which the scores were derived).
Finally, we investigated the joint association of PAEE and %MVPA with T2D in the whole sample. Both exposures were included as linear terms alongside an interaction term in a logistic regression model. Odds ratios for selected values of %MVPA (10%, 20%, 30%, and 40%) were displayed graphically across the corresponding observed range of PAEE, with tables showing the odds ratios for specific combinations.
We performed several sensitivity analyses on the linear association between PAEE and T2D in the whole sample: 1) a time-to-event analysis using Cox regression, 2) imputation of missing covariate data, 3) excluding participants whose estimated T2D event date occurred within the first 2 years post-accelerometry, 4) excluding participants who were underweight (BMI <18.5 kg/m2), and 5) excluding participants with HbA1c >48 mmoL/mol at study baseline (n = 386). HbA1c was obtained from blood samples collected at the initial recruitment visit. Further details on the assay used are provided on the UK Biobank website (36). We also repeated the PAEE-%MVPA analysis additionally adjusting for waist circumference to shed light on the potential role of abdominal adiposity (37) and using alternative cut points for MVPA (100 mg and 150 mg). We also included a supplementary analysis of the association between time spent in MVPA and incident T2D risk using cubic splines as described above.
All analyses performed in Stata v16.1 (StataCorp, College Station, TX). Figures were produced in R and BioRender.com.
Data and Resource Availability
UK Biobank data were obtained under application number 44448. Analysis code is available upon request to datasharing@mrc-epid.cam.ac.uk. UK Biobank data are available to researchers with an approved request (https://www.ukbiobank.ac.uk/register-apply/).
Results
Sample Descriptives
The main analytical sample consisted of n = 90,096 individuals (57% women; mean age at accelerometry baseline, 62 [SD 7.8] years). There were 2,018 incident T2D events. Table 1 presents the descriptive characteristics of the sample by tertile of PAEE. Supplementary Table 1 presents these characteristics by incident T2D status.
Table 1.
Descriptive characteristics by tertile of PAEE: UK Biobank (n = 90,096)
| PAEE | Whole sample | |||
|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | ||
| Sample size (% of total analysis sample) | 30,032 (33.3) | 30,032 (33.3) | 30,032 (33.3) | 90,096 (100.0) |
| PAEE range (kJ · kg−1 · day−1) | 2.8–36.1 | 36.1–45.4 | 45.4–129.2 | 2.8–129.2 |
| PAEE (kJ · kg−1 · day−1), mean (SD) | 29.9 (4.8) | 40.6 (2.6) | 54.1 (8.0) | 41.5 (11.4) |
| MVPA (h · day−1), mean (SD) | 0.7 (0.3) | 1.2 (0.3) | 1.9 (0.5) | 1.2 (0.6) |
| Diabetes incident events, n (%) | 1,111 (3.7) | 578 (1.9) | 329 (1.1) | 2,018 (2.2) |
| Age, years, mean (SD) | 64.5 (7.5) | 62.3 (7.7) | 60.1 (7.7) | 62.3 (7.8) |
| Age-group, years | ||||
| <60 | 7,982 (26.6) | 11,252 (37.5) | 14,476 (48.2) | 33,710 (37.4) |
| 60–70 | 14,112 (47.0) | 13,566 (45.2) | 12,385 (41.2) | 40,063 (44.5) |
| >70 | 7,938 (26.4) | 5,214 (17.4) | 3,171 (10.6) | 16,323 (18.1) |
| Female sex | 15,333 (51.1) | 17,623 (58.7) | 18,478 (61.5) | 51,434 (57.1) |
| Ethnicity | ||||
| White | 29,310 (97.6) | 29,213 (97.3) | 28,990 (96.5) | 87,513 (97.1) |
| Asian excluding Chinese | 245 (0.8) | 253 (0.8) | 262 (0.9) | 760 (0.8) |
| Chinese | 43 (0.1) | 57 (0.2) | 97 (0.3) | 197 (0.2) |
| Black | 177 (0.6) | 219 (0.7) | 310 (1.0) | 706 (0.8) |
| Mixed | 136 (0.5) | 141 (0.5) | 189 (0.6) | 466 (0.5) |
| Any other ethnic group | 121 (0.4) | 149 (0.5) | 184 (0.6) | 454 (0.5) |
| Townsend index of deprivation, mean (SD) | −1.7 (2.9) | −1.8 (2.8) | −1.8 (2.8) | −1.8 (2.8) |
| Highest education level achieved | ||||
| No qualification | 2,953 (9.8) | 2,189 (7.3) | 1,983 (6.6) | 7,125 (7.9) |
| Any other qualification | 14,328 (47.7) | 14,364 (47.8) | 14,606 (48.6) | 43,298 (48.1) |
| Degree level or above | 12,751 (42.5) | 13,479 (44.9) | 13,443 (44.8) | 39,673 (44.0) |
| Employment status | ||||
| Unemployed | 14,427 (48.0) | 11,519 (38.4) | 9,376 (31.2) | 35,322 (39.2) |
| In paid employment | 15,605 (52.0) | 18,513 (61.6) | 20,656 (68.8) | 54,774 (60.8) |
| Smoking status | ||||
| Never | 16,463 (54.8) | 17,542 (58.4) | 17,905 (59.6) | 51,910 (57.6) |
| Previous | 11,164 (37.2) | 10,656 (35.5) | 10,431 (34.7) | 32,251 (35.8) |
| Current | 2,405 (8.0) | 1,834 (6.1) | 1,696 (5.6) | 5,935 (6.6) |
| Alcohol drinking status | ||||
| Never | 1,838 (6.1) | 1,558 (5.2) | 1,538 (5.1) | 4,934 (5.5) |
| Less than twice a week | 14,131 (47.1) | 13,413 (44.7) | 13,316 (44.3) | 40,860 (45.4) |
| At least three times a week | 14,063 (46.8) | 15,061 (50.1) | 15,178 (50.5) | 44,302 (49.2) |
| Sleep duration (h) | ||||
| <7 | 6,503 (21.7) | 6,478 (21.6) | 6,587 (21.9) | 19,568 (21.7) |
| 7–8 | 20,981 (69.9) | 21,732 (72.4) | 22,123 (73.7) | 64,836 (72.0) |
| >8 | 2,548 (8.5) | 1,822 (6.1) | 1,322 (4.4) | 5,692 (6.3) |
| Fruit and vegetable intake score, mean (SD) | 1.6 (1.1) | 1.7 (1.1) | 1.8 (1.2) | 1.7 (1.1) |
| Parental history of diabetes | ||||
| No | 25,088 (83.5) | 24,934 (83.0) | 24,938 (83.0) | 74,960 (83.2) |
| Yes | 4,944 (16.5) | 5,098 (17.0) | 5,094 (17.0) | 15,136 (16.8) |
| BMI (kg/m2), mean (SD) | 27.8 (4.8) | 26.5 (4.2) | 25.3 (3.8) | 26.5 (4.4) |
| BMI (kg/m2) | ||||
| Underweight (<18.5) | 111 (0.4) | 150 (0.5) | 282 (0.9) | 543 (0.6) |
| Normal weight (18.5–25) | 8,677 (28.9) | 11,965 (39.8) | 15,332 (51.1) | 35,974 (39.9) |
| Overweight (25–30) | 13,217 (44.0) | 12,874 (42.9) | 11,175 (37.2) | 37,266 (41.4) |
| Obese (>30) | 8,027 (26.7) | 5,043 (16.8) | 3,243 (10.8) | 16,313 (18.1) |
| Prevalent CVD | ||||
| No | 21,144 (70.4) | 228,35 (76.0) | 24,240 (80.7) | 68,219 (75.7) |
| Yes | 8,647 (28.8) | 6,960 (23.2) | 5,598 (18.6) | 21,205 (23.5) |
| Prevalent cancer | ||||
| No | 25,695 (85.6) | 26,514 (88.3) | 27,203 (90.6) | 79,412 (88.1) |
| Yes | 4,334 (14.4) | 3,515 (11.7) | 2,829 (9.4) | 10,678 (11.9) |
| BMI genetic risk score tertile | ||||
| Lowest tertile | 9,386 (31.3) | 9,361 (31.2) | 9,334 (31.1) | 28,081 (31.2) |
| Middle tertile | 9,368 (31.2) | 9,319 (31.0) | 9,398 (31.3) | 28,085 (31.2) |
| Upper tertile | 9,430 (31.4) | 9,465 (31.5) | 9,190 (30.6) | 28,085 (31.2) |
| Insulin resistance risk score tertile | ||||
| Lowest tertile | 9,470 (31.5) | 9,277 (30.9) | 9,336 (31.1) | 28,083 (31.2) |
| Middle tertile | 9,457 (31.5) | 9,454 (31.5) | 9,173 (30.5) | 28,084 (31.2) |
| Upper tertile | 9,257 (30.8) | 9,414 (31.3) | 9,413 (31.3) | 28,084 (31.2) |
| T2D genetic risk score tertile | ||||
| Lowest tertile | 9,366 (31.2) | 9,394 (31.3) | 9,323 (31.0) | 28,083 (31.2) |
| Middle tertile | 9,434 (31.4) | 9,421 (31.4) | 9,229 (30.7) | 28,084 (31.2) |
| Upper tertile | 9,384 (31.2) | 9,330 (31.1) | 9,370 (31.2) | 28,084 (31.2) |
Data are n (%) unless otherwise indicated. Season of wear was modeled as two orthogonal spline variables; a visualization of these variables has been previously published in Strain et al. (12). Genetic risk scores were derived for those of White European ancestry only. A higher Townsend index score indicates greater deprivation.
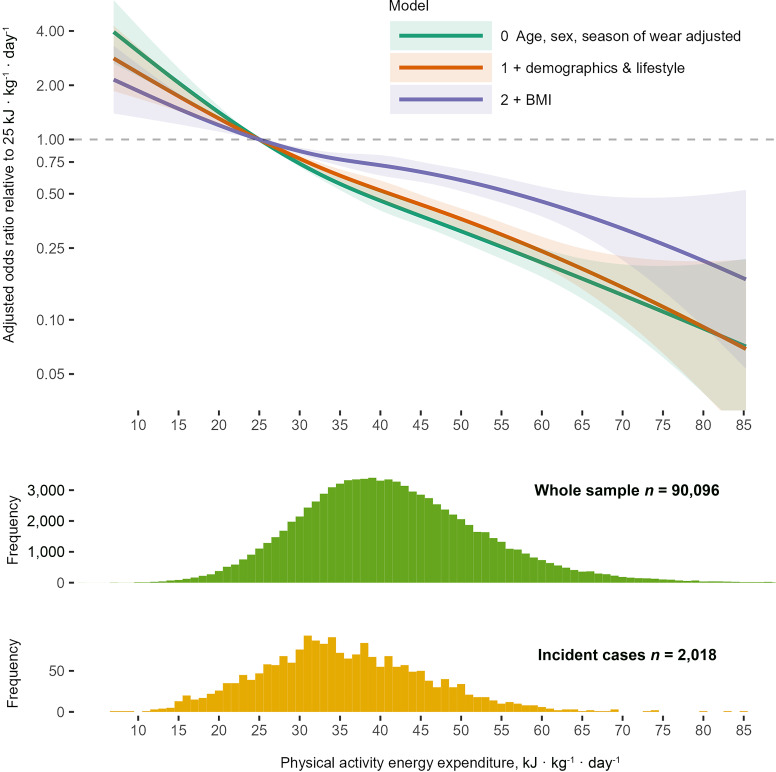
Cubic Spline–Modeled Associations of PAEE and Incident T2D
Figure 1 shows the cubic spline modeled association between PAEE and incident T2D for the three levels of model adjustment. The association was approximately linear; compared with a PAEE of 25 kJ · kg−1 · day−1, the odds ratios adjusted for demographic, lifestyle, and health-related confounders except BMI (model 1) were 0.78 (95% CI 0.75–0.82), 0.52 (0.46–0.59), and 0.36 (0.32–0.41) at 30, 40, and 50 kJ · kg−1 · day−1, respectively. The comparable odds ratios after additional adjustment for BMI (model 2) were 0.86 (0.82–0.90), 0.72 (0.63–0.82), and 0.59 (0.52–0.68), respectively (Supplementary Table 2).
Figure 1.
Cubic spline–modeled association between PAEE and incident T2D: UK Biobank (n = 90,096). Model 0 adjusted for age, sex, and season of accelerometry wear (using two orthogonal sine functions); model 1 additionally adjusted for ethnicity, Townsend index of deprivation, highest educational level achieved, employment status, parental history of diabetes, smoking status, alcohol drinking status, sleep duration, and fruit and vegetable intake. Model 2 additionally adjusted for BMI. Data presented for the observed range of PAEE amongst incident cases.
Linear Associations of PAEE and Incident T2D
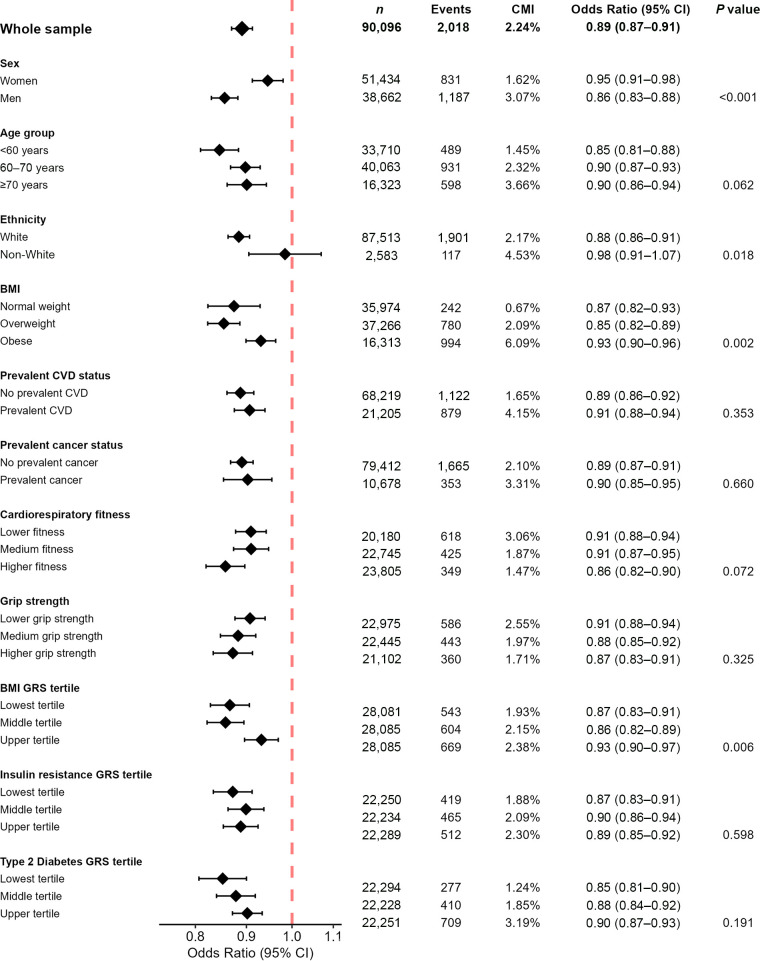
We observed 19% (17–21) lower odds of incident T2D per 5 kJ · kg−1 · day−1 higher PAEE in model 1 (Supplementary Fig. 5 and Supplementary Table 3). With further adjustment for BMI (model 2), odds were 11% (9–13) lower (Fig. 2).
Figure 2.
Odds ratios for incident T2D per 5 kJ · kg−1 · day−1 PAEE for the whole sample and in subgroups adjusted for BMI and other confounding factors (model 2): UK Biobank (n = 90,096). Model 2 adjusted for age, sex, season of accelerometry wear (using two orthogonal sine functions), ethnicity, Townsend index of deprivation, highest educational level achieved, employment status, parental history of diabetes, smoking status, alcohol drinking status, sleep duration, fruit and vegetable intake, and BMI. Genetic risk score–stratified analyses also adjusted for UK Biobank genotyping array and 10 genetic principal components but did not adjust for ethnicity as analyses were restricted to those of White European ancestry. P value for interaction between subgroups. CMI, cumulative incidence; GRS, genetic risk score.
The associations were stronger for men than for women. The model 1 odds ratios were 0.79 (0.77–0.82) and 0.83 (0.81–0.86), respectively, with a borderline significant interaction (P value for interaction, 0.033). The magnitude of the difference in the association between men and women was greater with further BMI adjustment (model 2 odds ratios 0.86 [0.83–0.88] for men and 0.95 [0.91–0.98] for women, with a P value for interaction <0.001).
The associations were weaker among the obese than the other BMI subgroups. The model 2 odds ratios (including adjustment for BMI within subgroup) were 0.87 (0.82–0.93) for those of normal weight, 0.85 (0.82–0.89) for those who were overweight, and 0.93 (0.90–0.96) for those who were obese (P value for interaction 0.002). This pattern of association was also observed for the tertiles of BMI genetic risk score (i.e., weaker associations in those at higher genetic risk of obesity).
There was some evidence that the association was stronger in White compared with non-White individuals, but this analysis is underpowered because of the small size of the non-White population subgroup in UK Biobank. There was no evidence of an interaction by age-group, prevalent CVD or cancer status, or genetic risk for T2D or insulin resistance. There were no differences in strength of association across tertiles of cardiorespiratory fitness or grip strength in the BMI-adjusted models, although the association was slightly stronger in the higher tertile of cardiorespiratory fitness in model 1.
There were negligible differences in the magnitude of the association between PAEE and incident T2D across the range of sensitivity analyses undertaken (Supplementary Table 4). The model 2 hazard ratio from Cox regression was 0.89 (0.87–0.91). The model 1 and model 2 odds ratios ranged between 0.81–0.82 and 0.89–0.90, respectively, when missing data were imputed and for different exclusion criteria (events estimated to occur within 2 years of accelerometry, those with BMI <18.5 kg/m2 were excluded, and those with HbA1c >48 mmoL/mol).
Joint Associations of PAEE and %MVPA With Incident T2D
The association between %MVPA and incident T2D was approximately linear (Supplementary Fig. 6).
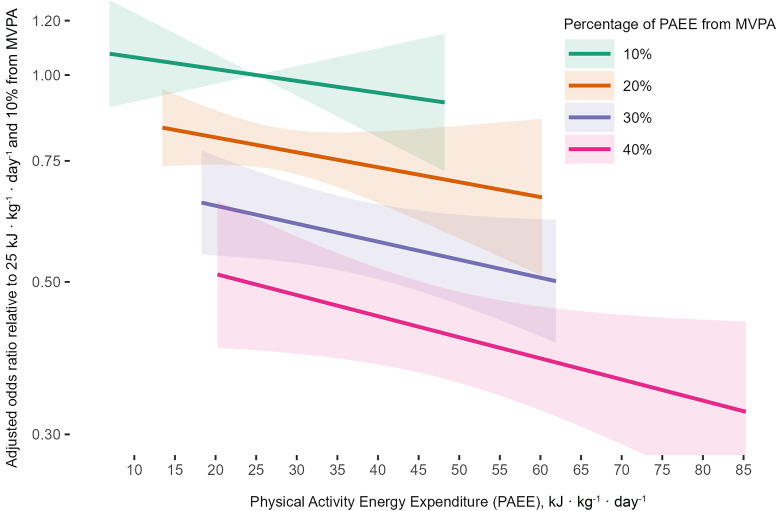
In the confounder and BMI-adjusted model (model 2) with a fixed PAEE of 25 kJ · kg−1 · day−1, a 20% contribution MVPA was associated with 21% (15–26) lower odds of incident T2D compared with a 10% contribution of MVPA (Fig. 3 and Supplementary Table 5). Meanwhile, 30% and 40% MVPA were associated with 37% (27–46) and 50% (38–60) lower odds, respectively. When %MVPA was fixed, higher volumes of PAEE were associated with lower odds of incident T2D. The greatest risk reductions were observed with a combination of high PAEE and higher %MVPA. For example, those with a PAEE of 50 kJ · kg−1 · day−1 and 40% MVPA had 58% (52–64) lower odds of incident T2D compared to those with a PAEE 15 kJ · kg−1 · day−1 and 10% MVPA. Supplementary Fig. 7 presents the BMI-adjusted odds ratios for further combinations of PAEE and %MVPA, grouping those with similar durations of MVPA.
Figure 3.
The joint association of PAEE and %MVPA with the odds of incident T2D adjusted for BMI and other confounding factors (model 2): UK Biobank (n = 90,096). Model 2 adjusted for age, sex, season of accelerometry wear (using two orthogonal sine functions), ethnicity, Townsend index of deprivation, highest educational level achieved, employment status, parental history of diabetes, smoking status, alcohol drinking status, sleep duration, fruit and vegetable intake, and body mass index. Data presented for the observed range of PAEE amongst incident cases for a range around the %MVPA value (±5%, extending to respective end of distributions for 10% and 40%).
The associations were stronger without adjustment for BMI (model 1; Supplementary Fig. 8). This was evident both with regards to the slope of the association between PAEE and incident T2D for a given %MVPA, and for the slope of the %MVPA association for a given PAEE, when compared with model 2. Also, for a given value of PAEE, the differences across selected %MVPA values were greater.
In sensitivity analyses, adjustment for waist circumference as well as BMI attenuated the odds ratios by up to 5 percentage points (Supplementary Table 5). The %MVPA associations tended to be slightly weaker using a lower movement intensity threshold for MVPA (100 mg) and stronger using a higher threshold (150 mg). The greatest differences in magnitude were evident at the higher end of the PAEE and %MVPA range (Supplementary Table 6).
Time Spent in MVPA
Supplementary Fig. 9 shows the cubic spline–modeled association between time spent in MVPA and incident T2D for the three levels of model adjustment. The association was approximately linear. Compared with a reference value of 0.5 h/day, the odds ratios for model 1 were 0.57 (0.52–0.63) and 0.39 (0.34–0.44) at 1 and 1.5 h/day, respectively. The comparable odds ratios after additional adjustment for BMI (model 2) were 0.74 (0.67–0.81) and 0.60 (0.53–0.68), respectively (Supplementary Table 7).
Conclusions
In this large prospective cohort study with objective measurement of physical activity, we found that estimated PAEE was inversely associated with incident T2D. Both without and with adjustment for BMI, the relationship between PAEE and risk of T2D was linear, with no observable attenuation in the association even at much higher PAEE levels. The magnitude of the association was 19% and 11% lower odds per 5 kJ · kg−1 · day−1 for the models without and with adjustment of BMI, a difference in PAEE equivalent to an additional 20-min brisk walk per day. These results suggest that the benefits of higher physical activity on T2D risk are constant, whatever the initial level of activity (i.e., “some is good, but more is better”). The strength of the association differed by sex, BMI, and genetic susceptibility to obesity. However, a linear inverse association between PAEE and incident T2D was evident among all subgroups investigated, except those of non-White ethnicity, which was underpowered.
We also found an association for moderate physical activity intensity, over and above total activity volume, with incident T2D risk. In other words, accumulating the same volume through higher intensity activity was associated with lower odds of T2D than accumulating through lower intensity activity. This is in line with our findings for all-cause mortality and CVD (12,15). It highlights the key message that health benefits can be achieved through a variety of combinations of volume and intensity, but that if practical and appealing, undertaking more intense activity should be encouraged.
Few studies have quantified the relationship between objectively measured physical activity and risk of T2D. Our estimates suggest a stronger association than has been typically observed in the literature. In a cohort of 16,415 Hispanic/Latino adults, Cuthbertson et al. (21) estimated a 2% (0–5) lower hazard of incident diabetes per 1,000 steps/day, with the association fully attenuated after adjustment for BMI. Similarly, Garduno et al. (19) found their estimated 12% (0–22) lower hazard of incident diabetes per 2,000 steps/day to be nonsignificant after BMI adjustment among a sample of 4,838 older U.S. women. Ballin et al. (22) found a nonlinear association between daily step count and incident diabetes among 3,055 older Swedish men and women. Compared with the sample median of 7,445 steps/day, the lowest extreme of the distribution (1,000 steps/day) had a threefold higher risk, and there were no differences in risk among the upper half of the exposure distribution. Both Cuthbertson et al. (21) and Garduno et al. (19) found the relationship to be approximately linear, while Ballin et al. (22) observed a nonlinear dose-response relationship typically observed between physical activity and other chronic health outcomes (19,21,22). Cuthbertson et al. (21) also found lower incidence of T2D.
As BMI is a known mechanism through which physical activity may influence the risk of T2D (38), adjustment likely produces a conservative estimate of association. However, as BMI also acts as a confounder (28), not adjusting for it likely results in an overestimation of the association. Our results tentatively suggest that more of the association between PAEE and T2D is mediated through BMI among women, as we observed a greater difference between models 1 and 2 than for men. However, the finding of a stronger association among men than women needs confirmation in further studies. We note this finding is opposite to the sex-specific meta-analytical results of self-reported data by Smith et al. (1) and the trends observed by Cuthbertson et al. (21).
We found no evidence of an interaction of PAEE and incident T2D with genetic predisposition to T2D or insulin resistance. We did observe a smaller effect size in individuals who were obese at baseline and in those who had higher genetic susceptibility to obesity. However, it is absolute rather than relative risk that determines the benefits of targeted prevention. There is an extremely strong relationship between obesity and T2D risk, as demonstrated by the distribution of cases in the different obesity strata in Fig. 2 (approximately three- and ninefold higher for overweight and obese compared with normal weight). Therefore, the absolute risk difference for a difference in PAEE in the subgroup of obese individuals will still be much larger than in nonobese subgroups despite the lower relative risk. Therefore, these results suggest that population-level approaches to increasing PAEE in all individuals should remain a public health priority.
Our finding that activity intensity plays a role over and above volume in T2D risk is interesting to consider from a mechanistic perspective. Our sensitivity analysis additionally adjusting for waist circumference showed further attenuation, potentially indicating visceral fat as an important factor. Previous research has suggested that higher-intensity activities may impact T2D risk through metabolic adaptations, while lower intensity activities may be mediated through changes in BMI (37). This is plausible as higher intensities require greater reliance on carbohydrate oxidation (39), which may increase the expression and activity of proteins related to glucose metabolism and insulin signaling. It is also possible that the greater stimulation of cardiovascular-related pathways (e.g., stroke volume, capillary density, red blood cell, and mitochondrial density) (40) leads to improved cardiorespiratory fitness, which in turn lowers the risk of T2D (41). The main strength of this study is the accurate quantification of PAEE at a large scale. This allows the investigation of dose-response relationships within subgroups and identification of interactions. We have also undertaken several sensitivity analyses indicating that the analytical assumptions made have a negligible impact on overall conclusions. A key limitation is the reliance on HES and mortality data for the ascertainment of T2D. Although it would be preferable to enhance ascertainment with information from other sources, particularly primary care records, these are not currently available for the whole cohort. However, it is important to note that 58% of our sample attended hospital in the follow-up period of 6 years and that diabetes is routinely recorded when admitted to hospital for other reasons in the U.K. Thus, the underascertainment that might be presumed through use of secondary care data may not be as consequential as it may appear (27), and the bias diminishes over time. Under the assumption that errors in the outcome classification are not associated with the exposure, the implication for our results of ascertainment error is to increase the uncertainty around the estimate of the association (42). To mitigate the issue of the likely diagnosis date being earlier than the first secondary care record, we used logistic regression rather than a time-to-event analysis method. That being said, our sensitivity analysis using Cox regression produced very similar estimates of association. Another potential limitation is that our estimate of PAEE relies on the accurate reflection of energy expenditure from dominant wrist acceleration. Given the method’s documented validity in a U.K. population (13,14), this is a reasonable assumption at a whole sample level. The distribution of estimated PAEE is narrower than PAEE measured with stable isotopes and resting metabolic rate assessment; this error would lead to the amplification of the dose-response relationship. However, individuals who engage primarily in activities such as resistance exercise or cycling may not be appropriately characterized by the wrist measure, which was also only done at a single time point, thus not accounting for variability in activity levels over time, all of which could attenuate the associations. Other limitations include the measurement of covariates 5 years prior to accelerometry, which may increase residual confounding in our estimates. However, we have previously shown that the majority of covariates are stable over this period, the exceptions being employment status and medication use (12). Also, the UK Biobank is not a representative national survey with a 5.5% response rate and respondents shown to be healthier and more affluent than the general population (43). However, our sample median PAEE of 42 kJ · kg−1 · day−1 is in line with nationally representative age-specific estimates (11).
In summary, we have shown a strong linear relationship between accelerometer-derived PAEE and incident T2D in a large sample of middle-aged adults. A difference in PAEE equivalent to an additional daily 20-min brisk walk was associated with 19% lower odds of T2D. The association was broadly similar across population subgroups, although slightly stronger in men than women and weaker in those with obesity and higher genetic susceptibility to obesity. These results support physical activity for the prevention of diabetes in the whole population. For a given level of PAEE, engaging in a greater proportion of moderate-to-vigorous activity was associated with additional benefits. Therefore, the role of activity intensity, over and above its contribution to PAEE, appears to be important for incident T2D.
Article Information
Funding. T.S., P.C.D., K.W., S.J.S., N.K., T.I.G., C.Li., E.W., C.La., S.B., and N.W. are supported by UK Medical Research Council (grants MC_UU_00006/1, MC_UU_00006/4, and MC_UU_12015/3). S.B. and N.W. are supported by the National Institute for Health Research (NIHR) Biomedical Research Centre Cambridge (IS-BRC-1215-20014). The NIHR Biomedical Research Centre Cambridge is a partnership between Cambridge University Hospitals NHS Foundation Trust and the University of Cambridge, funded by the NIHR. C.Li. is supported by a Jardine-Cambridge Graduate Scholarship.
The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR, or the Department of Health and Social Care. This work was funded by U.K. Research and Innovation grants listed above. For the purpose of open access, the author has applied a Creative Commons Attribution license to any Author Accepted Manuscript version arising.
Duality of Interest. No potential conflicts of interest relevant to this article were reported. E.W. is now an employee of AstraZeneca.
Author Contributions. T.S., P.C.D., K.W., S.J.S., T.I.G., C.La., S.B., and N.W. contributed to the analysis plan. T.S., P.C.D., N.K., T.I.G., C.Li., E.W., C.La., S.B., and N.W. contributed to the derivation of variables. T.S. takes responsibility for the data analysis with assistance, and checks were undertaken by S.J.S. All authors contributed to the interpretation of the results. T.S., P.C.D., S.B., and N.W. initially drafted the manuscript, and all authors contributed subsequently. T.S. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This study was presented in oral form at the 9th International Society for Physical Activity for Health Congress, Abu Dhabi, United Arab Emirates, 23–26 October 2022.
Footnotes
See accompanying article, p. 1132.
This article contains supplementary material online at https://doi.org/10.2337/figshare.21785603.
S.B. and N.W. are joint senior authors.
References
- 1. Smith AD, Crippa A, Woodcock J, Brage S. Physical activity and incident type 2 diabetes mellitus: a systematic review and dose-response meta-analysis of prospective cohort studies. Diabetologia 2016;59:2527–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aune D, Norat T, Leitzmann M, Tonstad S, Vatten LJ. Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur J Epidemiol 2015;30:529–542 [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization . WHO guidelines on physical activity and sedentary behaviour, 2020. Accessed 1 July 2022. Available from https://apps.who.int/iris/bitstream/handle/10665/336656/9789240015128-eng.pdf [PubMed]
- 4. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care 2016;39:2065–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kanaley JA, Colberg SR, Corcoran MH, et al. Exercise/physical activity in individuals with type 2 diabetes: a consensus statement from the American College of Sports Medicine. Med Sci Sports Exerc 2022;54:353–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tuomilehto J, Lindström J, Eriksson JG, et al.; Finnish Diabetes Prevention Study Group . Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 8. Gillies CL, Abrams KR, Lambert PC, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ 2007;334:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Warner ET, Wolin KY, Duncan DT, Heil DP, Askew S, Bennett GG. Differential accuracy of physical activity self-report by body mass index. Am J Health Behav 2012;36:168–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Westerterp KR. Doubly labelled water assessment of energy expenditure: principle, practice, and promise. Eur J Appl Physiol 2017;117:1277–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brage S, Lindsay T, Venables M, et al. Descriptive epidemiology of energy expenditure in the UK: findings from the National Diet and Nutrition Survey 2008-15. Int J Epidemiol 2020;49:1007–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Strain T, Wijndaele K, Dempsey PC, et al. Wearable-device-measured physical activity and future health risk. Nat Med 2020;26:1385–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. White T, Westgate K, Wareham NJ, Brage S. Estimation of physical activity energy expenditure during free-living from wrist accelerometry in UK adults. PLoS One 2016;11:e0167472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. White T, Westgate K, Hollidge S, et al. Estimating energy expenditure from wrist and thigh accelerometry in free-living adults: a doubly labelled water study. Int J Obes 2019;43:2333–2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dempsey PC, Rowlands AV, Strain T, et al. Association of physical activity volume and intensity with incident cardiovascular disease: a UK Biobank study. Eur Heart J 2022;43:4789–4800 [DOI] [PubMed] [Google Scholar]
- 16. Garcia L, Pearce M, Abbas A, et al. Non-occupational physical activity and risk of 22 cardiovascular disease, cancer, and mortality outcomes: a dose-response meta-analysis of large prospective studies. Br J Sports Med. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iso-Markku P, Kujala UM, Knittle K, Polet J, Vuoksimaa E, Waller K. Physical activity as a protective factor for dementia and Alzheimer’s disease: systematic review, meta-analysis and quality assessment of cohort and case-control studies. Br J Sports Med 2022;56:701–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pearce M, Garcia L, Abbas A, et al. Association between physical activity and risk of depression: a systematic review and meta-analysis. JAMA Psychiatry 2022;79:550–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garduno AC, LaCroix AZ, LaMonte MJ, et al. Associations of daily steps and step intensity with incident diabetes in a prospective cohort study of older women: the OPACH Study. Diabetes Care 2022;45:339–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen GC, Qi Q, Hua S, et al. Accelerometer-assessed physical activity and incident diabetes in a population covering the adult life span: the Hispanic Community Health Study/Study of Latinos. Am J Clin Nutr 2020;112:1318–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cuthbertson CC, Moore CC, Sotres-Alvarez D, et al. Associations of steps per day and step intensity with the risk of diabetes: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Int J Behav Nutr Phys Act 2022;19:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ballin M, Nordström P, Niklasson J, et al. Daily step count and incident diabetes in community-dwelling 70-year-olds: a prospective cohort study. BMC Public Health 2020;20:1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sudlow C, Gallacher J, Allen N, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Doherty A, Jackson D, Hammerla N, et al. Large scale population assessment of physical activity using wrist worn accelerometers: the UK Biobank Study. PLoS One 2017;12:e0169649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Hees VT, Fang Z, Langford J, et al. Autocalibration of accelerometer data for free-living physical activity assessment using local gravity and temperature: an evaluation on four continents. J Appl Physiol (1985) 2014;117:738–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Hees VT, Gorzelniak L, Leon ECD, et al. Separating movement and gravity components in an acceleration signal and implications for the assessment of human daily physical activity. PLoS One 2013;8:e61691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eastwood SV, Mathur R, Atkinson M, et al. Algorithms for the capture and adjudication of prevalent and incident diabetes in UK Biobank. PLoS One 2016;11:e0162388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jones PR, Ekelund U. Physical activity in the prevention of weight gain: the impact of measurement and interpretation of associations. Curr Obes Rep 2019;8:66–76 [DOI] [PubMed] [Google Scholar]
- 29. Bycroft C, Freeman C, Petkova D, et al. Genome-wide genetic data on ∼500,000 UK Biobank participants. 20 July 2017 [preprint]. bioRxiv:166298 [Google Scholar]
- 30. McCarthy S, Das S, Kretzschmar W, et al.; Haplotype Reference Consortium . A reference panel of 64,976 haplotypes for genotype imputation. Nat Genet 2016;48:1279–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang J, Howie B, McCarthy S, Memari Y, Walter K, et al.; UK10K Consortium . Improved imputation of low-frequency and rare variants using the UK10K haplotype reference panel. Nat Commun 2015;6:8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vujkovic M, Keaton JM, Lynch JA, et al.; HPAP Consortium; Regeneron Genetics Center; VA Million Veteran Program . Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat Genet 2020;52:680–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lotta LA, Gulati P, Day FR, et al.; EPIC-InterAct Consortium; Cambridge FPLD1 Consortium . Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat Genet 2017;49:17–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Locke AE, Kahali B, Berndt SI, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015;518:197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 2009;20:488–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. UK Biobank . Data-Field 30750: Glycated haemoglobin (HbA1c), 2022. Accessed 1 July 2022. Available from https://biobank.ndph.ox.ac.uk/showcase/field.cgi?id=30750
- 37. Lynch J, Helmrich SP, Lakka TA, et al. Moderately intense physical activities and high levels of cardiorespiratory fitness reduce the risk of non-insulin-dependent diabetes mellitus in middle-aged men. Arch Intern Med 1996;156:1307–1314 [PubMed] [Google Scholar]
- 38. Gill JMR, Cooper AR. Physical activity and prevention of type 2 diabetes mellitus. Sports Med 2008;38:807–824 [DOI] [PubMed] [Google Scholar]
- 39. Colberg SR, Sigal RJ, Fernhall B, et al.; American College of Sports Medicine; American Diabetes Association . Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care 2010;33:e147–e167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Booth FW, Thomason DB. Molecular and cellular adaptation of muscle in response to exercise: perspectives of various models. Physiol Rev 1991;71:541–585 [DOI] [PubMed] [Google Scholar]
- 41. Zaccardi F, O’Donovan G, Webb DR, et al. Cardiorespiratory fitness and risk of type 2 diabetes mellitus: A 23-year cohort study and a meta-analysis of prospective studies. Atherosclerosis 2015;243:131–137 [DOI] [PubMed] [Google Scholar]
- 42. Hutcheon JA, Chiolero A, Hanley JA. Random measurement error and regression dilution bias. BMJ 2010;340:c2289. [DOI] [PubMed] [Google Scholar]
- 43. Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol 2017;186:1026–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]