Abstract
OBJECTIVE
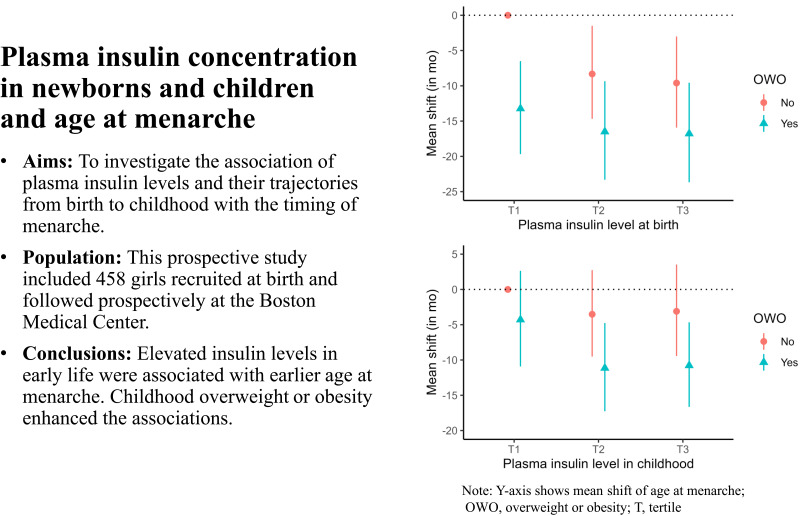
To investigate the association of plasma insulin levels and their trajectories from birth to childhood with the timing of menarche.
RESEARCH DESIGN AND METHODS
This prospective study included 458 girls recruited at birth between 1998 and 2011 and followed prospectively at the Boston Medical Center. Plasma nonfasting insulin concentrations were measured at two time points: at birth (cord blood) and in childhood (age 0.5–5 years). Age at menarche was obtained from a pubertal developmental questionnaire or abstracted from electronic medical records.
RESULTS
Three hundred six (67%) of the girls had reached menarche. The median (range) age at menarche was 12.4 (9–15) years. Elevated plasma insulin concentrations at birth (n = 391) and in childhood (n = 335) were each associated with an earlier mean age at menarche: approximately 2 months earlier per doubling of insulin concentration (mean shift, −1.95 months, 95% CI, −0.33 to −3.53, and −2.07 months, 95% CI, −0.48 to −3.65, respectively). Girls with overweight or obesity in addition to elevated insulin attained menarche about 11–17 months earlier, on average, than those with normal weight and low insulin. Considering longitudinal trajectories (n = 268), having high insulin levels both at birth and in childhood was associated with a roughly 6 months earlier mean age at menarche (mean shift, −6.25 months, 95% CI, −0.38 to −11.88), compared with having consistently low insulin levels at both time points.
CONCLUSIONS
Our data showed that elevated insulin concentrations in early life, especially in conjunction with overweight or obesity, contribute to the earlier onset of menarche, suggesting the need for early screening and intervention.
Graphical Abstract
Introduction
Menarche is a key milestone in female reproductive life, occurring in the late stages of pubertal development. Over the past few decades, a secular trend toward earlier occurring menarche has been observed (1,2). Early age at menarche has been associated with a wide range of adverse health consequences, including type 2 diabetes (3), cardiovascular disease (3,4), and breast cancer (5,6); thus, it is a major clinical and public health concern. While the precise mechanism underlying menarche onset remains elusive, it is well established that the hypothalamic-pituitary-gonadal (HPG) axis orchestrates the initiation and development of puberty (7). The HPG axis is transiently activated during the fetal and early postnatal periods, followed by a relative quiescent period in childhood (7), and then reactivated at the beginning of puberty, suggesting that early developmental periods are critical windows for pubertal development (8).
Insulin, an important metabolic hormone, plays a critical role in fetal growth and reproductive tract development (9). Insulin participates in the regulation of luteinizing hormone secretion (10), implicating its role in puberty onset. Previous studies have documented that type 1 diabetes, a condition characterized by insulin deficiency, was associated with some delay in the age of menarche (11), whereas obesity, characterized by insulin resistance coupled with hyperinsulinemia, has been linked to earlier menarche (7). Moreover, clinical randomized trials targeting insulin resistance using metformin, an insulin sensitizer, showed that improved insulin sensitivity may decrease the risk of early onset of puberty and menarche (12,13). Along the same lines, adiponectin and leptin levels, which have been associated with insulin sensitivity or insulin resistance, also play important roles in the onset of puberty (7). Taken together, the available biological evidence suggests that insulin is an additional modulator of sexual maturation, and insulin resistance may be an important risk factor for earlier menarche. However, to date, it is unknown whether insulin resistance during developmental periods has a long-term impact on age at menarche.
Using a long-running, racially diverse birth cohort, we aimed to examine age at menarche in relation to insulin levels at two time points: at birth (cord blood insulin) and in childhood (random insulin) among girls who were followed prospectively from birth onward. We also sought to examine whether elevated insulin concentrations in early life and childhood overweight or obesity (OWO) have combined associations with age at menarche.
Research Design and Methods
Study Participants
Participants in this study were a subset of the Boston Birth Cohort (BBC), which is an ongoing cohort that enrolled 8,623 mother-infant pairs between October 1998 and December 2019 at Boston Medical Center (BMC). From the BBC, 3,394 children (1,684 females, 1,710 males) who received primary care at BMC and consented to participate in the follow-up study were followed from birth onward. Of the 1,684 girls followed, 744 had plasma insulin concentrations measured. After excluding those with precocious puberty (i.e., the onset of secondary sexual characteristics before 8 years of age, n = 56), lost to follow-up (n = 38), older than 15 years of age but with missing information on age at menarche (n = 107), younger than 7 years old (n = 60), and with postnatal insulin measured at age 6 years or older (n = 25), this study included a total of 458 girls. From this group, 391 girls had cord blood insulin concentration measured, 335 girls had postnatal random insulin concentration measured, and 268 girls had insulin measured at both time points (Supplementary Fig. 1). The median duration of follow-up among the 458 girls was 11 years (range: 7.0–15.2 years). There were no remarkable differences between girls who were included in this study (n = 458) and girls enrolled in the BBC (n = 4,327) in terms of demographic and clinical variables, except that this study included a higher proportion of girls whose mothers self-identified as being of Black race or having obesity in prepregnancy (Supplementary Table 1). The BBC has been approved by the Institutional Review Boards of BMC, Boston, MA, and Johns Hopkins Bloomberg School of Public Health, Baltimore, MD. Written informed consent was obtained from the mother of each child at study entry and at follow-up, and child assent was obtained.
Determination of Plasma Insulin and Adipokines Concentrations
The collection and storage of cord blood and venous blood were in accordance with standardized operating procedures (14). Cord blood collected immediately after birth was used to ascertain insulin concentration at birth, while random venous blood collected at a median age of 1.5 years (range, 0.5–5 years) was used for quantifying insulin, adiponectin, and leptin concentrations in childhood. To minimize participants’ burden, BBC study visits were aligned with pediatric primary care visits, when childhood blood samples were collected. The detailed distribution of the hour of day at the study visit is presented in Table 1. Plasma insulin and leptin concentrations were determined using sandwich immunoassays based on flow metric xMAP technology on Luminex 200 machines (Luminex Corp., Austin, TX), and adiponectin was measured by ELISA. Intra-assay coefficients of variation were <8% for insulin, leptin, and adiponectin. A detailed description of the lab methods can be found in our previous publications (15,16). Plasma adiponectin concentrations and leptin/adiponectin ratio in childhood served as surrogates of insulin sensitivity (17) and insulin resistance (18), respectively.
Table 1.
Characteristics of the study population (n = 458)
| Maternal characteristics | N (%) |
|---|---|
| Maternal age at delivery (years)* | 28.1 (23.2–33.5) |
| Race and ethnicity | |
| Non-Hispanic Black | 282 (61.6) |
| Hispanic | 106 (23.1) |
| Other | 70 (15.3) |
| Education | |
| High school and below | 307 (67.0) |
| College and above | 151 (33.0) |
| Smoking status during pregnancy | |
| Nonsmoker | 380 (83.0) |
| Smoker | 78 (17.0) |
| Prepregnancy BMI (kg/m2) | 27.3 (6.9) |
| Prepregnancy obesity | |
| No | 339 (74.0) |
| Yes | 119 (26.0) |
| Diabetes during pregnancy | |
| Nondiabetic | 386 (84.3) |
| Gestational diabetes | 51(11.1) |
| Preexisting diabetes | 21 (4.6) |
| Hypertensive disorders in pregnancy | |
| No | 393 (85.8) |
| Yes | 65 (14.2) |
| Gestational weight gain | |
| Adequate | 129 (28.2) |
| Inadequate | 139 (30.3) |
| Excessive | 139 (30.4) |
| Missing data | 51 (11.1) |
| Maternal age at menarche (years)* | 13 (12–14) |
| Maternal age at menarche categories | |
| <12 years | 105 (22.9) |
| 12–13 years | 185 (40.4) |
| ≥14 years | 168 (36.7) |
| Child characteristics | N (%) |
|---|---|
| Fetal growth | |
| AGA | 353 (77.1) |
| SGA | 59 (12.9) |
| LGA | 46 (10.0) |
| Preterm birth | 136 (29.7) |
| Breastfeeding status | |
| Any | 347 (75.8) |
| None | 111 (24.2) |
| Timing of solid food introduction | |
| Early (1–3 months) | 43 (9.4) |
| Suggested (4–6 months) | 265 (57.9) |
| Delayed (>7 months) | 82 (17.9) |
| Missing data | 68 (14.8) |
| Rapid weight gain in the first year of life | |
| No | 148 (32.3) |
| Yes | 244 (53.3) |
| Missing data | 66 (14.4) |
| BMI z-score† | 0.9 (−3.4, 6.2) |
| OWO in childhood | 208 (45.4) |
| Hour of day at the study visit | |
| Before 9:00 a.m. | 17 (5.1) |
| 9.01–12:00 a.m. | 147 (43.9) |
| 12.01–2:00 p.m. | 63 (18.8) |
| 2:01–4:00 p.m. | 98 (29.2) |
| After 4:00 p.m. | 10 (3.0) |
| OWO trajectories (0–5 years) | |
| Stable normal weight | 71 (20.9) |
| Stable lean | 93 (27.3) |
| Early-onset OWO | 123 (36.2) |
| Late-onset OWO | 53 (15.6) |
| Age at menarche (years)† | 12.4 (9–15) |
| Attained menarche | 306 (66.8) |
Data are presented as mean (SD) or N (%). AGA, appropriate for gestational age; LGA, large for gestational age; SGA, small for gestational age.
Median (IQR).
Median (range).
Definition of Insulin Trajectories
We first determined the median of plasma insulin concentration at each time point (at birth [cord blood] and in childhood). Next, we identified four trajectories based on these medians, as follows: 1) consistently low (reference): stable below median at two time points; 2) increasing: below median at birth going up to above median in childhood; 3) decreasing: above median at birth falling to below median in childhood; and 4) consistently high: sustained at above median at both time points.
Ascertainment of Age at Menarche
After girls attained age 6 years, a questionnaire was used at least annually to gather menarche information. If a girl reached menarche, the month and year of the first menstrual period was recorded. Age at menarche reported at the first study visit after menarche occurred was used in the analyses. If a questionnaire response was not available (68.6%), age at menarche was abstracted from the electronic medical record (EMR). Of those who reached menarche, 139 (63%) reported age, 62 (29%) reported both age and year and month in which menarche occurred, and the other 19 reported both age and year. Given that year and month of menarche are more precise than reported age, we preferentially used year and month when available. If girls had multiple reports, the first report was used with the expectation of less recall bias given shorter intervals between the event and report. In the subset of girls with both reported age and year and month (n = 62), the correlation coefficient between the reported age and calculated age based on the reported year and month was 0.91. Age at menarche was further adjudicated using information on age at peak height velocity and Tanner stage of breast development.
Definition of Childhood OWO and BMI Trajectories
Child weight and height were measured by medical staff during clinic visits and documented in the EMR. BMI was calculated as weight in kilograms divided by the square of the height in meters (kg/m2), and BMI percentiles were calculated using World Health Organization reference values (19). OWO was defined as BMI ≥ 85th percentile for age. Childhood OWO status at a single time point (median [interquartile range (IQR)] age: 7.0 [6.5–7.3] years) was used for the analyses. In addition, BMI trajectories from birth to age 5 years were determined based on repeated BMI measurements using time series K-means clustering, with k set at four, as described previously (20,21). As a result, four BMI trajectory groups were generated: stable normal weight, stable lean, early-onset OWO, and late-onset OWO.
Prenatal and Postnatal Covariates
Maternal prepregnancy height and weight, race and ethnicity, educational attainment, and age at menarche were gathered from maternal interviews conducted at 24–72 h after delivery. Self-reported race and ethnicity were classified into mutually exclusive categories: non-Hispanic Black, Hispanic, and other (which included non-Hispanic White, Asian, Pacific Islander, more than one race, and other race). Educational attainment was categorized into high school and below versus college and above. Smoking status during pregnancy was classified into nonsmoker versus smoker (quitter or continuous smoker). Maternal age at menarche was grouped into <12, 12–13, and ≥14 years. Maternal BMI was calculated based on prepregnancy weight and height and then further classified into nonobese (<30 kg/m2) and obese (≥30 kg/m2). Maternal diabetes status during pregnancy (nondiabetic, gestational diabetes, and preexisting diabetes) was extracted from EMRs and verified by blood glucose profiles according to American Diabetes Association criteria (22). Maternal age at delivery, pregnancy complications, and child’s birth weight and gestational age at delivery were abstracted from EMRs. Hypertensive disorders in pregnancy included chronic/gestational hypertension, preeclampsia, and eclampsia, and hemolysis, elevated liver enzymes, and low platelets syndrome. Maternal gestational weight gain was calculated as the difference between pregestational or first trimester bodyweight and the last recorded bodyweight before delivery, and then categorized as adequate, inadequate, and excessive weight gain according to the Institute of Medicine recommendation (23). Preterm birth was defined as gestational age < 37 weeks. Fetal growth was classified into small for gestational age (birth weight for gestational age < 10th percentile), appropriate for gestational age (10th to 90th percentile), and large for gestational age (>90th percentile) (15). Infant breastfeeding history and the timing of first solid-food introduction were collected by questionnaire interview at follow-up visits (16,24). Breastfeeding status was grouped into any breastfeeding versus none. Timing of first solid-food introduction was categorized as ‘‘early’’ (within the first 3 months of age), ‘‘suggested’’ (within 4–6 months of age), or ‘‘delayed’’ (7 months of age or later). To identify rapid weight gain in the first year, we first calculated weight-for-age z-scores in the first year using World Health Organization reference values (19). Weight gain z-score was calculated as the change in weight-for-age z-scores from birth to 1 year of age, and further categorized into two groups: rapid (weight gain z-score > 0.67) versus nonrapid (≤0.67) (15).
Statistical Analysis
Since the distributions of plasma insulin and adipokine concentrations were skewed, wthey were log2-transformed to reduce the leverage of extreme values. Correlation coefficients were calculated between log2-transformed cord blood and postnatal insulin using Pearson correlation. To identify potential nonmonotonic dose-response relationships, we also modeled tertiles of plasma insulin concentration, adiponectin, leptin, and leptin/adiponectin ratio with age at menarche.
To start, we used accelerated failure time (AFT) models to assess the independent associations between plasma insulin concentrations at each time point (at birth and in childhood) and menarche timing using a Weibull distribution with adjustment for important covariates (Proc Lifereg, version 9.4; SAS Institute, Cary, NC). The time ratios (TRs) provided by the AFT model are interpreted as the ratio of the median time to event for a given level of plasma insulin to the reference level. Next, we converted the TR to a mean shift (in months) at age of menarche. The pertinent covariates were selected based on biological plausibility and relevant literature, including maternal age, educational attainment, race and ethnicity, smoking during pregnancy, prepregnancy BMI, diabetes status during pregnancy, hypertensive disorders in pregnancy, maternal age at menarche categories, child’s fetal growth, and preterm birth. The association model of childhood insulin concentration further included age of plasma insulin measurement and the hour of day at the study visit in addition to the abovementioned covariates. As a next step, we built AFT models to explore the association of longitudinal trajectories of plasma insulin from birth to childhood, adiponectin, leptin, and leptin/adiponectin ratio with age at menarche adjusted for the same covariates mentioned above. The median age at menarche was calculated using an unadjusted AFT model, which accounted for the censored individuals.
Next, we estimated the combined associations of plasma insulin concentrations, OWO, BMI trajectories, and adiponectin with menarche timing using AFT models. We also explored potential effect modification of childhood OWO by including insulin concentration, OWO, and their cross-product term in the AFT models.
At a 0.05 level of significance (two sides) with 80% power, we were able to detect a 1.5% difference in menarche timing among 186 participants who had reached menarche. All statistical analyses were performed using SAS version 9.4 (SAS Institute).
Results
Characteristics of the Study Population
The 458 girls included in this study were racially and ethnically diverse, with 62% non-Hispanic Black and 23% Hispanic. A relatively high percentage of the girls were born prematurely (30%) (Table 1). Most mothers (67%) did not attend college or beyond. More than one-fourth (26%) of mothers were obese at the time of their pregnancies, and 16% had preexisting or gestational diabetes. Maternal median (IQR) age at delivery was 28.1 (23.2–33.5) years. A high percentage (45%) of the girls had OWO in childhood, at median (IQR) ages of 7 (6.5–7.3) years. In all, 306 (67%) of the girls had reached menarche. Median age at menarche was 12.4 years, which was about 8.4 months earlier than their mothers (median = 13.0 years). The medians (IQR) of plasma insulin concentrations at birth and in childhood were 13.58 (8.23–21.36) µIU/mL and 12.56 (7.65–22.96) µIU/mL, respectively, and they were moderately correlated (ρ = 0.18, P = 0.004) (Supplementary Table 2).
Plasma Insulin at Birth and in Childhood, Its Trajectories, and Age at Menarche
The estimated mean age at menarche was lower among girls who had elevated insulin levels at each time point. As shown in Table 2, the mean age at menarche decreased by about 2 months for every doubling of plasma insulin concentration at birth (mean shift of age at menarche = −1.95; 95% CI −0.33 to −3.53) and in childhood (mean shift = −2.07; 95% CI −0.48 to −3.65). Compared with girls in the lowest tertile (T1), adjusted models suggested that menarche occurred approximately 7 months and 5 months earlier among girls in the top tertile (T3) of insulin levels at birth and in childhood, respectively (Table 2). The inclusion of childhood BMI z-score partially attenuated the effect estimates (Supplementary Table 3).
Table 2.
Association between plasma insulin levels (at birth, in childhood and longitudinal trajectories) with age at menarche
| Insulin concentration or tertile (µIU/mL) | n | TR (95% CI) | Mean shift (in mo) of age at menarche (95% CI) | P value |
|---|---|---|---|---|
| At birth (cord blood) | ||||
| T1: <9.8 | 130 | 1.00 | Reference | |
| T2: 9.9–18.4 | 131 | 0.97 (0.94, 1.00) | −5.42 (−0.15, −10.51) | 0.044 |
| T3: >18.4 | 130 | 0.96 (0.93, 0.99) | −6.58 (−1.37, −11.65) | 0.014 |
| A doubling of insulin level | 391 | 0.99 (0.98, 1.00) | −1.95 (−0.33, −3.53) | 0.018 |
| Childhood* | ||||
| T1: <9.4 | 111 | 1.00 | Reference | |
| T2: 9.5–18.2 | 112 | 0.97 (0.94, 1.00) | −5.00 (−0.19, −9.65) | 0.041 |
| T3: >18.3 | 112 | 0.97 (0.94, 1.00) | −4.95 (−0.25, −9.52) | 0.039 |
| A doubling of insulin level | 335 | 0.99 (0.98, 1.00) | −2.07 (−0.48, −3.65) | 0.011 |
| Trajectory* | ||||
| Consistently low | 82 | 1.00 | Reference | |
| Decreasing | 63 | 1.00 (0.96, 1.04) | 0.30 (−6.29, 7.17) | 0.931 |
| Increasing | 55 | 0.99 (0.95, 1.03) | −1.62 (−7.82, 4.85) | 0.620 |
| Consistently high | 68 | 0.96 (0.93, 1.00) | −6.25 (−0.38, −11.88) | 0.037 |
Adjusted for maternal age, educational attainment, race and ethnicity, smoking status during pregnancy, prepregnancy BMI, diabetes status during pregnancy, hypertensive disorders in pregnancy, maternal age at menarche categories, child’s fetal growth status, and preterm birth; mo, months; T, tertile.
Further adjusted for age at which plasma insulin was measured and the hour of day at the study visit in addition to the abovementioned covariates.
When modeling longitudinal insulin trajectories from birth to childhood, age at menarche occurred substantially earlier among girls with high insulin levels both at birth and in childhood compared with those with consistently low insulin levels at the two time points (aTR = 0.96; 95% CI 0.93 to 1.00), suggesting that menarche was occurring approximately 6 months earlier on average, 95%CI −0.38 to −11.88 (Table 2), among those girls.
Plasma Adipokines in Childhood and Age at Menarche
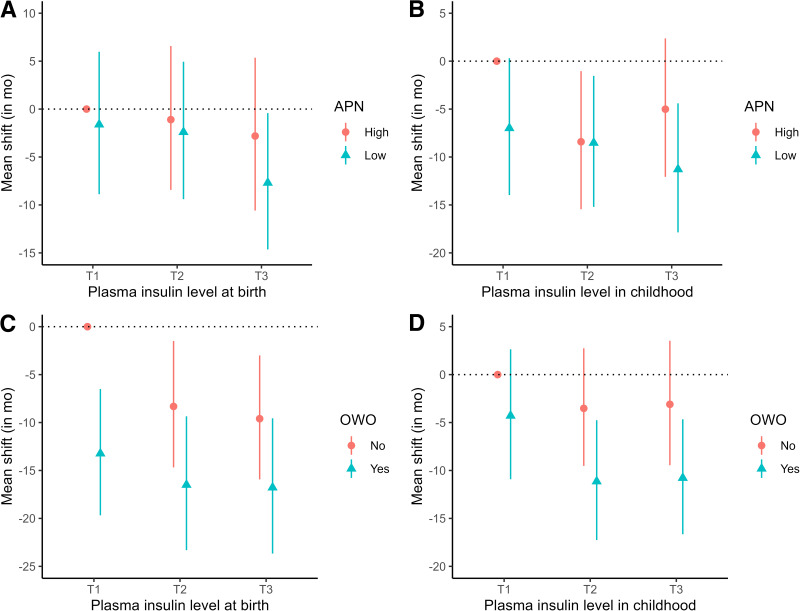
Childhood adiponectin was a predictor of age at menarche, with significantly later timing of menarche onset observed for those with T3 adiponectin levels versus T1 levels (mean shift = 6.40 months; 95% CI 1.71 to 11.24) (Supplementary Table 4). When adiponectin was modeled as a continuous variable, mean age at menarche occurred 4 months later for each doubling of adiponectin concentration (mean shift = 3.56, 95% CI 1.43 to 5.72). In contrast, an elevated leptin concentration and the leptin/adiponectin ratio were associated with earlier menarche (mean shift = −2.44 [95% CI −1.02 to −3.85] and −2.94 [95% CI −1.79 to −4.11], respectively). Compared with T1 levels, T3 leptin and T3 leptin/adiponectin ratios were associated with about a 9-month and 11-month earlier menarche on average, respectively (Supplementary Table 4). Furthermore, mean age at menarche occurred about 8 and 11 months earlier for girls with low adiponectin coupled with T3 cord blood and childhood insulin levels, respectively, compared with those with high adiponectin and T1 insulin levels (Fig. 1A and B).
Figure 1.
Combined associations of plasma insulin, OWO, and childhood adiponectin with age at menarche. A and B display the combined associations of early childhood adiponectin and plasma insulin at birth (A) and in childhood (B) with age at menarche. The y axis represents the mean shift of age at menarche (in months). C and D display the combined associations of childhood OWO and plasma insulin at birth (C) and in childhood (D) with age at menarche. The y axis represents the mean shift of age at menarche (in months). Low adiponectin was defined as plasma adiponectin concentrations in childhood below median, while high adiponectin was defined as above median. APN, adiponectin; mo, months; T, tertile.
Combined Associations of Insulin, OWO, and BMI Trajectories With Age at Menarche
Childhood OWO was associated with a roughly 9-month-earlier age at menarche (mean shift = −9.39; 95% CI −5.72 to −12.97) compared with normal weight (Table 3). Compared with stable normal weight from birth to age 5 years, both early-onset and late-onset OWO were associated with around a 9-month-earlier menarche. We also observed a substantial combined association of plasma insulin and OWO (Fig. 1C and D). As shown in Table 3, mean age at menarche occurred about 17 months earlier for girls with OWO and T3 cord blood insulin levels compared with those with normal weight and T1 insulin levels (mean shift = −16.78; 95% CI −9.56 to −23.67). A similar decrease was seen for girls with OWO and T3 insulin levels in childhood (mean shift = −10.78; 95% CI −4.66 to −16.65). The combination of insulin levels and BMI trajectories was strongly associated with earlier age at menarche.
Table 3.
Individual and joint associations of childhood OWO and insulin levels with age at menarche
| Insulin at birth | Insulin in childhood | ||||||
|---|---|---|---|---|---|---|---|
| INS | OWO | n | Mean shift (in mo) of age at menarche (95% CI) | P value | n | Mean shift (in mo) of age at menarche (95% CI) | P value |
| No | — | 250 | Reference | ||||
| Yes | — | 208 | −9.39 (−5.72, −12.97) | <0.001 | |||
| BMI-Z | — | 458 | −2.85 (−1.63, −4.05) | <0.001 | |||
| Combined | |||||||
| T1 | No | 70 | Reference | 69 | Reference† | ||
| T2 | No | 75 | −8.32 (−1.49, −14.90) | 0.018 | 58 | −3.52 (−9.54, 2.74) | 0.267 |
| T3 | No | 72 | −9.60 (−3.00, −15.93) | 0.005 | 51 | −3.10 (−9.45, 3.53) | 0.354 |
| T1 | Yes | 60 | −13.22 (−6.45, −19.69) | <0.001 | 43 | −4.29 (−10.92, 2.64) | 0.221 |
| T2 | Yes | 56 | −16.50 (−9.35, −23.31) | <0.001 | 54 | −11.14 (−4.76, −17.26) | <0.001 |
| T3 | Yes | 58 | −16.78 (−9.56, −23.67) | <0.001 | 61 | −10.78 (−4.66, −16.65) | <0.001 |
| Trajectory* | |||||||
| SNW | — | 71 | Reference | ||||
| SL | — | 93 | 1.06 (−5.28, 7.65) | 0.749 | |||
| EOWO | — | 123 | −8.86 (−3.27, −14.25) | 0.002 | |||
| LOWO | — | 53 | −9.50 (−2.84, −15.87) | 0.006 | |||
| Combined | |||||||
| T1 | No | 45 | Reference | 56 | Reference† | ||
| T2 | No | 49 | 3.50 (−5.54, 13.03) | 0.456 | 39 | −7.20 (−14.28, 0.20) | 0.057 |
| T3 | No | 50 | −2.90 (−12.18, 6.94) | 0.556 | 35 | −9.77 (−2.03, −17.16) | 0.014 |
| T1 | Yes | 47 | −6.91 (−15.37, 2.03) | 0.128 | 42 | −12.18 (−5.17, −18.91) | <0.001 |
| T2 | Yes | 54 | −8.66 (−17.57, 0.79) | 0.072 | 46 | −12.96 (−5.67, −19.93) | <0.001 |
| T3 | Yes | 45 | −15.46 (−5.96, −24.41) | 0.002 | 56 | −15.98 (−9.51, −22.17) | <0.001 |
Adjusted for maternal age, education attainment, race and ethnicity, smoking status during pregnancy, prepregnancy body mass index, diabetes status during pregnancy, hypertensive disorders in pregnancy, maternal age at menarche categories, child’s fetal growth status, and preterm birth. There was no evidence of interaction (all P values for interaction >0.05). BMI-Z, body mass index z-score; EOWO, early-onset OWO; INS, insulin; LOWO, late-onset OWO; mo, months; SL, stable lean; SNW, stable normal weight; T, tertile.
BMI trajectories.
Further adjusted for age at which plasma insulin was measured and the hour of day at the study visit in addition to the abovementioned covariates.
Sensitivity Analysis
When we additionally adjusted for rapid weight gain in the first year, timing of first solid-food introduction categories, breastfeeding status, or maternal gestational weight gain categories, the effect estimations were not substantially attenuated (Supplementary Tables 5–8). Furthermore, the associations between earlier menarche and elevated insulin concentrations at either time point (at birth, in childhood) were generally consistent when we restricted the analyses to major population subgroups: girls who self-identified as being non-Hispanic Black (Supplementary Table 9), girls with term births (Supplementary Table 10), girls with normal birth weight (≥2,500 g) (Supplementary Table 11), and girls with measurement of childhood insulin between ages 0.5 and 3 years (Supplementary Table 12). The estimated mean shift of age at menarche did not markedly change when stabilized inverse probability weighting was applied to account for potential selection bias due to exclusions (Supplementary Table 13), or when girls with precocious puberty were also included in the analysis (Supplementary Table 14).
Conclusions
To our knowledge, this is the first prospective birth cohort study to systematically investigate the associations of plasma insulin levels in early life (at birth and in childhood) and their longitudinal trajectories with age at menarche. We found that plasma insulin concentrations at birth or in childhood were independently associated with earlier mean ages at menarche, and a higher risk was particularly evident among girls with OWO. A consistently high plasma insulin level at birth and in childhood was strongly associated with earlier menarche, beyond established prenatal and postnatal risk factors. Our findings lend further support to the hypothesis that early life events may shape menarche timing and highlight that elevated insulin levels both at birth and in childhood may predict earlier age at menarche.
Our data revealed a strong consistent association between age at menarche and plasma insulin concentrations at two time points (at birth and in childhood), which suggests that any incremental increase in insulin levels at key developmental windows is associated with an increased risk of earlier menarche. The association was independent of known risk factors and childhood BMI. Our results are in agreement with previous reports that elevated insulin concentrations at age 6–8 years were associated with earlier menarche age (25,26). In support of our findings, one study showed that girls with central precocious puberty had higher plasma insulin levels than healthy controls (27). Since compensatory hyperinsulinemia is one of the consequences of insulin resistance, and the adverse effects related to insulin resistance are more likely mediated via compensatory hyperinsulinemia (28), our findings suggest that early-life insulin resistance may be implicated in advanced menarche timing.
Furthermore, when we examined the associations between longitudinal trajectories of insulin levels from birth to childhood and menarche timing, we found that girls who had consistently high plasma insulin levels both at birth and in childhood had the earliest menarche, suggesting that both at birth and the early postnatal period are susceptible risk time periods. Our findings also raise the possibility that early-life treatment of insulin resistance, via lifestyle changes, could prevent earlier menarche, and, in turn, reduce the risk of adverse health consequences related to earlier menarche, such as cardiovascular diseases, diabetes, and breast cancer in later life. Our results are in line with those of two clinical trials that used metformin, an insulin sensitizer, to treat girls with insulin resistance and showed a reduced risk of early onset of puberty and menarche (12,13). Therefore, some medications hold potential to help prevent earlier menarche.
Consistent with a body of studies that have established a link between childhood obesity and earlier onset of puberty and menarche (7), the current study showed that childhood OWO was significantly associated with earlier menarche. Importantly, our data further pinpointed that childhood OWO exacerbated the association between plasma insulin and earlier menarche. Since the combination of having both OWO and elevated insulin levels in early life results in earlier menarche than is seen among girls with one condition alone, it is likely that the intricate interplay between these two conditions is further implicated in the complex process of menarche. Given that early age at menarche is associated with a wide range of adverse health effects during puberty and beyond (29), our findings raise the possibility that appropriate screening and management of insulin resistance from birth to early childhood may reduce the considerable adverse effects of early menarche and could achieve long-lasting benefits for women of all ages.
Our data also showed that elevated adiponectin was associated with later menarche, whereas elevated leptin and the leptin/adiponectin ratio were associated with earlier menarche. These findings are in line with previous studies that reported a negative association between prepubertal leptin and age at menarche (25,26) but a positive association between prepubertal adiponectin and menarche (25). Given that plasma adiponectin concentrations and the leptin/adiponectin ratio in childhood have been shown to serve as surrogates of insulin sensitivity (17) and insulin resistance (18), respectively, these findings further support our hypothesis that early-life insulin resistance may contribute to earlier onset of menarche.
Higher insulin may contribute to an earlier menarche through several potential mechanisms: 1) neuroendocrine mechanisms, in which hyperinsulinemia stimulates the pulsatile luteinizing hormone secretion (30), which may regulate ovarian sex steroid production and trigger ovulation (31); and 2) peripheral mechanisms, in which insulin resistance brings about reduced hepatic production of sex hormone-binding globulin (32), which, in turn, results in increased sex steroid bioavailability (7) leading to earlier menarche. Since the HPG axis is vulnerable during developmental periods (7), it is possible that variations in insulin resistance during critical developmental windows may influence the HPG axis structures and functions. Some studies have provided evidence that epigenetic regulations contribute to female puberty (33).
Strengths and Limitations
Our study had several strengths. Plasma insulin concentrations were measured at two time points (at birth and in childhood). This study design offered the possibility of detecting an association between insulin levels during developmental windows and menarche timing. The prospective birth cohort study design also avoided the limitations of reverse causality. Several limitations also should be acknowledged. First, plasma insulin concentrations in childhood were measured in nonfasting samples that were collected randomly at any time during clinical hours. The random timing of blood sampling may have introduced background noise and, thus. biased our results toward the null. Second, we did not estimate insulin sensitivity by the hyperglycemic-euglycemic clamp method, the gold standard for determining insulin sensitivity, because it is challenging and impractical in large-scale epidemiological studies. Third, we did not measure neonatal venous blood insulin at birth; we measured cord blood insulin concentration at birth, which has been associated with birth weight (15,34), as a proxy. Fourth, we only measured total adiponectin; while high molecular weight adiponectin is strongly related to the role of insulin-sensitization (35), it was not measured. Five, age at menarche was obtained from the EMRs for more than two-thirds of the participants. Finally, given that our study participants had high BMI z-scores and a high rate of OWO in childhood, which may lead to high childhood insulin concentrations, caution should be used when generalizing our findings to lean populations.
In conclusion, in this prospective study, we demonstrated a strong association between elevated insulin in early life and earlier age at menarche. We found that childhood OWO enhanced the association. These findings may have important public health implications, including promoting the development of earlier screening and prevention strategies, especially given the wide range of adverse health consequences related to earlier menarche.
Article Information
Acknowledgments. The authors would like to thank the study participants, the nursing staff at Labor and Delivery of the BMC, and the field team for their contributions to the BBC.
Funding. The BBC (the parent study) is supported, in part, by the National Institutes of Health (Eunice Kennedy Shriver National Institute of Child Health and Human Development 2R01HD041702, R01HD086013, and R01HD098232 and National Institute of Environmental Health Sciences R01ES031272, U01ES034983, and R01ES031521), and the Health Resources and Services Administration of the US Department of Health and Human Services (UT7MC45949).
The contents of the manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the supporting agencies.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. G.W. and X.W. designed and conceptualized the study and drafted and revised the manuscript. C.P. and W.G.A. contributed to the acquisition of data, interpretation of the results, and revision of the manuscript. S.R., J.P.B., R.H., P.L.W., X.H., and X.W. interpreted the results, and reviewed and revised the manuscript. All authors approved the final version. All authors contributed to the discussion, reviewed/edited the manuscript, and approved the final manuscript. G.W. and X.W. are the guarantors of this work and, as such, had full access to all the study data and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.22294210.
References
- 1. Kaplowitz PB, Slora EJ, Wasserman RC, Pedlow SE, Herman-Giddens ME. Earlier onset of puberty in girls: relation to increased body mass index and race. Pediatrics 2001;108:347–353 [DOI] [PubMed] [Google Scholar]
- 2. Wyshak G, Frisch RE. Evidence for a secular trend in age of menarche. N Engl J Med 1982;306:1033–1035 [DOI] [PubMed] [Google Scholar]
- 3. Day FR, Elks CE, Murray A, Ong KK, Perry JR. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: the UK Biobank study. Sci Rep 2015;5:11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lakshman R, Forouhi NG, Sharp SJ, et al. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab 2009;94:4953–4960 [DOI] [PubMed] [Google Scholar]
- 5. Fuhrman BJ, Moore SC, Byrne C, et al. Association of the age at menarche with site-specific cancer risks in pooled data from nine cohorts. Cancer Res 2021;81:2246–2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Werneck AO, Coelho-E-Silva MJ, Padilha CS, et al. Age at menarche and cancer risk at adulthood. Ann Hum Biol 2018;45:369–372 [DOI] [PubMed] [Google Scholar]
- 7. Reinehr T, Roth CL. Is there a causal relationship between obesity and puberty? Lancet Child Adolesc Health 2019;3:44–54 [DOI] [PubMed] [Google Scholar]
- 8. Salgin B, Norris SA, Prentice P, et al. Even transient rapid infancy weight gain is associated with higher BMI in young adults and earlier menarche. Int J Obes 2015;39:939–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shifren JL, Osathanondh R, Yeh J. Human fetal ovaries and uteri: developmental expression of genes encoding the insulin, insulin-like growth factor I, and insulin-like growth factor II receptors. Fertil Steril 1993;59:1036–1040 [DOI] [PubMed] [Google Scholar]
- 10. Bucholtz DC, Chiesa A, Pappano WN, et al. Regulation of pulsatile luteinizing hormone secretion by insulin in the diabetic male lamb. Biol Reprod 2000;62:1248–1255 [DOI] [PubMed] [Google Scholar]
- 11. Codner E, Cassorla F. Puberty and ovarian function in girls with type 1 diabetes mellitus. Horm Res 2009;71:12–21 [DOI] [PubMed] [Google Scholar]
- 12. Ibáñez L, Ong K, Valls C, Marcos MV, Dunger DB, de Zegher F. Metformin treatment to prevent early puberty in girls with precocious pubarche. J Clin Endocrinol Metab 2006;91:2888–2891 [DOI] [PubMed] [Google Scholar]
- 13. Ibáñez L, Valls C, Ong K, Dunger DB, de Zegher F. Metformin therapy during puberty delays menarche, prolongs pubertal growth, and augments adult height: a randomized study in low-birth-weight girls with early-normal onset of puberty. J Clin Endocrinol Metab 2006;91:2068–2073 [DOI] [PubMed] [Google Scholar]
- 14. Austin MA, Ordovas JM, Eckfeldt JH, et al. Guidelines of the National Heart, Lung, and Blood Institute Working Group on Blood Drawing, Processing, and Storage for Genetic Studies. Am J Epidemiol 1996;144:437–441 [DOI] [PubMed] [Google Scholar]
- 15. Wang G, Divall S, Radovick S, et al. Preterm birth and random plasma insulin levels at birth and in early childhood. JAMA 2014;311:587–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang G, Hu FB, Mistry KB, et al. Association between maternal prepregnancy body mass index and plasma folate concentrations with child metabolic health. JAMA Pediatr 2016;170:e160845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brochu-Gaudreau K, Rehfeldt C, Blouin R, Bordignon V, Murphy BD, Palin MF. Adiponectin action from head to toe. Endocrine 2010;37:11–32 [DOI] [PubMed] [Google Scholar]
- 18. Frithioff-Bøjsøe C, Lund MAV, Lausten-Thomsen U, et al. Leptin, adiponectin, and their ratio as markers of insulin resistance and cardiometabolic risk in childhood obesity. Pediatr Diabetes 2020;21:194–202 [DOI] [PubMed] [Google Scholar]
- 19. World Health Organization . Child growth standards 2007. Accessed 12 September 2020. Available from https://www.who.int/toolkits/child-growth-standards/standards/body-mass-index-for-age-bmi-for-age
- 20. Hartigan JA, Wong MA. Algorithm AS 136: A k-means clustering algorithm. J R Stat Soc Ser C 1979;28:100–108. [Google Scholar]
- 21. Cao T, Zhao J, Hong X, et al. Cord blood metabolome and BMI trajectory from birth to adolescence: a prospective birth cohort study on early life biomarkers of persistent obesity. Metabolites 2021;11:739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. American Diabetes Association . 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes–2021. Diabetes Care 2021;44(Suppl. 1):S15–S33 [DOI] [PubMed] [Google Scholar]
- 23. Institute of Medicine . Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC, National Academies Press, 2009 [PubMed] [Google Scholar]
- 24. Hong X, Liang L, Sun Q, et al. Maternal triacylglycerol signature and risk of food allergy in offspring. J Allergy Clin Immunol 2019;144:729–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gavela-Pérez T, Navarro P, Soriano-Guillén L, Garcés C. High prepubertal leptin levels are associated with earlier menarcheal age. J Adolesc Health 2016;59:177–181 [DOI] [PubMed] [Google Scholar]
- 26. Thankamony A, Ong KK, Ahmed ML, Ness AR, Holly JM, Dunger DB. Higher levels of IGF-I and adrenal androgens at age 8 years are associated with earlier age at menarche in girls. J Clin Endocrinol Metab 2012;97:E786–E790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sørensen K, Aksglaede L, Petersen JH, Andersson AM, Juul A. Serum IGF1 and insulin levels in girls with normal and precocious puberty. Eur J Endocrinol 2012;166:903–910 [DOI] [PubMed] [Google Scholar]
- 28. Levy-Marchal C, Arslanian S, Cutfield W, et al.; Insulin Resistance in Children Consensus Conference Group . Insulin resistance in children: consensus, perspective, and future directions. J Clin Endocrinol Metab 2010;95:5189–5198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Janghorbani M, Mansourian M, Hosseini E. Systematic review and meta-analysis of age at menarche and risk of type 2 diabetes. Acta Diabetol 2014;51:519–528 [DOI] [PubMed] [Google Scholar]
- 30. Tanaka T, Nagatani S, Bucholtz DC, et al. Central action of insulin regulates pulsatile luteinizing hormone secretion in the diabetic sheep model. Biol Reprod 2000;62:1256–1261 [DOI] [PubMed] [Google Scholar]
- 31. Stamou MI, Balasubramanian R. Hypothalamic ceramides and the ovarian sympathetic system: at the crossroads of obesity and puberty. Cell Metab 2021;33:6–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Holly JM, Smith CP, Dunger DB, et al. Relationship between the pubertal fall in sex hormone binding globulin and insulin-like growth factor binding protein-I. A synchronized approach to pubertal development? Clin Endocrinol (Oxf) 1989;31:277–284 [DOI] [PubMed] [Google Scholar]
- 33. Lomniczi A, Wright H, Ojeda SR. Epigenetic regulation of female puberty. Front Neuroendocrinol 2015;36:90–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shields BM, Knight B, Hopper H, et al. Measurement of cord insulin and insulin-related peptides suggests that girls are more insulin resistant than boys at birth. Diabetes Care 2007;30:2661–2666 [DOI] [PubMed] [Google Scholar]
- 35. Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes 2006;55:249–259 [PubMed] [Google Scholar]