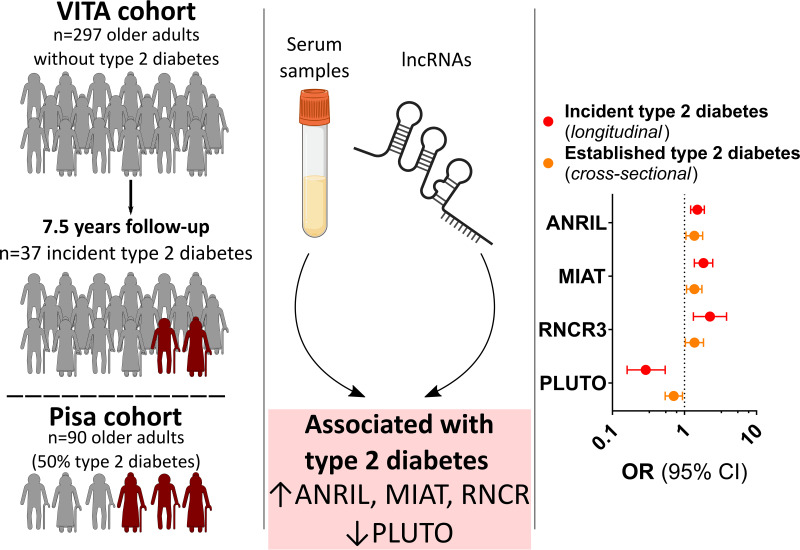
Abstract
OBJECTIVE
Long noncoding RNAs (lncRNAs) are involved in diabetogenesis in experimental models, yet their role in humans is unclear. We investigated whether circulating lncRNAs associate with incident type 2 diabetes in older adults.
RESEARCH DESIGN AND METHODS
A preselected panel of lncRNAs was measured in serum of individuals without diabetes (n = 296) from the Vienna Transdanube Aging study, a prospective community-based cohort study. Participants were followed up over 7.5 years. A second cohort of individuals with and without type 2 diabetes (n = 90) was used to validate our findings.
RESULTS
Four lncRNAs (ANRIL, MIAT, RNCR3, and PLUTO) were associated with incident type 2 diabetes and linked to hemoglobin A1c trajectories throughout the 7.5-year follow-up. Similar results (for MIAT and PLUTO also in combined analysis) were obtained in the validation cohort.
CONCLUSIONS
We found a set of circulating lncRNAs that independently portends incident type 2 diabetes in older adults years before disease onset.
Graphical Abstract
Introduction
Over the past few decades, the number of older adults with type 2 diabetes has markedly increased and now accounts for almost half of the affected individuals (1). Importantly, the high rate of chronic diabetic complications in newly diagnosed patients calls for early detection and highlights the need for accurate predictors of disease onset to tailor primary prevention efforts (2,3). Long noncoding RNAs (lncRNAs) are epigenetic regulators of gene expression, pre–messenger RNA splicing, RNA translation, and RNA stability, which have been found casually implicated in β-cell dysfunction and insulin resistance in preclinical models (4–6). However, their implication in diabetes development in healthy individuals is largely unknown. In the current study, we aimed to assess the relationship between circulating lncRNAs that are biologically linked to impaired glucose control and incident type 2 diabetes in older adults.
Research Design and Methods
A detailed description of the methodology is provided in the Supplementary Material. In the prospective community-based Vienna Transdanube Aging (VITA) cohort study (7,8), individuals of predominantly European descent aged 75 years at baseline were recruited based on the area of their residence (21st and 22nd districts of Vienna, Austria) (Supplementary Fig. 1). Exclusion criteria were a history of type 2 diabetes or type 1 diabetes or a hemoglobin A1c (HbA1c) concentration ≥6.5% (48 mmol/mol) (Supplementary Fig. 2). A total of 296 subjects without diabetes were included and assessed for incident type 2 diabetes at 2.5-, 5-, and 7.5-year follow-up visits. An independent study cohort from the Pisa University Hospital, Italy (9,10), including subjects with (n = 45) and without (n = 45) type 2 diabetes according to American Diabetes Association criteria (11) was used for a cross-sectional validation of our findings. The primary study end point was the development of type 2 diabetes within 7.5 years (VITA cohort) and the presence of type 2 diabetes at presentation (Pisa cohort).
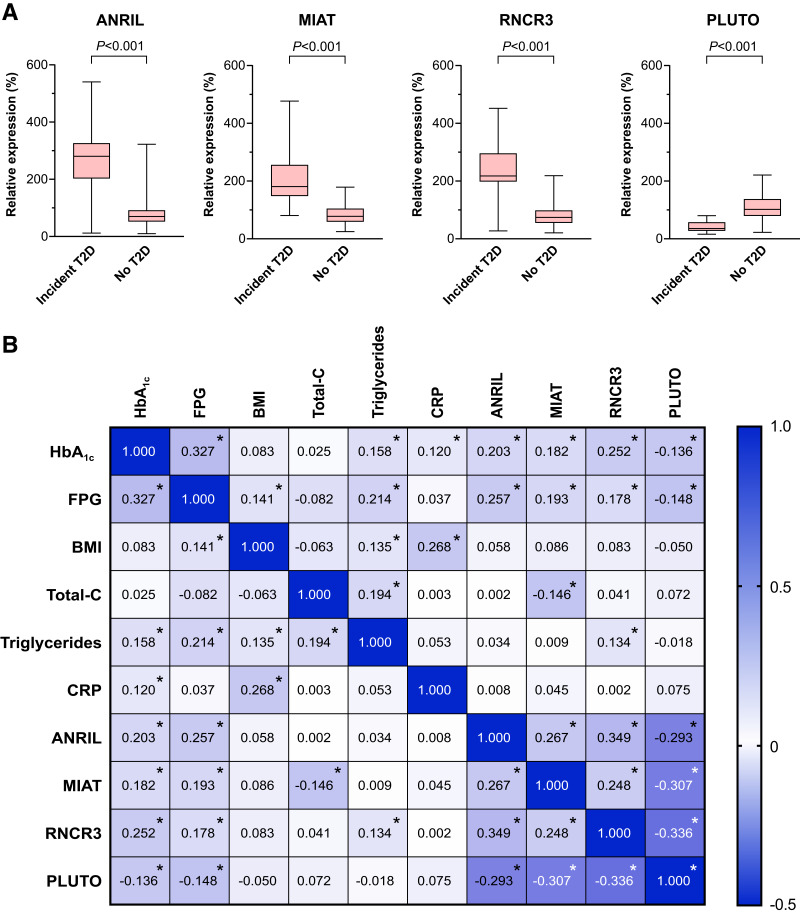
Profiling of lncRNAs was performed in serum samples collected at study start using a custom PCR array on Quant Studio 5 and 7 cyclers. A systematic literature search identified 15 lncRNAs with disease-specific differential expression and biological links to diabetes, which were included in the array (Supplementary Table 1). Four of these lncRNAs that were 1) differentially detected in individuals with type 2 diabetes, 2) present at levels conclusive with the literature (4,5,12), and 3) correlated with at least one parameter of glucose control (i.e., fasting plasma glucose [FPG] or HbA1c) were included in the final analyses: antisense noncoding RNA in the INK4 locus (ANRIL), retinal noncoding RNA 3 (RNCR3), myocardial infarction associated transcript (MIAT), and PDX1 associated lncRNA upregulator of transcription (PLUTO) (Fig. 1).
Figure 1.
Altered lncRNA profile is linked to impaired glycemic status in older adults. A: Box-and-whiskers plots of ANRIL, RNCR3, MIAT, and PLUTO in study participants of the VITA cohort with and without incident type 2 diabetes (T2D) during the 7.5-year follow-up period. Data were compared by the Mann-Whitney U test. B: Correlation matrix showing the strength of correlation between baseline variables of the study participants in the VITA cohort. ANRIL, MIAT, RNCR3, and PLUTO are correlated with each other as well as with HbA1c and FPG. C; cholesterol; CRP, C-reactive protein; *P < 0.05.
Comparisons of lncRNA levels were corrected for multiple testing by the Benjamini-Hochberg procedure. Odds ratios (ORs) for type 2 diabetes with 95% CIs were estimated using binary logistic regression. First, each lncRNA was examined separately in univariable analysis and in multivariable analysis adjusted for traditional risk factors and for the Cambridge Diabetes Risk Score (CDRS) (13) using Bonferroni correction. Two-way interactions for sex and each lncRNA were explored to evaluate potential sex differences in the association of each lncRNA with diabetes. Second, all variables included in univariable analyses were analyzed in a single multivariable regression model using stepwise backward selection. We used resampling techniques to internally validate the results in both cohorts.
Results
Baseline characteristics of the study population are presented in Table 1 and Supplementary Table 2. In individuals who developed type 2 diabetes, detected levels of ANRIL, RNCR3, and MIAT were significantly higher, while those of PLUTO were significantly lower (Fig. 1A, each P < 0.001). In line, ANRIL, MIAT, and RNCR3 positively correlated with HbA1c and FPG at baseline (Fig. 1B), while PLUTO displayed a negative correlation. The levels of all four lncRNAs showed a significant correlation with each other.
Table 1.
Baseline characteristics of patients of the VITA cohort
| Whole cohort (n = 296) | Incident type 2 diabetes (n = 37) | No type 2 diabetes (n = 259) | P value | |
|---|---|---|---|---|
| Age, years | 75.7 (75.3–76.1) | 75.9 (75.5–76.1) | 75.6 (75.3–76.1) | 0.191 |
| Female, n (%) | 181 (61.1) | 19 (51.4) | 162 (62.5) | 0.191 |
| BMI, kg/m2 | 26.6 (24.7–29.1) | 27.5 (25.6–30.4) | 26.6 (24.3–28.8) | 0.013 |
| Systolic blood pressure, mmHg | 140 (130–150) | 140 (128–150) | 140 (130–150) | 0.843 |
| Diastolic blood pressure, mmHg | 80 (70–85) | 80 (70–90) | 80 (70–85) | 0.430 |
| Heart rate, bpm | 71 (65–76) | 72 (65–78) | 70 (65–76) | 0.810 |
| Current smoker, n (%) | 31 (10.5) | 2 (5.4) | 29 (11.2) | 0.282 |
| History of hypertension, n (%) | 191 (64.5) | 26 (70.3) | 165 (63.7) | 0.435 |
| History of stroke, n (%) | 24 (8.1) | 3 (8.1) | 21 (8.1) | 1.000 |
| History of CAD, n (%) | 77 (26.0) | 10 (27.0) | 67 (25.9) | 0.881 |
| History of PAD, n (%) | 38 (12.8) | 6 (16.2) | 32 (12.4) | 0.511 |
| FPG, mmol/L | 5.6 (5.2–6.0) | 6.2 (5.6–6.8) | 5.6 (5.2–5.9) | <0.001 |
| HbA1c, % | 5.5 (5.3–5.8) | 6.0 (5.8–6.2) | 5.5 (5.2–5.8) | <0.001 |
| HbA1c, mmol/mol | 37 (34–40) | 42 (40–44) | 37 (33–40) | <0.001 |
| Triglyceride, mmol/L | 1.3 (1.0–1.8) | 1.3 (1.0–2.0) | 1.3 (1.0–1.8) | 0.333 |
| Total C, mmol/L | 6.1 (5.2–6.8) | 5.8 (5.1–6.3) | 6.2 (5.2–6.9) | 0.094 |
| LDL C, mmol/L | 3.8 (3.1–4.5) | 3.7 (3.0–4.2) | 3.8 (3.1–4.6) | 0.235 |
| HDL C, mmol/L | 1.5 (1.2–1.8) | 1.3 (1.1–1.5) | 1.5 (1.3–1.8) | 0.005 |
| Cobalamin, pg/mL | 444 (342–647) | 453 (304–576) | 442 (343–650) | 0.651 |
| Folic acid, ng/mL | 8.1 (5.9–11.3) | 7.5 (5.8–9.8) | 8.2 (5.9–11.5) | 0.122 |
| CRP, mg/L | 2 (1–5) | 3 (1–6) | 2 (1–5) | 0.294 |
| Creatinine, mg/dL | 1.1 (1.0–1.2) | 1.1 (1.0–1.2) | 1.1 (1.0–1.2) | 0.927 |
| ANRIL, % | 74.9 (55.3–108.3) | 283.1 (205.6–328.7) | 70.1 (52.4–92.0) | <0.001 |
| MIAT, % | 85.2 (61.8–121.0) | 183.0 (149.9–256.8) | 78.9 (59.9–105.6) | <0.001 |
| RNCR3, % | 79.6 (58.4–110.0) | 220.2 (198.6–300.7) | 75.0 (55.7–98.5) | <0.001 |
| PLUTO, % | 96.1 (70.2–126.1) | 36.3 (27.3–57.8) | 102.2 (79.1–137.3) | <0.001 |
| Beta-blockers, n (%) | 89 (41.0) | 12 (42.9) | 77 (40.7) | 0.832 |
| ACE-inhibitors, n (%) | 132 (60.8) | 18 (64.3) | 114 (60.3) | 0.688 |
| Antiplatelet agents, n (%) | 102 (34.6) | 16 (43.2) | 86 (33.3) | 0.236 |
| Oral anticoagulants, n (%) | 23 (7.8) | 5 (13.5) | 18 (7.0) | 0.165 |
| Calcium channel blockers, n (%) | 58 (26.7) | 10 (35.7) | 48 (25.4) | 0.250 |
| Diuretics, n (%) | 112 (51.6) | 13 (46.4) | 99 (52.4) | 0.556 |
| Statins, n (%) | 54 (18.2) | 9 (24.3) | 45 (17.4) | 0.306 |
Categorical data are shown as numbers (n) and percentages (%). Continuous data are presented as median and interquartile range. Groups were compared by χ2 test, Fisher exact test, and Mann-Whitney U test as appropriate. C, cholesterol; CAD, coronary artery disease; CRP, C-reactive protein; PAD, peripheral artery disease.
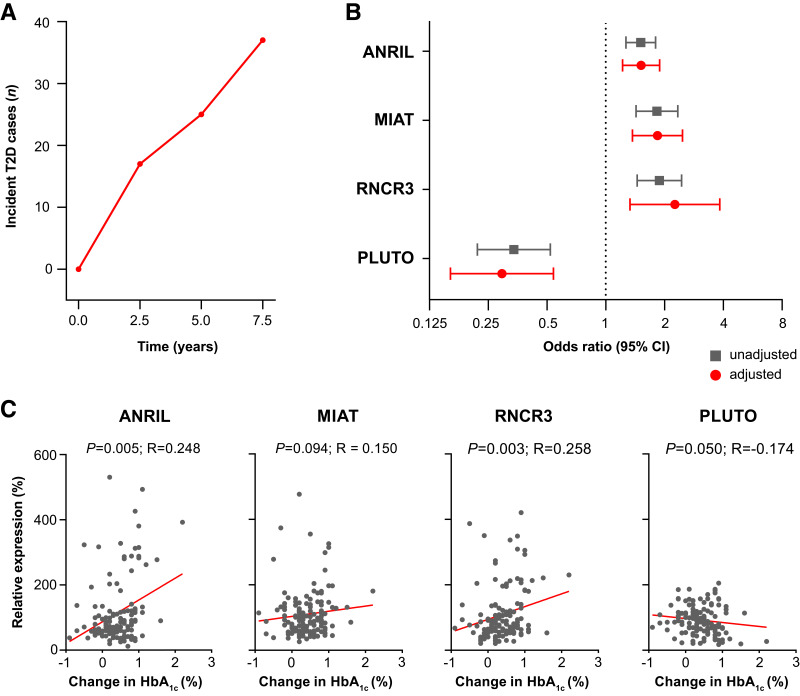
An incremental increase in HbA1c was detected throughout follow-up (Supplementary Fig. 3). In the VITA cohort, variables significantly associated with incident type 2 diabetes were HbA1c, FPG, BMI, HDL cholesterol, circulating levels of ANRIL (OR, 1.52; 95% CI, 1.27–1.80; P < 0.001), MIAT (OR, 1.83; 95% CI, 1.43–2.34; P < 0.001), RNCR3 (OR, 1.89; 95% CI, 1.45–2.45; P < 0.001), and PLUTO (OR, 0.34; 95% CI, 0.22–0.52; P < 0.001) (Supplementary Table 3). After adjustment for established risk factors, the findings remained consistent for ANRIL (adjusted [adj] OR, 1.51; 95% CI, 1.22–1.89; P < 0.001), MIAT (adj OR, 1.84; 95% CI, 1.37–2.47; P < 0.001), RNCR3 (adj OR, 2.26; 95% CI, 1.33–3.84; P < 0.001), and PLUTO (adj OR, 0.29; 95% CI, 0.16–0.54; P < 0.001) and were confirmed by internal validation (Fig. 2B and Supplementary Tables 4–8). There was no evidence of a sex-specific difference in the association of either lncRNA or incident type 2 diabetes (all Pinteraction > 0.05). Combined analysis of all four lncRNAs with traditional risk factors confirmed RNCR3 and PLUTO as independent risk factors of type 2 diabetes (Table 2). We performed an exploratory analysis to investigate the relationship between the lncRNA profile at baseline and HbA1c trajectories during the 7.5-year follow-up period (Fig. 2C). ANRIL, MIAT, and RNCR3 were linked to an increase in HbA1c throughout the 7.5-year follow-up period. In contrast, PLUTO negatively correlated with the absolute change in HbA1c.
Figure 2.
Development of type 2 diabetes (T2D) and changes in the glucose control profile over the 7.5-year follow-up in the VITA study cohort. A: Cumulative number of incident type 2 diabetes cases at 2.5-year, 5-year, and 7.5-year follow-up visits. B: Forest plot displaying crude (gray) and multivariable-adjusted (red) ORs for the development of type 2 diabetes at 7.5 years with models adjusted for HbA1c, FPG, BMI, HDL cholesterol, and the CDRS. Dots and squares represent ORs with line lengths indicating corresponding 95% CIs. C: Scatterplot showing the correlation of circulating lncRNA levels present in serum and the absolute change in HbA1c during 7.5 years of follow-up. ANRIL, MIAT, RNCR3, and PLUTO levels correlate with increasing HbA1c. Correlation was assessed using Spearman’s correlation coefficient.
Table 2.
Mutually adjusted ORs for incident type 2 diabetes in the VITA cohort
| Adjusted OR (95% CI) | P value | |
|---|---|---|
| HbA1c (%)* | 1.79 (1.03–3.10) | 0.040 |
| Total C (mmol/L)† | 0.95 (0.90–1.01) | 0.093 |
| HDL C (mmol/L)† | 1.10 (1.00–1.21) | 0.062 |
| PLUTO (%)‡ | 0.51 (0.26–0.99) | 0.047 |
| RNCR3 (%)‡ | 1.78 (1.16–2.72) | 0.008 |
Data represent the result of a backward selection process with the lncRNAs of interest and traditional risk factors included in the initial model. C, cholesterol.
Per 0.1% increment.
Per 0.0256 mmol/L increment.
Per 10% increment.
We validated our findings in an independent cohort of subjects with and without type 2 diabetes (Supplementary Table 9). Patients with type 2 diabetes showed higher levels of ANRIL, MIAT, and RNCR3 and lower levels of PLUTO (each P < 0.001) (Supplementary Fig. 4A). Accordingly, ANRIL, MIAT, and RNCR3 positively correlated with FPG and HbA1c, while PLUTO showed a negative correlation (HbA1c: each P < 0.001; FPG: P < 0.001 for MIAT, RNCR3, and PLUTO, and P = 0.007 for ANRIL) (Supplementary Fig. 4B and C). All lncRNAs were associated with type 2 diabetes in univariable and multivariable analyses (Supplementary Tables 10–12).
Conclusions
Here, we report for the first time that a set of circulating lncRNAs (ANRIL, MIAT, RNCR3, and PLUTO) associate with the development of type 2 diabetes in a prospective cohort of 296 older adults over a 7.5-year follow-up. Remarkably, these findings were confirmed in an external cohort (Supplementary Fig. 5). Furthermore, the identified set of lncRNAs was found to correlate with both baseline and follow-up HbA1c levels.
Very few studies have assessed circulating lncRNAs in human subjects with type 2 diabetes (14–16). While a small number of cross-sectional and short-term longitudinal reports showed higher expression levels of ANRIL (14,15) and MIAT (14–16) in the circulation of patients with advanced type 2 diabetes, previous work focused on the relationship of lncRNAs to established disease rather than future diabetes onset. The association between circulating lncRNAs and incident diabetes has important implications. First, their predictive potential and the wide availability of quantitative PCR devices might have relevance for risk stratification and disease prevention (17). Second, our results in humans support the notion that disease-specific epigenetic dysregulation precedes diabetes onset (18). Third, our study paves the way for mechanistic investigations of the identified lncRNAs in diabetes development and progression.
The link between lncRNA levels and impaired glycemic status is supported by their correlation with both 1) baseline FPG and HbA1c and 2) follow-up HbA1c values over the 7.5-year follow-up period. Our results suggest that ANRIL, RNCR3, MIAT, and PLUTO are part of a distinct lncRNA signature present several years before the clinical onset of type 2 diabetes and may be causally involved in the progressive loss of glucose homeostasis. Matching our results, PLUTO was found to be downregulated in pancreatic islets of patients with type 2 diabetes, where it modulates β-cell–specific transcriptional networks (12). However, in view of mechanistic evidence for a reciprocal modulation of lncRNAs and glucose levels (5,6,12,19,20), observational results from the current study need careful interpretation.
Our study has some limitations. First, it is limited by a relatively small sample size, yet benefits from its community-based study design with unique long-term follow-up data. Second, waist circumference and physical activity status were not available. Yet, our multivariable models were controlled for established confounders, including the CDRS, a validated diabetes risk assessment tool (13). Third, our literature search included primarily preclinical studies, given the limited evidence in humans. This may have affected the lncRNA selection. Fourth, given that no oral glucose tolerance test was performed, we cannot exclude the possibility that the selection criteria may have led to the inclusion of a small number of individuals with undetected preexisting diabetes. Finally, our study sample reflects a specific subgroup of the general population, and selection bias cannot be excluded with certainty. Caution is required when extrapolating the results to other age-groups and ethnicities.
Our proof-of-concept study shows for the first time that a set of lncRNAs is independently related to trajectories of impaired glucose control and future development of type 2 diabetes on top of HbA1c, FPG, BMI, and other established risk factors. Given the broad availability of quantitative PCR devices, our findings could have important clinical applications. Proactive approaches to risk stratification and early diabetes detection may improve patient outcomes and mitigate the socio-sanitary burden.
Article Information
Funding. F.A.W. is supported by the Lindenhof Foundation. A.Me. is the recipient of an International Grant from the Italian Society of Arterial Hypertension. S.A.M. and S.A. are the recipients of a Forschungskredit Candoc grant from the University of Zurich. P.R., P.F., M.H., and E.Gr. conducted the VITA study under the support of the Ludwig Boltzmann Institute of Aging Research Vienna, Austria. C.M.M. is funded by the Swiss National Science Foundation and the Swiss Heart Foundation. F.P. is the recipient of an H.H. Sheikh Khalifa bin Hamad Al Thani Foundation Assistant Professorship at the Faculty of Medicine, University of Zurich. This work was supported by the Swiss National Science Foundation (no. 310030_197557), the Swiss Heart Foundation (no. FF19045), the Stiftung für wissenschaftliche Forschung, the Olga Mayenfisch Foundation, the Swiss Life Foundation, the Kurt und Senta-Hermann Stiftung, the EMDO Stiftung and the Schweizerische Diabetes-Stiftung (to F.P.), the Holcim Foundation, the Swiss Heart Foundation, and the Swiss Life Foundation and the Gebauer Stiftung (to S.C.).
Duality of Interest. G.G.C. is coinventor on the International Patent WO/2020/226993 filed in April 2020. The patent relates to the use of antibodies which specifically bind IL-1α to reduce various sequelae of ischemia-reperfusion injury to the central nervous system. G.G.C. is a consultant to Sovida Solutions Limited. T.F.L. has, outside this work, received research and educational grants and in part honoraria from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Novartis, Sanofi, Servier, and CSL Vifor. C.M.M. has received research grants to the institution from Eli Lilly, AstraZeneca, Roche, Amgen, Novartis, Novo Nordisk, and MSD, including speaker or consultant fees. F.R. has not received personal payments from pharmaceutical companies or device manufacturers in the last three years (remuneration for the time spent in activities, such as participation as steering committee member of clinical trials and member of the Pfizer Research Award selection committee in Switzerland, were made directly to the University of Zurich). The Department of Cardiology (University Hospital of Zurich/University of Zurich) reports research, educational, and/or travel grants from Abbott, Amgen, AstraZeneca, Bayer, Berlin Heart, B. Braun, Biosense Webster, Biosensors Europe AG, Biotronik, Bristol Myers Squibb, Boehringer Ingelheim, Boston Scientific, Bracco, Cardinal Health Switzerland, Corteria, Daiichi Sankyo, Diatools AG, Edwards Lifesciences, Guidant Europe NV (BS), Hamilton Health Sciences, Kaneka Corporation, Kantar, Labormedizinisches Zentrum, Medtronic, MSD, Mundipharma Medical Company, Novartis, Novo Nordisk, Orion, Pfizer, Quintiles Switzerland Sarl, Sahajanand IN, Sanofi, Sarstedt AG, Servier, SIS Medical, SSS International Clinical Research, Terumo Deutschland, Trama Solutions, V-Wave, Vascular Medical, Vifor, Wissen.Plus, and ZOLL. The research and educational grants do not impact F.R.’s personal remuneration. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. F.P. and S.C. conceived the study. F.A.W., A.Me., F.P., and S.C. performed statistical analyses. S.A.M., A.Mo., E.Go., S.A., Y.P., and S.C. performed RNA measurements and generated the data. A.Me., N.R.P., P.R., P.F., M.H., G.P.F., A.V., S.M., F.R., and E.Gr. contributed to the project logistics. N.R.P., E.Go., P.R., P.F., M.H., T.F.L., G.G.C., C.M.M., G.P.F., A.V., S.M., F.R., and E.Gr. provided critical intellectual feedback during manuscript preparation. F.A.W. drafted the initial version of the manuscript. F.A.W., A.Me., F.P., and S.C. wrote the manuscript. All authors critically revised the manuscript and approved the final version. S.C. and F.P. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.22350793.
F.A.W., A.M., S.A.M., and N.R.P. contributed equally to this work.
F.P. and S.C. jointly directed the study.
References
- 1. Bellary S, Kyrou I, Brown JE, Bailey CJ. Type 2 diabetes mellitus in older adults: clinical considerations and management. Nat Rev Endocrinol 2021;17:534–548 [DOI] [PubMed] [Google Scholar]
- 2. Ahmad E, Lim S, Lamptey R, Webb DR, Davies MJ. Type 2 diabetes. Lancet 2022;400:1803–1820 [DOI] [PubMed] [Google Scholar]
- 3. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care 2012;35:2650–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dieter C, Lemos NE, Corrêa NRF, Assmann TS, Crispim D. The Impact of lncRNAs in diabetes mellitus: a systematic review and in silico analyses. Front Endocrinol (Lausanne) 2021;12:602597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Statello L, Guo CJ, Chen LL, Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol 2021;22:96–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. López-Noriega L, Rutter GA. Long non-coding RNAs as key modulators of pancreatic β-cell mass and function. Front Endocrinol (Lausanne) 2021;11:610213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fischer P, Jungwirth S, Krampla W, et al. Vienna Transdanube Aging “VITA”: study design, recruitment strategies and level of participation. J Neural Transm Suppl 2002:105–116 [DOI] [PubMed] [Google Scholar]
- 8. Jungwirth S, Zehetmayer S, Bauer P, Weissgram S, Tragl KH, Fischer P. Screening for Alzheimer’s dementia at age 78 with short psychometric instruments. Int Psychogeriatr 2009;21:548–559 [DOI] [PubMed] [Google Scholar]
- 9. Pugliese NR, Paneni F, Mazzola M, et al. Impact of epicardial adipose tissue on cardiovascular haemodynamics, metabolic profile, and prognosis in heart failure. Eur J Heart Fail 2021;23:1858–1871 [DOI] [PubMed] [Google Scholar]
- 10. Pugliese NR, Balletti A, Armenia S, et al. Ventricular-arterial coupling derived from proximal aortic stiffness and aerobic capacity across the heart failure spectrum. JACC Cardiovasc Imaging 2022;15:1545–1559 [DOI] [PubMed] [Google Scholar]
- 11. American Diabetes Association . 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020;43(Suppl. 1):S14–S31 [DOI] [PubMed] [Google Scholar]
- 12. Akerman I, Tu Z, Beucher A, et al. Human pancreatic β cell lncRNAs control cell-specific regulatory networks. Cell Metab 2017;25:400–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mendez CE, Walker RJ, Dawson AZ, Lu K, Egede LE. Using a diabetes risk score to identify patients without diabetes at risk for new hyperglycemia in the hospital. Endocr Pract 2021;27:807–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sathishkumar C, Prabu P, Mohan V, Balasubramanyam M. Linking a role of lncRNAs (long non-coding RNAs) with insulin resistance, accelerated senescence, and inflammation in patients with type 2 diabetes. Hum Genomics 2018;12:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Toraih EA, Abdelghany AA, Abd El Fadeal NM, Al Ageeli E, Fawzy MS. Deciphering the role of circulating lncRNAs: RNCR2, NEAT2, CDKN2B-AS1, and PVT1 and the possible prediction of anti-VEGF treatment outcomes in diabetic retinopathy patients. Graefes Arch Clin Exp Ophthalmol 2019;257:1897–1913 [DOI] [PubMed] [Google Scholar]
- 16. de Gonzalo-Calvo D, Kenneweg F, Bang C, et al. Circulating long-non coding RNAs as biomarkers of left ventricular diastolic function and remodelling in patients with well-controlled type 2 diabetes. Sci Rep 2016;6:37354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gadd DA, Hillary RF, McCartney DL, et al. Epigenetic scores for the circulating proteome as tools for disease prediction. eLife 2022;11:e71802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dayeh T, Tuomi T, Almgren P, et al. DNA methylation of loci within ABCG1 and PHOSPHO1 in blood DNA is associated with future type 2 diabetes risk. Epigenetics 2016;11:482–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cai R, Jiang J. LncRNA ANRIL silencing alleviates high glucose-induced inflammation, oxidative stress, and apoptosis via upregulation of MME in podocytes. Inflammation 2020;43:2147–2155 [DOI] [PubMed] [Google Scholar]
- 20. Yan B, Yao J, Liu JY, et al. lncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ Res 2015;116:1143–1156 [DOI] [PubMed] [Google Scholar]