Abstract
Purpose of review
Modern cancer therapies have allowed for a dramatic increase in the survival rates in both children and adults. However, a frequent and unfortunate side-effect of cancer therapy is a long-term decline in neurocognitive function. Specifically, cranial radiation therapy markedly alters memory processes, while chemotherapeutic agents are correlated with deficits in attention, concentration, and speed of information processing. Here, we describe the putative cellular etiologies of cancer treatment-induced cognitive decline, with an emphasis on the role of neural stem and precursor cell dysfunction.
Recent findings
New studies highlight the lasting effects of chemotherapy on memory, executive function, attention, and speed of information processing up to 20 years following chemotherapy. Cognitive decrements are associated with decreased white-matter integrity as well as alterations in stem cell function in humans and rodent models of cancer therapy. Genetic polymorphisms may underlie differential sensitivity of certain individuals to the neurological consequences of chemotherapy. Increasing data support the concept that disruption of normal neural stem and precursor cell function is an important causative factor for the cognitive deficits that result from cancer therapy in both children and adults.
Summary
Further studies are needed to elucidate the role of chemotherapy on cell-intrinsic processes and cellular microenvironments. Further, the effects of the new generation of targeted molecular therapies on neural stem and progenitor cell function remains largely untested. Understanding the mechanisms behind cancer therapy-induced damage to neural stem and precursor cell populations will elucidate neuroprotective and cell replacement strategies aimed at preserving cognition after cancer therapy.
Keywords: cancer, chemotherapy, cognition, neural stem cells, radiation
INTRODUCTION
Cancer therapy is profoundly beneficial; it saves, improves, and extends lives. Yet it is dangerous, causing cognitive side-effects in both children and adults that are lasting and debilitating. Advancements in cancer treatment are exemplified by the nearly 12 million survivors of cancer who live in the USA alone [1]. Although improvements in therapeutic protocols for childhood and adult cancers have resulted in a dramatic increase in cancer survivors, nearly 40% exhibit long-term sequelae of therapy [2]. The risk of chronic illness increases in those treated for central nervous system (CNS) tumors, with 91% reporting chronic conditions, including a debilitating neuropsychiatric syndrome characterized by impaired memory function, attention, speed of information processing, executive function, and multitasking [3].
COGNITIVE DYSFUNCTION IN CHILDREN AND ADULTS TREATED WITH RADIATION AND CHEMOTHERAPY
Children:
Many studies have demonstrated neurocognitive decrements associated with cranial radiation therapy, including increased incidence of impairments in memory, attention, and concentration in children treated for pediatric brain tumors or leukemia [4,5]. The long-term neurological impact of cancer therapy on survivors of childhood cancer is not surprising as much of the brain’s development occurs after birth. It has been recognized since the 1960s that the brain does not complete postnatal development until the end of the third decade of life, when the frontal lobes finish myelination [6,7]. Five-year survival rates of childhood acute lymphoblastic leukemia (ALL) are now over 80%, historically because of a combination of cranial irradiation and chemotherapy. (Most therapeutic protocols for ALL have eliminated prophylactic radiotherapy in an effort to reduce neurocognitive side-effects.) Survivors exhibit neurocognitive deficits in both processing speed and memory function, with those treated at younger ages exhibiting increased vulnerability to the neurocognitive effects of cancer treatments [8■]. High-risk patients requiring more aggressive therapy exhibit a moderate increase in neuropsychological deficits compared to standard risk patients, suggesting a dose dependency to cancer treatment-induced neurocognitive deficits [9]. Cranial radiation carries a particular risk to memory function, especially the process of forming new memories of events or facts that is subserved by the hippocampi in the medial temporal lobes. Chemotherapy exposure compounds this memory dysfunction by affecting another aspect of memory function – working memory, the conscious manipulation of information on short time scales necessary for reasoning and learning. Working memory function is highly dependent on certain frontal lobe regions and the integrity of white matter connections. Functional neuroimaging (fMRI) studies have shed light on the working memory dysfunction that follows chemotherapy. Neural activity is abnormal in the critical frontal lobes regions (prefrontal cortex and cingulate cortex) assessed by fMRI during a working memory task in survivors of childhood ALL treated with only chemotherapy compared to healthy controls, and the abnormal activity correlated with the difficulty of the task [10].
Adults:
The long-term effects of cancer therapy on adult cognition have been increasingly recognized. Radiation of the adult brain, particularly when the medial temporal lobes are involved in the radiation field, carries a high risk of memory dysfunction [11-14]. When patients are exposed only to cancer treatments limited to traditional chemotherapies, the symptoms of impaired speed of information processing, attention and concentration predominate [15,16]. Much of our understanding of the effects of chemotherapy on neurocognitive function in adults comes from breast cancer survivors. Five years after the cessation of breast cancer treatment, fMRI studies indicate that survivors had decreased activation of frontal lobe regions compared to controls. Those survivors treated with chemotherapy had less activation in the left caudal lateral prefrontal cortex and decreased processing speed compared to breast cancer survivors not exposed to chemotherapy. Interestingly, increased age and decreased education were correlated with the decrements in executive function seen in chemotherapy-treated survivors [17]. In another study assessing the impact of chemotherapy on breast cancer survivors 10 years following treatment, researchers similarly found decreased activity in frontal and medial temporal regions (the dorsolateral prefrontal cortex and parahippocampal gyrus) during executive function and episodic memory tasks, respectively, in participants who received chemotherapy and tamoxifen following local breast surgery and regional breast radiation compared to survivors without chemotherapy and tamoxifen treatments. Decreased cortical activity, assessed by fMRI, was also seen in bilateral posterior parietal cortex during the two neuropsychological tests, indicating possible deficits in visuospatial attention in addition to planning performance and memory function [18■]. Even more striking, a longer term follow-up study of breast cancer survivors treated with either cyclophophamide, methotrexate (MTX), or 5-fluorouracil (5-FU) demonstrated that chemotherapy treatment was associated with deficits in immediate and delayed verbal memory, processing speed, executive function, and psychomotor speed 20 years after therapy [19■].
Although many studies support the link between cancer therapy and deficits in hippocampal and subcortical white matter function, some fundamental questions remain to be addressed [20]. Many studies showing an association between neurocognitive deficits and chemotherapy are performed in humans in whom pretreatment baselines are not assessed. Noteworthy, a prospective, longitudinal study that compared cognition on pretreatment and post-treatment measures investigating the effects of 5-FU, doxorubicin, and cyclophosphamide on cognition in breast cancer patients found no significant group mean differences in cognition between pretreatment and post-treatment cognitive assessments. However, a significant decrease in learning, attention, and information processing speed following the cessation of treatment was found in a subset of women compared to their pretreatment levels [21]. One potential reason for the lack of group mean differences between pretreatment and post-treatment and further support for the necessity of pretreatment baseline testing is the evidence that tumors themselves can be associated with cognitive deficits in attention, executive function, and memory, especially in CNS tumors [22-24]. Many human studies also lack the proper control groups, whether healthy controls or non-chemotherapy-treated patients with the same tumor type [18■]. Further controls are needed for the influences of confounding variables, including hormonal treatments such as tamoxifen and glucocorticoid treatments, that independently affect learning, memory, and cell proliferation and differentiation [17,25,26]. Tamoxifen, a selective estrogen receptor modulator, is commonly used as part of breast cancer therapy but has been associated with deficits in verbal and visual memory, processing speed, and visuospatial ability in premenopausal women [27] and verbal memory and executive functioning in postmenopausal women [28], while glucocorticoids have been shown to suppress hippocampal cell proliferation and neurogenesis in rodent models [25,26].
The role of genetic markers in the predisposition of chemotherapeutic-induced cognitive dysfunction has become an important variable to consider. The presence of the human E4 allele of the apolipoprotein E gene in cancer survivors exposed to chemotherapeutic agents is associated with deficits in visual memory, spatial ability, and executive function compared to survivors without the E4 allele [29]. Breast cancer survivors who are carriers of the Val158Met variant of the gene for catechol-O-methyltransferase (COMT), an enzyme that plays a role in concentration of dopamine in the prefrontal cortex, exhibit decreased performance on tests of attention compared to healthy control individuals who are also carriers, suggesting an interaction between the polymorphism and chemotherapy [30■]. Providing further evidence of the importance of genetic variability in the association between chemotherapy and cognitive outcome, childhood survivors of ALL with polymorphisms in folate pathway genes have a 7.4-fold increase in developing attention-deficit disorder [31], and deficits in the ability to shift attention and processing speed [32■]. This is not surprising, given that MTX directly affects folate metabolism. These correlative studies suggest an association between cancer treatment and neurocognitive dysfunction that may be further mediated by genetic predispositions or environment (i.e. education).
CELLULAR ETIOLOGY
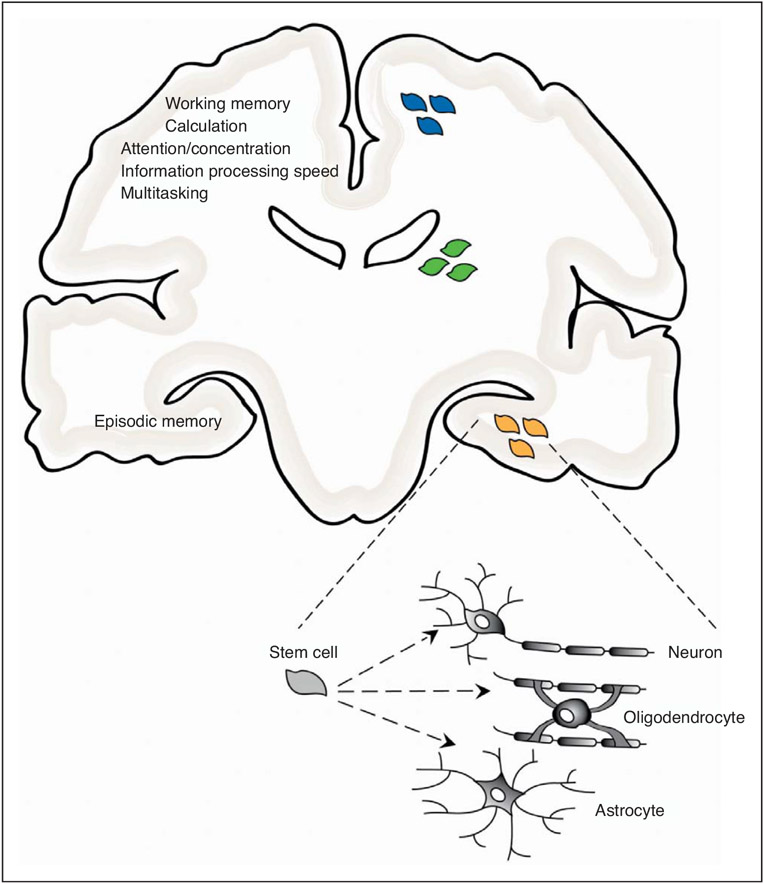
The constellation of neuropsychological symptoms attributed to cranial radiation and chemotherapy localize neuroanatomically to the hippocampus (memory) and subcortical white matter (attention, concentration and speed of information processing), two regions in which new cell production from neural precursor populations is critical to postnatal neurodevelopment and maintenance [33-35] (Fig. 1). Stem and progenitor cells, originating in the subventricular zone and dentate gyrus of the hippocampus, give rise to adult-born neurons and glial cells [36,37] (Fig. 1). Numerous lines of evidence suggest an association between postnatal neurogenesis and learning and memory function [38-40]. Learning improves the survival of postnatal-born neurons in the dentate gyrus [41,42]. Neurogenesis is also increased by exposure to enriched environments and voluntary exercise [43,44], two lifestyle variables associated with improved cognition [45]. Some of the strongest evidence implicating new neuron production in learning and memory processes is found in studies inhibiting neurogenesis. Using irradiation to abolish adult neurogenesis in rats during a critical period of training for a hippocampal-dependent task results in deficits in long-term memory formation, as tested using the Morris water maze [38]. Similarly, decreasing neurogenesis affects hippocampal-dependent tasks, such as trace memory tasks, but not hippocampal-independent tasks [46]. These studies point to a strong involvement of postnatally born neurons in hippocampal-dependent learning and memory function.
FIGURE 1.
Neural precursor populations in the postnatal brain include white matter oligodendrocyte progenitor cells (blue cells), subventricular zone stem and progenitor cells (green cells), and hippocampal stem and progenitor cells (orange cells). Cognitive functions putatively mediated by postnatal maintenance and maturation of neural precursor populations and negatively affected by cancer treatments are listed in the left half of the figure. Neural stem cells have the ability to differentiate into mature neurons, oligodendrocytes, or astrocytes as indicated in the lower right corner of the figure.
Subventricular zone neural stem cells also play a role in the production and maintenance of the glial progenitor cell population found in both white and gray matter. Oligodendroglial progenitor cells are found throughout the adult human brain and contribute to myelination during postnatal development and adult white matter maintenance and repair [47-49]. The marked impact of cancer therapies on neurocognitive function and the role of neural stem cells and glial progenitor cells in ongoing brain health and function highlight stem cells as a potential target for cancer therapy-related cognitive deficits.
ETIOLOGY OF CANCER TREATMENT-INDUCED MEMORY DYSFUNCTION
The molecular and cellular etiology of memory dysfunction following cranial radiation therapy has been thoroughly investigated. Cranial irradiation in rodents results in a specific blockade of hippocampal neurogenesis, which is mediated by neuroinflammatory disruption of the neurogenic microenvironment [50,51]. Of note, this decrease in neurogenesis is not attributed to the depletion of stem cell populations, but instead to perturbation of the microenvironment that normally supports neurogenesis. Radiation induces microglial inflammation and elaboration of proinflammatory cytokines that inhibit neuronal differentiation of stem cells [50-52]. In humans exposed to cranial radiation and systemic chemotherapy for acute myelogenous leukemia or medulloblastoma, similar inhibition of hippocampal neurogenesis and increases in microglial inflammation are also seen [53]. Promisingly, radiation-induced inflammatory inhibition of hippocampal neurogenesis can be mitigated by nonsteroidal anti-inflammatory therapy in preclinical rodent models [51,54]. On the basis of these findings, multiple clinical trials of nonsteroidal anti-inflammatory therapy or other proneurogenic strategies such as aerobic exercise [55,56] aimed at preserving memory function after cranial radiation therapy are currently underway in both children and adults.
Similarly, chemotherapeutic agents, such as cyclophosphamide [57], MTX [58], and doxorubicin [56], are associated with deficits in hippocampal-dependent memory and hippocampal neurogenesis. Cyclophosphamide and doxorubicin were found to decrease neurogenesis by 80–90%, and this decrease was associated with hippocampal-dependent memory deficits in a preclinical rodent model. Cyclophosphamide, but not doxorubicin, was also associated with a microglial inflammatory response [59■].
CANCER TREATMENT-INDUCED DEFICITS IN ATTENTION, CONCENTRATION, AND SPEED OF INFORMATION PROCESSING
The complex neural circuitry necessary for high-level cognitive and sensorimotor function matures throughout childhood and young adulthood. Central to the process of developing or strengthening a functional neural circuit is the generation of new glial cells for neuronal support, synapse formation, and myelination. It is well known that cancer therapy, especially during childhood, damages white matter integrity and that the degree of white matter damage correlates with cognitive deficits, such as impaired speed of information processing [60,61]. Recent advancements in technology have allowed researchers to more thoroughly investigate the impact of chemotherapy on the integrity of white matter tracts important to high-level cognition. Diffusion tensor imaging (DTI) allows for visualization and quantification of white matter integrity in the brain based on fractional anisotropy (directionality of water diffusion) and mean diffusivity (average amount of diffusion). White matter tracts associated with cognition are altered, as assessed by DTI, in chemotherapy-treated breast cancer patients compared to nontreated or healthy controls soon after cessation of treatment. This white matter degeneration of tracts in the frontal and temporal lobes is associated with deficits in neuropsychological tests of attention and information processing speed [62■].
Chemotherapeutic drugs, which selectively target dividing cells, disrupt normal neural precursor cell proliferation. The negative impact of chemotherapeutic agents on dividing stem and progenitor cells in the postnatal brain, including oligodendrocyte precursor cells (OPCs) in white and gray matter, may be especially detrimental to brain function. Glial progenitor cells are the major cycling cell population in the human brain and contribute to developmental myelination and likely to white matter maintenance throughout life [48,49,63,64].
The variability seen in human studies, as well as the difficulty of controlled, prospective human studies, points to the necessity of useful animal models to study the etiology of chemotherapy-induced neurocognitive dysfunction. Animal models have underscored the importance of stem cells in this process. It is known that pluripotent neural stem cells and multipotent or unipotent glial progenitor cells are exquisitely sensitive to traditional chemotherapeutic agents in rodents [65,66]. Many chemotherapeutic agents, including cisplatin [67] and 5-FU [68], decrease OPC proliferation and oligodendrogliogenesis. MTX is especially toxic to glial progenitor cells in frontal white matter that produce myelinating oligodendrocytes [65] and to neural stem cells [67,68] (Fig. 1). Short-term treatment with the antimetabolite chemotherapeutic agent, 5-FU, causes delayed CNS damage and degeneration that, unlike irradiation [50,51], is not associated with chronic inflammation or vascular damage. Instead, 5-FU appears to disrupt CNS progenitor cells and mature oligodendrocytes. Similarly, Schoenfeld et al. [69] found that oligodendrocytes are sensitive to rotenone, an inhibitor of mitochondrial oxidative phosphorylation, suggesting that cells with increased mitochondrial turnover, such as oligodendrocytes, may be selectively sensitive to antimetabolite chemotherapeutic agents. Indeed, antimetabolites, such as MTX and 5-FU, have been the chemotherapeutic agents most strongly linked clinically to poor cognitive outcome after chemotherapy. This targeted depletion of oligodendrocytes and their precursors by antimetabolite chemotherapeutic agents may play a role in the maldevelopment or degeneration of white matter tracts seen in subcortical structures of cancer survivors, especially in children who are still undergoing active developmental myelination.
CONCLUSION
Future studies aimed at elucidating the molecular and cellular etiology of neurocognitive dysfunction following cancer treatment are imperative to improving the lives of millions of cancer survivors. Little is known about the mechanisms of chemotherapy-induced damage to neural stem and precursor cell populations. Nor is it known whether the damage results from cell-intrinsic or microenvironmental effects of the chemotherapeutic agents. Furthermore, the effects of the new generation of targeted molecular therapies on neural stem and progenitor cell function are virtually untested, despite the concerning fact that these drugs frequently target signaling pathways critical not only to cancer cells, but also to neural stem and progenitor cells. Neuroprotective or cell replacement strategies are needed to address the long-term effects of cancer therapies on neural stem and precursor populations in the brains of both children and adults.
KEY POINTS.
Cancer therapy involving cranial radiation and chemotherapy is associated with neurocognitive deficits, including disruptions in memory, attention, and speed of information processing.
Cognitive dysfunction from chemotherapy alone is evident in some patient populations even 20 years after treatment.
Damage to neural stem and precursor cell function is believed to result in cancer therapy-induced cognitive dysfunction.
Genetic polymorphisms may predispose some individuals to more severe radiation or chemotherapy-associated cognitive dysfunction.
Understanding the mechanisms behind damage to neural stem or precursor cell populations and the neurogenic or gliogenic microenvironment are imperative to ultimately combat the negative effects of cancer therapy on brain integrity and function.
Acknowledgements
The authors gratefully acknowledge the support from the National Institute of Neurological Disorders and Stroke (5K08NS07092602), the Stanford NIH/NCRR CTSA (UL1 RR025744) and the Lucile Packard Foundation for Children’s Health.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 762).
- 1.American Cancer Society. Cancer Facts and Figures 2012. Atlanta: American Cancer Society; 2012. [Google Scholar]
- 2.Oeffinger KC, Nathan PC, Kremer LC. Challenges after curative treatment for childhood cancer and long-term follow up of survivors. Hematol Oncol Clin North Am 2010; 24:129–149. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong GT, Liu Q, Yasui Y, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst 2009; 101:946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langer T, Martus P, Ottensmeier H, et al. CNS late-effects after ALL therapy in childhood. Part III. Neuropsychological performance in long-term survivors of childhood ALL: impairments of concentration, attention, and memory. Med Pediatr Oncol 2002; 38:320–328. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong GT, Jain N, Liu W, et al. Region-specific radiotherapy and neuropsychological outcomes in adult survivors of childhood CNS malignancies. Neuro Oncol 2010; 12:1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yakovlev P The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain in early life. Oxford: Blackwell Scientific Publications; 1967. pp. 3–70. [Google Scholar]
- 7.Benes FM. Myelination of cortical-hippocampal relays during late adoles-cence. Schizophr Bull 1989; 15:585–593. [DOI] [PubMed] [Google Scholar]
- 8.■. Edelstein K, D’Agostino N, Bernstein LJ, et al. Long-term neurocognitive outcomes in young adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol 2011; 33:450–458. This study analyzed 24 adults who were treated with cranial radiation and chemotherapy following the diagnosis of acute lymphoblastic leukemia during childhood. The authors found that younger age of diagnosis and increased length of time since treatment were correlated with lower scores on neurocognitive tests, specifically if diagnosis of ALL occurred before the age of 5 years old.
- 9.Waber DP, Queally JT, Catania L, et al. Neuropsychological outcomes of standard risk and high risk patients treated for acute lymphoblastic leukemia on Dana-Farber ALL consortium protocol 95-01 at 5 years postdiagnosis. Pediatr Blood Cancer 2011; 58:758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson KE, Livesay KL, Campbell LK, et al. Working memory in survivors of childhood acute lymphocytic leukemia: functional neuroimaging analyses. Pediatr Blood Cancer 2010; 54:585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee PW, Hung BK, Woo EK, et al. Effects of radiation therapy on neuropsychological functioning in patients with nasopharyngeal carcinoma. J Neurol Neurosurg Psychiatry 1989; 52:488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abayomi OK. Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol 1996; 35:659–663. [DOI] [PubMed] [Google Scholar]
- 13.Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol 1994; 12:627–642. [DOI] [PubMed] [Google Scholar]
- 14.Surma-aho O, Niemela M, Vilkki J, et al. Adverse long-term effects of brain radiotherapy in adult low-grade glioma patients. Neurology 2001; 56:1285–1290. [DOI] [PubMed] [Google Scholar]
- 15.Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol 2011; 12:703–708. [DOI] [PubMed] [Google Scholar]
- 16.Meyers CA, Abbruzzese JL. Cognitive functioning in cancer patients: effect of previous treatment. Neurology 1992; 42:434–436. [DOI] [PubMed] [Google Scholar]
- 17.Kesler SR, Kent JS, O’Hara R. Prefrontal cortex and executive function impairments in primary breast cancer. Arch Neurol 2011; 68:1447–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.■. De Ruiter MB, Reneman L, Boogerd W, et al. Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum Brain Mapp 2011; 32:1206–1219. This study reports on the impactofchemotherapy on brain activation and cognition in breast cancer survivors 10 years after treatment. All patients received surgery and local radiation, but those who also received chemotherapy exhibited decreased activity in the prefrontal cortex, parahippocampal gyrus, and parietal cortex during neuropsychological tests associated with executive functioning and memory compared to nonchemotherapy-treated survivors.
- 19.■. Koppelmans V, Breteler MM, Boogerd W, et al. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol 2012; 30:1080–1086. In this study, women who received adjuvant chemotherapy for breast cancer were assessed for neurocognitive performance 20 years after the cessation of treatment. When compared to healthy noncancer controls, women who underwent chemotherapy treatment performed worse on immediate and delayed memory tests, executive functioning, and had slower processing and psychomotor speed.
- 20.Abrey LE. The impact of chemotherapy on cognitive outcomes in adults with primary brain tumors. J Neurooncol 2012; 108:285–290. [DOI] [PubMed] [Google Scholar]
- 21.Wefel JS, Lenzi R, Theriault RL, et al. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer 2004; 100:2292–2299. [DOI] [PubMed] [Google Scholar]
- 22.Correa DD, Maron L, Harder H, et al. Cognitive functions in primary central nervous system lymphoma: literature review and assessment guidelines. Ann Oncol 2007; 18:1145–1151. [DOI] [PubMed] [Google Scholar]
- 23.Hermelink K, Untch M, Lux MP, et al. Cognitive function during neoadjuvant chemotherapy for breast cancer: results of a prospective, multicenter, longitudinal study. Cancer 2007; 109:1905–1913. [DOI] [PubMed] [Google Scholar]
- 24.Seigers R, Pourtau L, Schagen SB, et al. Inhibition of hippocampal cell proliferation by methotrexate in rats is not potentiated by the presence of a tumor. Brain Res Bull 2010; 81:472–476. [DOI] [PubMed] [Google Scholar]
- 25.Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience 1994; 61:203–209. [DOI] [PubMed] [Google Scholar]
- 26.Gould E, Cameron HA, Daniels DC, et al. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J Neurosci 1992; 12:3642–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer JL, Trotter T, Joy AA, Carlson LE. Cognitive effects of tamoxifen in premenopausal women with breast cancer compared to healthy controls. J Cancer Surviv 2008; 2:275–282. [DOI] [PubMed] [Google Scholar]
- 28.Schilder CM, Seynaeve C, Beex LV, et al. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. J Clin Oncol 2010; 28:1294–1300. [DOI] [PubMed] [Google Scholar]
- 29.Ahles TA, Saykin AJ, Noll WW, et al. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology 2003; 12:612–619. [DOI] [PubMed] [Google Scholar]
- 30.■. Small BJ, Rawson KS, Walsh E, et al. Catechol-O-methyltransferase genotype modulates cancer treatment-related cognitive deficits in breast cancer survivors. Cancer 2011; 117:1369–1376. In this study, the authors compared breast cancer survivors treated with radiation and chemotherapy to healthy controls on tests of cognition and genetic variability in catechol-O-methyltransferase (COMT), a pathway related tothe concentrations of dopamine in the prefrontal cortex. Individuals with alleles (COMT−Val+) that result in increased degradation of dopamine exhibited deficits in attention, motor speed and verbal ability compared to carriers of alleles coding for normal processing of dopamine (COMT−Met). Interestingly, an interaction between COMT−Val+ genotype and chemotherapy exists, as carriers treated with chemotherapy performed worse on attention tests compared to healthy control carriers.
- 31.Krull KR, Okcu MF, Potter B, et al. Screening for neurocognitive impairment in pediatric cancer long-term survivors. J Clin Oncol 2008; 26:4138–4143. [DOI] [PubMed] [Google Scholar]
- 32.■. Kamdar KY, Krull KR, El-Zein RA, et al. Folate pathway polymorphisms predict deficits in attention and processing speed after childhood leukemia therapy. Pediatr Blood Cancer 2011; 57:454–460. This study reports an association between folate pathway polymorphisms and attention and processing speed deficits in children treated with chemotherapy for childhood acute lymphoblastic leukemia. Patients with folate pathway polymorphisms exhibited deficits in attention and processing speed, and survivors with greater than 6 folate pathway polymorphisms had lower neurocognitive scores than those with less than 6, suggesting multiple folate pathway risk alleles may predict neurocognitive disorders following chemotherapy treatment in ALL.
- 33.Brown MS, Stemmer SM, Simon JH, et al. White matter disease induced by high-dose chemotherapy: longitudinal study with MR imaging and proton spectroscopy. AJNR Am J Neuroradiol 1998; 19:217–221. [PMC free article] [PubMed] [Google Scholar]
- 34.Stemmer SM, Stears JC, Burton BS, et al. White matter changes in patients with breast cancer treated with high-dose chemotherapy and autologous bone marrow support. AJNR Am J Neuroradiol 1994; 15:1267–1273. [PMC free article] [PubMed] [Google Scholar]
- 35.Monje M, Dietrich J. Cognitive side effects of cancer therapy demonstrate a functional rolefor adult neurogenesis. Behav Brain Res 2011; 227:376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science 1977; 197:1092–1094. [DOI] [PubMed] [Google Scholar]
- 37.Kaplan MS, Bell DH. Mitotic neuroblasts in the 9-day-old and 11-month-old rodent hippocampus. J Neurosci 1984; 4:1429–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience 2005; 130:843–852. [DOI] [PubMed] [Google Scholar]
- 39.Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus 2006; 16:216–224. [DOI] [PubMed] [Google Scholar]
- 40.Clelland CD, Choi M, Romberg C, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 2009; 325:210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gould E, Beylin A, Tanapat P, et al. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci 1999; 2:260–265. [DOI] [PubMed] [Google Scholar]
- 42.Leuner B, Mendolia-Loffredo S, Kozorovitskiy Y, et al. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J Neurosci 2004; 24:7477–7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature 1997; 386:493–495. [DOI] [PubMed] [Google Scholar]
- 44.Van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA 1999; 96:13427–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Angevaren M, Aufdemkampe G, Verhaar HJ, et al. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev 2008; CD005381. [DOI] [PubMed] [Google Scholar]
- 46.Shors TJ, Miesegaes G, Beylin A, et al. Neurogenesis in the adult is involved in the formation of trace memories. Nature 2001; 410:372–376. [DOI] [PubMed] [Google Scholar]
- 47.Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci 2003; 24:476–488. [DOI] [PubMed] [Google Scholar]
- 48.Chang A, Nishiyama A, Peterson J, et al. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci 2000; 20:6404–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geha S, Pallud J, Junier MP, et al. NG2+/Olig2+ cells are the major cycle-related cell population of the adult human normal brain. Brain Pathol 2010; 20:399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med 2002; 8:955–962. [DOI] [PubMed] [Google Scholar]
- 51.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science 2003; 302:1760–1765. [DOI] [PubMed] [Google Scholar]
- 52.Ramanan S, Kooski M, Zhao W. The PPARα agonist fenofibrate preserves hippocampal neurogenesis and inhibits microglial activation after whole-brain irradiation. Int J Radiat Oncol Biol Phys 2009; 75:870–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Monje ML, Vogel H, Masek M, et al. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol 2007; 62:515–520. [DOI] [PubMed] [Google Scholar]
- 54.Voloboueva LA, Lee SW, Emery JF, et al. Mitochondrial protection attenuates inflammation-induced impairment of neurogenesis in vitro and in vivo. J Neurosci 2010; 30:12242–12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naylor AS, Bull C, Nilsson MK, et al. Voluntary running rescues adult hippocampal neurogenesis after irradiation of the young mouse brain. Proc Natl Acad Sci USA 2008; 105:14632–14637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong-Goodrich SJ, Pfau ML, Flores CT, et al. Voluntary running prevents progressive memory decline and increases adult hippocampal neurogenesis and growth factor expression after whole-brain irradiation. Cancer Res 2010; 70:9329–9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang M, Kim JS, Song MS, et al. Cyclophosphamide impairs hippocampus-dependent learning and memory in adult mice: Possible involvement of hippocampal neurogenesis in chemotherapy-induced memory deficits. Neurobiol Learn Mem 2010; 93:487–494. [DOI] [PubMed] [Google Scholar]
- 58.Seigers R, Schagen SB, Coppens CM, et al. Methotrexate decreases hippocampal cell proliferation and induces memory deficits in rats. Behav Brain Res 2009; 201:279–284. [DOI] [PubMed] [Google Scholar]
- 59.■. Christie LA, Acharya MM, Parihar VK, et al. Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin Cancer Res 2012; 18:1954–1965. Using a rat model, chronic administration of chemotherapy drugs was shown to impair hippocampal-dependent memory, as well as reduce neurogenesis by 80–90%. Activated microglia were associated with the administration of the chemotherapeutic agent cyclophosphamide but not doxorubicin.
- 60.Porto L, Preibisch C, Hattingen E, et al. Voxel-based morphometry and diffusion-tensor MR imaging of the brain in long-term survivors of childhood leukemia. Eur Radiol 2008; 18:2691–2700. [DOI] [PubMed] [Google Scholar]
- 61.Aukema EJ, Caan MW, Oudhuis N, et al. White matter fractional anisotropy correlates with speed of processing and motor speed in young childhood cancer survivors. Int J Radiat Oncol Biol Phys 2009; 74:837–843. [DOI] [PubMed] [Google Scholar]
- 62.■. Deprez S, Amant F, Yigit R, et al. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Hum Brain Mapp 2011; 32:480–493. Using diffusion tensor imaging and tests of cognitive performance, the authors found decreased myelin density in frontal andtemporal white mattertracts in breast cancer survivors treated with chemotherapy compared to healthy controls and nonchemotherapy-treated survivors. The decrements in white matter integritywere correlated with deficits in attention and motor processing speeds.
- 63.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell 2008; 132:645–660. [DOI] [PubMed] [Google Scholar]
- 64.Weiss S, Dunne C, Hewson J, et al. MultipotentCNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci 1996; 16:7599–7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morris GM, Hopewell JW, Morris AD. A comparison of the effects of methotrexate and misonidazole on the germinal cells of the subependymal plate of the rat. Br J Radiol 1995; 68:406–412. [DOI] [PubMed] [Google Scholar]
- 66.Rzeski W, Pruskil S, Macke A, et al. Anticancer agents are potent neurotoxins in vitro and in vivo. Ann Neurol 2004; 56:351–360. [DOI] [PubMed] [Google Scholar]
- 67.Dietrich J, Han R, Yang Y, et al. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol 2006; 5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han R, Yang YM, Dietrich J, et al. Systemic 5-fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. J Biol 2008; 7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schoenfeld R, Wong A, Silva J, et al. Oligodendroglial differentiation induces mitochondrial genes and inhibition of mitochondrial function represses oligodendroglial differentiation. Mitochondrion 2010; 10:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]