Abstract
Background
The benefits of breast conserving surgery for breast cancer patients are well established. To achieve adequate margins of excision, intraoperative management of breast margins is a critical factor through reducing reoperation for inadequate positive margin excision and associated morbidity and cost. Radiofrequency spectroscopy is a technology that could significantly reduce positive margins when used intraoperatively as an adjunct to other margin management methods.
Methods
A meta‐analysis was completed with 10 publications comparing use of radiofrequency spectroscopy technology (MarginProbe) with standard margin assessment procedures. Three randomized controlled studies and seven retrospective studies comparing MarginProbe to historical controls were included. The primary endpoint was reduction of re‐excision rates. Statistical significance level was set at the two‐sided 5% level corresponding to two‐sided 95% confidence intervals (CIs) of the pooled relative risk estimates.
Results
A total of 2335 patients from 10 publications were included in this meta‐analysis. The overall relative reduction in re‐excision rate was 0.49 (95% CI: 0.38–0.64, p < 0.001). Statistical methods were used to examine publication bias.
Conclusion
Despite the limited randomized controlled trials available comparing radiofrequency spectroscopy to standard operation procedures, the data from the 10 studies demonstrate a statistically significant reduction in re‐excision rate of 49% for MarginProbe usage, currently the only technology indicated for intraoperative identification of breast cancer tissue at the lumpectomy specimen margin.
Keywords: breast cancer, breast conserving surgery, MarginProbe, meta‐analysis, re‐excision
This meta‐analysis investigated whether radiofrequency spectroscopy technology (MagniProbe) reduces positive margins when used intraoperatively and contributes to fewer reoperations after breast conserving surgery. We concluded based on 10 studies, three randomized controlled and seven retrospective studies, that the MagniProbe usage provided significant reduction in re‐excision rate of 49% after lumpectomy and it is the only technology showing intraoperative breast cancer tissue at the lumpectomy specimen margin.
INTRODUCTION
Breast conservation therapy, including lumpectomy and sentinel lymph node biopsy followed by radiation therapy, is the best treatment plan for women with early‐stage breast cancer. The goal of lumpectomy is to completely excise the tumor with negative margins while maintaining acceptable cosmesis. 1 Positive surgical margins result in a two‐fold increased rate of local recurrence, a risk not mitigated by adjuvant therapy. 2 In an effort to facilitate clearance of surgical margins, surgeons have utilized a wide variety of intraoperative techniques with variable levels of success, such as specimen imaging, full cavity shave margins, pathological gross assessment, frozen section, and touch preparation. 3 Despite these efforts, surgical re‐excisions, as a result of positive margins, are still termed “the other breast cancer epidemic” with contemporary studies reporting rates exceeding 20% in patients with invasive ductal carcinoma (IDC), and often greater in invasive lobular carcinoma (ILC) and ductal carcinoma in situ (DCIS). 4 , 5 In 2014, the SSO‐ASTRO Margin Guideline was developed defining a positive margin for invasive cancer as “no tumor on ink.” 6 Although guideline adoption has resulted in a decrease of re‐excision rate, multiple studies continue to report re‐excision rates between 14% and 22% for invasive cancer. 7 , 8 , 9 More than this, a 2018 review of Medicare beneficiaries reported a 17.2% re‐excision rate among 5337 surgeons in the post‐guideline era, including a re‐excision rate more than 30% among 17.5% of surgeons. This study also found that overall re‐excision rates after breast conserving surgery (BCS) vary widely among surgeons from 0% to 91.7%. 10 All these findings emphasize the need for technologies that improve margin evaluation and clearance at the time of initial BCS.
With the goal of enhancing the surgeon's ability to achieve histologically clear margins at the initial operation, the MarginProbe device was developed as an adjunct device to provide real‐time intraoperative assessment of the excised breast specimen margins using radiofrequency spectroscopy technology. 11 While use of radiofrequency spectroscopy technology does not omit postoperative pathological assessment of the excised breast specimen, its use allows the surgeon to identify and immediately excise positive margins in the operating room, thereby reducing the need for reoperation due to positive surgical margins. 12 , 13 Until now, MarginProbe is the only FDA approved device for intraoperative margin assessment at the time of BCS.
Multiple studies have evaluated the impact of MarginProbe device on achieving negative surgical margins when combined with standard of care (SOC) procedures. 12 , 14 These studies demonstrated a 20%–80% relative reduction in the rate of margin re‐excision when compared to BCS performed without MarginProbe. However, despite the demonstrated ability of radiofrequency spectroscopy technology to decrease the rate of margin re‐excision, variance in the inclusion criteria, trial design, surgeons experience, and magnitude of benefit across various studies have held back rapid adoption of MarginProbe. To obtain a more precise estimate of the ability of the MarginProbe device reducing the need for margin re‐excision, we performed a systematic literature review and meta‐analysis of the randomized and “real‐world” studies of radiofrequency spectroscopy technology with the MarginProbe.
METHODS
Search strategy and selection criteria
We searched the PubMed MEDLINE database on December 31, 2022 using the search terms “MarginProbe,” “intraoperative margin assessment,” “radiofrequency spectroscopy,” and “breast cancer surgery.” Selection criteria included English language, peer‐reviewed publications, margin assessment using the MarginProbe and margin re‐excision rates must be reported. Repeated studies and studies without re‐excision rates or comparison groups were excluded. The PRISMA guideline was followed to report the including/excluding studies. 15
Data extraction
Two investigators reviewed and selected the papers independently (JW and LZ). If these two investigators had any disagreement, a third investigator (ZP) would review the publication again and make the final decision. Data extracted included the following items: First author's names, publication journal, year of publication, study design, number of patients, re‐excision rates of control group, re‐excision rate of MarginProbe group, and relative reduction rate. All the extracted data were also cross‐checked by JW and LZ.
Statistical analysis
Data were analyzed with the Metafor package in R Foundation for Statistical Computing, (Vienna, Austria) software version 4.2.1. 16 Statistical significance level was set at the two‐sided 5% level corresponding to two‐sided 95% confidence intervals of the pooled relative risk estimates.
Potential publication bias was examined using funnel plots and by Egger's test. 17 , 18 The funnel plot shows the effect size of the different studies on the x‐axis and an estimate of the sample size on the y‐axis. Small studies should have higher variability in estimates of relative risk compared with larger studies, and divergence from this pattern may indicate the presence of publication bias. Egger's test could test the asymmetry of the funnel plot. Sensitivity analyses were performed by excluding each single study from analyses, one at a time, and repeating the whole meta‐analysis. This shows how each individual study affects the overall estimate of the rest of the studies. 19
RESULTS
Characteristics of included studies
Searching PubMed using the search terms yielded a total of 7333 items: MarginProbe (25 items), intraoperative margin assessment (2363 items), radiofrequency spectroscopy (1806 items), and breast cancer surgery (3139). There was a total of 2940 duplicate publications across search terms, leaving 4393 items. Limiting articles to MarginProbe related, 28 items remained. A further 18 articles were excluded from the current analysis because they did not have a control group or were lacking experimental design or data information (Figure 1). Finally, 10 studies were included in the final meta‐analysis (Table 1).
FIGURE 1.
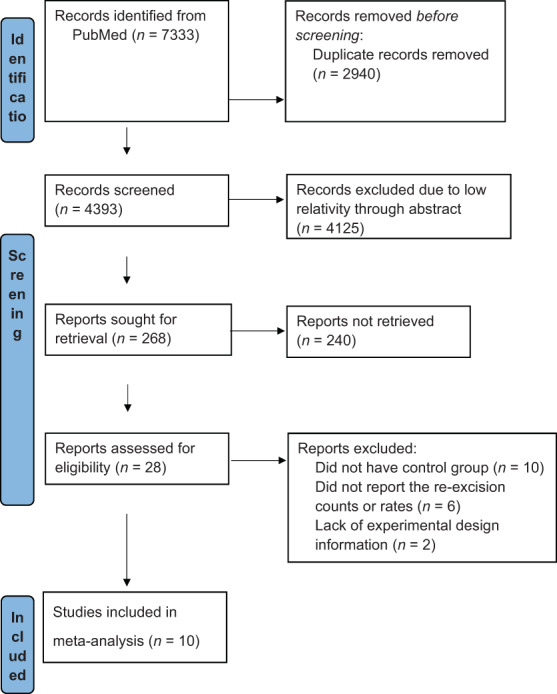
Publication selection flow chart following the PRISMA guideline.
TABLE 1.
Characteristics of all 10 eligible studies included in meta‐analysis.
| Publication | Study type | Total N | Control group | MarginProbe group | Relative reduction rate, % | ||||
|---|---|---|---|---|---|---|---|---|---|
| Event | Negative | Re‐excision rate, % | Event | Negative | Re‐excision rate, % | ||||
| Allweis et al. 21 | RCT | 293 | 19 | 131 | 12.7 | 8 | 135 | 5.6 | 55.8 |
| Schnabel et al. 20 | RCT | 596 | 77 | 221 | 25.8 | 59 | 239 | 19.8 | 23.4 |
| Thill et al. 26 | Treatment vs. historical data | 109 | 26 | 41 | 38.8 | 7 | 35 | 16.7 | 57.1 |
| Sebastian et al. 25 | Treatment vs. historical data | 351 | 48 | 138 | 25.8 | 16 | 149 | 9.7 | 62.4 |
| Blohmer et al. 23 | Treatment vs. historical data | 322 | 51 | 121 | 29.7 | 22 | 128 | 14.7 | 50.5 |
| Coble & Reid, 27 | Treatment vs. historical data | 336 | 30 | 169 | 15.1 | 9 | 128 | 6.6 | 56.4 |
| Kupstas et al. 24 | Treatment vs. historical data | 240 | 18 | 102 | 15.0 | 7 | 113 | 5.8 | 61.1 |
| Geha et al. 22 | RCT | 46 | 8 | 15 | 34.8 | 1 | 22 | 4.3 | 87.5 |
| LeeVan et al. 29 | Treatment vs. historical data | ‐ | ‐ | ‐ | 8.6 | 4 | 56 | 6.7 | 22.5 |
| Cen et al. 28 | Treatment versus historical data | 42 | 8 | 18 | 30.8 | 1 | 15 | 6.3 | 79.7 |
Abbreviation: RCT, randomized controlled trial.
Meta‐analysis of the primary endpoints
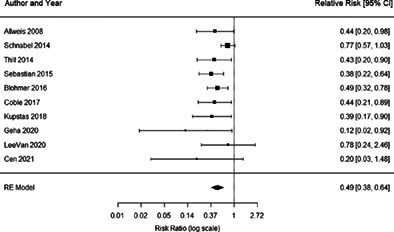
Ten studies were included in the meta‐analysis for analyzing the impact of the MarginProbe device on achievement of negative surgical margins. Of those, three studies are randomized controlled trials (RCTs) and seven are treatment with MarginProbe versus historical studies. Based on the meta‐analysis results, the overall relative reduction in re‐excision rate was 0.49 (95% CI: 0.38–0.64, p < 0.001) (Figure 2).
FIGURE 2.
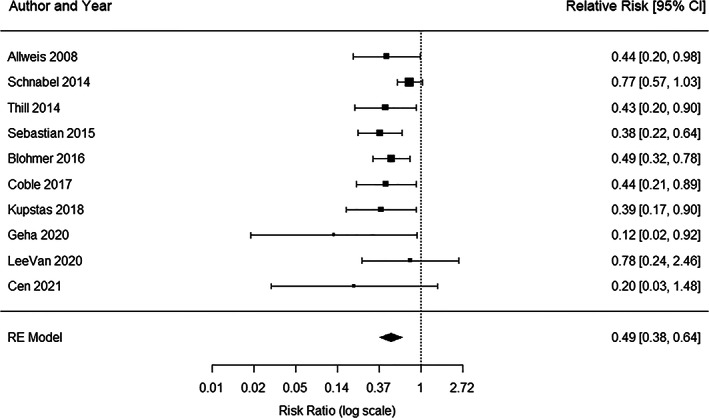
Forest plot results of all 10 studies used in meta‐analysis. Each black square represents the relative risk of having re‐excision, as calculated for each study and listed in the column “Relative Risk” on the right.The horizontal lines to the left and right of each square represent the lower and upper confidence limits, and the actual values are listed on the right. The pooled risk ratio is represented in the bottom row with black diamond. The left and right points of the diamond indicate the confidence limits.
A sensitivity analysis was performed to confirm the robustness of our findings and to confirm that the outcome of our meta‐analysis could not be attributed to the disproportionate weight of any single study. We recalculated the pooled risk estimates for the remainder of the studies by omitting one study at a time, which resulted in little change of the observed risk estimates from 0.43 (95% CI: 0.34–0.55) to 0.52 (95% CI: 0.39–0.69), all statistically significant less than 1. This confirms the findings and indicates that the effect of re‐excision is not substantially modified by any one study.
Publication bias was assessed using the Egger test, which suggested there is no publication bias (p = 0.0774) in current meta‐analysis. Visual inspection of contour‐enhanced funnel plots (Figure 3) also did not identify substantial asymmetry except for one study 20 which fell outside of the funnel.
FIGURE 3.
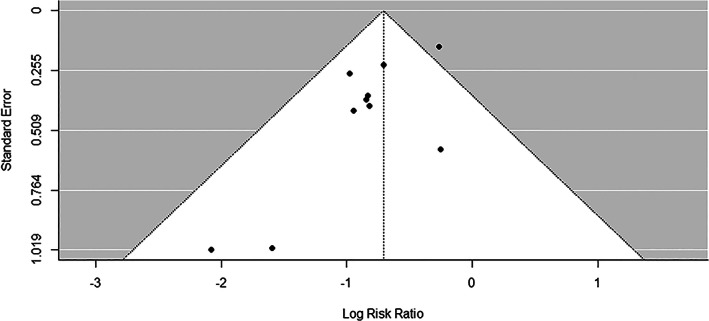
Funnel plot results of all 10 studies used in meta‐analysis. Each dot represents a study. The y‐axis is the standard error of the effect estimate. Larger studies with higher power are placed towards the top. Lower powered studies are placed towards the bottom. The x‐axis shows the risk ratio for the study. The plot should ideally resemble a pyramid funnel, with scatter due to sampling variation. The shape is expected because the studies have a wide range of standard errors.
DISCUSSION
Ten studies (three RCT and seven historical cohort studies) were examined through a meta‐analysis, which demonstrated the benefits of the MarginProbe for reducing the burden of margin re‐excision following BCS.
The MAST and Pivotal trials randomized patients to SOC alone versus SOC plus MarginProbe. 20 , 21 The MAST trial SOC protocol permitted intraoperative gross and microscopic examination of the primary specimen, while the Pivotal trial did not. Furthermore, the Pivotal trial was limited to patients with nonpalpable disease, whereas participants in the MAST trial had nearly equal distribution of palpable and nonpalpable disease. Geha et al. also reported RCT (SOC alone vs. SOC plus MarginProbe) results in their own institution, but with much smaller sample size. 22 Blohmer et al. Kupstas et al. and Sebastian et al. compared re‐excision rates with MarginProbe use to historical controls in patients with IDC, ILC, and DCIS, whereas Thill et al. restricted its single arm analysis to patients with DCIS alone. 23 , 24 , 25 , 26 Coble et al. was unique in its comparison of MarginProbe use to full cavity shave 27 and Cen et al. focused the comparison (with MarginProbe vs. without MarginProbe) on patients without complete response following neoadjuvant chemotherapy who underwent BCS. 28 LeeVan et al. compared MarginProbe surgery results to the prior year BCS results using just standard operating procedures. 29 All these studies also differ in participating surgeons, from as few as 1–5 surgeons in eight studies to between 35 and 53 surgeons in the two large RCTs, reflecting the potential for “real world” technical variability across surgeons and study sites. However, despite these differences, the sensitivity analysis confirms the robustness of the pooled data and demonstrates that intraoperative use of the MarginProbe achieves significant reductions in the rate of margin re‐excision than by other techniques for intraoperative margin assessment.
As is common with meta‐analyses, it is important to note that in these results there may be confounding factors that were not included in current analysis. Within the current meta‐analysis, the largest study, Schnabel et al. 20 showed some publication bias based on the Egger test, with a p‐value slightly over 0.05 (p = 0.0774). However, the sensitivity analysis demonstrated that the inclusion or exclusion of this study did not affect the results of the whole meta‐analysis. Additionally, there were several MarginProbe studies that could not be included in the analysis, due to the significant differences in risk factors, selection criteria and data availability. Despite these potential shortcomings, the results demonstrate the benefit of radiofrequency spectroscopy use in reducing the burden of margin re‐excision by 50%.
The need for re‐excision of surgical margins is driven by the inherent limitations of technology to efficiently assess the actual microscopic extent of malignancy intraoperatively. Although microscopic margin assessment practices, such as frozen section and touch preparation, can detect microscopic disease at the surgical margin, the technical limitations of these methods (e.g., issues with freezing fatty breast tissue, inability to evaluate the entire specimen margin surface, etc.) are resource intensive, costly, and not practical to perform in the span of most lumpectomy procedures. While not eliminating the need for microscopic margin analysis, radiofrequency spectroscopy overcomes several of these challenges by providing a portable, hand‐held device that can examine the entire face of the surgical specimen using a 7 mm sensor that provides immediate feedback from each measurement. The obvious advantage to the surgeon utilizing MarginProbe is that actionable information provided by the device can permit selective intraoperative excision of device‐detected positive margins, thereby reducing the need for reoperation.
Maintaining local control while maximizing cosmetic outcome is the essential of BCS. While wide excisions and full cavity shaving have demonstrated improvement in reducing reoperation rates, the increased volume of tissue resected to achieve negative margins can have implications on long‐term cosmetic outcomes. 4 Use of radiofrequency spectroscopy has raised a similar concern in that the potential of false positive readings may lead to unnecessary excision of surgical margins, risking an impact to breast cosmesis. 14 , 30 When assessing the impact of additional shaves taken with use of radiofrequency spectroscopy, multiple studies report little to no increase in total volume of tissue removed and no effect on cosmesis. 20 , 21
In addition to increasing the risk of developing breast cancer, high breast density poses a challenge to efforts to obtain clear lumpectomy margins. 31 Researchers at Memorial Sloan Kettering found that on multivariate analysis, high breast density was significantly associated with increased odds of re‐excision (OR 1.37, 95% CI: 1.00–1.86). 32 Although radiofrequency spectroscopy may be used to reduce re‐excision rates in all breast density types, false positive radiofrequency spectroscopy readings are more commonly observed in high density breast tissue due to similarities in the dielectric properties of fibrous and malignant tissue. Nonetheless, analysis of patients in the Pivotal trial stratified by breast density found that as a patient's density category increased, so too did the rate of histologically positive margins, underscoring the need for improved intraoperative margin assessment tools above and beyond traditionally utilized intraoperative techniques. 33 A separate study by Police et al. found that despite a higher risk of false positive device readings in dense breast tissue, MarginProbe provided the highest clinical benefit to those patients with higher breast density categories—the very population where the risk of true positive margins is greatest. 34 The review by Gray et al. suggested that due limited specificity, routine MarginProbe evaluation should not be recommended as long as the rates of positive margins are not exceedingly high (>40%). 35
Although we included all published studies as of December 2022 in the meta‐analysis, the main limitation of this study is the minority of RCTs and the heterogeneity and small to moderate size of some studies. While individually these studies represent use across varied institutional/hospital settings, surgeons, surgical volumes, tumor types, and margin assessment protocols, this meta‐analysis demonstrates that MarginProbe does achieve a statistically significant reduction in the burden of reoperation for margin re‐excision in women undergoing breast conserving surgery for breast cancer.
In conclusion, in an emerging era of value‐based health, the need to ensure negative margins and reduce the rate of reoperation will become increasing important. Although national consensus guidelines have standardized the definition of a positive margin with the goal of reducing the rate of arbitrary re‐excisions, re‐excision rates of 14%–22% continue to be reported, which emphasizes the need for technological innovations that could improve the detection of microscopic disease at the surgical margin. The present meta‐analysis demonstrates the ability of radiofrequency spectroscopy with MarginProbe, as an adjunct to SOC, to reduce the relative risk of margin re‐excision in women undergoing lumpectomy, potentially increasing the rate of breast conservation, improving breast cosmesis, reducing the morbidity of reoperation, and reducing related healthcare costs.
AUTHOR CONTRIBUTIONS
Jingzhen Wang searched databases, performed the statistical analysis and wrote the first draft of the manuscript. Lixia Zhang searched databases and extracted the data. Zezheng Pan designed the study and revised the article critically. All authors contributed to the article and approved the submitted version.
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grant no. 81860263) and the Natural Science Foundation of Jiangxi Province of China (grant no. 20192BAB205119).
Wang J, Zhang L, Pan Z. Evaluating the impact of radiofrequency spectroscopy on reducing reoperations after breast conserving surgery: A meta‐analysis. Thorac Cancer. 2023;14(16):1413–1419. 10.1111/1759-7714.14890
REFERENCES
- 1. Ananthakrishnan P, Balci FL, Crowe JP. Optimizing surgical margins in breast conservation. Int J Surg Oncol. 2012;2012:585670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cabioglu N, Hunt KK, Sahin AA, Kuerer HM, Babiera GV, Singletary SE, et al. Role for intraoperative margin assessment in patients undergoing breast‐conserving surgery. Ann Surg Oncol. 2007;14(4):1458–71. [DOI] [PubMed] [Google Scholar]
- 3. Nunez A, Jones V, Schulz‐Costello K, Schmolze D. Accuracy of gross intraoperative margin assessment for breast cancer: experience since the SSO‐ASTRO margin consensus guidelines. Sci Rep. 2020;10:17344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cody HS, Van Zee KJ. Reexcision — the other breast cancer epidemic. N Engl J Med. 2015;373(6):568–9. [DOI] [PubMed] [Google Scholar]
- 5. Landercasper J, Attai D, Atisha D, Beitsch P, Bosserman L, Boughey J, et al. Toolbox to reduce lumpectomy reoperations and improve cosmetic outcome in breast cancer patients: the American Society of Breast Surgeons Consensus Conference. Ann Surg Oncol. 2015;22(10):3174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morrow M, Abrahamse P, Hofer TP, Ward KC, Hamilton AS, Kurian AW, et al. Trends in reoperation after initial lumpectomy for breast cancer: addressing overtreatment in surgical management. JAMA Oncol. 2017;3(10):1352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Havel L, Naik H, Ramirez L, Morrow M, Landercasper J. Impact of the SSO‐ASTRO margin guideline on rates of re‐excision after lumpectomy for breast cancer: a meta‐analysis. Ann Surg Oncol. 2019;26(5):1238–44. [DOI] [PubMed] [Google Scholar]
- 8. Rosenberger LH, Mamtani A, Fuzesi S, Stempel M, Eaton A, Morrow M, et al. Early adoption of the SSO‐ASTRO consensus guidelines on margins for breast‐conserving surgery with whole‐breast irradiation in stage I and II invasive breast cancer: initial experience from memorial Sloan Kettering cancer center. Ann Surg Oncol. 2016;23(10):3239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schulman AM, Mirrielees JA, Leverson G, Landercasper J, Greenberg C, Wilke LG. Reexcision surgery for breast cancer: an analysis of the American Society of Breast Surgeons (ASBrS) MasterySM database following the SSO‐ASTRO “No ink on tumor” guidelines. Ann Surg Oncol. 2017;24(1):52–8. [DOI] [PubMed] [Google Scholar]
- 10. Kaczmarski K, Wang P, Gilmore R, Overton HN, Euhus DM, Jacobs LK, et al. Surgeon re‐excision rates after breast‐conserving surgery: a measure of low‐value care. J Am Coll Surg. 2019;228(4):504–512.e2. [DOI] [PubMed] [Google Scholar]
- 11. Gola S, Doyle‐Lindrud S. The MarginProbe® system: an innovative approach to reduce the incidence of positive margins found after lumpectomy. Clin J Oncol Nurs. 2016;20(6):598–9. [DOI] [PubMed] [Google Scholar]
- 12. Reid VJ, Falk JS, Police AM, Ridgeway CA, Cadena LL, Povoski SP. Minimizing re‐excision after breast conserving surgery—a review of radiofrequency spectroscopy for real‐time, intraoperative margin assessment. Expert Rev Med Devices. 2021;18(11):1057–68. [DOI] [PubMed] [Google Scholar]
- 13. Thill M. MarginProbe: intraoperative margin assessment during breast conserving surgery by using radiofrequency spectroscopy. Expert Rev Med Devices. 2013;10(3):301–15. [DOI] [PubMed] [Google Scholar]
- 14. Hoffman A, Ashkenazi I. The efficiency of MarginProbe in detecting positive resection margins in epithelial breast cancer following breast conserving surgery. Eur J Surg Oncol. 2022;48(7):1498–502. [DOI] [PubMed] [Google Scholar]
- 15. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Viechtbauer W. Conducting meta‐analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 17. Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iyengar S, Greenhouse JB. Selection models and the file drawer problem. Stat Sci. 1988;3(1):109–17. [Google Scholar]
- 19. Willis BH, Riley RD. Measuring the statistical validity of summary meta‐analysis and meta‐regression results for use in clinical practice. Stat Med. 2017;36(21):3283–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schnabel F, Boolbol SK, Gittleman M, Karni T, Tafra L, Feldman S, et al. A randomized prospective study of lumpectomy margin assessment with use of MarginProbe in patients with nonpalpable breast malignancies. Ann Surg Oncol. 2014;21(5):1589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Allweis TM, Kaufman Z, Lelcuk S, Pappo I, Karni T, Schneebaum S, et al. A prospective, randomized, controlled, multicenter study of a real‐time, intraoperative probe for positive margin detection in breast‐conserving surgery. Am J Surg. 2008;196(4):483–9. [DOI] [PubMed] [Google Scholar]
- 22. Geha RC, Taback B, Cadena L, Borden B, Feldman S. A single institution's randomized double‐armed prospective study of lumpectomy margins with adjunctive use of the MarginProbe in nonpalpable breast cancers. Breast J. 2020;26(11):2157–62. [DOI] [PubMed] [Google Scholar]
- 23. Blohmer JU, Tanko J, Kueper J, Groß J, Völker R, Machleidt A. MarginProbe© reduces the rate of re‐excision following breast conserving surgery for breast cancer. Arch Gynecol Obstet. 2016;294(2):361–7. [DOI] [PubMed] [Google Scholar]
- 24. Kupstas A, Ibrar W, Hayward RD, Ockner D, Wesen C, Falk J. A novel modality for intraoperative margin assessment and its impact on re‐excision rates in breast conserving surgery. Am J Surg. 2018;215(3):400–3. [DOI] [PubMed] [Google Scholar]
- 25. Sebastian M, Akbari S, Anglin B, Lin EH, Police AM. The impact of use of an intraoperative margin assessment device on re‐excision rates. SpringerPlus. 2015;4:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thill M, Dittmer C, Baumann K, Friedrichs K, Blohmer JU. MarginProbe®—final results of the German post‐market study in breast conserving surgery of ductal carcinoma in situ. Breast Edinb Scotl. 2014;23(1):94–6. [DOI] [PubMed] [Google Scholar]
- 27. Coble J, Reid V. Achieving clear margins. Directed shaving using MarginProbe, as compared to a full cavity shave approach. Am J Surg. 2017;213(4):627–30. [DOI] [PubMed] [Google Scholar]
- 28. Cen C, Chun J, Kaplowitz E, Axelrod D, Shapiro R, Guth A, et al. Margin assessment and re‐excision rates for patients who have Neoadjuvant chemotherapy and breast‐conserving surgery. Ann Surg Oncol. 2021;28(9):5142–8. [DOI] [PubMed] [Google Scholar]
- 29. LeeVan E, Ho BT, Seto S, Shen J. Use of MarginProbe as an adjunct to standard operating procedure does not significantly reduce re‐excision rates in breast conserving surgery. Breast Cancer Res Treat. 2020;183(1):145–51. [DOI] [PubMed] [Google Scholar]
- 30. Hermann N, Haas I, Malinger P, Kaufman Z. Margin assessment before intraoperative radiotherapy during breast conserving surgery‐does the addition of MarginProbe decrease the need for addition of fractionated whole breast radiation? Breast J. 2020;26(7):1343–6. [DOI] [PubMed] [Google Scholar]
- 31. Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–36. [DOI] [PubMed] [Google Scholar]
- 32. Walsh SM, Brennan SB, Zabor EC, Rosenberger LH, Stempel M, Lebron‐Zapata L, et al. Does breast density increase the risk of re‐excision for women with breast cancer having breast‐conservation therapy? Ann Surg Oncol. 2019;26(13):4246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schnabel FR, Allweis T. Breast density and the risk for positive lumpectomy margins. J Clin Oncol. 2015;33(28_suppl):50. [Google Scholar]
- 34. Police A, Lin E, Lane K. Intraoperative margin assessment with the MarginProbe at different mammographic breast densities. J Clin Oncol. 2015;33(28_suppl):47. [Google Scholar]
- 35. Gray RJ, Pockaj BA, Garvey E, Blair S. Intraoperative margin management in breast‐conserving surgery: a systematic review of the literature. Ann Surg Oncol. 2018;25(1):18–27. [DOI] [PubMed] [Google Scholar]


