Abstract
Inflammatory bowel diseases (IBD) are gastrointestinal disorders characterized by a breakdown in intestinal homeostasis by inflammatory immune responses to luminal antigens. Novel strategies for ameliorating IBD have been proposed in many studies using animal models. Our group has demonstrated that administration of Lactococcus lactis NCDO 2118 can improve clinical parameters of colitis induced by oral administration of dextran sulphate sodium (DSS). However, it is not clear whether other strains of L. lactis can yield the same effect. The objective of present study was to analyze the effects of three different L. lactis strains (NCDO2118, IL1403 and MG1363) in the development of DSS-induced colitis in C57BL/6 mice. Acute colitis was induced in C57/BL6 mice by the administration of 2% DSS during 7 consecutive days. Body weight loss and shortening of colon length were observed in DSS-treated mice, and none of L. lactis strains had an impact in these clinical signs of colitis. On the other hand, all strains improved the global macroscopical disease index and prevented goblet cells depletion as well as the increase of intestinal permeability. TNF-α production was reduced in gut mucosa of L. lactis DSS-treated mice indicating a modulation of a critical pro-inflammatory response by all strains tested. However, only L. lactis NCDO2118 and MG1363 induced a higher frequency of CD11c+CD11b−CD103+ tolerogenic dendritic cells in lymphoid organs of mice at steady state. We conclude that all tested strains of L. lactis improved the clinical scores and parameters of colitis, which confirm their anti-inflammatory properties in this model of colitis.
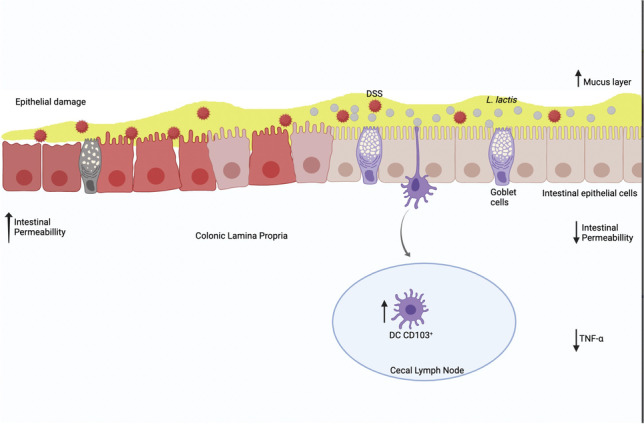
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-023-00928-0.
Keywords: Lactococcus lactis, Colitis, Cytokines, Probiotics
Introduction
Inflammatory bowel diseases (IBD) are defined as a group of gastrointestinal tract (GIT) disorders characterized by inflammation and epithelial injury [1]. Ulcerative colitis and Crohn’s disease are the two most frequent forms of IBD. Ulcerative colitis (UC) affects only the distal colon, while Crohn’s disease can affect several segments of the GIT. IBDs have been associated with increased mortality and morbidity in humans [2]. Severe changes commonly observed in these patients include weight loss, malnutrition, deficiency of various vitamins and minerals, altered body composition associated with loss of bone mineral mass, and high prevalence of osteopenia and osteoporosis, hypoalbuminemia, anemia, immune and metabolic disorders, and abdominal pain and bleeding, affecting drastically their quality of life [3].
It is known that an imbalance in the interactions between gut microbiota, immune system, and epithelial cells in genetically predisposed individuals triggers disruption of intestinal homeostasis and contribute to the onset of IBD [4]. Under physiological conditions, microbiota is a natural source of stimulation for the gut-associated lymphoid tissue (GALT). These local immune cells along with gut epithelial cells provide the first line of defense against potential noxious agents, but they also play a critical role in regulating immune responses to maintain gut homeostasis. Intestinal mechanisms controlling the entry of antigens and the immunological reactivity towards them are altered in pathological conditions such as IBD [5].
Although no animal model alone reproduces the complexity of human IBD, similar clinical signs can be observed in experimental models of ulcerative colitis. Oral administration of dextran sodium sulphate (DSS) to mice results in acute colitis with weight loss, diarrhea, rectal bleeding, colon shortening, and infiltration of inflammatory cells in the colonic mucosa. DSS is a negatively charged, water-soluble, sulfated polysaccharide with anticoagulant properties. In addition to aggravating rectal bleeding due to such anticoagulant effects, its high negative charge is toxic to the colon epithelium, resulting in mucosal erosion and ulceration, mucin destruction, increased exposure to luminal antigens, and increased intestinal permeability. Lesions caused by the DSS are restricted to the colon, especially to the distal portion that lodges a high concentration of microorganisms [6, 7]. It has been described that experimental colitis leads to changes in the intestinal microbiota increasing the abundance of anaerobic gram-negative bacteria. Okayasu and colleagues observed a significant increase in the population of Enterobacteraceae, Bacteroidaceae, and Clostridium spp. in mice with DSS-induced acute and chronic colitis [8].
Probiotic microorganisms have been proposed as a suitable therapeutic tool for IBD due to their anti-inflammatory effects demonstrated in animal models and clinical studies [9]. Probiotics are defined as living microorganisms that when administered orally result in benefits to the health of the host [10]. Some of them, especially the ones from Bifidobacterium and Lactobacillus genera, present local anti-inflammatory effects [11]. Administration of repeated doses of Lactobacillus casei strain Shirota to mice prevents indomethacin-induced intestinal injury [12]. Treatment of mice with a mixture of Bifidobacterium breve, L. acidophilus, Lactobacillus casei, and S. thermophilus reduced histological score and weight loss during experimental mucositis and improved animal survival [13]. Zhang and colleagues also observed anti-inflammatory effects of Lactobacillus plantarum during DSS-induced colitis development, with recovery of body weight, improvement of histological and macroscopic scores [14]. Oral administration of Bifidobacterium improved the intestinal barrier by increasing the concentration of mucin, frequency of goblet cells in the gut epithelium and by modulating pro-inflammatory cytokines such as IL-6 and TNF-α in mice with colitis [15].
In this scenario, bacteria of the Lactococcus genus have gained increasing attention due to their therapeutic, anti-inflammatory, and probiotic potential role in restoring intestinal barrier function and gut homeostasis [16]. Lactococcus lactis (L. lactis) is the most important bacteria among commercially used lactic acid bacteria (LAB) because they are naturally present in fermented dairy and vegetable products. Their high fermentation capacity generates final products that, in addition to their organoleptic and food preservation properties, constitute the major factor responsible for the development of an antimicrobial medium in the food matrix [17]. Although L. lactis it is not a comensal, it is a commonly consumed bacteria [18] that is classified as GRAS (Generally Regarded as Safe) by the United States Food and Drug Administration United States [10, 19, 20]. Some studies have demonstrated the efficacy of L. lactis subsp. FC in treating experimental colitis in mice [11, 21]. Our group has also shown that L. lactis subsp. lactis NCDO2118 has anti-inflammatory properties when administered during the remission phase of DSS-induced colitis in mice. This strain was able to improve the clinical signs of the disease, maintain epithelial integrity, reduce the production of some proinflammatory cytokines, as well as increase the frequency of tolerogenic CD11c+ CD11b−CD103+ dendritic cells (DCs) and LAP+ regulatory T cells (Treg) in spleens of treated mice [22]. Moreover, analysis of gut microbiota composition of mice treated with L. Lactis NCDO2118 using selective culture media indicated that L. lactis consumption leads to increased abundance of acid lactic bacteria (anaerobic) and reduction in bacteria associated with gut inflammatory conditions (aerobic bacteria such as Enterobacteria, for instance) [23]. It is known that L. lactis produce several types of bacteriocins, the main one called nisin, a broad spectrum bacteriocin that acts on gram-negative bacteria such as Clostridium botulinum and its spores, a microorganism that is highly present in IBD patients [24]. Therefore, studies on the effects of probiotic strains in inflammatory diseases is of paramount importance to identify which ones have therapeutic potential [25]. In this work, we investigated the anti-inflammatory effects of three strains of L. lactis that had already their genome sequenced in DSS-induced colitis: L. lactis subsp lactis NCDO2118 [26], L. lactis subsp lactis IL1403 [27], and L. lactis subsp cremoris MG1363 [28].
Material and methods
Animals
Female C57BL/6 mice, 8 to 10 weeks of age, were obtained from the vivarium of the Universidade Federal de Minas Gerais (UFMG), Brazil. Mice were kept in the experimental animal facility of Laboratório de Imunobiologia, Instituto de Ciências Biológicas, UFMG, in an environmentally controlled room with 12-h light–dark cycle. All procedures were approved by the local ethics committee for animal research (Comitê de Ética em Experimentação Animal (CETEA) from UFMG, Brazil- CEUA # 340/2017).
Experimental design
Bacterial strains and growth conditions
Three L. lactis strains were used in this study: L. lactis subsp. lactis NCDO 2118 [26], L. lactis subsp. lactis IL1403 [27], and L. lactis subsp. cremoris MG1363 [28]. They were grown at 30 °C in M17 medium (Difco) containing 0.5% glucose (Synth) without agitation until an OD600 of 1.5. Batches were prepared at a concentration of 1 × 109 UFC per mouse per day. Before gavage, batches were centrifuged at 4 °C, 2000 rpm to 10 min, and the pellet resuspended in physiological buffer.
Dextran sodium sulfate-induced colitis
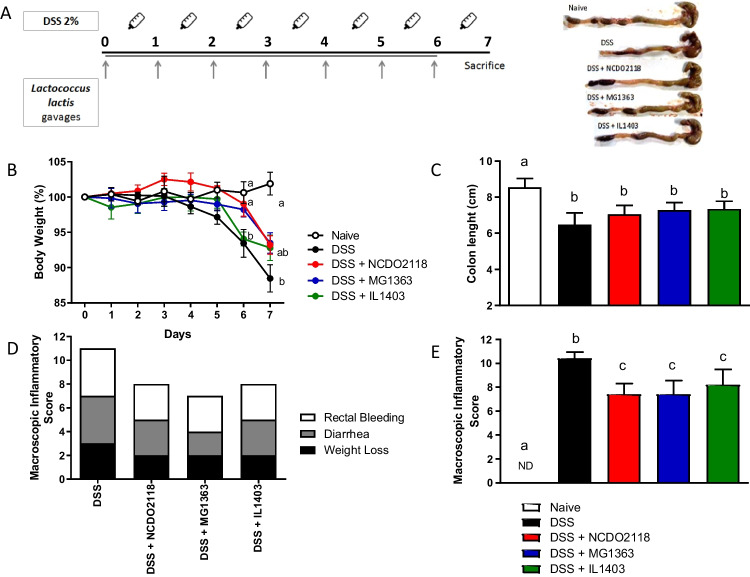
Acute ulcerative colitis was induced in mice by the ad libitum administration of 2% aqueous solution of dextran sodium sulfate (DSS, MP Biomedicals) during 7 consecutive days. The leftover solution was measured daily, and bottles were refilled every 24 h. Concomitantly with DSS administration, mice received a daily gavage of 1 × 109 L. lactis of different strains. Control groups received saline by gavage. At day 7, all mice were sacrificed. A schematic representation of the experimental procedure is shown in Fig. 1A.
Fig. 1.
Oral administration of L. lactis prevented body weight loss and improved macroscopic score of colitis. A Experimental protocol. C57BL/6 mice received continually 2% DSS concomitant with L. lactis gavage administration for 7 consecutive days. Mice of the control groups received saline by gavage. Mice were sacrificed at day 7. B Body weight percentage from day 0 to day 7. The body weight was measure daily. C Colon length measured in cm and representative photographs of the colon of one animal from each group. D, E Macroscopic inflammatory score of colitis, including scores related to body weight, diarrhea, and rectal bleeding. Bars are the mean of 5 mice/group, and the results are representative of 3 independent experiments. ANOVA, Tukey post-test
Macroscopic disease evaluation
Disease was assessed macropically by the measurement of three clinical parameters — weight loss, diarrhea, and rectal bleeding — at the last day of L. lactis administration (day 7), and graded as described by Cooper et al. [29]. Body weight loss was calculated as the difference between the initial and actual weight percentage. Diarrhea was determined by assessing mucus/fecal material adhering to anal fur and was confirmed by the presence or absence of fecal pellet formation and continuous fluid fecal material in the colon. Rectal bleeding was defined as diarrhea/feces containing visible blood and rectal bleeding. The three clinical parameters were scored separately: weight loss (0: normal; 1, 2, 3, and 4: 1–5%, 5–10%, 10–15%, and > 15% body weight loss, respectively), diarrhea (0: normal; 2: moderate; and 4: severe), and rectal bleeding (0: absent; 2: occult; and 4: visible). The macroscopic score was calculated from the score of the clinical signs using the following formula: (weight loss score) + (diarrhea score) + (rectal bleeding score).
Total colon length and colon weight were also measured.
Measurement of intestinal permeabillity
After 7 days of DSS treatment, all mice received 0.1 mL of a solution containing 18.5 Megabecquerel (MBq) of diethylenetriamine pentaacetic acid (DTPA) labeled with the radioisotope technetium (99mTc-DTPA) by gavage [30]. This same solution was used as a standard in the equipment. Four hours after gavage, all animals were anesthetized, and their blood was collected and placed in polystyrene tubes for radioactivity counting (to count the radioisotope 99 m technetium coupled to DPTA) in a gamma radiation counter device (Perkinelmer Wallac,1480 Wizard 3). Intestinal permeabillity was measured indirectly by the amount of radiation present in the blood [31, 32]. Data were expressed as a percentage of the radiation dose (% dose), calculated using the following equation: % dose/g = (cpm per g of blood /cpm of standard) × 100, where cpm represents counts of radioactivity per minute.
Histology
Samples of proximal colon were fixed in 10% formaldehyde and processed for histological analysis. Hematoxylin–eosin-stained (HE) sections were blindly scored based on a semiquantitative scoring system described previously [33] where the following features were graded: extent of destruction of normal mucosal architecture (0: normal; 1, 2, and 3: mild, moderate, and extensive damage, respectively), the presence and degree of cellular infiltration (0: normal; 1, 2, and 3: mild, moderate, and transmural infiltration, respectively), extent of muscle thickening (0: normal; 1, 2, and 3: mild, moderate, and extensive thickening, respectively), the presence or absence of crypt abscesses (0: absent; 1: present), and the presence or absence of goblet cell depletion (0: absent; 1: present). Scores for each feature were summed up to a maximum possible score of 11.
To analyze mucus production, histological sections were stained with Periodic Acid-Schiff (PAS), a reagent used to stain substances present in mucus. PAS oxidizes mucins and glycogen present in the tissue producing a magenta red color. Five pictures of histological sections for each animal were taken at 20 × magnification from different representative intestinal segments using a camera-coupled optical microscope. Images were analyzed using Image J software. Mucus production was measured by creating a binary image and calculating the total stained area [34]. Goblet cells were counted in HE stained sections.
Colon tissue preparation and cytokine assay
Colon samples were weighed and homogenized in PBS containing 0.05% (v/v) Tween-20, 0.1 mM phenylmethylsulphonyl fluoride, 0.1 mM benzethonium chloride, 10 mM EDTA, and 20 KIU Aprotinin A using a tissue homogenizer (100 mg tissue/ml buffer) [35]. Suspensions were centrifuged at 12,000 rpm for 20 min at 4 °C, and the supernatants were collected for the cytokine assay. Plates were coated with purified monoclonal antibodies reactive to the cytokines IL-6, IL-10, TNF-α, IL-1β, and TGF-β (BD-Pharmingen) overnight at 4 °C. On the following day, wells were washed, supernatants were added, and plates were incubated overnight at 4 °C. A serial dilution of the standard (BD-Pharmingen) for each cytokine was used to build a standard curve. On the third day, biotinylated monoclonal antibodies against the cytokines were added, and plates were incubated for 2 hat room temperature. Plates were washed again, and HRP-labelled Streptavidin (Southern Biotechnology) was added to detect biotin conjugated antibody. Color was developed at room temperature with 100 μl/well of orthophenylenediamine (1 mg/ml) and 0.04% (v/v) H2O2 substrate in sodium citrate buffer. Reaction was interrupted by the addition of 20 μl/well 2 N H2SO4. Absorbance was measured at 492 nm using a microplate reader (BIO-RAD). Results were reported in pg/ml using the standard curve as a reference.
Secretory IgA measurement in intestinal lavage fluid
Small intestines were washed with 10 ml PBS (137 mM/L NaCl, 2.7 mM/L KCl, 10 mM/L Na2HPO4 • 2 H2O, 2 mM/L KH2PO4) and centrifuged to extract secretory IgA (SIgA). Supernatants were collected. Levels of fecal SIgA were determined by ELISA. Ninety-six-well plates (NUNC) were coated with Ig goat anti-mouse UNLB antibody in coating buffer (pH 9.6) overnight at 4 °C. Wells were washed and blocked with 200 μl PBS containing 0.25% casein for 1 h at room temperature. Fecal supernants were added to the plates and incubated for 1 h at 37 °C. A serial dilution of purified mouse IgA (Southern Biothecnology) was used to build the standard curve. Subsequently, plates were washed, peroxidase-streptavidin goat anti-mouse IgA-HRP (Southern Biotechnology) diluted 1:10,000 was added, and plates were incubated for 1 h at 37 °C. Colour was developed at room temperature with 100 μl/well of orthophenylenediamine (1 mg/ml) (SIGMA) and 0.04% H2O2 substrate in sodium citrate buffer. The reaction was interrupted by the addition of 20 μl/well 2 N H2SO4. The absorbance was measured at 492 nm using an ELISA microplate reader (Bio-Rad). Results were reported in pg/ml using the standard curve as a reference.
Extraction and preparation of cells for flow cytometry
Cells from mesenteric lymph nodes (MLN) and spleens were extracted and placed in a tube containing complete RPMI medium (with 10% fetal bovine serum—FBS). Cells were grinded with the aid of a spleen grinder, centrifuged and resuspended in complete RPMI medium for plating. For spleenocytes, there was an additional step when red cells were lysed and removed from the samples by the addition 9 ml of distilled water 5 s before adding 1 ml of 10 × PBS to stop lysis. Spleen capsules and debris were removed with the aid of a fine-tipped pasteur pipette. Cells were counted in a Newbauer chamber and 1 × 106 cells/per well were plated in a 96-well plate and were subsequently labeled with antibodies for flow cytometry analysis.
Flow cytometry
Cell suspension of cecal lymph node, MLN and spleen were plated, washed with PBS wash (PBS + 0.05% Tween) and pre-incubated for 20 min in polysterene tubes with purified rat anti-mouse CD16 / CD32 (Fc Block, clone: 2.4G2, BD Biosciences Pharmingen) to avoid unspecific binding. Cells were washed and subsequently stained with specific monoclonal antibodies (mAb) conjugated with fluorochromes that are reactive to surface and intracellular molecules. For surface staining, cells were incubated for 30 min at 4 °C with fluorochrome-labelled mAbs for surface staining. Fluorescent-dye-conjugated Abs were purchased from eBioscience (USA; Anti-mouse LAP 46–9821-82, PERCP-efluor710; Anti mouse CD11b 47–0112-82, APC-eFluor 780; anti-CD11b, 47- 0112–82, APC; anti-mouse CD11c, 49–0114-80, e-fluor 450; anti-mouse CD4, 17–0041-82, APC); BD (USA; rat antimouse CD11b, 550,993; PerCP-Cy 5.5), or Biolegend (Anti-mouse CD4, 116,015, PE/Cy7; CD103 anti-mouse, 121,419, FITC). Cells were washed and, for intracellular labeling of the Foxp3 transcription factor, a commercial fixation/ permeabilization kit (eBioscience) was used according to the manufacturer’s instructions. Samples were incubated for another 30 min with rat anti-foxp3 mAb (Rat anti-FOXP3, 12–5773-82, PE; Rat anti-mouse FOXP3, 560,403, Alexa Fluor 488). Cells were washed again, resuspended in PBS, and analyzed by flow cytometry using a FACS CANTO II (BD Biosciences, San Jose, CA) and FACSCALIBUR. At least 50,000 events were acquired.
Individual controls (singles) containing only one labeled antibody in each tube, and also tubes with the FMO control (Fluorescence minus 1) were used to evaluate unespecific staining and adjust the fluorescence of positive cells. Cells without staining were used as a negative control. These controls were used to set the positive quadrants and to gate cells based on foward and side scatters (n = 5), as described by Herzenberg’s group [36]. The frequency (%) of positive cells and the mean fluorescence intensity were analyzed using FlowJo software, version 10.0 (Tree Star, Ashland, OR, USA).
Statistical analysis
Results were expressed as the mean ± standard error of the mean (SEM). Significance of differences among groups was determined by either Student’s t-test or ANOVA followed by Tukey’s post test. Differences were considered significant when P < 0.05.
Results
Oral administration of L. lactis ameliorated clinical signs of colitis
L. lactis strains were administered by gavage concomitantly with DSS administration in the drinking water for a period of 7 days (Fig. 1A) to test their effect on colitis development.
All mice consumed the same volume of DSS solution, indicating that disease induction was homogeneous in all groups (data not shown).
As expected, mice treated with DSS had a decrease in body weight when compared to mice from the Naive group (water-treated mice) (Fig. 1B). However, only at day 6, a statistical difference in body weight was observed between DSS group and both L. lactis-MG1363-treated and L. lactis-NCDO2118-treated groups, but not in L. lactis-IL1403-treated group. At day 7, mice treated with any of the three L. lactis strains presented weight loss similar to both Naïve and DSS groups. DSS treatment decreased about 10–15% of body weight, while the group treated with L. lactis showed weight loss of 5–10% at the end of the experimental protocol (day 7). Therefore, treatment with L. lactis MG1363 and L. lactis NCDO2118 was able to delay disease onset, but did not completely prevent weight loss, as observed on day 7 (Fig. 1B). In addition, mortality was observed in mice from the DSS-control group but not in mice from L. lactis-treated groups (data not shown).
Mice consuming L. lactis exhibited significant reduction in the main clinical symptoms of colitis: rectal bleeding, diarrhea, and weight loss (Fig. 1D, E), when compared to mice from the DSS group. Colon shortening caused by the disease was not prevented by treatment with any L. lactis strain (Fig. 1C), confirming previous data using L. lactis NCDO2118 [22].
L. lactis prevented colonic damage and the increase in intestinal permeability
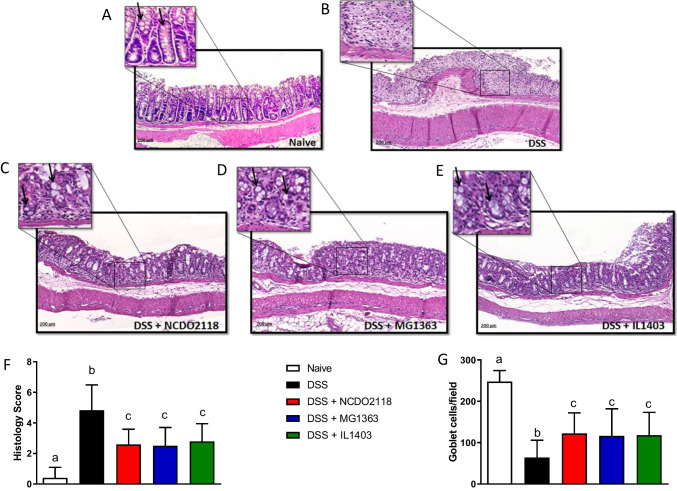
The ability of L. lactis to prevent and treat DSS-induced colonic damage was evaluated at the histological level. Colon sections from mice of the naive group showed an intact epithelium, a well-defined crypt length and no cellular infiltration in the mucosal and submucosal layers (Fig. 2A, F). In contrast, colon tissues from DSS-treated mice showed severe inflammatory lesions throughout the mucosa and submucosa with cellular infiltration, edema, increased thickness of the muscle layer, and loss of the crypts and of the normal mucosal architecture (Fig. 2B). The histological score was lower in groups treated with L. lactis suggesting the oral administration of L. lactis strains ameliorated all parameters of histological damage (Fig. 2C–F).
Fig. 2.
Oral administration of L. lactis prevented damage in the intestinal mucosa induced by colitis. Representative images (× 10) of H&E-stained paraffin sections of Control (A), DSS (B), DSS + L. Lactis NCDO2118 (C), DSS + L. Lactis MG1363 (D), and DSS + L. Lactis IL1403 (E) groups. F Histological scores of colon sections of DSS-colitis mice that received or not L. lactis. G Goblet cells were quantified per area. Each image in the 10 × magnification was considered an area. For each group, 15 images were obtained, and the cells were counted through the Image J program. Bars represent the mean ± sd (n = 5); ANOVA, Tukey post-test
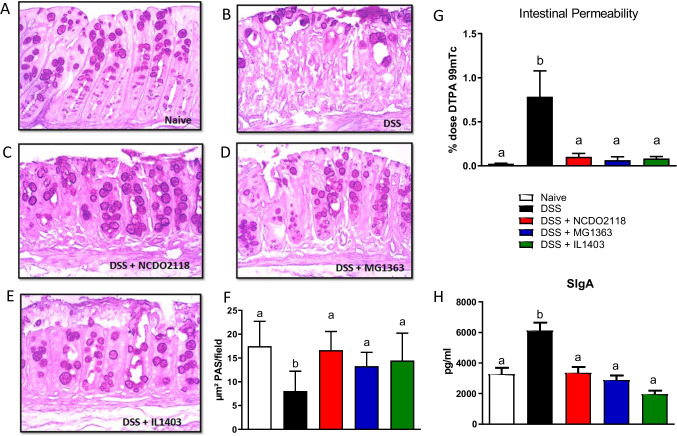
DSS-treated mice had a significant increase in intestinal permeability, but this pathological sign of colitis was completely prevented by treatment with any of the three strains of L. lactis (Fig. 3G).
Fig. 3.
L. lactis modulated mucus production and intestinal permeability in mice with DSS-colitis. Representative images (× 20) of PAS-stained paraffin sections of a colon from control (A), DSS (B), DSS + L. Lactis NCDO2118 (C), DSS + L. Lactis MG1363 (D), and DSS + L. Lactis IL1403 (E) groups. F Quantification of mucus area produced by the goblet cells through the Image J program. G Intestinal Permeability evaluation in mice that received a solution containing 99mTc-DTPA per gavage. After 4 h of 99mTc-DTPA administration, mice were sacrificed and the amount (%) of radiation presented in each sample was measured. H Total SIgA was measured by ELISA in small intestine fluid. Bars represent the mean ± dp (n = 5). ANOVA, Tukey post-test
L lactis treatment prevented depletion of goblet cells and preserved mucus and SIgA production in the colon of DSS-treated mice
As shown in Fig. 2G, DSS administration caused depletion in goblet cells when compared to naive group. Treatment with any of the three strains of L. lactis preserved the number of goblet cells in the colon mucosa during DSS administration. In line with this, L. lactis-treated mice preserved the production of mucus in the colonic mucosa (Fig. 3C–E) when compared to the DSS group (Fig. 3B). Statistical analysis confirmed that groups treated with L. lactis had mucus production similar to the production of mice from the naive group (Fig. 3F).
SIgA was measured in the intestinal lavage of mice at day 7. Mice that received only DSS had high levels of SIgA, whereas mice treated with L. lactis maintained SIgA production at its basal level (Fig. 3H).
L. lactis strains had distinct effects in dendritic cells and regulatory T cells of healthy and DSS-treated mice
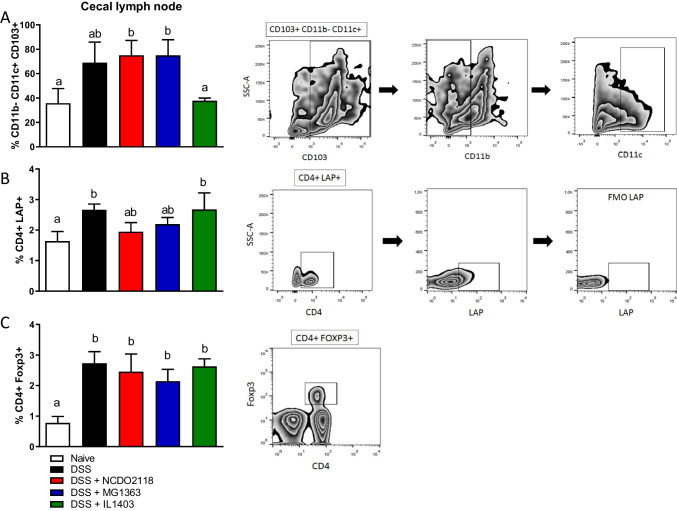
DSS-induced colitis in mice was associated with the increase in the frequency of LAP+ and Foxp3+ CD4+ Treg subsets in the cecal lymph nodes (Fig. 4B, C), but not in spleens and MLN (data not shown) when compared to the naive group. Interestingly, mice treated with L. lactis subsp. lactis NCDO2118 and L. lactis subsp. cremoris MG1363 during colitis development showed no increase in the frequency of LAP+CD4+ Treg cells but had a higher frequency of tolerogenic CD11c+CD11b−CD103+ DCs in the cecal lymph nodes (Fig. 4A–C). Treatment with L. lactis did not change the effect of DSS-induced increase in Foxp3+CD4+ Tregs in the cecal lymph nodes (Fig. 4C). Of note, L. lactis subsp. lactis NCDO2118 administration was able to increase the frequency of CD11c+CD11b−CD103+ DCs in the cecal lymph nodes (Supplementary Fig. 2A) and L. lactis subsp. cremoris MG1363 increased the frequency of this DC subset in spleens of control healthy mice (Supplementary Fig. 2C). Both strains had a similar effect on tolerogenic DCs in MLNs of control mice (Supplementary Fig. 2B).
Fig. 4.
Dendritic cells and regulatory T cells were differentially modulated in mice with DSS-colitis. Frequencies of CD11b−CD11c+CD103+ tolerogenic DCs (A), LAP+CD4+ Treg (B), and Foxp3+CD4+ Treg (C) in cecal lymph nodes, were evaluated by flow cytometry. Bars represent the mean of 5 mice/group, and the data is representative of two independent experiments. ANOVA, Tukey post-test
The L. lactis subsp. lactis IL1403 did not maintain the increase in CD11c+CD11b−CD103+ DCs induced by DSS treatment and it had no effect in DCs and Tregs in lymphoid organs of diseased or healthy mice (Fig. 4 and Supplementary Fig. 2).
L. lactis-modulated cytokine production in the colonic mucosa
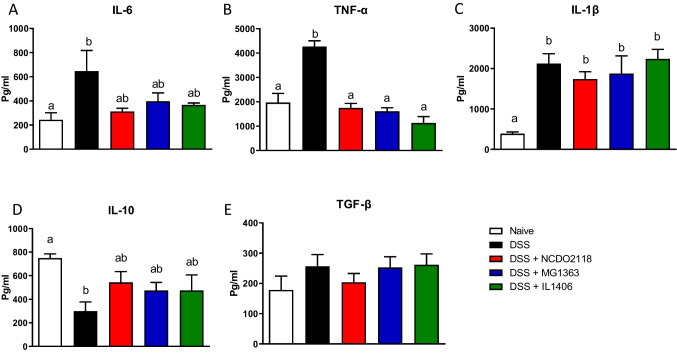
DSS administration increased the levels of pro-inflammatory cytokines such as IL-6, TNF-a, and IL-1β and decreased the production of the anti-inflammatory cytokine IL-10 in the colonic mucosa of mice (Fig. 5A–C). Conversely, treatment with L. lactis prevented the increase of IL-6 as well as TNF-a (Fig. 5A, B) and maintained the basal levels of IL-10 in the colon (Fig. 5D). None of the three strains of L. lactis could avoid the increase in IL-1β levels that was associated with colitis induction by DSS (Fig. 5C). TGF-β production was not affected by DSS-induced colitis (Fig. 5E).
Fig. 5.
L. lactis did not alter colonic IL-6 and IL-10, but reduced TNF-ɑ. IL-6 (A), TNF-α (B), IL-1β (C), IL-10 (D), and TGF-β (E) in extracts of colonic tissue of mice during with DSS-induced colitis were measured by ELISA. One representative result from two independent experiments is shown. Bars represent the mean ± SEM (n = 5). ANOVA, Tukey post-test
None of the three L. lactis strains were able to alter cytokine production in healthy mice (Supplementary Fig. 1C-E).
Discussion
DSS-induced colitis is a model characterized by clinical changes involving weight loss, diarrhea, rectal bleeding, histological, and cellular alterations, increased intestinal permeability, production inflammatory cytokines, among others. Thus, this model reproduces some aspects of human ulcerative colitis [8]. One of the relevant clinical parameters observed in colitis is weight loss, which occurs from day 3 to day 4 of DSS administration [6]. In this study, we observed that the three strains could not prevent completely the weight loss observed after 7 days of DSS intake. However, animals treated with L. lactis MG1363 or with L. lactis NCDO2118 lost weight slower than those treated with L. lactis IL1403. On day 6, L. lactis-IL1403-treated mice this group had a weight reduction similar to the DSS group, whereas L. lactis-MG1363-treated and L. lactis-NCDO2118-treated mice presented statistically significant differences in weight loss when compared to the DSS group (Fig. 1B). Thus, treatment with L. lactis MG1363 and with NCDO2118, but not with L. lactis IL1403, delayed the acute weight loss during colitis development.
In addition, we observed prostration and signs of weakness in mice with DSS-induced colitis, but not in L. lactis-treated mice (data not shown) that showed milder signs of colitis. Furthermore, we also observed mortality in mice from the DSS group, but not in the groups treated with L. lactis strains (data not shown). Although we could not observe a statistically significant improvement in weight loss on day 7, we observed less severe diarrhea and rectal bleeding in these animals (Fig. 1D). Previous studies from our group showed that administration of L. lactis NCDO2118 strain improved the clinical signs of DSS-induced colitis in mice [22]. We observed in the present study that the three strains of L. lactis had a similar effect in improving macroscopic (Fig. 1E) and histological (Fig. 2F) scores of the disease.
Despite these anti-inflammatory effects, we did not see changes in colon shortening in mice treated with L. lactis (Fig. 1C). Colonic shortening is a common alteration caused by DSS administration [6] and it was not prevented by the administration of these L. lactis bacteria.
DSS is a toxic agent that damages the gut epithelium and increases its permeability to the entry of luminal agents [7]. Indeed, intestinal permeability was increased in mice with DSS-induced colitis, whereas all groups treated with L. lactis preserved the intestinal barrier with a permeability similar to the one found in the naive group (Supplementary Fig. 1A).
The protective role of L. lactis strains in the gut barrier function can be due to their effect in critical elements of this barrier such as tight junction proteins, SIgA, and mucus production. Increased intestinal permeability is directly related to the loss of proteins from tight junctions, a damage caused by DSS treatment [37]. In this study, we did not evaluate the expression and integrity of tight junction proteins, but we did evalutate mucus and SIgA production. L. lactis administration significantly prevented epithelial destruction. Animals treated with the three L. lactis showed an intact mucosal architecture with less epithelial erosion, reduced inflammatory cell infiltrate, diminished edema, and preserved the number of goblet cells (Fig. 2A–G). The normal goblet cell numbers found in L. lactis-treated mice (Fig. 2G) when compared to mice from the DSS group was accompanied by maintenance of mucus production at levels similar to the ones found in the naive group (Fig. 3F). This suggests that one of the mechanisms by which L. lactis protects against the chemical aggression caused by DSS is through a preserved goblet cell production of mucus.
Concerning SIgA production, several probiotic bacteria induce an increase in SIgA concentration in feces [38]. This class of immunoglobulin is involved with the integrity of the mucosal barrier by limiting the penetration of commensal and pathogenic bacteria [39]. In our study, administration of L. lactis at steady state did not alter the production of SIgA in the small intestine of mice (Supplementary Fig. 1A). However, administration of DSS resulted in the increase in SIgA levels (Fig. 3H) probably as a protective reactive mechanism triggered by increased contact with luminal bacteria after DSS-induced damage in the epithelial barrier. Administration of L. lactis concomitantly with DSS did not involve induction of higher SIgA secretion. On the contrary, SIgA levels in L. lactis-treated mice with colitis were similar to the ones found in the naive group (Fig. 3H) probably because stimulation by luminal content in these mice was not as intense as in DSS-treated mice. Therefore, our results show that all L. lactis strains tested can maintain the physiological production of SIgA.
A previous study by our group [22] demonstrated that caco2 cells stimulated in-vitro with L. lactis and IL-1β had a reduction in IL-8 production. This finding together with the results reported here lead us to the hypothesis that L. lactis might control colitis development by two mechanisms: (a) via a direct action on goblet cells that produce mucus and protect the integrity of the intestinal epithelium; (b) via stimulation of immune cells, such as tolerogenic DCs and Treg cells. Therefore, we analyzed the frequency of these cells in mice treated with L. lactis.
Musaki et al. demonstrated that the severity of DSS-induced colitis increased in the absence of CD103+CD11b− DCs [40]. This DC population is involved in the differentiation of CD4+Foxp3+ Treg cells in MLN and in anti-inflammatory responses in intestinal models of inflammatory diseases [41, 42]. Therefore, we analyzed the frequency of this DC sub-population in mice treated or not with DSS and L. lactis. Both healthy groups and DSS-treated groups that received the administration of L. lactis subsp. lactis NCDO2118 and L. lactis subsp. cremoris MG1363 showed a higher frequency of tolerogenic CD11b−CD11c + CD103+ DCs in lymphoid organs (Fig. 4A–C, Supplementary Fig. 2A-C). This suggests that this increase may be an action of the bacteria and not a compensatory immunoregulatory mechanism caused by the DSS itself. The same results were not observed in mice treated with L. lactis IL1403. Surprisingly, treatment with this strain did not maintain the modulation of CD11b−CD11c + CD103+ DCs in lymphoid organs as observed for the other strains. It is possible that this bacterium produces a distinct set of mediators that can modulate mucus production, TNF-a secretion, and colitis development with a distinct effect on mucosal DCs.
Intestinal CD11b−CD11c + CD103+ DCs produce TGF-b and express Aldh, an enzyme that converts retinol (from ingested vitamin A) in retinoic acid (RA). These two products together, TGF-b and RA, enable the DCs to induce the differentiation of naive CD4 + T cells into Foxp3 + Treg cells in the MLNs [41, 42]. There was no increase in the population of CD4+Foxp3+ Treg cells in healthy animals treated with any of the three strains of L. lactis (Supplementary Fig. 2). However, there was an increase in the frequency of CD4+Foxp3+ and T CD4+LAP+ Treg cells in the cecal lymph nodes of mice treated with DSS only. Treatment with L. lactis did not change these augmented frequencies of Tregs (Fig. 4B, C). Therefore, our data suggest that administration of DSS increases the frequency of Treg cells, probably as another compensatory mechanism to regulate inflammation, and that L. lactis administration had no additional effect in it.
Curiously, tolerogenic DCs were increased by administration of L. lactis NCDO2118 and L. lactis MG1363 (Supplementary Fig. 2). It is likely that administration of L. lactis for 7 days had an effect in innate cells (such as DCs) but it was not enough to differentiate T cells. Thus, L. lactis strains seem to be controlling inflammation in the epithelial compartment by preserving protective responses such as mucus secretion by goblet cells. Administration of DSS for 7 consecutive days, on the other hand, induced increased in tolerogenic DCs and Tregs (Fig. 4). It is plausible that the stimulation provided by the inflammatory events triggered by DSS was strong enough to unleash anti-inflammatory circuits such as Treg differentiation. On the contrary, L. lactis stimulation is not an inflammatory stimulus and it is not expected to trigger additional Treg induction at steady state (Supplementary Fig. 2). Interestingly, in a chronic model of DSS-induced colitis, when L. lactis NCDO2118 was administered between two cycles of DSS treatment, an increase in the frequency of CD4+LAP+ Treg cells was observed in spleens and MLNs of L. lactis-treated mice [22]. It is plausible that in this inflammatory scenario, L. lactis can act as an adjuvant of Treg cell differentiation. Indeed, when administered in combination with alloantigens as an adjuvant therapeutic agent in a model of graft versus host disease (GVHD), L. lactis NCDO2118 was also able to increase the frequency of CD19+ LAP+ regulatory B cells in spleens of donor mice [43].
In addition to the effect in barrier function promoted by the three strains of L. lactis, a possible classic mechanism involved in the modulatory effect of L. lactis strains is the production of cytokines that modify the inflammatory response. In our study, oral treatment with the three strains of L. lactis had no modulatory effect in IL-6 production, but it reduced the levels of local TNF-α increased by DSS ingestion (Fig. 5A, B). Nishitani and collaborators [21] also identified a reduction in TNF-α after treatment with L. lactis subsp. cremoris FC. Of note, anti-TNF-a treatment is an effective therapeutic tool used in IBD patients and it seems to exert its effect through IL-10 signalling in macrophages (44).
Treatment with L. lactis during the development of colitis did not prevent the increase of IL-1β in the colon (Fig. 5C). In addition to activating transcription factors such as NF-kb, which induces increased expression of pro-inflammatory mediators, IL1β is involved in the process of wound healing and intestinal reepithelization. This cytokine may also be involved in the production of antimicrobial peptides and mucin, important factors in protecting the epithelial barrier [44]. Thus, IL-1b in the gut mucosa can exert inflammatory and repair functions.
IL-10 is probably one of the main cytokines that modulate immune responses in the intestinal mucosa. IL-10-deficient mice spontaneously develop intestinal inflammation [33, 35, 45]. In the present study, IL-10 levels did not change after L. lactis treatment in DSS-treated nor in healthy animals, although we observed a trend towards increase in IL-10 levels in L. lactis-treated mice (Fig. 5D). Among the cell types known to produce high levels of IL-10 in the colon are CD4+ regulatory Tr1 cells, macrophages with anti-inflammatory activities [46], and B-1 cells [47]. However, these cells were not analyzed in the present work and further studies are needed to verify the influence of L. lactis on their maintenance, proliferation and recruitment upon L. lactis treatment.
We did not observe changes in the production of TGF-β in DSS-treated animals (Fig. 5E) nor in those treated only with L. lactis (Supplementary Fig. 1C).
L. lactis bacteria are found on the ANVISA probiotic list although, according to the FAO/WHO guidelines, they cannot be included in the definition of probiotics since they are not capable of colonizing the GIT. However, a recent consensus established in 2014 after a debate organized by ISAPP (International Scientific Association for Probiotics and Prebiotics) with FAO and WHO researchers specialized in the field of probiotics concluded that bacteria that have anti-inflammatory effects may fall within the scope of probiotics even though they are not able to colonize the intestine [48]. Despite this, additional studies are still needed for L. lactis to be established as a probiotic.
No difference was identified between the anti-inflammatory effects of the three L. lactis strains in the development DSS-induced colitis, although L. lactis NCDO2118 and L. lactis MG1363 seem to act by different mechanisms when compared to L. lactis IL1403. Therefore, our study suggests that the anti-inflammatory role of L. lactis in this experimental model is not strain-specific even though some of their mechanisms of action might be. The protective effect against colonic inflammation produced by treatment with L. lactis may help to characterize this genus as a probiot bacterium with potential therapeutic use for inflammatory bowel diseases in humans.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Ilda Marçal de Souza and Hermes Oliveira for the excellent care of the animals.
Author contribution
Experimental procedures were performed by JLA, LL, NMR, MCC, PAVB, VBP and MG. Data analysis was done by JLA, LL, NMR, PAVB, DCC, TUM and ACGS. Bacteria Strains were provided by AM, VAA and VBP. Manuscript writing was prepared by JLA and AMCF. Manuscript revision was performed by JLA and AMCF.
Funding
This study was financially supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnologico), FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais), and PRPq-UFMG (Pró Reitoria de Pesquisa da UFMG), Brazil. Some of the authors are recipients of scholarships (J.L.A., L.L, M.C.C., A.C.G-S.) from CNPq and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), Brazil, and research fellowships (A.M.C.F., D.C.C., V.A., T.U.M.) from CNPq, Brazil.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
All authors consent to participate in this publication.
Consent for publication
All authors consent to publish the manuscript.
Conflict of interest
The authors declare no competing interests.
Footnotes
Responsible Editor: Mariana X Byndloss
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Juliana Lima Alves, Email: julianalima.nut@hotmail.com.
Ana Maria Caetano Faria, Email: anacaetanofaria@gmail.com.
References
- 1.Khor B, Gardet A, Xavier RJ (2011) Genetics and pathogenesis of inflammatory bowel disease. Nature [Internet]. 474:307–17. http://www.nature.com/articles/nature10209 [DOI] [PMC free article] [PubMed]
- 2.De Moreno De Leblanc A, Del Carmen S, Chatel JM, Miyoshi A, Azevedo V, Langella P et al (2015) Current review of genetically modified lactic acid bacteria for the prevention and treatment of colitis using murine models. Gastroenterol Res Pract 2015. 10.1155/2015/146972 [DOI] [PMC free article] [PubMed]
- 3.Melgar S, Bjursell M, Gerdin AK, Svensson L, Michaëlsson E, Bohlooly-Y M. Mice with experimental colitis show an altered metabolism with decreased metabolic rate. Am J Physiol - Gastrointest Liver Physiol. 2007;292:165–172. doi: 10.1152/ajpgi.00152.2006. [DOI] [PubMed] [Google Scholar]
- 4.Basso PJ, Fonseca MTC, Bonfá G, Alves VBF, Sales-Campos H, Nardini V, et al. Association among genetic predisposition, gut microbiota, and host immune response in the etiopathogenesis of inflammatory bowel disease. Brazilian J Med Biol Res. 2014;47:727–737. doi: 10.1590/1414-431X20143932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mowat AMI. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 6.Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2015;27:1–19. doi: 10.1002/0471142735.im1525s104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melgar S, Karlsson A, Michaëlsson E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: Correlation between symptoms and inflammation. Am J Physiol - Gastrointest Liver Physiol. 2005;288:1328–1338. doi: 10.1152/ajpgi.00467.2004. [DOI] [PubMed] [Google Scholar]
- 8.Okayasu ISA (1990) Reliable experimental acute and chronic 694–702 [DOI] [PubMed]
- 9.Abraham BP, Quigley EMM (2017) Probiotics in inflammatory bowel disease. Gastroenterol Clin North Am [Internet]. Elsevier Inc;46:769–82. 10.1016/j.gtc.2017.08.003 [DOI] [PubMed]
- 10.FAO/WHO (2002) Guidelines for the evaluation of probiotics in food 1–11
- 11.Ishida T, Yokota A, Umezawa Y, Toda T, Yamada K. Identification and characterization of lactococcal and Acetobacter strains isolated from traditional Caucasusian fermented milk. J Nutr Sci Vitaminol (Tokyo) 2005;51:187–193. doi: 10.3177/jnsv.51.187. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe T, Nishio H, Tanigawa T, Yamagami H, Okazaki H, Watanabe K, et al. Probiotic Lactobacillus casei strain Shirota prevents indomethacin-induced small intestinal injury: Involvement of lactic acid. Am J Physiol - Gastrointest Liver Physiol. 2009;297:506–513. doi: 10.1152/ajpgi.90553.2008. [DOI] [PubMed] [Google Scholar]
- 13.Tang Y, Wu Y, Huang Z, Dong W, Deng Y, Wang F et al (2017) Administration of probiotic mixture DM#1 ameliorated 5-fluorouracil–induced intestinal mucositis and dysbiosis in rats. Nutrition [Internet]. Elsevier Inc. 33:96–104. 10.1016/j.nut.2016.05.003 [DOI] [PubMed]
- 14.Zhang F, Li Y, Wang X, Wang S, Bi D (2019) The Impact of lactobacillus plantarum on the gut microbiota of mice with DSS-induced colitis. Biomed Res Int Hindawi 2019. 10.1155/2019/3921315 [DOI] [PMC free article] [PubMed]
- 15.Chen Y, Jin Y, Stanton C, Paul Ross R, Zhao J, Zhang H et al (2020) Alleviation effects of Bifidobacterium breve on DSS-induced colitis depends on intestinal tract barrier maintenance and gut microbiota modulation. Eur J Nutr [Internet]. Springer Berlin Heidelberg. 10.1007/s00394-020-02252-x [DOI] [PubMed]
- 16.Berlec A, Perše M, Ravnikar M, Lunder M, Erman A, Cerar A, et al. Dextran sulphate sodium colitis in C57BL/6J mice is alleviated by Lactococcus lactis and worsened by the neutralization of Tumor necrosis Factor α. Int Immunopharmacol. 2017;43:219–226. doi: 10.1016/j.intimp.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 17.Papadimitriou K, Pot B, Tsakalidou E (2015) How microbes adapt to a diversity of food niches. Curr Opin Food Sci [Internet]. Elsevier Ltd;;2:29–35. 10.1016/j.cofs.2015.01.001
- 18.Mancha-Agresti P, Drumond MM, Carmo FLR do, Santos MM, Santos JSC dos, Venanzi F et al (2017) A new broad range plasmid for DNA delivery in eukaryotic cells using lactic acid bacteria: in vitro and in vivo assays. Mol Ther - Methods Clin Dev [Internet]. Elsevier Ltd.;4:83–91. 10.1016/j.omtm.2016.12.005 [DOI] [PMC free article] [PubMed]
- 19.Donohue DC, Gueimonde M (2011) Some considerations for the safety of novel probiotic bacteria. Lact Acid Bact Microbiol Funct Asp Fourth Ed 423–38
- 20.Song AAL, In LLA, Lim SHE, Rahim RA. A review on Lactococcus lactis: from food to factory. Microb Cell Fact BioMed Central. 2017;16:1–15. doi: 10.1186/s12934-017-0669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishitani Y, Tanoue T, Yamada K, Ishida T, Yoshida M, Azuma T et al (2009) Lactococcus lactis subsp. cremoris FC alleviates symptoms of colitis induced by dextran sulfate sodium in mice. Int Immunopharmacol [Internet]. Elsevier B.V.;9:1444–51. 10.1016/j.intimp.2009.08.018 [DOI] [PubMed]
- 22.Luerce TD, Gomes-Santos AC, Rocha CS, Moreira TG, Cruz DN, Lemos L, et al. Anti-inflammatory effects of Lactococcus lactis NCDO 2118 during the remission period of chemically induced colitis. Gut Pathog. 2014;6:1–11. doi: 10.1186/1757-4749-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gusmao-Silva G, Aguiar SLF, Miranda MCG, Guimarães MA, Alves JL, Vieira AT, et al. Hsp65-producing lactococcocus lactis prevents antigen-induced arthritis in mice. Front Immunol. 2020;11:1–15. doi: 10.3389/fimmu.2020.562905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stiles ME, Holzapfel WH. Lactic acid bacteria of foods and their current taxonomy. Int J Food Microbiol. 1997;36:1–29. doi: 10.1016/S0168-1605(96)01233-0. [DOI] [PubMed] [Google Scholar]
- 25.de Moreno de LeBlanc A, del Carmen S, Zurita-Turk M, Santos Rocha C, van de Guchte M, Azevedo V et al (2011) Importance of IL-10 modulation by probiotic microorganisms in gastrointestinal inflammatory diseases. ISRN Gastroenterol 2011:1–11 [DOI] [PMC free article] [PubMed]
- 26.Oliveira LC, Saraiva TDL, Soares SC, Ramos RTJ, Sá PHCG, Carneiro AR, et al. Genome Sequence of Lactococcus lactis subsp. lactis NCDO 2118, a GABA-Producing Strain. Genome Announc. 2014;2:9–10. doi: 10.1128/genomeA.00980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolotin A, Wincker P, Mauger S, Jaillon O, Malarme K, Weissenbach J, et al. The complete genome sequence of the lactic acid bacterium lactococcus lactis ssp. lactis IL1403. Genome Res. 2001;11:731–53. doi: 10.1101/gr.169701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wegmann U, O’Connell-Motherway M, Zomer A, Buist G, Shearman C, Canchaya C, et al. Complete genome sequence of the prototype lactic acid bacterium Lactococcus lactis subsp. cremoris MG1363. J Bacteriol. 2007;189:3256–70. doi: 10.1128/JB.01768-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper HS, Murthy SN, Shah RS, Sedergran DJ (1993) Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest [Internet]. 69:238—249. http://europepmc.org/abstract/MED/8350599 [PubMed]
- 30.Diniz SOF, Barbosa AJA, Araújo ID, Nelson DL, da Machado LAS, Filho MB, et al. Assessment of bacterial translocation in obstructive jaundice using Tc-99m Escherichia coli. Brazilian Arch Biol Technol. 2005;48:45–9. doi: 10.1590/S1516-89132005000700006. [DOI] [Google Scholar]
- 31.Maioli TU, De Melo SB, Dias MN, Paiva NC, Cardoso VN, Fernandes SO, et al. Pretreatment with Saccharomyces boulardii does not prevent the experimental mucositis in Swiss mice. J Negat Results Biomed. 2014;13:1–8. doi: 10.1186/1477-5751-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Generoso SDV, Rodrigues NM, Trindade LM, Paiva NC, Cardoso VN, Carneiro CM et al (2015) Dietary supplementation with omega-3 fatty acid attenuates 5-fluorouracil induced mucositis in mice. Lipids Health Dis [Internet]. Lipids in Health and Disease.14:1–10. 10.1186/s12944-015-0052-z [DOI] [PMC free article] [PubMed]
- 33.McCafferty DM, Sihota E, Muscara M, Wallace JL, Sharkey KA, Kubes P. Spontaneously developing chronic colitis in IL-10/iNOS double-deficient mice. Am J Physiol - Gastrointest Liver Physiol. 2000;279:90–99. doi: 10.1152/ajpgi.2000.279.1.G90. [DOI] [PubMed] [Google Scholar]
- 34.De Matos OG, Amaral SS, Pereira Da Silva PEM, Perez DA, Alvarenga DM, Ferreira AVM et al (2012) Dietary supplementation with omega-3-pufa-rich fish oil reduces signs of food allergy in ovalbumin-sensitized mice. Clin Dev Immunol 2012. 10.1155/2012/236564 [DOI] [PMC free article] [PubMed]
- 35.Gomes-Santos AC, Moreira TG, Castro-Junior AB, Horta BC, Lemos L, Cruz DN et al (2012) New insights into the immunological changes in IL-10-deficient mice during the course of spontaneous inflammation in the gut mucosa. Clin Dev Immunol 2012. 10.1155/2012/560817 [DOI] [PMC free article] [PubMed]
- 36.Herzenberg LA, Tung J, Moore WA, Herzenberg LA, Parks DR. Interpreting flow cytometry data: a guide for the perplexed - Supplementary Information. Nat Immunol. 2006;7:681–685. doi: 10.1038/ni0706-681. [DOI] [PubMed] [Google Scholar]
- 37.Poritz LS, Garver KI, Green C, Fitzpatrick L, Ruggiero F, Koltun WA. Loss of the tight junction protein ZO-1 in Dextran sulfate sodium induced colitis. J Surg Res. 2007;140:12–19. doi: 10.1016/j.jss.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 38.O’Sullivan DJ. Screening of intestinal microflora for effective probiotic bacteria. J Agric Food Chem. 2001;49:1751–1760. doi: 10.1021/jf0012244. [DOI] [PubMed] [Google Scholar]
- 39.Brandtzaeg P (1998) Development and basic mechanisms of human gut immunity. Nutr Rev 56 [DOI] [PubMed]
- 40.Muzaki ARBM, Tetlak P, Sheng J, Loh SC, Setiagani YA, Poidinger M, et al. Intestinal CD103+ CD11b- dendritic cells restrain colitis via IFN-γ-induced anti-inflammatory response in epithelial cells. Mucosal Immunol. 2016;9:336–351. doi: 10.1038/mi.2015.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Annacker O, Coombes JL, Malmstrom V, Uhlig HH, Bourne T, Johansson-Lindbom B, et al. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051–1061. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coombes JL, Siddiqui KRR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β -and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mercadante ACT, Perobelli SM, Alves APG, Gonçalves-Silva T, Mello W, Gomes-Santos AC, et al. Oral combined therapy with probiotics and alloantigen induces B Cell–dependent long-lasting specific tolerance. J Immunol. 2014;192:1928–1937. doi: 10.4049/jimmunol.1301034. [DOI] [PubMed] [Google Scholar]
- 44.Forkel M, Mjösberg J (2016) Dysregulation of group 3 Innate lymphoid cells in the pathogenesis of inflammatory bowel disease. Curr Allergy Asthma Rep [Internet]. Curr Allergy Asthma Rep 16. 10.1007/s11882-016-0652-3 [DOI] [PMC free article] [PubMed]
- 45.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-P. [DOI] [PubMed] [Google Scholar]
- 46.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2009;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aziz M, Holodick NE, Rothstein TL, Wang P. The Role of B-1 Cells in Inflammation. Immunol Res. 2015;63:153–66. doi: 10.1007/s12026-015-8708-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 49.Koelink PJ, Bloemendaal FM, Li B, Westera L, Vogels EWM, van Roest M, Gloudemans AK, van’tWout AB, Korf H, Vermeire S, Te Velde AA, Ponsioen CY, D’Haens GR, Verbeek JS, Geiger TL, Wildenberg ME, van den Brink GR. Anti-TNF therapy in IBD exerts its therapeutic effect through macrophage IL-10 signalling. Gut. 2020;69(6):1053–1063. doi: 10.1136/gutjnl-2019-318264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.
Not applicable.