Abstract
Aims
There is considerable controversy regarding optimal management of patients with paraesophageal hiatus hernia (pHH). This survey aims at identifying recommended strategies for work-up, surgical therapy, and postoperative follow-up using Delphi methodology.
Methods
We conducted a 2-round, 33-question, web-based Delphi survey on perioperative management (preoperative work-up, surgical procedure and follow-up) of non-revisional, elective pHH among European surgeons with expertise in upper-GI. Responses were graded on a 5-point Likert scale and analyzed using descriptive statistics. Items from the questionnaire were defined as “recommended” or “discouraged” if positive or negative concordance among participants was > 75%. Items with lower concordance levels were labelled “acceptable” (neither recommended nor discouraged).
Results
Seventy-two surgeons with a median (IQR) experience of 23 (14–30) years from 17 European countries participated (response rate 60%). The annual median (IQR) individual and institutional caseload was 25 (15–36) and 40 (28–60) pHH-surgeries, respectively. After Delphi round 2, “recommended” strategies were defined for preoperative work-up (endoscopy), indication for surgery (typical symptoms and/or chronic anemia), surgical dissection (hernia sac dissection and resection, preservation of the vagal nerves, crural fascia and pleura, resection of retrocardial lipoma) and reconstruction (posterior crurorrhaphy with single stitches, lower esophageal sphincter augmentation (Nissen or Toupet), and postoperative follow-up (contrast radiography). In addition, we identified “discouraged” strategies for preoperative work-up (endosonography), and surgical reconstruction (crurorrhaphy with running sutures, tension-free hiatus repair with mesh only). In contrast, many items from the questionnaire including most details of mesh augmentation (indication, material, shape, placement, and fixation technique) were “acceptable”.
Conclusions
This multinational European Delphi survey represents the first expert-led process to identify recommended strategies for the management of pHH. Our work may be useful in clinical practice to guide the diagnostic process, increase procedural consistency and standardization, and to foster collaborative research.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00464-023-09933-8.
Keywords: Hiatus hernia, Paraesophageal hernia, Surgical technique, Mesh, Fundoplication, Delphi survey
Optimal treatment of paraesophageal hiatus hernia (pHH) remains a highly debated topic in upper-gastrointestinal (UGI) surgery. Numerous aspects regarding both the diagnostic work-up and the surgical management of this clinical entity are not broadly accepted, and even experts disagree on critical components including the application of surgical meshes for hiatal reinforcement, the indication for complementary sphincter augmentation and the diagnosis and treatment of short esophagus.
Based on our own clinical experience, we hypothesized that the current surgical practice may reflect those uncertainties. Since there are no uniform recommendations from national or international societies on this topic, we found it pertinent to perform a Delphi survey among recognized experts in UGI-surgery to identify recommended strategies.
Material and methods
Expert panel
Inclusion criteria for invited experts were ≥ 10 years of experience in UGI-surgery, an annual institutional caseload of ≥ 30 hiatal hernias, and a specialty interest in UGI-surgery as evidenced by recent (within the last 10 years) publications in the field. From their personal professional network, the lead authors of this project worked out a list of potential participants fulfilling the above criteria. This list was supplemented by board members of the European Foregut Society (EFS), a recently founded scientific society with a specific focus on benign esophago-gastric disease.
Delphi survey
To minimize bias, the focus was strictly on elective (planned) repair of non-revisional pHH; other hernia types, emergencies and recurrences were considered outside the scope of this work. PHH was defined as Skinner type II (true paraesophageal), type III (mixed sliding/axial and paraesophageal), and type IV (paraesophageal and herniation of other abdominal organs). The lead authors designed a 33-question survey to elicit respondent feedback on the following parameters: personal and institutional experience, diagnostic work-up, indications, technical details of hiatal repair (access routes, surgical dissection and reconstruction), and postoperative follow-up (Online Appendix 1). An online survey tool (SurveyMonkey, Palo Alto, CA, USA) was employed to disseminate the survey and to collect answers. In May 2021, experts were invited to participate via an email containing the study protocol, the expected number of Delphi rounds, and the anticipated time commitment. After agreeing to participate, experts were provided with access to each Delphi round via secure, institute-to-institute email. The attendees were also invited to leave comments on each question and to suggest changes to the wording. Throughout the Delphi survey, voting and commenting was conducted anonymously.
In both Delphi rounds, participants were asked to rank their agreement on each question using a 5-point Likert scale. Two scale variations were employed, the first indicated level of recommendation (1 = strongly recommended, 2 = recommended, 3 = neither recommended nor discouraged, 4 = discouraged, 5 = strongly discouraged) whilst the second informed the consent with which technical steps are performed by the participant (1 = a great deal, 2 = considerably, 3 = moderately, 4 = slightly, 5 = not at all).
After completion of Delphi round 1, the lead authors adapted the results according to the expert’s suggestions to create the next questionnaire. In Delphi round 2, the percentage of concordance (“strongly recommended” or “recommended” and “a great deal” or “considerably”) from the preceding round were visible, enabling experts to re-vote in consideration of previous results (Online Appendix 2).
Experts were given two weeks to complete each round. Two reminders were sent, the first one week after opening and the second two days before closing of each round. Data collection took place from May 2021 to September 2021.
Data analysis
Items of the questionnaire were defined as “recommended” if positive concordance among participants was > 75% (“strongly recommended” or “recommended” and “a great deal” or “considerably”) on a given question. Likewise, items were defined as “discouraged” if negative concordance among participants was > 75% (“discouraged” or “strongly discouraged” and “slightly” or “not at all”). Items with lower positive or negative concordance levels were categorized as “acceptable” (neither recommended nor discouraged). Data were analyzed using descriptive statistics and expressed as percentage of agreement and median (IQR) using SPSS version 26.0 (IBM Inc., Chicago, Ill, USA). No IRB approval or written consent was required for the paper.
Results
Participants
One hundred twenty-one European experts for upper-GI surgery were invited for round 1 and 2 of the Delphi, and 72 surgeons across 17 countries responded (response rate 60%). Details of participants are displayed in Table 1.
Table 1.
Details of the participants
| (%) | n | |
|---|---|---|
| Institution | ||
| University Hospital | 67 | 48 |
| Maximum Care Hospital | 8 | 6 |
| Teaching Hospital | 14 | 10 |
| General Hospital | 1 | 1 |
| Private Hospital | 10 | 7 |
| Position | ||
| Head of department | 39 | 28 |
| Senior consultant | 40 | 29 |
| Consultant | 11 | 8 |
| Attending surgeon | 3 | 2 |
| Retired | 7 | 5 |
| Institutional caseload per year | ||
| Mean | 52 | |
| Median (IQR) | 40 (28–60) | |
| Individual caseload per year | ||
| Mean | 23 | |
| Median (IQR) | 25 (15–36) |
Definition of paraesophageal hiatus hernia
There was agreement (91%) that pHH should be defined as the presence of a hernia sac extending from the abdominal cavity and/or bursa omentalis through the hiatus into the paraesophageal mediastinum and containing a variable portion of stomach. In contrast, the sole presence of a hernia sac or of gastric prolapse into the mediastinum was not considered as a suitable definition of pHH.
Preoperative diagnostic work-up and indication for surgery
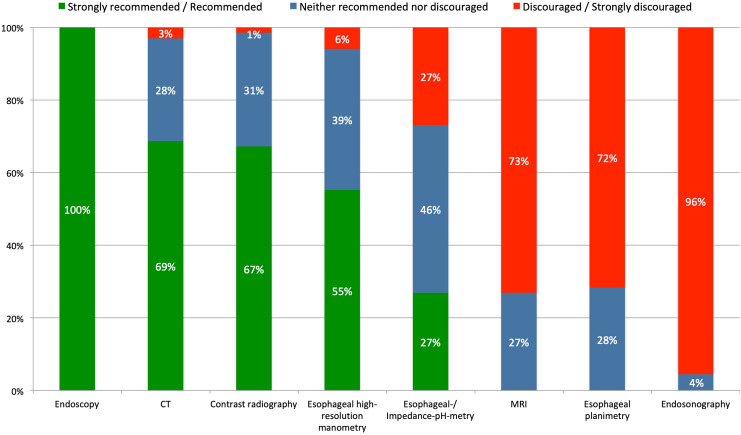
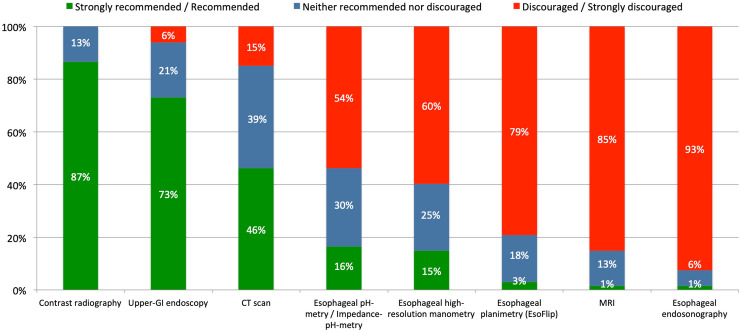
Upper-GI endoscopy was formally “recommended” and esophageal endosonography was “discouraged” as preoperative diagnostic tests. In contrast, most other diagnostic tools (CT scan, contrast radiography, esophageal manometry, (impedance-) pH-testing, MRI, and esophageal planimetry) were categorized as “acceptable” (Fig. 1).
Fig. 1.
Expert recommendations for diagnostic work-up for pHH
Surgery was the “recommended” therapeutic strategy in symptomatic and anemic patients independent of biological age (> or < 70 years). Conversely, indication for surgery was “acceptable” in patients with no or minor symptoms.
Access routes and steps of surgical dissection
Laparoscopy was the preferred access route of most (89%) participants. Other surgical access techniques (robotic-assisted laparoscopy (8%), thoracoscopy (1%), thoracotomy (1%)) were rarely used.
“Recommended” steps of the surgical dissection phase entailed resection of the hernia sac, visualization of both vagal nerves, resection of the retrocardiac lipoma, preservation of the crural fascia and of the pleural sac; all other details of surgical dissection were categorized as “acceptable” (Table 2).
Table 2.
Expert recommendations of surgical dissection for pHH repair
| Strongly recommended/Recommended (%) | Neither recommended nor discouraged (%) | Discouraged/Strongly discouraged (%) | Overall assessment | |
|---|---|---|---|---|
| Dissection of hernia sac from mediastinum | 100 | 0 | 0 | Recommended |
| Visualization of both vagal nerves | 96 | 4 | 0 | Recommended |
| Preservation of the crural fascia | 96 | 1 | 3 | Recommended |
| Resection/mobilization of retro-cardial lipoma | 93 | 4 | 3 | Recommended |
| Resection of hernia sac | 90 | 7 | 3 | Recommended |
| Preservation of pleural sac | 84 | 10 | 6 | Recommended |
| Repositioning of hernia sac contents during dissection of hernia sac from the mediastinum | 73 | 24 | 3 | Acceptable |
| Mobilization of gastric fundus | 67 | 21 | 12 | Acceptable |
| Preservation of aberrant left hepatic artery | 67 | 27 | 6 | Acceptable |
| Resection/mobilization of pre-cardial fat-pad | 66 | 21 | 13 | Acceptable |
| Repositioning of hernia sac contents as initial step of procedure | 63 | 24 | 13 | Acceptable |
| Intraoperative positioning of a large-bore esophageal tube | 37 | 37 | 25 | Acceptable |
| Preservation of hepatic branches of vagus nerves | 28 | 52 | 19 | Acceptable |
| Intraoperative endoscopy | 25 | 58 | 16 | Acceptable |
| Preservation of pulmonary branches of vagus nerves | 21 | 58 | 21 | Acceptable |
| Visualization of pulmonary veins | 6 | 48 | 46 | Acceptable |
Surgical reconstruction
“Recommended” steps of surgical reconstruction included suture repair of the hiatus and lower esophageal sphincter augmentation. All other steps of surgical reconstruction were categorized as “acceptable” (Table 3).
Table 3.
Expert recommendations for technical steps during reconstruction in pHH repair
| Strongly recommended/Recommended (%) | Neither recommended nor discouraged (%) | Discouraged/Strongly discouraged (%) | Overall assessment | |
|---|---|---|---|---|
| Suture repair | 100 | 0 | 0 | Recommended |
| Antireflux procedure | 96 | 4 | 0 | Recommended |
| In case of short esophagus: Esophageal lengthening procedure (Collis or other) | 51 | 36 | 13 | Acceptable |
| Positioning of large-bore esophageal tube | 45 | 37 | 18 | Acceptable |
| Gastropexy | 40 | 33 | 27 | Acceptable |
| Use of mesh | 25 | 52 | 22 | Acceptable |
| Postoperative wound drain | 24 | 25 | 51 | Acceptable |
| Postoperative gastric decompression tube | 16 | 25 | 58 | Acceptable |
| Postoperative chest drain | 6 | 25 | 69 | Acceptable |
| Ligamentum teres to reinforce hiatal repair | 4 | 43 | 52 | Acceptable |
| Use of relaxing diaphragmatic incisions | 4 | 39 | 57 | Acceptable |
| Left hepatic lobe (hepatic shoulder) to reinforce hiatal repair | 3 | 30 | 67 | Acceptable |
Hiatoplasty (crurorrhaphy and mesh augmentation)
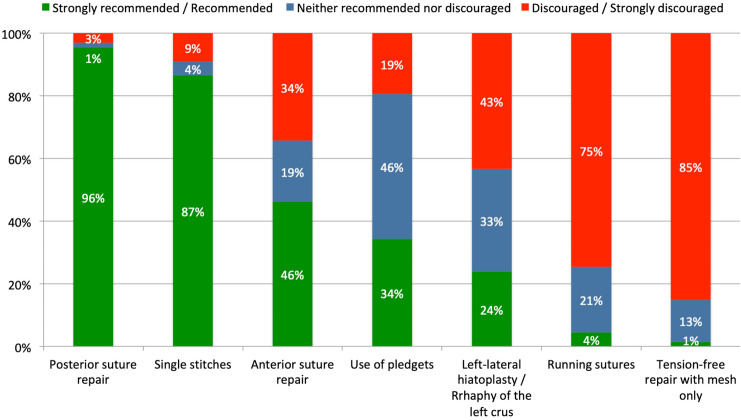
“Recommended” techniques for hiatoplasty were posterior crurorraphy, use of single stitches and non-resorbable braided suture material (size 0 or 2-0). In contrast, crurorrhaphy with running sutures and diaphragmatic relaxing incisions were “discouraged”. All other technical details of hiatoplasty (anterior and left-lateral crurorraphy, use of pledgets) were classified as “acceptable” (Fig. 2).
Fig. 2.
Expert recommendations for techniques for hiatoplasty in pHH repair
Most participants (72%) perform selective mesh augmentation (always 10%, never 18%), and there were no “recommended” or “discouraged” strategies regarding material, placement, shape, and fixation of mesh. Among selective mesh-users, indications were fragile diaphragmatic musculature (78%), large hiatus defects (73%), and recurrent hernia (73%). Preferred materials were synthetic absorbable, synthetic non- or partially absorbable, and biological meshes in 47%, 39%, and 25%, respectively, and mesh placement is performed posteriorly open (u-shape), anteriorly open (u-shape), completely encircling the esophagus (circular), and on posterior hiatoplasty only in 54%, 36%, 26%, and 5%, respectively. In contrast, most participants (81%) agreed to use sutures for mesh fixation, while other techniques (tacks 19%, fibrin glue 31%) did not reach concordance.
Of note, a relevant percentage of surgeons encountered mesh-related complications such as erosion, stenosis, mesh migration in own or referred patients. (Table 4).
Table 4.
Mesh-associated complications encountered by participants
| Complication | In own patients (%) | In referred patients (%) | Never (%) |
|---|---|---|---|
| Mesh erosion (esophagus, stomach, or esophago-gastric junction) | 19 | 72 | 19 |
| Mesh erosion to other organs (aorta, lung) | 3 | 27 | 72 |
| Stenosis distal esophagus/esophago-gastric junction | 15 | 71 | 24 |
| Mesh migration | 14 | 59 | 35 |
| Mesh infection | 10 | 35 | 61 |
| Pericardial tamponade | 6 | 7 | 89 |
| Pleural hemorrhage | 7 | 7 | 86 |
| Perioperative hemorrhage caused by mesh fixation | 6 | 10 | 85 |
| Pneumothorax | 27 | 13 | 68 |
| Chronic pain | 17 | 35 | 55 |
| Seroma formation | 31 | 22 | 56 |
Lower esophageal sphincter augmentation and management of short esophagus
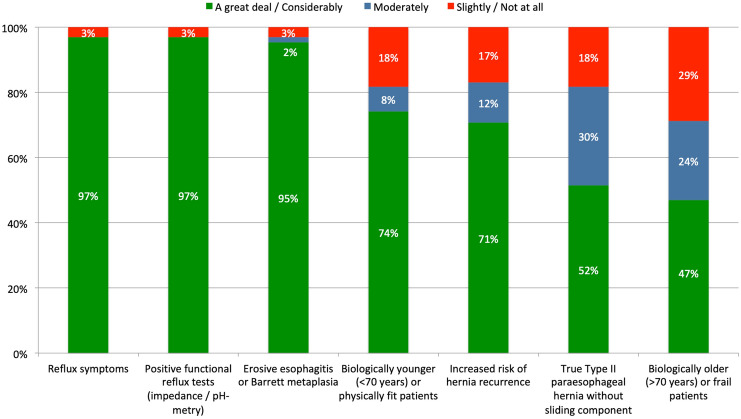
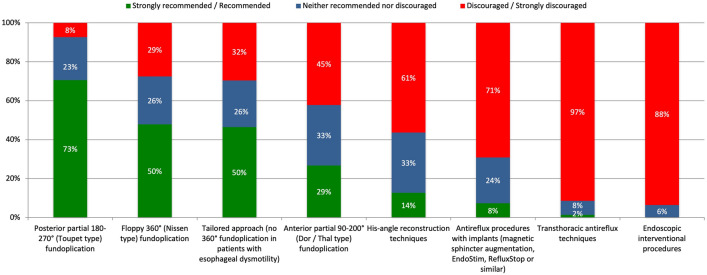
There was a high level of concordance (96%) among participants to perform an additional augmentation of the lower esophageal sphincter (LES) in all (64%) or selected (35%) patients. “Recommended” indications for LES augmentation were the presence of reflux symptoms (97%), erosive esophagitis or Barrett’s metaplasia (95%), and positive functional tests (97%). In contrast, biological age, increased risk for HH recurrence, and true Type II pHH did not impact the indication for LES augmentation (Fig. 3). Likewise, except “discouraged” transthoracic and interventional/endoscopic approaches, most LES augmentation techniques (total, partial and tailored fundoplication, techniques involving surgical implants) were categorized “acceptable” (Fig. 4).
Fig. 3.
Expert agreements for indications for LES augmentation in pHH repair
Fig. 4.
Expert recommendations for antireflux procedures in pHH repair
Only a minority (44%) of participants agreed that short esophagus (SE) is a relevant finding during pHH repair (not sure: 28%, disagree: 28%). Collis gastroplasty and simple gastropexy were the only “acceptable” surgical techniques; all other procedures were “discouraged”.
Follow up and clinical definition of recurrence
Contrast radiography was formally “recommended” as a diagnostic tool for clinical follow-up and “neutral” recommendation level was reached for upper-GI endoscopy, CT scan, esophageal manometry, pH-metry. In contrast, MRI, esophageal planimetry and endosonography were “discouraged” (Fig. 5).
Fig. 5.
Expert recommended diagnostic procedures to exclude recurrence after pHH repair
No recommendation was reached regarding the anatomical definition of recurrence and there was no negative or positive concordance > 75% to define clinical failure of pHH repair (Table 5).
Table 5.
Anatomical und clinical definition of recurrent hernia despite expert opinion
| Anatomical definition of recurrent hernia | (%) | n |
|---|---|---|
| Any evidence of gastric tissue above the diaphragm | 36 | 24 |
| At least 1 cm of gastric tissue above the diaphragm | 9 | 6 |
| At least 2 cm of gastric tissue above the diaphragm | 27 | 18 |
| At least 3 cm of gastric tissue above the diaphragm | 19 | 13 |
| At least 4 cm of gastric tissue above the diaphragm | 4 | 3 |
| Other | 4 | 3 |
| Clinical definition of recurrent hernia | (%) | n |
|---|---|---|
| Radiological and/or endoscopic evidence of gastric tissue above the diaphragm is sufficient to define recurrent hiatus hernia | 39 | 26 |
| Clinical evidence (symptoms) is sufficient to define recurrent hiatus hernia | 0 | 0 |
| Radiological and/or endoscopic (gastric tissue above the diaphragm) and clinical (symptoms) evidence is required to define recurrent hiatus hernia | 58 | 39 |
| Other | 3 | 2 |
Discussion
We performed this comprehensive survey among experts for UGI-surgery with the aim to identify recommended strategies for the treatment of pHH repair. As a result, we were able to identify high rates of concordance regarding indications for surgery, preoperative work-up, and several technical-surgical steps of hiatal dissection and reconstruction. However, an important lesson from this survey is that only few basic strategies for pHH are currently unanimously “recommended” or “discouraged”. Thus, huge uncertainties remain for many classical adjuncts such as the use of meshes, sphincter augmentation, and gastropexy, but also for the management of Short Esophagus and the correct definition and diagnosis of recurrence or failure. The limited concordance across elementary steps of pHH treatment observed in this European expert Delphi may reflect the influence of different surgical schools, but certainly mirrors a general therapeutic uncertainty caused by a notorious lack of reliable data and scientific evidence in the field.
The strengths of our survey include a large number of participating experts, a high response rate, and a well-defined index procedure (pHH). Furthermore, the modified Delphi approach enabled us to adapt and specify questions during the survey to achieve a sharper reflection of predominant recommendations.
The questionnaires were exclusively targeted at UGI surgeons with a high surgical caseload, the majority of whom holding appointments as chiefs or senior consultants in university or teaching hospitals. We selected pHH repair as the index procedure because this entity is highly prevalent (about 20% of all HH cases). In addition, exclusion of type I HH allowed for a more precise interpretation of results and conclusions. Our focus on pHH lies in contrast with some of the published literature, which comprises five surveys from the last decade, addressed to either members of the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) [1, 2], the European Association for Endoscopic Surgery (EAES), and members of the Swiss Society of Visceral Surgeons (SGVC) [3–5]. Of note, except for the European and the Swiss studies, which focused on type II-IV and type III HH [3] the SAGES surveys were designed to gather data on all types of HH including gastroesophageal reflux disease. Therefore, comparison with our results remains partly elusive. In addition, two retrospective population-based analyses on outcomes of mesh use in paraesophageal (type II–IV) HH repair using the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database have been recently reported [6, 7]. The prospective multi-national HERNIAMED data collection included 5462 paraesophageal hernia repairs and still remains another important source of information on the subject [8].
Conventional laparoscopy was the preferred surgical access route (92%), which compares favorably to data from the NSQIP and HERNIAMED databases [6, 8], as well as the SAGES and SGVC surveys [1, 2, 5] In accordance with the existing literature, our study confirms that transthoracic approaches for pHH have been largely abandoned. Of note, robotic-assisted laparoscopic surgery is still quite unpopular among European experts (8% preference), contrasting our recent survey among members of the SGVC (41% preference) [5].
The use of mesh to reinforce hiatal repairs remains a highly controversial subject, and a recent meta-analysis of RCT’s did not show any advantage of mesh augmentation over sutured hiatal closure [9]. Nevertheless,—as in the recent SGVC survey [5]—more than 80% of our participants use meshes in all or selected patients. Data from the HERNIAMED registry confirmed a rather constant, but much lower utilization rate of meshes in paraesophageal hernia repair in Austria, Germany, and Switzerland (33.0% and 38.9% in 2013 and 2019, respectively) [8], whereas in the US, this rate even decreased between from 45% in 2010 to 36% in 2017 [7]. It must be kept in mind that the current scientific evidence regarding meshes is extremely fragmented owing to different materials, shapes, fixation techniques, and follow-up periods, and the exact incidence of the much-feared mesh-related complications is not precisely known and estimated to 1–2% according to a large systematic review [10–17]. Nevertheless, more than 80% of our participants stated that they have encountered patients with mesh complications such as erosion. In contrast to earlier research, biological meshes play a minor role in the current surgical armamentarium, probably owing to the disappointing long-term results from two RCT’s [14, 16]. Thus, most of our participants chose synthetic non-absorbable mesh, which is in line with other recent surveys [3–5]. In this context, the significance of synthetic long-term absorbable materials remains unclear. Recent retrospective cohort studies have shown promising results, but long-term follow-up is currently not available [18–20].
Augmentation of the lower esophageal sphincter is a frequently performed adjunct to pHH repair and was formally “recommended” by our participants. Our results confirm recent data from the multi-institutional HERNIAMED registry reporting additional sphincter augmentation in paraesophageal hernia repair in 60–70% [8, 21]. However, routine and selective sphincter augmentation is performed by 64% and 35% of our participants, respectively, which contrasts the 84% (routine) and 9% (selective) sphincter augmentation rates in the EAES survey [3]. We assume that these differences reflect the rather weak scientific evidence for additional sphincter augmentation in the literature, which is mainly based on a single RCT [17], and a number of case series and small cohort studies [22].
As in a previous survey [5], there was no concordance among our experts regarding gastro- or fundo-phrenicopexy, probably due to the limited and conflicting evidence for this surgical adjunct in pHH repair [22–25]. Similarly, we found a mixed attitude towards short esophagus: 56% of participants were either unsure or disagreed that esophageal shortening represents a relevant finding during pHH repair. Of those confirming the importance of esophageal foreshortening, 67% agreed that esophageal lengthening (Collis) procedure and fundoplication around the neo-esophagus should be performed in this situation, which is in line with current expert recommendations [5, 26]
There are certain limitations associated with our study. First, as in other surveys on the subject [1–4], our questionnaire did not undergo a formal validation process before dissemination. Second, by reporting on results from the preceding round 1, peer pressure may have led to changing results in the second Delphi round to conform. Nevertheless, results from the previous round were presented in an anonymized form, thus eliminating the impact of dominant opinion leaders. Third, bias in our process of expert selection cannot be excluded and despite a very high response rate compared with other surveys, only seventy-two European experts in UGI-surgery participated, which potentially limits the relevance of our results.
Other limitations include the definition of the index procedure. Although classification of HH into four types according to Skinner and Belsey [27, 28] is broadly accepted, major uncertainties remain, particularly regarding an inconsistent and synonymous use of the terms “type II or III HH”, “mixed HH”, “large HH”, “pHH”, “upside-down stomach”, and “(intra)thoracic stomach”. Thus, in the US, the term “pHH” generally refers to all large HH (types I-IV) with migration of the fundus into the mediastinum, whereas many European surgeons strictly reserve this term for pHH type II (without any sliding component) independent of hernia size and of reducibility of the hernia sac [29–33]. Therefore, despite our effort to adequately define the index procedure of our survey, we cannot guarantee that all participants share a similar understanding of pHH.
In conclusion, consensus amongst European experts in UGI-surgery is limited to just a few basic components of surgical management for pHH. Whilst the observed therapeutic polypragmatism regarding many details of the procedure may simply manifest the clinical necessity to adapt to the clinical variability and complexity of pHH, it may also reflect a lack of standardization. Therefore, also considering the rapidly increasing prevalence of pHH in the ageing Western world, it may be a great opportunity for international surgical associations like the European Foregut Society (EFS) to promote well-designed clinical trials and guidelines.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
For the Hiatus Hernia Delphi Collaborative Group: Suzanne Sarah Gisbertz6, Ferdinand Köckerling7, Thorsten G. Lehmann8, Dietmar Lorenz9, Frank Alexander Granderath10, Riccardo Rosati11, Christoph Wullstein12, Lars Lundell13,56, Edward Cheong14, Philippe Nafteux15, Stefano Olmi16, Stefan Mönig17, Matthias Biebl18, Jessica Leers19, Joerg Zehetner20, Ivan Kristo21, Richard George Berrisford22, Ognjan M. Skrobić23, Aleksandar P. Simić23, Manuel Pera24, Peter Philipp Grimminger25, Ines Gockel26, Konstantinos Zarras27, Vincent Bernard Nieuwenhuijs28, James A. Gossage29, Mark i. van Berge Henegouwen30,57, Hubert J. Stein31, Sheraz R. Markar32, Willem Eduard Hueting33, Eduardo M. Targarona34, Jan Johansson35, Graeme D. Macaulay36, Bas P.L. Wijnhoven37, Frank Benedix38, Stephen E. Attwood39, Arnulf Heinrich Hölscher40, Pablo Priego41, Karl-Hermann Fuchs42, Misha D.P. Luyer43, Ewen A. Griffiths44, Torgeir Thorson Søvik45, Dimitrios Theodorou46, Bruno Sgromo47, Jarmo A. Salo48, Rishi Singhal49, Anders Thorell50, Giovanni Zaninotto51, Marko Bitenc52, Xavier Benoit D’journo53, Grant M. Fullarton54, Thomas Horbach55
Author contributions
SS, LB, NB, BM: study design, performing the experiments, critical revision of the manuscript. SG, CAG: performing the experiments, statistical analysis, interpretation of data, drafting the manuscript. CAG: study design, performing the experiments, interpretation of data, drafted the manuscript. All authors gave their final approval.
Funding
Open access funding provided by University of Zurich.
Declarations
Disclosure
Christian A. Gutschow received consulting fees and/or honoraria from Medtronic, B. Braun, BD/Bard medical and Micro-Tech Europe GmbH, and third party funding for his institution from B.Braun. Stephan Gerdes, Sebastian F. Schoppmann and Beat P. Müller-Stich have no conflict of interest or financial ties to disclose. Nicholas Boyle received consulting fees and/or honoraria from Johnson & Johnson and BD/Bard medical. Luigi Bonavina received third party funding for his institution from BD/Bard medical and participated on data safety boards with BD/Bard medical and Johnson & Johnson.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Christian A. Gutschow, Email: christian.gutschow@usz.ch
the Hiatus Hernia Delphi Collaborative Group:
Suzanne Sarah Gisbertz, Ferdinand Köckerling, Thorsten G. Lehmann, Dietmar Lorenz, Frank Alexander Granderath, Riccardo Rosati, Christoph Wullstein, Lars Lundell, Edward Cheong, Philippe Nafteux, Stefano Olmi, Stefan Mönig, Matthias Biebl, Jessica Leers, Joerg Zehetner, Ivan Kristo, Richard George Berrisford, Ognjan M. Skrobić, Aleksandar P. Simić, Manuel Pera, Peter Philipp Grimminger, Ines Gockel, Konstantinos Zarras, Vincent Bernard Nieuwenhuijs, James A. Gossage, Mark i. van Berge Henegouwen, Hubert J. Stein, Sheraz R. Markar, Willem Eduard Hueting, Eduardo M. Targarona, Jan Johansson, Graeme D. Macaulay, Bas P.L. Wijnhoven, Frank Benedix, Stephen E. Attwood, Arnulf Heinrich Hölscher, Pablo Priego, Karl-Hermann Fuchs, Misha D.P. Luyer, Ewen A. Griffiths, Torgeir Thorson Søvik, Dimitrios Theodorou, Bruno Sgromo, Jarmo A. Salo, Rishi Singhal, Anders Thorell, Giovanni Zaninotto, Marko Bitenc, Xavier Benoit D’journo, Grant M. Fullarton, and Thomas Horbach
References
- 1.Frantzides CT, Carlson MA, Loizides S, Papafili A, Luu M, Roberts J, Zeni T, Frantzides A. Hiatal hernia repair with mesh: a survey of SAGES members. Surg Endosc. 2010;24:1017–1024. doi: 10.1007/s00464-009-0718-6. [DOI] [PubMed] [Google Scholar]
- 2.Pfluke JM, Parker M, Bowers SP, Asbun HJ, Daniel Smith C. Use of mesh for hiatal hernia repair: a survey of SAGES members. Surg Endosc. 2012;26:1843–1848. doi: 10.1007/s00464-012-2150-6. [DOI] [PubMed] [Google Scholar]
- 3.Furnée EJB, Smith CD, Hazebroek EJ. The use of mesh in laparoscopic large hiatal hernia repair: a survey of European surgeons. Surg Laparoscopy, Endoscopy & Percutaneous Techniques. 2015;25:307–311. doi: 10.1097/SLE.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 4.Huddy JR, Markar SR, Ni MZ, Morino M, Targarona EM, Zaninotto G, Hanna GB. Laparoscopic repair of hiatus hernia: does mesh type influence outcome? A meta-analysis and European survey study. Surg Endosc. 2016;30:5209–5221. doi: 10.1007/s00464-016-4900-3. [DOI] [PubMed] [Google Scholar]
- 5.Gerdes S, Vetter D, Müller PC, Kapp JR, Gutschow CA. Current surgical concepts for type III hiatal hernia: a survey among members of the Swiss Society of Visceral Surgery. Swiss Med Wkly. 2021;151:W30052. doi: 10.4414/smw.2021.w30052. [DOI] [PubMed] [Google Scholar]
- 6.Schlosser KA, Maloney SR, Prasad T, Augenstein VA, Heniford BT, Colavita PD. Mesh reinforcement of paraesophageal hernia repair: Trends and outcomes from a national database. Surgery. 2019;166:879–885. doi: 10.1016/j.surg.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Schlottmann F, Strassle PD, Patti MG. Laparoscopic paraesophageal hernia repair: utilization rates of mesh in the USA and short-term outcome analysis. J Gastrointest Surg. 2017;21:1571–1576. doi: 10.1007/s11605-017-3452-8. [DOI] [PubMed] [Google Scholar]
- 8.Köckerling F, Simon T, Hukauf M, Hellinger A, Fortelny R, Reinpold W, Bittner R. The importance of registries in the postmarketing surveillance of surgical meshes. Ann Surg. 2018;268:1097–1104. doi: 10.1097/SLA.0000000000002326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petric J, Bright T, Liu DS, Wee Yun M, Watson DI. Sutured versus mesh-augmented hiatus hernia repair: a systematic review and meta-analysis of randomized controlled trials. Ann Surg. 2022;275:e45–e51. doi: 10.1097/SLA.0000000000004902. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Cheng T. Mesh erosion after hiatal hernia repair: the tip of the iceberg? Hernia. 2019;23:1243–1252. doi: 10.1007/s10029-019-02011-w. [DOI] [PubMed] [Google Scholar]
- 11.Frantzides CT. A prospective, randomized trial of laparoscopic polytetrafluoroethylene (PTFE) patch repair vs simple cruroplasty for large hiatal hernia. Arch Surg. 2002;137:649. doi: 10.1001/archsurg.137.6.649. [DOI] [PubMed] [Google Scholar]
- 12.Granderath FA. Laparoscopic Nissen fundoplication with prosthetic hiatal closure reduces postoperative intrathoracic wrap herniation: preliminary results of a prospective randomized functional and clinical study. Arch Surg. 2005;140:40. doi: 10.1001/archsurg.140.1.40. [DOI] [PubMed] [Google Scholar]
- 13.Ilyashenko VV, Grubnyk VV, Grubnik VV. Laparoscopic management of large hiatal hernia: mesh method with the use of ProGrip mesh versus standard crural repair. Surg Endosc. 2018;32:3592–3598. doi: 10.1007/s00464-018-6087-2. [DOI] [PubMed] [Google Scholar]
- 14.Oelschlager BK, Pellegrini CA, Hunter JG, Brunt ML, Soper NJ, Sheppard BC, Polissar NL, Neradilek MB, Mitsumori LM, Rohrmann CA, Swanstrom LL. biologic prosthesis to prevent recurrence after laparoscopic paraesophageal hernia repair: long-term follow-up from a multicenter, prospective, randomized trial. J Am Coll Surg. 2011;213:461–468. doi: 10.1016/j.jamcollsurg.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Oor JE, Roks DJ, Koetje JH, Broeders JA, van Westreenen HL, Nieuwenhuijs VB, Hazebroek EJ. Randomized clinical trial comparing laparoscopic hiatal hernia repair using sutures versus sutures reinforced with non-absorbable mesh. Surg Endosc. 2018;32:4579–4589. doi: 10.1007/s00464-018-6211-3. [DOI] [PubMed] [Google Scholar]
- 16.Watson DI, Thompson SK, Devitt PG, Aly A, Irvine T, Woods SD, Gan S, Game PA, Jamieson GG. Five year follow-up of a randomized controlled trial of laparoscopic repair of very large hiatus hernia with sutures versus absorbable versus nonabsorbable mesh. Ann Surg. 2020;272:241–247. doi: 10.1097/SLA.0000000000003734. [DOI] [PubMed] [Google Scholar]
- 17.Müller-Stich BP, Kenngott HG, Gondan M, Stock C, Linke GR, Fritz F, Nickel F, Diener MK, Gutt CN, Wente M, Büchler MW, Fischer L. Use of mesh in laparoscopic paraesophageal hernia repair: a meta-analysis and risk-benefit analysis. PLoS ONE. 2015;10:e0139547. doi: 10.1371/journal.pone.0139547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdelmoaty WF, Dunst CM, Filicori F, Zihni AM, Davila-Bradley D, Reavis KM, Swanstrom LL, DeMeester SR. Combination of surgical technique and bioresorbable mesh reinforcement of the crural repair leads to low early hernia recurrence rates with laparoscopic paraesophageal hernia repair. J Gastrointest Surg. 2020;24:1477–1481. doi: 10.1007/s11605-019-04358-y. [DOI] [PubMed] [Google Scholar]
- 19.Panici Tonucci T, Asti E, Sironi A, Ferrari D, Bonavina L. Safety and efficacy of crura augmentation with Phasix ST mesh for large hiatal hernia: 3-year single-center experience. J Laparoendosc Adv Surg Tech. 2020;30:369–372. doi: 10.1089/lap.2019.0726. [DOI] [PubMed] [Google Scholar]
- 20.Aiolfi A, Cavalli M, Sozzi A, Lombardo F, Lanzaro A, Panizzo V, Bonitta G, Mendogni P, Bruni PG, Campanelli G, Bona D. Medium-term safety and efficacy profile of paraesophageal hernia repair with Phasix-ST® mesh: a single-institution experience. Hernia. 2022;26:279–286. doi: 10.1007/s10029-021-02528-z. [DOI] [PubMed] [Google Scholar]
- 21.Köckerling F, Zarras K, Adolf D, Kraft B, Jacob D, Weyhe D, Schug-Pass C. What Is the Reality of Hiatal Hernia Management?—A Registry Analysis. Front Surg. 2020;7:584196. doi: 10.3389/fsurg.2020.584196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohn GP, Price RR, DeMeester SR, Zehetner J, Muensterer OJ, Awad Z, Mittal SK, Richardson WS, Stefanidis D, Fanelli RD, for the SAGES Guidelines Committee Guidelines for the management of hiatal hernia. Surg Endosc. 2013;27:4409–4428. doi: 10.1007/s00464-013-3173-3. [DOI] [PubMed] [Google Scholar]
- 23.Diaz S. Laparoscopic paraesophageal hernia repair, a challenging operation, medium-term outcome of 116 patients. J Gastrointest Surg. 2003;7:59–67. doi: 10.1016/S1091-255X(02)00151-8. [DOI] [PubMed] [Google Scholar]
- 24.Malm J, Rosen M, Ponsky J, Fanning A. Anterior gastropexy may reduce the recurrence rate after laparoscopic paraesophageal hernia repair. Surg Endosc. 2003;17:1036–1041. doi: 10.1007/s00464-002-8765-2. [DOI] [PubMed] [Google Scholar]
- 25.Poncet G, Robert M, Roman S, Boulez J-C. Laparoscopic repair of large hiatal hernia without prosthetic reinforcement: late results and relevance of anterior gastropexy. J Gastrointest Surg. 2010;14:1910–1916. doi: 10.1007/s11605-010-1308-6. [DOI] [PubMed] [Google Scholar]
- 26.Durand L, De Antón R, Caracoche M, Covián E, Gimenez M, Ferraina P, Swanström L. Short esophagus: selection of patients for surgery and long-term results. Surg Endosc. 2012;26:704–713. doi: 10.1007/s00464-011-1940-6. [DOI] [PubMed] [Google Scholar]
- 27.Altorki NK, Yankelevitz D, Skinner DB. Massive hiatal hernias: The anatomic basis of repair. J Thorac Cardiovasc Surg. 1998;115:828–835. doi: 10.1016/S0022-5223(98)70363-0. [DOI] [PubMed] [Google Scholar]
- 28.Skinner DB, Belsey RH. Surgical management of esophageal reflux and hiatus hernia. Long-term results with 1,030 patients. J Thorac Cardiovasc Surg. 1967;53:33–54. doi: 10.1016/S0022-5223(19)43239-X. [DOI] [PubMed] [Google Scholar]
- 29.Cheverie JN, Lam J, Neki K, Broderick RC, Lee AM, Matsuzaki T, Cubas R, Sandler BJ, Jacobsen GR, Fuchs K-H, Horgan S. Paraesophageal hernia repair: a curative consideration for chronic anemia? Surg Endosc. 2020;34:2243–2247. doi: 10.1007/s00464-019-07014-3. [DOI] [PubMed] [Google Scholar]
- 30.Kahrilas PJ, Kim HC, Pandolfino JE. Approaches to the diagnosis and grading of hiatal hernia. Best Pract Res Clin Gastroenterol. 2008;22:601–616. doi: 10.1016/j.bpg.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The SAGES, Committee G, Stefanidis D, Hope WW, Kohn GP, Reardon PR, Richardson WS, Fanelli RD. Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc. 2010;24:2647–2669. doi: 10.1007/s00464-010-1267-8. [DOI] [PubMed] [Google Scholar]
- 32.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R, the Global Consensus Group The montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 33.DeMeester TR. Shackelford’s surgery of the alimentary tract. Amsterdam: Elsevier; 2019. Etiology and natural history of gastroesophageal reflux disease and predictors of progressive disease; pp. 204–220. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.