Abstract
Purpose of Review
The addition of lateral extra-articular tenodesis (LET) to anterior cruciate ligament (ACL) reconstruction (ACLR) has become increasingly popular to address residual rotatory knee instability. The purpose of this article is to review the anatomy and biomechanics of the anterolateral complex (ALC) of the knee, outline different LET techniques, and provide biomechanical and clinical evidence for its use as an augmentation procedure with ACLR.
Recent Findings
Rotatory knee instability has been identified as a common contributor to ACL rupture in both the primary and revision settings. Several biomechanical studies have shown that LET reduces strain on the ACL by decreasing excess tibial translation and rotation. Additionally, in vivo studies have demonstrated restoration of side-to-side differences in anterior-posterior knee translation, higher rates of return to play, and overall increased patient satisfaction following combined ACLR and LET. As a result, various LET techniques have been developed to help offload the ACL graft and lateral compartment of the knee. However, conclusions are limited by a lack of concrete indications and contraindications for use of LET in the clinical setting.
Summary
Recent studies have shown that rotatory knee instability contributes to native ACL and ACL graft rupture and LET may provide further stability to reduce rates of failure. Further investigation is needed to establish concrete indications and contraindications to determine which patients would most benefit from added stability of the ALC.
Keywords: Anterior cruciate ligament, Anterolateral complex, Lateral extra-articular tenodesis, Rotatory knee instability
Introduction
Recent population-based studies demonstrated an increase in the incidence of anterior cruciate ligament (ACL) reconstruction (ACLR), especially among females and younger athletes [1–3]. Despite advancements in understanding of ACL anatomy and arthroscopic surgical techniques, graft failure rates remain high, occurring in up to 20% of ACLR [4]. There are three main etiologies of ACLR failure: trauma, technical failures, and biology [5].
Rotatory knee instability is a complex, three-dimensional problem thought to be the result of damage to the ACL and other extra-articular structures. Over the past decade, rotatory knee instability has become increasingly implicated as a cause of graft failure, reported in up to 25% of patients who have undergone ACLR [6, 7]. Recently, there is an increased interest in injury to the extra-articular structures of the anterolateral knee, known as the anterolateral complex (ALC), as a cause of ACLR failure. Lateral extra-articular tenodesis (LET) has become a popular augmentation procedure for providing added stability to the ALC and addressing persistent rotatory knee instability.
Many questions exist surrounding the role of the ALC and who may benefit from LET with ACLR. Answering these questions requires knowledge of the anatomy of the ALC, its biomechanical role in both ACL-intact and ACL-deficient knees, and an understanding of results of ACLR after lateral-based augmentation. Thus, the purpose of this review is to provide an overview of the ALC, followed by a discussion of the biomechanical basis of ALC augmentation, different surgical techniques, and investigations supporting augmentation of the ALC. Lastly, the review will conclude with expert-level recommendations for management of the ALC as a source of rotatory knee instability.
Anatomy of the Anterolateral Complex
Broadly, the ALC is a collection of soft tissue structures that provide dynamic rotatory stability to the knee [8]. These soft tissue structures, first described by Seebacher et al. [9••], are characterized as distinct layers of tissue. Layer one is made up of the iliotibial band (ITB), comprised of superficial, middle, and deep bands [10]. The superficial ITB spans from Gerdy’s tubercle anteriorly to the anterolateral and lateral tibia posteriorly [11].
Additionally, fibers of the superficial ITB run in confluence with the lateral patella and patellar tendon anteriorly and reinforce the fascia of the biceps femoris posteriorly [11] (Fig. 1). The middle layer is less distinct, and is identified as oblique fibers at the supracondylar femur region that are visible only with sharp dissection from the superficial layer [11].The deep ITB is made up of Kaplan fibers more distally, which anchor to the distal femur and are thought to function as a static restraint against internal tibial rotation [12] (Fig. 2). Layer two is comprised of the retinacula and aponeurosis of the lateral patellofemoral ligaments and the quadriceps [10]. Lastly, layer three is made up of the joint capsule, also called the capsulo-osseous layer [10] (Fig. 3).
Fig. 1.
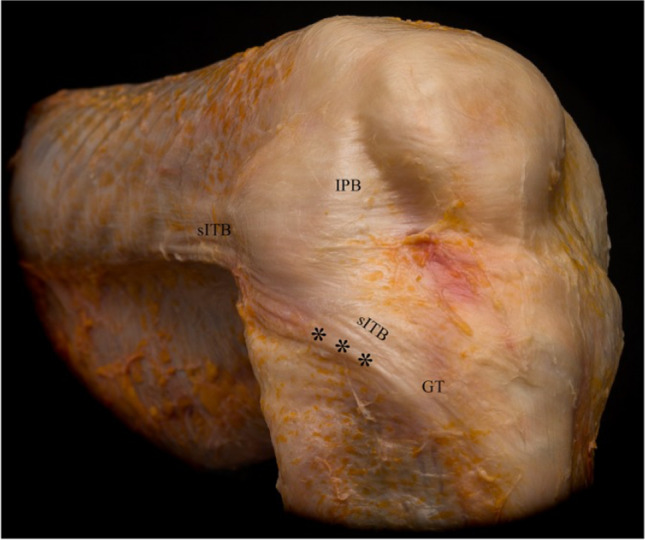
Cadaveric dissection illustrating the first layer of the anterolateral complex, including the superficial iliotibial band (sITB) and iliopatellar band (IPB). *** = fold at the posterior aspect of the sITB observed at increased angles of knee flexion. GT = Gerdy’s tubercle. Picture reproduced from Herbst et al. [11] with permission from SAGE Publications, ©2017
Fig. 2.
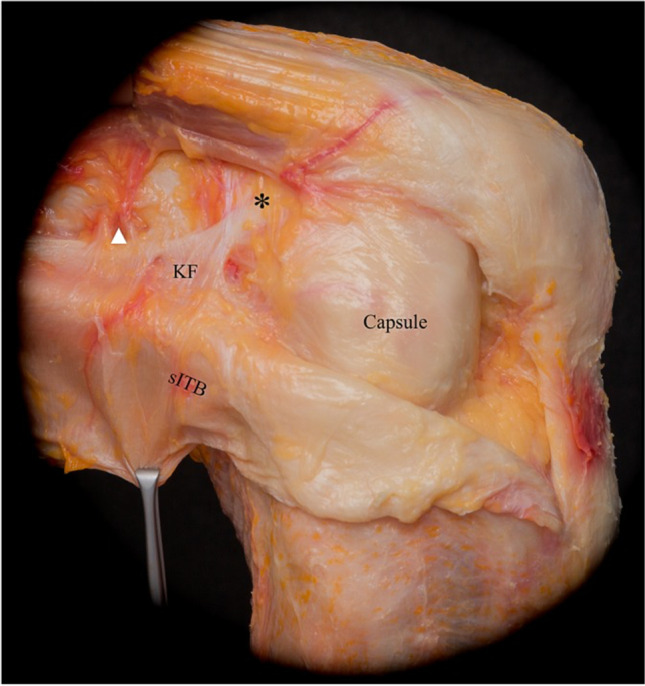
Cadaveric dissection illustrating reflection of the superficial layers of anterolateral complex to demonstrate the confluence of the reflected superficial iliotibial band (sITB) and Kaplan fibers (KF), allowing for attachment to the distal femoral metaphysis. Also demonstrated in this layer are branches of the superior genicular artery (white arrowhead), and accessory insertions of KF anterior and proximal to the femoral epicondyle (*). Additionally, at this layer the anterolateral capsule is now visible. Picture reproduced from Herbst et al. [11] with permission from SAGE Publications, ©2017
Fig. 3.
Deep dissection of the anterolateral complex. A Capsulo-osseous layer (black arrowhead) is visualized with posterior reflection of the superficial iliotibial band (sITB). Note that this structure is separate from the Kaplan fibers (KF) and its accessory insertions (*). White arrowhead = branches of superficial genicular artery. B With deeper, more proximal dissection, it is possible to delineate the KF from the intermuscular septum (IS). Additionally, further retraction of the sITB reveals the deep iliotibial band, merging with the sITB distally. Pictures reproduced from Herbst et al. [11] with permission from SAGE Publications, ©2017
The three layers of the ALC are largely agreed upon; however, controversy surrounds the anterolateral capsular thickening of the ALC, referred to by some as the “anterolateral ligament” (ALL). In 2013, Claes et al. published a cadaveric study identifying the ALL as originating from the femoral epicondyle and inserting just posterior to Gerdy’s tubercle [13••]. In the wake of the identification of this structure, there was a large movement to identify its function. In 2017, an expert group reached consensus on the ALL’s function as a resistor of forced tibial internal rotation and rotatory knee instability, seen clinically with the “pivot-shift” phenomenon [14].
While the identification of the ALL seemed to provide an anatomical structure that could be reconstructed or augmented to confer further stability, more recent studies have challenged its existence as a distinct ligament. A 2017 biomechanical study evaluating the tissue properties of the knee found that the force distribution of the anterolateral capsule represented a fibrous sheet of tissue rather than a distinct ligament [15•]. In a separate cadaveric study, dissections of 20 fresh-frozen cadavers identified a mid-third capsular ligament at the layer of the anterolateral capsule. However, this “ligament” was only found in 35% of specimens, and authors concluded that it was unlikely to be a true anatomic structure like other traditional soft tissue stabilizers of the knee [10].
Biomechanical Role of the ALC
As early as 1981, biomechanical studies determined that the ACL is the primary restraint against anterior–posterior translation and pathologic tibial internal rotation [16]. However, recent studies have focused on the contributing role of the ALC on rotatory knee stability and have found varying results. A 2015 cadaveric study measured strain to evaluate the role of the thickening of the lateral complex identified by the authors as the ALL, and found that the structure lengthened with knee flexion and internal rotation and shortened with external rotation [17]. Additional studies evaluating the effect of sectioning ALC structures on knee motion have found that in ACL-deficient knees, loss of the ALC resulted in further increases in anterior translation and internal tibial rotation [18–20], suggesting that these structures are involved in anterolateral stability of the knee.
Furthermore, a 2016 cadaveric robotic study compared the relative contribution of anterolateral stability for different structures of the ALC, and found that the Kaplan fibers of the deep layer of the ITB played a greater role than the ALL/anterolateral capsule in resisting pathologic levels of tibial anterior subluxation and internal tibial rotation [21]. A 2018 cadaveric study reinforced the role of the Kaplan fibers and anterolateral capsule as resistors of internal tibial rotation and anterior translation and found that these structures become more important in resisting these pathologic motions, as well as the pivot shift, in ACL-deficient knees [22]. The findings of these biomechanical studies provide an impetus for surgical reconstruction and supplementation of the anterolateral complex, which will be the focus of the remainder of the review.
History of the LET
The use of the ITB to reinforce the lateral knee joint capsule was described as early as the late 1800s [23]. In the 1930s, lateral extra-articular reconstructions utilized fascia in a combined, intra-articular loop-like configuration to restore the anterolateral capsule [24]. In the 1960s, the LET was first described by Dr. Lemaire as an isolated extra-articular procedure to restore rotatory knee stability in ACL-deficient knees [25•]. Several modifications then followed, which will be subsequently discussed.
Popular Surgical Techniques
Over the last 50 years, numerous techniques for LET have been described. Although the type of graft and method of fixation vary by technique, all reconstructions involve the creation of a ligament between the lateral femoral condyle and anterolateral tibial plateau. Each technique can be separated into one of three categories: LET with or without separate ACLR, combined intraarticular ACLR and LET using the same graft, and ALL reconstruction. The present review will focus on the first two categories, which are more commonly performed in practice.
Isolated LET
Initially described as standalone procedures for ACL deficiency, the following LET techniques have been adapted for use in conjunction with contemporary ACLR. While each technique has been modified over the years, all remain non-anatomic in design.
Lemaire Procedure
The Lemaire procedure, first described in 1967 [25•, 26], employs a strip of ITB 18 cm in length and 1.5 cm in width. The graft is harvested with the distal attachment to Gerdy’s tubercle left intact. Two osseous tunnels are drilled; one just proximal to the lateral collateral ligament (LCL) insertion on the lateral epicondyle, and the second through Gerdy’s tubercle. The graft is then passed under the LCL, through the femoral tunnel, back under the LCL, and into the tibial tunnel. The graft is subsequently fixed at 30° of knee flexion in neutral rotation.
Modified Lemaire Procedure
A modification of the Lemaire procedure was described in 2002 by Christel and Dijan [27]. Here, a 1.2 × 7.5 cm strip of ITB is harvested, left intact distally, and twisted 180° on top of the LCL. It is then fixed proximal to the LCL insertion with either a screw and washer, or within an osseous femoral tunnel. The authors’ proposed benefits include shorter incision length, decreased morbidity due to shorter ITB harvest, and improved isometry due to twisting of the graft and localization of the femoral fixation point (“Krackow’s point”) with calipers.
Since 2002, several other modifications of Lemaire’s original technique have been reported [28, 29]. While these techniques describe ITB harvest of a similar size (often 1 × 8 cm), the graft is left flat, passed deep to the LCL, and fixed proximal and posterior to the femoral insertion of the LCL between 30° [29] and 60° [28] of knee flexion in neutral rotation.
Ellison Procedure
In contrast to the prior procedures in which the ITB is left attached to Gerdy’s tubercle, Ellison described a distal transfer of the ITB in 1979 [30]. A 1.5 cm sliver of bone is elevated from Gerdy’s tubercle, after which a 1.5 cm strip of ITB is mobilized proximally. The capsule beneath the LCL is then vertically incised and plicated. Finally, the ITB with attached bone block is passed deep to the LCL and secured anterior to the harvest site in a bony trough with the knee flexed to 90°.
Combined LET and ACLR
Although the prior procedures were developed in isolation to address ACL deficiency, such techniques failed to restore anteroposterior stability, with associated poor outcomes [31–33]. This resulted in adaptation of the above techniques for use in combination with ACLR, as well as the development of combined intra- and extra-articular techniques.
MacIntosh Lateral Substitution with Over-the-Top Technique
MacIntosh described a combined extra- and intra-articular reconstruction for ACL deficiency, the results of which were published in 1985 [34]. In this procedure, a distally based, 25 × 4 cm ITB graft is harvested. The graft is passed deep to the LCL from distal to proximal, and subsequently shuttled through a subperiosteal tunnel to exit anterior to the lateral intermuscular septum. The graft is then passed around the lateral femoral condyle, into the knee joint, and through a tibial tunnel back to its insertion on Gerdy’s tubercle. It is fixed with suture at Gerdy’s tubercle, the tibial tubercle, the aperture of the subperiosteal condylar tunnel, and the proximal aspect of the LCL.
Marcacci Procedure
Since the introduction of the combined MacIntosh technique, several modifications have been described [35, 36]. One of the more commonly performed is the Marcacci procedure, published in 1998 [37]. Rather than using an ITB graft, this technique employs a hamstring harvest. The semitendinosus and gracilis tendons are procured, left attached at the pes insertion, and sutured together. A tibial tunnel is drilled, and the graft is passed intraarticularly through the tibial tunnel, around the lateral femoral condyle in “over-the-top” fashion, and out laterally through the ITB. The graft is then tensioned and fixed proximal to the flare of the lateral condyle with two staples in 90° of knee flexion. Finally, the graft is passed deep to the fascia and fixed just inferior to Gerdy’s tubercle to complete the lateral tenodesis.
Biomechanical Effect of LET
With renewed interest in the anterolateral structures of the knee and their role in rotatory knee instability, the biomechanical effects of LET procedures have been meticulously examined. This contribution is highlighted by multiple biomechanical cadaveric studies demonstrating that in the presence of combined ACL and anterolateral lesions, ACLR alone resulted in residual anterior translation and internal rotation laxity. This laxity was significantly decreased when ACLR was combined with ALL reconstruction or LET [38–40].
In addition to eliminating rotatory knee instability, LET procedures offload the strain sustained by ACLR grafts. During simulated pivoting loads, the addition of LET resulted in decreased ACL graft force with up to 80% decreased load seen at 30° of flexion [41]. These findings were replicated in a subsequent study of six cadaveric knees, which found that the modified Lemaire technique reduced ACL graft forces by 61% [42]. The authors concluded that the LET could be considered protective of the ACL graft and suggested that it be considered for use in high-risk patients.
Technical aspects of the procedure have also been biomechanically studied. One cadaveric study examined the knee flexion angle at which the graft was fixed. The LET (modified Lemaire) procedure restored native knee kinematics when fixed at 0, 30, and 60° of knee flexion [43]. In comparison, ALL reconstruction only restored knee kinematics when fixed in full extension. Regarding femoral attachment sites, Kittl et al. examined various femoral attachment sites and graft courses and found favorable graft length changes with a graft deep to the LCL and attached proximal to the lateral femoral epicondyle [44]. These findings, when evaluated in conjunction with the length change measurements reported in the original anatomic study of Claes et al. [13••], are notable, as they suggest the described ALL was lax in lower flexion angles, where the pivot shift most commonly occurs.
While the LET may be successful in reducing tibial translation and excessive tibial rotation, there is some concern that the procedure may constrict anteroposterior tibial translation and internal tibial rotation. Jette et al. found that, while both ALL reconstruction and LET restored native internal rotation, there was decreased internal tibial rotation as the knee flexion angle increased [38]. Marom et al. analyzed cadaveric specimens and found that the addition of LET to ACLR resulted in anteriorization of the center of contact stress in the lateral compartment [45]. While this may be beneficial in patients with anterior subluxation of the lateral tibia, it was also noted that both the mean and the peak lateral compartment contact stresses were increased. Xu et al. studied the effect of cortical fixation on knee kinematics and found that femoral tunnel fixation decreased internal rotation and anterior tibial translation more than femoral cortical fixation in cadaveric models [46].
The biomechanical effects of LET have been studied in vivo as well. Multiple kinematic studies using gait analysis have demonstrated the restoration of pre-injury gait dynamics and baseline knee translation with the addition of LET to ACLR [47, 48]. Another recent in vivo analysis examined the change in cartilage contact pressure with and without LET and found that while LET resulted in a larger lateral compartment cartilage contact center at 6 months, there was no significant difference at 12 months post-operatively [49].
Indications and Contraindications
LET has been employed in both the primary and revision ACLR setting. Criteria for the addition of LET to primary ACLR that have shown favorable outcomes include patients with one or more of the following: younger individuals < 25 years old [50••] with goals of return to contact, pivoting sports [51], high-grade anterolateral rotatory laxity with grade 2 or higher pivot shift [52], generalized ligamentous laxity (Beighton score > 4) [50••, 53], knee hyperextension > 10° [50••, 53], lateral coronal plane laxity [54], increased posterior tibial slope [55•], concomitant lateral meniscal deficiency [56], and MRI evidence of anterolateral capsular injury [50••, 57, 58]. Furthermore, in the revision setting, LET may aid in prevention of subsequent failure, particularly in high-risk patients with the characteristics discussed above [59, 60]. While these indications have been cited extensively in the literature, it is important to note that their development originates from studies limited by their low level of evidence (expert opinion or retrospective studies with short-term follow-up and no direct comparison group) and by the exclusive use of hamstring autograft, limiting the generalizability of LET with different ACLR grafts.
Conversely, the addition of an LET is contraindicated in ACL-deficient knees with concomitant posterolateral corner injury/laxity, as the lateral augmentation may fix the tibia in a subluxated position. Lastly, LET should be cautiously considered in those with lateral compartment knee osteoarthritis as well as in the skeletally immature due to risk of injury to the femoral physis [28, 61]. Table 1 lists common indications and contraindications.
Table 1.
Indications and contraindications for LET augmentation with ACLR, based on previous literature [28, 50••, 51–54, 55•, 56–61] and authors’ preference
| Indications and contraindications for LET | |
|---|---|
| When to consider adding LET | When to consider avoiding LET |
| Younger individuals (<25 years of age) | Concomitant posterolateral corner injury/laxity |
| High grade anterolateral rotatory laxity (Grade 2 or higher pivot shift) | Pre-existing lateral compartment knee osteoarthritis |
| Generalized ligamentous laxity (Beighton score >4) | Skeletally immature individuals |
| Knee hyperextension (>10°) | |
| Lateral coronal plane laxity | |
| Increased posterior tibial slope | |
| Concomitant lateral meniscal deficiency | |
| MRI evidence of anterolateral capsular injury |
MRI, magnetic resonance imaging
Clinical Outcomes
The original LET was performed in isolation and led to suboptimal clinical outcomes with persistent inability to return to preinjury level of sport in over half of cases [33, 62]. However, after the standardization of concomitant intra-articular ACLR, reported clinical and functional results have improved.
A systematic review from Hewison et al. found that the addition of LET to ACLR results in a statistically significant reduction in rotatory knee laxity as measured by the pivot shift test [63]. However, despite this reduction, there was no difference in KT-1000/2000 measured anterior translation, suggesting the dominant role of the ACL graft in resisting anterior translation [63]. Moreover, there was no difference in clinical outcomes, though the means by which patient reported clinical outcomes were measured varied considerably and were difficult to analyze [63]. The results from this review are consistent with subsequent systematic reviews [64, 65], which also found improvement in the pivot shift test but no statistical improvement in patient outcome scores. It should be noted that the subsequent studies again did not attempt to pool clinical scoring systems due to the small number of individual studies utilizing each one, and that a prospectively collected database of patients treated with LET in addition to ACLR did show improved patient reported outcomes at 2 years post-operatively [66].
Clinical outcomes have also been examined in high-risk patients, such as those undergoing revision ACLR [67–69]. In the revision ACL setting with high grade knee laxity (defined by high grade pivot shift or side-to-side difference greater than 6 mm), Alm et al. reported that the addition of LET led to decreased failure rates as well as increased postoperative functional scores [68]. These benefits may be present through mid-term follow-up as well, as a systematic review from Grassi et al. exhibited improved subjective outcome scores and high return to play rates at 5-year follow-up in a similar high-risk cohort of revision patients [67]. These findings may be confined to patients with high preoperative laxity, however, as a case series from Eggeling et al. found no difference in graft failure rate with the addition of LET in revision ACLRs with low preoperative anterior laxity (less than 5 mm) [69].
Another group of high-risk patients are female athletes who participate in cutting and pivoting sports. Guzzini et al. reported a case series of sixteen elite female soccer players and found high subjective clinical outcomes with no residual laxity or graft failures following combined LET with ACLR [70].
Perhaps, the best clinical evidence in high-risk patients to date stems from the STABILITY trial, a multicenter randomized controlled trial comparing hamstring autograft ACLR with and without LET [71••]. The trial includes patients aged 14–25 years with ACL deficiency and two of the following: a high-grade pivot shift (grade two or higher), desire to return to high-risk sports, generalized ligamentous laxity, or knee recurvatum exceeding 10°. In one of the earliest STABILITY I studies, the addition of LET to ACLR significantly reduced graft rupture and rotatory knee instability at 2 years post-operatively [50••]. In a 2022 case-control study utilizing STABILITY I data, a logistic regression analysis found once again that LET was protective of graft rupture, while younger age, increased posterior tibial slope, early return to sport, and high-grade knee laxity had increased odds of graft rupture [55•]. Finally, while the addition of LET resulted in slightly increased pain and reduced subjective functional recovery up to six months post-operatively, these differences resolved by 12 months [72].
Despite overall positive patient outcomes, the increased lateral compartment pressure observed in biomechanical studies has led to clinical concern for the development of osteoarthritis. Devitt et al. [73] performed a systematic review of studies examining this and found insufficient evidence to suggest there is an increased rate of osteoarthritis with the addition of LET. Long term follow-up studies have also examined the potential correlation. Prospective case series consisting of 20-year [74•] and 24.5-year [75] follow-up both show high subjective patient satisfaction and no association of osteoarthritis development with LET. An additional recent cohort study also reported a significantly higher risk of osteoarthritis with ACLR alone compared to LET augmentation at 15-year follow-up [76].
Future Directions
As discussed, the current literature supports the efficacy of LET in reducing failure of hamstring autograft ACLR [50••]. However, there is limited evidence to suggest that these same benefits are present with the use of other commonly used grafts such as bone-patellar tendon-bone (BTB) or quadriceps tendon autograft. The STABILITY II trial [77], currently underway, is a high-level, prospective, randomized study that will compare BTB and quadriceps tendon autograft ACLR with and without LET. This study, including others focusing on long-term, prospective outcomes evaluating the role of LET on ACL graft rupture in the primary and revision settings, will help to further elucidate the utility of LET as a tool for restoring knee laxity and preventing ACL graft re-rupture.
Authors’ Preferred Approach
The authors perform primary autograft ACLR with BTB or quadriceps tendon, reserving hamstring autograft for cases of extensor mechanism insufficiency or patient preference. As there currently exists no high-level prospective evidence to guide the use of lateral augmentation procedures in primary ACLR with quadriceps or BTB autograft, at present, the authors perform combined primary ACLR and LET predominantly in patients enrolled in the STABILITY II trial. LET is also considered adjunctively in revision ACL cases with no other identifiable causes of failure (tunnel malposition, increased posterior tibial slope, posterolateral laxity, coronal malalignment). The preferred technique is a modified Lemaire extraarticular tenodesis, due to the procedure’s simplicity, low complication rate, and excellent clinical outcomes [50••, 78].
Surgical Technique
The patient is positioned supine with a tourniquet on the proximal thigh. A leg holder is placed around the tourniquet, and the contralateral leg is placed in the lithotomy position. The foot of the bed is then dropped, allowing the operative extremity to hang free at 90° of knee flexion. The extremity is prepared and draped in sterile fashion.
Following completion of knee arthroscopy and ACLR, attention is turned to the LET. A 3-cm incision is made over the lateral aspect of the thigh, starting just posterior to the lateral epicondyle and extending proximally in line with the fibers of the ITB. Dissection is carried to the level of the ITB, and the posterior border is identified. A 0.8 × 1 cm strip of ITB is incised, centered slightly posteriorly yet taking care to preserve the posterior Kaplan fibers. A graft 8 cm in length is harvested and left attached distally to Gerdy’s tubercle. The free end of the graft is then whipstitched with a braided, absorbable size 0 suture. The anterior and posterior borders of the LCL are identified and marked with electrocautery. A tonsil is then placed deep to the LCL, and the ITB graft is passed deep to the LCL from distal to proximal. The point of graft fixation is subsequently marked with electrocautery just proximal to the flare of the lateral condyle, and proximal and posterior to the lateral epicondyle. The site is debrided of soft tissue with a rongeur and electrocautery. The knee is then flexed to 60° with neutral rotation, and the graft is fixed with a Richards staple at approximately 20 Newtons of tension. Staple position is confirmed with intraoperative fluoroscopy, and any remaining graft is sutured onto itself. The wound is then irrigated, and the ITB closed with absorbable size 0 suture. The skin and subcutaneous tissue is then closed in a layered fashion. Table 2 lists pearls and pitfalls of the authors’ preferred approach of ACLR and LET.
Table 2.
Pearls and pitfalls of LET with ACLR, based on authors’ preferred approach
| Pearls and pitfalls of LET with ACLR | |
|---|---|
| Pearls | Pitfalls |
| Center ITB incision slightly posteriorly to identify and preserve posterior Kaplan fibers | Avoid too posterior and deep of incision during ITB graft harvest to protect posterior Kaplan fibers |
| Mark the anterior and posterior borders of the LCL with electrocautery for easy identification | Fixation with Richards staple in too much knee flexion/extension and non-neutral rotation of foot – doing so may result in over constraint or laxity of graft at final fixation |
| Pass the ITB deep to the LCL to prevent excess movement of the graft | |
| Graft fixation with Richards staple at 20 Newtons of tension | |
LET, lateral extra-articular tenodesis; ACLR, anterior cruciate ligament reconstruction; ITB, iliotibial band; LCL, lateral collateral ligament
Full weightbearing and range of motion is permitted immediately, with rehabilitation and restrictions dictated by concomitant meniscal pathology.
Conclusion
The anterolateral complex of the knee works synergistically with the ACL to prevent rotational instability. While ALC augmentation procedures were initially developed to address ACL deficiency in isolation, biomechanical and clinical data have led to use of the LET as a procedure that both augment and protect the ACL graft. Recent studies highlight the utility of adjunctive LET in high-risk patients undergoing primary and revision ACLR, as it appears to decrease failure rates and improve patient outcomes. However, further high-quality studies are needed to elucidate the precise indications for and outcomes of combined ACLR and LET.
Data Availability
All relevant data generated or analyzed during this study are included in this published article.
Declarations
Competing Interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ehab M. Nazzal, Email: nazzalem2@upmc.edu
Laura E. Keeling, Email: keelingle@upmc.edu
Patrick M. Ryan, Email: pmryan2020@gmail.com
Zachary J. Herman, Email: hermanz@upmc.edu
Jonathan D. Hughes, Email: hughesjd3@upmc.edu
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Longo UG, Nagai K, Salvatore G, Cella E, Candela V, Cappelli F, et al. Epidemiology of anterior cruciate ligament reconstruction surgery in italy: a 15-year nationwide registry study. J Clin Med. 2021;10(2). 10.3390/jcm10020223. [DOI] [PMC free article] [PubMed]
- 2.Longo UG, Salvatore G, Ruzzini L, Risi Ambrogioni L, de Girolamo L, Viganò M, et al. Trends of anterior cruciate ligament reconstruction in children and young adolescents in Italy show a constant increase in the last 15 years. Knee Surg Sports Traumatol Arthrosc. 2021;29(6):1728–1733. doi: 10.1007/s00167-020-06203-1. [DOI] [PubMed] [Google Scholar]
- 3.Paudel YR, Sommerfeldt M, Voaklander D. Increasing incidence of anterior cruciate ligament reconstruction: a 17-year population-based study. Knee Surg Sports Traumatol Arthrosc. 2022 doi: 10.1007/s00167-022-07093-1. [DOI] [PubMed] [Google Scholar]
- 4.Laboudie P, Douiri A, Bouguennec N, Biset A, Graveleau N. Combined ACL and ALL reconstruction reduces the rate of reoperation for graft failure or secondary meniscal lesions in young athletes. Knee Surg Sports Traumatol Arthrosc. 2022;30(10):3488–3498. doi: 10.1007/s00167-022-06956-x. [DOI] [PubMed] [Google Scholar]
- 5.Wright RW, Huston LJ, Spindler KP, Dunn WR, Haas AK, Allen CR, et al. Descriptive epidemiology of the Multicenter ACL Revision Study (MARS) cohort. Am J Sports Med. 2010;38(10):1979–1986. doi: 10.1177/0363546510378645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chahla J, Moatshe G, Geeslin AG, LaPrade RF. Biomechanical role of lateral structures in controlling anterolateral rotatory laxity: the anterolateral ligament. Oper Tech Orthop. 2017;27(2):102–106. doi: 10.1053/j.oto.2017.02.004. [DOI] [Google Scholar]
- 7.Sonnery-Cottet B, Thaunat M, Freychet B, Pupim BHB, Murphy CG, Claes S. Outcome of a combined anterior cruciate ligament and anterolateral ligament reconstruction technique with a minimum 2-year follow-up. Am J Sports Med. 2015;43(7):1598–1605. doi: 10.1177/0363546515571571. [DOI] [PubMed] [Google Scholar]
- 8.Brockmeyer M, Orth P, Höfer D, Seil R, Paulsen F, Menger MD, et al. The anatomy of the anterolateral structures of the knee - a histologic and macroscopic approach. Knee. 2019;26(3):636–646. doi: 10.1016/j.knee.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 9.•• Seebacher JR, Inglis AE, Marshall JL, Warren RF. The structure of the posterolateral aspect of the knee. J Bone Joint Surg Am. 1982;64(4):536-41. First anatomic description of the layered anatomy of the anterolateral complex of the knee. [PubMed]
- 10.Herbst E, Burnham JM, Albers M, Musahl V, Fu FH. Layer-by-layer anatomy of the anterolateral complex of the knee. Oper Tech Orthop. 2017;27(2):91–95. doi: 10.1053/j.oto.2017.02.002. [DOI] [Google Scholar]
- 11.Herbst E, Albers M, Burnham JM, Fu FH, Musahl V. The anterolateral complex of the knee. Orthop J Sports Med. 2017;5(10):2325967117730805. doi: 10.1177/2325967117730805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marom N, Greditzer HGt, Roux M, Ling D, Boyle C, Pearle AD, et al. The incidence of Kaplan fiber injury associated with acute anterior cruciate ligament tear based on magnetic resonance imaging. Am J Sports Med. 2020;48(13):3194–9.10.1177/0363546520956302. [DOI] [PubMed]
- 13.•• Claes S, Vereecke E, Maes M, Victor J, Verdonk P, Bellemans J. Anatomy of the anterolateral ligament of the knee. J Anat. 2013;223(4):321-8. 10.1111/joa.12087. First article to distinctly define the anterolateral ligament of the anterolateral knee. [DOI] [PMC free article] [PubMed]
- 14.Sonnery-Cottet B, Daggett M, Fayard J-M, Ferretti A, Helito CP, Lind M, et al. Anterolateral Ligament Expert Group consensus paper on the management of internal rotation and instability of the anterior cruciate ligament - deficient knee. J Orthop Traumatol. 2017;18(2):91–106. doi: 10.1007/s10195-017-0449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.• Guenther D, Rahnemai-Azar AA, Bell KM, Irarrázaval S, Fu FH, Musahl V, et al. The Anterolateral capsule of the knee behaves like a sheet of fibrous tissue. Am J Sports Med. 2017;45(4):849-55. 10.1177/0363546516674477. Biomechanical study refuting the anterolateral ligament as a distinct ligament, but instead describing the anterolateral complex as a fibrous sheet of tissue. [DOI] [PubMed]
- 16.Lipke JM, Janecki CJ, Nelson CL, McLeod P, Thompson C, Thompson J, et al. The role of incompetence of the anterior cruciate and lateral ligaments in anterolateral and anteromedial instability. A biomechanical study of cadaver knees. J Bone Joint Surg Am. 1981;63(6):954–60. [PubMed]
- 17.Zens M, Niemeyer P, Ruhhammer J, Bernstein A, Woias P, Mayr HO, et al. Length changes of the anterolateral ligament during passive knee motion: a human cadaveric study. Am J Sports Med. 2015;43(10):2545–2552. doi: 10.1177/0363546515594373. [DOI] [PubMed] [Google Scholar]
- 18.Spencer L, Burkhart TA, Tran MN, Rezansoff AJ, Deo S, Caterine S, et al. Biomechanical analysis of simulated clinical testing and reconstruction of the anterolateral ligament of the knee. Am J Sports Med. 2015;43(9):2189–2197. doi: 10.1177/0363546515589166. [DOI] [PubMed] [Google Scholar]
- 19.Rasmussen MT, Nitri M, Williams BT, Moulton SG, Cruz RS, Dornan GJ, et al. An in vitro robotic assessment of the anterolateral ligament, part 1: secondary role of the anterolateral ligament in the setting of an anterior cruciate ligament injury. Am J Sports Med. 2016;44(3):585–592. doi: 10.1177/0363546515618387. [DOI] [PubMed] [Google Scholar]
- 20.Nitri M, Rasmussen MT, Williams BT, Moulton SG, Cruz RS, Dornan GJ, et al. An in vitro robotic assessment of the anterolateral ligament, part 2: anterolateral ligament reconstruction combined with anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(3):593–601. doi: 10.1177/0363546515620183. [DOI] [PubMed] [Google Scholar]
- 21.Kittl C, El-Daou H, Athwal KK, Gupte CM, Weiler A, Williams A, et al. The role of the anterolateral structures and the ACL in controlling laxity of the intact and ACL-deficient knee. Am J Sports Med. 2016;44(2):345–354. doi: 10.1177/0363546515614312. [DOI] [PubMed] [Google Scholar]
- 22.Geeslin AG, Chahla J, Moatshe G, Muckenhirn KJ, Kruckeberg BM, Brady AW, et al. Anterolateral knee extra-articular stabilizers: a robotic sectioning study of the anterolateral ligament and distal iliotibial band Kaplan fibers. Am J Sports Med. 2018;46(6):1352–1361. doi: 10.1177/0363546518759053. [DOI] [PubMed] [Google Scholar]
- 23.Segond P. Recherches cliniques et expérimentales sur les épanchements sanguins du genou par entorse. Prog Med. 1879;1–85.
- 24.Strickler FP. A satisfactory method of repairing crucial ligaments. Ann Surg. 1937;105(6):912–916. doi: 10.1097/00000658-193706000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.• Lemaire M. Rupture anciennes du ligament croisé antérieur. Frequence-clinique traitement. J Bone Jt Surg Br B. 1967;58:142. Earliest description of Lemaire procedure of lateral extra-articular tenodesis.
- 26.Lemaire M. [Chronic knee instability. Technics and results of ligament plasty in sports injuries]. J Chir (Paris). 1975;110(4):281–94 [PubMed]
- 27.Christel P, Djian P. Anterio-lateral extra-articular tenodesis of the knee using a short strip of fascia lata. Rev Chir Orthop Reparatrice Appar Mot. 2002;88(5):508–513. [PubMed] [Google Scholar]
- 28.Jesani S, Getgood A. Modified Lemaire lateral extra-articular tenodesis augmentation of anterior cruciate ligament reconstruction. JBJS Essent Surg Tech. 2019;9(4).10.2106/jbjs.St.19.00017. [DOI] [PMC free article] [PubMed]
- 29.Schlichte LM, Aitchison AH, Green DW, Cordasco FA. Modified Lemaire lateral extra-articular tenodesis in the pediatric patient: an adjunct to anterior cruciate ligament reconstruction. Arthrosc Tech. 2020;9(1):e111–e116. doi: 10.1016/j.eats.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ellison AE. Distal iliotibial-band transfer for anterolateral rotatory instability of the knee. J Bone Joint Surg Am. 1979;61(3):330–337. doi: 10.2106/00004623-197961030-00002. [DOI] [PubMed] [Google Scholar]
- 31.Draganich LF, Reider B, Miller PR. An in vitro study of the Müller anterolateral femorotibial ligament tenodesis in the anterior cruciate ligament deficient knee. Am J Sports Med. 1989;17(3):357–362. doi: 10.1177/036354658901700308. [DOI] [PubMed] [Google Scholar]
- 32.Amis AA, Scammell BE. Biomechanics of intra-articular and extra-articular reconstruction of the anterior cruciate ligament. J Bone Joint Surg Br. 1993;75(5):812–817. doi: 10.1302/0301-620x.75b5.8376447. [DOI] [PubMed] [Google Scholar]
- 33.Ireland J, Trickey EL. Macintosh tenodesis for anterolateral instability of the knee. J Bone Joint Surg Br. 1980;62(3):340–345. doi: 10.1302/0301-620x.62b3.7410466. [DOI] [PubMed] [Google Scholar]
- 34.Bertoia JT, Urovitz EP, Richards RR, Gross AE. Anterior cruciate reconstruction using the MacIntosh lateral-substitution over-the-top repair. J Bone Joint Surg Am. 1985;67(8):1183–1188. doi: 10.2106/00004623-198567080-00006. [DOI] [PubMed] [Google Scholar]
- 35.Marshall JL, Warren RF, Wickiewicz TL, Reider B. The anterior cruciate ligament: a technique of repair and reconstruction. Clin Orthop Relat Res. 1979;143:97–106. [PubMed] [Google Scholar]
- 36.Ekstrand J. Reconstruction of the anterior cruciate ligament in athletes, using a fascia lata graft: a review with preliminary results of a new concept. Int J Sports Med. 1989;10(4):225–232. doi: 10.1055/s-2007-1024907. [DOI] [PubMed] [Google Scholar]
- 37.Marcacci M, Zaffagnini S, Iacono F, Neri MP, Loreti I, Petitto A. Arthroscopic intra- and extra-articular anterior cruciate ligament reconstruction with gracilis and semitendinosus tendons. Knee Surg Sports Traumatol Arthrosc. 1998;6(2):68–75. doi: 10.1007/s001670050075. [DOI] [PubMed] [Google Scholar]
- 38.Jette C, Gutierrez D, Sastre S, Llusa M, Combalia A. Biomechanical comparison of anterolateral ligament anatomical reconstruction with a semi-anatomical lateral extra-articular tenodesis. A cadaveric study. Knee. 2019;26(5):1003-9. 10.1016/j.knee.2019.07.005. [DOI] [PubMed]
- 39.Inderhaug E, Stephen JM, Williams A, Amis AA. Biomechanical comparison of anterolateral procedures combined with anterior cruciate ligament reconstruction. Am J Sports Med. 2017;45(2):347–354. doi: 10.1177/0363546516681555. [DOI] [PubMed] [Google Scholar]
- 40.Delaloye JR, Hartog C, Blatter S, Schläppi M, Müller D, Denzler D, et al. Anterolateral ligament reconstruction and modified Lemaire lateral extra-articular tenodesis similarly improve knee stability after anterior cruciate ligament reconstruction: a biomechanical study. Arthroscopy. 2020;36(7):1942–1950. doi: 10.1016/j.arthro.2020.03.027. [DOI] [PubMed] [Google Scholar]
- 41.Marom N, Ouanezar H, Jahandar H, Zayyad ZA, Fraychineaud T, Hurwit D, et al. Lateral extra-articular tenodesis reduces anterior cruciate ligament graft force and anterior tibial translation in response to applied pivoting and anterior drawer loads. Am J Sports Med. 2020;48(13):3183–3193. doi: 10.1177/0363546520959322. [DOI] [PubMed] [Google Scholar]
- 42.Mayr R, Sigloch M, Coppola C, Hoermann R, Iltchev A, Schmoelz W. Modified Lemaire tenodesis reduces anterior cruciate ligament graft forces during internal tibial torque loading. J Exp Orthop. 2022;9(1):45. doi: 10.1186/s40634-022-00484-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inderhaug E, Stephen JM, Williams A, Amis AA. Anterolateral tenodesis or anterolateral ligament complex reconstruction: effect of flexion angle at graft fixation when combined with ACL reconstruction. Am J Sports Med. 2017;45(13):3089–3097. doi: 10.1177/0363546517724422. [DOI] [PubMed] [Google Scholar]
- 44.Kittl C, Halewood C, Stephen JM, Gupte CM, Weiler A, Williams A, et al. Length change patterns in the lateral extra-articular structures of the knee and related reconstructions. Am J Sports Med. 2015;43(2):354–362. doi: 10.1177/0363546514560993. [DOI] [PubMed] [Google Scholar]
- 45.Marom N, Jahandar H, Fraychineaud TJ, Zayyad ZA, Ouanezar H, Hurwit D, et al. Lateral extra-articular tenodesis alters lateral compartment contact mechanics under simulated pivoting maneuvers: an in vitro study. Am J Sports Med. 2021;49(11):2898–2907. doi: 10.1177/03635465211028255. [DOI] [PubMed] [Google Scholar]
- 46.Xu J, Qiao Y, Han K, Xu C, Dong S, Zhao J. Modified Lemaire lateral extra-articular tenodesis with the iliotibial band strip fixed on the femoral cortical surface reduces laxity and causes less overconstraint in the anterolateral lesioned knee: a biomechanical study. Arthroscopy. 2022;38(12):3162–3171. doi: 10.1016/j.arthro.2022.06.016. [DOI] [PubMed] [Google Scholar]
- 47.Chiba D, Gale T, Nishida K, Suntaxi F, Lesniak BP, Fu FH, et al. Lateral extra-articular tenodesis contributes little to change in vivo kinematics after anterior cruciate ligament reconstruction: a randomized controlled trial. Am J Sports Med. 2021;49(7):1803–1812. doi: 10.1177/03635465211003298. [DOI] [PubMed] [Google Scholar]
- 48.Di Benedetto P, Buttironi MM, Mancuso F, Roman F, Vidi D, Causero A. Kinetic and Kinematic analysis of ACL reconstruction in association with lateral-extrarticular tenodesis of the knee in revision surgery: a pilot study. Acta Biomed. 2021;92(S3):e2021027.10.23750/abm.v92iS3.11776. [DOI] [PMC free article] [PubMed]
- 49.Nishida K, Gale T, Chiba D, Suntaxi F, Lesniak B, Fu F, et al. The effect of lateral extra-articular tenodesis on in vivo cartilage contact in combined anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2022;30(1):61–70. doi: 10.1007/s00167-021-06480-4. [DOI] [PubMed] [Google Scholar]
- 50.•• Getgood AMJ, Bryant DM, Litchfield R, Heard M, McCormack RG, Rezansoff A, et al. Lateral extra-articular tenodesis reduces failure of hamstring tendon autograft anterior cruciate ligament reconstruction: 2-year outcomes from the STABILITY study randomized clinical trial. Am J Sports Med. 2020;48(2):285-97.10.1177/0363546519896333. Multicenter prospective randomized clinical trial demonstrating lateral extra-articular tenodesis with hamstring tendon autograft anterior cruciate ligament reconstruction reduces graft failure and persistent rotatory knee instability. [DOI] [PubMed]
- 51.Borque KA, Jones M, Laughlin MS, Balendra G, Willinger L, Pinheiro VH, et al. Effect of lateral extra-articular tenodesis on the rate of revision anterior cruciate ligament reconstruction in elite athletes. Am J Sports Med. 2022;50(13):3487–3492. doi: 10.1177/03635465221128828. [DOI] [PubMed] [Google Scholar]
- 52.Musahl V, Kopf S, Rabuck S, Becker R, van der Merwe W, Zaffagnini S, et al. Rotatory knee laxity tests and the pivot shift as tools for ACL treatment algorithm. Knee Surg Sports Traumatol Arthrosc. 2012;20(4):793–800. doi: 10.1007/s00167-011-1857-6. [DOI] [PubMed] [Google Scholar]
- 53.Larson CM, Bedi A, Dietrich ME, Swaringen JC, Wulf CA, Rowley DM, et al. Generalized hypermobility, knee hyperextension, and outcomes after anterior cruciate ligament reconstruction: prospective, case-control study with mean 6 years follow-up. Arthroscopy. 2017;33(10):1852–1858. doi: 10.1016/j.arthro.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Getgood A. Editorial commentary: indications for lateral extra-articular tenodesis in primary anterior cruciate ligament reconstruction. Arthroscopy. 2022;38(1):125–127. doi: 10.1016/j.arthro.2021.07.005. [DOI] [PubMed] [Google Scholar]
- 55.• Firth AD, Bryant DM, Litchfield R, McCormack RG, Heard M, MacDonald PB, et al. Predictors of graft failure in young active patients undergoing hamstring autograft anterior cruciate ligament reconstruction with or without a lateral extra-articular tenodesis: the stability experience. Am J Sports Med. 2022;50(2):384-95.10.1177/03635465211061150. Case control with predictive analysis to define high-risk patients who would benefit from addition of lateral extra-articular tenodesis with hamstring tendon autograft. [DOI] [PMC free article] [PubMed]
- 56.Floyd ER, Carlson GB, LaPrade RF. Arthroscopic-assisted lateral meniscal allograft transplantation with open ligamentous extra-articular tenodesis. Arthrosc Tech. 2021;10(3):e903–e908. doi: 10.1016/j.eats.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mahmoud A, Torbey S, Honeywill C, Myers P. Lateral extra-articular tenodesis combined with anterior cruciate ligament reconstruction is effective in knees with additional features of lateral, hyperextension, or increased rotational laxity: a matched cohort study. Arthroscopy. 2022;38(1):119–124. doi: 10.1016/j.arthro.2021.04.068. [DOI] [PubMed] [Google Scholar]
- 58.Musahl V, Getgood A, Neyret P, Claes S, Burnham JM, Batailler C, et al. Contributions of the anterolateral complex and the anterolateral ligament to rotatory knee stability in the setting of ACL Injury: a roundtable discussion. Knee Surg Sports Traumatol Arthrosc. 2017;25(4):997–1008. doi: 10.1007/s00167-017-4436-7. [DOI] [PubMed] [Google Scholar]
- 59.Porter MD, Shadbolt B, Pomroy S. The augmentation of revision anterior cruciate ligament reconstruction with modified iliotibial band tenodesis to correct the pivot shift: a computer navigation study. Am J Sports Med. 2018;46(4):839–845. doi: 10.1177/0363546517750123. [DOI] [PubMed] [Google Scholar]
- 60.Redler A, Iorio R, Monaco E, Puglia F, Wolf MR, Mazza D, et al. Revision anterior cruciate ligament reconstruction with hamstrings and extra-articular tenodesis: a mid- to long-term clinical and radiological study. Arthrosc J Arthrosc Relat Surg. 2018;34(12):3204–13. 10.1016/j.arthro.2018.05.045. [DOI] [PubMed]
- 61.Orduna S, Yordi NA. Lateral extraarticular tenodesis in combination with ACL reconstruction: indications, technique description. Glob J Ortho Res. 1 (4): 2019. GJOR MS ID. 2019;520.
- 62.Neyret P, Palomo JR, Donell ST, Dejour H. Extra-articular tenodesis for anterior cruciate ligament rupture in amateur skiers. Br J Sports Med. 1994;28(1):31–34. doi: 10.1136/bjsm.28.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hewison CE, Tran MN, Kaniki N, Remtulla A, Bryant D, Getgood AM. Lateral extra-articular tenodesis reduces rotational laxity when combined with anterior cruciate ligament reconstruction: a systematic review of the literature. Arthroscopy. 2015;31(10):2022–2034. doi: 10.1016/j.arthro.2015.04.089. [DOI] [PubMed] [Google Scholar]
- 64.Song GY, Hong L, Zhang H, Zhang J, Li Y, Feng H. Clinical outcomes of combined lateral extra-articular tenodesis and intra-articular anterior cruciate ligament reconstruction in addressing high-grade pivot-shift phenomenon. Arthroscopy. 2016;32(5):898–905. doi: 10.1016/j.arthro.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 65.Agarwal N, Monketh J, Volpin A. Clinical and mechanical outcomes in isolated anterior cruciate ligament reconstruction vs additional lateral extra-articular tenodesis or anterolateral ligament reconstruction. World J Orthop. 2022;13(7):662–675. doi: 10.5312/wjo.v13.i7.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rowan FE, Huq SS, Haddad FS. Lateral extra-articular tenodesis with ACL reconstruction demonstrates better patient-reported outcomes compared to ACL reconstruction alone at 2 years minimum follow-up. Arch Orthop Trauma Surg. 2019;139(10):1425–1433. doi: 10.1007/s00402-019-03218-3. [DOI] [PubMed] [Google Scholar]
- 67.Grassi A, Zicaro JP, Costa-Paz M, Samuelsson K, Wilson A, Zaffagnini S, et al. Good mid-term outcomes and low rates of residual rotatory laxity, complications and failures after revision anterior cruciate ligament reconstruction (ACL) and lateral extra-articular tenodesis (LET) Knee Surg Sports Traumatol Arthrosc. 2020;28(2):418–431. doi: 10.1007/s00167-019-05625-w. [DOI] [PubMed] [Google Scholar]
- 68.Alm L, Drenck TC, Frosch KH, Akoto R. Lateral extra-articular tenodesis in patients with revision anterior cruciate ligament (ACL) reconstruction and high-grade anterior knee instability. Knee. 2020;27(5):1451–1457. doi: 10.1016/j.knee.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 69.Eggeling L, Drenck TC, Frings J, Krause M, Korthaus A, Krukenberg A, et al. Additional lateral extra-articular tenodesis in revision ACL reconstruction does not influence the outcome of patients with low-grade anterior knee laxity. Arch Orthop Trauma Surg. 2022;142(2):291–299. doi: 10.1007/s00402-021-04145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guzzini M, Mazza D, Fabbri M, Lanzetti R, Redler A, Iorio C, et al. Extra-articular tenodesis combined with an anterior cruciate ligament reconstruction in acute anterior cruciate ligament tear in elite female football players. Int Orthop. 2016;40(10):2091–2096. doi: 10.1007/s00264-016-3261-9. [DOI] [PubMed] [Google Scholar]
- 71.•• Getgood A, Bryant D, Firth A. The Stability study: a protocol for a multicenter randomized clinical trial comparing anterior cruciate ligament reconstruction with and without Lateral Extra-articular Tenodesis in individuals who are at high risk of graft failure. BMC Musculoskelet Disord. 2019;20(1):216.10.1186/s12891-019-2589-x. Established protocol for the STABILITY trial evaluating addition of lateral extra-articular tenodesis with hamstring tendon autograft anterior cruciate ligament reconstruction. [DOI] [PMC free article] [PubMed]
- 72.Getgood A, Hewison C, Bryant D, Litchfield R, Heard M, Buchko G, et al. No difference in functional outcomes when lateral extra-articular tenodesis is added to anterior cruciate ligament reconstruction in young active patients: the stability study. Arthroscopy. 2020;36(6):1690–1701. doi: 10.1016/j.arthro.2020.02.015. [DOI] [PubMed] [Google Scholar]
- 73.Devitt BM, Bouguennec N, Barfod KW, Porter T, Webster KE, Feller JA. Combined anterior cruciate ligament reconstruction and lateral extra-articular tenodesis does not result in an increased rate of osteoarthritis: a systematic review and best evidence synthesis. Knee Surg Sports Traumatol Arthrosc. 2017;25(4):1149–1160. doi: 10.1007/s00167-017-4510-1. [DOI] [PubMed] [Google Scholar]
- 74.• Zaffagnini S, Marcheggiani Muccioli GM, Grassi A, Roberti di Sarsina T, Raggi F, Signorelli C, et al. Over-the-top ACL reconstruction plus extra-articular lateral tenodesis with hamstring tendon grafts: prospective evaluation with 20-year minimum follow-up. Am J Sports Med. 2017;45(14):3233–42. 10.1177/0363546517723013. Long-term prospective follow up study of hamstring tendon ACL reconstruction using over-the-top technique with lateral extra-articular tenodesis. [DOI] [PubMed]
- 75.Pernin J, Verdonk P, Si Selmi TA, Massin P, Neyret P. Long-term follow-up of 24.5 years after intra-articular anterior cruciate ligament reconstruction with lateral extra-articular augmentation. Am J Sports Med. 2010;38(6):1094–102.10.1177/0363546509361018. [DOI] [PubMed]
- 76.Viglietta E, Ponzo A, Monaco E, Iorio R, Drogo P, Andreozzi V, et al. ACL reconstruction combined with the Arnold-Coker modification of the MacIntosh lateral extra-articular tenodesis: long-term clinical and radiological outcomes. Am J Sports Med. 2022;50(2):404–414. doi: 10.1177/03635465211062609. [DOI] [PubMed] [Google Scholar]
- 77.Getgood A. STABILITY 2: Anterior cruciate ligament reconstruction +/- lateral tenodesis with patellar vs quad tendon (STABILITY 2). 2022. clinicaltrials.gov/ct2/show/NCT03935750. Accessed 3 Jan 2023.
- 78.Perelli S, Costa GG, Terron VM, Formagnana M, Bait C, Espregueira-Mendes J, et al. Combined anterior cruciate ligament reconstruction and modified Lemaire lateral extra-articular tenodesis better restores knee stability and reduces failure rates than isolated anterior cruciate ligament reconstruction in skeletally immature patients. Am J Sports Med. 2022;50(14):3778–3785. doi: 10.1177/03635465221128926. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data generated or analyzed during this study are included in this published article.



