Abstract
Quorum sensing (QS) is an inter- and intracellular communication mechanism that regulates gene expression in response to population size. Autoinducer-2 (AI-2) signaling is a QS signaling molecule common to both Gram-negative and Gram-positive bacteria. Enterococcus faecalis is one of the leading causes of nosocomial infections worldwide. There has been an increasing interest in controlling infectious diseases through targeting the QS mechanism using natural compounds. This study aimed to investigate the effect of nisin and p-coumaric acid (pCA), on biofilm formation and AI-2 signaling in E. faecalis. Their effect on the expression of the QS-regulated virulence encoding gene sprE was also investigated. Nisin exhibited a MIC ranging from 0.25 to 0.5 mg/mL, while the MIC of pCA was 1 mg/mL. The luminescence-based response of the reporter strain Vibrio harveyi BB170 was used to determine AI-2 activity in E. faecalis strains. Nisin was not effective in inhibiting AI-2 activity, while pCA reduced AI-2 activity by ≥ 60%. Moreover, pCA and nisin combination showed higher inhibitory effect on biofilm formation of E. faecalis, compared to the treatment of pCA or nisin alone. qRT-PCR analysis showed that nisin alone and the combination of nisin and pCA, at their MIC values, led to a 32.78- and 40.22-fold decrease in sprE gene expression, respectively, while pCA alone did not have a significant effect. Considering the demand to explore new therapeutic avenues for infectious bacteria, this study was the first to report that pCA can act like a quorum sensing inhibitor (QSI) against AI-2 signaling in E. faecalis.
Keywords: Enterococcus faecalis, Quorum sensing, Biofilm, Nisin, p-Coumaric acid
Introduction
Quorum sensing (QS) is a cell density-dependent inter- and intracellular communication mechanism that regulates gene expression in response to population size. During this process, bacteria communicate with other cells, interact with their surrounding environment, and control their cellular functions through synthesizing, detecting and reacting to chemical signal molecules called autoinducers (AI) [1, 2]. The components of QS mechanism are (i) the AI, (ii) the signal synthase, (iii) the signal receptor, (iv) the signal response regulator, and (v) the regulated genes [3]. This system is used by both Gram-negative and Gram-positive bacteria in response to various environmental stress conditions such as nutrient deficiency, the inhibitory effect of temperature, and host defense mechanisms [4]. The autoinducing molecules affect the expression of wide range of genes involved in various processes including adaptation, virulence, toxicity, sporulation, bioluminescence, food spoilage, plasmid transformation, antibiotic resistance, and biofilm formation in bacteria [5, 6].
Autoinducer-2 (AI-2) is a QS signaling molecule common to both Gram-negative and Gram-positive bacteria [7]. Unlike other bacterial signaling mechanisms, AI-2 signaling has evolved to enable interaction between different strains of bacteria within the same environmental niche [8]. Several bacterial strains, including enterococci, are known to have the ability to produce AI-2 [9].
Enterococcus faecalis is one of the leading causes of nosocomial infections worldwide [10]. This pathogen has both natural and acquired resistance to a variety of antibiotics [11]. Enterococci species are clinically important as they form biofilms on permanent medical devices such as venous and urinary catheters, orthopedic implants, or infective endocarditis, causing biofilm-related persistent infections [12, 13]. Since the antibiotic resistance of biofilm-embedded bacteria is 1000 times higher compared to their planktonic counterparts, it is necessary to develop new combat strategies [14]. During this process and to overcome drug resistance bottlenecks, the newly developed approaches should not cause selective pressure on bacteria and end up with the emergence of novel resistance problems. From a biofilm perspective, there has been an increasing interest in averting or curing infectious diseases by targeting the QS mechanism using natural compounds which are safe and effective with no side effects [15].
Quorum sensing inhibition (QSI) is considered a promising therapeutic approach to control bacterial biofilms [16]. By targeting the QS mechanism, infections can be prevented through interrupting the communication process between bacteria by (a) interruption of signal molecules synthesis, (b) degradation of signal molecules, and (c) interference with signal receptors all while reducing the tendency of bacteria to develop [17, 18].
Nisin, produced by Lactococcus lactis, is the most important member of group I bacteriocins, also known as lantibiotics [19]. It is approved by the US Food and Drug Administration (FDA) as “generally recognized as safe” and confirmed by the EU as a safe food preservative (E234), due to its non-toxicity and its bacteriolytic and bactericidal activities [20]. Its antimicrobial activity relies on its interaction with the cell membrane precursor lipid II, implicating the inhibition of bacterial cell wall biosynthesis and pore formation in the membrane and resulting in the leakage of intracellular components and therefore cell death [21]. Besides its usage for food shelf-life extension in the food industry [22], nisin usages has expanded to healthcare [23]. In vitro evidence suggested that nisin can significantly enhance the antibacterial and antibiofilm activities of many antibiotics against drug-resistant pathogens when used in combination [24]. Therefore, nisin can be used in combination with other natural compounds to enhance their activity and decrease the use of antibiotics while impeding further antibiotic resistance [25].p-Coumaric acid (pCA), also known as 4-hydroxycinnamic acid, is an important class of secondary metabolites produced by plants both as an intermediate product of the lignin pathway and in response to food stress and injury [26]. In addition to its antioxidative [27], anticancer [28], and antibacterial [29] effects, pCA possesses the ability to inhibit QS signal in Gram-negative bacteria through inhibiting the synthesis of AHL [30], and it is considered as a promising antibiofilm agent [31].
To date, the QSI activity of nisin and pCA combination against E. faecalis has not been explored. Furthermore, there have been no studies on the effect of nisin and pCA combination on E. faecalis biofilm formation abilities. This work aimed to assess the effect of these two natural molecules, nisin and p-coumaric acid, on biofilm formation and AI-2 signaling in E. faecalis. The effect of these compounds, when used individually and combined, on the expression of E. faecalis QS-regulated and virulence encoding gene sprE, was also investigated.
Materials and methods
Chemicals
Nisin from L. lactis was purchased from Sigma-Aldrich/USA (N5764). For experimental use, the nisin powder was dissolved in 0.02N hydrochloric acid (HCl), sterilized using a 0.22-μm pore size filter and stored at − 20 °C. p-Coumaric acid was purchased from Sigma-Aldrich/USA (C9008). For experimental use, pCA was dissolved by vortexing in ethanol (Sigma-Aldrich/USA) at a concentration of 40 mg/ml until a homogeneous solution was obtained. pCA solution was sterilized by filtration using a 0.22-μm pore size filter. A fresh solution was prepared by diluting the stock solution, according to the determined minimum inhibitory concentration (MIC) values for each experiment.
Bacteria and growth conditions
The strains used in this study are Enterococcus faecalis isolates (74 encoded food isolate and 114 encoded clinical isolate), provided by Ankara University Biology Department culture collection, and E. faecalis OG1RF ATCC 47,077, provided by American Type Culture Collection, Manassas, VA. For planktonic growth, E. faecalis strains were inoculated in tryptic soy broth (TSB) and incubated at 37 °C under static conditions. For biofilm formation, E. faecalis strains were grown in 1% glucose supplemented TSB. Reporter Vibrio harveyi BB170, which responds to AI-2 concentrations, was obtained from ATCC and grown in complete autoinducer bioassay (AB) medium at 30 °C for 20–24 h, without agitation. AB medium was prepared according to the ATCC medium 2746 preparation instructions.
Determination of MICs
The minimum inhibitory concentration (MIC) of nisin and p-coumaric acid were determined by the microdilution method, in accordance with Kitazaki et al. [32]. Briefly, strains were incubated in Mueller–Hinton II broth (MHB-II) for overnight at 37 °C. The cells were diluted in MHB-II to the turbidity of 0.5 McFarland standard. Fifty microliters of MHB-II were transferred to each well of the 96-well microtiter plate. Then, the antimicrobial agent was dispensed into the first well of the plate. Then serial twofold dilutions were made in order to obtain final concentrations of samples ranging from 0.031 to 2 mg/ml. Afterward, 50 µl bacterial suspension was dispensed into each well. The plate was then incubated at 37 °C for 24 h. MIC was determined as the lowest concentration at which bacterial growth was inhibited. Experiments were carried out as 3 replicates on different days for each strain.
AI-2 activity of E. faecalis strains
Supernatants from log-phase growing E. faecalis strains in TSB were collected by centrifugation at 10,000 g for 2 min, filtered (0.22 μm pore sized filter) to remove cell residues and kept at –20 °C until used [33]. In order to determine the autoinducer activity of E. faecalis test and control strains, reporter V. harveyi BB170 strain was inoculated into AB broth and incubated at 30 °C for 18 h with 200 rpm shaking conditions. Afterward, the overnight active V. harveyi BB170 culture was diluted 1:5000 in sterile AB medium, and 90 μl of the diluted bacterial culture was transferred to the each well of 96-well polystyrene microdilution plates. Cell-free supernatants (CFSs) of test strains were transferred into the wells containing V. harveyi BB170 strain at the ratio of 9:1 (vol/vol, V. harveyi BB170: CFSs). Control wells contained AB broth instead of CFS. The plates were incubated at 30 °C for 3 h, with agitation at 200 rpm. Luminescence was measured by quantifying the light production of V. harveyi with Perkin-Elmer Victor V3. The luminescence values, representing the autoinducer-2 (AI-2) activity, were calculated as a percent (%) fold change by the ratios of the luminescence of the test samples (reporter strain with E. faecalis CFSs) to the control (reporter strain without CFSs, with AB Broth). Results were reported as relative AI-2 activity [34].
The effect of nisin and p-coumaric acid on the AI-2 activity of E. faecalis strains
First, the inhibitory effects of nisin and p-coumaric acid on V. harveyi BB170 strain were examined in order to prevent any further misleading results. For this purpose, overnight BB170 culture was diluted (1:5000) with fresh AB medium. Nisin and pCA were adjusted to the most effective inhibitory concentrations and transferred to a 96-well microplate. The growth control wells contained only AB broth. Diluted BB170 culture was added to the wells at the ratio of 9:1 (vol/vol, V. harveyi BB170/antimicrobial agents) and incubated at 30 °C with agitation for 3 h [34]. After incubation, the suspension was transferred to the Marine Agar to determine viable cell number. The living cell numbers of the control and test groups were evaluated by a Student’s t test. A p value of 0.05 was used for statistical significance. After detecting no inhibition effect of nisin and p-coumaric acid on V. harveyi, it was used for further experiments.
Nisin, p-coumaric acid, and combinations of nisin + pCA were prepared at concentrations determined from MIC assays. Overnight V. harveyi BB170 strain was diluted (1:5000) in AB medium, and 90 μl of the diluted reporter strain was transferred to each well of the 96-well cell culture microplate. Cell-free Enterococcus supernatants and 10 μl of the nisin, pCA, and nisin + pCA at different concentrations were added. Control wells contained only CFSs. The microdilution plate containing the antimicrobial agents was incubated at the same conditions as the AI-2 assay. Fold change between luminometric values of control wells and wells containing nisin, pCA, and nisin + pCA combinations were calculated as the percent (%) of inhibition.
The effect of nisin and p-coumaric acid on biofilm formation
The biofilm inhibition effect of nisin (0.5 mg/ml nisin), pCA (1 mg/ml pCA), and nisin + pCA combination (0.5 mg/ml nisin + 1 mg/ml pCA) on E. faecalis biofilms was determined. Tested nisin, p-coumaric acid, and combinations of nisin + pCA were prepared at concentrations determined from MIC assays. Solutions were prepared by diluting stock solutions using ddH2O to reach the final concentration. Overnight E. faecalis strains were diluted 1:100 (adjusted to OD595 = 0.07) in TSB medium supplemented with 1% glucose, mixed with antimicrobial agents, and 200 µL of bacteria + agent combination was transferred on to wells. Plates were incubated at 37 °C for 24–48 h for biofilm production.
After incubation, the supernatants from the wells were removed while avoiding the deterioration of the biofilm structure and wells were rinsed by PBS. Cells attaching to the wells were dissolved in 200 µl PBS, and 10 µl of the suspensions were transferred to TSB agar and incubated overnight at 37 °C. The number of viable cells was counted, and the cell number of treated samples was compared with the cell number of untreated biofilm samples. Inhibition percentage was calculated using the following formula: % inhibition = [(CFU/ml control − CFU/ml treat) / CFU/ml control] × 100.
Effect of nisin and p-coumaric acid on virulence encoding gene sprE
Total RNA isolation from E. faecalis strains was performed using the High Pure RNA Isolation Kit (Roche, Germany) following the manufacturer’s instructions. Total RNAs were used to synthesize cDNA, according to a standard operating procedure of Transcriptor First Strand cDNA Synthesis Kit (Roche, Germany). 16S rRNA gene was used as an internal control to normalize expression values. 5 × HOT FIREPol EvaGreen qPCR Supermix (08–36-00,001; Solis BioDyne, Estonia) was used as master mix for PCR. The real-time PCR reaction mixture (10 µl) contained 1 × HOT FIREPol EvaGreen qPCR Supermix, 1 µl of template cDNA, and the appropriate forward and reverse PCR primers. The qRT-PCR reactions were carried out using a Light Cycler 480 (Roche, Germany) device. PCR conditions were as follows: 15 min at 95 °C, followed by 40 cycles of 95 °C for 15 min, 60 °C for 20 s, and 72 °C for 20 s. Primers used for sprE amplification were forward primer 5′-CGACCATTGCGTGTGGTTTT-3′ and reverse primer 5′-ATTGCGGTAGTGACTGTCGG-3′. All experiments were performed in triplicates. The difference (fold change) in the initial concentration of each transcript (normalized to 16S rRNA) was calculated using the 2ΔΔCt method [35].
Statistical analysis
Data values are expressed as mean values ± standard deviations. SPSS (26.0) statistics software was used for the statistical analysis of the values we obtained. The dependent samples t test was performed. Results with a p value of < 0.05 were considered statistically significant. All experiments were repeated at least 3 times in triplicate. Data obtained as CFU was converted to log10 values for normalization.
Results
MICs of nisin and p-coumaric acid
Nisin exhibited higher antibacterial efficacy against 114 and 74 encoded strains (MIC = 0.25 mg/ml) than OG1RF strain (MIC = 0.5 mg/ml). On the other hand, the effect of p-coumaric acid on E. faecalis strains remained the same in all strains (MIC = 1 mg/ml).
The effect of nisin and p-coumaric acid on the AI-2 activity
The effect of nisin and p-coumaric acid alone and in combination (0.5 mg/ml nisin, 1 mg/ml pCA vs 0.5 mg/ml nisin + 1 mg/ml pCA) on V. harveyi strain BB170 was investigated to verify if the changes in AI-2 activity were due to the growth inhibition of the reporter strain V. harveyi BB170. No significant differences between the control stains’ growth level and the treated strains’ growth level were detected (log CFU/ml was 5.6 ± 0.3, 5.6 ± 0.6, 5.9 ± 0.7, and 5.7 ± 0.3 for control, nisin alone treatment, pCA alone treatment, and nisin + pCA combination, respectively). Therefore, the agents did not exhibit any inhibitory effect on the reporter strain, and the changes in AI-2 activity were caused by the tested antimicrobials.
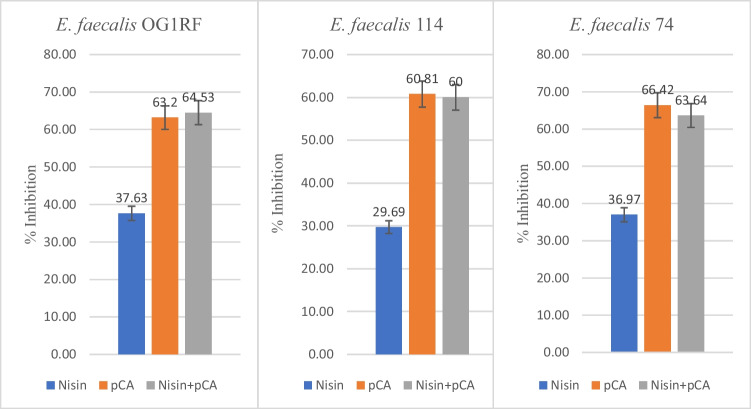
OG1RF, 74, 114 encoded strains of E. faecalis produced the relative AI-2-like activity of 249, 285, 253 relative light units, respectively, which was significantly high in comparison to control (p < 0.05). Nisin alone, at its respective MIC for each strain, was not effective in inhibiting AI-2 activity (p > 0.05) in the three strains (E. faecalis 74, 114, and control strain OG1RF), while pCA (1 mg/ml) alone reduced AI-2 activity by ≥ 60%. Furthermore, the combination of pCA and nisin (at respective MIC for each strain) decreased AI-2 activity slightly more than pCA alone. However, this increase was not statistically significant (p > 0.05) (Fig. 1).
Fig. 1.
The effects of nisin and pCA alone and in combination on AI-2 activity of E. faecalis test strains
Inhibitory effect of nisin and p-coumaric acid on biofilm formation of E. faecalis test strains
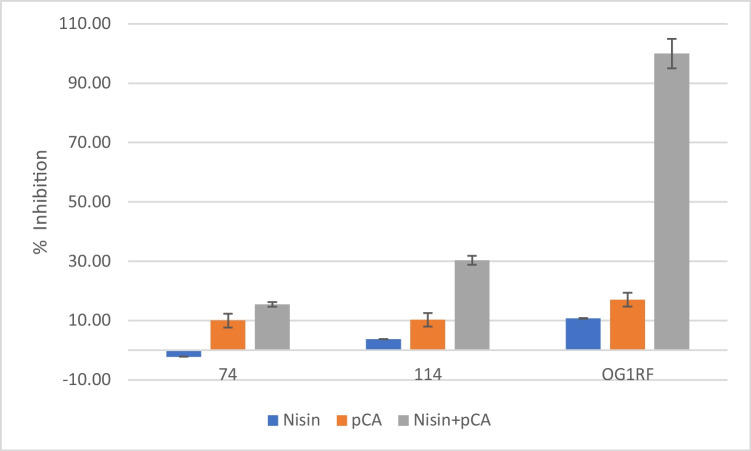
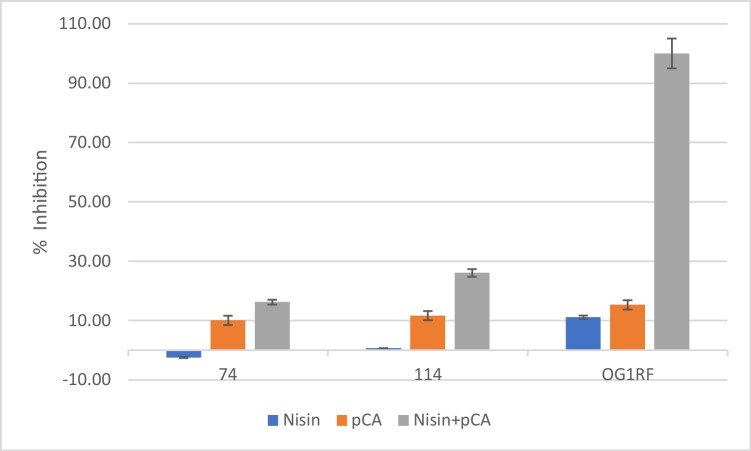
The ability of nisin and pCA alone and in combination to inhibit the biofilm formation of E. faecalis test strains after 24 h and 48 h was investigated (Fig. 1 and Fig. 2). pCA and nisin combination showed the highest inhibitory effect on E. faecalis test strains’ biofilm formation Fig. 3. One mg/ml pCA + 0.5 mg/ml nisin combination completely inhibited the biofilm formation of OG1RF strain after 24 and 48 h. One mg/ml pCA + 0.25 mg/ml nisin combination showed 30.31 ± 2.47% and 26.08 ± 3.24% E. faecalis 114 biofilm inhibition after 24 and 48 h, respectively. However, E. faecalis strain 74 was the most resistant strain that 1 mg/ml pCA + 0.25 mg/ml nisin showed the least biofilm inhibition percentage (15.46 ± 2.25% and 16.24 ± 5.39% after 24 and 48 h, respectively). pCA alone treatment resulted in 17.05 ± 0.56% (after 24 h) and 15.32 ± 0.54% (after 48 h) inhibition of OG1RF strain’s biofilm, 10.30 ± 0.41% (after 24 h) and 11.65 ± 3.38% (after 48 h) inhibition of 114 strain’s biofilm, and 10.02 ± 0.47% (after 24 h) and 10.99 ± 0.75% (after 48 h) inhibition of 74 strain’s biofilm.
Fig. 2.
The effects of nisin and pCA alone and in combination on 24-h biofilm formation of E. faecalis test strains
Fig. 3.
The effects of nisin and pCA alone and in combination on 48-h biofilm formation of E. faecalis test strains
Nisin alone treatment inhibited the biofilm formation of OG1RF strain by 10.75 ± 0.87% and 11.16 ± 4.16% after 24 and 48 h, respectively, while it had the lowest inhibitory effect on 114 strain’s biofilm formation (3.76 ± 0.90% and 0.66 ± 3.70% after 24 and 48 h, respectively). Unlike other strains, nisin alone had no inhibitory effect on the biofilm formation of the 74 encoded strain, and an increase in the number of living bacteria was observed. This positive effect (− 2.13 ± 0.22%) of nisin on the cell vitality after 24 h of incubation was statistically significant, while its effect after 48 h (− 2.47 ± 2.07%) was not significant. All other reported results were statistically significant (p < 0.05).
Effect of nisin and p-coumaric acid on E. faecalis virulence gene sprE
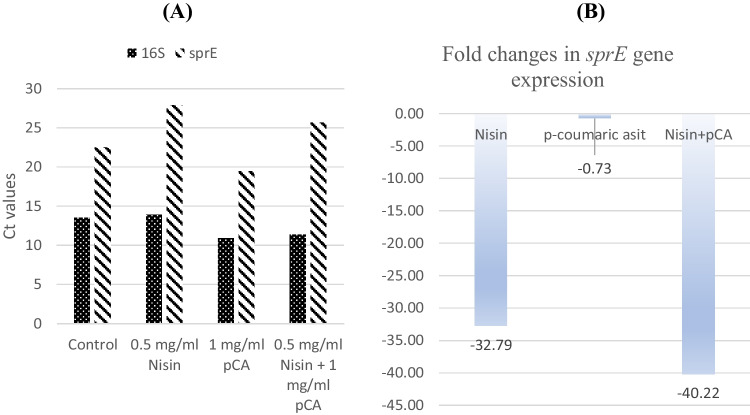
OG1RF strain was the only strain harboring sprE gene in its genome, as a result of PCRs using gene-specific primers. Therefore, only OG1RF strain was used to investigate the effect of antimicrobials on expression of sprE gene. The effect of nisin and p-coumaric acid alone and in combination on the sprE virulence gene was obtained from qRT-PCR, and the Ct values are given in Fig. 4A. The expression levels of the sprE gene were normalized to the 16S rRNA gene. Nisin (0.5 mg/ml) alone and the combination of nisin and pCA (0.5 mg/ml nisin + 1 mg/ml pCA) led to a 32.78- and 40.22-fold decrease in expression of the sprE gene, respectively, while pCA alone did not have a significant effect (− 0.73-fold) on sprE expression (Fig. 4B).
Fig. 4.
A Ct values of sprE gene expression in OG1RF strain’s test groups. B Fold changes in sprE gene expression in OG1RF strain’s test groups
Discussion
E. faecalis is one of the most important nosocomial pathogens, characterized by its high antibiotic resistance against widely used antibiotics, its tendency to spread antibiotic resistance genes, and its ability to form strong biofilms in clinical and industrial settings, leading to serious healthcare problems and economic drawbacks [36]. Considering these consequences and the need to effectively treat biofilm-related infections caused by E. faecalis, the development of new strategies against E. faecalis biofilms is a critical research topic. Since the QS system regulates biofilm formation, antibiotic resistance, and virulence [37], this current study investigated the effect of nisin and p-coumaric acid on the AI-2 signaling, biofilm formation, and virulence genes expression of E. faecalis.
The present study showed that p-coumaric acid exerted an antimicrobial effect against E. faecalis food and clinical isolate and E. faecalis OG1RF with MIC of 1 mg/ml, which is in accordance with of the study conducted by Toafiq et al. [38]. Consistent with the current literature, nisin showed an antimicrobial effect against E. faecalis test strains with MICs ranging from 0.25 to 0.5 mg/ml [24, 39]. The QSI activity of pCA and nisin on AI-2 signaling was investigated, and results reported that pCA (1 mg/ml) alone reduced AI-2 activity by ≥ 60%. Previous studies investigating the effects of pCA on QS mechanism have been conducted only on Gram-negative bacteria where pCA was demonstrated to inhibit the AHLs signal molecules. Myszka et al. [30] showed that pCA (120 and 240 μmol/L) inhibited the AHL synthesis in Pseudomonas fluorescens which led to the decrease in the expression of flagella gene flgA and thus the inhibition of biofilm formation. Bodini et al. [40] reported that pCA led to the inhibition of the QS responses of Chromobacterium violaceum 5999, Agrobacterium tumefaciens NTL4, and Pseudomonas chlororaphis. The current study was the first to report that pCA exhibits QSI effect against AI-2 signaling in E. faecalis. The results of this study showed that nisin did not affect the inhibition of AI-2 signaling and no positive or negative contribution to pCA activity when applied in combination. However, Gui et al. [41] reported that nisin enhanced the activity of AHL lactonases AiiAAI96, which are quorum-quenching enzymes, when used in combination to prevent sturgeon spoilage.
In this study, pCA and nisin combination showed significantly higher inhibitory effect on the biofilm formation of E. faecalis test strains, compared to the treatment of pCA or nisin alone. The inhibitory effect of nisin and pCA combination was strain specific. The most effective reduction was determined at OG1RF encoded test strain both at AI-2 and biofilm inhibition. However, AI-2 activity was important, but not the only one mechanism controls the biofilm production, it was determined that biofilm inhibition level was not as high as the inhibition of AI-2 activity. In line with these results, Bag and Chattopadhyay [42] showed that pCA and nisin had a synergistic antibiofilm activity, and the antibiofilm effect of nisin was significantly enhanced (more than twofold) when used in combination with pCA against Bacillus cereus and Salmonella Typhimurium biofilms. In addition, Kot et al. [31] reported that pCA inhibited Escherichia coli biofilm formation on the internal urinary catheter surface and significantly reduced mature biofilm biomass leading to its partial eradication. The strong antibiofilm effect of nisin and pCA against Enterococcus faecalis biofilm structures described in this study is most likely due to the fact that pCA disrupts quorum sensing signaling and increases the bactericidal activity of nisin against the dispersed biofilm cells [21] by further affecting the membrane permeability of these cells [31].
The serine protease gene sprE encodes an extracellular serine protease that was demonstrated to be involved in E. faecalis pathogenesis and biofilm formation [43, 44]. Considering that sprE gene is controlled by the fsr quorum sensing system [45], the effect of the potential QSI nisin and pCA on sprE expression level was investigated. Data showed that nisin (0.5 mg/ml) alone and its combination with pCA (0.5 mg/ml nisin + 1 mg/ml pCA) led to significant decrease in the expression of sprE gene, while pCA alone did not have a remarkable effect on its expression. In another study investigating the effect of natural QSI on QS-regulated virulence genes, Ahmed et al. [46] showed that trans-cinnamaldehyde and salicylic acid remarkably decreased the expression of extracellular virulence factors, such as protease, elastase, and pyocyanin in Pseudomonas aeruginosa.
Increasing antibiotic resistance and the demand for developing new therapeutic avenues to control infectious bacteria, this study aimed at investigating the QSI properties of the natural products nisin and p-coumaric acid against E. faecalis. It was reported that pCA inhibited AI-2 signaling in E. faecalis and that nisin + pCA combinations showed higher inhibitory effect on E. faecalis biofilm formation and reduced the expression of the QS-regulated extracellular virulence factor serine protease. Together with these promising findings and the literature data summarized above are evaluated together, it is highly recommended to use nisin and pCA combination in industrial and clinical process to combat with E. faecalis biofilms.
Funding
This work was financially supported by the Scientific and Technological Research Council of Turkey (grant 118Z697). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Data availability
All data generated or analyzed during this study are included in this published article.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Responsible Editor: Ilana Camargo
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Miller MB, Bassler BL. Quorum sensing in bacteria. Annu Rev Microbiol. 2001;55:165–199. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- 2.Ng WL, Bassler BL. Bacterial quorum-sensing network architectures. Annu Rev Genet. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nazzaro F, Fratianni F, Coppola R. Quorum sensing and phytochemicals. Int J Mol Sci. 2013;14(6):12607–12619. doi: 10.3390/ijms140612607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 5.Bassler BL. How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr Opin Microbiol. 1999;2(6):582–587. doi: 10.1016/S1369-5274(99)00025-9. [DOI] [PubMed] [Google Scholar]
- 6.Kendall MM, Sperandio V (2014) Cell-to-cell signaling in Escherichia coli and Salmonella. EcoSal Plus 6(1). 10.1128/ecosalplus.ESP-0002-2013 [DOI] [PMC free article] [PubMed]
- 7.Pereira CS, Thompson JA, Xavier KB. AI-2-mediated signalling in bacteria. FEMS Microbiol Rev. 2013;37(2):156–181. doi: 10.1111/j.1574-6976.2012.00345.x. [DOI] [PubMed] [Google Scholar]
- 8.Xavier KB, Bassler BL. Interference with AI-2-mediated bacterial cell-cell communication. Nature. 2005;437(7059):750–753. doi: 10.1038/nature03960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schauder S, et al. The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol Microbiol. 2001;41(2):463–476. doi: 10.1046/j.1365-2958.2001.02532.x. [DOI] [PubMed] [Google Scholar]
- 10.Richards MJ, et al. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000;21(8):510–515. doi: 10.1086/501795. [DOI] [PubMed] [Google Scholar]
- 11.Haghi F, Lohrasbi V, Zeighami H. High incidence of virulence determinants, aminoglycoside and vancomycin resistance in enterococci isolated from hospitalized patients in Northwest Iran. BMC Infect Dis. 2019;19(1):744. doi: 10.1186/s12879-019-4395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunny GM, LE Hancock, Shankar N (2014) Enterococcal biofilm structure and role in colonization and disease. In: Gilmore MS et al. (eds) Enterococci: from commensals to leading causes of drug resistant infection. Massachusetts Eye and Ear Infirmary, Boston [PubMed]
- 13.Mohamed JA, Huang DB. Biofilm formation by enterococci. J Med Microbiol. 2007;56(Pt 12):1581–1588. doi: 10.1099/jmm.0.47331-0. [DOI] [PubMed] [Google Scholar]
- 14.Costerton JW, Stewart PS, Greenberg E. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Q, et al. Quorum sensing: a prospective therapeutic target for bacterial diseases. Biomed Res Int. 2019;2019:2015978. doi: 10.1155/2019/2015978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santhakumari S, Ravi AV. Targeting quorum sensing mechanism: an alternative anti-virulent strategy for the treatment of bacterial infections. S Afr J Bot. 2019;120:81–86. doi: 10.1016/j.sajb.2018.09.028. [DOI] [Google Scholar]
- 17.Hentzer M, Givskov M. Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J Clin Invest. 2003;112(9):1300–1307. doi: 10.1172/JCI20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalia VC, Raju SC, Purohit HJ. Genomic analysis reveals versatile organisms for quorum quenching enzymes: acyl-homoserine lactone-acylase and -lactonase. Open Microbiol J. 2011;5:1–13. doi: 10.2174/1874285801105010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin JM, et al. Biomedical applications of nisin. J Appl Microbiol. 2016;120(6):1449–1465. doi: 10.1111/jam.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gharsallaoui A, et al. Nisin as a food preservative: part 1: physicochemical properties, antimicrobial activity, and main uses. Crit Rev Food Sci Nutr. 2016;56(8):1262–1274. doi: 10.1080/10408398.2013.763765. [DOI] [PubMed] [Google Scholar]
- 21.Prince A, et al. Lipid-II independent antimicrobial mechanism of nisin depends on its crowding and degree of oligomerization. Sci Rep. 2016;6:37908. doi: 10.1038/srep37908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oshima S, et al. Nisin A extends the shelf life of high-fat chilled dairy dessert, a milk-based pudding. J Appl Microbiol. 2014;116(5):1218–1228. doi: 10.1111/jam.12454. [DOI] [PubMed] [Google Scholar]
- 23.Joo NE, et al. Nisin, an apoptogenic bacteriocin and food preservative, attenuates HNSCC tumorigenesis via CHAC1. Cancer Med. 2012;1(3):295–305. doi: 10.1002/cam4.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong Z, et al. An in vitro study on the effects of nisin on the antibacterial activities of 18 antibiotics against Enterococcus faecalis. PLoS ONE. 2014;9(2):e89209. doi: 10.1371/journal.pone.0089209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathur H, et al. Fighting biofilms with lantibiotics and other groups of bacteriocins. NPJ Biofilms Microbiomes. 2018;4:9. doi: 10.1038/s41522-018-0053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferreira PS, et al. A review of analytical methods for p-coumaric acid in plant-based products, beverages, and biological matrices. Crit Rev Anal Chem. 2019;49(1):21–31. doi: 10.1080/10408347.2018.1459173. [DOI] [PubMed] [Google Scholar]
- 27.Abdel-Wahab MH, et al. Influence of p-coumaric acid on doxorubicin-induced oxidative stress in rat's heart. Pharmacol Res. 2003;48(5):461–465. doi: 10.1016/S1043-6618(03)00214-7. [DOI] [PubMed] [Google Scholar]
- 28.Pei K, et al. p-Coumaric acid and its conjugates: dietary sources, pharmacokinetic properties and biological activities. J Sci Food Agric. 2016;96(9):2952–2962. doi: 10.1002/jsfa.7578. [DOI] [PubMed] [Google Scholar]
- 29.Lou Z, et al. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control. 2012;25(2):550–554. doi: 10.1016/j.foodcont.2011.11.022. [DOI] [Google Scholar]
- 30.Myszka K, et al. Role of gallic and p-coumaric acids in the AHL-dependent expression of flgA gene and in the process of biofilm formation in food-associated Pseudomonas fluorescens KM120. J Sci Food Agric. 2016;96(12):4037–4047. doi: 10.1002/jsfa.7599. [DOI] [PubMed] [Google Scholar]
- 31.Kot B, et al. Antibiofilm activity of trans-cinnamaldehyde, p-coumaric, and ferulic acids on uropathogenic Escherichia coli. Turk J Med Sci. 2015;45(4):919–924. doi: 10.3906/sag-1406-112. [DOI] [PubMed] [Google Scholar]
- 32.Kitazaki K, et al. In vitro synergistic activities of cefazolin and nisin A against mastitis pathogens. J Vet Med Sci. 2017;79(9):1472–1479. doi: 10.1292/jvms.17-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jesudhasan PR, et al. Transcriptome analysis of genes controlled by luxS/autoinducer-2 in Salmonella enterica serovar Typhimurium. Foodborne Pathog Dis. 2010;7(4):399–410. doi: 10.1089/fpd.2009.0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu L, Hume ME, Pillai SD. Autoinducer-2-like activity associated with foods and its interaction with food additives. J Food Prot. 2004;67(7):1457–1462. doi: 10.4315/0362-028X-67.7.1457. [DOI] [PubMed] [Google Scholar]
- 35.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 36.Anderson AC, et al. Enterococcus faecalis from food, clinical specimens, and oral sites: prevalence of virulence factors in association with biofilm formation. Front Microbiol. 2015;6:1534. doi: 10.3389/fmicb.2015.01534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao X, Z Yu, Ding T (2020) Quorum-sensing regulation of antimicrobial resistance in bacteria. Microorganisms 8(3):425 [DOI] [PMC free article] [PubMed]
- 38.Taofiq O, et al. Phenolic acids, cinnamic acid, and ergosterol as cosmeceutical ingredients: stabilization by microencapsulation to ensure sustained bioactivity. Microchem J. 2019;147:469–477. doi: 10.1016/j.microc.2019.03.059. [DOI] [Google Scholar]
- 39.Grenier D, et al. Biocompatible combinations of nisin and licorice polyphenols exert synergistic bactericidal effects against Enterococcus faecalis and inhibit NF-kappaB activation in monocytes. AMB Express. 2020;10(1):120. doi: 10.1186/s13568-020-01056-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bodini SF, et al. Quorum sensing inhibition activity of garlic extract and p-coumaric acid. Lett Appl Microbiol. 2009;49(5):551–555. doi: 10.1111/j.1472-765X.2009.02704.x. [DOI] [PubMed] [Google Scholar]
- 41.Gui M, et al. Effect of AHL-lactonase and nisin on microbiological, chemical and sensory quality of vacuum packaged sturgeon storage at 4ºC. Int J Food Prop. 2021;24(1):222–232. doi: 10.1080/10942912.2021.1872621. [DOI] [Google Scholar]
- 42.Bag A, Chattopadhyay RR. Synergistic antibacterial and antibiofilm efficacy of nisin in combination with p-coumaric acid against food-borne bacteria Bacillus cereus and Salmonella typhimurium. Lett Appl Microbiol. 2017;65(5):366–372. doi: 10.1111/lam.12793. [DOI] [PubMed] [Google Scholar]
- 43.Engelbert M, et al. Contribution of gelatinase, serine protease, and fsr to the pathogenesis of Enterococcus faecalis endophthalmitis. Infect Immun. 2004;72(6):3628–3633. doi: 10.1128/IAI.72.6.3628-3633.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qin X, et al. Effects of Enterococcus faecalis fsr genes on production of gelatinase and a serine protease and virulence. Infect Immun. 2000;68(5):2579–2586. doi: 10.1128/IAI.68.5.2579-2586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin X, et al. Characterization of fsr, a regulator controlling expression of gelatinase and serine protease in Enterococcus faecalis OG1RF. J Bacteriol. 2001;183(11):3372–3382. doi: 10.1128/JB.183.11.3372-3382.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmed S, et al. Natural quorum sensing inhibitors effectively downregulate gene expression of Pseudomonas aeruginosa virulence factors. Appl Microbiol Biotechnol. 2019;103(8):3521–3535. doi: 10.1007/s00253-019-09618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.