Abstract
Fungal sinusitis is a widespread infection that affects both healthy and immunocompromised individuals. Reports of sinus fungal infections have increased due to recent advances in diagnosis. Furthermore, susceptible and immune-compromised patients play an important role in increasing the number of reported cases. Infections with lesser-known fungi have been reported infrequently around the world. This paper describes a Cladosporium tenuissimum infection caused by chronic fungal sinusitis in a woman who had traveled to several countries. We used morphological and molecular methods to confirm the infection. The infection is most likely caused by the use of sulfasalazine, which is related to the patient’s rheumatism. Sulfasalazine inhibits neutrophilic chemoattractant lipid synthesis in neutrophils, which play a key role in antifungal immunity. The patient is also undergoing root canal therapy and has several upper jaw implants, which may have contributed to the development of sinusitis.
Keywords: Sinusitis, Cladosporium, Disorder, Fungus ball
Introduction
Fungi are all around us, and we inhale them in with every breath. However, only a small percentage of them cause pathological inflammatory responses in the airways, and invasive fungal sinusitis is uncommon. Fungal species are pathogenic in humans, causing a wide range of diseases in the airways. The term “fungal rhino sinusitis” refers to inflammatory and infectious conditions of the nose and paranasal sinuses. It ranges in severity from harmless colonization to potentially fatal invasive disease. This condition is heavily mediated by the patient and significantly influences the nature of the fungal disease in the respiratory tract. Rhinosinusitis refers to a group of conditions marked by inflammation of the paranasal sinuses and nasal mucosa. Meanwhile, chronic rhinosinusitis (CRS) is a group of disorders characterized by nasal mucosa and paranasal sinus inflammation for at least 12 weeks [1–3]. This inflammation may be due to germs, bacteria, fungi, or allergic or non-allergic causes. The prevalence of fungal rhinosinusitis (FRS) is increasing. It causes severe physical symptoms that impair the quality of life. The disease appears in five clinical-pathological forms, each with its own set of diagnostics, therapeutic, and prognostic criteria, acute invasive FRS with invasive forms, chronic and invasive granulomatosis. The non-invasive form is characterized by a fungus ball and allergic FRS, as well as mucosal changes ranging from inflammatory thickening to the formation of impure nasal polyps [4]. The fungus is found in 3 to 5% of cultured sinus samples. The most common causes of fungal sinusitis are Aspergillus and Zygomycetes; in the meantime, some fungi such as Cladosporium agents also cause fungal sinusitis [5, 6].
CRS is a chronic public health concern affecting the quality of life in more than 5% of people [1]. CRS affects 12.5% of the population in the USA at some point in their lives. Health care and working days incur significant costs. CRS is thought to be caused by bacteria, viruses, anatomical abnormalities, and fungi. The role of fungi in CRS is still a topic of intense research. FRS is histopathologically classified into two types based on the fungus’ tissue invasion: invasive and non-invasive [7, 8]. Invasive disease is divided into acute invasive, chronic invasive, and granulomatous. This non-invasive disease is classified into three different clinical forms: localized colonization, fungus ball, and eosinophil-related FRS, including AFRS [9]. A fungus ball is described as the presence of a non-invasive accumulation of a dense mass of fungal hyphae in a sinus cavity [7].
Fungus balls are dense collections of fungal hyphae that are typically formed in the sinuses by fungal spores and cause little inflammation or mucosal reaction. Fungus balls are most commonly found in one of the maxillary sinuses (94%), but they can also appear in other sinuses. The majority of the remainder occur in the sphenoid sinus. Fungus balls, unlike invasive fungal sinusitis, are more common in immunocompromised individuals and have been reported to be more common in the middle-aged female population [10, 11]. The pathogenesis of fungal ball formation is unknown. As with saprophytic fungal infection, it is widely accepted that fungal entry into the sinuses occurs through spore inhalation [11]. Previous surgery/mucosal injury, as previously mentioned, may play a role. Furthermore, maxillary fungal balls have been linked to previous dental treatments, specifically dental fillings and root canal therapy, and have been found in 89.2% of patients with prior dental work in previous studies [12, 13] However, fungal infections may take years to appear after initial intervention [3, 11].
A fungus ball’s presence is usually non-specific and can be asymptomatic [12]. Facial and head pain (or retro-orbital pain in sphenoid disease) are some of the symptoms caused by the two disease processes. Endoscopic examination of the nasal cavity reveals important information about the type and location of sinus damage, as well as the shape and location of fungal pellets, and can range from completely normal to scaling, purulent discharge, and edematous mucosa with the formation of a polyp or fungal ball [14]. A computerized tomography (CT) scan of the paranasal sinuses raises the possibility of a fungal infection or fungus ball. CT scans have a sensitivity and specificity of 62% and 99% for detecting fungal pellets, respectively. This is caused by an opacified sinus with increased intralesional density or calcification [12, 13].
Specifically, phaeohyphomycotic refers to unusual and opportunistic infections caused by skin fungi that are distinguished from other hyphomycetes in the tissue by melanin-containing cell walls. Infections with these fungi have been reported in amphibians, reptiles, birds, fish, humans, and domestic animals, resulting in a variety of clinical conditions [15]. Cladosporium genus, Ascomycota family, is a cosmopolitan filamentous fungus that rots in soil, water, wind, and trees. Cladosporium classification and differentiation are extremely difficult due to the abundance of complexity in size, shape, pigmentation, surface ornamentation, and conidiophore structure. Cladosporium behavior and properties are heavily influenced by external factors and influences such as weather, soil type, and various latitude influences [16]. The species of this genus are commonly thought of as environmental contaminants, but they can also cause infections in different individuals as superficial or invasive infections. Cladosporium tenuissimum are somewhat differentiated using colony color and microscopic shapes, and the final differentiation is done by molecular tests [1].
Materials and methods
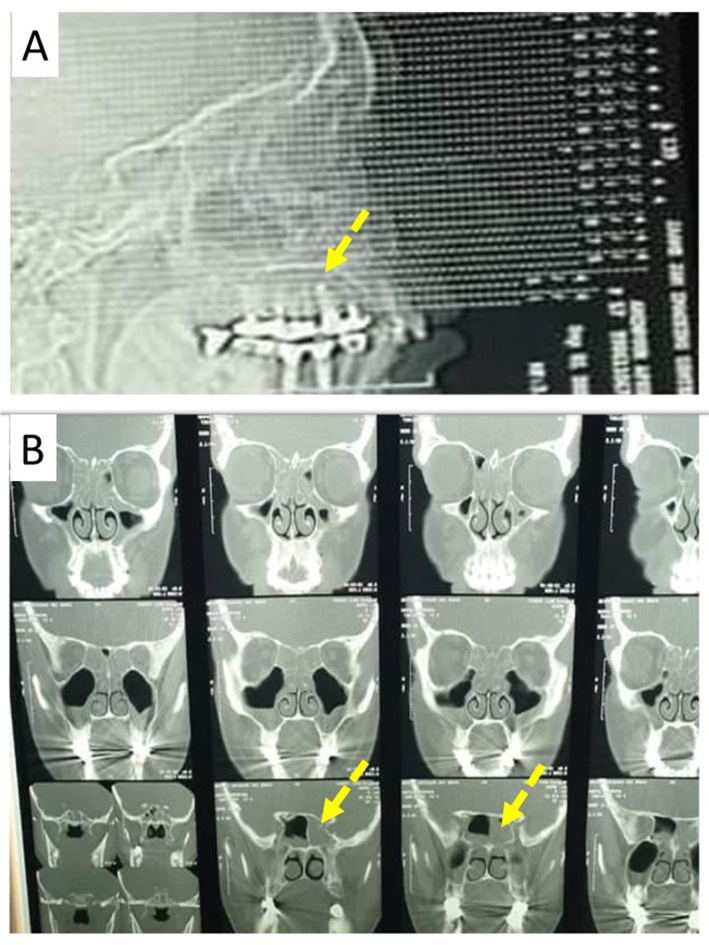
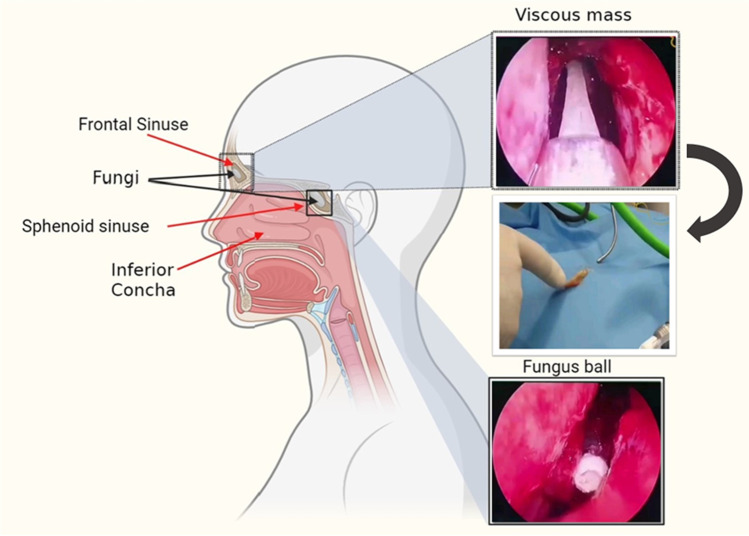
A 65-year-old woman suffering from back pain and leg pain for a long time was diagnosed with rheumatoid arthritis and was treated with sulfasalazine. During the treatment of rheumatism, she traveled to several countries, including the UK, America, Canada, and Mexico. Due to decay of the upper jaw teeth over the years, the patient has also had one case of root canal treatment and several cases of implants. After receiving sulfasalazine for 3–4 months, she gradually and finally lost her sense of smell and taste. She experienced other symptoms including nasal congestion, headache, sinus pain, colored discharge, severe allergies, runny nose, and rarely fever. She visited an otolaryngologist to evaluate her sense of smell and taste as well as her problems. Then it was subjected to radiological and laboratory tests. In the radiological examination and CT scan, the otolaryngologist suspected the existence of two masses in the sphenoid sinus and frontal sinus (Fig. 1). Finally, the patient underwent surgery to remove a fungus ball from the sphenoid sinus and a viscous and stretchy mass from the frontal sinus. The schematic figure from sphenoid sinuses and frontal sinuses is shown in Fig. 2.
Fig. 1.
Computed Tomography (CT) scan of sinuses. A The flash indicates the dental implant in the upper jaw of the patient. B The flashes indicate the fungal mass in the left sphenoid sinus of the patient
Fig. 2.
Fungus ball from the sphenoid sinus and viscous mass from the frontal sinus of the case
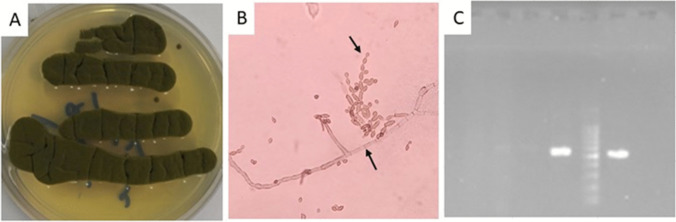
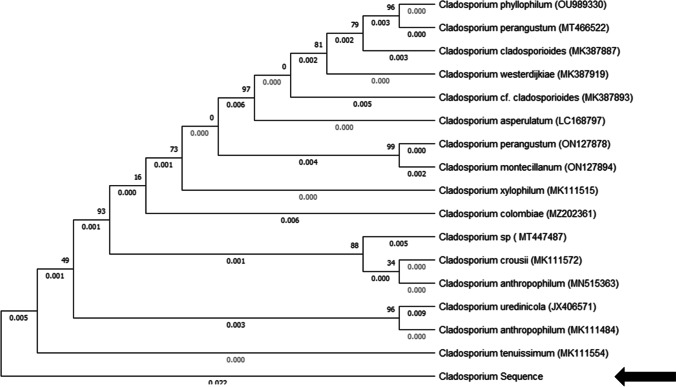
In the laboratory, the sample (fungus ball and slimy mass) was subjected to direct microscopic examination and cultured on Sabouraud dextrose agar medium. Fungal culture from specimen referred to the laboratory (Fig. 3A) and direct smear from culture (Fig. 3B). After a few days, fungus growth was also observed in the culture medium. The colonies were olive-green to olive-brown with a velvety or powdery appearance. Slide culture observed hyphae, conidiophores, and ovoid conidia. The fungal agent was identified using polymerase chain reaction (PCR) method, followed by nucleotide sequence analysis of the amplified product. DNA was extracted from culture media according to instruction of the DNA extraction kit (Yekta Tajhize). In brief, isolates were grown on Sabouraud agar plates at 30°C for 48 h. Colonies were harvested using an inoculation loop and resuspend in 1 ml fungal saline (0.9% w/v NaCl) to obtain a suspension containing 1–5 × 106 cells (measured) photometrically at A530, or McFarland 0.5. After that, the tubes were centrifuged and the cell pellets were collected. A total of 500 μl lyticase solution was added to the tube and incubate at 37°C for 30 min to produce spheroplasts. Lyticase solution contained 10 U/ml lyticase, 50 mM Tris, pH 7.5, 10 mM EDTA, and 28 mM β-mercaptoethanol. After that, the mixture was centrifuged at full speed for 10 min and the supernatant was discarded. Subsequently, the pellet was resuspended in 180 μl buffer ATL and 20 μl proteinase K stock solution. The mixture was incubated at 55°C for 15 min and vortexed for 15 s. A total of 200 μl of buffer AL was added to the sample, and was mixed thoroughly by vortexing. The mixture was pipetted into the DNeasy Mini spin column placed in a 2-ml collection tube, and centrifuged at ≥6000 × g for 1 min, and flow-through and collection tube was discarded. DNeasy Mini spin column was placed in a new 2-ml collection tube in which 500 μl Buffer AW1 was added, and centrifuged for 1 min at ≥6000 × g. The flow-through and collection tube was discarded. The DNeasy Mini spin column was placed in a new 2-ml collection tube and 500 μl Buffer AW2 was added, and to dry the DNeasy membrane it was centrifuged for 3 min at 20,000 × g. After that, the flow-through and collection tube were discarded. The DNeasy Mini spin column was placed in a clean 1.5-ml or 2-ml microcentrifuge tube and 200 μl Buffer AE was pipetted directly onto the DNeasy membrane. Subsequently, the tube was incubated at room temperature for 1 min, and then centrifuged for 1 min at ≥6000 × g (8000 rpm) to elute. Gene was amplified using the primers P-ITS1: 5′-TCCGTAGGTGAACCTGCGG-3′; and P-ITS4: 5′-TCCTCCGCTTATTGATATGC-3′ [17]. The PCR amplification was performed in 20 μL reaction mixtures. An initial denaturation step at 95 °C for 5 min was applied prior to 40 cycles of denaturation for 20 s at 95 °C, hybridization for 30 s at 60 °C, and extension for 60 s at 72 °C, followed by a final extension at 72 °C for 5 min. PCR products were analyzed using electrophoresis on 1.5% agarose gel stained with safe green (Yekta Tajhize). Figure 3 C shows the PCR product that was investigated by electrophoresis. The PCR product was sent to a genomic company (Iran) for sequencing. The sequence was checked to correct ambiguities. Homologies with available data in GenBank were checked using the basic local alignment search tool (www.ncbi.nlm.nih.gov/BLAST). Nucleotide sequence analysis identified the amplified fungal agent fragment as Cladosporium tenuissimum (Fig. 4). The sequence was deposited in GenBank under the accession number ON965336. The patient was treated with itraconazole after surgery to prevent a recurrence. No postoperative complications were reported and the patient was screened after 6 months. After follow-up, the patient’s signs and symptoms as well as the results were satisfactory. And the patient’s sense of smell and taste had returned to normal.
Fig. 3.
A Fungal culture from Cladosporium tenuissimum. B Fungal filaments in direct examination by methylene blue staining. C PCR product on 1.5% gel agarose. The 1000-bp ladder is located in the center of the sample which is loaded in the gel
Fig. 4.
One of 600 equally most parsimonious and equally looking phylogenetic trees based on a heuristic tree search using aligned sequences of the internal transcribed spacer regions 1 and 2 and the 5.8S rDNA. The tree was randomly selected. Support based on 10 000 replicates of a fast step-wise addition bootstrap analysis is indicated near the branches. Trees were rooted with the strains of Cladosporium salinae. Most monophyletic species clades received high, but some deeper branches moderate, bootstrap support
Results and discussion
We report a rare case of CRS with fungus balls in the sphenoid sinus and adhesive mass in the frontal sinus with Cladosporium tenuissimum. The simultaneous removal of two foreign bodies from the sphenoid and frontal sinuses shows that probably after fungal spores enter the sinus space due to the weakness of the immune system, these spores are allowed to grow and reproduce and finally they have created fungal bodies. Many of these fungal infections remain undiagnosed for various reasons and cause physical and economic damage to patients for a long time before diagnosis. A long delay in diagnosis causes damage to the tissues around the sinuses and even the surrounding bones and damages the patient’s sense of smell and taste; so, it is very important to quickly diagnose these infections. This fungal infection also had the potential to cause infection in healthy people.
Fungus balls are usually very difficult to treat and there is no other way than surgery and removal. For this reason, it is very necessary to carry out detailed radiological examinations and identify the fungal agent in the laboratory in the early stages and treat the infection in time and to some extent, it will prevent the formation of large fungal masses, and save the patient from suffering injuries and complications of surgery. Accurate history with radiological findings and molecular and microbiological diagnosis may help diagnose these types of infections. Demonstration of fungal hyphae and spores in direct vision with a positive culture from correctly collected samples as well as cellular response and molecular identification could be useful in initial diagnosis and treatment.
In the present case, we studied a patient who was taking sulfasalazine for the treatment of rheumatoid arthritis. Lipoxygenase, cycloxygenase, and other folate-metabolizing enzymes are inhibited by sulfasalazine. Sulfasalazine inhibits TNF-alpha-induced adhesion molecule upregulation on monocytes and granulocytes; it also inhibits T cell proliferation and B cell activation [18]. Since Cladosporium is a saprophytic fungus in the soil, the patient may have developed chronic fungal sinusitis by inhaling fungal spores during numerous trips to different countries. In addition, it is likely that fungal spores were transmitted to the upper jaw and sinuses due to root canal therapy or dental implants. Examples of chronic fungal sinusitis, cutaneous, and phaeohyphomycosis infections caused by Cladosporium agents can be seen in Table 1 [6, 19, 20]. In the study of Swam et al., a case of CRS in a smoking male farmer with Paecilomyces variotii was mentioned, that there was a deviation of the nasal septum, nasal congestion, bleeding from the nose when sneezing, and a bulge on the right side of the soft palate. There was no visible mass in the sinus space, and a diagnosis of chronic sinusitis with secondary fungal infection was made, and treatment with itraconazole was performed [1]. Ahmad Alroqi also reported a case of FRS in a 20-year-old Saudi man with a complaint of progressive bilateral nasal obstruction caused by Aspergillus nidolans [21]. In recent years, fungal infections of the sinuses have become more common in different parts of the world for the following reasons: (1) greater recognition and understanding among physicians; (2) the use of modern tools and methods of diagnosis; and (3) patient concern and follow-up. Successful treatment of such fungal infections largely depends on accurate identification of the pathogen and early and appropriate intervention with surgery and sinus drainage along with antifungal drugs based on the standard diet [22, 23].
Table 1.
Cladosporium tenuissimum infections case reports
| Case | Age in years/gender | Year | Country | Predisposing factors | Treatment | Site of infection |
|---|---|---|---|---|---|---|
| 1 | 57/male | 2001 | Japan | Healthy | Steroids and fluconazole | Sinus |
| 2 | 25/male | 2001 | India | Cervical tubercular lymphadenitis | Fluconazole | Sinus |
| 3 | 18/ male | 2002 | Iran | Wegener’s granulomatosis | Surgery and amphotericin B | Sinus |
| 4 | 30/female | 2006 | India | Healthy | Potassium iodide | Knee skin |
| 5 | 62/ male | 2015 | Asian | Healthy | Amphotericin B and fluconazole | Eye |
| 6 | 69/female | 2020 | Japan | Diabetic mellitus | Surgery and voriconazole | Eye |
| 7 | 68/ male | 2021 | China | CARD9 mutation | Voriconazole | Lung |
Conclusion
We describe rare cases of CRS with Cladosporium tenuissimum. It is possible that susceptible patients are affected by saprophytic and low-risk fungi such as these. A detailed description of the patient’s conditions along with radiological and laboratory findings such as microbiological diagnosis and showing fungal hyphae with specific cell response or positive fungal culture in correctly collected sinus contents can be useful in correct diagnosis.
Authors’ contribution
Abozar Nasiri Jahrodi: study design, sample preparation, first draft preparation.
Fateme-Maryam Sheikholeslami: molecular investigation.
Mehdi Barati: manuscript revision and image preparing.
Declarations
Consent for publication
A copy of the written consent of the case is available for review by the journal editor upon request.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Swami T et al (2016) Chronic invasive fungal rhinosinusitis by Paecilomyces variotii: a rare case report. Indian J Med Microbiol 34(1) [DOI] [PubMed]
- 2.Alotaibi NH, et al. Chronic invasive fungal rhinosinusitis in immunocompetent patients: a retrospective chart review. Front Surg. 2020;7:608342. doi: 10.3389/fsurg.2020.608342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mensi M, et al. Risk of maxillary fungus ball in patients with endodontic treatment on maxillary teeth: a case-control study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(3):433–436. doi: 10.1016/j.tripleo.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee SS, Chakrabarti A. Epidemiology and medical mycology of fungal rhinosinusitis. Otorhinolaryngol Clin Int J. 2009;1(1):1–13. doi: 10.5005/jp-journals-10003-1001. [DOI] [Google Scholar]
- 5.Greval R, et al. Incidence of fungal infections in chronic maxillary sinusitis. Indian J Pathol Microbiol. 1990;33(4):339–343. [PubMed] [Google Scholar]
- 6.Sood N, Makkar R. Case report. Subcutaneous phaeohyphomycosis due to Cladosporium cladosporioides. Mycoses. 2000;43(1-2):85–87. doi: 10.1046/j.1439-0507.2000.00545.x. [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarti A, et al. Epidemiology of chronic fungal rhinosinusitis in rural India. Mycoses. 2015;58(5):294–302. doi: 10.1111/myc.12314. [DOI] [PubMed] [Google Scholar]
- 8.Buzina W, et al. Fungal biodiversity–as found in nasal mucus. Med Mycol. 2003;41(2):149–161. doi: 10.1080/mmy.41.2.149.161. [DOI] [PubMed] [Google Scholar]
- 9.Chakrabarti A, et al. Fungal rhinosinusitis: a categorization and definitional schema addressing current controversies. Laryngoscope. 2009;119(9):1809–1818. doi: 10.1002/lary.20520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deutsch PG, Whittaker J, Prasad S. Invasive and non-invasive fungal rhinosinusitis—a review and update of the evidence. Medicina. 2019;55(7):319. doi: 10.3390/medicina55070319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watkinson JC, Clarke RW. basic sciences, endocrine surgery, rhinology. CRC Press; 2018. Scott-Brown's otorhinolaryngology and head and neck surgery: volume 1. [Google Scholar]
- 12.Dufour X, et al. Paranasal sinus fungus ball: epidemiology, clinical features and diagnosis. A retrospective analysis of 173 cases from a single medical center in France, 1989–2002. Sabouraudia. 2006;44(1):61–67. doi: 10.1080/13693780500235728. [DOI] [PubMed] [Google Scholar]
- 13.Dhong H-J, Jung J-Y, Park JH. Diagnostic accuracy in sinus fungus balls: CT scan and operative findings. Am J Rhinol. 2000;14(4):227–232. doi: 10.2500/105065800779954446. [DOI] [PubMed] [Google Scholar]
- 14.Montone KT. Pathology of fungal rhinosinusitis: a review. Head Neck Pathol. 2016;10(1):40–46. doi: 10.1007/s12105-016-0690-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seyedmousavi S, et al. Exophiala sideris, a novel black yeast isolated from environments polluted with toxic alkyl benzenes and arsenic. Fungal Biol. 2011;115(10):1030–1037. doi: 10.1016/j.funbio.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Bensch K, et al. The Genus Clad Stud Mycol. 2012;72:1–401. doi: 10.3114/sim0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White TJ, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc: Guide To Methods Appl. 1990;18(1):315–322. [Google Scholar]
- 18.Kočí M. Synthesis and in vitro evaluation of bispyridinium type acetylcholinesterase inhibitors. [Pharmacy] 2012. [Google Scholar]
- 19.Basiri Jahromi S, Khaksar A, Iravani K. Phaeohyphomycosis of the sinuses and chest by cladosporium bantianum. Med J The Islamic Republic of Iran (MJIRI) 2002;16(1):55–58. [Google Scholar]
- 20.Symersky T, et al. Faecal elastase-I: helpful in analysing steatorrhoea. Neth J Med. 2004;62(8):286–289. [PubMed] [Google Scholar]
- 21.Alroqi A. A case report of rare fungal pathogens causing recurrent allergic fungal rhinosinusitis and literature review. Sci Prog. 2021;104(4):00368504211053512. doi: 10.1177/00368504211053512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeShazo RD, Chapin K, Swain RE. Fungal sinusitis. N Engl J Med. 1997;337(4):254–259. doi: 10.1056/NEJM199707243370407. [DOI] [PubMed] [Google Scholar]
- 23.Veress B, et al. Further observations on the primary paranasal aspergillus granuloma in the Sudan: a morphological study of 46 cases. The American J Trop Med Hyg. 1973;22(6):765–772. doi: 10.4269/ajtmh.1973.22.765. [DOI] [PubMed] [Google Scholar]