Dear Editor,
Assisted reproductive technology (ART) has been used for more than 45 years, by which more than 9 million infants were born. One of the key factors to determine the success rate of the ART is embryo selection.1,2 Due to the existence of aneuploidy in 20%–30% of blastocysts, preimplantation genetic screening (PGS) has been widely used to increase live birth rate. However, the live birth rate remains around 50% even with the help of PGS.2 DNA methylation is known to play an important role during embryogenesis.3–5 A previous study showed that a large proportion of human embryos have abnormal DNA methylome, and indicated that preimplantation DNA methylation screening (PIMS) can analyze both copy number variation (CNV) and global DNA methylation level.5 However, whether DNA methylation patterns can affect the clinical outcome of ART has not been investigated in clinics. In this regard, we performed a clinical trial of PIMS (trial number: NCT03642574). We aimed to examine the relationship between embryo methylome and the clinical outcome of ART.
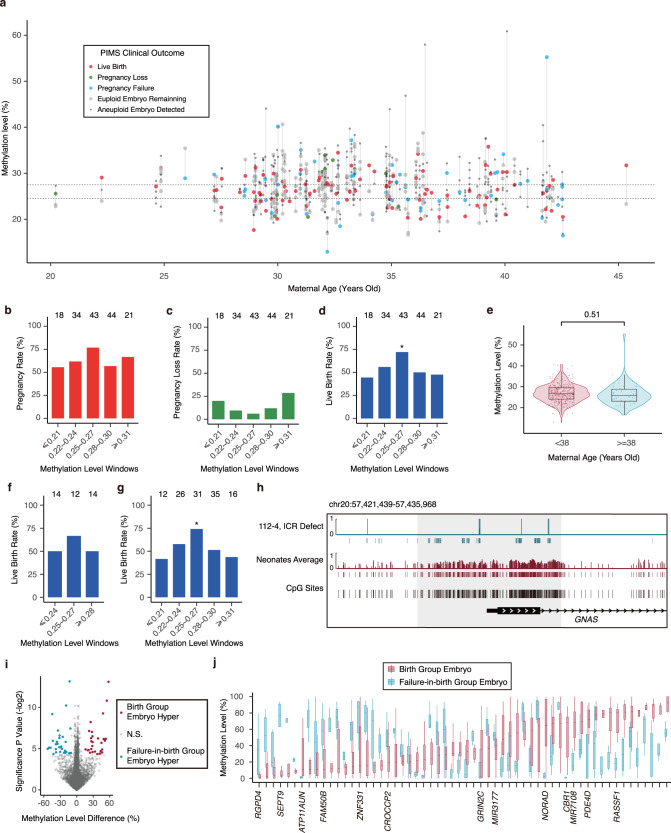
182 families including 800 blastocysts were enrolled in PIMS. 3–5 biopsied cells from trophectoderm of each blastocyst were measured with whole genome bisulfite sequencing. Using methylome data, we analyzed CNV and global average methylation levels (Supplementary information).5 The global methylation level is the average of all sequencing-covered CpGs. Our data show that the methylation level variation of different trophectoderm cells in the same embryo is similar (Supplementary information, Fig. S1a). Since PIMS can simultaneously provide information on CNV and methylation, there is no need to perform PGS to analyze CNV anymore. Not knowing what kind of methylome can produce the best clinical outcome of ART, we selected embryos only based on CNV instead of DNA methylome information. In total, 163 euploid embryos underwent elective single embryo transfers (Fig. 1a; Supplementary information, Fig. S2a), and 3 cases of twin pregnancy were excluded from the downstream analysis. The clinical data show 57 pregnancy failures, 13 pregnancy losses and 90 (56.25%) live birth neonates (Supplementary information, Table S1, S2).
Fig. 1. The clinical outcomes of PIMS.
a Overview of enrolled participants and the clinical outcomes of their embryos in PIMS. Embryos from the same participant were presented in the same column. Dashed lines indicate the methylation level window between 0.25–0.27. b–d Barplots showing pregnancy rate (b), pregnancy loss rate (c) and live birth rate (d) of the embryos with different methylation levels. e Methylation levels of the embryos for younger and AMA women. Significance of two-sided t-test was indicated above. f, g Barplots showing live birth rate of embryos with different methylation levels for younger (f) or AMA women (g). * refers to P < 0.05 and the total number of embryos transferred in each methylation level window was indicated in each column in (b–d, f, g). h Visual track of the embryos with unmethylated GNAS ICR (P = 0.016). Genomic (black) and sequencing covered (red or cyan) CpG sites were indicated by the vertical bars. The gray shaded box indicates GNAS ICR. i Differential methylated regions between the birth and failure groups. j Methylation level distribution of embryos in the birth and failure groups for the identified DMR in (i). Genes whose promoter overlapped with DMR were indicated in their corresponding columns in (j). Boxes and whiskers in (e) and (j) represent the 25th/75th percentiles and 1.5× interquartile range, respectively.
To study whether embryos with different methylation levels have different clinical outcomes, we assigned embryos into different groups according to their DNA methylation level. Notably, the embryos with DNA methylation levels between 0.25 and 0.27 produce significantly higher live birth rates than the embryos with other methylation levels (odds ratio [OR], 2.52; 95% confidence interval [CI], 1.13–5.95; P = 0.02). The pregnancy rate shows similar trends (OR, 2.21; CI, 0.95–5.51; P = 0.06), while the pregnancy loss rate shows the opposite trends (OR, 0.35; CI, 0.04–1.75; P = 0.22) (Fig. 1b–d). Overall, the higher difference of the methylation value from the window of 0.25–0.27 is, the lower the birth rate and pregnancy rate become (Fig. 1b, c); meanwhile, the pregnancy loss rate becomes higher also (Fig. 1d). Therefore, euploid embryos with DNA methylation level closest to 0.25–0.27 should be preferentially selected for transferring.
The proportion of aneuploid blastocysts in younger women is significantly less than that in women with advanced maternal age (AMA, ≥ 38 year old).6 During ART practice, PGS can increase the live birth rate for AMA women, while it has a limited effect on younger women.2,6,7 In contrast, the DNA methylation level variance of embryos is similar between both younger and AMA women (Fig. 1e). Further analysis shows that the association between DNA methylation level and the clinical outcome can be observed in both younger and AMA women (Fig. 1f, g; Supplementary information, Fig. S3a–d). We further checked the distribution of the age in each methylation level window, which shows there are no significant differences in maternal age among different windows (Supplementary information, Fig. S4a). These data indicate that the DNA methylation level affects the clinical outcome in both younger and AMA women.
DNA methylation abnormalities in imprinted control regions (ICRs) can cause imprinting disorders. The birth defect of imprinting disorders occurs in about 0.2% of the human population, and this rate is doubled in ART-born babies.8–10 Unfortunately, imprinting disorders cannot be avoided during current ART practice. Here, we checked the methylation status of known germline ICRs.11–13 As expected, all these germline ICRs are middle methylated. In addition, about half of the reads in ICRs are fully methylated reads, and about half reads are unmethylated (Supplementary information, Fig. S5a). Furthermore, our data show that some embryos have abnormal methylation states in germline ICR. For example, the methylation level of GNAS ICR in an unused embryo is unmethylated with a significant absence of hypermethylated reads (methylation level: 0.07, P = 0.016) (Fig. 1h). Our data suggest that we can use PIMS to exclude the embryos with methylation mutations in ICRs, which can potentially decrease the rate of imprinting disorders during ART practice.
We noticed that some embryos with methylation in the 0.25–0.27 window could not lead to live birth, suggesting that some important regions with abnormal methylation states might lead to the failure of live birth. To test whether the embryos can lead to live birth or not, we divided the embryos into live birth group and failure birth group. Fifty eight differential methylated regions (DMRs) between these two groups were identified with a P value of less than 0.05 (Fig. 1i). Our data show that 64 genes locate within 10 kb of these DMRs, and the promoters of 13 genes overlap with DMRs (Fig. 1j; Supplementary information, Table S3). Some of these genes are known to regulate embryo development and be affected by DNA methylation. For example, DNA methylation of SEPT9 promoter is associated with cervical cancer.14
Taken together, the PIMS method can examine both CNV and methylation information of embryos, so it can replace the method of preimplantation genetic testing for aneuploidy. Embryos with better methylation states can produce better clinical outcomes during ART. Therefore, PIMS can potentially increase the live birth rate of ART, and decrease the birth defect rate.
Supplementary information
Acknowledgements
This work was supported by the grants from the National Key R&D Program of China (2022YFC2703200, 2021YFC2700600, 2021YFC2700500); Research Unit of Gametogenesis and Health of ART-Offspring, the Chinese Academy of Medical Sciences; Special Foundation of President of the Chinese Academy of Sciences; the National Natural Science Foundation of China (31871454 to J.L., 32121001to J.L.)
Author contributions
J.L., Z.C., and Y.G. conceived and designed the study. L.Y., J.Z., L.W., X.Y., J.Y., S.J., L.G., M.F., M.G., Y.Z., X.G., and K.W. conducted the experiments. L.Y. and J.Z. performed the bioinformatics analyses. J.L. and J.Z. wrote the manuscript.
Data availability
The data that support the findings in this study are deposited in the Genome Sequence Archive (GSA) for human under the accession number HRA002940. Our data are available with a signed data use agreement; please contact liuj@big.ac.cn. The downstream reuse of the controlled access datasets is restricted to non-profitable usage.
Competing interests
X.Y. and J.L. are shareholders of Nvwa life technology. The other authors declare no competing financial interests.
Footnotes
These authors contributed equally: Yuan Gao, Lizhi Yi, Jianhong Zhan, Lijuan Wang, Xuelong Yao.
Contributor Information
Yuan Gao, Email: gaoyuan@sduivf.com.
Jiang Liu, Email: liuj@big.ac.cn.
Zi-Jiang Chen, Email: chenzijiang@hotmail.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41422-023-00809-z.
References
- 1.Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Fertil. Steril. 2000;73:1155–1158. doi: 10.1016/S0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 2.Munné S, et al. Fertil. Steril. 2019;112:1071–1079.e7. doi: 10.1016/j.fertnstert.2019.07.1346. [DOI] [PubMed] [Google Scholar]
- 3.Jiang L, et al. Cell. 2013;153:773–784. doi: 10.1016/j.cell.2013.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L, et al. Cell. 2014;157:979–991. doi: 10.1016/j.cell.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li G, et al. J. Genet. Genomics. 2017;44:475–481. doi: 10.1016/j.jgg.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Glick I, Kadish E, Rottenstreich M. Int. J. Womens Health. 2021;13:751–759. doi: 10.2147/IJWH.S283216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan J, et al. N. Engl. J. Med. 2021;385:2047–2058. doi: 10.1056/NEJMoa2103613. [DOI] [PubMed] [Google Scholar]
- 8.Fauque P, et al. Clin. Epigenetics. 2020;12:191. doi: 10.1186/s13148-020-00986-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vermeiden JP, Bernardus RE. Fertil. Steril. 2013;99:642–651. doi: 10.1016/j.fertnstert.2013.01.125. [DOI] [PubMed] [Google Scholar]
- 10.Cortessis VK, et al. J. Assist. Reprod. Genet. 2018;35:943–952. doi: 10.1007/s10815-018-1173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Court F, et al. Genome Res. 2014;24:554–569. doi: 10.1101/gr.164913.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zink F, et al. Nat. Genet. 2018;50:1542–1552. doi: 10.1038/s41588-018-0232-7. [DOI] [PubMed] [Google Scholar]
- 13.Joshi RS, et al. Am. J. Hum. Genet. 2016;99:555–566. doi: 10.1016/j.ajhg.2016.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiao X, et al. Clin. Epigenetics. 2019;11:120. doi: 10.1186/s13148-019-0719-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings in this study are deposited in the Genome Sequence Archive (GSA) for human under the accession number HRA002940. Our data are available with a signed data use agreement; please contact liuj@big.ac.cn. The downstream reuse of the controlled access datasets is restricted to non-profitable usage.