Abstract
The rapid proliferation of smokeless tobacco (SLT) in India has occurred without adequate information on the possible dangers and toxicity of these products. Tobacco flavors as well as nicotine (both protonated and un-protonated) are responsible for health dangers and addiction. The study aimed to offer information on the physical characteristics of commonly used smokeless tobacco products (including microscopic analysis), along with nicotine content (both total and un-protonated), pH, moisture, and flavors. The Standard Operating Procedures (SOPs) validated by the World Health Organization (WHO) recognized Tobacco Testing Laboratory TobLabNet) were applied for the analysis of various constituents of the SLTs. The microscopic analysis indicated that some of the SLT products like khaini were finely processed and available in filter pouches for users’ convenience and prolonged use leading to prolonged retention and addiction potential. Nicotine absorption and availability (both protonated and un-protonated) are affected by moisture and pH. Essences provide a pleasant aroma and flavor, with an increased risk of misuse and other health problems. Few chewing tobacco and Zarda had the lowest levels of un-protonated nicotine (0.10–0.52% and 0.15–0.21%, respectively), whereas Gul, Gudhaku, and Khaini had the highest levels, ranging from 95.33 to 99.12%. Moisture and pH ranged from 4.54 to 50.19% and 5.25–10.07 respectively. Menthol (630.74–9681.42 µg/g) was the most popular flavour, followed by Eucalyptol (118.16–247.77 µg/g) and camphor (148.67 and 219.317 µg/g). SLT’s health concerns and addiction dangers are exacerbated by the high proportion of bioavailable nicotine coupled with flavors. The findings of this study have important implications for the regulation and use of SLT in countries where use of SLT is prevalent.
Subject terms: Chemical biology, Health care, Medical research, Chemistry
Introduction
Smokeless tobacco is a complex chemical mixture that contains a variety of chemicals and additives, including flavors, areca nut, and slaked lime, and used with betel leaves1. Smokeless Tobacco (SLT) products are extremely complex, containing almost 4000 compounds, many of which are hazardous, mutagenic, and carcinogenic2 in nature. The alkaloid nicotine, the primary addictive substance in tobacco3–5, exists in protonated and un-protonated forms6. The addition of slaked lime in the preparation of SLT enhances nicotine bioavailability7,8.
Betel quid with tobacco, Khaini, Gutka, Pan Masala with tobacco, Zarda, Mishri, Mawa, Gul, Bajjar, Gudhaku, and other SLT products are widely available and used in India. These items can be chewed, sucked, or placed between the cheek, gum, or teeth9,10. Bangladesh, Bhutan, India, Myanmar, Nepal, Sri Lanka, and Timor-Leste are among the countries in the South-East Asian Region (SEAR) with the highest prevalence of SLT use11. Prevalence of current SLT use among men is highest in Myanmar (62.2%), and among women in Timor-Leste (26.8%)11. As per a recent study, during 2015–2019, there were 165 803 900 SLT users across SEAR, with 479 466 attributable deaths annually, of which India accounted for 79.9%, with 383 248 deaths.
According to the Global Adult Tobacco Survey-2, (GATS 2), every third adult in rural India and every fifth adult in urban India consumes tobacco in some form or another. Thus, 28.6% (266.8 million) of adults in India aged 15 and above use tobacco in some form. The prevalence of tobacco usage in India is 42.4 percent among men and 14.2 percent among women12. The most popular tobacco product is Khaini (tobacco and lime mixture), which is used by one in every nine adults (11.2%), followed by bidi, which is smoked by 7.7% of adult Indians11 Gutkha (a mixture of tobacco, lime, and areca nut) is ranked third (6.8%), and betel quid with tobacco is ranked fourth (5.8%). In India, 18.4% of women use SLT, and because smoking is typically a socially taboo (GATS 2), SLT is used as an alternate and more acceptable form of tobacco intake12,13. Affordability and accessibility lead to increased use of smokeless tobacco products, including through illicit trade. Even in instances of jurisdictional ban, the sale and possession of smokeless tobacco products, continue through illicit means (10).
The vast majority of SLT products are typically blended with herbs, spices, areca nuts, betel leaves, and slaked lime and made in the unorganized sector, where they are poorly regulated14 and contain significant quantities of tobacco, increasing the possibility of abuse and long-term dependence15–17. Nicotine is a basic alkaloid that remains un-ionized at an alkaline pH and is responsible for tobacco addiction. Nicotine absorption is influenced by several factors, including concentration, moisture content, flavorings, and pH. The pH of the product influences nicotine absorption; a higher pH accelerates the production of free-base nicotine (the most potent and easily absorbed form of nicotine), resulting in better absorption via the oral mucosa. As a result, pH information, in addition to nicotine, is a crucial indicator of bioavailable free nicotine. Moisture content affects nicotine absorption; a product with a higher moisture content absorbs more nicotine than one with a lower moisture content18–20.
Due to its distinctive scent, taste, and appeal, flavor additives are an essential aspect of SLT products21,22. Mint, spearmint, and wintergreen have been around for a long time, and menthol is used to soften the harshness of tobacco23,24 and make it more appealing to young people and beginners25. The health dangers of SLT are unknown because they have not been thoroughly explored, and professionals do not have much evidence on which to base their opinions. The aim of this study was to investigate the chemical composition and microscopic examination of frequently used SLT products in India in order to produce evidence regarding the potential health risks, hazards, and toxicity of these products. Microscopical analysis and signature data on the chemical components of well-known products are both necessary to ascertain the physical characteristics and quality of tobacco used. Microscopic analysis is considered a traditional, rapid, well-approved, and cost-effective approach for the identification of plant products. Since SLT products contain various species/ flavors and ingredients, it helps to identify products containing tobacco. Thus, simple microscopic techniques were used to identify the tobacco ingredients in SLT products. For the examination of the chemical contents of SLT products, gas chromatography (GC) with various detectors (Flame Ionization, Mass detector) is favored and is the suggested standard approach26,27. In this research we describe an easy-to-use and quick analytical process for determining nicotine utilizing gas chromatography-flame ionization detection, developed by WHO TobLabNet 1228. For the chemical ingredient analysis, GC–MS-based techniques were applied to identify, list and quantify the possible flavors added to the SLT products18,28.
Material and methods
All chemicals used for analysis were of analytical grade. Solvents and routine chemicals were procured from SISCO research laboratory (Mumbai, India). Nicotine, Quinoline (Internal standard), flavors (Eucalyptol, Camphor, Menthol, Methyl Salicylate, Ethyl salicylate, Cinnamaldehyde, Eugenol, Diphenyl ether, and Coumarin) and its internal standard (3′, 4′-(Methylenedioxy) acetophenone—MDA) were procured from Sigma Aldrich, USA having purity of ≥ 99.0%).
Patient and public involvement
Patients were not involved in this research.
Samples
A total of twenty-one brands of SLT and Pan masala were randomly sourced from retail shops or vendors selling tobacco products in India's north, east, west, and central regions for this study. The sample included one brand each sample of Khaini, Gudhaku, and Kharra, two of Zarda and Mawa, six of chewing tobacco, three of Gul, and five of Pan Masala. As per IARC, International Agency for Research on Cancer, Pan Masala is a ready-to-eat commercially available sachet containing areca nut crushed into very small pieces, slaked lime, catechu, and condiments with or without powdered tobacco). The samples were transported in airtight sealed packs, to the Drug Toxicology Laboratory, Centre for Addiction Medicine (CAM), National Institute of Mental Health and Neurosciences (NIMHANS), Bengaluru, India. The samples were stored in plastic bags and stored in a Thermo Scientific Ultra Low deep freezer at – 20 °C. Prior to analysis, samples were refrigerated for 24 h for comprehensive re-equilibration, followed by 2 h of equilibration to ambient conditions. The University of Kentucky’s College of Agriculture provided Coresta Reference Tobacco Products: CRP1.1, CRP2.1, CRP3.1, and CRP4.1 as reference material for method validation. Ethics clearance for method standardization and quantification of commonly abused substances was obtained.
SLT products and Pan Masala used in this study are classified and tabulated based on the way in which they are consumed by the user (chewing, sucking by placing between gums and cheek for a gradual release of the ingredients and applied on teeth and gums as dentifrice) tabulated in Table 1.
Table 1.
Types and mode of ingestion of Smokeless Tobacco Products.
| Sucking | Chewing | Applied on teeth and gums |
|---|---|---|
| Kharra | Tobacco (for chewing) | Gudakhu |
| Khaini | Kharra | Gul |
| Loose Tobacco | Mawa | |
| Zarda | ||
| Pan Masala |
pH, moisture and nicotine analysis
The state-of-the-art Drug Toxicology Laboratory at NIMHANS, Bangalore, is a world-class facility equipped with high-end and sophisticated instruments for tobacco analysis, as well as a member of the World Health Organization (WHO) commissioned Tobacco Laboratory Network (TobLabNet). TobLabNet aims to validate analytical approaches and Standard Operating Procedures (SOP) for evaluating smokeless tobacco constituents and smoked tobacco emissions across the globe.
Moisture and pH were evaluated using (TobLabNet) SOP 13 and 14 on a Thermo Scientific Heratherm oven QM5180 and an Orion Star A211 pH meter. Nicotine quantification was performed in accordance with SOP12, which permitted the use of FID detector in conjunction with a 7890A gas chromatography. The flavors were quantified using Agilent Technologies' 5975C mass selective detectors (GC MSD)29.
Nicotine stock standard solution (2 g/L) was made by dissolving nicotine in a 2:1:4:1 ratio of water, extraction solution, and 2 M sodium hydroxide. Internal standard, n-heptadecane diluted in n-hexane, was included in the extraction solution (0.5 mg/ml). To mix the nicotine stock standard solution, it was shaken for roughly 60 min in an orbital shaker. Following phase separation, the supernatant organic solution was used to make nicotine working standards in concentrations of 50, 250, 500, 750, 1000 and 1500 mg/L, which were serially diluted with the extraction solution. The produced solutions were kept at 4–8 °C and shielded from light.
Flavor analysis
Nine different flavors (Eucalyptol, Camphor, Menthol, Methyl salicylate, Ethyl salicylate, Cinnamaldehyde, Eugenol, Diphenyl ether, and Coumarin) were analyzed according to the work done by Stanfill SB, 2018 (29, 30) and CDC TL-Method 060.
Microscopic examination
The microscopic images were captured on a Leica digital microscope DM6 B, Germany and Leica digital microscope DVM 6, Singapore on Leica LAS X 3.0.8, Microsystems CMS GmbH. A Leica digital microscope DM6 B was used to undertake microscopic cellular examinations30,31. A small amount of the sample was combined with enough water to make a fragment, which was then extracted for microscopic analysis. Samples were inspected directly using a Leica digital microscope DVM 6 for 3-D imaging.
Ethics approval and consent to participate
This work does not involve human samples and hence ethics exemption for this particular study was obtained.
Results
Simple microscopic analysis focused on identifying the unique characteristics of the tobacco plant. Trichomes were found to be unicellular or multicellular epidermal appendages (Figs. 1, 2) with distinct morphologies (31). Multicellular glandular trichomes resemble epidermis outgrowths with a head of cells (Fig. 2) and secrete or store significant amounts of particular metabolites for plant's chemical defense mechanism30. Microscopic examination confirmed the presence of tobacco in SLT, which also includes a number of spices and flavors.
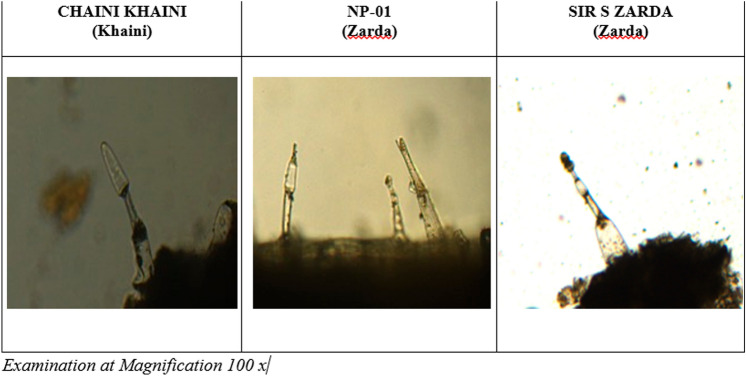
Figure 1.
3-D Images of smokeless tobacco products (SLT).
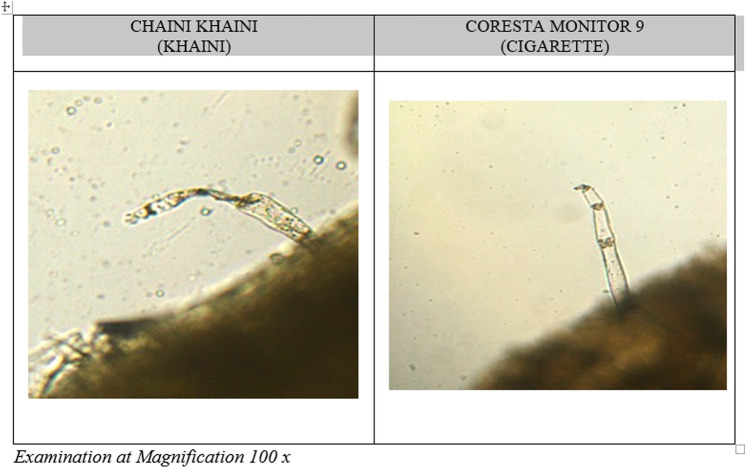
Figure 2.
Cellular Microscopic Images of Tobacco Trichrome for 3 smokeless tobacco products.
Similarly, control samples, Chaini Khaini reference product, and CM 9 (Coresta Monitor Cigarette) were studied under comparable conditions and revealed Chaini Khaini was finely processed as compared to Zarda samples (NP-01 and Sir S Zarda) in a 3-D inspection with Leica digital microscope DVM 6 to acquire an in-depth study on the physical content of the products. The Chaini Khaini 3-D capture revealed nicely cut material, however the Zarda items were coarsely cut and sized irregularly (Fig. 1).
The Chaini Khaini appeared flakier than the reference material, implying that the product has been thoroughly processed or that other ingredients have been added. The pH of Khaini, on the other hand, is in the alkaline range (pH 8–10).
The results of nicotine, un-protonated nicotine, pH, and moisture of the SLT products studied are presented in Table 2.
Table 2.
Mean of moisture, pH, nicotine (total and unprotonated μg/gm) measured in triplicate in Smokeless Tobacco Products.
| Smokeless tobacco type | Name of the product | Moisture content % | pH | *Nicotine content mg/g | *Un-protonated nicotine content mg/g | % Un-protonated nicotine |
|---|---|---|---|---|---|---|
| Gudhaku (Tobacco paste-like preparation) | Tota chaap | 15.48 | 9.62 | 5.81 | 5.67 | 97.55 |
| Kharra (Mixture of areca nut and tobacco) | Golden babu | 8.49 | 8.17 | 7.37 | 4.31 | 58.55 |
| Khaini (Sun-dried/fermented tobacco leaves) | Chaini khaini | 22.78 | 9.33 | 4.67 | 4.45 | 95.33 |
| Gul (Oral tobacco powder) | AR chand gul | 8.64 | 8.59 | 28.23 | 22.24 | 78.79 |
| Jora panja gul | 4.54 | 10.07 | 22.02 | 21.82 | 99.12 | |
| Gulab marka Gul | 5.98 | 9.63 | 31.25 | 30.50 | 97.60 | |
| Zarda (Moist/dry chewing tobacco with spice essences, and perfumes) | NP-01 | 11.61 | 5.20 | 21.72 | 0.03 | 0.15 |
| Sir S zarda | 12.24 | 5.35 | 17.91 | 0.04 | 0.21 | |
| Chewing tobacco (Cured tobacco leaves) | No brand- Loose tobacco | 17.73 | 8.58 | 17.26 | 13.53 | 78.41 |
| P P tobacco | 14.78 | 5.41 | 17.24 | 0.04 | 0.24 | |
| Kuber tobacco | 50.19 | 8.42 | 6.99 | 5.00 | 71.53 | |
| Madhu chewing tobacco | 8.76 | 5.74 | 14.28 | 0.07 | 0.52 | |
| BHR chewing tobacco | 13.74 | 5.25 | 26.13 | 0.03 | 0.10 | |
| Double black royal touch | 12.1 | 5.43 | 23.08 | 0.06 | 0.26 | |
| Pan masala (Mixture of areca nut with slaked lime, catechu and other flavoring agents with or witout tobacco.) | Kamala pasand pan masala | 8.19 | 8.90 | 0.00 | 0.00 | 88.35 |
| Pan parag premium | 5.67 | 8.79 | 0.00 | 0.00 | 85.48 | |
| Rajnigandha pan masala | 7.47 | 8.97 | 0.00 | 0.00 | 89.91 | |
| Vimal pan masala | 5.37 | 8.83 | 0.00 | 0.00 | 86.59 | |
| Raj niwas premium | 6.26 | 8.70 | 0.00 | 0.00 | 82.72 |
*Dry weight.
The moisture content among the SLT products (n = 19) ranged from 4.54 to 50.19%. The average moisture percentage was 12.63 ± 10.28. The coefficient of variation was 83.96%, indicating wide dispersion across brands. Kuber tobacco had the highest moisture content (50.19%), while Gul of the brand Jora Panja had the lowest moisture content (4.54%). For the Coresta standards, moisture contents were within the mentioned range (Table 3). Gudakhu, Kharra, and Khaini have low to high moisture content. We found that moisture content was inversely related to nicotine, particularly Gul products, where unprotonated nicotine was 78.79–99.12% and moisture was 4.43–8.64%. pH is another major component that affects nicotine pharmacokinetics. As pH rises, the fraction of unprotonated nicotine increases and is easily absorbed through the buccal mucosa. Altering the pH of a product can greatly increase nicotine absorption and influence its abuse potential. The pH of the products analyzed ranged from 5.25 to 10.07, the average was 7.84 ± 1.77 the coefficient of variation was 22.52%. pH for Coresta standards was 6.08–8.30, well within the range (Table 3).
Table 3.
Moisture, pH and nicotine analysis in Coresta reference standards.
| Name of the product | Moisture content % | pH | Nicotine content (mg/g) |
|---|---|---|---|
| CRP1.1 | 54.64 | 7.42 | 7.07 |
| CRP2.1 | 51.10 | 7.94 | 9.75 |
| CRP3.1 | 5.78 | 6.99 | 16.39 |
| CRP4.1 | 22.29 | 5.89 | 9.24 |
The nicotine concentrations in SLT products ranged from 4.67 to 28.23 mg/g (Table 1), average was 12.84 and the coefficient of variation was 83.96%, The unprotonated nicotine was calculated by putting the product pH and appropriate Pka into the Henderson-Haselbalch equation29 it ranged from 0.10 to 99.1% and the coefficient of variation was 71.38%, indicating great heterogeneity across brands. Products with an acidic pH (5.20–5.74) had 0.12–0.26% unprotonated nicotine (P.P, Madhu, BHR, and Double Black Royal Touch) while the products with basic pH (8.17–10.07) had unprotonated nicotine levels ranging from 58.55 to 99.12%. The Chaini Khaini with pH 9.33 had unprotonated nicotine of 95.33%. Gul sample had unprotonated nicotine in the range of 78.79%-99.12%. Jora panga Gul had a high level of unprotonated nicotine. The Pan Masala brands we tested did not contain any nicotine. The nicotine levels for the Coresta standards were 7.07–16.34 (Table 3).
Nine distinct flavors were detected in the tested SLT products (Table 4). The findings revealed that menthol was the most consistently present ingredient. Menthol concentrations in Zarda samples ranged from 4145.40 to 9681.42 µg/g, in chewing tobacco from 296.52 to 6617.37 µg/g, and in Pan Masala from 2371.62 to 5156.51 µg/g. The menthol content of the Khaini and Kharra samples was 5377.51 and 630.74 µg/g, respectively. Menthol has the ability to improve the delivery of nicotine. The amount of eucalyptol discovered in Zarda, Pan Masala, and Khaini was between 118.16 and 247.77 µg/g. Only Zarda and chewing tobacco had camphor, with concentrations of 148.67 and 219.32 µg/g, respectively. Coumarin was identified in a range of concentrations in Zarda, chewing tobacco, and Khaini, ranging from 112.33 to 244.25 µg/g. In one of the chewing tobacco samples, diphenyl ether was detected at a concentration of 10.89 µg/g. AR Chand and Jora Panja Gul had high nicotine levels without flavors, whereas Gulab Marka Gul contained coumarin 189.26 µg/g. We also looked for methyl salicylate, ethyl salicylate, cinnamaldehyde, and eugenol, but they were not detected.
Table 4.
Concentration (μg/g, wet) of flavor -related compounds (n = 3) in Smokeless Tobacco Products.
| Smokeless tobacco type | Name of the product | Eucalyptol | Camphor | Menthol | Diphenyl ether | Coumarin |
|---|---|---|---|---|---|---|
| Gudhaku | Tota chaap | – | – | – | – | – |
| Kharra | Golden babu | – | – | 630.74 | – | – |
| Khaini | Chaini khaini | 244.59 | – | 5377.51 | – | 145.28 |
| Gul | A R chand Gul | – | – | – | – | – |
| Jora panja Gul | – | – | – | – | – | |
| Gulab marka Gul | – | – | – | – | 189.26 | |
| Zarda | Np-01 | 165.81 | 148.67 | 9681.42 | – | 244.25 |
| Sir S zarda | – | – | 4145.40 | – | – | |
| Chewing Tobacco | No brand-Loose tobacco | – | – | – | – | – |
| P P tobacco | – | – | 296.52 | - | 112.33 | |
| Kuber tobacco | – | 219.17 | - | 10.89 | – | |
| Madhu chewing tobacco | – | – | 2023.99 | – | – | |
| BHR chewing tobacco | – | – | 1826.44 | – | 164.26 | |
| Pan Masala | Double black royal touch | – | – | 6617.37 | – | – |
| Kamala pasand pan masala | 118.16 | – | 2371.62 | – | – | |
| Pan parag premium | – | – | 4554.58 | – | – | |
| Rajnigandha pan masala | 247.77 | – | 6513.97 | – | – | |
| Vimal pan masala | 178.96 | – | 5148.41 | – | – | |
| Raj niwas premium | 186.56 | – | 5156.96 | – | – |
Discussion
The current research study thoroughly examined pH, moisture, nicotine (protonated and unprotonated), and flavors in SLT available in India. It was found that the majority of commonly used SLT products had high levels of un-protonated nicotine and a basic pH state. Microscopic examination indicated that the Chaini Khaini was a finely ground tobacco product made with pasteurized air- or sun-cured tobacco that comes in tiny teabag-like sachets, while other Khaini brands were not finely processed. SLT had tobacco as reference material, therefore the photos were illustrative regardless of brand and type. The pH of SLT products is critical in determining the amount of un-protonated (or “free base”) nicotine, which impacts nicotine bioavailability32. Nicotine is found in both protonated (charged) and un-protonated (uncharged) forms in tobacco and tobacco smoke. Un-protonated nicotine is rapidly absorbed in the mouth, and the rate of absorption is a primary factor of addiction for nicotine, as it is for other substances33,34. Using an alkalinizing agent, such as the addition of ammonium bicarbonate, to change the pH level of the product and increase the amount of un-protonated nicotine is one way to manage nicotine delivery35 (Fig. 3).
Figure 3.
Cellular Microscopic Images showing trichomes in Khaini Ref Product and CM 9 Cigarette.
Use of an ingredient that enhances salivation, like acetic acid, is another way to improve nicotine absorption of SLT products. Saliva production increases moistening of the tobacco held in the mouth (the plug) and facilitates nicotine extraction. Ammonium bicarbonate and acetic acid are used in the production of SLT.
The total and un-protonated nicotine levels of SLT products were consistent with our findings for Zarda, Gutkha, and Khaini in a prior investigation29. Gul, Kharra, and loose tobacco products, which are extensively consumed in India are also included in the current study. Gul, Kharra, and loose tobacco products included high levels of un-protonated nicotine, which delivered a considerable amount of nicotine to the user and were linked to health risks and addiction. Gulab Marka Gul with pH 9.63 had the greatest nicotine content (both total and un-protonated) of 31.25 mg/g. Similar Bangladeshi yields36 had mean nicotine levels of 31 mg/g, which were three times greater than SLT brands from Pakistan. (10 mg/g powder)29,37. Although nicotine was not detected in the Pan Masala samples, data from the National Tobacco Testing Labs (NTTL, India) (The Hindu, April 8, 2015) reveals that it is a leading cause of oral submucous fibrosis that often progresses to oral cancer10,24.
The nicotine, un-protonated nicotine, and flavour concentrations described in this paper are provided on a wet-weight basis. Nicotine has a bitter and disagreeable taste, thus flavouring is used to conceal it and make the product more palatable and appealing. Eucalyptol, camphor, methyl salicylate, ethyl salicylate, menthol, eugenol, cinnamaldehyde, coumarin, and diphenyl ether were among the nine regularly used flavourings. The additional flavour components in Zarda and Khaini were higher than the rest of the SLT products (Table 4). Stanfill et al.27 studied flavours in SLT products' and reported Menthol (range: 160–21,700 µg/g) was the most commonly used ingredient, followed by diphenyl ether (7.05–7.380 µg/g), coumarin (5.94–1,420 µg/g), eugenol (25.2–1250 µg/g), and camphor (6.94–1160 µg/g). Methyl salicylate (8.31–75.0 µg/g), pulegone (6.40–74.0 µg/g), and ethyl salicylate (10.5–16.0 µg/g) were among the compounds with lower amounts. According to our findings, menthol levels were 296.52–9681.42 µg/g, coumarin 112.33–244.25 µg/g, camphor (148.67–219.17 µg/g, eucalyptol 118.16–247.77 µg/g and diphenyl ether 10.89 µg/g was present in one product only. Khaini had high pH, nicotine, and un-protonated nicotine, but Zarda had very high total nicotine concentrations (21.9–32.9 mg/g). Menthol, camphor, coumarin eucalyptol, and diphenyl ether were the most commonly used flavors. Few SLT products had methyl salicylate, pulegone, or ethyl salicylate (18–20). We found no ethyl, methyl salicylate, cinnamaldehyde, or eugenol among the tested SLT products.
Despite the fact that SLT poses a serious health risk, little research has been done on the effects of oral exposure to these substances. Microscopic examination revealed that khaini samples went through fine processing. Customers may be drawn to these expertly packaged and refined products. Zarda has a lot of additives, which may be because it contains a variety of flavors, spices, and other ingredients, including tobacco and areca nut29,31. Even though SLTs are illegal in some Indian states and jurisdictions, the products are still sold and smuggled into the market because the bans'/regulations are not strictly enforced. In addition, SLT products like Gutkha and Pan Masala contain additional ingredients like areca nut, cardamom, and slaked lime, which increases the toxicity and addictiveness of the product. These products become more appealing because of certain flavoring ingredients' more seductive aromas and tastes, but they can also be toxic and dangerous. The effects of SLT product use on health need to be described through further study.
Conclusion
Unlike cigarettes, the contents of SLT products are poorly researched and documented, especially in the South-East Asia Region, which has the highest prevalence of use of these products. Many countries with high prevalence of SLT use face regulatory challenges in the absence of testing facilities and relevant evidence regarding the contents, addiction potential, toxicity and health effects of these products. The microscopic examination in this study provided an added level of information to aid enforcement activities, in identifying SLTs, especially where such products are prohibited in some jurisdictions. The findings of this study would have important ramifications for regulating the use of SLT products as the evidence could drive and strengthen policies, legislations, extant bans as well as the implementation of WHO FCTC Articles to pave the way for effective tobacco control in the countries where SLT use is prevalent.
Acknowledgements
Joyce Kiang Kin Har from the Singapore Health Sciences Authority, as well as Gayathri C and Manjesh G from the Toxicology Laboratory, Department of Psychiatry, NIMHANS, Bengaluru, are appreciated for their contributions to this work.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the views of the Institutes involved National Institute of Mental Health and Neurosciences, India, Health Sciences Authority, Singapore, and the World Health Organization.
Abbreviations
- SLT
Smokeless tobacco
- GCMS
Gas chromatograph, and mass spectrometer
- FID
Flame ionization detector
- SEAR
South-East Asia Region
- SOP
Standard operating procedure
- WHO TobLabNet
World Health Organization Tobacco Laboratory Network
- WHO FCTC
World Health Organization Framework Convention on Tobacco Control
Author contributions
This idea was conceptualized by P.M. and J.K. All experimental work was carried out at NIMHANS, Bengaluru, India and HSA, Singapore by S.S.K., V.R., F.A.M. and M.Y.B.C. P.S. and N.P.C. prepared main manuscript text. All authors have reviewed the manuscript.
Funding
This project was funded by the WHO SEARO under grant WHO/001/108/2019/01255.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to confidential issues but are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Richter P, Spierto FW. Surveillance of smokeless tobacco nicotine, pH, moisture, and un-protonated nicotine content. Nicotine Tob. Res. 2003;5(6):885–889. doi: 10.1080/14622200310001614647. [DOI] [PubMed] [Google Scholar]
- 2.Bethesda M. Smokeless tobacco and public health: A global perspective. Cancer Institute, Natl Dis Control Centers Dis Control Prev Dep Heal Us Serv Hum 2014;14–7983.
- 3.CORESTA Guide N° 11,Technical Guide for Sample Handling of Smokeless Tobacco and Smokeless Tobacco Products May 2020 - second edition .
- 4.Henningfield JE, Radzius A, Cone EJ. Estimation of available nicotine content of six smokeless tobacco products. Tob. Control. 1995;4(1):57–61. doi: 10.1136/tc.4.1.57. [DOI] [Google Scholar]
- 5.Addicott MA. Tobacco addiction. In: Cognition and Addiction [Internet] Elsevier; 2020: 129–41. https://linkinghub.elsevier.com/retrieve/pii/B9780128152980000095.
- 6.Stanfill S, Tran H, Robert T, Fernandez C, Zhu W, Marynak K, King B, Blasini L, Blount BC, Watson C. Characterization of total and un-protonated (free) nicotine content of nicotine pouch products. Nicotine Tob. Res. 2021;23(9):1590–1596. doi: 10.1093/ntr/ntab030. [DOI] [PubMed] [Google Scholar]
- 7.Bhisey RA. Chemistry and toxicology of smokeless tobacco. Indian J. Cancer. 2012;49(4):364–372. doi: 10.4103/0019-509X.107735. [DOI] [PubMed] [Google Scholar]
- 8.Roshni RS. Estimation of magnesium carbonate, calcium carbonate and pH of pan masala and smokeless tobacco products. Tob. Induced Dis. 2021;19(1):164. doi: 10.18332/tid/140914. [DOI] [Google Scholar]
- 9.Muthukrishnan A, Warnakulasuriya S. Oral health consequences of smokeless tobacco use. Indian J. Med. Res. 2018;148(1):35–40. doi: 10.4103/ijmr.IJMR_1793_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaur J, Rinkoo AV, Richardson S. Trends in smokeless tobacco use and attributable mortality and morbidity in the South-East Asia Region: Implications for policy. Tob. Control. 2023 doi: 10.1136/tc-2022-057669. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization (WHO); SEAR Tobacco Atlas Perspectives from the South-East Asia Region [Internet]. 2020. https://www.who.int/publications/i/item/sear-tobacco-atlas
- 12.Tata Institute of Social Sciences (TISS), Mumbai and Ministry of Health and Family Welfare, Government of India. Global Adult Tobacco Survey GATS 2 India 2016–17. https://ntcp.nhp.gov.in/assets/document/surveys-reports-publications/Global-Adult-Tobacco-Survey-Second-Round-India-2016-2017.
- 13.Ravishankar PL, Nadkerney P, Pramod V, Soni A, Jaiswal R, Kumar A. Effect of Gudakhu (smokeless tobacco) on periodontal health: A case-control study. Int. J. Oral Care Res. 2017;5(2):87–90. [Google Scholar]
- 14.Mehrotra R, Yadav A, Sinha DN, Parascandola M, John RM, Ayo-Yusuf O, et al. Smokeless tobaccoSLT control in 180 countries across the globe: Call to action for full implementation of WHO FCTC measures. Lancet Oncol. 2019;20(4):e208–e217. doi: 10.1016/S1470-2045(19)30084-1. [DOI] [PubMed] [Google Scholar]
- 15.London ED. Patterns of nicotine action in the brain. Tob. Control. 1994;3(2):101–101. doi: 10.1136/tc.3.2.101. [DOI] [Google Scholar]
- 16.Henningfield JE, Heishman SJ. The addictive role of nicotine in tobacco use. Psychopharmacology. 1995;117:11–13. doi: 10.1007/BF02245089. [DOI] [PubMed] [Google Scholar]
- 17.Benowitz NL, Porchet H, Sheiner L, Jacob P. Nicotine absorption and cardiovascular effects with smokeless tobacco use: Comparison with cigarettes and nicotine gum. Clin. Pharmacol. Ther. 1988;44(1):23–28. doi: 10.1038/clpt.1988.107. [DOI] [PubMed] [Google Scholar]
- 18.IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans - Smokeless Tobacco and some Tobacco-speicific N-Nitrosamines. IARC Monogr Eval Carcinog Risks to Humans [Internet]. 2007;89. https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono89-6.pdf. [PMC free article] [PubMed]
- 19.Standard operating procedure for determination of the pH of smokeless tobacco products. WHO TobLabNet Official Method SOP14 2022. https://www.who.int/publications/i/item/9789240044708.
- 20.Standard operating procedure for determination of moisture content in smokeless tobacco products. WHO TobLabNet Official Method SOP13; 2022. https://www.who.int/publications/i/item/9789240044685.
- 21.Oliver AJ, Jensen JA, Vogel RI, Anderson AJ, Hatsukami DK. Flavored and nonflavored smokeless tobacco products: Rate, pattern of use, and effects. Nicotine Tob. Res. 2013;15(1):88–92. doi: 10.1093/ntr/nts093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kostygina G, Ling PM. Tobacco industry use of flavourings to promote smokeless tobacco products. Tob. Control. 2016;25:i40–i49. doi: 10.1136/tobaccocontrol-2016-053212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C, Isabelle LM, Pickworth WB, Pankow JF. Levels of mint and wintergreen flavorants: Smokeless tobacco products vs. confectionery products. Food Chem Toxicol. 2010;48(2):755–763. doi: 10.1016/j.fct.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Harrell M, Loukas A, Jackson C, Marti CN, Perry C. Flavored tobacco product use among youth and young adults: What if flavors didn’t exist? Tob. Regul. Sci. 2017;3(2):168–173. doi: 10.18001/TRS.3.2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rose SW, Johnson AL, Glasser AM, Villanti AC, Ambrose BK, Conway K, et al. Flavour types used by youth and adult tobacco users in wave 2 of the Population Assessment of Tobacco and Health (PATH) Study 2014–2015. Tob. Control. 2020;29(4):432–446. doi: 10.1136/tobaccocontrol-2018-054852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stepanov I, Hecht SS, Ramakrishnan S, Gupta PC. Tobacco-specific nitrosamines in smokeless tobacco products marketed in India. Int. J. Cancer. 2005;116(1):16–19. doi: 10.1002/ijc.20966. [DOI] [PubMed] [Google Scholar]
- 27.Stanfill SB, Jia LT, Ashley DL, Watson CH. Rapid and chemically selective nicotine quantification in smokeless tobacco products using GC-MS. J. Chromatogr. Sci. 2009;47(10):902–909. doi: 10.1093/chromsci/47.10.902. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization (WHO). WHO TobLabNet Official Method SOP 04 - Standard operating procedure for determination of nicotine in cigarette tobacco filler. 2014;18.
- 29.Standard operating procedure for determination of nicotine content in smokeless tobacco products. WHO TobLabNet Official Method SOP12; 2022. https://www.who.int/publications-detail-redirect/9789240044661.
- 30.Amme S, Rutten T, Melzer M, Sonsmann G, Vissers JPC, Schlesier B, et al. A proteome approach defines protective functions of tobacco leaf trichomes. Proteomics. 2005;5:2508–2518. doi: 10.1002/pmic.200401274. [DOI] [PubMed] [Google Scholar]
- 31.Antony JVM, Ramani P, Ramasubramanian A, Sukumaran G. Particle size, penetration rate and effects of smoke and smokeless tobacco products—An invitro analysis. Heliyon. 2021;7(3):e06455. doi: 10.1016/j.heliyon.2021.e06455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomar SL, Henningfield JE. Review of the evidence that pH is a determinant of nicotine dosage from oral use of smokeless tobacco. Tob. Control. 1997;6(3):219–225. doi: 10.1136/tc.6.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armitage AK, Turner DM. Absorption of nicotine in cigarette and cigar smoke through the oral mucosa. Nature. 1970;226(5252):1231–1232. doi: 10.1038/2261231a0. [DOI] [PubMed] [Google Scholar]
- 34.Henningfield JE, Fant RV, Tomar SL. Smokeless tobacco: An addicting drug. Adv. Dent. Res. 1997;11:330–335. doi: 10.1177/08959374970110030401. [DOI] [PubMed] [Google Scholar]
- 35.Pickworth WB. Nicotine absorption from smokeless tobacco modified to adjust pH. J. Addict Res. Ther. 2014 doi: 10.4172/2155-6105.1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stanfill SB, Connolly GN, Zhang L, Jia LT, Henningfield JE, Richter P, et al. Global surveillance of oral tobacco products: Total nicotine, unionised nicotine and tobacco-specific N-nitrosamines. Tob. Control. 2011;20(3):1–10. doi: 10.1136/tc.2010.037465. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization 2018; Regulation of flavoured smokeless tobacco in the South-East Asia Region ISBN: 978-92-9022-631-4; https://creativecommons.org/licenses/by-nc-sa/3.0/igo.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to confidential issues but are available from the corresponding author on reasonable request.