Abstract
Purpose
We present our experience of titanium-milled two-piece patient-specific implants (PSIs) for primary reconstructions of extensive orbital floor and medial wall fractures (EOFMFs) and evaluate their postoperative functional and aesthetic outcomes in relation to commercially available implants.
Methods
We included all patients with primary reconstructions (< 22 days from injury) of EOFMFs treated in our department between January 2011 and October 2020. Extensive orbital floor and medial wall fracture was defined as involvement of orbital floor, medial wall and maxilloethmoidal junction; a fracture defect 5 mm or more; defect size more than a third of both inferior and medial walls; and Jaquiéry classification III or more. Patient characteristics, details of fracture defects and surgeries, postoperative outcomes and implant positions were retrospectively evaluated and compared between study groups.
Results
Nineteen patients were included: 5 with two-piece PSIs and 14 with commercial implants. Implant position was good in 4/5 patients with two-piece PSIs and 2/14 with commercial implants. Revision surgery, globe malposition (GMP) > 2 mm, significant diplopia and poor implant position were more frequent in patients with commercial implants than two-piece PSIs. None of the patients with a good overall implant position had any significant postoperative symptoms.
Conclusion
Extensive orbital fracture reconstructions are somewhat rare, and surgical treatment is associated with a high rate of complications and postoperative symptoms. Titanium-milled two-piece PSIs are well suited for primary reconstructions of EOFMFs, as they lead to more precise reconstructions and fewer postoperative symptoms than commercially available implants.
Keywords: Orbital fracture, Patient-specific implant, Computer-aided design and manufacturing, CAD-CAM, Diplopia, Globe malposition
Introduction
Orbital wall fractures may be blow-out fractures or fractures that involve the orbital rim in addition to the orbital walls [1]. Orbital fractures usually present with clinical symptoms including swelling and hematoma around the orbit, eye motility disorders, globe malposition (GMP) and diplopia [2]. These findings tend to be more pronounced in cases with extensive orbital wall defects involving both the floor and medial wall (EOFMF) [3], which often require surgical treatment aiming to restore eye function and achieve acceptable cosmesis [4].
Surgical reconstructions of EOFMFs are known to be challenging, especially if the maxilloethmoidal junction is involved as it functions as the anatomical cornerstone of the orbital cavity [5–7]. Although various surgical approaches and methods have been described to treat EOFMFs, persistent postoperative symptoms, including GMP and diplopia, are still relatively common [8]. Precise anatomical restoration of the orbital walls is considered as the main goal of orbital fracture surgery, even though the position of the implant is known not to be the only factor influencing postoperative clinical outcomes [8, 9].
Three-dimensional (3D) technology, including computer-aided design and manufacturing (CAD-CAM) techniques, are increasingly used in the treatment of orbital fractures (10). Orbital implants may be preoperatively prepared by bending them manually on a personalized skull-model or by making a virtual reconstruction by mirroring the unaffected contralateral side on the affected side as a reference [10]. Patient-specific implants (PSI), here defined as implants prepared by the latter method, enable more precise reconstructions than reconstructions with commercial implants [11].
As mentioned, the precision of the bony orbital cavity reconstruction is not the only factor that affects postoperative outcome. In EOFMFs, extensive surgical approaches and substantial surgical handling of the orbital soft tissue may cause marked iatrogenic soft tissue injury. However, if the surgeon attempts to avoid this soft tissue injury, a smaller implant may be chosen and, therefore, a part of the defect may be left uncovered. Both of these situations may lead to residual postoperative symptoms. Thus, two-piece PSIs may help the surgeon to perform a precise anatomical reconstruction, to reduce the iatrogenic orbital soft tissue injury [12] and to decrease the duration of surgery and the requirement for secondary surgery.
The aim of this retrospective study was to present our experience of titanium-milled two-piece PSIs for primary reconstructions of EOFMFs. In addition, we sought to evaluate their postoperative functional and aesthetic outcomes and postoperative complications compared to commercially available implants.
Materials and methods
Study design
We sought to compare the outcomes of titanium-milled two-piece PSIs and commercial implants in orbital fracture reconstructions. We retrospectively evaluated all patients with primary orbital fracture reconstruction treated in the Department of Oral and Maxillofacial Diseases, Helsinki University Hospital between January 2011 and October 2020.
Inclusion criteria and study variables
We included patients, who underwent a primary reconstruction of EOFMF with preoperative and postoperative high-resolution 16-slice computed tomography (CT) imaging into the study. Delay from injury to fracture reconstruction was required to be ≤ 21 days.
Extensive orbital floor and medial wall fracture was defined according to five criteria: orbital floor and medial wall fracture; fracture of maxilloethmoidal junction; ≥ 5 mm fracture defect dislocation, defect size more than a third of both inferior and medial walls; and Jaquiéry classification ≥ III [13].
We collected the following variables and compared them between the study groups: age, sex, delay from injury to surgery, facial fracture type (i.e., isolated orbital fracture without involvement of the orbital rim or impure orbital fractures with involvement of the orbital rim), orbital fracture defect type (i.e., Jaquiéry classification) [13], occurrence of other associated injuries, injury mechanism, surgical approach, duration from injury to surgery and duration of the follow-up period.
Variables regarding clinical postoperative outcomes included the following: requirement of orbital revision surgery; occurrence of GMP > 2 mm, eyelid malposition, any diplopia, diplopia that interfered with daily activities and diplopia that required strabismus evaluation by an ophthalmologist; requirement of additional procedures due to eyelid malposition.
Evaluation of implant position
The authors evaluated the positions of the implants based on postoperative CT scan images. The overall anterior, medial and posterior positions of the plates were classified as good (i.e. implant resting on sound bony margins, restoring normal contour), acceptable (i.e. implant resting on sound bony margins but not restoring normal contour) and poor (i.e. edge of implant in sinus, not restoring normal contour) as previously described [14]. In addition, we analyzed the defect coverage by the implant (defect fully covered by the implant/maximum defect size left uncovered in mm in any of the CT scan images), location of the defect area left uncovered (medial or lateral to the implant or both) and the resemblance of the implant form to the orbit (good resemblance/implant positioned above the surface of the orbital wall/head of implant pointing into the orbit, or extraocular muscle/implant positioned partly in the ethmoidal or maxillary sinus).
Virtual planning and manufacturing of patient-specific implants
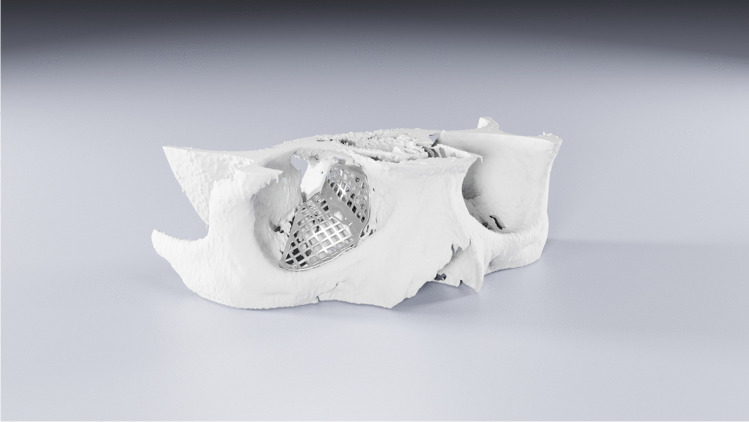
The two-piece PSIs (Fig. 1) were designed preoperatively by one of the authors (J.S.) and engineered using CAD in the Planmeca ProModel™ system (Planmeca Ltd). Mirroring of the unaffected contralateral side was used as a reference for the virtual reconstruction [10]. Two-piece PSIs were designed to rely on at least three intact shelf structures of the orbital structures (anteromedial, anterolateral and posterior): anteriorly on the inner surface of the anterior orbital rim, laterally on the infraorbital groove but not in or over it and medially and posteriorly over the whole fracture defect when possible. The virtually planned design and fit of the implant was tested on a printed 3D model preoperatively to confirm precise fit and to improve the orientation of the surgeon for surgery. The lateral piece of the implant was first placed over the orbital floor. The medial piece was then placed over the fracture defect of the medial wall and connected to the designed groove of the medial edge in floor part implant, where the implants were locked by two or three conical small hooks. The patient-specific implants were computer numerical control (CNC)-milled from titanium (grade 2) alloy blocks to a thickness of 0.3 to 0.4 mm by Planmeca Ltd.
Fig. 1.
The titanium-milled two-piece implant for right-side orbital fracture was designed virtually. The shape of the orbit was reconstructed by mirroring of the unfractured orbit. The implant consisted of two parts, of which the medial piece was connected to the lateral piece by a groove and conical small hooks
Statistical analyses
Data were analyzed with IBM SPSS Statistics for Mac (version 24, IBM Corp). The results are shown as means (range) and numbers of named cases (percentages).
Results
Of the 266 orbital fracture patients requiring primary fracture reconstruction, 19 patients (7%) fulfilled the inclusion criteria and received reconstructions of unilateral EOFMFs (Fig. 2). Five patients had two-piece PSIs and 14 commercial implants (10 patients had prebent orbital implants (MatrixORBITAL, DePuySynthes) and 4 had unprebent orbital meshes (DePuySynthes, MatrixMidface)) (Table 1).
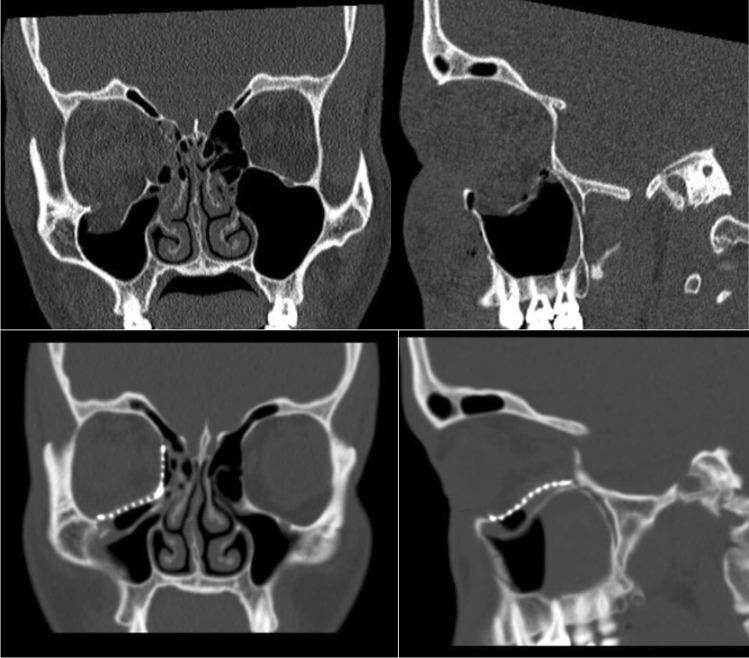
Fig. 2.
The patient had an extensive right-sided orbital fracture due to assault. The patient’s eye movements were restricted due to soft tissue entrapment on the edges of the fracture. Computer-tomography images showed fracture extension to the posterior orbital third in both the orbital floor and medial wall. The customized two-piece implant was preplanned (Fig. 1), and surgery was conducted 11 days after injury. Postoperative images showed excellent implant fitting
Table 1.
Patient characteristics and details of trauma and surgery
| Variable | PSI (n = 5) | Non-PSI (n = 14) |
|---|---|---|
| Median age, years (range) | 42 (24–57) | 52 (23–77) |
| Median delay from injury to surgery, days (range) | 11 (4–13) | 6 (0–14) |
| Isolated orbital fracture | 2 (40) | 8 (57) |
| Orbital fracture extending to orbital rim(s) | 3 (60) | 6 (43) |
| Associated injuries, any | 3 (60) | 6 (43) |
| Intracranial injuries | 1 (20) | 5 (36) |
| Cervical spine fractures | 0 | 0 |
| Cervical vein injury | 0 | 1 (7) |
| Treatment delay as days, mean (range) | 11 (4–17) | 6 (0–14) |
| Implants used | ||
| Two-piece PSI | 5 (100) | 0 |
| Prebent implant | 0 | 10 (71) |
| Unbent mesh | 0 | 4 (29) |
| Implant fixed with a screw | 0 | 5 (36) |
| Surgical approach | ||
| Lower eyelid | 1 (20) | 9 (64) |
| Transconjunctival | 0 | 3 (21) |
| Combined transconjunctival and retrocaruncular | 4 (80) | 2 (14) |
| Lateral cantholysis associated with the surgical approach | 1 (20) | 3 (21) |
All values are n (%) unless otherwise indicated
PSI patient-specific implant
Surgical details are presented in Table 1. The delay between diagnosis and primary surgery was slightly greater in patients with a two-piece PSI (mean 11 (4–17) days) compared to other patients (mean 6 (0–14) days). A transconjunctival approach was combined with a retrocaruncural approach in a third of all patients. A lateral cantholysis or canthotomy was performed in a fifth of all patients.
The implants tended to be in a better position in patients with two-piece PSIs (Table 2; Fig. 2) than in patients with commercial implants; none of the two-piece PSIs were positioned poorly. Most of the suboptimal and poor positionings of the commercial implants were located in the posterior and medial parts of the fracture defect (Table 2). Moreover, only one patient with a two-piece PSI (20%) had 5 mm or more of the fracture defect left uncovered; the respective number was 93% in patients with commercial implants (Table 2).
Table 2.
Positions of the implants on the postoperative CT scan
| Variable | Two-piece PSI (n = 5) | Non-PSI (n = 14) |
|---|---|---|
| Overall | ||
| Good | 4 (80) | 2 (14) |
| Suboptimal | 1 (20) | 10 (71) |
| Poor | 0 | 2 (24) |
| Anteriorly | ||
| Good | 5 (100) | 14 (100) |
| Medially | ||
| Good | 5 (100) | 8 (57) |
| Suboptimal | 0 | 4 (29) |
| Poor | 0 | 2 (14) |
| Posteriorly | ||
| Good | 4 (80) | 7 (50) |
| Suboptimal | 1 (20) | 6 (43) |
| Poor | 0 | 1 (7) |
| Implant covering the fracture defect | ||
| Defect fully covered by the implant | 2 (40) | 0 |
| ≥ 5 mm of the defect left uncovered | 1 (20) | 13 (93) |
| 1–4 mm of the defect left uncovered | 2 (40) | 2 (14) |
| 5–10 mm of the defect left uncovered | 1 (20) | 8 (57) |
| > 10 mm of the defect left uncovered | 0 | 5 (36) |
| Location of the uncovered fracture defect | ||
| Medial to the implant | 3 (60) | 10 (71) |
| Lateral to the implant | 0 | 0 |
All values are n (%) unless otherwise indicated
PSI patient-specific implant
Postoperative long-term follow-up data were available from 14 patients (Table 3). Overall, at the final follow-up, eight patients (57%) experienced any diplopia, which disturbed daily activities in two patients (14%) and required prism glasses in one patient (7%). Moreover, of patients with commercial implants, five (50%) had any lower lid malposition, two (14%) had significant globe malposition, and two (14%) required revision surgery. Postoperative clinical complications were more frequent in patients with commercial implants than two-piece PSIs (Table 3). Patients with two-piece PSIs did not require revision surgery or suffer from GMP > 2 mm (Table 3).
Table 3.
Clinical postoperative outcomes of patients with extensive orbital fracture reconstruction
| Variable | two-piece PSI (n = 4) | Non-PSI (n = 10) |
|---|---|---|
| Follow ups actualized as planned | 4/5 (80) | 10/14 (71) |
| Length of the follow-up period among the patients who participated in the follow-ups, days (range) | 99 (43–160) | 273 (24–804) |
| Postoperative complications | ||
| Revision surgery | 0 | 2 (14) |
| Significant globe malposition (> 2 mm) | 0 | 2 (14) |
| Any lower lid malposition | 0 | 5 (50) |
| Lower lid malposition requiring surgical procedures | 0 | 2 (20) |
| Any diplopia, n (%) | 2 (50) | 6 (60) |
| Diplopia disturbing daily activities | 0 | 2 (20) |
| Diplopia requiring evaluation at the strabismus policlinic | 1 (20) | 1 (10) |
| Diplopia requiring strabismus surgery | 0 | 0 |
| Strabismus requiring prism glasses | 0 | 1 (10) |
All values are n (%) unless otherwise indicated
PSI patient-specific implant
Patients with a poor implant position tended to have significant postoperative clinical symptoms; all of these patients had both significant GMP and some degree of diplopia (Table 4).
Table 4.
Implant position vs postoperative clinical symptoms
| Variable | Significant globe malposition (n = 2) | Any diplopia (n = 8) | Diplopia disturbing daily activities (n = 2) |
|---|---|---|---|
| Overall | |||
| Good (n = 5) | 0 | 2 (40) | 0 |
| Suboptimal (n = 7) | 0 | 4 (57) | 1 (14) |
| Poor (n = 2) | 2 (100) | 2 (100) | 1 (50) |
| Medially | |||
| Good (n = 10) | 0 | 6 (60) | 1 (10) |
| Suboptimal (n = 2) | 0 | 0 | 0 |
| Poor (n = 2) | 2 (100) | 2 (100) | 1 (50) |
| Posteriorly | |||
| Good (n = 8) | 1 (13) | 3 (38) | 0 |
| Suboptimal (n = 5) | 0 | 4 (80) | 1 (20) |
| Poor (n = 1) | 1 (100) | 1 (100) | 1 (100) |
All values are n (% of the total number of patients in each category) unless otherwise indicated
Discussion
In this single-centre retrospective study, we presented a series of cases with reconstructions of EOFMFs. We found excellent postoperative outcomes in patients with two-piece PSIs; they had anatomically more precise reconstructions with less postoperative symptoms compared to patients with commercial implants despite their larger orbital defects. As EOFMFs are rare and may be challenging to reconstruct, our results suggest that two-piece PSIs can be recommended for their primary reconstructions to avoid further revision surgeries and other suboptimal outcomes.
The aims of orbital reconstructions are to restore the anatomy of the orbital cavity [9, 15], retrieve orbital soft tissue content, eliminate unstable bony fragments and identify stable bone platforms while causing minimal iatrogenic injury [3, 16]. This is essential to achieve acceptable postoperative outcomes and to avoid complications [3, 16, 17]. As verified by our results, most of the technical challenges related to reconstructions of EOFMFs are located in the medial and posterior walls and in the inferomedial strut of the orbital cavity, as recognizing these structures post-traumatically is difficult. Moreover, dissection of these structures must be carefully performed due to the proximity of several important structures, including the medial palpebral ligament, lacrimal system, ethmoidal arteries and optic nerve [3].
In our series, most of the patients with commercial implants had malpositioned implants. In contrast, this was observed in only one patient with a two-piece PSI and was not clinically relevant. These findings reflect the challenges related to the process of fitting and aligning commercial implants, which may be time consuming and operator dependent. Importantly, these results also emphasize the advantages of CAD-CAM technology in reconstructing EOFMFs, as personalized CAD-CAM implants lead to more precise orbital volumetric reconstruction compared with traditional reconstruction methods [18, 19]. Additionally, CAD-CAM implants can be modelled preoperatively, which may further decrease the possibility of errors in implant positioning and reduce surgery duration [11].
Enophthalmos, restricted extraocular muscle motility and diplopia are a typical postoperative complication related to extensive orbital wall fractures [3, 9, 20, 21]. Postoperative symptoms may be caused not only by the fracture defect and changes in the volume of the orbital cavity [3], but also by orbital soft tissue injury and herniation via compromised soft tissue function and eye movements [20, 22, 23]. Some previous studies [8, 23, 24] revealed that the development of unfavourable clinical outcomes was not associated with any of the radiological predictors regarding the fracture or the implant type or position, which our results partly support; postoperative clinical symptoms were not only related to implant position. However, poor implant position tended to lead to more pronounced postoperative symptoms when compared with good or suboptimal position. This indicates that anatomically precise reconstruction is still a relevant goal for orbital fracture surgery, for which 3D technology is a beneficial tool.
The delay between fracture diagnosis and primary surgery was longer in patients with PSIs than commercial implants. However, this difference was not clinically significant, as orbital fracture surgery can be safely performed within the first weeks of the trauma without leading to inferior postoperative outcomes [25].
There is debate that the transconjunctival-retrocaruncular approach may cause postoperative complications, including persistent inferior oblique malfunction, inferior canalicular obstruction and scarring, which may result in diplopia [26]. In our series, no signs of complications related to the surgical approach were found, which is consistent with some previous studies [7, 27]. On the contrast, transconjunctival-retrocaruncular approach provides good visibility to the narrow operative field with potential avoidance of excessive tissue stretching, lateral canthotomy or cantholysis or skin incisions during the surgery when used with two-piece PSIs.
In our centre, the Helsinki protocol of two-piece PSIs is fairly simple and quick; the implant can be acquired within 1 day of order and thus does not cause a significant delay in surgery. Even if the manufacturing costs of PSIs for primary orbital reconstructions are evidently higher than using commercial implants, they lead to more precise implant positioning and better clinical outcomes. Thus, the costs of the overall treatment may eventually decrease and lead to less morbidity and stress to the patient. However, due to the importance of cost-effectiveness, the performance of primary orbital reconstruction with PSIs could be started in patients with large fractures, as they generally have poorer postoperative outcomes than patients with single-wall fractures.
The correct placement of PSIs was easily evaluated without intraoperative navigation by the good fitting of the PSI over the fracture defect and the proper fixation between the two parts of the implant. Thus, two-piece PSIs may at least partially replace intraoperative navigation and imaging, which were not available in the present study. Despite the screwless fixation of two-piece PSIs, no failures of the attachment between the implant parts occurred.
The greatest limitation of this study was the low number of patients, which precludes definite conclusions. Additionally, the retrospective and non-comparative study design and the limited rate of actualized clinical follow ups were also weaknesses. Moreover, due to the low number of patients and the variety of procedures performed during the primary surgery of the fracture reconstruction, this study did not assess the effect of PSIs on surgery duration.
Conclusions
Titanium-milled two-piece PSIs are well suited for primary reconstructions of EOFMFs. These patients had fewer postoperative clinical symptoms than patients with commercial implants. This was possibly due to the less invasive surgical approaches, reduced iatrogenic soft tissue injury and greater precision of reconstruction. Therefore, we recommend the described two-piece PSI method for these most challenging primary orbital fracture reconstructions. However, to achieve even better postoperative outcomes for all orbital fracture patients, future research should focus on the role of damage to intraorbital soft tissues [23, 28] and the importance of atraumatic surgical techniques in addition to the precision of the reconstruction.
Author contribution
All the authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Malla Salli, Matti Nikunen and Johanna Snäll. The first draft of the manuscript was written by Malla Salli, and all the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Funding
Open Access funding provided by University of Helsinki including Helsinki University Central Hospital. M.S., M.N. and J.S. received grants from the Helsinki University Hospital Fund and J.S. received a grant from the Paulo Foundation.
Declarations
Ethics approval
The study was approved by the Internal Review Board of the Head and Neck Centre, Helsinki University Central Hospital, Finland. The Internal Review Board of the Head and Neck Centre waived the requirement of informed consent due to the retrospective nature of this study. The guidelines of the Declaration of Helsinki were followed in this study.
Consent to participate
Patient consent was not required because of the retrospective nature of the study. Consent has been obtained from the patient cases presented in patient/computer tomography images.
Consent for publication
The patients’ anonymity has not been endangered in this study. However, informed consent was obtained from all individual participants who could be theoretically identified from the images.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gonzalez MO, Durairaj VD. Indirect orbital floor fractures: a meta-analysis. Middle East Aft J Ophtalmol. 2010;17(2):138–141. doi: 10.4103/0974-9233.63076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young SM, Kim Y-D, Kim SW, et al. Conservatively treated orbital blowout fractures: spontaneous radiologic improvement. Opthalmology. 2018;125(6):938–944. doi: 10.1016/j.ophtha.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Ordon AJ, Kozakiewicz M, Wilczynski M, Loba P. The influence of concomitant medial wall fracture on the results of orbital floor reconstruction. J Craniomaxillofac Surg. 2018;46:573–577. doi: 10.1016/j.jcms.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Homer N, Huggins A, Durairaj VD. Contemporary management of orbital blowout fractures. Curr Opin Otolaryngol Head Neck Surg. 2019;27(4):310–316. doi: 10.1097/MOO.0000000000000550. [DOI] [PubMed] [Google Scholar]
- 5.Cho RI, Davies BW. Combined orbital floor and medial wall fractures involving the inferomedial strut: repair technique and case series using preshaped porous polyethylene/titanium implants. Craniomaxillofac Trauma Reconstr. 2013;6:161–170. doi: 10.1055/s-0033-1343785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hur SW, Kim SE, Chung KJ, et al. Combined orbital fractures: surgical strategy of sequential repair. Arch Plast Surg. 2015;42:424–430. doi: 10.5999/aps.2015.42.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su GW, Harris GJ. Combined inferior and medial surgical approaches and overlapping thin implants for orbital floor and medial wall fractures. Ophtal Plast Recontr Surg. 2006;22:420–423. doi: 10.1097/01.iop.0000242163.03589.0e. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerer RM, Gellrich N-C, von Bulow S, et al. Is there more to the clinical outcome in posttraumatic reconstruction of the inferior and medial orbital walls than accuracy of implant placement and implant surface contouring? A prospective multicenter study to identify predictors of clinical outcome. J Craniomaxillofac Surg. 2018;46:578–587. doi: 10.1016/j.jcms.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Snäll J, Narjus-Sterba M, Toivari M, et al. Does postoperative orbital volume predict postoperative globe malposition after blow-out fracture reconstruction? A 6-month clinical follow-up study. Oral Maxillofac Surg. 2019;23:27–34. doi: 10.1007/s10006-019-00748-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kärkkäinen M, Wilkman T, Mesimäki K, Snäll J. Primary reconstruction of orbital fractures using patient-specific titanium milled implants: the Helsinki protocol. Br J Oral Maxillofac Surg. 2018;56:791–796. doi: 10.1016/j.bjoms.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Sigron GR, Ruedi N, Chammartin F et al (2020). Three-dimensional analysis of isolated orbital floor fractures pre- and post-reconstruction with standard titanium meshes and “hybrid” patient-specific implants. J Clin Med 9:1579; doi:10.3390/jcm9051579 [DOI] [PMC free article] [PubMed]
- 12.Tikkanen J, Mesimäki K, Snäll J. Patient-specific two-piece screwless implant for the reconstruction of a large orbital fracture. Br J Oral Maxillofac Surg. 2020;588(1):112–113. doi: 10.1016/j.bjoms.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Jaquiéry C, Aeppli C, Cornelius P, et al. Reconstruction of orbtital wall defects: critical review of 72 patients. Int J Oral Maxillofac Surg. 2007;36(3):193–199. doi: 10.1016/j.ijom.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Ellis E, Tan Y. Assessment of internal orbital reconstructions for pure blowout fractures: cranial bone grafts titanium mesh. J Oral Maxillofac Surg. 2003;61:442–453. doi: 10.1053/joms.2003.50085. [DOI] [PubMed] [Google Scholar]
- 15.Jung HN, Suh S-I, Kim H-J, Ryoo I. Comparison of clinicoradiological findings between patients with recovering diplopia and those with residual diplopia after surgery for pure orbital blowout fracture. J Craniomaxillofac Surg. 2018;46:375–380. doi: 10.1016/j.jcms.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Kim YH, Kim TG, Lee JH, et al. Inlay implanting technique for the correction of medial orbital wall fracture. Plast Reconstr Surg. 2011;127:321–326. doi: 10.1097/PRS.0b013e3181f95cfd. [DOI] [PubMed] [Google Scholar]
- 17.Park J, Huh J, Lee J, et al. Reconstruction of large orbital posterior floor wall fracture considering orbital floor slope using endoscope. J Craniofac Surg. 2017;28:947–950. doi: 10.1097/SCS.0000000000003461. [DOI] [PubMed] [Google Scholar]
- 18.Pedemonte C, Sáez F, Vargas I, et al. Can customized implants correct enophtalmos and delayed diplopia in posttraumatic orbital deformities? A volumetric analysis. Int J Oral Maxillofac Surg. 2016;45:1086–1094. doi: 10.1016/j.ijom.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Kim YC, Jeong WS, Park TK, et al. The accuracy of patient specific implant prebented with 3D-printed rapid prototype model for orbital wall reconstruction. J Craniomaxillofac Surg. 2017;45(6):928–936. doi: 10.1016/j.jcms.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Choi J, Park SW, Kim J, et al. Predicting late enophthalmos: differences between medial and inferior orbital wall fractures. J Plast Reconstr Aesthet surg. 2016;69(12):238–244. doi: 10.1016/j.bjps.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Kasaee A, Mirmohammadsadeghi A, Kazemnezhad F, et al. The predictive factors of diplopia and extraocular movement limitations in isolated pure orbital fractures. J Curr Ophthalmol. 2017;29(1):54–58. doi: 10.1016/j.joco.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z, Zhang Y, He Y, et al. Correlation between the volume of herniated orbital contents and the amount of enophthalmos in the orbital floor and wall fractures. J Oral Maxillofac Surg. 2012;70(1):68–73. doi: 10.1016/j.joms.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 23.Jung H, Byun JY, Kim HJ, et al. Prognostic CT findings of diplopia after surgical repair of pure orbital blowout fracture. J Cranio Maxillo Fac Surg. 2016;44:1479–1484. doi: 10.1016/j.jcms.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 24.Schönegg D, Wagner M, Schumann P, et al. Correlation between increased orbital volume and enophthalmos and diplopia in patients with fractures of the orbital floor or the medial orbital wall. J Craniomaxillofac Surg. 2018;46:1544–1549. doi: 10.1016/j.jcms.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Simon GJ, Syed HM, McCann JD, Goldberg RA. Early versus late repair of orbital blowout fractures. Ophthalmic Surg Lasers Imaging. 2009;40:141–148. doi: 10.3928/15428877-20090301-05. [DOI] [PubMed] [Google Scholar]
- 26.Malhotra R, Saleh GM, de Sousa JL, et al. The transcaruncular approach to orbital fracture repair: Ophthalmic sequelae. J Craniofac Surg. 2007;18(2):420–426. doi: 10.1097/scs.0b013e31803384c2. [DOI] [PubMed] [Google Scholar]
- 27.Lee CS, Yoon JS, Lee SY. Combined transconjunctival and transcaruncural approach for repair of large medial orbital fractures. Arch Opthalmol. 2009;127:291–296. doi: 10.1001/archophthalmol.2009.5. [DOI] [PubMed] [Google Scholar]
- 28.Pilanci O, Ceran F, Sagir M, et al. Evaluation of the retro-orbital fatty tissue volume in delayed orbital blow-out fractures. Opthal Plast Reconstr Surg. 2016;32:207–210. doi: 10.1097/IOP.0000000000000467. [DOI] [PubMed] [Google Scholar]