Abstract
Objective
Free flaps are widely used for the repair of soft tissue defects in the lower limbs, but there is still a specific rate of necrosis. Few clinical retrospective studies have analyzed the nontechnical risk factors for lower limb free flap necrosis. This study aimed to analyze the nontechnical causes of flap necrosis in lower limb soft tissue reconstruction in order to identify risk factors and improve the survival rate of free flaps.
Methods
Clinical data from 244 cases of soft tissue defects of the leg or foot that were repaired with a free flap from January 2011 to June 2020 were retrospectively analyzed. The flap results were divided into complete survival and necrosis groups. The patients' general information, smoking history, soft tissue defect site, Gustilo‐Anderson classification, shock after injury, type and size of the flap, and time from injury to flap coverage were recorded. A logistic regression model was used to analyze the correlations between flap necrosis and possible risk factors.
Results
Of the 244 flaps, 32 suffered from partial or total necrosis, and 212 completely survived. Univariate analysis showed that age, smoking history, soft tissue defect site, and time from injury to flap coverage were significantly correlated with flap necrosis (p ≤ 0.2). Multivariate logistic regression analysis showed that moderate‐to‐severe smoking history (p < 0.001, odds ratio [OR] = 10.259, 95% confidence interval [CI] = 2.886–36.468), proximal leg defect (p = 0.006, OR = 7.095, 95% CI = 1.731–29.089), and time from injury to flap coverage >7 days (p = 0.003, OR = 12.351, 95% CI = 2.343–65.099) were statistically significant risk factors for flap necrosis (p < 0.05), and age was excluded (p = 0.666; p = 0.924).
Conclusion
The risk of flap necrosis was significantly increased when the soft tissue defect was located in the proximal leg, the time from injury to flap coverage was >7 days, and the patient had a moderate‐to‐severe smoking history. These three risk factors have an increased influence on flap necrosis and have guiding significance in predicting flap prognosis.
Keywords: Free Flap, Multivariate Logistic Regression Analysis, Risk Factors, Soft Tissue Defect
The risk of flap necrosis was significantly increased when the soft tissue defect was located in the proximal leg, the time from injury to flap coverage was >7 days, and the patient had a moderate‐to‐severe smoking history. These three risk factors have an increased influence on flap necrosis and have guiding significance in predicting flap prognosis.

Introduction
Open fractures of the lower limbs are usually caused by major traffic accidents, mostly involving direct high‐energy violence. Fractures are often combined with severe soft tissue injuries, resulting in extensive soft tissue defects. 1 , 2 , 3 Improper treatment can lead to bone infection, osteonecrosis, bone nonunion, and amputation. For large soft tissue defects of the leg and foot, local flaps are limited because of the usually larger size of the defects encountered, such that their donor area is typically compromised. With the development of microsurgical technology, free flaps are widely used to repair soft tissue defects of the leg and foot. However, the risk is relatively high, and there is a certain necrosis rate.
Most studies on flap necrosis risk factors have focused on pedicled flap risk factors. Bekara et al. 4 found that age >60 years, diabetes, and arteriopathy were significant risk factors for perforator‐pedicled propeller flap complications in the lower extremities. Gong et al. 5 concluded that the soft tissue defect site, flap size, and postoperative wound infection were risk factors for pedicled flap necrosis in hand soft tissue defect reconstruction. Peng et al. 6 reported that the width of the skin island was an independent risk factor affecting partial necrosis of the posterior tibial artery perforator‐plus fasciocutaneous flap. Furthermore, Mlodinow et al. 7 stated that smoking status and increased age raised the mastectomy flap necrosis risk. However, Qiu et al. 8 reported that injury cause, length/width ratio of the wound, thickness of the pedicle, operation time, injury site, direction of blood perfusion in the flap, and operating methods, rather than age, sex, and flap size, were significant risk factors for necrosis of flap‐like wounds.
Previous studies on factors associated with free flap necrosis usually included technical and nontechnical aspects. Technological aspects include 9 , 10 , 11 , 12 : (i) hematoma formation due to inadequate intraoperative hemostasis. Inadequate drainage will lead to obstruction of venous return and increase the chance of infection; (ii) poor angulation of vessel placement. Before the vascular anastomosis, we should find the ideal anastomosis position in the recipient vessel, and keep the vessel anastomosis in mild tension; (iii) misidentification of arterial and venous. In some exceptional cases, we may find the walls of the arteries and veins are similar in thickness. An intraoperative misidentification will result in the absence of venous return after arterial anastomosis; and (iv) poor‐quality vascular anastomosis. The vascular anastomosis technique is the primary technique for flap repair surgery, and the level of vascular anastomosis technique will directly affect the outcome of flap surgery. Poor anastomosis technique is manifested by inconsistent stitch spacing at the anastomosis, vascular epithelial invagination, or even vascular counter‐stitching. Few studies have been conducted specifically on nontechnical factors of free flap necrosis, especially with large samples and complete data. Analysis of nontechnical risk factors affecting free flap necrosis has significance for improving flap viability and prognosis.
Few studies have reported the total necrotic rate of free flaps and the nontechnical risk factors for flap necrosis in leg or foot tissue defect reconstructions. We conducted a retrospective study of 244 patients who underwent lower extremity free flap reconstruction at our institution to summarize the nontechnical causes of the vascular crisis and provide a reference for orthopaedic and plastic surgeons.
This study aimed to (i) explore the effects of sex, age, smoking history, soft tissue defect site, Gustilo‐Anderson classification, shock after injury, type and size of free flap, and time from injury to flap coverage on free flap necrosis and (ii) identify risk factors to reduce the necrosis rate of free flaps.
Methods
Inclusion and Exclusion Criteria
Inclusion criteria included (i) patients with unilateral open fractures with complex soft tissue defects of the leg or foot who had undergone reconstructive surgery using a free flap and (ii) patients who underwent the initial flap surgery (not the secondary surgery). Exclusion criteria included flap necrosis due to technical factors, such as poor vascular anastomosis (subcutaneous hematoma, vascular tortuosity, or improper anastomotic location). Flap necrosis >60% was regarded as complete necrosis, and flap necrosis ≤60% was considered as partial necrosis. 13 Partial necrosis here refers to conditions requiring further surgical intervention, not those that eventually heal with minor wound management.
From January 2011 to June 2020, 254 patients with open fractures (Gustilo II–IIIC) of the lower limbs underwent free flap procedures at our hospital. After screening, 10 patients with flap necrosis due to vascular anastomosis were excluded. Of the 244 flaps, 32 had partial or complete necrosis and were included in the flap necrosis group; 212 flaps survived completely and were included in the complete survival group.
This study was approved by the Medical Ethics Committee of Wuxi Ninth People's Hospital (LW2020019). Informed consent was obtained from all patients for data to be documented and published in the present study.
Risk Factor Analysis
We performed a univariate analysis of the factors affecting the prognosis of flaps, including gender (0 = female, 1 = male), age at the time of surgery (0 = “Y < 30,” 1 = “30 ≤ Y ≤ 50”, 2 = “Y > 50”), smoking history (0 = no, 1 = mild, 2 = moderate‐to‐severe), soft tissue defect site (0 = proximal leg, 1 = mid‐leg, 2 = distal leg, 3 = foot and ankle), Gustilo‐Anderson classification (0 = II, 1 = IIIA, 2 = IIIB, 3 = IIIC), shock after injury(0 = no,1 = yes), type (0 = anterolateral thigh flap, 1 = latissimus dorsi myocutaneous flap) and size of flap, and time from injury to flap coverage (0 = “T < 72 h,” 1 = “72 h ≤ T ≤ 7 days,” 2 = “T > 7 days”). Independent variables with statistically significant differences in univariate analysis were incorporated into the multivariate logistic regression model analysis.
Age was divided into three groups: <30 years old, 30–50 years old, and >50 years old, according to the criteria of the Mangled Extremity Severity Score. 14 , 15 Patients' smoking history was divided into mild and moderate‐to‐severe smoking history according to the smoking index. 16 Smoking index = average number of cigarettes smoked per day × years of smoking. In this study, a smoking index <200 was defined as mild smoking history, and a smoking index >200 was defined as moderate‐to‐severe smoking history. The degree of limb soft tissue injury was divided into four groups: types II, IIIA, IIIB, and IIIC, according to the Gustilo‐Anderson classification. The time from injury to flap coverage was divided into three stages according to the study by Wood 17 , 18 : (1) early reconstruction, within 72 h of injury; (2) intermediate reconstruction, performed between 72 h and 7 days; and (3) late reconstruction, performed >7 days after injury. The flap size was included in the statistical analysis as a continuous variable.
Statistical Methods
All statistical analyses were performed using IBM SPSS software (version 23.0; IBM Corp., Armonk, NY, USA). Flap size was a continuous variable and expressed as an average. The remaining variables were categorical variables and expressed as frequencies. To identify the risk factors for flap necrosis, a univariate analysis was first performed for the initial screening. To avoid missing important risk factors, we selected all variables with p ≤ 0.2 in univariate analysis to be included in the multivariate analysis. The correlation between flap necrosis and the risk factors was analyzed using a binary logistic regression model. Statistical significance was defined as p < 0.05.
Results
General Results
Among the 244 patients, 179 were male and 65 were female. Their ages ranged from 1 to 71 years, with an average age of 42.4 ± 14.8 years. Among them, 50 patients were <30 years old, 112 were 30–50 years old, and 82 were >50 years old. Eighty‐seven patients with a smoking history were classified according to the smoking index 16 : 18 cases of mild smoking history, that is, smoking index <200, and 69 cases of moderate to severe smoking history, that is, smoking index >200. All patients with a history of smoking were advised to quit smoking. Soft tissue defects were mainly located in the proximal leg, middle section, distal section, and foot or ankle in 29, 35, 68, and 112 cases, respectively. According to the Gustilo‐Anderson classification of open fracture, 19 there were 38 cases of type II, 61 cases of type IIIA, 80 cases of type IIIB, and 65 cases of type IIIC fractures. Hemorrhagic shock after the injury occurred in 28 cases. The time from injury to flap coverage was within 72 h in 67 cases, from 72 h to 7 days in 96 cases, and more than 7 days in 81 cases. The types of free flaps included anterolateral thigh flaps (145 cases) and latissimus dorsi myocutaneous flaps (99 cases). End‐to‐end vascular microanastomosis was adopted in all cases.
Among the 244 patients, 35 flaps had vascular crises after surgery, of which three were saved after vascular exploration, and 32 had flap necrosis. These patients were divided into two groups: 212 completely survived flaps were included in the complete survival group and 32 necrotic flaps were included in the flap necrosis group. The flap necrosis group included 25 males and seven females. Among them, two cases were <30 years old, 15 were 30–50 years old, and 15 were >50 years old. According to the smoking index, two patients in the flap necrosis group had a mild smoking history and 22 had a moderate‐to‐severe smoking history. Patients who experienced flap necrosis underwent secondary flap surgery (n = 24) or skin grafting (n = 8), and soft tissue defects were repaired in all patients. The causes of flap necrosis did not include hematoma formation, improper placement of blood vessels, or low‐quality vascular anastomosis. The nine factors analyzed included sex, age, smoking history, soft tissue defect site, Gustilo‐Anderson classification, shock after injury, type and size of free flap, and time from injury to flap coverage. The univariate and multivariate analysis results are shown in Tables 1 and 2, respectively.
TABLE 1.
Univariate analysis of free flap necrosis in repairing soft tissue defects of the lower limbs
| Factors | Cases | Completely survive | Flap necrosis | χ 2/t | p value |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 179 | 154 | 25 | 0.428 | 0.513 |
| Female | 65 | 58 | 7 | ||
| Age | |||||
| Y < 30 | 50 | 48 | 2 | ||
| 30 ≤ Y ≤ 50 | 112 | 97 | 15 | 5.582 | 0.061 |
| Y > 50 | 82 | 67 | 15 | ||
| Smoking history | |||||
| No | 157 | 149 | 8 | ||
| Mild | 18 | 16 | 2 | 30.256 | <0.001 |
| Moderate to severe | 69 | 47 | 22 | ||
| Defect site | |||||
| Proximal leg | 29 | 17 | 12 | ||
| Middle leg | 35 | 31 | 4 | 24.033 | <0.001 |
| Distal leg | 68 | 60 | 8 | ||
| Foot and ankle | 112 | 104 | 8 | ||
| Gustilo‐ Anderson classification | |||||
| II | 38 | 35 | 3 | 3.143 | 0.370 |
| IIIA | 61 | 55 | 6 | ||
| IIIB | 80 | 69 | 11 | ||
| IIIC | 65 | 53 | 12 | ||
| Post‐traumatic shock | |||||
| No | 216 | 189 | 27 | 0.624 | 0.429 |
| Yes | 28 | 23 | 5 | ||
| Time from injury to flap coverage | |||||
| T < 72 h | 67 | 65 | 2 | 18.879 | <0.001 |
| 72 h ≤ T ≤ 7 days | 96 | 87 | 9 | ||
| T > 7 days | 81 | 60 | 21 | ||
| Type of free flap | |||||
| Anterolateral thigh flap | 145 | 127 | 18 | 0.154 | 0.695 |
| Latissimus dorsi myocutaneous flap | 99 | 85 | 14 | ||
| Size of flaps | ‐ | 179.47 ± 68.80 | 181.34 ± 55.38 | 0.022 | 0.883 |
| Total | 244 | 212 | 32 | ‐ | ‐ |
TABLE 2.
Results of multivariate regression analysis of free flap necrosis in repairing soft tissue defects of the lower limbs
| Factors | β | Standard error | Wald | p value | OR | 95% CI | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Age | |||||||
| Y < 30 | |||||||
| 30 ≤ Y ≤ 50 | 0.392 | 0.907 | 0.187 | 0.666 | 1.480 | 0.250 | 8.752 |
| Y > 50 | −0.091 | 0.957 | 0.009 | 0.924 | 0.913 | 0.140 | 5.956 |
| Smoking history | |||||||
| No | |||||||
| Mild | 0.653 | 1.041 | 0.393 | 0.531 | 1.921 | 0.249 | 14.784 |
| Moderate to severe | 2.328 | 0.647 | 12.945 | <0.001 | 10.259 | 2.886 | 36.468 |
| Defect site | |||||||
| Proximal leg | 1.959 | 0.720 | 7.408 | 0.006 | 7.095 | 1.731 | 29.089 |
| Mid‐leg | 0.472 | 0.772 | 0.374 | 0.541 | 1.603 | 0.353 | 7.282 |
| Distal leg | 0.012 | 0.624 | <0.001 | 0.985 | 1.012 | 0.298 | 3.436 |
| Foot and ankle | |||||||
| Time from injury to flap coverage | |||||||
| T < 72 h | |||||||
| 72 h ≤ T ≤ 7 days | 1.848 | 0.893 | 4.282 | 0.039 | 6.346 | 1.102 | 36.525 |
| T > 7 days | 2.514 | 0.848 | 8.786 | 0.003 | 12.351 | 2.343 | 65.099 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Results of Univariate Analysis
The univariate analysis showed that sex (p = 0.513), Gustilo‐Anderson classification (p = 0.370), shock after injury (p = 0.429), and type (p = 0.695) and size (p = 0.883) of the flap had no effect on the flap prognosis, and the difference was not statistically significant (p > 0.2). Age, smoking history, soft tissue defect site, and time from injury to flap coverage were significantly correlated with flap necrosis (p ≤ 0.2). The above four factors were included in the multivariate logistic regression analysis.
Results of Multivariate Regression Analysis
The results showed no correlation between age (p = 0.666; p = 0.924) and flap necrosis (p > 0.05). There was a positive correlation between smoking history and flap necrosis (p < 0.001, odds ratio [OR] = 10.259, 95% confidence interval [CI] = 2.886–36.468). This showed that the longer the smoking history and the greater the number of cigarettes consumed, the more likely the flap was to undergo necrosis. Soft tissue defect sites were associated with flap necrosis. The incidence of flap necrosis was higher (p = 0.006, OR = 7.095, 95% CI = 1.731–29.089) when the soft tissue defect was located in the proximal leg. The time from injury to flap coverage was positively correlated with flap necrosis. The longer the interval between the injury and flap coverage, the higher the likelihood of flap necrosis (p = 0.003, OR = 12.351, 95% CI = 2.343–65.099).
The importance of each screened risk factor was estimated by the partial chi‐square statistics minus the predicted degrees of freedom (χ 2‐df). Moderate‐to‐severe smoking history was the most critical risk factor, followed by the time from injury to flap coverage >7 days and the defect site located in the proximal leg.
Case Report
To illustrate the concept of orthoplastic surgery mentioned below, we included a brief description of a typical case, a 19‐year‐old man with an open right leg injury caused by a machine (Figure 1). The patient suffered an open fracture of the right tibia caused by a machine injury and was classified as type IIIB according to Gustilo‐Anderson's classification. His right leg suffered a soft tissue defect and a comminuted tibia fracture. We performed emergency surgery: debridement of the right leg, open reduction and internal fixation of the tibia and fibula, filling the tibial defect with PMMA (polymethylmethacrylate) cement, and repairing the wound with free anterolateral thigh flap transplantation. The flap survived successfully. At 6 weeks after surgery, we removed the PMMA cement, implanted autogenous cancellous bone in the induced membrane, and added another plate to the medial tibia for enhanced stability. It can be seen from this case that the application of orthoplastic surgery in the treatment of open fractures of the leg dramatically shortens the treatment cycle.
Fig. 1.
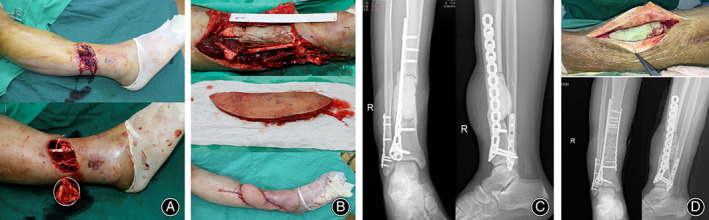
A typical case: 19‐year‐old male with an open right leg injury caused by a machine. (A) The appearance of the injured limb showed anterior tibial soft tissue defect and a large number of free bone fragments; (B) Limited internal fixation, bone cement filling in the bone defect, and free anterolateral thigh flap transplantation were operated on urgently; (C) X‐ray film after the emergency operation; (D) In the second stage, the bone cement was removed, the bone graft was implanted, and another plate was added.
Discussion
The major finding of this study was the identification of the nontechnical causes of flap necrosis in lower limb soft tissue reconstruction. In this study, we found that the risk of flap necrosis was significantly increased when the soft tissue defect was located in the proximal leg, the time from injury to flap coverage was more than 7 days, and the patient had a moderate‐to‐severe smoking history.
Correlation between Shock and Flap Necrosis
According to the modified extremity severity score, shock duration and limb ischemia after injury are determinants of one‐stage amputation. 20 Post‐injury shock implies a high degree of injury and is often associated with major arterial injury of the lower extremities. 21 Theoretically, post‐injury shock is associated with free flap necrosis. However, in this study, there was no significant correlation between shock after injury and flap necrosis. The authors have not found other studies on the relationship between post‐injury shock and free flap necrosis. With the development of damage control surgery and microsurgical techniques, most severely injured limbs can survive emergency reconstruction of blood circulation, and satisfactory results can be obtained by secondary flap coverage. 22 , 23 , 24 The Department of Orthopedic Trauma in our hospital implements a 24‐h emergency duty system and has skilled microsurgical techniques, which are key factors in improving the survival rate of limb salvage and free flaps.
Smoking Has a Negative Prognosis for Flaps
Smoking can cause nicotine to quickly enter the blood circulation to stimulate sympathetic excitement and release a large number of adrenocortical active factors, which increase vasoactive elements and angiotensin, thereby promoting vasoconstriction. After smoking, hypoxia and hypercapnia cause a rapid increase in erythrocyte volume, and a large number of aggregated platelets increase blood viscosity and affect blood circulation. Increased smoking can increase the risk of vascular embolism and vascular crisis in the free flap. 25 Simultaneously, smoking can increase nitric oxide (NO) in the blood, and NO can competitively bind to hemoglobin, decreasing blood oxygen. 26 Mlodinow 7 found that a long smoking history significantly increases the risk of flap necrosis after mastectomy. Hwang 27 studied the relationship between smoking and flap necrosis and suggested that smoking reduces oxygen pressure in the alveoli and oxygen in the subcutaneous wound tissue. Kalmar 28 conducted a retrospective study of breast reconstruction using free flaps in North America between 2015 and 2020. In multivariate regression analysis, breast free flap failure was significantly higher in patients who had smoked within the past year (p = 0.030; AOR, 1.7). Nicotine can also induce vasoconstriction. Smokers are more likely to experience flap loss, hematoma, or fat necrosis than nonsmokers. Therefore, smoking should be prohibited during treatment, and patients should be encouraged to correct this harmful habit.
Correlation between Defect Site and Flap Necrosis
It is important to select suitable blood vessels in the recipient area for free flap transplantation. For soft tissue defects in the leg, because the descending branch of the lateral circumflex femoral artery carried by the anterolateral thigh flap and the thoracodorsal artery carried by the latissimus dorsi myocutaneous flap usually have a large caliber, the most suitable recipient vessel, in theory, is the anterior or posterior tibial vessel. However, in the proximal leg, due to injury to the main blood vessels and the need to retain the main blood vessels, we used the descending genicular vessels 29 and medial inferior genicular vessels 30 as the recipient vessels for the free flaps. The caliber of these blood vessels is narrower, which is quite different from that of the descending branch of the lateral circumflex femoral and thoracodorsal vessels, thus increasing the risk of vascular crisis. Therefore, in this study, soft tissue defects in the proximal leg were more prone to flap necrosis than those in the middle and distal legs and ankles. The well‐known nontrunk arteries around the knee include the articular and saphenous branches of the descending genicular artery, medial and lateral superior genicular arteries, medial and lateral inferior genicular arteries, middle genicular artery, anterior tibial recurrent artery, sural artery, and semimembranosus artery. These well‐known arteries and their branches are connected to form a blood supply network around the knee. 31 The main blood vessels of the leg can be preserved to the maximum extent by using them as the blood vessels in the recipient area, and the functional damage caused by flap transplantation can be greatly reduced. However, there is a high demand for microsurgical techniques. Liu 32 performed free flap reconstruction in 14 patients with soft tissue defects around the knee between 2015 and 2019, and selected the descending genicular artery as the recipient vessel. In two of these cases, necrosis occurred in the distal tip of the flaps. The skin flaps entirely survived in the other 12 patients. Doctors with microsurgery skills are recommended to use the well‐known nontrunk arteries around the knee as blood vessels in the recipient area.
Effect of the Time from Injury to Flap Coverage on Prognosis
This study showed that the initial free flap reconstruction performed within the first 72 h reduced the rate of free flap failure. The advantages of early flap coverage include light scarring around the wound, clear tissue structure, mild inflammatory reaction, and good tissue tension during suturing. 33 , 34 , 35 Based on the concept of orthoplastic surgery, 36 , 37 , 38 , 39 we advocate emergency surgery with limited internal fixation combined with free flap coverage if the damage condition is acceptable. In 1986, Godina 40 published a report on 532 cases of limb salvage with severe trauma, of which 134 cases completed soft tissue repair within 72 h after injury. His team included orthopaedic trauma and plastic surgeons who could perform joint bone and soft tissue repair operations. The results showed that early wound repair could significantly reduce infection, flap failure, and reoperation rates. The earlier the repair, the better the effect. Godina's practice of early repair and combined repair pioneered the concept of orthoplastic surgery. 41 Boriani 42 conducted a prospective multi‐center cohort study which include a total of 160 patients. Of these, 70% were treated with an orthoplastic approach, whereas 30% were treated by an orthopaedic team. All outcome measures were statistically improved by the orthoplastic approach. Our hospital has a group of doctors who can treat fractures and repair soft tissues, forming an orthoplastic team that can perform complete debridement, fixation, and coverage simultaneously. The utility model had the advantages of fewer operations, shorter treatment cycles, rapid functional recovery, and satisfactory clinical outcomes. 43 , 44 We believe that the advantages of emergency microsurgical repairs are significant. First, it prevents persistent ischemic necrosis in some tissues. Emergency soft tissue coverage with an abundant blood supply can effectively prevent persistent necrosis caused by ischemia. This can improve the prognostic function of the affected limb and reduce the occurrence of complications. Second, it reduces the number of surgeries and rehabilitation cycles needed. Patients can start functional exercise in the early stages, and the braking time after surgery is reduced, which avoids the occurrence of complications such as joint contracture and muscle atrophy. Finally, it reduces the psychological impact of severe trauma on patients. Emergency microsurgical repair can help patients to quickly recover from acute trauma and reduce the psychological impact of repeated surgeries.
Strengths and Limitations of the Study
The main strength of this study is the summary of nontechnical causes of flap necrosis in soft tissue reconstruction of the leg and foot by univariate analysis and multifactorial logistic regression analysis. The results of the study showed that soft tissue defect site, time from injury to flap coverage, and smoking history were nontechnical risk factors for flap necrosis. The findings of the study are instructive in predicting flap prognosis. We strictly screened the positive group (flap necrosis) cases by reviewing the surgical records of vascular re‐exploration to ensure the reliability of our conclusions. Although studies related to the analysis of risk factors for flap necrosis have been reported, the present study differs from them in terms of subjects, sample size, and type of independent variables.
However, there are certain limitations for this study: (i) the sample size of this study was not large enough, especially with regard to the number of patients with flap necrosis; (ii) the sample size of the “positive group” (flap necrosis) in this study was only 32 cases, which led to a limitation in the number of variables included in the binary logistic regression analysis; and (iii) the factors of critical medical comorbidities, such as diabetes, were not included in the regression analysis as risk factors. However, we will collect more cases and increase the sample size of the flap necrosis group to include more risk factors such as diabetes in future studies.
Conclusion
Soft tissue defect site, time from injury to flap coverage, and smoking history were nontechnical risk factors for free flap necrosis of the lower limbs. The risk of flap necrosis significantly increased when the soft tissue defect was located in the proximal leg, the time from injury to flap coverage was more than 7 days, and the patient had a moderate‐to‐severe smoking history. These three risk factors have an increased influence on flap necrosis and have guiding significance in predicting the flap prognosis.
Author Contributions
Hao Liu and Yongjun Rui conceived the idea for this manuscript. Ming Zhou and Yuan Xue performed statistical analysis. Hao Liu and Jun Liu wrote the primary manuscript. Yongwei Wu and Yunhong Ma edited the manuscript. All authors have reviewed, read, and approved the final version of this paper.
Conflict of Interest
The authors declare no conflicts of interest in this study.
Ethics Statement
The study was approved by the Medical Ethics Committee of Wuxi Ninth People's Hospital (LW2020019). Written informed consent was obtained from all participants prior to study commencement.
Acknowledgement
This study was supported by Wuxi Top Medical Expert Team of Taihu Talent Program.
Hao Liu and Jun Liu are the two first authors who contributed equally to this work.
References
- 1. Chan JK, Aquilina AL, Lewis SR, Rodrigues JN, Griffin XL, Nanchahal J. Timing of antibiotic administration, wound debridement, and the stages of reconstructive surgery for open long bone fractures of the upper and lower limbs. Cochrane Database Syst Rev. 2022;4(4):Cd013555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ndlovu S, Naqshband M, Masunda S, Ndlovu K, Chettiar K, Anugraha A. Clinical effectiveness of the ganga hospital open injury severity score for limb salvage versus amputation in patients with complex limb injuries: a systematic review and meta‐analysis. Bone Joint J. 2023;105‐b(1):21–8. [DOI] [PubMed] [Google Scholar]
- 3. Nicolaides M, Pafitanis G, Vris A. Open tibial fractures: an overview. J Clin Orthop Trauma. 2021;20:101483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bekara F, Herlin C, Mojallal A, Sinna R, Ayestaray B, Letois F, et al. A systematic review and meta‐analysis of perforator‐pedicled propeller flaps in lower extremity defects: identification of risk factors for complications. Plast Reconstr Surg. 2016;137(1):314–31. [DOI] [PubMed] [Google Scholar]
- 5. Gong X, Cui J, Jiang Z, Lu L, Li X. Risk factors for pedicled flap necrosis in hand soft tissue reconstruction: a multivariate logistic regression analysis. ANZ J Surg. 2018;88(3):E127–e31. [DOI] [PubMed] [Google Scholar]
- 6. Peng P, Dong Z, Wei J, Liu L, Luo Z, Zheng L. Risk factors related to the partial necrosis of the posterior tibial artery perforator‐plus fasciocutaneous flap. Eur J Trauma Emerg Surg. 2022;48(2):1247–53. [DOI] [PubMed] [Google Scholar]
- 7. Mlodinow AS, Fine NA, Khavanin N, Kim JY. Risk factors for mastectomy flap necrosis following immediate tissue expander breast reconstruction. J Plast Surg Hand Surg. 2014;48(5):322–6. [DOI] [PubMed] [Google Scholar]
- 8. Qiu D, Wang X, Wang X, Jiao Y, Li Y, Jiang D. Risk factors for necrosis of skin flap‐like wounds after ED debridement and suture. Am J Emerg Med. 2019;37(5):828–31. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Y, Gazyakan E, Bigdeli AK, Will‐Marks P, Kneser U, Hirche C. Soft tissue free flap for reconstruction of upper extremities: a meta‐analysis on outcome and safety. Microsurgery. 2019;39(5):463–75. [DOI] [PubMed] [Google Scholar]
- 10. Stranix JT, Azoury SC, Lee ZH, Kozak G, Plana N, Thanik VD, et al. Matched comparison of microsurgical anastomoses performed with loupe magnification versus operating microscope in traumatic lower extremity reconstruction. Plast Reconstr Surg. 2020;145(1):235–40. [DOI] [PubMed] [Google Scholar]
- 11. Iamaguchi RB, Takemura RL, Silva GB, de Oliveira Alves JA, Torres LR, Cho AB, et al. Peri‐operative risk factors for complications of free flaps in traumatic wounds ‐ a cross‐sectional study. Int Orthop. 2018;42(5):1149–56. [DOI] [PubMed] [Google Scholar]
- 12. Iamaguchi RB, Macedo LS, Cho AB, Rezende MR, Mattar R, Wei TH. Microsurgical reconstruction in an orthopedic hospital: indications and outcomes in adults. Rev Bras Ortop (Sao Paulo). 2022;57(5):772–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gupta A, Lakhiani C, Lim BH, Aho JM, Goodwin A, Tregaskiss A, et al. Free tissue transfer to the traumatized upper extremity: risk factors for postoperative complications in 282 cases. J Plast Reconstr Aesthet Surg. 2015;68(9):1184–90. [DOI] [PubMed] [Google Scholar]
- 14. Gratl A, Kluckner M, Gruber L, Klocker J, Wipper S, Enzmann FK. The mangled extremity severity score (MESS) does not predict amputation in popliteal artery injury. Eur J Trauma Emerg Surg. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schechtman DW, Walters TJ, Kauvar DS. Utility of the mangled extremity severity score in predicting amputation in military lower extremity arterial injury. Ann Vasc Surg. 2021;70:95–100. [DOI] [PubMed] [Google Scholar]
- 16. Frost‐Pineda K, Polster M. Heaviness of smoking index in menthol and non‐menthol smokers. J Addict Dis. 2022:1–8. [DOI] [PubMed] [Google Scholar]
- 17. Wood T, Sameem M, Avram R, Bhandari M, Petrisor B. A systematic review of early versus delayed wound closure in patients with open fractures requiring flap coverage. J Trauma Acute Care Surg. 2012;72(4):1078–85. [DOI] [PubMed] [Google Scholar]
- 18. Coombs J, Billow D, Cereijo C, Patterson B, Pinney S. Current concept review: risk factors for infection following open fractures. Orthop Res Rev. 2022;14:383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alıç T, Hassa E. Open fractures from Gustilo and Anderson to the present: a bibliometric analysis with global productivity and research trends. Indian J Orthop. 2022;56(12):2119–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Suriyakumar S, Saluja SS, Ramanujam M, Mancheri MN, Jambu N. Management of grade 3C compound injury of lower limb with floating knee ‐ salvage versus amputation (case series). J Orthop Case Rep. 2021;11(2):119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee CH, Chang YJ, Li TS, Chen YC, Hsieh YK. Vascular trauma in the extremities: factors associated with the outcome and assessment of amputation indexes. Acta Cardiol Sin. 2022;38(4):455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boucher J, Guerre E, Duquennoy‐Martinot V, Guerreschi P, Pasquesoone L. Free flap for lower limb salvage in infectious purpura fulminans. Ann Chir Plast Esthet. 2021;66(6):420–8. [DOI] [PubMed] [Google Scholar]
- 23. Mcculloch I, Valerio I. Lower extremity reconstruction for limb salvage and functional restoration ‐ the combat experience. Clin Plast Surg. 2021;48(2):349–61. [DOI] [PubMed] [Google Scholar]
- 24. Perkins ZB, Kersey AJ, White JM, Lauria AL, Propper BW, Tai NRM, et al. Impact of ischemia duration on lower limb salvage in combat casualties. Ann Surg. 2022;276(3):532–8. [DOI] [PubMed] [Google Scholar]
- 25. Lv L, Wu S, Yang Y, Yue X. Modified effect of active or passive smoking on the association between age and abdominal aortic calcification: a nationally representative cross‐sectional study. BMJ Open. 2021;11(10):e047645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kobayashi J, Ohtake K, Murata I, Sonoda K. Nitric oxide bioavailability for red blood cell deformability in the microcirculation: a review of recent progress. Nitric Oxide. 2022;129:25–9. [DOI] [PubMed] [Google Scholar]
- 27. Hwang K, Son JS, Ryu WK. Smoking and flap survival. Plast Surg (Oakv). 2018;26(4):280–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kalmar CL, Drolet BC, Kassis S, Thayer WP, Higdon KK, Perdikis G. Breast reconstruction free flap failure: does platelet count matter? Ann Plast Surg. 2022;89(5):523–8. [DOI] [PubMed] [Google Scholar]
- 29. Kobayashi Y, Uchiyama Y, Ishii T, Nakajima D, Yanagisawa S, Saito I, et al. Three cases of free flap anastomoses to the descending genicular artery for knee joint trauma with severe soft tissue injury. J Orthop Sci. 2022;27(3):734–8. [DOI] [PubMed] [Google Scholar]
- 30. Shen YM, Yu DN, Hu XH, Qin FJ, Li M, Ning FG. Repairing proximal and middle lower‐leg wounds with retrograde sartorius myocutaneous flap pedicled by perforating branches of medial inferior genicular artery or posterior tibial artery. J Plast Reconstr Aesthet Surg. 2012;65(9):1158–64. [DOI] [PubMed] [Google Scholar]
- 31. Fu J, Qing L, Wu P, Tang J. Customized reconstruction of a complex soft‐tissue defect around the knee with a free perforator flap. Am J Transl Res. 2021;13(5):4401–11. [PMC free article] [PubMed] [Google Scholar]
- 32. Liu J, Wu Y, Zhou M, Liu H, Kang Y, Wang Y, et al. Repair of severe peri‐knee soft tissue defect using an anterolateral thigh flap with the descending genicular vessels as the recipient pedicle: a case series of 14 patients. Ann Palliat Med. 2021;10(5):5341–50. [DOI] [PubMed] [Google Scholar]
- 33. Cao Z, Li C, He J, Qing L, Yu F, Wu P, et al. Early reconstruction delivered better outcomes for severe open fracture of lower extremities: a 15‐year retrospective study. J Clin Med. 2022;11(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee ZH, Stranix JT, Levine JP. The optimal timing of traumatic lower extremity reconstruction: current consensus. Clin Plast Surg. 2021;48(2):259–66. [DOI] [PubMed] [Google Scholar]
- 35. Radtke C, Meyer‐Marcotty M. Reconstruction of soft tissue defects of the lower extremities. Unfallchirurg. 2021;124(10):782–8. [DOI] [PubMed] [Google Scholar]
- 36. Azoury SC, Kovach SJ, Levin LS. Reconstruction options for lower extremity traumatic wounds. J Am Acad Orthop Surg. 2022;30(16):735–46. [DOI] [PubMed] [Google Scholar]
- 37. Mansour AM, Jacobs A, Raj MS, Lee FG, Terrasse W, Wallace SJ, et al. Lower extremity soft tissue reconstruction review article. Orthop Clin North Am. 2022;53(3):287–96. [DOI] [PubMed] [Google Scholar]
- 38. Maruccia M, Vicenti G, Carrozzo M, Caizzi G, di Summa PG, Moretti B, et al. The free tissue transfer‐Masquelet‐reamer‐irrigator‐aspirator bone graft Orthoplastic approach for lower extremity reconstruction. Plast Reconstr Surg. 2022;149(6):1203e–8e. [DOI] [PubMed] [Google Scholar]
- 39. Müller SLC, Morgenstern M, Kuehl R, Muri T, Kalbermatten DF, Clauss M, et al. Soft‐tissue reconstruction in lower‐leg fracture‐related infections: an orthoplastic outcome and risk factor analysis. Injury. 2021;52(11):3489–97. [DOI] [PubMed] [Google Scholar]
- 40. Godina M. Early microsurgical reconstruction of complex trauma of the extremities. Plast Reconstr Surg. 1986;78(3):285–92. [DOI] [PubMed] [Google Scholar]
- 41. Levin LS. From replantation to transplantation: the evolution of orthoplastic extremity reconstruction. J Orthop Res. 2022. [DOI] [PubMed] [Google Scholar]
- 42. Boriani F, Ul Haq A, Baldini T, Urso R, Granchi D, Baldini N, et al. Orthoplastic surgical collaboration is required to optimise the treatment of severe limb injuries: a multi‐centre, prospective cohort study. J Plast Reconstr Aesthet Surg. 2017;70(6):715–22. [DOI] [PubMed] [Google Scholar]
- 43. Kang Y, Wu Y, Ma Y, Liu J, Gu J, Zhou M, et al. "Primary free‐flap tibial open fracture reconstruction with the Masquelet technique" and internal fixation. Injury. 2020;51(12):2970–4. [DOI] [PubMed] [Google Scholar]
- 44. Aljawadi A, Islam A, Jahangir N, Niazi N, Elmajee M, Reid A, et al. One‐stage combined "fix and flap" approach for complex open Gustilo‐Anderson IIIB lower limbs fractures: a prospective review of 102 cases. Arch Orthop Trauma Surg. 2022;142(3):425–34. [DOI] [PubMed] [Google Scholar]


