Abstract
Metabolic dysfunction increases with age and is a contributing factor to Alzheimer’s disease (AD) development. We have previously observed impaired insulin sensitivity and glucose homeostasis in the APP/PS1 model of AD. To improve these parameters, we chronically exposed male and female mice to mild hypothermic environmental temperature (eT), which positively modulates metabolism. Although a hypothermic eT normalized insulin sensitivity, glucose tolerance was still impaired in both sexes of AD mice. We observed increased plasma glucagon and B-cell activating factor in both sexes, but additional sexually dimorphic mechanisms may explain the impaired glucose homeostasis in AD mice. Hepatic Glut2 was decreased in females while visceral adipose tissue TNFα was increased in male APP/PS1 mice. A mild hypothermic eT did not improve spatial learning and memory in either sex and increased amyloid plaque burden in male APP/PS1 mice. Overall, plasma markers of glucose homeostasis and AD pathology were worse in females compared to male APP/PS1 mice suggesting a faster disease progression. This could affect the therapeutic outcomes if interventional strategies are administered at the same chronological age to male and female APP/PS1 mice. Furthermore, this data suggests a dichotomy exists between mechanisms to improve metabolic function and cognitive health that may be further impaired in AD.
Keywords: Amyloid-beta, Cognitive decline, Dementia, Metabolism, Preadipocyte/adipocyte
Graphical Abstract
Graphical Abstract.
Alzheimer’s disease (AD) is an age-related neurodegenerative disorder characterized by progressive anterograde amnesia and eventual death. Despite decades of pharmaceutical development and clinical trials, current medication is symptomatic, and purported disease-modifying drugs have shown discordant results in clinical trials (1). Increasing evidence supports preventive lifestyle strategies are key to combating this greatest medical challenge of the 21st century.
Basal metabolic rate naturally declines with age and is attributed to deficits in muscle mass, vasoconstriction, glucose metabolism, insulin signaling, and thermoregulation (2). This can cause dyslipidemia, hyperglycemia, and insulin resistance, which has been observed in more than half of adults aged 70 years or older (3). Metabolic dysregulation impairs cognition (4) and contributes to AD pathogenesis (5). Observational, longitudinal, and prospective studies indicate that insulin resistance in midlife is associated with an increased risk of developing dementia and AD later in life (6). This reduced insulin sensitivity and the corresponding thermoregulatory deficits with age may precipitate the pathophysiological changes associated with AD progression (7,8).
Thermoregulation is modulated through hypothalamic insulin signaling to increase heat production by uncoupling mitochondrial respiration from adenosine triphosphate synthesis. Facultative induction of nonshivering thermogenesis improves insulin sensitivity and glucose metabolism (9,10) and likely has additional benefits on metabolic health (11). Although chronic exposure to a hypothermic environmental temperature (eT) causes adaptive responses to improve metabolism, it is also detrimental to information encoding. Mild hypothermia impairs cognitive performance in rats (12,13), nonhuman primates (14), and humans (15). Alertness and cognition are strongly correlated with body temperature, whereby maximal performance is observed at higher temperatures (16). Additionally, working, short-term, and long-term memory performance is decreased in humans during endogenous periods of lower body temperature (16–18). Thus, the beneficial metabolic effects of cold induced thermogenesis may also be detrimental to cognitive health.
In vitro, amyloid fibril formation and tau hyperphosphorylation (the pathological hallmarks associated with AD) are accelerated in lower temperatures (19,20), suggesting a lower eT may accelerate disease progression. This effect has been observed in the 3xTg model of AD when exposed to a hypothermic eT (21,22). We sought to elucidate the impact of eT reduction on glucose homeostasis as well as learning and memory recall in the APP/PS1 mouse model of progressive cerebral amyloidosis. Double transgenic APP/PS1 mice express a chimeric mouse/human amyloid precursor protein (Swe695) and a mutant human presenilin 1 (PS1) lacking exon 9 (ΔE9). These mutations overexpress APP with preferential cleavage of amyloid-β (Aβ)42 isoforms (23). Amyloid accumulation and subtle cognitive impairments are observed at 6 months of age and become prominent by 12 months (24). We have previously shown APP/PS1 mice develop impairments to insulin sensitivity and glucose homeostasis that contribute to their cognitive deficits (25). Starting at 6 months of age APP/PS1 and C57BL/6 littermate control mice were chronically housed in a 16°C controlled eT for 6 months. Separate cohorts of male and female APP/PS1 mice were maintained at ambient (23°C) conditions to examine temperature effects in AD mice. Translationally, this time frame corresponds to conversion from mild cognitive impairment to AD, allowing us to determine the effects of mild hypothermia on metabolism as well as spatial learning and memory recall during disease progression.
Method
Animals
Protocols for animal use were approved by the Institutional Animal Care and Use Committee at Southern Illinois University School of Medicine and in accordance with the ARRIVE guidelines. Male and female APP/PS1 and littermate control C57BL/6 mice used for this study were maintained in our animal colony and originated from founder C57BL/6J (RRID:IMSR_JAX:000664) and APP/PS1 (RRID:MMRRC_034832-JAX) mice from Jackson Laboratory (Bar Harbor, ME). A 5 mm tail snip was sent to TransnetYX®, Inc (Cordova, TN) to confirm genotypes. Mice were group-housed according to sex and genotype on a 12:12 hour light:dark cycle, and food and water were available ad libitum. One week post cognitive assessment, mice were deeply anesthetized with isoflurane and a cardiac puncture for blood chemistry analysis was performed. Immediately following, mice were euthanized by decapitation. Tissue was extracted and stored at −80°C until processing.
Chemicals
All chemicals were prepared and stored according to manufacturer recommendations unless otherwise noted.
Environmental Conditions
From 6 to 12 months of age, mouse cages were moved into an environmental chamber (Powers Scientific Cat: RIS33SD) maintained at 16 ± 1°C. Mice were only removed from this chamber for cage cleanings and to measure body weight (bw) and food consumption. A separate cohort of male and female age-matched APP/PS1 mice were kept at ambient room temperature (23 ± 1°C) and underwent the same testing paradigm.
Mouse Weight and Food Consumption
BW and food consumption were monitored weekly during the final month of chronic mild hypothermia exposure (11–12 months of age).
Intraperitoneal (ip) Insulin Tolerance Test (ITT) and Glucose Tolerance Test (GTT)
To determine insulin sensitivity, an initial blood glucose measurement (time = 0) was taken from the tail vein of 4 hours fasted mice and measured using a Presto® glucometer (AgaMatrix, Salem, NH) followed by ip injection of 1 IU/kg bw Humulin® R (Henry Schein, Melville, NY: Cat: 1238578). To determine glucose tolerance, an initial blood glucose measurement was taken (time = 0) from 15-hour fasted mice followed by an ip injection of 2 g of dextrose/kg bw (Fisher Scientific Cat: D15). Following either injection, blood glucose levels were measured sequentially at 15, 30, 45, 60, and 120 minutes (25).
Morris Water Maze (MWM) Training and Probe Challenge
At approximately 12 months of age, mice underwent cognitive assessment using the MWM spatial learning and memory recall paradigm. The MWM tests spatial learning and memory by requiring the mouse to utilize visual cues to repeatedly swim to a static, submerged hidden platform (26). The MWM paradigm consisted of 5 consecutive learning days with 3, 90-second trials/day and a minimum intertrial interval of 20 minutes. After 2 days without testing, the platform was removed, and mice were given a single, 60-second probe challenge to test long-term memory recall. The ANY-maze video tracking system (Stoelting Co., Wood Dale, IL; RRID:SCR_014289) was used to record navigational parameters and data analysis. The 3 trials for each training day were averaged for each mouse for analysis. Variables extracted from ANY-maze and utilized for data analysis include platform latency, swimming speed, platform crosses and occupancy, annulus 40 crosses and occupancy, and quadrant occupancy.
RT-PCR
RNA was extracted from the tissue by homogenization in Trizol Reagent and separated by centrifugation at 12 000 × g for 15 min at 4°C with chloroform. Next, RNA was isolated by centrifugation at 12 000 × g for 15 min at 4°C in 100% isopropanol. A final wash step was performed using 75% EtOH with centrifugation at 7 500 × g for 5 min. The pellet was resuspended in RNAse free water and quantified using a NanoDrop Spectrophotometer. cDNA was synthesized using candidate primers (Integrated DNA Technologies; Coralville, IA; Table 1) and an iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Relative mRNA expression was analyzed by quantitative RT-PCR as previously described using the QuantStudio PCR System (Applied Biosystems, Waltham, MA) and SYBR Green MasterMix (Bio-Rad). Beta-2-microglobulin (B2M) was used as the internal housekeeping gene.
Table 1.
A List of Forward and Reverse mRNA Primers
| Gene | Forward | Reverse |
|---|---|---|
| ADIPOQ | 5′-TGTTCCTCTTAATCCTGCCCA-3′ | 5′-CCAACCTGCACAAGTTCCCTT-3′ |
| AKT1, Protein kinase B | 5′-ATGAACGACGTAGCCATTGTG-3′ | 5′-TTGTAGCCAATAAAGGTGCCA-3′ |
| B2M | 5′-AAGTATACTCACGCCACCCA-3′ | 5′-AGGACCAGTCCTTGCTGAAG-3′ |
| G6PC | 5′-CACAGTGGACGACATCCGAAA-3′ | 5′-AGCTACATAGGAATTACGGGCAA-3′ |
| GLK, Glucokinase | 5′-TGAGCCGGATGCAGAAGG-3′ | 5′-GCAACATCTTTACACTGGCCT-3′ |
| GLUT2 | 5′-TCAGAAGACAAGATCACCGGA-3′ | 5′-GCTGGTGTGACTGTAAGTGGG-3′ |
| IL6 | 5′-CTGCAAGAGACTTCCATCCAG-3′ | 5′-AGTGGTATAGACAGGTCTGTTGG-3′ |
| INSR | 5′-CCTGGTTATCTTCGAGATGGTCC-3′ | 5′-CCCCACATTCCTCGTTGTCA-3′ |
| PI3K | 5′-TAGCTGCATTGGAGCTCCTT-3′ | 5′-TACGAACTGTGGGAGCAGAT-3′ |
| PPARGC1A, Peroxisome proliferator-activated receptor gamma coactivator 1-alpha | 5′-TATGGAGTGACATAGAGTGTGCT-3′ | 5′-GTCGCTACACCACTTCAATCC-3′ |
| TNFA | 5′-CAGGCGGTGCCTATGTCTC-3′ | 5′-CGATCACCCCGAAGTTCAGTAG-3′ |
| UCP1 | 5′-AGGCTTCCAGTACCATTAGGT-3′ | 5′-CTGAGTGAGGCAAAGCTGATTT-3′ |
Notes: ADIPOQ = adiponectin; B2M = beta-2-microglobulin; G6PC = glucose-6-phosphatase; GLUT2 = glucose transporter 2; IL6 = interleukin 6; InsR = insulin receptor; PI3K = phosphatidylinositol 3-kinase; TNFA = tumor necrosis factor α; UCP1 = uncoupling protein 1.
Soluble Aβ42 Determination
The hippocampus from one hemisphere was dissected and stored at −80°C until tissue processing. Soluble Aβ 42 concentrations were determined using the Human/Rat β amyloid ELISA kits (WAKO Chemicals; Cat: 292-64501) according to the manufacturer recommended protocols.
Amyloid Plaque Staining and Semiquantification
A hemibrain was fixed in 4% paraformaldehyde for 24–48 hours, then transferred into 30% sucrose in 0.1M phosphate buffer for at least 24 hours prior to sectioning. Twenty micron coronal sections through the hippocampus were obtained using a cryostat (Model HM525 NX, Thermo Fisher Scientific, Waltham, MA). Every sixth serial section was stained for plaques using Amylo-Glo RTD with EtBr as a nucleic acid-specific fluorescing counter stain (1:100; Biosensis, Temecula, CA; Cat: TR-400) according to the manufacturer-recommended protocols. Stained slices were mounted and coverslipped using ProLong Gold Antifade Mountant (Thermo Fisher Scientific; Cat# P36930) (25). Imaging and quantification were conducted using a BZX-810 fluorescent microscope (Keyence Corp., Itasca, IL) with a UV filter. The accompanying software was used to manually outline the entire hippocampus and automatically determine the number of plaques per hippocampal area (mm2) and plaque burden as a percentage of total plaque area in relation to the hippocampal area. Four hippocampal slices per mouse were averaged to obtain a single value for each subject. Amyloid plaques were identified by a dense spherical core of intense staining surrounded by a less compact spherical halo.
Blood Chemistry
A cardiac puncture was used to collect blood in EDTA-coated tubes (Sarstedt Inc., Newton, NC; Microvette CB 300) on wet ice until centrifugation at 1 500 × g for 10 min at 4°C. The plasma supernatant was collected and stored at −80°C until analysis with a multiplex assay kit (Meso Scale Discovery, Rockville, MD) according to the manufacturer’s recommended protocols.
Statistical Analysis
A power calculation for a 2-way ANOVA measures statistical design (genotype × sex) was calculated with an average effect size of 0.35 using previously published data (25). Prism software (GraphPad Software, Inc., La Jolla, CA; RRID:SCR_002798) was used for all statistical analyses. A 2-way ANOVA was used to test for the significance of sex, genotype, and their interactions. Temperature treatment differences between APP/PS1 mice within the same sex were determined using a 2-tailed Student’s t test. Potential outliers were determined with a single Grubb’s test (α = 0.05). Data are represented as mean ± standard error of the mean (SEM), and significance was defined as p < .05.
Results
Body Weight and Food Consumption
Weekly bw and food consumption (Supplementary Figure 1) was averaged at 11–12 months of age. BW was similar between genotypes within a sex, but female mice weighed less than genotype-matched males. Both sexes of APP/PS1 mice housed at 16°C had reduced bw compared to those kept at 23°C, which was likely a result of increased nonshivering thermogenesis to maintain body temperature. Surprisingly, food consumption was markedly reduced in female APP/PS1 mice, particularly when housed at ambient eT. No food consumption differences were observed in male mice nor between sexes of the same genotype.
Blood Glucose Levels in APP/PS1 Mice After Chronic Hypothermia
We have previously demonstrated 12-month-old male APP/PS1 mice are less insulin sensitive and glucose tolerant compared to age-matched C57BL/6 mice (25). Exposure to hypothermic conditions improves insulin sensitivity and increases glucose uptake into tissue (9). After 6 months of chronic 16°C exposure, the peripheral blood glucose response to an ip injection of insulin was improved in both sexes of APP/PS1 mice resulting in a similar profile compared with temperature- and sex-matched C57BL/6 mice (Figure 1A and B). The area under the curve (AUC) of blood glucose concentration was similar between sexes of C57BL/6, but decreased in female APP/PS1 mice (Figure 1C), indicating improved sensitivity to insulin. Fed blood glucose levels were similar across the sexes of mice (Figure 1D). Although chronic exposure to a 16°C eT normalized insulin sensitivity in APP/PS1 mice to that of temperature-matched C57BL/6 littermates, this was not true for glucose tolerance. Glucose tolerance was impaired in both male and female APP/PS1 compared to sex-matched littermate C57BL/6 mice (Figure 1E and F). The blood glucose AUC for the GTT (Figure 1G) as well as fasting blood glucose levels (Figure 1H) were increased in female APP/PS1 mice compared to littermate controls housed at 16°C. Similar blood glucose responses were observed between APP/PS1 mice housed at different eT. For C57BL/6 mice, females showed better glucose tolerance after chronic mild hypothermia. Overall, chronic exposure to 16°C in APP/PS1 mice improved insulin sensitivity, but not glucose tolerance, particularly in females.
Figure 1.
Percent change of blood glucose levels after an ip injection of 1 IU/kg bw of insulin at t = 0 minutes in male (A) and female (B) mice. The AUC was calculated for the duration of the ITT and compared for genotype and sex effects (C). Fed blood glucose levels (D) were determined prior to ip injection of insulin. Blood glucose levels after an ip injection of 2 g/kg bw of glucose at t = 0 minutes in male and female APP/PS1 and littermate C57BL/6 mice (E and F). The AUC was calculated for the duration of the GTT and compared for genotype and sex effects (G). Fasted blood glucose levels (H) were determined prior to ip injection of glucose. The 2-way ANOVA factor analysis is reported above each graph and the number of animals is inset on the bar graphs. *p < .05, **p < .01; male genotype comparisons are indicated with a blue asterisk while purple is used for females. #p < .05 for temperature differences between female APP/PS1 mice. §§p < .01, §§§§p < .0001 for sex differences within C57BL/6 mice and ‡p < .05 for sex differences within APP/PS1 mice. Ip = intraperitoneal; bw = body weight; AUC = area under the curve; ITT = insulin tolerance test; GTT = glucose tolerance test; ANOVA = analysis of variance.
Plasma Analysis of Peptides Associated With Glucose Regulation
Circulating plasma levels of hormones and peptides associated with glucose metabolism were measured by multiplex analysis. Glucagon is released by the pancreas to promote liver glycogenolysis, thereby increasing circulating glucose levels. Hyperglucagonemia was observed in both sexes of APP/PS1 mice housed at ambient eT that was not improved after chronic exposure to 16°C. Higher glucagon levels were observed in females regardless of genotype (Figure 2A). Glucagon-like peptide 1 (GLP1) is released by the gastrointestinal tract to reduce circulating glucose levels by promoting satiety, suppressing glucagon secretion, and stimulating insulin release. Despite the hyperglucagonemic profile of female APP/PS1 mice, their GLP1 plasma levels were also elevated, and eT had no effect. (Figure 2B). An effect of temperature was seen with male APP/PS1 mice. Those housed at 16°C eT had lower plasma GLP1 levels that may support their elevated plasma glucagon (Figure 2A and B). Plasma insulin was similar in male mice, but exposure to 16°C eT increased levels in female APP/PS1 mice compared to those housed at ambient eT. B-cell activating factor (BAFF) is a member of the tumor necrosis factor (TNF) ligand family that regulates adipose tissue inflammation and induces insulin resistance (27). Similar to glucagon, BAFF plasma levels were increased in females regardless of genotype (Figure 2D). Chronic exposure to mild hypothermia increased BAFF plasma levels in both sexes of APP/PS1 mice compared to temperature-matched C57BL/6 and APP/PS1 mice housed at ambient eT. Fibroblast growth factor 21 (FGF21) regulates metabolism and is important for the thermogenic recruitment of white adipose tissue (WAT) during cold exposure. A 16°C eT decreased female APP/PS1 plasma FGF21 levels compared to those housed at ambient conditions, while similar values were observed in male mice (Figure 2E). Six months of chronic cold eT altered the plasma profile of several peptides in a manner that would contribute to the impaired glucose tolerance of APP/PS1 mice.
Figure 2.
Plasma expression levels of glucagon (A), glucagon-like peptide 1 (GLP1; B), insulin (C), B-cell activating factor (BAFF, D), and fibroblast growth factor 21 (FGF21, E) are shown for both sexes of C57BL/6 and APP/PS1 mice. The 2-way ANOVA factor analysis is reported above each graph and the number of animals is inset on the bar graphs. *p < .05, **p < .01, ***p < .001; male genotype comparisons are indicated with a blue asterisk while purple is used for females. #p < .05, ###p < .001; APP/PS1 male temperature comparisons are indicated with a blue hashtag while purple is used for female. §p < .05, §§p < .01, ‡p < .05, ‡‡p < .01 for sex differences between C57BL/6 mice and APP/PS1 mice, respectively. ANOVA = analysis of variance.
Hepatic Glucose Metabolizing Genes
The liver is responsible for maintaining a readily available supply of energy in the form of glucose and glycogen. Insulin receptor (InsR) signaling through phosphatidylinositol 3-kinase (PI3K)/Akt pathways mediates the translocation of glucose transporters (Glut) to the membrane surface, thereby increasing glucose uptake. Once taken up into the cell, glucose is phosphorylated by glucokinase (Gck) to glucose-6-phosphate for use in either glycolysis or glycogenesis. Glucose-6-phosphatase (G6PC) hydrolyzes glucose-6-phosphate back to glucose. Chronic cold exposure mostly altered hepatic transporter expression levels, but in a sexually dimorphic manner. InsR was increased in both temperature cohorts of male APP/PS1 mice, but no change to downstream signaling was observed (Supplementary Figure 2A–C). While APP/PS1 female mice housed at 16°C had similar insulin signaling gene expression levels, GLUT2 was decreased compared to C57BL/6 littermate controls (Supplementary Figure 2D). Changes to hepatic glucose metabolizing genes were not seen for either sex (Supplementary Figure 2E and F). Considering Glut2 acts as a hepatic glucose sensor, the decreased GLUT2 mRNA expression in APP/PS1 female mice may impair their glucose tolerance and cause elevated fasting blood glucose levels (Figure 1F–H). Temperature effects between APP/PS1 cohorts were only observed in male mice where G6PC expression was reduced after chronic exposure to 16°C eT, suggesting the majority of the hepatic mRNA expression resulted from AD phenotype rather than eT.
Visceral Adipose Tissue (VAT) mRNA Levels
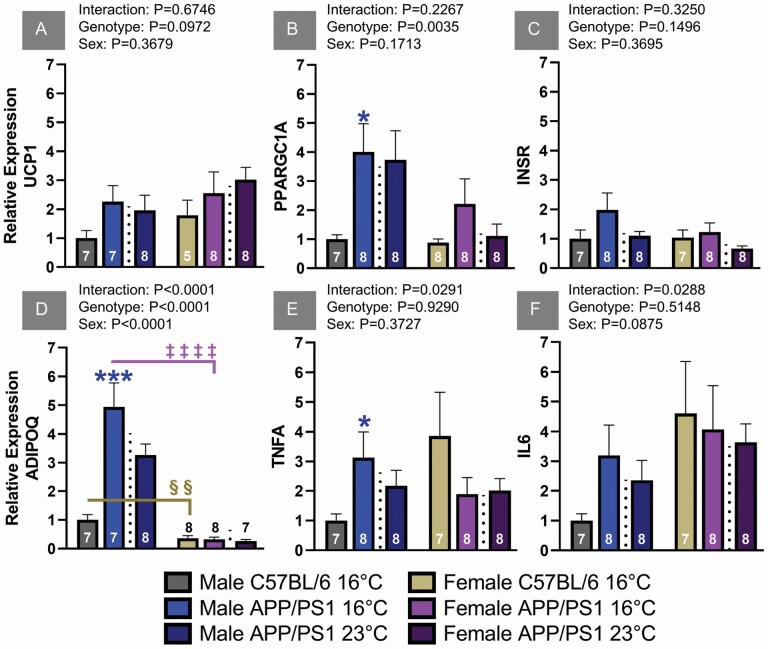
VAT accumulation is associated with metabolic dysregulation and insulin resistance mediated through cytokine and adipokine release. Cold exposure is known to activate thermogenic responses in VAT that reduce proinflammatory cytokines and improves whole body metabolism. Uncoupling protein (UCP) 1 is a mitochondrial protein present in brown and beige adipose tissue responsible for generating heat during cold exposure. VAT UCP1 mRNA was similar between genotypes for both sexes of mice, indicating similar cold acclimation responses (Figure 3A). Peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1α is a cold-inducible regulator of mitochondria that leads to the browning of WAT through FGF21 signaling. Although PGC-1α mRNA expression was increased in male APP/PS1 mice exposed to 16°C, these levels were similar to littermates housed at ambient eT (Figure 3B). This suggests AD phenotype rather than temperature is responsible for this increased expression. While hepatic InsR expression was increased in male APP/PS1 mice (Supplementary Figure 2A), the observed expression levels in VAT were similar between sex, genotype, and treatment (Figure 3C). Adiponectin (ADIPOQ) has insulin-sensitizing effects and positively regulates glucose metabolism. ADIPOQ expression was elevated in both housing conditions of male APP/PS1 mice and was higher compared to females regardless of genotype (Figure 3D). Female mice had similar expression levels across housing conditions and genotypes. The 16°C APP/PS1 male cohort did have the largest ADIPOQ expression, which may have played a role in their improved insulin sensitivity (Figure 1A). Proinflammatory cytokines are known to systemically impair glucose homeostasis. TNFα and interleukin (IL)-6 mRNA expression were elevated in APP/PS1 male mice independent of temperature (Figure 3E and F) and despite elevated ADIPOQ levels. Inflammatory markers in females were similar regardless of genotype or eT. Overall, chronic cold exposure did not improve thermogenic, metabolic, or inflammatory VAT markers in either sex of APP/PS1 mice compared to their ambient eT controls.
Figure 3.
Visceral adipose tissue mRNA relative expression levels of uncoupling protein 1 (UCP1; A), PGC-1α (PPARGC1A; B), insulin receptor (InsR; C), adiponectin (ADIPOQ; D), tumor necrosis factor α (TNFA; E), and interleukin 6 (IL6; F) relative to B2M. The 2-way ANOVA factor analysis is reported above each graph and the number of animals is inset on the bar graphs. Gene names are used to label the ordinate. *p < .05, ***p < .001; male genotype comparisons are indicated with a blue asterisk. §§p < .01, ‡‡‡‡p < .0001 for sex differences between C57BL/6 and APP/PS1 mice, respectively. ANOVA = analysis of variance.
Spatial Learning and Memory Recall in APP/PS1 Mice
Learning and memory recall deficits in APP/PS1 mice are routinely observed by 12 months of age (24,25). While a mild hypothermic eT is known to improve metabolic function, this may not benefit memory formation. At 12 months of age, spatial learning and memory were assessed using the MWM. During the 5 training sessions, male and female C57BL/6 mice took less time to locate the hidden escape platform compared to sex-matched APP/PS1 mice housed at 16°C (Figure 4A and B). The AUC for the training sessions indicated 6 months of mild hypothermia-impaired learning in both sexes of APP/PS1 mice (Figure 4C). The learning profiles between APP/PS1 mice housed at different eT showed similar deficits (Figure 4A–C). Swimming speed was equivalent among all groups of mice during the delayed probe challenge, indicating this factor does not account for navigational differences (Figure 4D). The number of crosses and the percentage of time spent searching the former location of the escape platform was reduced in both sexes of APP/PS1 mice (Figure 4E and F). When expanding the spatial search criteria to an area slightly larger than the former escape platform, the number of crosses and the percentage of time in the annulus 40 was also reduced in APP/PS1 mice (Figure 4G and H). Additionally, APP/PS1 mice spent less time in the target quadrant (Figure 4I). Analogous to learning, memory recall impairments were similar between APP/PS1 mice regardless of eT. Learning and memory recall was similar between sex-matched genotypes. Chronic exposure to mild hypothermic conditions does not ameliorate spatial learning and memory deficits in either sex of APP/PS1 mice.
Figure 4.
Average latency to find the submerged platform over the 5 training sessions for APP/PS1 and C57BL/6 littermate control male (A) and female (B) mice. The platform latency AUC was calculated for the 5 training sessions and compared for genotype and sex effects (C). Parameters for the 60 second probe challenge are shown in Figures D–I. The 2-way ANOVA factor analysis is reported above each graph and the number of animals is inset on the bar graphs. *p < .05, **p < .01, ***p < .001; male comparisons are indicated with a blue asterisk while purple is used for females. AUC = area under the curve; ANOVA = analysis of variance.
Chronic Hypothermic Conditions Did Not Reduce Hippocampal Plaque Burden
To determine the effects of eT on insoluble amyloid deposition, immunofluorescent imaging was used. Representative images are shown in Figure 5A, where plaques fluoresced a dense blue, and the hippocampal cytoarchitecture was outlined in red. No plaques were observed in either sex of C57BL/6 mice (top), so APP/PS1 mice exposed to either 16°C (middle) or 23°C (bottom) eT were directly compared. Female APP/PS1 mice had a higher number of plaques and a larger plaque burden compared to temperature-matched males (Figure 5B and C). A mild hypothermic eT had no effect on plaques in females but increased both measures in male APP/PS1 mice compared with sex-matched littermates housed at ambient eT (Figure 5B and C). Soluble Aβ 42 is considered the neurotoxic species associated with cognitive dysfunction in AD and is prevalent in APP/PS1 mice due to transgene insertion. Hippocampal soluble Aβ 42 levels were similar within sexes between APP/PS1 mice exposed to either eT (Figure 5D). Chronic housing in mild hypothermic conditions did not reduce soluble or insoluble Aβ 42.
Figure 5.
Immunoflourescent staining (A) was used to determine insoluble hippocampal amyloid plaques (blue) in APP/PS1 mice (scale bars = 200 µm). The number of plaques per mm2 and the plaque burden for APP/PS1 are shown in B and C because plaques were not present in either sex of C57BL/6 mice. An ELISA was used to measure soluble hippocampal Aβ 42 concentrations. C57BL/6 mice are shown as a negative control with a segmented ordinate. The 2-way ANOVA factor analysis is reported above the graph while the bar graph insets refer to the number of animals. ‡p < .05, ‡‡p < .01, ‡‡‡p < .001 for sex differences across similar eT and ##p < .01; APP/PS1 male temperature comparisons are indicated with a blue hashtag. eT = environmental temperature; ANOVA = analysis of variance.
Discussion
Exposure to eT lower than the thermoneutral zone increases metabolic expenditure for heat production in order to maintain body temperature. Chronic exposure to colder eT causes adaptive responses, including body temperature as a potential survival mechanism limiting metabolic demands during periods of food scarcity (28,29). Some of these mechanisms include neuroendocrine responses known to improve insulin sensitivity and glucose homeostasis in both mice (30,31) and humans (9). Considering the evidence that metabolic dysregulation impairs cognition (4) and contributes to AD pathogenesis (5), we wanted to determine if exposure to a mild hypothermic environment would improve the insulin sensitivity and glucose homeostasis of APP/PS1 mice (25). The age of the mice for this study was chosen based on the pathology and cognitive aspects of disease progression. Amyloid accumulation and subtle cognitive impairments are observed at 6 months of age and rapidly progress to 12 months in APP/PS1 mice (24). This time frame translationally corresponds to mild cognitive impairment, which is the opportune window to initiate disease-modifying treatment to prevent conversion to AD. However, in our present study, a mild hypothermic environment did not improve glucose homeostasis, reduce soluble and insoluble Aβ 42, or delay cognitive decline compared to that of age and sex-matched littermate controls. Sexually dimorphic responses were detected with greater effects on blood glucose, plasma hormones, and plaque accumulation in female APP/PS1 mice compared to males.
Six months of chronic mild hypothermic conditions improved insulin sensitivity and plasma insulin levels to that of temperature-matched controls in both sexes of AD mice. However, AD mice were less glucose tolerant, and females had elevated fasted blood glucose levels despite consuming less food. This suggests other metabolic factors were contributing to the hyperglycemia. Plasma glucagon, a counterregulatory hormone responsible for glucose release from tissues, was elevated in both sexes of APP/PS1 mice, particularly in females. GLP1 decreases blood glucose levels by stimulating insulin release and inhibiting glucagon secretion. GLP1 was increased in both male and female AD mice without a concomitant change in plasma insulin concentration. This suggests GLP1 was elevated as a potential compensatory mechanism to inhibit glucagon secretion and normalize peripheral blood glucose levels. BAFF is a TNF cytokine family member that regulates the proliferation and differentiation of B cells for innate immune responses. Increased BAFF contributes to WAT inflammation and hyperglycemia (27) and attenuates thermogenic responses to cold exposure (32). The elevated plasma BAFF concentration in APP/PS1 mice housed at 16°C possibly contributed to the male VAT inflammation and prevented UCP1 expression despite elevated PGC-1α after cold exposure in APP/PS1 mice.
Glucose absorption, storage, and production are tightly controlled through various liver metabolic pathways to regulate energy substrates for use in other tissues, including skeletal muscle and WAT. Changes to liver genes in AD mice were associated with transporters rather than glucose metabolizing genes. Both cohorts of male APP/PS1 mice had increased hepatic InsR mRNA expression, however, housing at 16°C improved insulin sensitivity without a change in plasma insulin concentrations. This suggests additional mechanisms may have been responsible such as increased VAT ADIPOQ expression. Both cohorts of female APP/PS1 mice had reduced hepatic Glut2 and similar InsR expression. InsR interacts with Glut2 to regulate bidirectional glucose transport across hepatocytes. The reduced hepatic Glut2 transporter expression coupled with hypergluconemia in female APP/PS1 mice suggests efflux rather than an influx of hepatic glucose. Overall, this would contribute to the glucose intolerance and higher fasting blood glucose observed in female APP/PS1 mice after mild hypothermic eT.
Exposure to colder eT generates hyperthermic responses mediated through sympathetic excitation of brown and beige adipose tissue to induce nonshivering thermogenesis. This improves the metabolic function of WAT through a browning process that reduces proinflammatory cytokines and positively regulates successful aging (11). Thermogenic effects of adipose tissue are mediated by FGF21, UCP1, and PGC-1α. UCP1 VAT expression was similar in all groups of mice studied. PGC-1α expression was increased in male APP/PS1 regardless of eT, suggesting this was influenced by AD phenotype. Considering cold-induced thermogenesis is attenuated by BAFF (32), its increased plasma concentration in all APP/PS1 mice would diminish the thermogenic response.
Effective AD treatments are contingent upon delaying or ameliorating cognitive decline. Reducing eT improves metabolism through insulin signaling and glucose homeostasis, which also provide procognitive benefits. In our present study, exposure to a cooler eT in both sexes normalized insulin sensitivity between genotypes, but this did not improve spatial learning and memory. This is particularly evident in female APP/PS1 mice that had better insulin sensitivity, but worse performance on both the learning and memory recall aspects of the MWM compared to males. The impaired spatial learning of female APP/PS1 mice also affected their memory recall on the probe challenge. Considering insoluble plaques and soluble Aβ 42 levels were elevated in APP/PS1 females, this may suggest disease progression is more advanced compared to males of the same chronological age. The role temperature plays in memory encoding may also contribute to the impaired spatial navigation of female APP/PS1 mice. Exposure to colder eT or a reduction in body temperature are known to impair cognitive performance in mammals (12–15). Reducing food consumption lowers body temperature, and the decreased feeding seen in APP/PS1 females could cause a double hit that negatively affected cognitive performance. A paradoxical relationship of eT may exist between metabolic performance and cognitive health, particularly during the progression of AD. The thermoregulatory decline observed in natural aging is thought to be a compensatory mechanism to increase survival through metabolic efficiency (33,34). However, this may also cause the natural cognitive deterioration observed with aging and could be a compounding risk factor for dementia (7).
Part of this increased dementia risk may be attributed to accelerated amyloid fibril formation and tau phosphorylation observed at lower temperatures in vitro (20,35). This has also been observed experimentally in 3xTg AD mice that had increased tau phosphorylation and soluble Aβ 42 after 24-hour exposure to a 4°C environment (21,22). Our present study shows exposing APP/PS1 mice to 16°C for 6 months did not alter soluble hippocampal Aβ 42 levels, but amyloid plaques were increased in males compared to age-matched APP/PS1 mice maintained at 23°C eT. We also observed greater amyloid plaque accumulation in female APP/PS1 mice. B-cell activation increases AD pathology (36), so the elevated plasma BAFF concentration in female mice may have caused more hippocampal plaque deposition. Despite the increased plaque accumulation, spatial learning and memory deficits were similar between eT cohorts of APP/PS1 male mice. Female APP/PS1 mice had more hippocampal soluble Aβ 42 and plaque burden independent of eT that would account for their slower learning curve compared to males.
Conclusion
Metabolic dysfunction increases with age and is a contributing factor to AD development. While exposure to a colder eT improves metabolism, this also impairs cognitive performance. Although chronic exposure to mild hypothermia positively modulated peripheral insulin sensitivity, this did not lead to a concomitant improvement in glucose tolerance or spatial learning and memory recall in APP/PS1 mice. Sexually dimorphic responses were observed whereby plasma markers of glucose homeostasis and AD pathology were worse in females compared to males suggesting faster disease progression. This has potential implications for sex-specific therapeutic outcomes when interventions are administered at the same chronological age. Finally, a dichotomy exists between mechanisms to improve metabolic function and cognitive health that may be enhanced in AD. Considering that age-related thermogenic decline may drive AD progression, future studies will examine possible beneficial effects of chronic exposure to thermoneutral eT.
Supplementary Material
Acknowledgments
We would like to thank Melissa Roberts for conducting plasma multiplex analysis.
Contributor Information
Samuel McFadden, Department of Neurology, Dale and Deborah Smith Center for Alzheimer’s Research and Treatment, Neurosciences Institute, Southern Illinois University School of Medicine, Springfield, Illinois, USA.
Lindsey N Sime, Department of Neurology, Dale and Deborah Smith Center for Alzheimer’s Research and Treatment, Neurosciences Institute, Southern Illinois University School of Medicine, Springfield, Illinois, USA.
MaKayla F Cox, Department of Neurology, Dale and Deborah Smith Center for Alzheimer’s Research and Treatment, Neurosciences Institute, Southern Illinois University School of Medicine, Springfield, Illinois, USA.
Caleigh A Findley, Department of Neurology, Dale and Deborah Smith Center for Alzheimer’s Research and Treatment, Neurosciences Institute, Southern Illinois University School of Medicine, Springfield, Illinois, USA; Department of Pharmacology, Southern Illinois University School of Medicine, Springfield, Illinois, USA.
Mackenzie R Peck, Department of Neurology, Dale and Deborah Smith Center for Alzheimer’s Research and Treatment, Neurosciences Institute, Southern Illinois University School of Medicine, Springfield, Illinois, USA.
Kathleen Quinn, Department of Neurology, Dale and Deborah Smith Center for Alzheimer’s Research and Treatment, Neurosciences Institute, Southern Illinois University School of Medicine, Springfield, Illinois, USA.
Yimin Fang, Department of Neurology, Dale and Deborah Smith Center for Alzheimer’s Research and Treatment, Neurosciences Institute, Southern Illinois University School of Medicine, Springfield, Illinois, USA.
Andrzej Bartke, Department of Internal Medicine, Southern Illinois University School of Medicine, Springfield, Illinois, USA; Department of Medical Microbiology, Immunology and Cell Biology, Southern Illinois University School of Medicine, Springfield, Illinois, USA.
Erin R Hascup, Department of Neurology, Dale and Deborah Smith Center for Alzheimer’s Research and Treatment, Neurosciences Institute, Southern Illinois University School of Medicine, Springfield, Illinois, USA; Department of Pharmacology, Southern Illinois University School of Medicine, Springfield, Illinois, USA.
Kevin N Hascup, Department of Neurology, Dale and Deborah Smith Center for Alzheimer’s Research and Treatment, Neurosciences Institute, Southern Illinois University School of Medicine, Springfield, Illinois, USA; Department of Pharmacology, Southern Illinois University School of Medicine, Springfield, Illinois, USA.
Funding
This work was supported by the National Institutes of Health (NIA R01AG057767 and NIA R01AG061937), Dale and Deborah Smith Center for Alzheimer’s Research and Treatment, Kenneth Stark Endowment (S.M., L.N.S., M.F.C., C.A.F., M.R.P., K.Q., Y.F., E.R.H., and K.N.H.), Illinois Department of Public Health (03282005H; K.N.H.), Illinois Health Improvement Association (K.N.H.), and NIA R21-AG062985 (A.B.).
Conflict of Interest
None declared.
Author Contributions
S.M., L.N.S., M.F.C., C.A.F., M.R.P., K.Q., and Y.F. assisted with colony maintenance and experimental assays. A.B. and E.R.H. assisted with experimental design and manuscript revision. K.N.H. conceived the study, supervised the experiments, analyzed the data, and wrote the manuscript. All authors approved the final version of the manuscript.
Data Availability
All data is available upon reasonable request.
References
- 1. Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimer’s Dis. 2022;9:197–210. doi: 10.14283/jpad.2022.30 [DOI] [PubMed] [Google Scholar]
- 2. Basu R, Breda E, Oberg AL, et al. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003;52:1738–1748. doi: 10.2337/diabetes.52.7.1738 [DOI] [PubMed] [Google Scholar]
- 3. Moore JX, Chaudhary N, Akinyemiju T. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, national health and nutrition examination survey, 1988–2012. Prev Chronic Dis. 2017;14:1–16. doi: 10.5888/pcd14.160287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neergaard JS, Dragsbæk K, Christiansen C, et al. Metabolic syndrome, insulin resistance, and cognitive dysfunction: does your metabolic profile affect your brain? Diabetes. 2017;66:1957–1963. doi: 10.2337/db16-1444 [DOI] [PubMed] [Google Scholar]
- 5. Hascup ER, Hascup KN. Toward refining Alzheimer’s disease into overlapping subgroups. Alzheimer’s Dement Transl Res Clin Interv. 2020;6:1–10. doi: 10.1002/TRC2.12070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimer’s Dement. 2015;11:718–726:1-10. doi: 10.1016/J.JALZ.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 7. Whittington R A, Papon M-A, Chouinard-Decorte F, Planel E. Hypothermia and Alzheimers disease neuropathogenic pathways. Curr Alzheimer Res. 2010;7:717–725. doi: 10.2174/156720510793611646 [DOI] [PubMed] [Google Scholar]
- 8. Holtzman A, Simon EW. Body temperature as a risk factor for Alzheimer’s disease. Med Hypotheses. 2000;55:440–444. doi: 10.1054/mehy.2000.1085 [DOI] [PubMed] [Google Scholar]
- 9. Hanssen MJW, Hoeks J, Brans B, et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med. 2015;21:863–865. doi: 10.1038/nm.3891 [DOI] [PubMed] [Google Scholar]
- 10. Lee P, Smith S, Linderman J, et al. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes. 2014;63:3686–3698. doi: 10.2337/db14-0513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bartke A, Brannan S, Hascup E, Hascup K, Darcy J. Energy metabolism and aging. World J Mens Health. 2020;38:222–232. doi: 10.5534/WJMH.200112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rauch TM, Welch DI, Gallego L. Hypothermia impairs performance in the Morris water maze. Physiol Behav. 1989;46:315–320. doi: 10.1016/0031-9384(89)90273-4 [DOI] [PubMed] [Google Scholar]
- 13. Panakhova E, Burešová O, Bure J. The effect of hypothermia on the rat’s spatial memory in the water tank task. Behav Neural Biol. 1984;42:191–196. doi: 10.1016/S0163-1047(84)91059-8 [DOI] [PubMed] [Google Scholar]
- 14. Bauer RH, Fuster JM. Delayed matching and delayed response deficit from cooling dorsolateral prefrontal cortex in monkeys. J Comp Physiol Psychol. 1976;90:293–302. doi: 10.1037/h0087996 [DOI] [PubMed] [Google Scholar]
- 15. Coleshaw SRK, van Someren RNM, Wolff AH, Davis HM, Keatinge WR. Impaired memory registration and speed of reasoning caused by low body temperature. J Appl Physiol Respir Environ Exerc Physiol. 1983;55:27–31. doi: 10.1152/jappl.1983.55.1.27 [DOI] [PubMed] [Google Scholar]
- 16. Wright KP, Hull JT, Czeisler CA. Relationship between alertness, performance, and body temperature in humans. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1370-R1377. doi: 10.1152/ajpregu.00205.2002 [DOI] [PubMed] [Google Scholar]
- 17. Johnson MP, Duffy JF, Dijk DJ, Ronda JM, Dyal CM, Czeisler CA. Short-term memory, alertness and performance: a reappraisal of their relationship to body temperature. J Sleep Res. 1992;1:24–29. doi: 10.1111/j.1365-2869.1992.tb00004.x [DOI] [PubMed] [Google Scholar]
- 18. Schmidt C, Collette F, Cajochen C, Peigneux P. A time to think: circadian rhythms in human cognition. Cogn Neuropsychol. 2007;24:755–789. doi: 10.1080/02643290701754158 [DOI] [PubMed] [Google Scholar]
- 19. Cohen SIA, Cukalevski R, Michaels TCT, et al. Distinct thermodynamic signatures of oligomer generation in the aggregation of the amyloid-β peptide. Nat Chem. 2018;10:523–531. doi: 10.1038/s41557-018-0023-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carrettiero DC, Santiago FE, Motzko-Soares ACP, Almeida MC. Temperature and toxic Tau in Alzheimer’s disease: new insights. Temperature. 2015;2:491–498. doi: 10.1080/23328940.2015.1096438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vandal M, White PJ, Tournissac M, et al. Impaired thermoregulation and beneficial effects of thermoneutrality in the 3×Tg-AD model of Alzheimer’s disease. Neurobiol Aging. 2016;43:47–57. doi: 10.1016/j.neurobiolaging.2016.03.024 [DOI] [PubMed] [Google Scholar]
- 22. Tournissac M, Vandal M, François A, Planel E, Calon F. Old age potentiates cold-induced tau phosphorylation: linking thermoregulatory deficit with Alzheimer’s disease. Neurobiol Aging. 2017;50:25–29. doi: 10.1016/j.neurobiolaging.2016.09.024 [DOI] [PubMed] [Google Scholar]
- 23. Jankowsky JL, Fadale DJ, Anderson J, et al. Mutant presenilins specifically elevate the levels of the 42 residue β-amyloid peptide in vivo: evidence for augmentation of a 42-specific γ secretase. Hum Mol Genet. 2004;13:159–170. doi: 10.1093/hmg/ddh019 [DOI] [PubMed] [Google Scholar]
- 24. Webster SJ, Bachstetter AD, Nelson PT, Schmitt FA, Van Eldik LJ. Using mice to model Alzheimer’s dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front Genet. 2014;5:1–14. doi: 10.3389/fgene.2014.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hascup ER, Broderick SO, Russell MK, et al. Diet-induced insulin resistance elevates hippocampal glutamate as well as VGLUT1 and GFAP expression in AβPP/PS1 mice. J Neurochem. 2019;148:219–237. doi: 10.1111/jnc.14634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bromley-Brits K, Deng Y, Song W. Morris Water Maze test for learning and memory deficits in Alzheimer’s disease model mice. J Vis Exp. 2011;53:e2920. doi: 10.3791/2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hamada M, Abe M, Miyake T, et al. B cell-activating factor controls the production of adipokines and induces insulin resistance. Obesity. 2011;19:1915–1922. doi: 10.1038/oby.2011.165 [DOI] [PubMed] [Google Scholar]
- 28. Castellani JW, Young AJ. Human physiological responses to cold exposure: acute responses and acclimatization to prolonged exposure. Auton Neurosci Basic Clin. 2016;196:63–74. doi: 10.1016/j.autneu.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 29. Glanville EJ, Seebacher F. Advantage to lower body temperatures for a small mammal (Rattus fuscipes) experiencing chronic cold. J Mammal. 2010;91:1197–1204. 10.1644/10-mamm-a-003.1 [DOI] [Google Scholar]
- 30. Wang Z, Ning T, Song A, Rutter J, Wang QA, Jiang L. Chronic cold exposure enhances glucose oxidation in brown adipose tissue. EMBO Rep. 2020;21:e50085. doi: 10.15252/embr.202050085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang TY, Liu C, Wang A, Sun Q. Intermittent cold exposure improves glucose homeostasis associated with brown and white adipose tissues in mice. Life Sci. 2015;139:153–159. doi: 10.1016/j.lfs.2015.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim B, Hyun CK. B-cell-activating factor depletion ameliorates aging-dependent insulin resistance via enhancement of thermogenesis in adipose tissues. Int J Mol Sci. 2020;21:1–13. doi: 10.3390/ijms21145121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Keil G, Cummings E, de Magalhães JP. Being cool: how body temperature influences ageing and longevity. Biogerontology. 2015;16:383–397. doi: 10.1007/s10522-015-9571-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Waalen J, Buxbaum JN. Is older colder or colder older? The association of age with body temperature in 18,630 individuals. J Gerontol Ser A Biol Sci Med Sci. 2011;66A:487–492. doi: 10.1093/gerona/glr001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Törnquist M, Michaels TCT, Sanagavarapu K, et al. Secondary nucleation in amyloid formation. Chem Commun. 2018;54:8667–8684. doi: 10.1039/c8cc02204f [DOI] [PubMed] [Google Scholar]
- 36. Kim K, Wang X, Ragonnaud E, et al. Therapeutic B-cell depletion reverses progression of Alzheimer’s disease. Nat Commun. 2021;12:1–11. doi: 10.1038/s41467-021-22479-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data is available upon reasonable request.