Abstract
Background:
Glomerular filtration rate (GFR) is a key measure of kidney function, but often inaccurately ascertained by serum creatinine and cystatin C in pediatrics. In this pilot trial, we evaluated the relationship between GFR derived using phase-contrast MRI (PC-MRI) biomarkers and GFR by 125I-iothalamate clearance in youth undergoing bone marrow transplantation (BMT).
Methods:
A total of twenty-one pediatric BMT candidates (8-21 years of age) were recruited for a research kidney PC-MRI. After completion of 125I iothalamate clearance, same day PC-MRI measurements were completed of the kidney circulation without gadolinium-based contrast agent. MRI included a non-contrast balanced-SSFP triggered angiography to position ECG-gated breath-held 2D PC-MRI flow measurements (1.2×1.2×6 mm3). A multivariate model of MRI biomarkers estimating GFR (GFR-MRI) was selected using the elastic net approach.
Results:
GFR-MRI variables selected by elastic net included: average heart rate during imaging (bpm), peak aorta flow below the kidney artery take-offs (ml/s), average kidney artery blood flow, average peak kidney vein blood flow, and average kidney vein blood flow (ml/s). The GFR-MRI model demonstrated strong agreement with GFR by 125I iothalamate (R2 = 0.65), which was stronger than what was observed with eGFR by the Full Age Spectrum and Chronic Kidney Disease in Children under 25 (CKiD U25) approaches.
Conclusion:
In this pilot study, non-invasive GFR-MRI showed strong agreement with gold-standard GFR in youth scheduled for BMT. Further work is needed to evaluate whether non-contrast GFR-MRI holds promise to become a superior alternative to eGFR and GFR by clearance techniques.
Keywords: GFR, eGFR, PC-MRI, Bone Marrow Transplant, blood flow
Introduction:
Glomerular filtration rate (GFR) is central to the assessment kidney function, and is often used to diagnose kidney disease as well as determine dosing of medications cleared by the kidney [1–3]. In clinical practice, estimated GFR (eGFR) is frequently calculated by serum concentrations of endogenous filtration markers, including creatinine and cystatin C [1–3]. The pediatric equations used to calculate eGFR are derived from cohorts of young persons with chronic kidney disease, and are posited to be inaccurate at the normal-to-elevated GFR ranges, which is problematic in early disease when GFR is preserved or often elevated (hyperfiltration) [4]. The alternative is to directly measure GFR by tracking serum or urine clearance of intravenously (IV) administered exogenous filtration markers. Such measures are arduous and potentially invasive. Accordingly, use of methods to directly measure GFR remain limited to patient populations in whom the benefits of accurate ascertainment of kidney function outweigh their invasiveness, risks, and costs. Yet, several pediatric populations, including youth with obesity, diabetes mellitus, hypertension, and bone marrow transplant (BMT) recipients, are at high risk of kidney disease and may experience delay in diagnosis and treatment with inaccurate GFR ascertainment. Thus, more accurate non-invasive methods to determine GFR accurately are urgently needed.
Previous imaging studies have estimated GFR using MRI and gadolinium-based contrast media (GBCM) to model glomerulus filtration with tracer kinetic models and shown promising results [5]. However, evidence of GBCM brain deposition and the perception of non-minimal risk of nephrogenic systemic fibrosis (NSF) may cause hesitancy concerning use of gadolinium-based contrast agents in the presence of kidney disease, especially in the pediatric setting [6]. Given that kidney blood flow (KBF) is a key physiological determinant of GFR, and phase-contrast MRI (PC-MRI) is a quick noninvasive technique capable of accurately quantifying KBF (without exogenous IV contrast), we aim to assess blood flow using PC-MRI measurements to estimate GFR in children being evaluated for bone marrow transplant. Young persons scheduled to receive a BMT undergo GFR ascertainment by 125I-iothalamate clearance at our hospital and thus represent an ideal participant population to test the performance of PC-MRI to estimate GFR. We hypothesized in this pilot and feasibility trial that a non-contrast agent approach using PC-MRI biomarkers would strongly associate with GFR by 125I-iothalamate clearance in young BMT candidates.
Methods:
A total of twenty-one pediatric BMT candidates were prospectively recruited for a research kidney MRI. Participants 8 to 21 years of age who were scheduled to undergo a BMT and an 125I iothalamate clearance study to quantify GFR were eligible to enroll in the study. Before the MRI, fasting urine was collected to assess albumin-to-creatinine ratio (ACR) and blood to serum creatinine and cystatin C. Serum creatinine and cystatin C were used to estimate GFR by Full Age Spectrum (FAS), and Chronic Kidney Disease in Children under 25 (CKiD U25) equations [7]. All laboratory assays were performed by the University of Colorado CTRC Core Labs. Creatinine was assessed enzymatically (Beckman Coulter, California, US) and cystatin C immunoturbidimetrically (Kamiya Biomedical, Washington, US). A Siemens Nephelometry instrument was used to measure urine microalbumin (Siemens Healthineers, Erlangen, Germany).
After completion of 125I iothalamate clearance, same day PC-MRI measurements were completed of the kidney circulation. MRI included balanced-SSFP TRiggered ANgiography non-Contrast Enhanced (B-TRANCE) imaging to position ECG-gated breath-held 2D PC-MRI flow measurements (1.2×1.2×6 mm3). PC-MRI was positioned orthogonal to the left and right kidney artery at the takeoff of the descending aorta. When venous kidney flow was visible, additional images were acquired of the venous return. In all cases, velocity sensitivity (venc≈100 cm/s) was adjusted to avoid velocity aliasing. Bilateral, time-resolved arterial and venous KBF were measured using region of interests drawn in Circle CVI42 (Calgary). Right, left, and total arterial and venous net flow, peak flow, and peak velocity were computed. Total bilateral blood flow was computed as the sum of the left and right kidney vessels.
Statistical Analysis:
A final multivariate model was selected using the elastic net approach with the shrinkage parameters lambda and alpha selected using leave one out cross-validation (R packages glmnet and caret) [8]. Lambda and alpha were chosen to produce the most parsimonious model with root mean squared error (RMSE) within 1 standard error of the minimum obtained during cross-validation. Rather than using a stepwise approach based on statistical significance, elastic net model selection is based on minimizing prediction error. After model selection with elastic net, simple linear models were fit using the selected markers as predictors. MRI models to estimate GFR were compared to eGFR calculations by serum creatinine and cystatin C based on the coefficient of determination (R2) between predicted values and gold standard GFR measurement (Figure 1).
Figure 1.
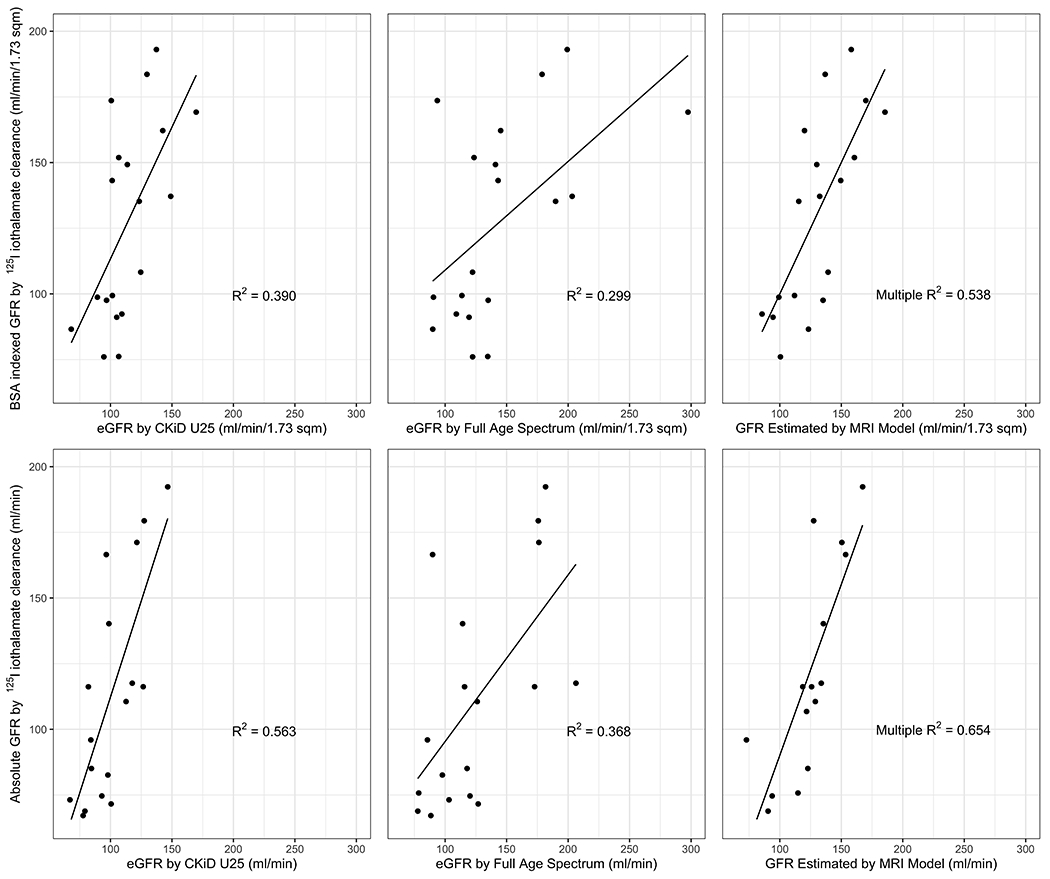
Agreements among iothalamate-based GFR, eGFR and GFR-MRI. Correlations between iothalamate based GFR and estimated GFR (BSA indexed and non-indexed) are shown, as well as GFR estimated by MRI model. The MRI model predicting indexed iothalamate based GFR included average heart rate, peak aorta flow below the kidney artery take-offs, average kidney artery blood flow, average peak kidney vein blood flow, age, and sex. The MRI model predicting absolute iothalamate based GFR includes average heart rate, peak aorta flow below kidney artery take-offs, average kidney vein blood flow, average peak kidney vein blood flow, age, and sex. Note: CKiD U25 and FAS were calculated according to Pottel et al., NDT 2015, PMID: 26932693.
MRI variables selected by the elastic net included average heart rate during imaging (bpm), peak aorta flow below the kidney artery take-offs (ml/s), average kidney artery blood flow, average peak kidney vein blood flow, and average kidney vein blood flow (ml/s). Average kidney artery blood flow was calculated by summing the average superior and inferior kidney artery blood flow (if present) on the right and left sides and taking the mean of the two sides. Average peak kidney vein blood flow and average kidney vein blood flow were calculated similarly.
Results:
Twenty of twenty-one research participants completed the study protocol. One participant was excluded due to claustrophobia on commencement of the research MRI and an inability to complete the scan. Supplementary Table 1 summarizes the participant characteristics. Briefly, 43% of the participants were female with a median age of 15 years, median GFR-iothalamate of 136 ml/min. None of the participants exhibited elevated albuminuria.
Figure 1 shows the agreements among GFR-iothalamate, eGFR and GFR-MRI. The agreements between BSA-indexed and non BSA-indexed GFR-iothalamate and GFR-MRI models were superior to those observed with eGFR by CKiD U25 and FAS. For example, the BSA indexed R2 correlations to GFR-iothalamate for eGFR by CKiD U25 and FAS were 0.390 and 0.299, respectively, as compared to R2=0.538 for the GFR-MRI model. Additionally, the non-BSA indexed R2 was 0.654 for the GFR-MRI model.
Discussion:
GFR is vital to the assessment of kidney function, and is used to define and stage chronic kidney disease (CKD) [1–3]. Even with improved methods to estimate GFR, the use of endogenous filtration markers lack precision and accuracy before CKD stage 3 [9–13]. Racial disparities in equation performance have also been recently highlighted [12, 14]. Furthermore, longitudinal changes in estimated GFR may not reflect changes in measured GFR [15]. These shortcomings are of particular concern in pediatric populations at high risk for kidney disease, as the disconnect between eGFR and measured GFR can cause misclassification of early kidney disease as well as flawed longitudinal trajectories of kidney function [16].
Previous MRI-based studies have investigated GBCM-based tracer kinetic models with a two-compartment model and compelling correlations to gold standard GFR measurements were found (R2 = 0.97) [5]. However, these studies require exogenous agents with non-minimal risk surrounding GBCM brain deposition and concerns surrounding NSF in individuals with impaired kidneys [6]. Here we show a non-contrast method which better predicts GFR than eGFR approaches using creatinine and cystatin C.
This pilot and feasibility study has several constraints worth mentioning, including the small sample size and the lack of test-retest reliability of the GFR-MRI. Therefore, the data should be considered hypothesis generating. Strengths of the study include gold-standard measurements of GFR by 125I-iothalamate clearance. Future studies will need to consider the cost/benefit of GFR-MRI as compared to laboratory-based approaches.
In conclusion, the poor performance of eGFR by creatinine and cystatin C in youth is problematic because inaccurate estimates of kidney function can lead to inconsistent treatment, intervention, and increased costs. Here, we found GFR estimated by the MRI model correlated strongly with GFR by iothalamate, the gold standard. However, GFR determined by clearance techniques are impractical and too invasive for widespread clinical use. Thus, non-invasive and, ideally non-GBCM, methods are needed that can accurately detect changes in GFR where interventions may be the best option to preserve kidney structure and function. Although data from this pilot and feasibility study should be limited to hypothesis generating, we contend that GFR-MRI represents a technological advancement of clinical importance and holds promise to become a superior alternative to eGFR and GFR by clearance techniques in the future.
Supplementary Material
Acknowledgements:
This brief report is limited to 6 co-authors, and thus we would like to acknowledge Kristen J. Nadeau, M.D., M.S. in Pediatric Endocrinology, Laura Pyle, Ph.D. in Biostatistics, Takashi Fujiwara, Ph.D, and Nicholas Stence, M.D. in Radiology, and the entire Bone Marrow Transplant team at Children’s Hospital Colorado, and in particular Amy Keating, M.D., Michael Verneris, M.D., Hesham Eissa, M.D., Chris McKinney, M.D., Taizo Nakano, M.D., and Roger Giller, M.D.
Funding Acknowledgement
This project was funded by a Novel Method Development grant from the CCTSI at University of Colorado Anschutz Medical Campus (CTSA Grant UL1 TR002535, KL2 TR002534, & TL1 TR002533). P.B. receives salary and research support from NIDDK (R01 DK129211, R01 DK132399, R21 DK129720, K23 DK116720, UC2 DK114886), NHLBI (R01 HL165433), JDRF (3-SRA-2022-1097-M-B, 3-SRA-2022-1243-M-B, 3-SRA-2022-1230-M-B), Boettcher Foundation, American Heart Association (20IPA35260142), Ludeman Family Center for Women’s Health Research at the University of Colorado, the Department of Pediatrics, Section of Endocrinology and Barbara Davis Center for Diabetes at University of Colorado School of Medicine. AJB receives funding from NHLBI (R01 HL133504).
Footnotes
Conflicts of interest: P.B. reports serving as a consultant for AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Eli-Lilly, LG Chemistry, Sanofi, Novo Nordisk, and Horizon Pharma. P.B. also serves on the advisory boards and/or steering committees of AstraZeneca, Bayer, Boehringer Ingelheim, Novo Nordisk, and XORTX. The other authors have no relationships relevant to the contents of this paper to disclose.
Data Availability:
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Levey AS, Inker LA (2016) GFR as the “Gold Standard”: Estimated, Measured, and True. Am J Kidney Dis 67:9–12 [DOI] [PubMed] [Google Scholar]
- 2.(2013) Kidney Disease: Improving Global Outcomes (KDIGO): Clinical Practise Guidelines for the Evaluation and Management of Chronic Kidney Disease. Kidney International Supplements 3:1–150 [Google Scholar]
- 3.Gronroos MH, Bolme P, Winiarski J, Berg UB (2007) Long-term renal function following bone marrow transplantation. Bone Marrow Transplant 39:717–723 [DOI] [PubMed] [Google Scholar]
- 4.Gaspari F, Ruggenenti P, Porrini E, Motterlini N, Cannata A, Carrara F, Jimenez Sosa A, Cella C, Ferrari S, Stucchi N, Parvanova A, Iliev I, Trevisan R, Bossi A, Zaletel J, Remuzzi G, Investigators GFRS (2013) The GFR and GFR decline cannot be accurately estimated in type 2 diabetics. Kidney Int 84:164–173 [DOI] [PubMed] [Google Scholar]
- 5.Kurugol S, Afacan O, Lee RS, Seager CM, Ferguson MA, Stein DR, Nichols RC, Dugan M, Stemmer A, Warfield SK, Chow JS (2020) Prospective pediatric study comparing glomerular filtration rate estimates based on motion-robust dynamic contrast-enhanced magnetic resonance imaging and serum creatinine (eGFR) to (99m)Tc DTPA. Pediatr Radiol 50:698–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinreb JC, Rodby RA, Yee J, Wang CL, Fine D, McDonald RJ, Perazella MA, Dillman JR, Davenport MS (2021) Use of Intravenous Gadolinium-Based Contrast Media in Patients With Kidney Disease: Consensus Statements from the American College of Radiology and the National Kidney Foundation. Kidney Med 3:142–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pierce CB, Munoz A, Ng DK, Warady BA, Furth SL, Schwartz GJ (2021) Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int 99:948–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman J, Hastie T, Tibshirani R (2010) Regularization Paths for Generalized Linear Models via Coordinate Descent. Journal of Statistical Software 33:1–22 [PMC free article] [PubMed] [Google Scholar]
- 9.Bjornstad P, Cherney DZ, Maahs DM (2015) Update on Estimation of Kidney Function in Diabetic Kidney Disease. Curr Diab Rep 15:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Souza V, Cochat P, Rabilloud M, Selistre L, Wagner M, Hadj-Aissa A, Dolomanova O, Ranchin B, Iwaz J, Dubourg L (2015) Accuracy of different equations in estimating GFR in pediatric kidney transplant recipients. Clin J Am Soc Nephrol 10:463–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fadrowski JJ, Neu AM, Schwartz GJ, Furth SL (2011) Pediatric GFR estimating equations applied to adolescents in the general population. Clin J Am Soc Nephrol 6:1427–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey AS, Tighiouart H, Titan SM, Inker LA (2020) Estimation of Glomerular Filtration Rate With vs Without Including Patient Race. JAMA Intern Med 180:793–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS (2012) Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367:20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eneanya ND, Yang W, Reese PP (2019) Reconsidering the Consequences of Using Race to Estimate Kidney Function. JAMA 322:113–114 [DOI] [PubMed] [Google Scholar]
- 15.de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, Zinman B, Steffes MW (2014) Longitudinal changes in estimated and measured GFR in type 1 diabetes. J Am Soc Nephrol 25:810–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou H, Hastie T (2005) Regularization and variable selection via the elastic net. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 67:301–320 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


