Abstract
Background.
Although maternal stressor exposure has been associated with shorter telomere length (TL) in offspring, this literature is based largely on White samples. Furthermore, timing of maternal stressors has rarely been examined. Here, we examined how maternal stressors occurring during adolescence, pregnancy, and across the lifespan related to child TL in Black and White mothers.
Method.
Mothers (112 Black; 110 White; Mage = 39) and their youngest offspring (n = 222; Mage = 8) were part of a larger prospective cohort study, wherein mothers reported their stressors during adolescence (assessed twice during adolescence for the past year), pregnancy (assessed in midlife for most recent pregnancy), and across their lifespan (assessed in midlife). Mother and child provided saliva for TL measurement. Multiple linear regression models examined the interaction of maternal stressor exposure and race in relation to child TL, controlling for maternal TL and child gender and age. Race-stratified analyses were also conducted.
Results.
Neither maternal adolescence nor lifespan stressors interacted with race in relation to child TL. In contrast, greater maternal pregnancy stressors were associated with shorter child TL, but this effect was present for children of White but not Black mothers. Moreover, this effect was significant for financial but not social pregnancy stressors. Race-stratified models revealed that greater financial pregnancy stressors predicted shorter telomeres in offspring of White, but not Black mothers.
Conclusions.
Race and maternal stressors interact and are related to biological aging across generations, but these effects are specific to certain races, stressors, and exposure time periods.
Keywords: Chronic stress, STRAIN, Pregnancy, Cellular aging, Race, Intergenerational stress
Introduction
Racial disparities in health have reached a critical juncture, particularly between Black/African and White individuals, with lower life expectancy and earlier onset of chronic diseases of aging for Black relative to White individuals (Mays, Cochran, & Barnes, 2007; Murray et al., 2006; Williams & Mohammed, 2009). Evidence points to the influence of racism-related stressor exposures and coping strategies that can have long-term health consequences (Turner, 2013). In terms of stressor exposure, African Americans experience more acute and chronic life stressors as compared to their White counterparts, even after adjusting for socioeconomic status (SES) (Brown, Mitchell, & Ailshire, 2020; Sternthal, Slopen, & Williams, 2011). In terms of coping, Black Americans may have adopted unique coping resources to withstand chronic stressor exposures that are the result of oppressive socio-historical contexts.
One commonly used strategy by Black Americans is termed John Henryism, a style characterized by a high-effort, active coping that might have detrimental physical health effects due to the amounting physiological burden (Robinson & Thomas Tobin, 2021). Another coping mechanism at the intersection of race and gender is the Superwoman Schema, which reflects a desire on the behalf of Black women to present an image of strength, suppress emotions, avoid vulnerability, succeed regardless of resources, and help others (Woods-Giscombé, 2010). Although initially conceptualized as a positive coping mechanism to defy gendered racism, this may have negative health consequences (Woods-Giscombé et al., 2019). Formulations such as the ‘weathering’ hypothesis suggest that the rate of biological aging of African American women may be accelerated as a consequence of the cumulative impact of racism-related stressor exposures and the need for the development of unique coping mechanisms that may have long-term health consequences (Forde, Crookes, Suglia, & Demmer, 2019).
The negative health effects of experiencing major life stressors can also span multiple generations. Maternal stressor exposure during the perinatal period can have important long-term health consequences for the offspring, which has led to models such as the Developmental Origins of Health and Disease (DOHaD) hypothesis (Barker, 2007). Black mothers report more pregnancy stressors as compared to White mothers (Liu, Giallo, Doan, Seidman, & Tronick, 2016; Lu & Chen, 2004). The prenatal period is thus an important time during which racial health disparities might originate, though research is scarce in marginalized communities (Conradt, Carter, & Crowell, 2020). Furthermore, maternal stressor exposure during her own childhood/adolescence or during her lifetime can confer health risks on her offspring such as child psychopathology (Bödeker et al., 2019; Rijlaarsdam et al., 2014). Understanding when during the life course (adolescence v. pregnancy v. lifespan) maternal stressors are most impactful for offspring health is an important research question. Here, we focused on immune cellular age as a key outcome.
Telomere dynamics have been proposed to be a key mechanism in translating psychosocial stressors into biological changes that increase risk for health problems within and across generations (Epel, 2020). In this context, telomere length (TL) may be a potentially useful biomarker for understanding intergenerational stress effects and race/racism-related health disparities (Selvaraju, Phillips, Fouty, Babu, & Geetha, 2021). Telomeres are DNA-protein complexes that serve as protective caps at the ends of chromosomes, maintaining chromosomal integrity. With each cell division, telomeres are not fully replicated and shorten with age, constituting a biological marker for aging (Blackburn, Epel, & Lin, 2015). Critically short telomeres can trigger apoptosis or cellular senescence cascades. However, the enzyme telomerase, a ribonucleoprotein reverse transcriptase, can add repetitive nucleotide sequences to the ends of the DNA, thereby counteracting telomere shortening (Blackburn et al., 2015).
Deterioration of the telomere/telomerase maintenance system is implicated in physical (Haycock et al., 2014; Ma et al., 2011) and psychiatric diseases (Darrow et al., 2016), and has been linked to stressor exposure, particularly for childhood stressors (Epel & Prather, 2018). Notably, race-based differences in telomere dynamics have been shown, with most studies finding that Black individuals have longer telomeres (Brown, Needham, & Ailshire, 2017; Codd et al., 2021; c.f. Geronimus et al., 2010) and higher levels of telomerase (Kroenke et al., 2012).
Research also has suggested the presence of intergenerational stressor effects on TL, with most studies primarily investigating the sensitive period of pregnancy. In particular, maternal stressors occurring during pregnancy have been associated with shorter TL in newborns (Entringer et al., 2013) and young adults (Entringer et al., 2011). These effects likely occur via stress-related maternal-placental-fetal biological (e.g. oxidative, immune, endocrine, metabolic) pathways that may exert a ‘programming’ effect on the developing telomere biology system (Entringer, de Punder, Buss, & Wadhwa, 2018). Several independent cohort studies have now replicated the effects of maternal pregnancy stress on offspring TL (Carroll, Mahrer, Shalowitz, Ramey, & Schetter, 2020; Marchetto et al., 2016; Send et al., 2017; Verner et al., 2021), though some studies have not found an association (Ämmälä et al. 2020; Slykerman et al. 2019). A striking feature of this literature is the scarcity of non-White, particularly Black samples. Indeed, the three largest studies conducted to date included all-White, European participants (Ämmälä et al., 2020; Slykerman et al., 2019; Verner et al., 2021). The few studies that have enrolled Black participants either had small samples (Carroll et al., 2020; Entringer et al., 2013; Marchetto et al., 2016) or did not test for pregnancy stress-by-race interactions (Enlow et al., 2018; Esteves et al., 2020).
It has been proposed that stressors interact with race to shape offspring health outcomes, though this is rarely examined (Giscombé & Lobel, 2005). Racial differences in prenatal stressor exposure (Liu et al., 2016; Lu & Chen, 2004), culturally relevant coping mechanisms (John Henryism; Superwoman Schema), and racial differences in TL (Brown et al., 2017; Codd et al., 2021) may yield different associations. Examining the complex interactive effects of maternal race and stressor exposure with offspring TL may advance our understanding of racial disparities in offspring health and longevity.
Furthermore, maternal stressor exposure occurring outside the pregnancy period has rarely been examined in relation to offspring TL, despite new developments that extended the DOHaD hypothesis to the preconception period (Keenan, Hipwell, Class, & Mbayiwa, 2018). The DOHaD extension argues that pregnancy health depends on the integrity of parental physiological systems prior to conception and thus includes maternal childhood/adolescence or cumulative experiences across the lifespan. Adolescence, particularly late adolescence, is an important developmental period during which time stressor exposures can shape the responsiveness of stress-related physiological systems that may in turn impact maternal-fetal processes in pregnancy (Keenan et al., 2018). Similarly, maternal lifelong stressor exposure may contribute to fetal programming of the offspring’s telomere biology system via long-term alterations of stress response systems as well as oocyte quality and mitochondrial function across oocyte development (Entringer et al., 2018). To our knowledge, however, no study has examined the cumulative effects of maternal stressor exposure occurring across the entire lifespan on offspring TL.
One study examined maternal stress occurring in the year prior to conception and did not find associations with offspring buccal TL (Carroll et al., 2020). In addition, two studies have investigated the effects of childhood adversity on offspring TL, showing that maternal childhood adversity was associated with shorter infant TL across infancy (Esteves et al., 2020). Furthermore, retrospective reports of mother’s familial emotional support and sexual abuse in childhood have been associated with newborn TL in male infants (Enlow et al., 2018). It is important to replicate these findings and to widen the stressor assessment window to span the entire life course. Lastly, when possible, it is important to test how the intersection of race and stressor exposure impacts offspring TL. Black women are not only exposed to more cumulative stressors, but they also perceive and cope with them differently, which might have differential effects on offspring TL.
To address these issues, we examined how maternal stressor exposure occurring during late childhood/adolescence, pregnancy, and across the life course related to child TL in Black and White mothers. Based on the literature summarized above, we hypothesized that maternal stressor exposure occurring during adolescence, pregnancy, and across the life course would be associated with shorter offspring TL, particularly for offspring of Black mothers.
Method
Overview of the National Heart, Lung and Blood Institute Growth & Health Study
The National Heart, Lung and Blood Institute Growth & Health Study (NGHS) is a prospective cohort study that recruited Black and White girls at 9–10 years of age from Richmond (CA, USA), Cincinnati (OH, USA), and Washington (D.C., USA). A total of 2379 girls (1209 Black; 1166 White) were enrolled in 1987–88 and followed prospectively from 9/10 to 19/20 years old (for an overview of the NGHS, see Morrison, 1992). The original aim of the NGHS was to examine factors associated with racial disparities in the onset and development of obesity and cardiovascular disease, specifically in Black and White females. After the initial ten-year study, a follow-up study was conducted in midlife (Mage = 39) that re-enrolled participants from the Richmond (CA) site, which was chosen based on census data showing approximately equal percentages of Black and White children with the least degree of income and occupational disparity between the races. We re-recruited 624 (307 Black; 317 White) of the original 887 participants (459 Black; 428 White) from the Richmond site (73.8% retention rate among eligible women). The follow-up study was further enriched by inviting biological children to participate with their mothers (the original participants) to examine the intergenerational transmission of stress and disease risk.
Participants
Eligibility criteria for adult women were (a) an original NGHS participant from the Richmond, CA site; (b) not pregnant at the time of recruitment and had not experienced a pregnancy, miscarriage, or abortion within the last three months; and (c) neither living abroad nor incarcerated or otherwise institutionalized. Participants’ biological children were eligible if they were between the ages of 2 and 17. Of the 645 children who participated, 262 provided valid TL data. For the present study, we focused on data of the youngest (most recent born) child, for which we had measures of stress exposure during pregnancy. Of the 262 children with valid TL data, 222 children were the youngest. In total, there were 222 mother-youngest child dyads with offspring TL data.
Study procedures
Multiple recruitment strategies were used to re-recruit original NGHS participants from the Richmond site (e.g. mailing and telephone follow-up, social media outreach). Eligible participants provided written informed consent and participated in a baseline survey and home/clinic visit, including saliva collection for TL assessment. This study was approved by the Institutional Review Board.
Self-report measures
Sociodemographic information
The survey obtained in midlife (Mage = 39) assessed sociodemographic information, including maternal race (‘Black’ or ‘White’), ethnicity, age, highest level of education completed, annual household income, child age, and child gender.
Maternal stressor measures
For a timeline of maternal stressor assessments in NGHS, see Fig. 1. Briefly, major life stressors that occurred over the past 12 months were assessed at age 17/18 and 19/20, indicating maternal stressor exposure during adolescence. In addition, during midlife (Mage = 39), mothers retrospectively reported on the major life stressors that occurred during their most recent pregnancy as well as major life stressors that had occurred across their lifespan.
Fig. 1.
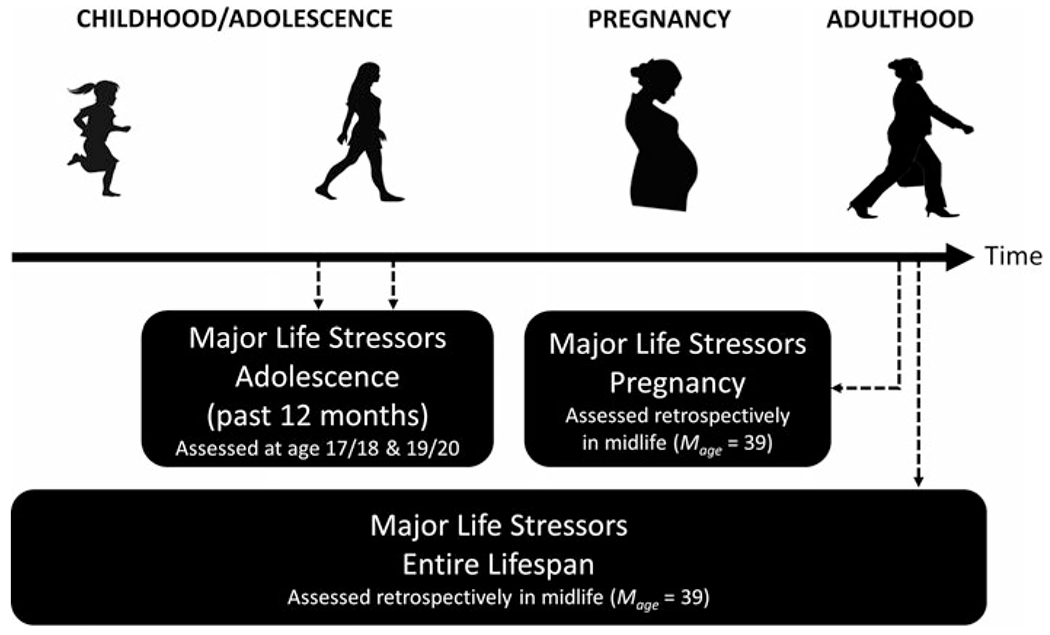
Timeline of maternal stressor assessments. Note: Dotted arrows indicate the assessment time.
Maternal adolescence stressors
Prospective measures of maternal life stressors occurring during adolescence were available as part of the historical NGHS. Specifically, a Life Events Scale (LES; Franko et al., 2004) was administered at age 17/18 (40 items) and 19/20 (45 items), assessing if the participant experienced a variety of stressors over the past 12 months. A total LES score was calculated by summing all items across both time points.
Maternal pregnancy stressors
Stressors occurring during the most recent pregnancy were measured retrospectively in midlife (Mage = 39) using a list of 14 major life stressors (Centers for Disease Control and Prevention, 2005). A total sum was computed for all 14 stressors. Stressors were further categorized into total social stressors and financial stressors.
Maternal lifespan stressors
Mothers’ exposure to major stressors occurring over the entire life course was assessed in midlife (Mage = 39) using the Stress and Adversity Inventory for Adults (Adult STRAIN), which is an online system for assessing cumulative lifetime stressor exposure that has demonstrated very good validity and excellent test-retest reliability (Slavich & Shields, 2018).
Maternal depressive symptoms
Current mood may influence retrospective reports of maternal stressor exposure (Maughan & Rutter, 1997), so sensitivity analyses were conducted controlling for depressive symptoms at the time of stressor assessment. Depressive symptoms were assessed in midlife (Mage = 39) using the 20-item Center for Epidemiological Studies Depression Scale (CES-D), which measured symptoms over the past week on a 0–3 scale (Radloff, 1977).
Maternal and offspring telomere length measurement
The TL measurement assay was adapted from the published original method by Cawthon (Cawthon, 2002; Lin, Epel, & Blackburn, 2012). Additional details can be found in the online Supplement. The inter-assay coefficient of variation for this study was 2.2% ± 1.6%.
Statistical analysis
All analyses were conducted with IBM SPSS Statistics version 27. Maternal TL was natural log transformed, which improved skewness (Statistic = −0.38, s.e. = 0.17) and kurtosis (Statistic = 1.27, s.e. = 0.33). Offspring TL values above mean + 3 × s.d. were winsorized (6 participants) and then ln transformed, which improved skewness (Statistic = 0.59, s.e. = 0.17) and kurtosis (Statistic = 1.74, s.e. = 0.33).
Although there is no clear guidance on how to model the intersection of maternal race and stressor exposure best quantitatively, the most common statistical methods have been regression models with interaction terms and stratified analyses (Bauer et al., 2021; Guan et al., 2021; Wu, 2021). A series of multiple linear regression models examined the interaction of maternal stress exposure and race on offspring TL, controlling for covariates that are known to predict offspring TL, including child age and gender (Factor-Litvak et al., 2016), and maternal TL (Broer et al., 2013). Specifically, we controlled for maternal TL to examine environmental exposure effects while controlling for heritable factors such as genetic variants or direct transmission from germ line telomeres (Haussmann & Heidinger, 2015). Given race-related experiential theories and race-based TL differences, we also examined the effect of maternal stressors on offspring TL in models stratified by maternal race (Shenassa, Rossen, Cohen, Morello-Frosch, & Payne-Sturges, 2017; Williams, Shenassa, Slopen, & Rossen, 2018).
Results
Sample characteristics
Descriptive statistics are shown in Table 1. Mothers had a narrow age range (37–42) due to the cohort design. The majority of participants (63%) experienced at least one pregnancy stressor, with 57% experiencing at least one social stressor and 38% reporting at least one financial stressor. Lower maternal education and household income were associated with greater maternal adolescence, pregnancy, and lifespan stressor exposure (all ps < 0.05). Offspring were on average 8 years old (range: 2–17) with the majority of children (70%) being 10 years old or younger. Thus, children clustered around a similar developmental stage.
Table 1.
Descriptive statistics for sociodemographic information, adolescence, pregnancy, and lifespan stressors, as well as telomere length (TL) of mother-child dyads
| Entire sample | Black mothers | White mothers | |||
|---|---|---|---|---|---|
| (n = 222) | (n = 112) | (n = 110) | |||
| Category | Variable | Mean (s.d.) or No. (%) | Mean (s.d.) or No. (%) | Mean (s.d.) or No. (%) | |
| Maternal | Sociodemographics | Hispanic, Latino, or Spanish origin, No. (%) yes | 8 (3.6) | 3 (2.7) | 5 (4.6) |
|
| |||||
| Age, years | 39.31 (1.14) | 39.37 (1.09) | 39.25 (1.20) | ||
|
| |||||
| Education, No. (%) less than college degree | 138 (62%)* | 87 (78%) | 51 (46%) | ||
|
| |||||
| Annual household income, No. (%) < $ 60 000 | 90 (43%)* | 63 (60%) | 27 (25%) | ||
|
| |||||
| Adolescence stressors | Total count of stressors during adolescence | 14.81 (8.29) | 15.64 (8.83) | 13.98 (7.65) | |
|
| |||||
| Pregnancy stressors | Total count of stressors during pregnancy | 1.87 (2.07)* | 2.43 (2.25) | 1.31 (1.72) | |
|
| |||||
| Total count of social stressors during pregnancy | 1.28 (1.52)* | 1.72 (1.68) | 0.85 (1.22) | ||
|
| |||||
| Total count of financial stressors during pregnancy | 0.59 (0.86)* | 0.72 (0.92) | 0.46 (0.78) | ||
|
| |||||
| Lifespan stressors | Total count of stressors across the lifespan | 26.89 (15.68) | 27.90 (16.40) | 25.80 (14.89) | |
|
| |||||
| Cellular aging marker | TL (T/S ratio) | 1.19 (0.24)* | 1.24 (0.24) | 1.13 (0.22) | |
|
| |||||
| Child | Sociodemographics | Age, years | 8.12 (3.98) | 8.41 (4.05) | 7.83 (3.91) |
|
| |||||
| Gender, No. (%) female | 119 (54%) | 58 (52%) | 61 (56%) | ||
|
| |||||
| Cellular aging marker | TL (T/S ratio) | 1.57 (0.44) | 1.60 (0.47) | 1.53 (0.39) | |
Indicates significant race differences (p < 0.05).
Notably, demographic characteristics differed across races (Table 1), such that Black mothers reported lower SES as compared to White mothers. However, unlike other studies that are often characterized by structural confounding of race and SES, in the present sample, there was a sufficient representation of higher-SES Black participants and lower SES White participants. Black mothers were exposed to more pregnancy stressors but had longer telomeres than White mothers.
Effects of sociodemographic variables on offspring telomere length
As hypothesized, maternal TL was positively correlated with child TL, r(208) = 0.27, p < 0.001. There were no significant relations between child TL with maternal race, t(220) = −1.30, p = 0.194, age, r(220) = −0.001, p = 0.990, education, t(220) = −1.89, p = 0.060, and annual household income, r(210) = 0.005, p = 0.940. Child age was not related to child TL, r(220) = −0.054, p = 0.425, which was expected given that children clustered around a similar developmental stage. Female gender was marginally associated with longer child TL, t(220) = −1.91, p = 0.058.
Effects of maternal stressors and race on offspring telomere length
Linear regression models and statistics are presented in Table 2 (models with stress-by-race interactions)†,1 and Table 3 (models stratified by maternal race). First, we examined the effect of maternal adolescence stressors and race on child TL (Table 2, Model a). Results showed that count of stressors occurring during mothers’ adolescence did not interact with maternal race in relation to offspring TL (p = 0.720). Stratified analyses by maternal race (Table 3) also showed no effect of maternal adolescence stressors on child TL within Black and White mothers separately.
Table 2.
Maternal adolescence, pregnancy, and lifespan stressors predicting offspring telomere length (TL)
| Model | Variable | B | Std. Error | Beta | t | p | |
|---|---|---|---|---|---|---|---|
| Adolescence stressorsa | Adolescence stressors | −0.002 | 0.00 | −0.065 | −0.62 | 0.538 | 0.002 |
| Maternal race (0 = White; 1 = Black) | 0.014 | 0.03 | 0.033 | 0.48 | 0.634 | 0.001 | |
| Adolescence stressors-by-race interaction | 0.001 | 0.00 | 0.038 | 0.36 | 0.720 | 0.001 | |
| Maternal TL | 0.262 | 0.07 | 0.262 | 3.78 | <0.001 | 0.064 | |
| Child age | 0.000 | 0.004 | −0.005 | −0.07 | 0.947 | 0.000 | |
| Child gender (0 = boy; 1 = girl) | 0.047 | 0.03 | 0.114 | 1.67 | 0.096 | 0.013 | |
| Total pregnancy stressorsb | Total pregnancy stressors | −0.022 | 0.01 | −0.217 | −1.80 | 0.074 | 0.015 |
| Maternal race (0 = White; 1 = Black) | 0.018 | 0.03 | 0.044 | 0.61 | 0.544 | 0.002 | |
| Total pregnancy stressors-by-race interaction | 0.031 | 0.02 | 0.237 | 2.02 | 0.044 | 0.019 | |
| Maternal TL | 0.259 | 0.07 | 0.257 | 3.67 | <0.001 | 0.062 | |
| Child age | 0.000 | 0.004 | 0.008 | 0.12 | 0.907 | 0.000 | |
| Child gender (0 = boy; 1 = girl) | 0.046 | 0.03 | 0.112 | 1.64 | 0.104 | 0.012 | |
| Social pregnancy stressorsc | Social pregnancy stressors | −0.017 | 0.02 | −0.128 | −1.03 | 0.307 | 0.005 |
| Maternal race (0 = White; 1 = Black) | 0.014 | 0.03 | 0.034 | 0.47 | 0.641 | 0.001 | |
| Social pregnancy stressors-by-race interaction | 0.028 | 0.02 | 0.164 | 1.36 | 0.176 | 0.009 | |
| Maternal TL | 0.258 | 0.07 | 0.255 | 3.62 | <0.001 | 0.061 | |
| Child age | 0.000 | 0.004 | −0.009 | −0.13 | 0.898 | 0.0001 | |
| Child gender (0 = boy; 1 = girl) | 0.049 | 0.03 | 0.119 | 1.73 | 0.085 | 0.014 | |
| Financial pregnancy stressorsd | Financial pregnancy stressors | −0.062 | 0.03 | −0.256 | −2.33 | 0.021 | 0.025 |
| Maternal race (0 = White; 1 = Black) | 0.016 | 0.03 | 0.040 | 0.57 | 0.573 | 0.001 | |
| Financial pregnancy stressors-by-race interaction | 0.079 | 0.04 | 0.249 | 2.27 | 0.024 | 0.024 | |
| Maternal TL | 0.255 | 0.07 | 0.253 | 3.64 | <0.001 | 0.060 | |
| Child age | 0.001 | 0.004 | 0.022 | 0.32 | 0.751 | 0.0005 | |
| Child gender (0 = boy; 1 = girl) | 0.040 | 0.03 | 0.097 | 1.42 | 0.158 | 0.009 | |
| Lifespan stressorse | Lifespan stressors | 0.000 | 0.002 | −0.038 | −0.32 | 0.748 | 0.001 |
| Maternal race (0 = White; 1 = Black) | 0.019 | 0.03 | 0.048 | 0.61 | 0.545 | 0.002 | |
| Lifespan stressors-by-race interaction | 0.001 | 0.002 | 0.047 | 0.40 | 0.693 | 0.001 | |
| Maternal TL | 0.273 | 0.08 | 0.275 | 3.47 | <0.001 | 0.070 | |
| Child age | −0.001 | 0.004 | −0.027 | −0.34 | 0.731 | 0.001 | |
| Child gender (0 = boy; 1 = girl) | 0.051 | 0.03 | 0.125 | 1.63 | 0.105 | 0.016 |
Outcome: Offspring TL (winsorized and natural log transformed).
Note: = Squared semipartial (or ‘part’) correlation (individual predictor).
Bold values indicate p < .05.
Model fit R = 0.300; R2 = 0.090; F(6, 202) = 3.33; p = 0.004.
Model fit R = 0.320; R2 = 0.103; F(6, 195) = 3.72; p = 0.002.
Model fit R = 0.304; R2 = 0.092; F(6, 195) = 3.30; p = 0.004.
Model fit R = 0.333; R2 = 0.111; F(6, 195) = 4.04; p < 0.001.
Model fit R = 0.330; R2 = 0.109; F(6, 152) = 3.09; p = 0.007.
Table 3.
The impact of maternal adolescence, pregnancy, and lifespan stressors on offspring telomere length (TL), stratified by maternal race
| Stratified by | Model | Variable | B | Std. Error | Beta | t | p | |
|---|---|---|---|---|---|---|---|---|
| White mothers | Adolescence stressorsa | Adolescence stressors | −0.001 | 0.003 | −0.043 | −0.44 | 0.663 | 0.002 |
| Maternal TL | 0.256 | 0.10 | 0.248 | 2.56 | 0.012 | 0.060 | ||
| Child age | −0.004 | 0.01 | −0.077 | −0.76 | 0.450 | 0.005 | ||
| Child gender (0 = boy; 1 = girl) | 0.032 | 0.04 | 0.075 | 0.77 | 0.444 | 0.005 | ||
| Total pregnancy stressorsb | Total pregnancy stressors | −0.020 | 0.01 | −0.158 | −1.59 | 0.116 | 0.023 | |
| Maternal TL | 0.271 | 0.10 | 0.262 | 2.70 | 0.008 | 0.066 | ||
| Child age | −0.003 | 0.01 | −0.048 | −0.47 | 0.643 | 0.002 | ||
| Child gender (0 = boy; 1 = girl) | 0.035 | 0.04 | 0.082 | 0.85 | 0.399 | 0.007 | ||
| Social pregnancy stressorsc | Social pregnancy stressors | −0.015 | 0.02 | −0.086 | −0.87 | 0.386 | 0.007 | |
| Maternal TL | 0.266 | 0.10 | 0.257 | 2.62 | 0.010 | 0.064 | ||
| Child age | −0.004 | 0.01 | −0.075 | −0.74 | 0.461 | 0.005 | ||
| Child gender (0 = boy; 1 = girl) | 0.039 | 0.04 | 0.090 | 0.92 | 0.360 | 0.008 | ||
| Financial pregnancy stressorsd | Financial pregnancy stressors | −0.060 | 0.03 | −0.214 | −2.13 | 0.036 | 0.040 | |
| Maternal TL | 0.263 | 0.10 | 0.254 | 2.66 | 0.009 | 0.063 | ||
| Child age | −0.001 | 0.01 | −0.027 | −0.26 | 0.794 | 0.001 | ||
| Child gender (0 = boy; 1 = girl) | 0.025 | 0.04 | 0.058 | 0.59 | 0.553 | 0.003 | ||
| Lifespan stressorse | Lifespan stressors | −0.0003 | 0.002 | −0.020 | −0.17 | 0.867 | 0.0004 | |
| Maternal TL | 0.239 | 0.11 | 0.244 | 2.13 | 0.037 | 0.057 | ||
| Child age | −0.007 | 0.01 | −0.126 | −1.07 | 0.290 | 0.014 | ||
| Child gender (0 = boy; 1 = girl) | 0.034 | 0.05 | 0.084 | 0.74 | 0.464 | 0.007 | ||
| Black mothers | Adolescence stressorsa | Adolescence stressors | −0.0004 | 0.002 | −0.017 | −0.17 | 0.864 | 0.0003 |
| Maternal TL | 0.257 | 0.10 | 0.253 | 2.62 | 0.010 | 0.063 | ||
| Child age | 0.003 | 0.00 | 0.054 | 0.56 | 0.574 | 0.003 | ||
| Child gender (0 = boy; 1 = girl) | 0.055 | 0.04 | 0.138 | 1.40 | 0.164 | 0.018 | ||
| Total pregnancy stressorsb | Total pregnancy stressors | 0.009 | 0.01 | 0.101 | 1.03 | 0.308 | 0.010 | |
| Maternal TL | 0.235 | 0.10 | 0.229 | 2.31 | 0.023 | 0.052 | ||
| Child age | 0.003 | 0.005 | 0.056 | 0.57 | 0.569 | 0.003 | ||
| Child gender (0 = boy; 1 = girl) | 0.055 | 0.04 | 0.136 | 1.37 | 0.174 | 0.018 | ||
| Social pregnancy stressorsc | Social pregnancy stressors | 0.011 | 0.01 | 0.091 | 0.92 | 0.360 | 0.008 | |
| Maternal TL | 0.236 | 0.10 | 0.230 | 2.32 | 0.022 | 0.052 | ||
| Child age | 0.002 | 0.005 | 0.051 | 0.51 | 0.608 | 0.003 | ||
| Child gender (0 = boy; 1 = girl) | 0.056 | 0.04 | 0.138 | 1.39 | 0.166 | 0.019 | ||
| Financial pregnancy stressorsd | Financial pregnancy stressors | 0.018 | 0.02 | 0.083 | 0.83 | 0.410 | 0.007 | |
| Maternal TL | 0.235 | 0.10 | 0.229 | 2.30 | 0.023 | 0.051 | ||
| Child age | 0.003 | 0.005 | 0.062 | 0.63 | 0.532 | 0.004 | ||
| Child gender (0 = boy; 1 = girl) | 0.054 | 0.04 | 0.133 | 1.34 | 0.183 | 0.017 | ||
| Lifespan stressorse | Lifespan stressors | 0.0003 | 0.00 | 0.025 | 0.23 | 0.816 | 0.001 | |
| Maternal TL | 0.302 | 0.11 | 0.290 | 2.69 | 0.009 | 0.083 | ||
| Child age | 0.002 | 0.01 | 0.045 | 0.42 | 0.673 | 0.002 | ||
| Child gender (0 = boy; 1 = girl) | 0.058 | 0.04 | 0.144 | 1.33 | 0.187 | 0.020 |
Outcome: Offspring TL (winsorized and natural log transformed).
Note: = squared semipartial (or ‘part’) correlation (individual predictor).
Bold values indicate p < .05.
Model fit: White Mothers R = 0.289; R2 = 0.084; F(4, 100) = 2.28; p = 0.066; Black Mothers: R = 0.304; R2 = 0.093; F(4, 99) = 2.53; p = 0.046.
Model fit: White Mothers R = 0.329; R2 = 0.108; F(4, 98) = 2.97; p = 0.023; Black Mothers: R = 0.304; R2 = 0.093; F(4, 94) = 2.40; p = 0.055.
Model fit: White Mothers R = 0.304; R2 = 0.092; F(4, 98) = 2.49; p = 0.048; Black Mothers: R = 0.301; R2 = 0.091; F(4, 94) = 2.35; p = 0.060.
Model fit: White Mothers R = 0.354; R2 = 0.126; F(4, 98) = 3.52; p = 0.010; Black Mothers: R = 0.299; R2 = 0.089; F(4, 94) = 2.30; p = 0.064.
Model fit: White Mothers R = 0.313; R2 = 0.098; F(4, 72) = 1.96; p = 0.111; Black Mothers: R = 0.344; R2 = 0.118; F(4, 77) = 2.58; p = 0.044.
Next, we examined the effect of maternal pregnancy stressors and race on child TL. Results showed that stressors occurring during mothers’ pregnancy and race significantly interacted in predicting offspring TL (p = 0.044; Table 2, Model b), such that more maternal pregnancy stressors were associated with shorter offspring TL but only for children of White mothers (Fig. 2). Stratified analyses by maternal race (Table 3) paralleled the overall pattern of a negative association for offspring of White mothers (p = 0.116), but not for children of Black mothers (p = 0.308), although the effect in offspring of White mothers was not statistically significant.
Fig. 2.
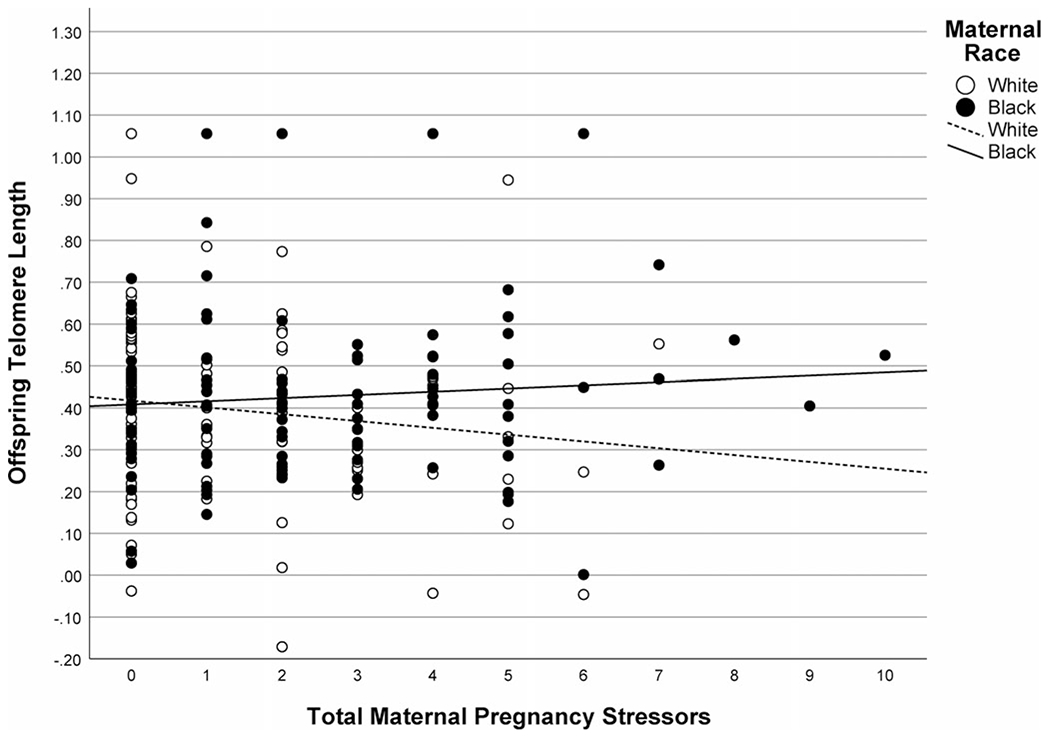
Association between maternal stressors occurring during pregnancy and offspring telomere length (TL) in children of Black and White mothers. Mothers who experienced more stressors during pregnancy had offspring with shorter TL, but this relation was only significant for children of White but not Black mothers. Note: Offspring TL was winsorized and natural log transformed.
Based on prior research showing differential effects for financial v. social stressors (Puterman et al., 2016), we investigated whether the above-described findings differed by pregnancy stressor type. Only maternal financial pregnancy stressors (Table 2, Model d), and not social pregnancy stressors (Table 2, Model c), significantly interacted with maternal race in predicting offspring TL (p = 0.024). Stratified analyses by maternal race (Table 3) showed that experiencing more financial stressors during pregnancy was associated with shorter TL for children of White mothers (p = 0.036) but not Black mothers (p = 0.410). Sensitivity analyses further stratified by both maternal race and child gender showed that greater exposure to financial pregnancy stressors was associated with shorter TL only for boys of White mothers (p = 0.010; online Supplementary Table S1).
Finally, we examined the effect of maternal lifespan stressors and race on offspring TL (Table 2, Model e). Mother’s stressors occurring over the entire life course did not significantly interact with maternal race in predicting offspring TL (p = 0.693). Stratified analyses by maternal race (Table 3) also did not show an effect of maternal lifespan stressors on child TL within each race group.
Other sensitivity analyses
The models above that used retrospective maternal stressor questionnaires were re-run while controlling for maternal depressive symptoms at the time of stressor assessment to account for possible mood-dependent reporting (Maughan & Rutter, 1997). Importantly, controlling for depressive symptoms did not alter any of the findings (see online Supplemental sensitivity analysis and Table S2). Furthermore, non-significant results for the associations between maternal adolescence and lifespan adversity with race on offspring TL remained non-significant when maternal TL was not included in the regression models (online Supplementary Table S3).
Discussion
This is the first study we are aware of to examine stressor exposure timing effects and race in the transmission of maternal life stressor exposure on offspring TL. Results showed that experiencing more maternal pregnancy stressors was related to shorter offspring TL but only for children of White mothers. Post-hoc analyses further revealed that this effect was only significant for financial pregnancy stressors and for boys of White mothers. Neither maternal adolescence nor lifespan stressors interacted with race in predicting offspring TL. Race-stratified models paralleled the overall pattern, showing that greater financial pregnancy stressors predicted shorter telomeres in offspring of White, but not Black mothers.
Our findings point to pregnancy stressors as influential social-environmental exposures that may shape TL for offspring of White women. It is under intrauterine conditions – when there is the closest overlap between the mother’s and the child’s biological and psychosocial environment – that maternal stressor effects may be more impactful for offspring health relative to maternal stress occurring prior to conception. This finding has important public health implications as adult TL, which is a function of the initial set point and attrition over time (Aviv, 2008), has been linked to stress-related diseases (Darrow et al., 2016; Haycock et al., 2014; Ma et al., 2011). Therefore, the foundation for later disease susceptibility might be laid during the prenatal period (Entringer et al., 2018). Reducing maternal stressor exposure occurring during the sensitive period of pregnancy may promote lifelong health, and this may occur in part by protecting offspring telomere shortening.
Multiple mechanisms may link maternal prenatal stressors and offspring TL. Maternal epigenetic patterning/marking does likely not transmit strongly, so transmission is not likely due to epigenetics (Entringer et al., 2018). Since all analyses controlled for observed maternal TL – a proxy for biological inheritance – our findings are less likely to be the results of mothers’ genetics, and of direct transmission of shorter TL through the germline. This strengthens our interpretation that the transmission occurred through the indirect impact of maternal stress exposure on offspring telomere biology via non-heritable pathways (Haussmann & Heidinger, 2015), such as through maternal health behaviors that might affect offspring stress regulation and ultimately impact telomere biology, or through multiple stress-related endocrine, inflammatory, oxidative and metabolic pathways during intrauterine life (Entringer et al., 2018). Evidence for the latter comes from studies examining stress-related biological processes that mediate the effect of stress on telomere biology during adult life. For example, biological markers of inflammation, such as C-Reactive Protein, Interleukin-6, and tumor necrosis factor-α have been linked to telomere shortening (Bekaert et al., 2007; Kiecolt-Glaser et al., 2011; Osler, Bendix, Rask, & Rod, 2016) and telomerase activity (Akiyama et al., 2004). Furthermore, studies that have examined associations in the context of pregnancy reported links between dehydroepiandrosterone sulfate (DHEAS), proinflammatory states (Lazarides et al., 2019), reactive oxygen species (Liu et al., 2017), and maternal estrogen levels (Entringer et al., 2015) with newborn TL.
Our results further highlight the need for a deeper understanding of the intergenerational transmission of stressor effects in Black mothers. The relation between pregnancy stressor exposure and shorter offspring TL was not observed in the offspring of Black mothers, nor maternal stressors at any other time point. Prior studies that have reported links have been primarily performed in White/European samples (Entringer et al., 2011; Send et al., 2017; Verner et al., 2021). Prior studies that included Black participants were either underpowered (e.g. Entringer et al. 2013; Marchetto et al. 2016) or did not examine interactions between pregnancy stressors and race (Enlow et al., 2018; Esteves et al., 2020). Clearly, further research on race effects in the transmission of pregnancy stressor effects across generations is needed.
We can only speculate about the lack of findings for offspring of Black mothers. Race-based experiential theories may offer an explanation. It is possible that Black mothers – despite their greater stressor exposure – did not perceive the stressors as threatening or upsetting (see Brown et al., 2020), which might buffer the effects of maternal stress on offspring TL. Furthermore, the development of culturally relevant coping strategies adapted by Black women, such as John Henryism (Robinson & Thomas Tobin, 2021) and Supeiwoman Schema (Woods-Giscombé, 2010), could have mitigated maternal stress effects. These coping styles are likely a ‘double-edged sword’ (Perez et al., 2022). Although prior studies have found detrimental effects of John Henryism on physical health and allostatic load (Robinson & Thomas Tobin, 2021), this coping style has also been shown to be protective for Black women’s mental health (Bronder, Speight, Witherspoon, & Thomas, 2014), which could have exerted protective effects. Similarly, Superwoman Schema has been linked to detrimental health (Woods-Giscombé et al., 2019), but recent research suggests that effects vary based on the specific Superwoman Schema dimensions, with some aspects being protective (e.g. presenting strength, emotion suppression) and others exacerbating the impact of racial discrimination stress on allostatic load (motivation to succeed, obligation to help others; Allen et al., 2019). Unfortunately, race-based experiential theories that could explain the lack of finding in offspring of Black women could not be directly tested in these data. Examining stress perceptions (in addition to exposures), protective factors and unique coping resources and experiences of Black women warrant further investigation.
Another explanation for the lack of findings for offspring of Black mothers is drawing on race-based differences in telomere dynamics. Most studies have found that Black individuals have longer TL (e.g. Brown et al. 2017; Codd et al. 2021). Black American individuals have been shown to have a higher genetic loading for longer telomeres, in part through genes for telomerase (Hamad, Tuljapurkar, & Rehkopf, 2016). Black individuals also have been found to have higher levels of telomerase, tested in at least one study (Kroenke et al., 2012). Therefore, Black mothers in the present study may not only have longer telomeres, but also higher telomerase, which might be protective in the face of stressor exposure. Assessing both TL and telomerase as well as tracking trajectories over time may provide insights into racial differences in the intergenerational transmission of maternal stressor effects.
Post-hoc analyses examining type of pregnancy stressors in relation to offspring TL showed that maternal financial (but not social) stressors were associated with shorter TL in children of White mothers. These findings advance prior studies that did not distinguish between financial and social stressors occurring during pregnancy or that assessed overall stress perceptions. The three most common financial pregnancy stressors in our sample involved partner’s job loss, self unwanted job loss, and inability to pay bills. Therefore, our results are consistent with a systematic review that concluded that lower parental SES (often measured by household income) is associated with shorter TL in children (Coimbra, Carvalho, Moretti, Mello, & Belangero, 2017). The stressors assessed here represented chronic financial difficulties and acute life events (job loss), which can both induce a physiological chronic stress response during pregnancy. The resulting financial and employment insecurity and related difficulties to provide for the child may have contributed to a chronic threat state for the pregnant mother, worried about stability and wellbeing of herself and her child – although this interpretation is speculative as the subjective severity of financial difficulties was not directly measured. Furthermore, financial and employment-related stressors might pose long-term chronic stressors that cannot be offset easily by psychosocial resources. Our findings suggest that financial stressors (e.g. financial insecurity, unemployment) may be particularly influential in shaping offspring health and thus important to address in interventions. Future research may also investigate the subjective severity of financial and employment difficulties in addition to whether the stressor occurred or not.
Post-hoc analyses revealed that maternal pregnancy stressors may have sex-specific effects on offspring telomere biology. Specifically, the relation between financial pregnancy stressors and offspring TL was significant for boys, but not girls, of White mothers. Females have longer telomeres than males (Gardner et al., 2014), perhaps due to the protective effects of endogenous estrogen on biomarkers of aging (e.g. upregulating telomerase activity; Lulkiewicz, Bajsert, Kopczynski, Barczak, and Rubis, 2020). Greater exposure to estrogen during pregnancy might make girls more resistant to maternal stress exposure effects. Males are also exposed to higher cortisol levels across trimesters, perhaps mediating sex-specific effects of maternal stressor exposure on newborn TL (Enlow et al., 2019). In sum, our finding is consistent with prior studies showing that males may be more susceptible to maternal exposure effects on offspring TL (Enlow et al., 2018; Enlow, Petty, Hacker, & Burris, 2021), but conclusions are limited by our relatively small cell sizes. Examining sex effects in the maternal transmission of stress on telomere biology remains an open research area.
We did not find an effect of maternal stressors occurring during late childhood/adolescence or across the life course on offspring TL. One explanation is that maternal exposures might have the greatest impact on offspring TL in utero – a sensitive period when the shared socio-biological environment is maximized. Alternatively, it is possible that maternal adolescence and lifespan adversity are less relevant for offspring TL and that preconception stressors in closer proximity to conception (e.g. within 3 months) should be assessed in future studies (Carroll et al., 2020). Although this is the first study to examine the effects of maternal lifespan stressor exposure, prior studies have seen a relation of maternal childhood adversity with offspring TL (Enlow et al., 2018; Esteves et al., 2020). Given evidence that particularly the early developmental years are important for TL (Mayer et al., 2019), it is also possible that our prospective measure captured stressor exposure too late in childhood/adolescence (stressors before age 16 were not assessed). Nevertheless, both adolescence and lifespan stressor exposure can shape stress physiology and ultimately impact pregnancy health (Entringer et al., 2018; Keenan et al., 2018). Therefore, maternal stressors occurring during adolescence or across the lifespan might still directly influence offspring TL mediated by effects on parental germline telomeres pre-fertilization and its effects on the inherited TL by the offspring (Entringer et al., 2018; Haussmann & Heidinger, 2015). In this case, controlling for maternal TL might have obscured effects. Nevertheless, non-significant results for the associations between maternal adolescence and lifespan adversity with race on offspring TL remained when maternal TL was not included in the regression models. In sum, this remains an open research question and our null findings require replication in future studies.
Limitations and strengths
Limitations include that pregnancy and lifetime stressor exposure was based on retrospective self-report, which can be influenced by recall and reporting biases (Hardt & Rutter, 2004), and current mood states (Maughan & Rutter, 1997). Therefore, it is possible that the maternal stressor measures may measure negative biases in autobiographical memory or current affective states rather than actual stressor exposure (Danese & Widom, 2020). However, the stress assessment instruments used both measure stressors that are moderate-to-severe in nature, which are less impacted by recall biases (Krinsley, Gallagher, Weathers, Kutter, & Kaloupek, 2003). Furthermore, lifespan stressor count assessed by the STRAIN has been shown to be robust to recall bias as well as mood and social desirability effects (Slavich & Auerbach, 2018; Slavich & Shields, 2018). Furthermore, to account for mood biases with retrospective measures at least partly, we conducted sensitivity analyses that adjusted for depressive symptoms at the time of assessment, which did not affect the results. Moreover, prior research has shown that interview-based assessments of major life stressors can be reliably recalled across the lifespan (Brown & Harris, 1978). Nevertheless, assessment measures have been largely developed and normed on White samples, so it is possible that unique stressors and experiences of Black women are not fully captured by these state-of-the-art instruments.
Another limitation is that we did not have information regarding the timing of pregnancy stressors, despite evidence that the effect of pregnancy stress on child development is time dependent (e.g. see Project Ice Storm findings; Cao, Laplante, Brunet, Ciampi, & King, 2014; King & Laplante, 2005). Specifically, prior research showed that higher maternal perceived stress in the 3rd trimesters was predictive of shorter child buccal TL (Carroll et al., 2020), so this remains an open research question.
In addition, this study only enrolled individuals who identified as Black and White, capitalizing on the original aims of the NGHS to examine factors associated with racial disparities in the onset and development of obesity and cardiovascular disease, specifically in Black and White females. This design provided a unique opportunity to understand the intersection of maternal race and stressor exposure leveraging a population-based study that was designed to enroll a relatively large sample of Black and White women who were within a similar range of SES (sufficient representation of higher-SES Black participants and lower SES White participants). Nevertheless, given the dearth of research in non-White samples (Conradt et al., 2020) and the complexities involved in the analysis of the intertwined effects of social, historical and biological factors on health (Kaufman & Cooper, 2008), future studies designed to do so should specifically examine the intergenerational transmission of stress within Black samples as well as other understudied racial/ethnic communities. Understanding how racial disparities in health originate and transmit across generations is a critical public health problem.
A further limitation is that TL was assessed from saliva samples. This may limit direct comparison with findings obtained from other tissue types given that telomere attrition rates vary by cell types. However, TL obtained from salivary DNA is frequently used in population-based cohorts and correlated with TL obtained from blood samples (Mitchell et al., 2014; Stout et al., 2017). Furthermore, telomeres were measured using quantitative PCR, which may be vulnerable to measurement error. However, we extracted DNA from all samples in the same batch and assayed samples in the same assay batch to minimize potential. The coefficient of variation for this study was therefore low. Another limitation is that TL was only assessed at one time point, limiting conclusions about how maternal stressor exposure and race are associated with offspring telomere attrition rates over time. Furthermore, we did not have a measure of TL at birth. It is plausible that differences in TL were present at birth (e.g. in offspring of Black mothers), but compensated over time through telomere repair mechanisms (Salvador et al., 2016; Zhao, Pan, Liu, & Liu, 2014).
Nevertheless, this study had several strengths including a comprehensive, multi-time point stressor assessment that enabled us to examine how different types of maternal stressors, occurring during different time periods, were related to offspring TL. Additionally, the large sample size of Black and White women enabled us to test stressor-by-race interactions in the intergenerational transmission. Moreover, this study is among the few that controlled for maternal TL (c.f. Send et al., 2017), decreasing the likelihood that mothers directly transmitted shorter TL to their offspring. Finally, we collected data on life stressors in later childhood/adolescents prospectively, before pregnancy, contributing to existing literature using retrospective reports of maternal childhood adversity.
Conclusion
In conclusion, these data show that maternal stressors occurring during pregnancy, and in particular financial stressors occurring during this time period, are associated with offspring TL, but only for children of White mothers. Given the scarcity of research with diverse samples, understanding the intersection of race and the transmission of stress effects across generations remains an important area of research. In terms of targeting interventions, it may be beneficial to identify women with elevated life stressor exposure occurring during the critical period of pregnancy. Interventions could target food insecurity, financial strain and housing instability, and increase social support. Such intervention efforts could improve both maternal and child health outcomes across the lifespan, including aging-related diseases.
Supplementary Material
Acknowledgments.
We thank the Nutrition Policy Institute who provided consultation and support with historical study data. Most of all, we thank our incredible study participants.
Financial support.
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development grant R01HD073568, the National Heart, Lung, and Blood Institute grant R56HL141878, the National Institute on Aging grants R56AG059677 & R01AG059677, as well as the National Institute on Aging grants K99AG062778 and R00AG062778 (principal investigator Stefanie Mayer, PhD). This work was further supported by the Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) K12 award, grant number K12 HD051958 (principal investigators Ellen Gold, PhD 2013–2019; Nancy Lane, MD 2020) funded by the National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development, Office of Research on Women’s Health, Office of Dietary Supplements, and the National Institute on Aging. George M. Slavich was supported by grant #OPR21101 from the California Governor’s Office of Planning and Research/California Initiative to Advance Precision Medicine. These organizations had no role in designing or planning this study; in collecting, analyzing, or interpreting the data; in writing the article; or in deciding to submit this article for publication.
Footnotes
Supplementary material. The supplementary material for this article can be found at https://doi.org/10.1017/S0033291722003397
Linear regression models using Hayes (2017) PROCESS Model 1 (with 5000 bootstrap resamples) yielded the same results.
The notes appear after the main text.
Conflict of interest. All authors declare no conflict of interest.
References
- Akiyama M, Yamada O, Hideshima T, Yanagisawa T, Yokoi K, Fujisawa K, … Anderson KC (2004). TNFα induces rapid activation and nuclear translocation of telomerase in human lymphocytes. Biochemical and Biophysical Research Communications, 316(2), 528–532. [DOI] [PubMed] [Google Scholar]
- Allen AM, Wang Y, Chae DH, Price MM, Powell W, Steed TC, … Woods-Giscombe CL (2019). Racial discrimination, the superwoman schema, and allostatic load: Exploring an integrative stress-coping model among African American women. Annals of the New York Academy of Sciences, 1457(1), 104–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ämmälä A-J, Vitikainen EI, Hovatta I, Paavonen J, Saarenpää-Heikkilä O, Kylliäinen A, … Paunio T (2020). Maternal stress or sleep during pregnancy are not reflected on telomere length of newborns. Scientific Reports, 10(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A (2008). The epidemiology of human telomeres: Faults and promises. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 63(9), 979–983. [DOI] [PubMed] [Google Scholar]
- Barker DJ (2007). The origins of the developmental origins theory. Journal of Internal Medicine, 261(5), 412–417. [DOI] [PubMed] [Google Scholar]
- Bauer GR, Churchill SM, Mahendran M, Walwyn C, Lizotte D, & Villa-Rueda AA (2021). Intersectionality in quantitative research: A systematic review of its emergence and applications of theory and methods. SSM Population Health, 14, 100798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekaert S, De Meyer T, Rietzschel ER, De Buyzere ML, De Bacquer D, Langlois M, … Cassiman P (2007). Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell, 6(5), 639–647. [DOI] [PubMed] [Google Scholar]
- Blackburn EH, Epel ES, & Lin J (2015). Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science (New York, N.Y.), 350(6265), 1193–1198. [DOI] [PubMed] [Google Scholar]
- Bödeker K, Fuchs A, Führer D, Kluczniok D, Dittrich K, Reichl C, … Möhler E (2019). Impact of maternal early life maltreatment and maternal history of depression on child psychopathology: Mediating role of maternal sensitivity? Child Psychiatry & Human Development, 50(2), 278–290. [DOI] [PubMed] [Google Scholar]
- Broer L, Codd V, Nyholt DR, Deelen J, Mangino M, Willemsen G, … De Geus EJ (2013). Meta-analysis of telomere length in 19 713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. European Journal of Human Genetics, 21(10), 1163–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronder EC, Speight SL, Witherspoon KM, & Thomas AJ (2014). John Henryism, depression, and perceived social support in Black women. Journal of Black Psychology, 40(2), 115–137. [Google Scholar]
- Brown GW, & Harris T (1978). Social origins of depression: A study of psychiatric disorder in women (1st American ed.). New York: Free Press. [Google Scholar]
- Brown LL, Mitchell UA, & Ailshire JA (2020). Disentangling the stress process: Race/ethnic differences in the exposure and appraisal of chronic stressors among older adults. The Journals of Gerontology: Series B, 75(3), 650–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LL, Needham B, & Ailshire J (2017). Telomere length among older US adults: Differences by race/ethnicity, gender, and age. Journal of Aging and Health, 29(8), 1350–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Laplante DP, Brunet A, Ciampi A, & King S (2014). Prenatal maternal stress affects motor function in 5½-year-old children: Project ice storm. Developmental Psychobiology, 56(1), 117–125. [DOI] [PubMed] [Google Scholar]
- Carroll JE, Mahrer NE, Shalowitz M, Ramey S, & Schetter CD (2020). Prenatal maternal stress prospectively relates to shorter child buccal cell telomere length. Psychoneuroendocrinology, 121, 104841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM (2002). Telomere measurement by quantitative PCR. Nucleic Acids Research, 30(10), e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2005). Phase 5 core questionnaire–pregnancy stressful life events. Pregnancy risk assessment monitoring system (PRAMS). Washington, DC: CDC. www.cdc.gov/prams/pdf/phase5_corequestions.pdf. [Google Scholar]
- Codd V, Denniff M, Swinfield C, Warner SC, Papakonstantinou M, Sheth S, … Bountziouka V (2021). A major population resource of 474074 participants in UK Biobank to investigate determinants and biomedical consequences of leukocyte telomere length. medRxiv. [Google Scholar]
- Coimbra BM, Carvalho CM, Moretti PN, Mello MF, & Belangero SI (2017). Stress-related telomere length in children: A systematic review. Journal of Psychiatric Research, 92, 47–54. [DOI] [PubMed] [Google Scholar]
- Conradt E, Carter SE, & Crowell SE (2020). Biological embedding of chronic stress across two generations within marginalized communities. Child Development Perspectives, 14(4), 208–214. [Google Scholar]
- Danese A, & Widom CS (2020). Objective and subjective experiences of child maltreatment and their relationships with psychopathology. Nature Human Behaviour, 4(8), 811–818. [DOI] [PubMed] [Google Scholar]
- Darrow SM, Verhoeven JE, Révész D, Lindqvist D, Penninx BW, Delucchi KL, … Mathews CA (2016). The association between psychiatric disorders and telomere length: A meta-analysis involving 14,827 persons. Psychosomatic Medicine, 78(7), 776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enlow MB, Bollati V, Sideridis G, Flom JD, Hoxha M, Hacker MR, & Wright RJ (2018). Sex differences in effects of maternal risk and protective factors in childhood and pregnancy on newborn telomere length. Psychoneuroendocrinology, 95, 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enlow MB, Petty CR, Hacker MR, & Burris HH (2021). Maternal psychosocial functioning, obstetric health history, and newborn telomere length. Psychoneuroendocrinology, 123, 105043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enlow MB, Sideridis G, Bollati V, Hoxha M, Hacker MR, & Wright RJ (2019). Maternal cortisol output in pregnancy and newborn telomere length: Evidence for sex-specific effects. Psychoneuroendocrinology, 102, 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, de Punder K, Buss C, & Wadhwa PD (2018). The fetal programming of telomere biology hypothesis: An update. Philosophical Transactions of the Royal Society B: Biological Sciences, 373(1741), 20170151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Epel ES, Kumsta R, Lin J, Hellhammer DH, Blackburn EH, … Wadhwa PD (2011). Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proceedings of the National Academy of Sciences, 108(33), E513–E518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Epel ES, Lin J, Blackburn EH, Buss C, Simhan HN, & Wadhwa PD (2015). Maternal estriol concentrations in early gestation predict infant telomere length. Journal of Clinical Endocrinology & Metabolism, 100(1), 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Epel ES, Lin J, Buss C, Shahbaba B, Blackburn EH, … Wadhwa PD (2013). Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. American Journal of Obstetrics and Gynecology, 208(2), 134–e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES (2020). Can childhood adversity affect telomeres of the next generation? Possible mechanisms, implications, and next-generation research. American Journal of Psychiatry, 177(1), 7–9. [DOI] [PubMed] [Google Scholar]
- Epel ES, & Prather AA (2018). Stress, telomeres, and psychopathology: Toward a deeper understanding of a triad of early aging. Annual Review of Clinical Psychology, 14, 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves KC, Jones CW, Wade M, Callerame K, Smith AK, Theall KP, & Drury SS (2020). Adverse childhood experiences: Implications for offspring telomere length and psychopathology. American Journal of Psychiatry, 177(1), 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Factor-Litvak P, Susser E, Kezios K, McKeague I, Kark JD, Hoffman M, … Aviv A (2016). Leukocyte telomere length in newborns: Implications for the role of telomeres in human disease. Pediatrics, 137(4), e20153927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde AT, Crookes DM, Suglia SF, & Demmer RT (2019). The weathering hypothesis as an explanation for racial disparities in health: A systematic review. Annals of Epidemiology, 33, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franko D, Striegel-Moore R, Brown K, Barton B, McMahon R, Schreiber GB, … Daniels SR (2004). Expanding our understanding of the relationship between negative life events and depressive symptoms in black and white adolescent girls. Psychological Medicine, 34, 1319–1330. [DOI] [PubMed] [Google Scholar]
- Gardner M, Bann D, Wiley L, Cooper R, Hardy R, Nitsch D, … Ben-Shlomo Y (2014). Gender and telomere length: Systematic review and meta-analysis. Experimental Gerontology, 51, 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Hicken AT, Pearson JA, Seashols SJ, Brown KL, & Cruz TD (2010). Do US Black women experience stress-related accelerated biological aging? Human Nature, 21(1), 19–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giscombé CL, & Lobel M (2005). Explaining disproportionately high rates of adverse birth outcomes among African Americans: The impact of stress, racism, and related factors in pregnancy. Psychological Bulletin, 131(5), 662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan A, Thomas M, Vittinghoff E, Bowleg L, Mangurian C, & Wesson P (2021). An investigation of quantitative methods for assessing intersectionality in health research: A systematic review. SSM-Population Health, 16, 100977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamad R, Tuljapurkar S, & Rehkopf DH (2016). Racial and socioeconomic variation in genetic markers of telomere length: A cross-sectional study of US older adults. EBioMedicine, 11, 296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt J, & Rutter M (2004). Validity of adult retrospective reports of adverse childhood experiences: Review of the evidence. Journal of Child Psychology and Psychiatry, 45(2), 260–273. [DOI] [PubMed] [Google Scholar]
- Haussmann MF, & Heidinger BJ (2015). Telomere dynamics may link stress exposure and ageing across generations. Biology Letters, 11(11), 20150396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, & Willeit P (2014). Leucocyte telomere length and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ, 349, g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2017). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: Guilford publications. [Google Scholar]
- Kaufman JS, & Cooper RS (2008). Telomeres and race: What can we learn about human biology from health differentials? Aging Cell, 7(4), 448–450. [DOI] [PubMed] [Google Scholar]
- Keenan K, Hipwell AE, Class QA, & Mbayiwa K (2018). Extending the developmental origins of disease model: Impact of preconception stress exposure on offspring neurodevelopment. Developmental Psychobiology, 60(7), 753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin J-P, Weng N-P, Malarkey WB, Beversdorf DQ, & Glaser R (2011). Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosomatic Medicine, 73(1), 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S, & Laplante DP (2005). The effects of prenatal maternal stress on children’s cognitive development: Project ice storm. Stress (Amsterdam, Netherlands), 8(1), 35–45. [DOI] [PubMed] [Google Scholar]
- Krinsley KE, Gallagher JG, Weathers FW, Kutter CJ, & Kaloupek DG (2003). Consistency of retrospective reporting about exposure to traumatic events. Journal of Traumatic Stress, 16(4), 399–409. [DOI] [PubMed] [Google Scholar]
- Kroenke CH, Pletcher MJ, Lin J, Blackburn E, Adler N, Matthews K, & Epel E (2012). Telomerase, telomere length, and coronary artery calcium in black and white men in the CARDIA study. Atherosclerosis, 220(2), 506–512. [DOI] [PubMed] [Google Scholar]
- Lazarides C, Epel ES, Lin J, Blackburn EH, Voelkle MC, Buss C, … Entringer S (2019). Maternal pro-inflammatory state during pregnancy and newborn leukocyte telomere length: A prospective investigation. Brain Behavior Immunity, 80, 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Epel E, & Blackburn E (2012). Telomeres and lifestyle factors: Roles in cellular aging. Mutation Research, 730(1–2), 85–89. [DOI] [PubMed] [Google Scholar]
- Liu CH, Giallo R, Doan SN, Seidman LJ, & Tronick E (2016). Racial and ethnic differences in prenatal life stress and postpartum depression symptoms. Archives of Psychiatric Nursing, 30(1), 7–12. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhou G, Chen Q, Ouyang F, Little J, Zhang J, & Chen D (2017). Impact of dehydroepiandrosterone sulfate on newborn leukocyte telomere length. Scientific Reports, 7, 42160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MC, & Chen B (2004). Racial and ethnic disparities in preterm birth: The role of stressful life events. American Journal of Obstetrics and Gynecology, 191(3), 691–699. [DOI] [PubMed] [Google Scholar]
- Lulkiewicz M, Bajsert J, Kopczynski P, Barczak W, & Rubis B (2020). Telomere length: How the length makes a difference. Molecular Biology Reports, 47(9), 7181–7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Zhou Z, Wei S, Liu Z, Pooley KA, Dunning AM, … Shen M (2011). Shortened telomere length is associated with increased risk of cancer: A meta-analysis. PLoS One, 6(6), e20466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetto NM, Glynn RA, Ferry ML, Ostojic M, Wolff SM, Yao R, & Haussmann MF (2016). Prenatal stress and newborn telomere length. American Journal of Obstetrics and Gynecology, 215(1), 94–e1 [DOI] [PubMed] [Google Scholar]
- Maughan B, & Rutter M (1997). Retrospective reporting of childhood adversity: Issues in assessing long-term recall. Journal of Personality Disorders, 11(1), 19–33. [DOI] [PubMed] [Google Scholar]
- Mayer SE, Prather AA, Puterman E, Lin J, Arenander J, Coccia M, … Epel ES (2019). Cumulative lifetime stress exposure and leukocyte telomere length attrition: The unique role of stressor duration and exposure timing. Psychoneuroendocrinology, 104, 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays VM, Cochran SD, & Barnes NW (2007). Race, race-based discrimination, and health outcomes among African Americans. Annual Review of Psychology, 58, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C, Hobcraft J, McLanahan SS, Siegel SR, Berg A, Brooks-Gunn J, … Notterman D (2014). Social disadvantage, genetic sensitivity, and children’s telomere length. Proceedings of the National Academy of Sciences, 111(16), 5944–5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J (1992). Obesity and cardiovascular disease risk factors in black and white girls: The NHLBI growth and health study. American Journal of Public Health, 82(12), 1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJ, Kulkarni SC, Michaud C, Tomijima N, Bulzacchelli MT, Iandiorio TJ, & Ezzati M (2006). Eight Americas: Investigating mortality disparities across races, counties, and race-counties in the United States. PLoS Medicine, 3(9), e260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osler M, Bendix L, Rask L, & Rod NH (2016). Stressful life events and leucocyte telomere length: Do lifestyle factors, somatic and mental health, or low grade inflammation mediate this relationship? Results from a cohort of Danish men born in 1953. Brain Behavior Immunity, 58, 248–253. [DOI] [PubMed] [Google Scholar]
- Perez AD, Dufault SM, Spears EC, Chae DH, Woods-Giscombe CL, & Allen AM (2022). Superwoman Schema and John Henryism among African American women: An intersectional perspective on coping with racism. Social Science & Medicine, 115070. [DOI] [PubMed] [Google Scholar]
- Puterman E, Gemmill A, Karasek D, Weir D, Adler NE, Prather AA, & Epel ES (2016). Lifespan adversity and later adulthood telomere length in the nationally representative US health and retirement study. Proceedings of the National Academy of Sciences, 113(42), E6335–E6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. [Google Scholar]
- Rijlaarsdam J, Stevens GW, Jansen PW, Ringoot AP, Jaddoe VW, Hofman A, … Tiemeier H (2014). Maternal childhood maltreatment and offspring emotional and behavioral problems: Maternal and paternal mechanisms of risk transmission. Child Maltreatment, 19(2), 67–78. [DOI] [PubMed] [Google Scholar]
- Robinson MN, & Thomas Tobin CS (2021). Is John Henryism a health risk or resource?: Exploring the role of culturally relevant coping for physical and mental health among Black Americans. Journal of Health and Social Behavior, 62(2), 136–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador L, Singaravelu G, Harley CB, Flom P, Suram A, & Raffaele JM (2016). A natural product telomerase activator lengthens telomeres in humans: A randomized, double blind, and placebo controlled study. Rejuvenation Research, 19(6), 478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraju V, Phillips M, Fouty A, Babu JR, & Geetha T (2021). Telomere length as a biomarker for race-related health disparities. Genes, 12(1), 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Send TS, Gilles M, Codd V, Wolf I, Bardtke S, Streit F, … Sütterlin MW (2017). Telomere length in newborns is related to maternal stress during pregnancy. Neuropsychopharmacology, 42(12), 2407–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenassa ED, Rossen LM, Cohen J, Morello-Frosch R, & Payne-Sturges DC (2017). Income inequality and US children’s secondhand smoke exposure: Distinct associations by race–ethnicity. Nicotine & Tobacco Research, 19(11), 1292–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, & Auerbach RP (2018). Stress and its sequelae: depression, suicide, inflammation, and physical illness. [Google Scholar]
- Slavich GM, & Shields GS (2018). Assessing lifetime stress exposure using the stress and adversity inventory for adults (Adult STRAIN): An overview and initial validation. Psychosomatic Medicine, 80(1), 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slykerman RF, Joglekar MV, Hardikar AA, Satoor SN, Thompson JM, Jenkins A, … Murphy R (2019). Maternal stress during pregnancy and small for gestational age birthweight are not associated with telomere length at 11 years of age. Gene, 694, 97–101. [DOI] [PubMed] [Google Scholar]
- Sternthal MJ, Slopen N, & Williams DR (2011). Racial disparities in health: How much does stress really matter? Du Bois Review, 8(1), 95–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout SA, Lin J, Hernandez N, Davis EP, Blackburn E, Carroll JE, & Glynn LM (2017). Validation of minimally-invasive sample collection methods for measurement of telomere length. Frontiers in Aging Neuroscience, 9, 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RJ (2013). Understanding health disparities: The relevance of the stress process model. Society and Mental Health, 3(3), 170–186. [Google Scholar]
- Verner G, Epel E, Lahti-Pulkkinen M, Kajantie E, Buss C, Lin J, … Entringer S (2021). Maternal psychological resilience during pregnancy and newborn telomere length: A prospective study. American Journal of Psychiatry, 178(2), 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AD, Shenassa E, Slopen N, & Rossen L (2018). Cardiometabolic dysfunction among US adolescents and area-level poverty: Race/ethnicity-specific associations. Journal of Adolescent Health, 63(5), 546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, & Mohammed SA (2009). Discrimination and racial disparities in health: Evidence and needed research. Journal of Behavioral Medicine, 32(1), 20–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods-Giscombé CL (2010). Superwoman schema: African American women’s views on stress, strength, and health. Qualitative Health Research, 20(5), 668–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods-Giscombé CL, Allen AM, Black AR, Steed TC, Li Y, & Lackey C (2019). The Giscombe superwoman schema questionnaire: Psychometric properties and associations with mental health and health behaviors in African American women. Issues in Mental Health Nursing, 2019, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu E (2021). Learning from “racism, not race” for intersectionality research and the research enterprise. Social Work Research, 45(3), 220–224. [Google Scholar]
- Zhao Z, Pan X, Liu L, & Liu N (2014). Telomere length maintenance, shortening, and lengthening. Journal of Cellular Physiology, 229(10), 1323–1329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


