Abstract
Infections are known to trigger flares of autoimmune diseases in humans and serve as an inciting cause of autoimmunity in animals. Evidence suggests a causative role of infections in triggering antigen-specific autoimmunity, previous thought mainly through antigen mimicry. However, an infection can induce bystander autoreactive T and B cell polyclonal activation, believed to result in non-pathogenic and pathogenic autoimmune responses. Lastly, epitope spreading in autoimmunity is a mechanism of epitope changes of autoreactive cells induced by infection, promoting the targeting of additional self-epitopes. This review highlights recent research findings, emphasizes infection-mediated autoimmune responses, and discusses the possible mechanisms involved.
Keywords: Autoimmunity, infectious disease, molecular mimicry, microbes, autoantibodies, bystander activation
1. Introduction
Infectious diseases provoke acute damage directly by pathogens in hosts; however, they may secondarily promote autoimmunity in humans and other animals (Figure 1). Infections are known to trigger flares of autoimmune diseases in humans and serve as an inciting cause of autoimmunity in animals. There is an intricate interplay between the pathogen and the immune response, impacting the maintenance of immune homeostasis and autoimmune development. Several mechanisms are involved in viral, bacterial, fungal, and parasitic infection-mediated autoimmunity. The autoimmunity induced by infections can be through antigen mimicry, the release of hidden self-antigens, epitope spread, and dysregulated innate immune responses. Overall, the mechanisms of infection-mediated autoimmunity can be stratified by antigen-specific and non-specific (bystander) pathways. Examples of specific pathogens, their involvement in autoimmune diseases, and their proposed mechanisms of autoimmune contributions are summarized in Table 1.
Figure 1.
Infection-mediated autoimmunity: Normal immunity vs. Autoimmunity. Normal immunity involves degradation of infectious agents with pathogenic antigen epitope presentation by APCs (1). APCs present pathogen epitopes to B and T cells (2), resulting in the production of cytokines, antibodies, or cytotoxic T cell activity (3). In infection-mediated autoimmunity, autoreactive T and/or B cells can be activated by self-antigen epitopes presented by APCs or through bystander activation (4-6). The activation of autoreactive cells promotes an autoimmune response through the production of an autoimmune cytokine storm or pathogenic autoantibodies that target host cells/tissues.
Table 1.
Summary table of infectious agents linked to autoimmune disease(s).
| Infectious agent | Organismal Group |
Organism Infected |
Autoimmune Disease(s) |
Proposed Mechanism |
Study |
|---|---|---|---|---|---|
| Epstein-Barr virus | Virus | Humans | Sjogren’s syndrome, SLE, MS | Molecular mimicry | 43-46 |
| Coxsackievirus and MCMV | Virus | Humans and mice | Myocarditis | Molecular mimicry | 54,55 |
| HBV | Virus | Humans and mice | Hepatitis | Molecular mimicry | 41,58 |
| Influenza A virus | Virus | Humans | T1DM Henoch-Schönlein purpura | Molecular mimicry | 62-64,66,68,69 |
| TMEV | Virus | Mice | MS-like | Epitope spreading | 76 |
| SARS-CoV-2 | Virus | Humans | MS, Severe dermatitis, Acute disseminated encephalomyelitis | Molecular mimicry, Bystander activation | 91-97 |
| HIV | Virus | Humans | RA, SLE | Bystander activation | 113- 115,118 |
| Campylobacter jejuni | Bacteria | Humans | GBS | Molecular mimicry | 134,137 |
| Pseudomonas aeruginosa | Bacteria | Humans | Arthritic complication in cystic fibrosis | Molecular mimicry | 139 |
| Staphylococcus aureus | Bacteria | Humans and mice | SLE | Bystander activation | 163,171 |
| Aspergillus genera | Fungi | Humans | MS | - | 190 |
| Saccharomyces genera | Fungi | Humans | MS | - | 190 |
| Candida albicans | Fungi | Humans | T1 DM | - | 196 |
| Gliocladium fimbriatum | Fungi | Mice | EAE | - | 197 |
| Aspergillus fumigatus | Fungi | Mice | EAE | - | 197 |
| Trypanosoma brucei | Parasite | Humans and mice | Anemia | Bystander activation | 206 |
1.1. Mechanisms of pathogen-mediated autoimmune responses.
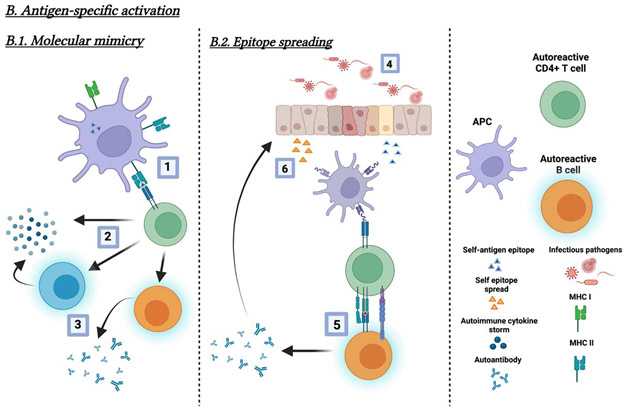
Injection of pristane (a mineral oil component) results in autoimmunity in mice 1, suggesting that an environmental factor can induce autoimmune disease. Antigens from pathogens could trigger autoimmunity by 1) antigenic similarity between microbial molecules and host antigens (antigenic mimicry); 2) innate immune signals triggered by pathogens or pathogen products could enhance the immunogenicity of host antigens or host-mimicking epitopes, thereby perturbing regulatory immunity and resulting in the breakdown of tolerance 2; or 3) the autoimmunity targets self-epitopes or self-antigens released during pathogen infections3 (Figure 2).
Figure 2.
Model of molecular and cellular mechanisms of infection-mediated autoimmunity. (A) Bystander (non-specific) activation mechanism. Bystander activation in infection or inflammation-mediated autoimmunity primarily occurs via cytokines (e.g., IL-6) and cytokine receptors on autoreactive cells. Part of this model (1) depicts a “leaky” gut and microbial product translocation to circulation involving APC presentation of self-antigens to autoreactive cells (2). Or, microbial products (e.g., LPS) directly activate autoreactive B cells (3) via their receptors (e.g., TLR4) without APC presentation and T cell help. The process overall involves (a) T cell dependent or (b) T cell independent autoreactive B cell activation, which may result in the production of autoantibodies. (B.1) Molecular mimicry. In molecular mimicry, self-epitopes structurally similar to pathogenic antigen epitopes are presented by APCs (4); APCs present them to autoreactive B and T cells (5), resulting in the production of autoantibodies or autoreactive T cell mediated autoimmunity (6). Additionally, the immune responses against self-epitope mimicry may accelerate the cellular and tissue damage, resulting in the release of new self-antigens. (B.2) Epitope spreading. Infection-mediated autoimmunity via epitope spreading refers to the development of autoimmunity to self-epitopes distinct from the initial pathogen-specific epitope during infections. Infection mediated damage (4) results in APC presentation of self-antigen epitopes and the activation of autoreactive cells (5). The development of T and B cell autoimmunity results from the production of new autoantibodies and self-antigens from further host cell damage (6). Subsequent autoreactive T cell responses occur following further presentation by APCs.
The exact mechanisms of infection-mediated bystander autoimmune activation are not fully understood. For instance, short-term and especially long-term repeated stimulation of pathogens or their components via acute and chronic infection or colonization may trigger pathogenic autoimmune responses (e.g., pathogenic anti-double stranded DNA [anti-dsDNA] autoantibody production 4,5) via innate immune response-mediated breakdown of tolerance. This review discusses several potential mechanisms of bystander immune activation mediated autoimmunity following infections by pathogens or microbe colonization.
1.2. Possible risk factors.
Sex. Antigen-specific antibody responses against self-antigens and foreign antigens in response to pathogens are generally higher in females than males 6,7. X-chromosome-related genes, toll-like receptor 7 (TLR7) responsiveness, T regulatory cells (Treg), and sex hormones play a role in the sex differences in T cell and B cell antibody responses 6,8-11. In a previous study by Grimaldi et al. using mouse B cells, estrogen stimulation resulted in the upregulation of several genes (e.g., cd22, shp-1, bcl-2, and vacm-1) through estrogen receptors on B cells and as a consequence of B cell activation and survival12, suggesting a role of estrogen in threshold alteration for B cell apoptosis. Additionally, X-chromosome dosing is a phenomenon generally associated with sex differences in autoimmunity. A higher risk of autoimmunity was associated with the presence of two or more X chromosomes due to increased expression of X-linked immune-response genes (e.g., TLR7)13,14. TLR7 recognizes endogenous RNA-containing autoantigens and induces the expression of type I IFN, a central pathogenic cytokine in SLE15. Using samples from patients with systemic lupus erythematous (SLE) under 16-years-old, one study reported TLR7 levels with notice of more than 2 copies of TLR7 in ~22% of female patients compared with healthy female controls16. Another study in patients with SLE noted that a more pronounced IFN signature was associated with overexpression of specific TLR7 transcripts17. Although various methods of gene dosage compensation are used to equalize the differences of sex chromosome gene number18-20, a subset of individuals maintain varying gene dosages likely due to the breakdown of X chromosome inactivation, contributing to autoimmunity21. Genetic factors. Certain genetic factors are associated with different autoimmune diseases, including specific HLA-DR types 22, deficiency of complement components 23, functional variants in the B cell genes (e.g., BANK1) 24, and gene expression in transcription factors related to the innate immune pathway (e.g., interferon (IFN)-α signaling 25,26). For example, genetic analysis results show specified genetic overlap shared loci between SLE and 16 different autoimmune and inflammatory diseases (i.e., Celiac disease, Rheumatoid arthritis, Crohn’s disease, and others). In brief, genome-wide association studies showed IL23R, TNFAIP3, and IL2RA as loci largely associated with autoimmune diseases27. The autoimmunity-related genetic loci were supported by other genetic studies in autoimmune diseases28-30. These findings reveal the importance of T cell response and innate immunity in autoimmunity development while demonstrating the role of genetic predisposition in inflammation and autoimmunity. Innate immunity and inflammation play a key role in autoimmunity 31. TLRs are pattern recognition receptors (PRRs); their ligand recognition and engagement can initiate innate immunity 31. TLRs (TLR2, TLR4, TLR7, and TLR9) and ligands have been linked to autoimmunity 32‘33. Inflammation, especially long-term inflammation, is triggered by infection, microbial components, or other stimuli as known drivers for polyclonal T and B cell activation and many autoantibodies. Numerous physiologic roles are attributed to translocated bacterial cell wall components following colonization 34-37. Less is known regarding their role in autoimmunity. Others. The other risk factors include but are not limited to age and race 38.
2. Virus and autoimmunity
2.1. Virus-induced autoimmune responses via molecular mimicry (antigen specific)
Infectious diseases have long been considered as one of the triggers for autoimmunity, mainly via molecular mimicry39. Molecular mimicry (Figure 2B.1) occurs when an antigen shares a similar sequence or structure to self-antigens, resulting in T and B cells recognizing the self-antigens as foreign40,41. Initially discovered using mouse antibodies to the measles virus and herpes simplex virus, the antibodies reacted to the proteins of the measles and herpes simplex viruses and to the intermediate filaments of conventional cells42. Studies continue to investigate the interplay between host and viral antigens contributing to the immune-mediated attack on hosts.
Epstein-Barr virus (EBV) is associated with various autoimmune disorders (e.g., Sjögren’s syndrome, SLE, and multiple sclerosis [MS])43,44. An early investigation sought to understand the association between multiple sclerosis and EBV reactivation in genetically susceptible patients45. Later, a shared sequence homology was determined between the open reading frames of several viruses (not limited to EBV) and myelin epitopes, which initiated the presentation of myelin antigens through MHCI/II in the central nervous system (CNS) and induced MS46. The molecular mimicry was between EBV DNA polymerase (EBV 627-641) and myelin antigens, namely the myelin basic protein (MBP) MBP 85-9947; antigen presentation activated EBV-specific CD4+ T cells cross-reactive with self-peptides from myelin in the CNS and results in demyelination 48. Additionally, MS-mediated systemic inflammation was associated with chronic/recurrent viral infection of B cells47, likely resulting from inflammation-mediated viral replication and reactivation.
The most common cause of myocarditis is a viral infection. Coxsackievirus, parvovirus, human herpes virus, adenovirus, murine cytomegalovirus (MCMV), and several other viruses have been reported to play a role in the development of myocarditis49-52. The perturbation of host immune responses to viral infection promotes the induction of myocarditis, including infection-induced inflammation, natural killer and antigen-presenting cell (APC) cytotoxicity, and autoreactive B cell activation and pathogenic autoantibody production53. The induction of autoimmune myocarditis is reported as a consequence of coxsackievirus and MCMV infections; infection-mediated myocardial injury mediates cell-associated self-antigens released from tissue damage which can activate autoreactive B cells to produce tissue-specific autoantibodies 54. Two classic phases are detailed involving 1) viral-induced cardiomyocyte damage and inflammation followed by 2) an autoimmune-mediated phase correlated with myocarditis auto-immunity. Specifically, the major target antigen for the T helper cell-mediated attack was the cardiac α-myosin heavy chain (myhcα) in mice55. Section examination of mouse hearts suggests a direct anti-myhcα IgG deposition, triggering cardiomyocyte damage via autoantibody deposition on cardiomyocytes and complement activation in mice 56-57. Moreover, some host myocardial cellular antigens share similar epitopes with viral antigens that can induce chronic inflammation and autoimmunity 49 53.
Hepatitis B virus (HBV) polymerase cross-reacts with myelin protein and can trigger CNS inflammatory lesions 41,58,59. Previous computational analysis examined the encephalitogenic site of MBP and found MBP and HBV-associated autoimmunity41. Sequences of HBV viral proteins were compared with the encephalitogenic site of MBP, followed by immunologic studies to determine cross-reactivity and induction of CNS autoimmune disease in vitro and in vivo with mouse models. Furthermore, many animal viruses and MBP showed amino acid homologies, suggesting the generation of an inflammatory response at the location containing the specific self-protein. Reports of extrahepatic diseases associated with HBV infection are relatively uncommon. However, they show a strong association between HBV infection and autoimmunity, suggesting genetic background of autoimmunity is likely involved in host’s susceptibility 60.
Influenza infection outcomes vary dependent on acute versus long-term infection with a late autoimmune response observed in some individuals 61. In clinical case reports of prolonged infection, dendritic cell (DC) mediated innate immune response activates autoreactive T cells and autoreactive B cells resulting in autoantibody production 62. Although the mechanism leading to influenza-associated autoimmune disease is ill-defined, there is a noticeable increase in autoantibody production in response to influenza infections and vaccinations in reports63-66. Specifically, there is an increased production of antiphospholipid (aPL) antibodies (e.g., anticardiolipin [aCL] and/or anti-β2 glycoprotein I [β2-GPI] antibodies) following infection or vaccination, probably through the mechanism of molecular mimicry between peptide domain of various bacteria/viruses and a β2-GPI molecule (i.e., hexapeptide [TLRVYK])67. Other autoimmune responses associated with an influenza infection include diabetes mellitus type I, Henoch-Schoenlein purpura, and schizophrenia in rare cases 64,68,69. Furthermore, there were case reports of autoimmune development of the peripheral nervous system autoimmune disease, Guillain-Barre syndrome (GBS), an aftereffect of the 1976 swine influenza vaccine 70.
2.2. Virus-induced bystander autoimmune responses (antigen non-specific)
While viral infection-mediated antigen-specific autoimmunity is one mechanism leading to immune system dysregulation, autoimmune responses can also be triggered non-specifically through bystander activation 40,71. Bystander activation (e.g., cytokines) can stimulate autoreactive T and B cells non-specifically following pathogen recognition, leading to autoimmune responses (Figure 2A). Autoimmune responses can be induced by activated autoreactive T and B cells in response to a number of self-antigens released from tissue damage following infection and pathogen recognition 72. In most cases, autoimmunity via a bystander pathway is believed as short-term with no clinical pathogenic effects. However, bystander pathway-mediated autoimmune responses can be harmful in hosts, especially presenting with the genetic background of autoimmune diseases.
Viral infection not only leads to the activation of APCs and innate and adaptive immunities but also may activate autoreactive T cells72. Specifically, virus specific CD8+ T cells can mediate the death of infected cells through cytotoxicity, which is critical for controlling infections. However, CD8+ memory T cells may induce bystander effects through cytotoxic factors, including IFN-γ, granzyme B, or other mediators (e.g., IL-12, IL-15, and IL-18)73. Allegedly, the contact between bystander responses and infected cells may induce heterologous immune activation, an immune response to a different antigen due to activation from the original pathogen/antigen 74. Through dysregulated cGAS-STING pathway, immunogenic antigen binding to pathogen DNA can lead to type I IFN production from bystander-activated cells, promoting heightened inflammation and autoimmunity 75.
Using influenza hemagglutinin as a model viral antigen and transgenic myelin oligodendrocyte glycoprotein-specific B cells, a previous study showed that these B cells could capture cognate antigen from cell membranes along with small quantities of co-expressed bystander antigens and enable B cell escape from tolerance. The study suggests a mechanism of bystander effects on autoimmune CNS diseases like acute disseminated encephalomyelitis. This mechanism offers a link between infection and autoimmunity76.
Polyclonal hypergammaglobulinemia often presents during the chronic inflammatory conditions, such as chronic viral infections and autoimmune diseases. A previous study revealed that lymphocytic choriomeningitis virus infection induced polyclonal B cell activation and hypergammaglobulinemia in mice, which was dependent on the help of CD4+ T cells to the B cells presented lymphocytic choriomeningitis virus peptides. Thus, polyclonal B cell activation can drive hypergammaglobulinemia resulting from recognizing specific helper T cells to B cells that have processed viral antigens irrespective of the B cell receptor specificity77. The B cells as APCs activate non-specifically and may contribute to antibody-mediated autoimmunity.
Adjuvant effects or innate immune responses.
Besides adaptive immune response triggered autoimmune diseases following viral infection, innate immunity plays a critical role in promoting autoreactivity. Several TLR ligands (e.g., TLR7 agonist) are used as vaccine adjuvants. Most vaccine adjuvants can activate an innate immune response. The innate immune cells express PRRs such as TLRs to identify pathogens and distinguish self versus non-self. However, through binding to TLRs, pathogen-associated molecular patterns (PAMPs)-mediated innate immune responses can lead to inflammation and autoimmune pathogenesis. Moreover, damage-associated molecular patterns (DAMPs) are fragments released from cells damaged due to infection or trauma from a pathogen 78. DAMPs can also illicit inflammation via TLRs (i.e., TLR7) and shape the anti-viral adaptive immune response following enhanced cytokine and chemokine production. The innate immune response is involved with the development of autoimmunity through bystander activation of autoreactive T and B cells and/or adjacent damage of neighboring tissues 79-80. For example, type I IFN mediated innate immune response can have a dual effect in enhancing anti-viral immunity and accelerating chronic disease pathogenesis (e.g., chronic viral infections and autoimmune diseases). In vivo mouse studies using demyelinating TMEV indicates that heightened innate immune response contributes to the development of demyelinating disease 81. Viral PAMPs induced innate immune response and production of type I IFN, allowing infiltration of immune cells into the CNS and increased adaptive immunity (e.g., CD4+ and CD8+ T cell activation) in mice triggers autoimmunity following administration of TMEV 81. Notably, the study demonstrates an association between demyelinating and innate immune response to viral infection. These findings also highlight the innate immune system as a potential therapeutic target for those suffering from MS, which is under continued investigation 82-83.
2.3. Viral-Induced epitope spreading (antigen specific)
The etiologies of many autoimmune disorders are incompletely understood, with various factors contributing to the development of autoimmunity. Aside from the molecular mimicry and bystander activation mechanisms purportedly contributing to autoimmune disease, epitope spreading (Figure 2B.2) is a third proposed mechanism involving viral antigen recognition into antigen-specific self-epitope responses84,85. The epitope-spreading mechanism’s involvement is associated with human diseases such as rheumatoid arthritis, SLE, and symmetric polyarthritis86-88. Epitope spreading is characterized by intramolecular spreading in which an immune response is expanded from an initiating antigenic epitope to different epitopes on the same molecule, or by intermolecular spreading for expansion to epitopes on a different antigenic molecule 89. Viral infection-mediated epitope spreading is supported by the study that intracranial infection with Theiler's murine encephalomyelitis virus (TMEV) causes an MS-like lesion in the susceptible mouse brain due to epitope spreading 89. TMEV infection induced a TMEV-specific CD4+ T cell response which targets viral antigens in the CNS and initiates myelin damage. Later, myelin-specific CD4+ T cells are triggered via epitope spreading and contribute to disease pathogenesis 89. Epitope spreading demonstrates the harmful downstream immunologic consequences following viral infections.
2.4. SARS-CoV-2 infection and autoimmunity.
Recently, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) raised the concern of autoimmune responses, especially in individuals with genetic backgrounds of autoimmune diseases. Acute manifestations were of particular concern due to fatal respiratory failure90. Moreover, there is a growing concern about COVID-19 being an environmental trigger of autoimmunity through molecular mimicry and bystander cell activation91-97-98. Acute infection with COVID-19 stimulates an innate immune response resulting in a proinflammatory cytokine storm (i.e., IL-1β, TNF-α, and IFNγ) and chemokines as a non-specific anti-viral immune response. The non-specific proinflammatory cytokine response can promote tissue damage and release of hidden self-antigens; APCs uptake the self-antigens and present them to autoreactive T and B cells, triggering polyclonal autoreactive T and B cell activation and autoimmune responses during COVID-19 infection. Specific tissues such as the blood-brain barrier (BBB) and myelin sheath are harmed by the hyperinflammatory response, leading to the development of autoimmune features in COVID-19 infected patients99-102. SARS-CoV-2 viral antigens are also reported to mediate autoimmunity via molecular mimicry92. For instance, polyneuropathies (e.g., myasthenia gravis, GBS, and MS) are associated with human autoantigen production103. Using comparative sequence analysis between SARS-CoV-2 and host factors, viral hexapeptides (i.e., KDKKKK and EIPKEE) showed shared homology with human heat shock proteins (Hsps) (e.g., Hsps 90 and 60), where autoantibodies target Hsps and contribute to autoimmunity 104.
Two studies published by Trahtemberg et al. in 2021 provided contemporary understanding on autoantibody production in patients with or without COVID-19 infection in comparably ill respiratory failure 105,106. Both being longitudinal studies, their first study analyzed the production of ANAs, antigen-specific autoantibodies, myositis-related autoantibodies and anti-cytokine autoantibodies in ICU patients who were COVID-19+ or COVID-19−. Similarly, in their second study they analyzed serum levels of aPL, aCL, and other antiphospholipid antibodies due to their relevant roles in antiphospholipid syndrome. Antiphospholipid syndrome has similar clinical manifestations to COVID-19 with venous and arterial thrombosis and multiple organ involvement. Interestingly, their findings show no significant differences between COVID-19+ and COVID-19− groups or the stratified patient groups for any of the autoantibodies tested, which may result from non-functional autoantibody production following COVID-19 infection or the small sample size. This is similar to recently published findings demonstrating that bystander polyclonal autoreactive B cell activation is associated with COVID-19 infection compared to healthy controls, but not linked to disease severity107. Acute infections and cytokine storms (e.g., IL-6 108) can induce polyclonal autoreactive B cell activation and various autoantibody production, and COVID-19 infection seems to exhibit the polyclonal activation as well 109. However, infection-mediated polyclonal B cell activation and autoantibody production may result in the development or flare of autoimmune diseases due to the proliferation of progenitor pathogenic autoantibody producing B cells in the host with genetic backgrounds of autoimmune diseases. It’s evident that COVID-19 infection is concerning for population health both acute and chronically, however, the exact mechanism of autoimmunity is under continued investigation.
2.5. HIV infection and autoimmunity.
Some acute infectious diseases are associated with polyclonal B cell activation and autoantibody production, likely resulting from infection-mediated cytokine storm 98. Acute HIV infection is associated with increases in a number of autoantibodies; the majority is normalized to undetectable levels or levels similar to healthy controls following antiretroviral therapy (ART) and viral suppression in the circulation likely due to reduced inflammation 110-112. We have conducted a protein array to evaluate 87 autoantibodies in plasma samples from untreated HIV patients, HIV patients on suppressive ART, and healthy controls. We found that HIV infection was associated with a number of increased IgG autoantibodies (40%), and ART-treated HIV patients had normal autoreactive IgG levels except for the anti-CD4 and anti-prothrombin (manuscript submitted). We isolated anti-CD4 IgGs from plasma of patients with poor CD4+ T cell recovery following ART and viral suppression, and found that anti-CD4 IgGs mediated CD4+ T cell death through antibody-mediated cytotoxicity in vitro 113-115. This autoimmunity phenomenon exhibits its pathogenic activity for preventing CD4+ T cell immune reconstitution from ART in the absence of autoimmune diseases.
During the 1980s-1990s, increased anti-CD4 IgG was reported in HIV patients, but no pathogenic activity was proven, likely due to bystander autoreactive B cell activation via cytokine storm during the pre-ART era 116,117. Recently, a study showed that plasma levels of anti-CD4 IgGs increased during pre-ART but decreased after suppressive ART; however, a subgroup of HIV patients during post-ART still maintained higher levels of anti-CD4 IgGs compared to healthy controls 118. Moreover, plasma anti-CD4 IgG levels and CD4+ T cell counts were inversely correlated only in the patients after long-term ART (48-96 weeks), but neither short-term ART (0-24 weeks) nor pre-ART 118. Again, inflammation can induce low-affinity nonfunctional autoantibodies 77, a potential mechanism for higher autoantibody levels observed in HIV patients during pre-ART. In HIV, autoimmune features often exhibited after ART initiation 110,111, suggesting pathogenic autoantibodies may be developed through ART-improved immunity except controlling of tolerance. Moreover, HIV gp120 can bind to human CD4 protein and enhance antigenicity, which may contribute to the pathogenic activity of anti-CD4 autoantibody in HIV disease 119. Nonetheless, it may take many years to develop a pathogenic autoantibody due to disordered somatic hypermutation and class switch recombination 120.
3. Bacteria and autoimmunity
Autoantibodies occur years prior to clinical disease in certain autoimmune diseases (e.g., SLE); thus, chronic infections or microbial product translocation to the system may contribute to a very early step toward developing clinical disease features. Notably, autoantibodies like anti-nuclear antibodies occur in ~20% of healthy women. However, only a small proportion develops autoimmune diseases after many years following up; the mechanism remains unknown 121.
It is clear that bacterial infection, bacterial components or products, bacteria-related TLRs, (i.e., TLR2, TLR4, TLR9), and TLR-downstream cytokines (e.g., IFN-α) are associated with etiology and pathogenesis of autoimmunity 32,122-133. Both antigen-specific and non-specific autoimmune responses is involved in bacteria-mediated autoimmunity.
3.1. Bacteria-induced antigen-specific autoimmune responses.
GBS is reportedly associated with Campylobacter jejuni infection due to molecular mimicry 134. Specifically, infection-induced production of anti- ganglioside antibodies, targeting the antigen belonging to a major myelin component. Damage to the gangliosides is associated with the development of axonal neuropathy and motor nerve disorders 135,136. Research findings have shown an increased presence of IgG autoantibodies in GBS patients. These autoantibodies can bind to complement, suggesting the role of anti-ganglioside IgGs in GBS pathogenesis 137. Following C. jejuni infection, Yuki et al. found that bacterial lipopolysaccharides (LPS) from C. jejuni resembled molecules of the motor axolemma (e.g., GM1, GM1b, and GD1a), and the sequence similarity between C. jejuni LPS and host ganglioside molecules results in pathogenic autoantibody production and development of GBS.
Hsps are a large family of proteins essential for chaperoning protein maturation, degradation, and re-folding138. Much is known about their upregulation in response to stressful stimuli, including their ability to regulate both innate and adaptive immune responses. However, anti-Hsp antibodies are associated with autoimmune disease progression. Notably, an arthritic complication in cystic fibrosis is related to increased levels of anti-human Hsps and colonization of Pseudomonas aeruginosa139. Increased serum levels of IgG anti-human Hsp 27 and Hsp 90 autoantibodies in patients with P. aeruginosa lung colonization was found compared to individuals without lung infection. The amino acid sequences of antigenic bacterial components are similar to Hsp sequences found in human tissues. Through the production of anti-Hsp antibodies, the immune system is perturbed resulting in the release of interleukins and the development of autoimmunity.
3.2. Bacteria-induced bystander autoimmune responses.
B cells express various levels of PRRs such as TLRs and can respond to bacterial products (e.g., LPS, lipoteichoic [LTA], peptidoglycan [PGN]) directly without antigen-specific presentation. Recent studies suggest that a compromised mucosal barrier and increased systemic bacteria or bacterial product (e.g., LPS, LTA, PGN) translocation are linked to autoantibody production and etiology and pathogenesis of autoimmune diseases 140-142. In the presence of a compromised barrier (e.g., gut, skin, oral cavity, or genital tract), pathogens or non-pathogenic microbes colonized in the mucosal or epithelial barrier may directly translocate to the blood system as a whole microbiome or components of the bacterial cell wall that are either enzymatically released or bud off as vesicles 142,143. Of note, prior studies of gut microbes inducing SLE by Silverman and Kriegel indicate it is bacterial non-protein components that were detectable in the blood from patients with autoimmune diseases in the physiological condition without a clinical infection 143,144. Translocated non-protein components of the bacteria cell wall were implicated as a trigger in autoimmune diseases in humans 143,145.
Recent studies demonstrate that PGN and LPS can be detected in the blood of healthy adult humans 113,115,146-148. LPS and PGN are major components of bacteria that are highly immunogenic due to conserved structural molecular motifs unique to a bacterium and detected by the human immune system (i.e., TLR2, TLR4). Administration of bacterial LPS can induce autoantibodies and autoimmunity in animals 149,150 and variant in LPS contributes to autoimmunity in humans 151. A recent study revealed that PGN from Enterococcus has an immune regulatory activity and impact on responses to immune checkpoint inhibitor therapy for cancer 39. Thus, there is precedent that microbial products, PGN and LPS, found systemically, can have profound effects on immunity. In our recent studies 115,152, intraperitoneal injection of whole Staphylococcus aureus and isolated S. aureus PGN induced autoantibody production and accelerated autoimmune renal disease mediated by autoantibodies in C57/B6 and/or MRL/lpr mice 145. S. aureus PGN induced pathogenic autoantibody production and class switch recombination through TLR2, whereas Bacillus subtilis PGN drove the production of natural non-pathogenic autoantibodies 145. PGNs from different bacterial species vary based on the length of sugar polymers. For example, B. subtilis PGN exhibits an average of 50-250 disaccharides whereas S. aureus PGN exhibits ~18 disaccharides 153-156. Notably, S. aureus colonization is associated with SLE development in humans, although the mechanisms are unknown 157,158. 50% of lupus patients with skin rashes contained high levels of S. aureus colonization in the skin with resultant type I IFN induced skin barrier disruption in lupus, likely allowing S. aureus translocation 158. Our findings may support therapies targeting S. aureus PGN, such as small molecule inhibitors, probiotics 159, anti-bacterial peptides 160, or a TLR2 inhibitor 161 to prevent autoimmune disease development and progression. In case of infection, whole bacteria and bacterial proteins can present systemically; the other components of bacteria, such as staphylococcal enterotoxin B (SEB) and Staph Protein A, can also be immune regulatory proteins; intravenous injection of SEB led to the development of a lupus like disease in mice162,163. and administration of Staph Protein A reduced lupus nephritis in mice 164. Thus, the differential modulatory effects of the bacterial components depend on the variant structure of individual bacterial components (e.g., LPS, PGN).
Bacterial TLR signals are involved in autoimmunity and autoimmune disease pathogenesis. TLRs, especially TLR2, TLR4, and TLR9 have been reported to play a role in bystander autoimmune responses and autoimmunity 32,165. TLR is expressed on a number of different immune cells. Each B cell subset responds differently to TLR signals, and most sensitive TLR signals are observed in marginal zone B cells (MZ) and B1 cells which express high levels of TLR2 and TLR4 166. Increased B-1 B cell counts and enlarged MZ B cell population are reported in patients with SLE or lupus-prone mice 166. Germinal center B cells and memory B cells do not express significant amounts of TLR2 166. Besides B cells, TLRs are upregulated on T cells upon engagement of T cell receptor, produce proinflammatory cytokines such as IL-17 in response to TLR ligands, and contribute to autoreactive B cell activation 167. TLRs recognize microbial structures and detect foreign ones, but also recognize the danger molecular patterns such as host extracellular matrix proteins (i.e., Hsps, high mobility group box-1 protein, beta amyloid, and endogenous nucleic acids) released from necrotic and apoptotic cells167. Thus, perturbations of TLR signals and responses could contribute to autoimmunity via bystander autoreactive immune activation.
Bacterial components or products in the CNS contribute to the development of demyelinating plaques and chronic neuroinflammation, contributing to neuropathogenesis 168-170. Following bacterial infection and BBB crossing of bacterial metabolites into the CNS, an extended immune response leads to neuronal modulation 171-175. Bacterial metabolites (e.g., short-chain fatty acids, trimethylamines, and others) are BBB-permeable and are shown to modulate microglial maturation 176,177. Although the mechanism is yet to be determined, a study using SCFA supplementation for water in germ free mice influenced the microglial morphological and genetic phenotype of microglia 178. Additionally, bacterial-mediated neuroinflammation may stem from its neuroactive metabolites. Specifically, modulation of cellular production of serotonin is primarily influenced by bacterial presence, as shown in cell culture in vitro 179,180. By binding to microglial 5-HT receptors, serotonin induce microglial activation, triggering a release of cytokine-carrying exosomes and modulating neuroinflammation 181. These findings suggest a role of bacteria in modulating CNS function directly as well as indirectly through bacterial metabolites.
Other mechanisms also account for bystander autoimmune activation besides PPRs and its ligands, such as cytokines, bacterial proteins and toxins 182. The excess cytokines or dysregulation of cytokine signaling (e.g., IL-17/IL-23, GM-CSF, IL-6) mediated by pathogen or microbes including bacteria, virus, and other pathogens may breakdown the tolerance and induce non-specific polyclonal autoreactive T and/or B cell activation and a number of autoantibody production34,36. However, these cytokine-mediated bystander autoreactive B cell activation and autoantibodies are thought to be non-functional with low affinity and would not promote to autoimmune diseases in the general population; their roles in autoimmune disease in the hosts with autoimmune disease genetic backgrounds are not fully understood. In terms of bacterial superantigens (i.e., toxin), staphylococcal enterotoxin B can induce lupus like autoimmunity in mice 182.
4. Other pathogens and autoimmune responses (i.e., Fungi, parasites)
4.1. Mycology and autoimmunity
The fungal element of the microbiome, also known as the mycobiome, plays an essential role in maintaining microbiota community structure, metabolic function, and immune response to pathogens183. Studies show pathogenic and synergistic interactions between fungi and bacteria, influencing the host physiology184-186. The impact on host cells is due to the increased prevalence of certain fungal species alone or through mixed infections caused by cross-kingdom interactions. Each factor is essential in understanding immune system modulation and the onset of autoimmunity in immunocompromised individuals.
Gut-associated fungi may contribute to the development of MS. A conserved fungi epitope elicits a host autoimmune response through molecular mimicry or epitope spreading mechanisms187. Additionally, infection or colonization of commensal fungus Aspergillus fumigatus was related to HLA-DRB1*15 allele group sensitivity, which is a critical MS genetic risk factor31. In another study, altered mycobiome is observed related to the pathogenesis of MS188. Using a diet-based longitudinal study, higher alpha diversity in the gut mycobiome composition was shown in people with MS compared to healthy controls; an increased abundance of two commensal fungal genera, Saccharomyces and Aspergillus, was observed in MS patients188. Notably, Saccharomyces was positively correlated with the frequency of basophils with a negative correlation with regulatory B cells 189. Conversely, Aspergillus was positively correlated with activated DCs and total B cell number 188. Overall, the study findings suggest interplay between fungal and host immunity which may relate to autoimmune development.
Type 1 Diabetes mellitus (T1DM) is characterized by the destruction of pancreatic β-cells. β-cells produce insulin, a hormone vital in regulating glucose levels in the blood. In T1DM, dysregulated host immune system can result in β-cells destruction, leading to complications of abnormal glucose levels, β-cells are capable to repair their own damage 190, however, T1DM patients have impaired ability of β-cell repair, leading to treatment difficulties 191-193. A mycobiome-based study194 discovered that T1DM pediatric patients had a higher prevalence of autoantibodies against islet cells and a higher abundance of intestinal Candida albicans compared to healthy controls. Cytokine levels were not tested in this study194. Nonetheless, these studies provide evidence of a correlation between fungal infection/colonization and pathogenic autoantibodies that damage the pancreatic β-cells leading to the early onset of diabetes.
Aside from direct interactions with immune cells, fungal infections can result in the production of mycotoxins which may accelerate disease. Specifically, gliotoxin is a mycotoxin produced by various fungal species, including Gliocladium fimbriatum and Aspergillus fumigatus195. Experimental autoimmune encephalomyelitis (EAE) is used as a model for MS. A previous study showed that several weeks of injection of the gliotoxin promoted EAE pathogenesis 196. Interestingly, injection of gliotoxin derived from G. fimbriatum enhanced neuroinflammation and the injury of microglial cells, astrocytes, and oligodendrocytes, as well as demyelination due to the oligodendrocytes and astrocytes damage196. These findings are significant in demonstrating the development of autoimmunity not only from fungal cells directly but also through fungal-associated mycotoxins.
4.2. Parasitology and autoimmunity
A fourth significant group of infectious diseases are those caused by parasites. Parasites are typically recognized by the innate immune system (e.g., mast cells, eosinophils, and basophils), and may play a role in immune system modulation197-201. Autoimmune development may result from infection-mediated heightened inflammation and further activation of the adaptive immune response. For example, in response to parasitic infection, phagocytes (i.e., eosinophils) induce classic and piecemeal degranulation, cytolysis from granule release, and eosinophil extracellular traps202. Other phagocytes such as DCs have been shown to recognize lipid antigen containing phosphatidyl serine from Schistosoma mansoni through TLR2 while recognizing S. mansoni lacto-N-fucopentaosa III (LNFPIII) through TLR4203. However, perturbations in immune responses by these phagocytes double as APCs, may result in activation of an adaptative immune response against self-antigens and further damage to host cells, a mechanism of parasite-mediated development of autoimmunity204.
One implication of autoimmunity concerning parasitic infection involves the progression of anemia in those infected with Trypanosoma brucei. Data from in vivo mouse studies and African patient samples infected with T. brucei parasites show the production of anti-phosphatidylserine autoantibodies from autoreactive B-cells205. These autoantibodies were found to bind to uninfected erythrocytes, resulting in increased erythrocytic lysis. Additionally, parasitic infections (e.g., T. brucei and T. cruzi) were compared in mouse models, demonstrating an increased level of plasma autoantibodies against PS and erythrocyte lysate as well as an expansion of atypical B cells designated as Age-Associated B-cells in mice infected with T. brucei206. Overall, findings suggested a relationship between PS and autoimmunity as a mechanism underlying anemia and acute African trypanosomiasis.
The “hygiene hypothesis” contributes to understanding the influence of parasite-associated microbiome on allergic hypersensitive reactions and autoimmune diseases207. This hypothesis suggests that a subset of parasitic infections shape the regression of allergies and autoimmune diseases in countries with frequent exposures to helminths and parasites208. Some parasites are postulated to suppress the immune function to improve certain autoimmune diseases. One example involves inflammatory bowel disease (IBD), characterized by chronic inflammation in the gastrointestinal system. IBD is reportedly more common in countries with a lower prevalence of chronic gut infestation209. In vivo mouse models were used to examine the magnitude of colonic damage following Trichinella spiralis infection210, and demonstrated a reduction in dinitrobenzenesulfonic acid colitis and the down-regulation of IFN-γ and myeloperoxidase activities. Continued studies are necessary to understand the influence of various parasites on the mechanisms underlying allergies and autoimmunity. Nonetheless, a relationship between parasitic infection and immune system modulation is evident.
5. Conclusions and future perspectives
While there is much to learn on the etiology of the various forms of autoimmunity, infectious disease cannot be ruled out. As highlighted in our review, the presence of infectious agents including viruses, bacteria, fungi, and parasites can trigger an immunogenic response in individuals. Moreover, through molecular mimicry, bystander activation, and epitope spreading, it can be gathered that infection clearance is not enough to deter autoimmune development. The prolonged presence of proinflammatory molecules, targeting of self-antigens and self-epitopes, similar molecular sequence between host and microbe molecules, and more, contributes to the dysregulation of the immune function, causing host cell or tissue damage through autoimmunity. Further careful investigation should provide valuable insight into the role of pathogens or microbes and their interactions with the host, which may be critical for developing new therapeutic strategies for autoimmune diseases.
Highlights:
Pathogenic infection can induce normal immunity and autoimmunity
Infection mediated autoimmune bystander (non-specific) and antigen specific mechanisms contribute to autoimmunity
ACKNOWLEDEMENT
This work was supported by grants from the National Institutes of Health R03DA057164 (Wei Jiang), by Merit Review Award Number CX002422 (Wei Jiang) from the United States (U.S.) Department of Veterans Affairs Office of Research and Development (CSR&D) Service, and by NIH National Institute of General Medical Sciences grant R25GM072643 (Cynthia F. Wright). Figures were created using BioRender at BioRender.com.
ABBREVIATIONS
- ANA
anti-nuclear antibodies
- aPL
antiphospholipid
- aCL
anticardiolipin
- APC
antigen-presenting cell
- ART
anti retroviral therapy
- BBB
Brain-brain barrier
- CNS
Central Nervous System
- DAMPs
Damage Associated Molecular Pattern
- DC
Dendritic Cell
- EBV
Epstein Barr Virus
- GBS
Guillain-Barre Syndrome
- HBV
Hepatitis B virus
- HIV
Human Immunodeficiency Virus
- Hsp
Heat shock protein
- IFN
Interferon
- LPS
Lipopolysaccharide
- LTA
Lipoteichoic Acid
- MBP
Myelin Basic Protein
- MCMV
Murine cytomegalovirus
- MHC
Major Histocompatibility Complex
- MS
Multiple Sclerosis
- PAMPs
Pathogen Associated Molecular Pattern
- PGN
Peptidoglycan
- PRR
Pattern Recognition Receptor
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SLE
Systemic Lupus Erythematous
- T1DM
Type 1 Diabetes Mellitus
- TCR T
cell Receptor
- TLR
Toll-like receptor
- TMEV
Theiler’s murine encephalomyelitis virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial or non-financial interests.
References
- 1.Satoh M & Reeves WH Induction of lupus-associated autoantibodies in BALB/c mice by intraperitoneal injection of pristane. J Exp Med 180, 2341–2346 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wraith DC, Goldman M & Lambert PH Vaccination and autoimmune disease: what is the evidence? Lancet 362, 1659–1666 (2003). 10.1016/S0140-6736(03)14802-7 [DOI] [PubMed] [Google Scholar]
- 3.McRae BL, Vanderlugt CL, Dal Canto MC & Miller SD Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. The Journal of experimental medicine 182, 75–85 (1995). 10.1084/jem.182.1.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamen DL et al. Autoantibody prevalence and lupus characteristics in a unique African American population. Arthritis Rheum 58, 1237–1247 (2008). 10.1002/art.23416 [DOI] [PubMed] [Google Scholar]
- 5.Langkilde H, Voss A, Heegaard N & Laustrup H Autoantibodies persist in relatives to systemic lupus erythematosus patients during 12 years follow-up. Lupus 26, 723–728 (2017). 10.1177/0961203316676378 [DOI] [PubMed] [Google Scholar]
- 6.Klein SL, Jedlicka A & Pekosz A The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis 10, 338–349 (2010). 10.1016/S1473-3099(10)70049-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li QZ et al. Risk factors for ANA positivity in healthy persons. Arthritis research & therapy 13, R38 (2011). 10.1186/ar3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Afshan G, Afzal N & Qureshi S CD4+CD25(hi) regulatory T cells in healthy males and females mediate gender difference in the prevalence of autoimmune diseases. Clinical laboratory 58, 567–571 (2012). [PubMed] [Google Scholar]
- 9.Pisitkun P et al. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science 312, 1669–1672 (2006). 10.1126/science.1124978 [DOI] [PubMed] [Google Scholar]
- 10.Berghofer B et al. TLR7 ligands induce higher IFN-alpha production in females. J Immunol 177, 2088–2096 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Laffont S et al. X-Chromosome complement and estrogen receptor signaling independently contribute to the enhanced TLR7-mediated IFN-alpha production of plasmacytoid dendritic cells from women. J Immunol 193, 5444–5452 (2014). 10.4049/jimmunol.1303400 [DOI] [PubMed] [Google Scholar]
- 12.Grimaldi CM, Cleary J, Dagtas AS, Moussai D & Diamond B Estrogen alters thresholds for B cell apoptosis and activation. The Journal of clinical investigation 109, 1625–1633 (2002). 10.1172/JCI14873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Invernizzi P, Pasini S, Selmi C, Gershwin ME & Podda M Female predominance and X chromosome defects in autoimmune diseases. Journal of autoimmunity 33, 12–16 (2009). 10.1016/j.jaut.2009.03.005 [DOI] [PubMed] [Google Scholar]
- 14.Scofield RH et al. Klinefelter’s Syndrome, 47,XXY, in Male Systemic Lupus Erythematosus Supports a Gene Dose Effect from the X Chromosome. Arthritis and rheumatism 58, 2511–2517 (2008). 10.1002/art.23701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banchereau J & Pascual V Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity 25, 383–392 (2006). 10.1016/j.immuni.2006.08.010 [DOI] [PubMed] [Google Scholar]
- 16.García-Ortiz H et al. Association of TLR7 copy number variation with susceptibility to childhood-onset systemic lupus erythematosus in Mexican population. Annals of the rheumatic diseases 69, 1861–1865 (2010). 10.1136/ard.2009.124313 [DOI] [PubMed] [Google Scholar]
- 17.Shen N et al. Sex-specific association of X-linked Toll-like receptor 7 (TLR7) with male systemic lupus erythematosus. Proceedings of the National Academy of Sciences of the United States of America 107, 15838–15843 (2010). 10.1073/pnas.1001337107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deane JA et al. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity 27, 801–810 (2007). 10.1016/j.immuni.2007.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Coz C et al. CD40LG duplication-associated autoimmune disease is silenced by nonrandom X-chromosome inactivation. The Journal of allergy and clinical immunology 141, 2308–2311.e2307 (2018). 10.1016/j.jaci.2018.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subramanian S et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proceedings of the National Academy of Sciences 103, 9970–9975 (2006). 10.1073/pnas.0603912103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brooks WH & Renaudineau Y Epigenetics and autoimmune diseases: the X chromosome-nucleolus nexus. Front Genet 6, 22 (2015). 10.3389/fgene.2015.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terao C et al. Association between antinuclear antibodies and the HLA class II locus and heterogeneous characteristics of staining patterns: the Nagahama study. Arthritis & rheumatology 66, 3395–3403 (2014). 10.1002/art.38867 [DOI] [PubMed] [Google Scholar]
- 23.Nishino H, Shibuya K, Nishida Y & Mushimoto M Lupus erythematosus-like syndrome with selective complete deficiency of C1q. Ann Intern Med 95, 322–324 (1981). [DOI] [PubMed] [Google Scholar]
- 24.Kozyrev SV et al. Corrigendum: Functional variants in the B-cell gene BANK1 are associated with systemic lupus erythematosus. Nature genetics 40, 484 (2008). 10.1038/ng0408-484 [DOI] [PubMed] [Google Scholar]
- 25.Abelson AK et al. STAT4 associates with systemic lupus erythematosus through two independent effects that correlate with gene expression and act additively with IRF5 to increase risk. Annals of the rheumatic diseases 68, 1746–1753 (2009). 10.1136/ard.2008.097642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Remmers EF et al. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N Engl J Med 357, 977–986 (2007). 10.1056/NEJMoa073003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramos PS et al. A comprehensive analysis of shared loci between systemic lupus erythematosus (SLE) and sixteen autoimmune diseases reveals limited genetic overlap. PLoS genetics 7, e1002406 (2011). 10.1371/journal.pgen.1002406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alarcón-Segovia D et al. Familial aggregation of systemic lupus erythematosus, rheumatoid arthritis, and other autoimmune diseases in 1,177 lupus patients from the GLADEL cohort. Arthritis and rheumatism 52, 1138–1147 (2005). 10.1002/art.20999 [DOI] [PubMed] [Google Scholar]
- 29.Gateva V et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nature genetics 41, 1228–1233 (2009). 10.1038/ng.468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramos PS, Brown EE, Kimberly RP & Langefeld CD Genetic Factors Predisposing to Systemic Lupus Erythematosus and Lupus Nephritis. Semin Nephrol 30, 164–176 (2010). 10.1016/j.semnephrol.2010.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chauhan SK, Singh VV, Rai R, Rai M & Rai G Distinct autoantibody profiles in systemic lupus erythematosus patients are selectively associated with TLR7 and TLR9 upregulation. J Clin Immunol 33, 954–964 (2013). 10.1007/s10875-013-9887-0 [DOI] [PubMed] [Google Scholar]
- 32.Lartigue A et al. Critical role of TLR2 and TLR4 in autoantibody production and glomerulonephritis in lpr mutation-induced mouse lupus. Journal of immunology 183, 6207–6216 (2009). 10.4049/jimmunol.0803219 [DOI] [PubMed] [Google Scholar]
- 33.Simchoni N & Cunningham-Rundles C TLR7- and TLR9-Responsive Human B Cells Share Phenotypic and Genetic Characteristics. J Immunol 194, 3035–3044 (2015). 10.4049/jimmunol.1402690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamilton JA Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol 8, 533–544 (2008). 10.1038/nri2356 [DOI] [PubMed] [Google Scholar]
- 35.Harris KM, Fasano A & Mann DL Cutting edge: IL-1 controls the IL-23 response induced by gliadin, the etiologic agent in celiac disease. J Immunol 181, 4457–4460 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Yoshimura A, Suzuki M, Sakaguchi R, Hanada T & Yasukawa H SOCS, Inflammation, and Autoimmunity. Front Immunol 3, 20 (2012). 10.3389/fimmu.2012.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furman D et al. Chronic inflammation in the etiology of disease across the life span. Nat Med 25, 1822–1832 (2019). 10.1038/s41591-019-0675-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pons-Estel GJ, Alarcon GS, Scofield L, Reinlib L & Cooper GS Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum 39, 257–268 (2010). 10.1016/j.semarthrit.2008.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griffin Matthew E., E J., Becker Jessica L., Luo Ji-Dung, Carroll Thomas S., Jha Jyoti K., Fanger Gary R., Hang Howard C.. Enterococcus peptidoglycan remodeling promotes checkpoint inhibitor cancer immunotherapy. Scinece 373, 1040–1046 (2021). 10.1126/science.abc9113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cusick MF, Libbey JE & Fujinami RS Molecular Mimicry as a Mechanism of Autoimmune Disease. Clinical Reviews in Allergy & Immunology 42, 102–111 (2012). 10.1007/s12016-011-8294-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujinami RS & Oldstone MB Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunity. Science (New York, N.Y.) 230, 1043–1045 (1985). 10.1126/science.2414848 [DOI] [PubMed] [Google Scholar]
- 42.Fujinami RS, Oldstone MB, Wroblewska Z, Frankel ME & Koprowski H Molecular mimicry in virus infection: crossreaction of measles virus phosphoprotein or of herpes simplex virus protein with human intermediate filaments. Proceedings of the National Academy of Sciences of the United States of America 80, 2346–2350 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Houen G & Trier NH Epstein-Barr Virus and Systemic Autoimmune Diseases. Frontiers in Immunology 11, 587380 (2021). 10.3389/fimmu.2020.587380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pender MP, Csurhes PA, Lenarczyk A, Pfluger CMM & Burrows SR Decreased T cell reactivity to Epstein-Barr virus infected lymphoblastoid cell lines in multiple sclerosis. Journal of Neurology, Neurosurgery, and Psychiatry 80, 498–505 (2009). 10.1136/jnnp.2008.161018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wandinger K et al. Association between clinical disease activity and Epstein-Barr virus reactivation in MS. Neurology 55, 178–184 (2000). 10.1212/wnl.55.2.178 [DOI] [PubMed] [Google Scholar]
- 46.Jakhmola S et al. A plausible contributor to multiple sclerosis; presentation of antigenic myelin protein epitopes by major histocompatibility complexes. Comput Biol Med 148, 105856 (2022). 10.1016/j.compbiomed.2022.105856 [DOI] [PubMed] [Google Scholar]
- 47.Lang HLE et al. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nature immunology 3, 940–943 (2002). 10.1038/ni835 [DOI] [PubMed] [Google Scholar]
- 48.Holmøy T, Kvale EØ & Vartdal F Cerebrospinal fluid CD4+ T cells from a multiple sclerosis patient cross-recognize Epstein-Barr virus and myelin basic protein. Journal of Neurovirology 10, 278–283 (2004). 10.1080/13550280490499524 [DOI] [PubMed] [Google Scholar]
- 49.Andréoletti L, Lévêque N, Boulagnon C, Brasselet C & Fornes P Viral causes of human myocarditis. Arch Cardiovasc Dis 102, 559–568 (2009). 10.1016/j.acvd.2009.04.010 [DOI] [PubMed] [Google Scholar]
- 50.Andréoletti L et al. Active Coxsackieviral B infection is associated with disruption of dystrophin in endomyocardial tissue of patients who died suddenly of acute myocardial infarction. J Am Coll Cardiol 50, 2207–2214 (2007). 10.1016/j.jacc.2007.07.080 [DOI] [PubMed] [Google Scholar]
- 51.Gamba D, Dolivo G & Bozic C [Cases of viral myocarditis in newborn infants]. Rev Med Suisse Romande 86, 206–215 (1966). [PubMed] [Google Scholar]
- 52.Kuhl U et al. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation 112, 1965–1970 (2005). 10.1161/CIRCULATIONAHA.105.548156 [DOI] [PubMed] [Google Scholar]
- 53.Feldman AM & McNamara D Myocarditis. The New England journal of medicine 343, 1388–1398 (2000). 10.1056/NEJM200011093431908 [DOI] [PubMed] [Google Scholar]
- 54.Krebs P et al. Molecular mapping of autoimmune B cell responses in experimental myocarditis. Journal of autoimmunity 28, 224–233 (2007). 10.1016/j.jaut.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 55.Pummerer C, Grässl G & Neu N Cellular immune mechanisms in myosin-induced myocarditis. European Heart Journal 16, 71–74 (1995). 10.1093/eurheartj/16.suppl_O.71 [DOI] [PubMed] [Google Scholar]
- 56.Liao L et al. Antibody-mediated autoimmune myocarditis depends on genetically determined target organ sensitivity. The Journal of experimental medicine 181, 1123–1131 (1995). 10.1084/jem.181.3.1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fairweather D, Kaya Z, Shellam GR, Lawson CM & Rose NR From Infection to Autoimmunity. Journal of autoimmunity 16, 175–186 (2001). 10.1006/jaut.2000.0492 [DOI] [PubMed] [Google Scholar]
- 58.Mao Y-S, Lu C-Z, Wang X & Xiao B-G Induction of experimental autoimmune encephalomyelitis in Lewis rats by a viral peptide with limited homology to myelin basic protein. Experimental Neurology 206, 231–239 (2007). 10.1016/j.expneurol.2007.04.015 [DOI] [PubMed] [Google Scholar]
- 59.Shaw S-Y, Laursen RA & Lees MB Analogous amino acid sequences in myelin proteolipid and viral proteins. FEBS Letters 207, 266–270 (1986). 10.1016/0014-5793(86)81502-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei J & Duan S Severe Guillain-Barré syndrome associated with chronic hepatitis B: A case report and literature review. Medicine 100, e27989 (2021). 10.1097/MD.0000000000027989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prevention and Control of Influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP).
- 62.Toplak N & Avcin T Influenza and autoimmunity. Annals of the New York Academy of Sciences 1173, 619–626 (2009). 10.1111/j.1749-6632.2009.04759.x [DOI] [PubMed] [Google Scholar]
- 63.Avcin T & Toplak N Antiphospholipid antibodies in response to infection. Current Rheumatology Reports 9, 212–218 (2007). 10.1007/s11926-007-0034-x [DOI] [PubMed] [Google Scholar]
- 64.Mormile R, D'Alterio V, Treccagnoli G & Sorrentino P Henoch-Schonlein purpura with antiphospholipid antibodies after influenza vaccination: how fearful is it in children? Vaccine 23, 567–568 (2004). 10.1016/j.vaccine.2004.07.029 [DOI] [PubMed] [Google Scholar]
- 65.Tarján P et al. Influenza vaccination and the production of anti-phospholipid antibodies in patients with systemic lupus erythematosus. Scandinavian Journal of Rheumatology 35, 241–243 (2006). 10.1080/03009740500474552 [DOI] [PubMed] [Google Scholar]
- 66.Watanabe T & Onda H Henoch-Schonlein purpura with antiphospholipid antibodies following an influenza vaccination. Pediatr Nephrol 16, 458–459; discussion 460-462 (2001). 10.1007/s004670100569 [DOI] [PubMed] [Google Scholar]
- 67.Blank M et al. Bacterial induction of autoantibodies to beta2-glycoprotein-I accounts for the infectious etiology of antiphospholipid syndrome. The Journal of clinical investigation 109, 797–804 (2002). 10.1172/JCI12337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fruntes V & Limosin F Schizophrenia and viral infection during neurodevelopment: a pathogenesis model? Med Sci Monit 14, RA71–77 (2008). [PubMed] [Google Scholar]
- 69.Sano H, Terasaki J, Tsutsumi C, Imagawa A & Hanafusa T A case of fulminant type 1 diabetes mellitus after influenza B infection. Diabetes Res Clin Pract 79, e8–9 (2008). 10.1016/j.diabres.2007.10.030 [DOI] [PubMed] [Google Scholar]
- 70.Schonberger LB et al. Guillain-Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976--1977. American Journal of Epidemiology 110, 105–123 (1979). 10.1093/oxfordjournals.aje.a112795 [DOI] [PubMed] [Google Scholar]
- 71.McCoy L, Tsunoda I & Fujinami RS Multiple sclerosis and virus induced immune responses: autoimmunity can be primed by molecular mimicry and augmented by bystander activation. Autoimmunity 39, 9–19 (2006). 10.1080/08916930500484799 [DOI] [PubMed] [Google Scholar]
- 72.Pacheco Y et al. Bystander activation and autoimmunity. Journal of autoimmunity 103, 102301 (2019). 10.1016/j.jaut.2019.06.012 [DOI] [PubMed] [Google Scholar]
- 73.Boyman O Bystander activation of CD4+ T cells. European journal of immunology 40, 936–939 (2010). 10.1002/eji.201040466 [DOI] [PubMed] [Google Scholar]
- 74.Sun L, Wu J, Du F, Chen X & Chen ZJ Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science (New York, N.Y.) 339, 786–791 (2013). 10.1126/science.1232458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Decout A, Katz JD, Venkatraman S & Ablasser A The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nature reviews. Immunology 21, 548–569 (2021). 10.1038/s41577-021-00524-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sanderson NS et al. Cocapture of cognate and bystander antigens can activate autoreactive B cells. Proc Natl Acad Sci U S A 114, 734–739 (2017). 10.1073/pnas.1614472114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hunziker L et al. Hypergammaglobulinemia and autoantibody induction mechanisms in viral infections. Nat Immunol 4, 343–349 (2003). 10.1038/ni911 [DOI] [PubMed] [Google Scholar]
- 78.Roh JS & Sohn DH Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Network 18, e27 (2018). 10.4110/in.2018.18.e27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brubaker SW, Bonham KS, Zanoni I & Kagan JC Innate immune pattern recognition: a cell biological perspective. Annual review of immunology 33, 257–290 (2015). 10.1146/annurev-immunol-032414-112240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Herrada AA et al. Innate Immune Cells' Contribution to Systemic Lupus Erythematosus. Frontiers in Immunology 10, 772 (2019). 10.3389/fimmu.2019.00772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Olson JK & Miller SD The innate immune response affects the development of the autoimmune response in Theiler's virus-induced demyelinating disease. J Immunol 182, 5712–5722 (2009). 10.4049/jimmunol.0801940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Elieh-Ali-Komi D & Cao Y Role of Mast Cells in the Pathogenesis of Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. Clinical Reviews in Allergy & Immunology 52, 436–445 (2017). 10.1007/s12016-016-8595-y [DOI] [PubMed] [Google Scholar]
- 83.Van Kaer L, Postoak JL, Wang C, Yang G & Wu L Innate, innate-like and adaptive lymphocytes in the pathogenesis of MS and EAE. Cellular & Molecular Immunology 16, 531–539 (2019). 10.1038/s41423-019-0221-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Getts DR, Chastain EML, Terry RL & Miller SD Virus infection, antiviral immunity, and autoimmunity. Immunological reviews 255, 197–209 (2013). 10.1111/imr.12091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smatti MK et al. Viruses and Autoimmunity: A Review on the Potential Interaction and Molecular Mechanisms. Viruses 11, E762 (2019). 10.3390/v11080762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen J et al. Correlation between systemic lupus erythematosus and cytomegalovirus infection detected by different methods. Clinical rheumatology 34, 691–698 (2015). 10.1007/s10067-015-2868-3 [DOI] [PubMed] [Google Scholar]
- 87.Goupil BA & Mores CN A Review of Chikungunya Virus-induced Arthralgia: Clinical Manifestations, Therapeutics, and Pathogenesis. The Open Rheumatology Journal 10 (2016). 10.2174/1874312901610010129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pera A, Broadley I, Davies KA & Kern F Cytomegalovirus as a Driver of Excess Cardiovascular Mortality in Rheumatoid Arthritis. Circulation Research 120, 274–277 (2017). 10.1161/CIRCRESAHA.116.309982 [DOI] [PubMed] [Google Scholar]
- 89.Tompkins SM, Fuller KG & Miller SD Theiler's virus-mediated autoimmunity: local presentation of CNS antigens and epitope spreading. Annals of the New York Academy of Sciences 958, 26–38 (2002). [PubMed] [Google Scholar]
- 90.Knight JS et al. The intersection of COVID-19 and autoimmunity. The Journal of clinical investigation 131, e154886 (2021). 10.1172/JCI154886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gupta M & Weaver DF COVID-19 as a Trigger of Brain Autoimmunity. ACS Chemical Neuroscience, acschemneuro.1c00403 (2021). 10.1021/acschemneuro.1c00403 [DOI] [PubMed] [Google Scholar]
- 92.Liu Y, Sawalha AH & Lu Q COVID-19 and autoimmune diseases. Current opinion in rheumatology 33, 155–162 (2021). 10.1097/BOR.0000000000000776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anand P, Puranik A, Aravamudan M, Venkatakrishnan AJ & Soundararajan V SARS-CoV-2 strategically mimics proteolytic activation of human ENaC. eLife 9, e58603 (2020). 10.7554/eLife.58603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lucchese G & Flöel A Molecular mimicry between SARS-CoV-2 and respiratory pacemaker neurons. Autoimmunity reviews 19, 102556 (2020). 10.1016/j.autrev.2020.102556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lucchese G & Flöel A SARS-CoV-2 and Guillain-Barré syndrome: molecular mimicry with human heat shock proteins as potential pathogenic mechanism. Cell Stress Chaperones 25, 731–735 (2020). 10.1007/s12192-020-01145-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marino Gammazza A et al. Human molecular chaperones share with SARS-CoV-2 antigenic epitopes potentially capable of eliciting autoimmunity against endothelial cells: possible role of molecular mimicry in COVID-19. Cell Stress Chaperones 25, 737–741 (2020). 10.1007/s12192-020-01148-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Venkatakrishnan AJ et al. Benchmarking evolutionary tinkering underlying human–viral molecular mimicry shows multiple host pulmonary–arterial peptides mimicked by SARS-CoV-2. Cell Death Discov. 6, 1–14 (2020). 10.1038/s41420-020-00321-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiang W et al. COVID-19 is associated with bystander polyclonal autoreactive B cell activation as reflected by a broad autoantibody production, but none is linked to disease severity. J Med Virol (2022). 10.1002/jmv.28134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baig AM, Khaleeq A, Ali U & Syeda H Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS chemical neuroscience 11, 995–998 (2020). 10.1021/acschemneuro.0c00122 [DOI] [PubMed] [Google Scholar]
- 100.Burks JS, DeVald BL, Jankovsky LD & Gerdes JC Two coronaviruses isolated from central nervous system tissue of two multiple sclerosis patients. Science (New York, N.Y.) 209, 933–934 (1980). 10.1126/science.7403860 [DOI] [PubMed] [Google Scholar]
- 101.Yeh EA, Collins A, Cohen ME, Duffner PK & Faden H Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics 113, e73–76 (2004). 10.1542/peds.113.1.e73 [DOI] [PubMed] [Google Scholar]
- 102.Zhou L, Zhang M, Wang J & Gao J Sars-Cov-2: Underestimated damage to nervous system. Travel Med Infect Dis 36, 101642 (2020). 10.1016/j.tmaid.2020.101642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Romi F, Helgeland G & Gilhus NE Heat-Shock Proteins in Clinical Neurology. ENE 66, 65–69 (2011). 10.1159/000329373 [DOI] [PubMed] [Google Scholar]
- 104.Moudgil KD, Thompson SJ, Geraci F, De Paepe B & Shoenfeld Y Heat-Shock Proteins in Autoimmunity. Autoimmune Diseases 2013, 621417 (2013). 10.1155/2013/621417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Trahtemberg U & Fritzler MJ COVID-19-associated autoimmunity as a feature of acute respiratory failure. Intensive Care Medicine 47, 801–804 (2021). 10.1007/s00134-021-06408-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Trahtemberg U et al. Anticardiolipin and other antiphospholipid antibodies in critically ill COVID-19 positive and negative patients. Annals of the rheumatic diseases 80, 1236–1240 (2021). 10.1136/annrheumdis-2021-220206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jiang W et al. COVID-19 is associated with bystander polyclonal autoreactive B cell activation as reflected by a broad autoantibody production, but none is linked to disease severity. J Med Virol (2022). 10.1002/jmv.28134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Szczepek AJ, Belch AR & Pilarski LM Expression of IL-6 and IL-6 receptors by circulating clonotypic B cells in multiple myeloma: potential for autocrine and paracrine networks. Exp Hematol 29, 1076–1081 (2001). 10.1016/s0301-472x(01)00682-8 [DOI] [PubMed] [Google Scholar]
- 109.Fairweather D, Kaya Z, Shellam GR, Lawson CM & Rose NR From infection to autoimmunity. Journal of autoimmunity 16, 175–186 (2001). 10.1006/jaut.2000.0492 [DOI] [PubMed] [Google Scholar]
- 110.Zandman-Goddard G & Shoenfeld Y HIV and autoimmunity. Autoimmun Rev 1, 329–337 (2002). [DOI] [PubMed] [Google Scholar]
- 111.lordache L et al. Autoimmune diseases in HIV-infected patients: 52 cases and literature review. Autoimmun Rev 13, 850–857 (2014). 10.1016/j.autrev.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 112.Tanko RF et al. Effect of Antiretroviral Therapy on the Memory and Activation Profiles of B Cells in HIV-Infected African Women. J Immunol 198, 1220–1228 (2017). 10.4049/jimmunol.1601560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Luo Z et al. Variation in blood microbial lipopolysaccharide (LPS) contributes to immune reconstitution in response to suppressive antiretroviral therapy in HIV. EBioMedicine 80, 104037 (2022). 10.1016/j.ebiom.2022.104037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Luo Z et al. A link between IL-23 and anti-CD4 autoantibody production in antiretroviral- treated HIV-infected individuals. J Virol (2021). 10.1128/JVI.00271-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Luo Z et al. Systemic translocation of Staphylococcus drives autoantibody production in HIV disease. Microbiome 7, 25 (2019). 10.1186/s40168-019-0646-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chams V, Idziorek T & Klatzmann D Biological properties of anti-CD4 autoantibodies purified from HIV-infected patients. AIDS 5, 565–569 (1991). [PubMed] [Google Scholar]
- 117.Sekigawa I et al. Characterization of autoantibodies to the CD4 molecule in human immunodeficiency virus infection. Clinical immunology and immunopathology 58, 145–153 (1991). [DOI] [PubMed] [Google Scholar]
- 118.Song A et al. Effects of Early and Delayed Antiretroviral Therapy on Plasma Anti-CD4 Autoreactive IgG and Its Association With CD4(+) T-Cell Recovery in Acute HIV-Infected Individuals. Front Pharmacol 11, 449 (2020). 10.3389/fphar.2020.00449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Furci L et al. Human immunodeficiency virus type 1 glycoprotein 120-specific T lymphocytes provide intermolecular help for anti-CD4 autoantibody production in exposed uninfected subjects. AIDS research and human retroviruses 13, 1461–1469 (1997). 10.1089/aid.1997.13.1461 [DOI] [PubMed] [Google Scholar]
- 120.Elkon K & Casali P Nature and functions of autoantibodies. Nat Clin Pract Rheumatol 4, 491–498 (2008). 10.1038/ncprheum0895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Slight-Webb S et al. Autoantibody-positive healthy individuals with lower lupus risk display a unique immune endotype. J Allergy Clin Immunol 146, 1419–1433 (2020). 10.1016/j.jaci.2020.04.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ferrand J & Gantier MP Assessing the Inhibitory Activity of Oligonucleotides on TLR7 Sensing. Methods Mol Biol 1390, 79–90 (2016). 10.1007/978-1-4939-3335-8_5 [DOI] [PubMed] [Google Scholar]
- 123.Gotoh M & Matsuda J Prevalence of antibodies to lipid A, lipopolysaccharide and lipoteichoic acid in systemic lupus erythematosus patients with antiphospholipid antibodies. Lupus 6, 678–679 (1997). 10.1177/096120339700600810 [DOI] [PubMed] [Google Scholar]
- 124.Gotoh M & Matsuda J Induction of anticardiolipin antibody and/or lupus anticoagulant in rabbits by immunization with lipoteichoic acid, lipopolysaccharide and lipid A. Lupus 5, 593–597 (1996). 10.1177/096120339600500606 [DOI] [PubMed] [Google Scholar]
- 125.Licht R, van Bruggen MC, Oppers-Walgreen B, Rijke TP & Berden JH Plasma levels of nucleosomes and nucleosome-autoantibody complexes in murine lupus: effects of disease progression and lipopolyssacharide administration. Arthritis and rheumatism 44, 1320–1330 (2001). [DOI] [PubMed] [Google Scholar]
- 126.Niewold TB, Hua J, Lehman TJ, Harley JB & Crow MK High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun 8, 492–502 (2007). 10.1038/sj.gene.6364408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li QZ et al. Interferon signature gene expression is correlated with autoantibody profiles in patients with incomplete lupus syndromes. Clin Exp Immunol 159, 281–291 (2010). 10.1111/j.1365-2249.2009.04057.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Segal Y, Calabro M, Kanduc D & Shoenfeld Y Human papilloma virus and lupus: the virus, the vaccine and the disease. Current opinion in rheumatology 29, 331–342 (2017). 10.1097/BOR.0000000000000398 [DOI] [PubMed] [Google Scholar]
- 129.Ercolini AM & Miller SD The role of infections in autoimmune disease. Clinical and experimental immunology 155, 1–15 (2009). 10.1111/j.1365-2249.2008.03834.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gilkeson GS, Pippen AM & Pisetsky DS Induction of cross-reactive anti-dsDNA antibodies in preautoimmune NZB/NZW mice by immunization with bacterial DNA. The Journal of clinical investigation 95, 1398–1402 (1995). 10.1172/JCI117793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gilkeson GS et al. Modulation of renal disease in autoimmune NZB/NZW mice by immunization with bacterial DNA. J Exp Med 183, 1389–1397 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ehlers M & Ravetch JV Opposing effects of Toll-like receptor stimulation induce autoimmunity or tolerance. Trends Immunol 28, 74–79 (2007). 10.1016/j.it.2006.12.006 [DOI] [PubMed] [Google Scholar]
- 133.Moreth K et al. The proteoglycan biglycan regulates expression of the B cell chemoattractant CXCL13 and aggravates murine lupus nephritis. J Clin Invest 120, 4251–4272 (2010). 10.1172/JCI42213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yuki N Ganglioside mimicry and peripheral nerve disease. Muscle Nerve 35, 691–711 (2007). 10.1002/mus.20762 [DOI] [PubMed] [Google Scholar]
- 135.Cats EA et al. Multifocal motor neuropathy: association of anti-GM1 IgM antibodies with clinical features. Neurology 75, 1961–1967 (2010). 10.1212/WNL.0b013e3181ff94c2 [DOI] [PubMed] [Google Scholar]
- 136.Susuki K et al. Anti-GM1 antibodies cause complement-mediated disruption of sodium channel clusters in peripheral motor nerve fibers. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 27, 3956–3967 (2007). 10.1523/JNEUROSG.4401-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hafer-Macko C et al. Acute motor axonal neuropathy: an antibody-mediated attack on axolemma. Annals of Neurology 40, 635–644 (1996). 10.1002/ana.410400414 [DOI] [PubMed] [Google Scholar]
- 138.Miller DJ & Fort PE Heat Shock Proteins Regulatory Role in Neurodevelopment. Frontiers in Neuroscience 12, 821 (2018). 10.3389/fnins.2018.00821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.al-Shamma MR, McSharry C, McLeod K, McCruden EA & Stack BH Role of heat shock proteins in the pathogenesis of cystic fibrosis arthritis. Thorax 52, 1056–1059 (1997). 10.1136/thx.52.12.1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shi L et al. The SLE transcriptome exhibits evidence of chronic endotoxin exposure and has widespread dysregulation of non-coding and coding RNAs. PLoS One 9, e93846 (2014). 10.1371/journal.pone.0093846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ogunrinde E et al. A link between plasma microbial translocation, microbiome, and autoantibody development in first-degree relatives of systemic lupus erythematosus patients. Arthritis & rheumatology (2019). 10.1002/art.40935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Manfredo Vieira S et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science 359, 1156–1161 (2018). 10.1126/science.aar7201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Azzouz D et al. Lupus nephritis is linked to disease-activity associated expansions and immunity to a gut commensal. Annals of the rheumatic diseases 78, 947–956 (2019). 10.1136/annrheumdis-2018-214856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zegarra-Ruiz DF et al. A Diet-Sensitive Commensal Lactobacillus Strain Mediates TLR7-Dependent Systemic Autoimmunity. Cell host & microbe 25, 113–127 e116 (2019). 10.1016/j.chom.2018.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ning W et al. Staphylococcus aureus peptidoglycan (PGN) induces pathogenic autoantibody production via autoreactive B cell receptor clonal selection, implications in systemic lupus erythematosus. Journal of autoimmunity 131, 102860 (2022). 10.1016/j.jaut.2022.102860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huang Z et al. Antibody neutralization of microbiota-derived circulating peptidoglycan dampens inflammation and ameliorates autoimmunity. Nat Microbiol 4, 766–773 (2019). 10.1038/s41564-019-0381-1 [DOI] [PubMed] [Google Scholar]
- 147.Molinaro R, Mukherjee T, Flick R, Philpott DJ & Girardin SE Trace levels of peptidoglycan in serum underlie the NOD-dependent cytokine response to endoplasmic reticulum stress. J Biol Chem 294, 9007–9015 (2019). 10.1074/jbc.RA119.007997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Brenchley JM et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 12, 1365–1371 (2006). 10.1038/nm1511 [DOI] [PubMed] [Google Scholar]
- 149.Izui S, Kobayakawa T, Louis J & Lambert PH Induction of thymocytotoxic autoantibodies after injection of bacterial lipopolysaccharides in mice. European journal of immunology 9, 338–341 (1979). 10.1002/eji.1830090416 [DOI] [PubMed] [Google Scholar]
- 150.Huugen D et al. Aggravation of anti-myeloperoxidase antibody-induced glomerulonephritis by bacterial lipopolysaccharide: role of tumor necrosis factor-alpha. Am J Pathol 167, 47–58 (2005). 10.1016/s0002-9440(10)62952-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vatanen T et al. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell 165, 842–853 (2016). 10.1016/j.cell.2016.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ning W, C D, Howe PH, Bian C, Kamen DL, Luo Z, Fu X, Ogunrinde E, Yang L, Wang X, Li QZ, Oates J, Zhang W, White D, Wan Z, Gilkeson GS, Jiang W. Staphylococcus aureus peptidoglycan (PGN) induces pathogenic autoantibody production via autoreactive B cell receptor clonal selection, implications in systemic lupus erythematosus. Journal of Autoimmunity (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wolf AJ & Underhill DM Peptidoglycan recognition by the innate immune system. Nat Rev Immunol 18, 243–254 (2018). 10.1038/nri.2017.136 [DOI] [PubMed] [Google Scholar]
- 154.Atrih A, Zollner P, Allmaier G & Foster SJ Structural analysis of Bacillus subtilis 168 endospore peptidoglycan and its role during differentiation. J Bacteriol 178, 6173–6183 (1996). 10.1128/jb.178.21.6173-6183.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Popham DL, Helin J, Costello CE & Setlow P Analysis of the peptidoglycan structure of Bacillus subtilis endospores. J Bacteriol 178, 6451–6458 (1996). 10.1128/jb.178.22.6451-6458.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vollmer W, Blanot D & de Pedro MA Peptidoglycan structure and architecture. FEMS Microbiol Rev 32, 149–167 (2008). 10.1111/j.1574-6976.2007.00094.x [DOI] [PubMed] [Google Scholar]
- 157.Conti F et al. Association between Staphylococcus aureus nasal carriage and disease phenotype in patients affected by systemic lupus erythematosus. Arthritis research & therapy 18, 177 (2016). 10.1186/s13075-016-1079-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sirobhushanam S et al. Staphylococcus aureus Colonization Is Increased on Lupus Skin Lesions and Is Promoted by IFN-Mediated Barrier Disruption. J Invest Dermatol 140, 1066–1074 e1064 (2020). 10.1016/j.jid.2019.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nanda Kumar NS et al. Probiotic administration alters the gut flora and attenuates colitis in mice administered dextran sodium sulfate. J Gastroenterol Hepatol 23, 1834–1839 (2008). 10.1111/j.1440-1746.2008.05723.x [DOI] [PubMed] [Google Scholar]
- 160.Johansson ME & Hansson GC Microbiology. Keeping bacteria at a distance. Science 334, 182–183 (2011). 10.1126/science.1213909 [DOI] [PubMed] [Google Scholar]
- 161.Mistry P et al. Inhibition of TLR2 signaling by small molecule inhibitors targeting a pocket within the TLR2 TIR domain. Proc Natl Acad Sci U S A 112, 5455–5460 (2015). 10.1073/pnas.1422576112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fujiki M et al. Bacterial superantigen staphylococcal enterotoxin B induces interstitial pneumonia in SCID mice reconstituted with peripheral blood mononuclear cells from collagen vascular disease patients. Clinical immunology 96, 38–43 (2000). 10.1006/clim.2000.4872 [DOI] [PubMed] [Google Scholar]
- 163.Principato M & Qian BF Staphylococcal enterotoxins in the etiopathogenesis of mucosal autoimmunity within the gastrointestinal tract. Toxins (Basel) 6, 1471–1489 (2014). 10.3390/toxins6051471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kim C, Siminovitch KA & Ochi A Reduction of lupus nephritis in MRL/lpr mice by a bacterial superantigen treatment. J Exp Med 174, 1431–1437 (1991). 10.1084/jem.174.6.1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Krieg AM & Vollmer J Toll-like receptors 7, 8, and 9: linking innate immunity to autoimmunity. Immunol Rev 220, 251–269 (2007). 10.1111/j.1600-065X.2007.00572.x [DOI] [PubMed] [Google Scholar]
- 166.Ma K, Li J, Fang Y & Lu L Roles of B Cell-Intrinsic TLR Signals in Systemic Lupus Erythematosus. Int J Mol Sci 16, 13084–13105 (2015). 10.3390/ijms160613084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jimenez-Dalmaroni MJ, Gerswhin ME & Adamopoulos IE The critical role of toll-like receptors--From microbial recognition to autoimmunity: A comprehensive review. Autoimmun Rev 15, 1–8 (2016). 10.1016/j.autrev.2015.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hercher C, Chopra V & Beasley CL Evidence for morphological alterations in prefrontal white matter glia in schizophrenia and bipolar disorder. J Psychiatry Neurosci 39, 376–385 (2014). 10.1503/jpn.130277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hsiao EY et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463 (2013). 10.1016/j.cell.2013.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shaw W Increased urinary excretion of a 3-(3-hydroxyphenyl)-3-hydroxypropionic acid (HPHPA), an abnormal phenylalanine metabolite of Clostridia spp. in the gastrointestinal tract, in urine samples from patients with autism and schizophrenia. Nutr Neurosci 13, 135–143 (2010). 10.1179/147683010X12611460763968 [DOI] [PubMed] [Google Scholar]
- 171.Girschick HJ et al. Bacterial triggers and autoimmune rheumatic diseases. Clinical and experimental rheumatology 26, S12–17 (2008). [PubMed] [Google Scholar]
- 172.Matyszak MK Inflammation in the CNS: balance between immunological privilege and immune responses. Progress in Neurobiology 56, 19–35 (1998). 10.1016/s0301-0082(98)00014-8 [DOI] [PubMed] [Google Scholar]
- 173.Svenningsson A, Petersson AS, Andersen O & Hansson GK Nitric oxide metabolites in CSF of patients with MS are related to clinical disease course. Neurology 53, 1880–1882 (1999). 10.1212/wnl.53.8.1880 [DOI] [PubMed] [Google Scholar]
- 174.Conn AR, Fell DI & Steele RD Characterization of alpha-keto acid transport across blood-brain barrier in rats. Am J Physiol 245, E253–260 (1983). 10.1152/ajpendo.1983.245.3.E253 [DOI] [PubMed] [Google Scholar]
- 175.Rothhammer V et al. Microglial control of astrocytes in response to microbial metabolites. Nature 557, 724–728 (2018). 10.1038/s41586-018-0119-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chen PS et al. Valproic acid and other histone deacetylase inhibitors induce microglial apoptosis and attenuate lipopolysaccharide-induced dopaminergic neurotoxicity. Neuroscience 149, 203–212 (2007). 10.1016/j.neuroscience.2007.06.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Parker A, Fonseca S & Carding SR Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 11, 135–157 (2019). 10.1080/19490976.2019.1638722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Erny D et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nature Neuroscience 18, 965–977 (2015). 10.1038/nn.4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Roshchina VV in Microbial Endocrinology: Interkingdom Signaling in Infectious Disease and Health (eds Lyte Mark & Freestone Primrose P. E.) 17–52 (Springer, 2010). [Google Scholar]
- 180.Yano JM et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276 (2015). 10.1016/j.cell.2015.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Glebov K et al. Serotonin stimulates secretion of exosomes from microglia cells. Glia 63, 626–634 (2015). 10.1002/glia.22772 [DOI] [PubMed] [Google Scholar]
- 182.Chowdhary VR, Tilahun AY, Clark CR, Grande JP & Rajagopalan G Chronic exposure to staphylococcal superantigen elicits a systemic inflammatory disease mimicking lupus. J Immunol 189, 2054–2062 (2012). 10.4049/jimmunol.1201097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pflughoeft KJ & Versalovic J Human Microbiome in Health and Disease. Annual Review of Pathology: Mechanisms of Disease 7, 99–122 (2012). 10.1146/annurev-pathol-011811-132421 [DOI] [PubMed] [Google Scholar]
- 184.Allison DL et al. Candida-Bacteria Interactions: Their Impact on Human Disease. Microbiology Spectrum 4 (2016). 10.1128/microbiolspec.VMBF-0030-2016 [DOI] [PubMed] [Google Scholar]
- 185.Arvanitis M & Mylonakis E Fungal-bacterial interactions and their relevance in health. Cellular Microbiology 17, 1442–1446 (2015). 10.1111/cmi.12493 [DOI] [PubMed] [Google Scholar]
- 186.Trejo-Hernández A, Andrade-Domínguez A, Hernández M & Encarnación S Interspecies competition triggers virulence and mutability in Candida albicans-Pseudomonas aeruginosa mixed biofilms. The ISME journal 8, 1974–1988 (2014). 10.1038/ismej.2014.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dendrou CA, Fugger L & Friese MA Immunopathology of multiple sclerosis. Nat Rev Immunol 15, 545–558 (2015). 10.1038/nri3871 [DOI] [PubMed] [Google Scholar]
- 188.Shah S et al. Alterations of the gut mycobiome in patients with MS. EBioMedicine 71, 103557 (2021). 10.1016/j.ebiom.2021.103557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hoffmann C et al. Archaea and fungi of the human gut microbiome: correlations with diet and bacterial residents. PloS one 8, e66019 (2013). 10.1371/journal.pone.0066019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Eizirik DL, Sandler S & Palmer JP Repair of Pancreatic β-cells: A Relevant Phenomenon in Early IDDM? Diabetes 42, 1383–1391 (1993). 10.2337/diab.42.10.1383 [DOI] [PubMed] [Google Scholar]
- 191.Faradji RN et al. Long Term Insulin Independence and Improvement in Insulin Secretion after Supplemental Islet Infusion under Exenatide and Etanercept. Transplantation 86, 1658–1665 (2008). 10.1097/TP.0b013e31818fe448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Halban PA, German MS, Kahn SE & Weir GC Current Status of Islet Cell Replacement and Regeneration Therapy. The Journal of Clinical Endocrinology and Metabolism 95, 1034–1043 (2010). 10.1210/jc.2009-1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Martin-Pagola A et al. Insulin protein and proliferation in ductal cells in the transplanted pancreas of patients with type 1 diabetes and recurrence of autoimmunity. Diabetologia 51, 1803–1813 (2008). 10.1007/s00125-008-1105-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gürsoy S et al. Autoimmunity and intestinal colonization by Candida albicans in patients with type 1 diabetes at the time of the diagnosis. Korean Journal of Pediatrics 61, 217–220 (2018). 10.3345/kjp.2018.61.7.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Scharf DH, Brakhage AA & Mukherjee PK Gliotoxin – bane or boon? Environmental Microbiology 18, 1096–1109 (2016). 10.1111/1462-2920.13080 [DOI] [PubMed] [Google Scholar]
- 196.Fraga-Silva T. F. d. C. et al. Gliotoxin Aggravates Experimental Autoimmune Encephalomyelitis by Triggering Neuroinflammation. Toxins 11, 443 (2019). 10.3390/toxins11080443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cooke A et al. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol 21, 169–176 (1999). 10.1046/j.1365-3024.1999.00213.x [DOI] [PubMed] [Google Scholar]
- 198.Motran CC et al. Helminth Infections: Recognition and Modulation of the Immune Response by Innate Immune Cells. Frontiers in Immunology 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sabin EA, Araujo MI, Carvalho EM & Pearce EJ Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. The Journal of infectious diseases 173, 269–272 (1996). 10.1093/infdis/173.1.269 [DOI] [PubMed] [Google Scholar]
- 200.Savino W et al. Cytokines and cell adhesion receptors in the regulation of immunity to Trypanosoma cruzi. Cytokine & Growth Factor Reviews 18, 107–124 (2007). 10.1016/j.cytogfr.2007.01.010 [DOI] [PubMed] [Google Scholar]
- 201.Zaccone P, Fehervari Z, Phillips JM, Dunne DW & Cooke A Parasitic worms and inflammatory diseases. Parasite Immunol 28, 515–523 (2006). 10.1111/j.1365-3024.2006.00879.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Folci M, Ramponi G, Arcari I, Zumbo A & Brunetta E Eosinophils as Major Player in Type 2 Inflammation: Autoimmunity and Beyond. Advances in Experimental Medicine and Biology 1347, 197–219 (2021). 10.1007/5584_2021_640 [DOI] [PubMed] [Google Scholar]
- 203.Thomas PG et al. Maturation of dendritic cell 2 phenotype by a helminth glycan uses a Toll-like receptor 4-dependent mechanism. J Immunol 171, 5837–5841 (2003). 10.4049/jimmunol.171.11.5837 [DOI] [PubMed] [Google Scholar]
- 204.Mu Y, McManus DP, Hou N & Cai P Schistosome Infection and Schistosome-Derived Products as Modulators for the Prevention and Alleviation of Immunological Disorders. Frontiers in Immunology 12, 619776 (2021). 10.3389/fimmu.2021.619776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Rivera-Correa J et al. Autoimmunity to phosphatidylserine and anemia in African Trypanosome infections. PLoS Negl Trop Dis 15, e0009814 (2021). 10.1371/journal.pntd.0009814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Rivera-Correa J et al. Plasmodium DNA-mediated TLR9 activation of T-bet+ B cells contributes to autoimmune anaemia during malaria. Nature Communications 8, 1282 (2017). 10.1038/s41467-017-01476-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Papazahariadou M et al. Autoimmunity and parasites. Int J Immunopathol Pharmacol 21, 5–9 (2008). 10.1177/039463200802100102 [DOI] [PubMed] [Google Scholar]
- 208.Bach J-F The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nature reviews. Immunology 18, 105–120 (2018). 10.1038/nri.2017.111 [DOI] [PubMed] [Google Scholar]
- 209.Canavan C, West J & Card T The epidemiology of irritable bowel syndrome. Clin Epidemiol 6, 71–80 (2014). 10.2147/CLEP.S40245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Khan WI et al. Intestinal Nematode Infection Ameliorates Experimental Colitis in Mice. Infection and Immunity 70, 5931–5937 (2002). 10.1128/IAI.70.11.5931-5937.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]