Abstract
To maintain asepsis in production environments, contamination must be constantly controlled. To this end, microbiological monitoring is constantly used with the objective of evaluating the incidence of microorganisms prevalent in the sampling of air, surface, and people, in the area of an environment considered aseptic, isolated, and identified using the rapid and automated phenotypic microbiological methodology, highlighting the MALDI-TOF mass spectrometry analysis technique (MS), being identified at the level of genus and/or species. For that purpose, microbiological control of environmental monitoring of environments considered aseptic in a pharmaceutical industry was conducted for 12 months. The isolated microorganisms were identified using the mass spectrometry identification method (MALDI-TOF). In area classification A, the most prevalent microorganisms were bacteria in the sampling person. The microbial population was composed of bacteria of the genus Micrococcus sp. and Staphylococcus sp. Based on the results, it is possible to observe that in an environment where the process requires human operations, possible microbial contamination is inevitable and requires the identification of microorganisms at least at the level of species and/or genus. The microorganisms identified and found in the sampling of the aseptic environment must be evaluated with frequency to ensure that the productive environment guarantees the quality of the product produced.
Supplementary information
The online version contains supplementary material available at 10.1007/s42770-023-00987-3.
Keywords: Microbiological control, Clean rooms, Microorganisms, Mass spectrometry
Introduction
A high-quality product is of essential importance to the pharmaceutical industry. This is influenced by the microbiome and may be either from feedstock or acquired during the production process [1]. Thus, product sterility may be assured by cleaning the manufacturing environment to maintain an appropriate cleaning standard [2]. Microorganism occurrence on pharmaceutical preparations for a product has the potential to change organoleptic properties and reduce and even inactivate its therapeutic activity, leading to a risk for patient health, either for microbe contamination or an increase in toxic metabolites that present contamination [3].
Microorganism contamination can be from pharmaceutical inputs, water, and even the production environment. Thus, identification and quantification are necessary, and if overlimit levels are observed, asepsis actions must be taken to improve cleaning and product quality. It is important that preventive attention be paid to microorganisms contaminating pharmaceutical products, especially if it is a pathogenic agent or its presence may interfere with produced drug stability and efficacy [4]. We highlight that if production is aiming for exportation, worldwide market standards must be observed, with those becoming stricter over time. These results indicate the necessity of fast and automated microbiological methods for the constant reevaluation of microorganism identification processes [5].
Clean area of a productive process is ordered according to environment-requested characteristics for sterile production, being classified on “degree” A, B, C, and D based on parameters established by current laws. Degree A is defined as an area that cannot have viable particles and a high operational risk zone. Degree B are areas around the A-area that are not sterile. However, B-area cleaning is important to avoid product contamination from the A-area. Areas C and D represent clean areas, where fewer critical steps on sterile product fabrication are carried out, in which a higher microorganism tolerance can be established.
To assure product sterility, the pharmaceutical industry adopts actions of environmental monitoring and microbiological control in drug production environments. This monitoring consists of quantification of the number of colony-forming units (CFUs) to identify viable microorganisms and establish actions to minimize or eliminate contamination [6]. Samplings on classified areas in the pharmaceutical industry are carried out through techniques of active collection, which is the first parameter for area qualification. Passive environmental sampling on the surface, which includes walls, machines, equipment, ground, and people, is carried out through the process of monitoring clean rooms, with people considered the higher focus of contamination [7].
Limits for alert and actions are established to control microbiological monitoring for each degree established at the area, and results are expressed on colony-formator units (CFU). When the limit for microbiological level is crossed, a revision for the documentation and investigation must be carried out, being adopted, among the actions, for identification of contaminating microorganism and its probable source [1].
Microbiome analysis of clean rooms in the pharmaceutical industry is a useful tool to identify and measure the microbiome. From these results, it is possible to observe the frequency of these incidents as establish strategies to minimize or eliminate these occurrences [8]. To assure result quality, microbiologists may need methods to allow efficient quantification and identification of microorganisms [5]. The system matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) analyze intact bacteria, cell lysates, or bacterial extracts, which are detected by molecular biomarkers (in general peptides or ribosomal proteins) [9, 10]. This equipment results in less-time analysis and has a lower cost, which assures higher efficiency in identification and increases productivity for the pharmaceutical industry. Microorganism identification is fundamental for establishing efficient disinfection methods to reduce their frequency. This also allows the correct follow-up of contamination processes, confirming microorganisms in validation and in promotion of culture media for environmental monitoring [5]. These procedures are important to fulfill practices, such as the adoption of preventive and corrective operational actions, validation of cleaning processes, sanitization of areas, and personal training [6].
Considering these aspects and due to the requirement of fast tests to identify microorganisms, this research has the objective of characterizing the productive environment of a biopharmaceutical and identifying the microorganisms using the phenotypic microbiologic methodology and automatized by mass spectrometry MALDI-TOF MS, thus contributing to the establishment of preventive actions on the productive environment to avoid contamination.
Material and methods
This research was carried out in an aseptic biopharmaceutical A-degree area. Once elevated-risk operations are carried out, contamination monitoring is essential. For this area, tanks for formulation and bottling of biopharmaceutical products are carried, which are also the place for environmental sampling points. Sampling places and frequency were established according to the Intern Environmental Program from the institution. Microbiological monitoring was carried out under conditions of rest (static) and in operation (dynamic) for sampling of air (active air sampling), surface, and people in A-degree areas in a pharmaceutical industry in a 12-month period (2018–2019) as an internal part of the environmental monitoring program. For this study, other areas (B-, C-, and D-degree) were not considered for sampling.
Samples of air were carried for one hour (approximately a thousand liters of air) through fixation of 9 cm Petri dishes with medium trypticase in soy agar (TSA) irradiation. These were coupled on an air active sampling system Silt Sample ®. Rodac plates (Replicate Organism Detection Counting) with 6 cm containing AST medium were used to collect material from the surface and people. For these last, the collection is consisted of clothes, accessory, and body parts. All plates were incubated at a temperature of 30 to 35 °C for 72 h, when colonies were counted and registered [11].
Sampling on surfaces was performed by using a swab and was carried out by fractioning the swab over the areas and applying it in Petri dishes. For people, the same system was used for collecting samples on chest, legs, and in between fingers. Additionally, the tips of the fingers were also collected by touching the Petri dishes. It must be noted that although people and surface samplings were collected on distinct parts in each, samples were considered one for analysis.
Identification was carried out through mass spectrometry. The process was executed with a Bruker MALDI Biotyper following the manufacturer’s instructions. Analysis consisted of protein profile obtainment in duplicate from microbiological cultures (bacteria, filamentous fungi, and yeasts). Data were registered on the industry Environmental Monitoring Program for the 2019 report. All data were organized using Microsoft Office Excel ® software. From MALDI-TOF mass spectrometry data, microorganisms were identified at the species and/or genus level to quantify microbiological contamination.
Results
The results for genus and/or species for microorganisms isolated from air and surface samples can be observed in Tables 1 and 2. For the results from people, due to the number of observations, they were organized into the genus level (Table 3); however, the results for species can be observed in Supplementary Table 1. We carried 36652 samplings for active air for the study area. Of the eight microorganisms observed, six were identified at the genus level, while the others were identified at the genus level, with coccus being the prevalent microorganism group.
Table 1.
Microorganisms found on active air sampling on A-degree biopharmaceutical production area
| Microorganisms | Level* | Group | Occurrence |
|---|---|---|---|
| Paenibacillus woosongensis | Species | Bacillus | 1 |
| Corynebacterium sp. | Genus | Bacillus | 1 |
| Micrococcus luteus | Species | Coccus | 6 |
| Kocuria palustris | Species | Coccus | 2 |
| Kocuria sp. | Genus | Coccus | 1 |
| Staphylococcus warneri | Species | Coccus | 1 |
| Corynebacterium tuberculostearicum | Species | Bacillus | 1 |
| Micrococcus terreus | Species | Coccus | 1 |
| Total | 14 | ||
*Level which occurrence was observed
Table 2.
Microorganisms found on surface sampling on A-degree biopharmaceutical production area
| Microorganisms | Level* | Group | Occurence |
|---|---|---|---|
| Bacillus sp. | Genus | Bacillus | 2 |
| Burkholderia cepacia | Species | Bacillus | 1 |
| Micrococcus luteus | Species | Coccus | 1 |
| Staphylococcus sp. | Genus | Coccus | 1 |
| Total | 5 | ||
*Level which occurrence was observed
Table 3.
Resumed list of microorganisms found on surface sampling on A-degree biopharmaceutical production area
| Genus | Type | Level* | Occurrences | |
|---|---|---|---|---|
| Genus | Species | |||
| Acinetobacter | Bacillus | 1 | 5 | 17 |
| Aerococcus | Coccus | 1 | 1 | 12 |
| Arthrobacter | Bacillus | 1 | 2 | 11 |
| Aspergillus | Filamentous fungi | 1 | 2 | 17 |
| Aureimonas | Bacillus | 1 | 1 | 6 |
| Aureobasidium | Filamentous fungi | 1 | 0 | 1 |
| Bacillus | Bacillus | 1 | 4 | 35 |
| Brachybacterium | Bacillus | 0 | 1 | 1 |
| Brevibacillus | Bacillus | 1 | 0 | 1 |
| Brevibacterium | Bacillus | 1 | 1 | 7 |
| Brevundimonas | Bacillus | 1 | 0 | 1 |
| Burkholderia | Bacillus | 1 | 0 | 1 |
| Candida | Yeast | 1 | 1 | 2 |
| Cellulosimicrobium | Bacillus | 0 | 1 | 4 |
| Chaetomium | Filamentous fungi | 1 | 0 | 4 |
| Corynebacterium | Bacillus | 0 | 14 | 229 |
| Curtobacterium | Bacillus | 1 | 0 | 2 |
| Curvularia | Filamentous fungi | 1 | 1 | 4 |
| Dermabacter | Bacillus | 0 | 1 | 2 |
| Dietzia | Bacillus | 1 | 0 | 3 |
| Escherichia | Bacillus | 1 | 0 | 1 |
| Exiguobacterium | Bacillus | 1 | 1 | 2 |
| Gordonia | Bacillus | 1 | 0 | 1 |
| Kocuria | Bacillus | 1 | 3 | 64 |
| Kytococcus | Bacillus | 1 | 1 | 8 |
| Leclercia spp. | Bacillus | 1 | 0 | 1 |
| Malassezia | Bacillus | 0 | 1 | 1 |
| Massilia | Bacillus | 1 | 0 | 1 |
| Methylobacterium | Bacillus | 1 | 0 | 1 |
| Microbacterium | Bacillus | 1 | 2 | 5 |
| Micrococcus | Coccus | 1 | 3 | 614 |
| Moniliella | Yeast | 1 | 0 | 5 |
| Moraxella | Bacillus | 1 | 1 | 17 |
| Paenibacillus | Bacillus | 1 | 0 | 1 |
| Paracoccus | Bacillus | 1 | 0 | 1 |
| Pediococcus | Coccus | 0 | 1 | 1 |
| Penicillium | Filamentous fungi | 1 | 2 | 8 |
| Propionibacterium | Anaerobic bacillus | 1 | 1 | 7 |
| Pseudomonas | Bacillus | 1 | 2 | 11 |
| Rhodotorula | Yeast | 0 | 1 | 1 |
| Roseomonas | Bacillus | 0 | 1 | 2 |
| Rothia | Bacillus | 0 | 1 | 5 |
| Scopulariopsis | Filamentous fungi | 1 | 1 | 20 |
| Serratia | Bacillus | 0 | 1 | 1 |
| Staphylococcus | Coccus | 1 | 11 | 939 |
| Streptococcus | Coccus | 2 | 1 | 4 |
| Trichosporon | Coccus | 1 | 0 | 1 |
| Total | 38 | 70 | 2083 | |
*Level which occurrence was observed
For surfaces, 8318 samples were collected. From those, two microorganisms were identified at the genus level, while the other two were identified at the species level. All species observed on surfaces were from bacteria (bacillus and coccus) (Table 2).
For people, we carried out 93,027 samplings, with 110 microorganisms identified at the species (73) and genus (37) levels. We observed higher diversity for these samples, including bacteria (bacillus and coccus), filamentous fungi, and yeasts. Table 3 shows the short microorganism list observed, with the complete list (separated from species) found in Supplementary Table 1.
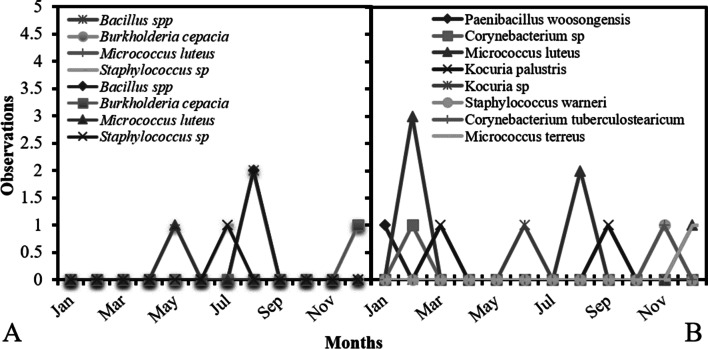
In Fig. 1, we can observe incidence data for microorganisms on active air sampling and surfaces in the year of study. A higher concentration of those microorganisms was observed in active air sampling in February (Fig. 1B) with a higher incidence of Micrococcus luteus. For surfaces, a higher incidence was observed in August, with Bacillus sp. the microorganism with higher number (Fig. 1A).
Fig. 1.
Number of microorganisms founded on sampling of surfaces (A) and active air (B) on a A-degree clean area during the study period
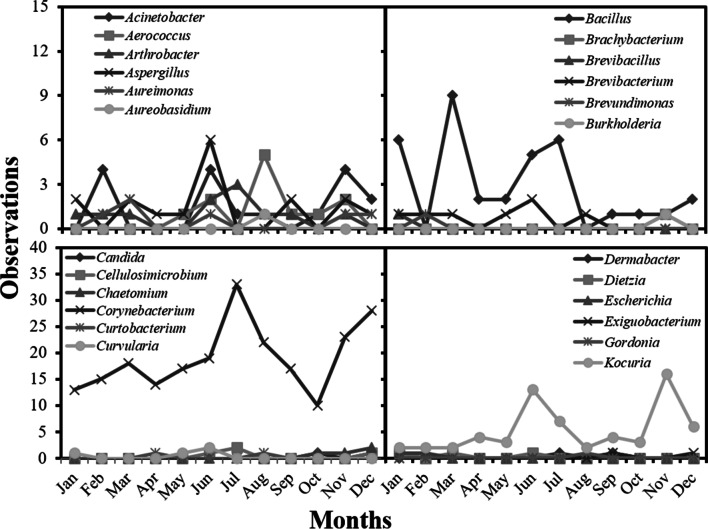
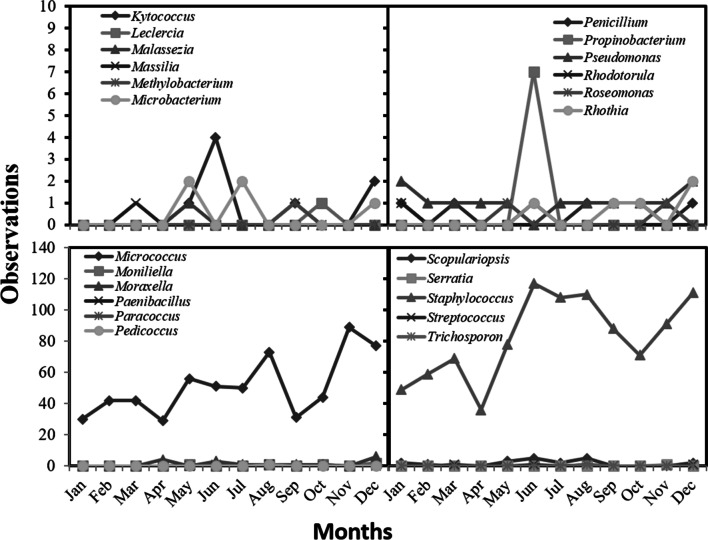
We observed more than 230 observations/month for people for the months of June, August, November, and December. The results detailed by species can be observed in the supplementary Figs 1, 2 , 3, and data sorted by genus can be observed in Figs. 2 and 3. Higher incidence of microorganisms were Micrococcus luteus, Staphylococcus epidermidis, Staphylococcus hominis, and Staphylococcus spp.
Fig. 2.
Number of microorganisms founded on sampling people on a A-degree clean area during the study period sorted by genus
Fig. 3.
Number of microorganisms founded on sampling people on a A-degree clean area during the study period sorted by genus
Discussion
Monitoring both the number and species in clean rooms is important to comprehend the way contamination occurs and to establish containment actions [8]. For efficient industrial production, fast and accurate results are essential; thus, the use of MALDI-TOF mass spectrometry can be a useful tool to ensure fast and accurate microorganism identification and reduce costs.
According to our results, a higher microbial incidence on people was found than those from surfaces and air (Figs. 1, 2 and 3). In the areas monitored, a higher prevalence of the genus Micrococcus spp. and Staphylococcus spp. was observed. Micrococcus luteus is among the bacteria mostly found in industrial system water and has high potential to establish biofilms, resulting in severe damage to production [12]. Additionally, strains of Micrococcus luteus highly resistant to radiation have been isolated and reported in the literature, compromising the sterilization of industrial and hospital environments [13].
The Staphylococcus genus, from the Micrococcaceae family, has 33 species, of which 17 can be found in human biological samples. They are facultative anaerobic or aerobic organisms that are catalase positive. Staphylococcus is a genus highly susceptible to mutation, acquiring resistance to antimicrobial and sanitizing agents. Thus, it is of extreme importance to rotate these products for effective disinfection of either the hospital or industrial environment [14].
Essentially, human-free environments in general have lower contamination and lower microbial levels. Studies such as the present show a conclusive way that operators, even when carefully prepared, allow viable microorganisms to enter the environment [14–16]. Predominance of bacteria coccus gran-positive found on operators even fully dressed require an extensive training on aseptic techniques. Bacteria from human skin, as these can indicate inefficient aseptic techniques for dressing, the lack of knowledge, and process execution. Additionally, it can be an indication of extensive human intervention in the aseptic production place [17]. In addition to the higher prevalence of bacteria on people sampling for this research, fungi were also isolated (Figs. 2 and 3).
At a lower rate, bacteria were found on active air sampling and surfaces. These findings corroborate the study from Sandle [8], which described gram-positive cocci more frequently on air. This author also correlated this result to the presence of people as the source of this contamination. Skin fragments from operators can be the most likely source of these microorganisms to be found in air.
Although zero contamination is unreal, maintaining this factor as close as possible to zero is of interest for higher quality. However, contamination cannot be avoided by a single method, and some microorganisms may be more resistant to some cleaning agents than others, requiring different strategies [18–21]. Additionally, there are contaminant organisms that are able to develop into biofilms [22–26], which requires differential strategies for decontamination. In this case, microorganism identification is essential to allow the establishment of appropriate strategies not only for decontamination but also to prevent it. If the process of identification is slow, production may be delayed, and batches may be retained until microorganisms are identified and/or eliminated. In this case, a fast tool for identification is important. MALDI-TOFF MS can be considered a high-speed method for identification [27, 28], and this technique is applicable for monitoring and identifying the microorganisms contaminating the production area.
Rapid microorganism identification associated with the biology of each organism can also be essential for decontamination strategies. Once it is possible to identify the microorganism, we can, based on its biology, understand from which sources it can be associated. We can mention some fungi as Trichosporum sp. and Malassezia sp. and Candida sp., related to skin infections [29–31], as well as Aspergillus sp., Penicillium sp., and Curvularia sp., related to nail infections [32, 33]. As those microorganisms are identified faster, through MALDI-TOFF MS, the source of contamination may be identified and prevented. It must be highlighted that the MALDI-TOFF MS system can be used for microorganism identification by using the material from the first collection, with no need for additional spikes for sampling and identification by the micologist. The use of a database is also advantageous because identification will not be faster but will avoid human errors [27, 28, 34].
In any environment in which human operation is present, microbial contamination is inevitable at some level. Thus, a zero tolerance for contamination on any local is not technically possible and unreal. Thus, the results from environmental monitoring must be frequently revised to ensure that installation is a valid state for releasing the product [35]. For this, the established limits must be observed, and microorganism detection must be executed in a fast and accurate way, as could be observed here, through MALDI-TOF MS.
Conclusions
Under the conditions of this research, 49 microorganisms were found, mostly from people.
Higher prevalence of Micrococcus spp. and Staphylococcus spp. was found.
MALDI-TOF mass spectrometry can be a fast and accurate tool for microorganism monitoring.
Supplementary information
Below is the link to the electronic supplementary material.
Author contribution
Conceptualization: MLGuimarães, MABezerra-Júnior, WVSPereira. Methodology: MLGuimarães, MABezerra-Júnior, VM de Almeida. Formal Analysis and investigation: MLGuimarães. Writing—original draft preparation: ML Guimarães, VM de Almeida. Writing—review and editing: WVSPereira. Supervision: WVSPereira, MABezerra-Júnior.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Responsible Editor: Gisele Monteiro
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brasil (2010) Resolução RDC No17 de 16 de Abril de 2010 – Boas Práticas de Fabricação de Medicamentos [RDC Resolution No. 17 of April 16, 2010 - Good Drug Manufacturing Practices]. Ministério da Saúde. Agência Na¬cional de Vigilância Sanitária
- 2.EUROPEAN-COMMISSION (2008) The rules governing medicinal products in the European Union. Volume 4 – EU guidelines for good manufacturing practice for medicinal products for human and veterinary use. Published online
- 3.Hatcher RA (1908) The United States Pharmacopeia. Jama, L(1):30–34. 10.1001/jama.1908.25310270030002f
- 4.Assis D. A espectrometria de massas aplicada na classificação e identificação de microorganismos [Mass spectrometry applied to the classification and identification of microorganisms] Rev da Univ Val do Rio Verde. 2011;9(2):344–355. doi: 10.5892/ruvrv.2011.92.344355. [DOI] [Google Scholar]
- 5.Pacheco FLC, Pinto TDJA (2010) The bacterial diversity of pharmaceutical clean rooms analyzed by the fatty acid methyl ester technique. PDA J Pharm Sci Technol 64(2):156–166. Accessed April 8, 2022. https://journal.pda.org/content/64/2/156.short [PubMed]
- 6.M. C (2012) Monitoramento e controle microbiológico [Microbiological monitoring and control]. Rev SBCC 55:10–14
- 7.Utescher CLDA, Franzolin MR, Trabulsi LR, Gambale V. Microbiological monitoring of clean rooms in development of vaccines. Brazilian J Microbiol. 2007;38(4):710–716. doi: 10.1590/S1517-83822007000400023. [DOI] [Google Scholar]
- 8.Sandle T. A review of cleanroom microflora: types, trends, and patterns. PDA J Pharm Sci Technol. 2011;65(4):392–403. doi: 10.5731/pdajpst.2011.00765. [DOI] [PubMed] [Google Scholar]
- 9.Schapoval EES. Controle biológico de qualidade de produtos farmacêuticos, correlatos e cosméticos [Biological quality control of pharmaceuticals, related products and cosmetics] Rev Bras Ciências Farm. 2005;41(2):279–280. doi: 10.1590/s1516-93322005000200018. [DOI] [Google Scholar]
- 10.Lay JO. MALDI-TOF mass spectrometry and bacterial taxonomy. TrAC - Trends Anal Chem. 2000;19(8):507–516. doi: 10.1016/S0165-9936(00)00027-3. [DOI] [Google Scholar]
- 11.MP X, HS N, MA SX, ARE de OX. Monitoramento microbiológico de áreas grau A e grau B de uma produção asséptica. Rev Unimontes Científica. 2017;19(2):112–125. Accessed April 8, 2022. https://www.periodicos.unimontes.br/index.php/unicientifica/article/view/1184
- 12.Cyranka B. Otimização do processo de descontaminação no sistema isolador de Bio-Manguinhos [Optimization of the decontamination process in the BiomBio-manguinhos isolator system]. Published online 2011. Accessed April 8, 2022. https://www.arca.fiocruz.br/handle/icict/5910
- 13.Deng W, Yang Y, Gao P, Chen H, Wen W, Sun Q. Radiation-resistant Micrococcus luteus SC1204 and its proteomics change upon gamma irradiation. Curr Microbiol. 2016;72(6):767–775. doi: 10.1007/s00284-016-1015-y. [DOI] [PubMed] [Google Scholar]
- 14.C PC. Identificação da microbiota presente em áreas classificadas de produção de uma indústria farmacêutica [Identification of the microbiota present in classified production areas of a pharmaceutical industry]. Published online 2009
- 15.Wu GF, Liu XH. Characterization of predominant bacteria isolates from clean rooms in a pharmaceutical production unit. J Zhejiang Univ Sci B. 2007;8(9):666–672. doi: 10.1631/jzus.2007.B0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shukla A, Vishnoi G, Das DR. Current good manufacturing guidelines for medicinal product. J Drug Deliv Ther. 2016;6(2):57. doi: 10.22270/jddt.v6i2.1201. [DOI] [Google Scholar]
- 17.Cundell AM. Microbial identification strategies in the pharmaceutical industry. PDA J Pharm Sci Technol. 2006;60(2):111–123. Accessed April 8, 2022. https://journal.pda.org/content/60/2/111.full [PubMed]
- 18.Arini A, Feurtet-Mazel A, Maury-Brachet R, Pokrovsky OS, Coste M, Delmas F. Recovery potential of periphytic biofilms translocated in artificial streams after industrial contamination (Cd and Zn) Ecotoxicology. 2012;21(5):1403–1414. doi: 10.1007/s10646-012-0894-3. [DOI] [PubMed] [Google Scholar]
- 19.Mandal R, Singh A, Pratap SA. Recent developments in cold plasma decontamination technology in the food industry. Trends Food Sci Technol. 2018;80:93–103. doi: 10.1016/j.tifs.2018.07.014. [DOI] [Google Scholar]
- 20.Goodburn C, Wallace CA. The microbiological efficacy of decontamination methodologies for fresh produce: a review. Food Control. 2013;32(2):418–427. doi: 10.1016/j.foodcont.2012.12.012. [DOI] [Google Scholar]
- 21.Anand S, Singh D. Resistance of the constitutive microflora of biofilms formed on whey reverse-osmosis membranes to individual cleaning steps of a typical clean-in-place protocol. J Dairy Sci. 2013;96(10):6213–6222. doi: 10.3168/jds.2013-7012. [DOI] [PubMed] [Google Scholar]
- 22.de Macêdo JAB. Biofilmes bacterianos, uma preocupação da indústria de farmacêutica [Bacterial biofilms, a pharmaceutical industry concern] Rev Fármacos Medicam. 2000;2(7):19–24. [Google Scholar]
- 23.Sardi JDCO, Pitangui NDS, Rodríguez-Arellanes G, Taylor ML, Fusco-Almeida AM, Mendes-Giannini MJS. Highlights in pathogenic fungal biofilms. Rev Iberoam Micol. 2014;31(1):22–29. doi: 10.1016/j.riam.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Miranda AC, Leães GF, Copetti MV. Fungal biofilms: insights for the food industry. Curr Opin Food Sci. 2022;46:100846. doi: 10.1016/j.cofs.2022.100846. [DOI] [Google Scholar]
- 25.Junqueira JC, Jorge AOC, Barbosa JO, et al. Photodynamic inactivation of biofilms formed by Candida spp., Trichosporon mucoides, and Kodamaea ohmeri by cationic nanoemulsion of zinc 2,9,16,23-tetrakis(phenylthio)-29H, 31H-phthalocyanine (ZnPc) Lasers Med Sci. 2012;27(6):1205–1212. doi: 10.1007/s10103-012-1050-2. [DOI] [PubMed] [Google Scholar]
- 26.Matsuura K, Asano Y, Yamada A, Naruse K. Detection of Micrococcus luteus biofilm formation in microfluidic environments by pH measurement using an ion-sensitive field-effect transistor. Sensors (Switzerland) 2013;13(2):2484–2493. doi: 10.3390/s130202484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassagne C, Normand AC, L’Ollivier C, Ranque S, Piarroux R. Performance of MALDI-TOF MS platforms for fungal identification. Mycoses. 2016;59(11):678–690. doi: 10.1111/myc.12506. [DOI] [PubMed] [Google Scholar]
- 28.Chalupová J, Raus M, Sedlářová M, Šebela M. Identification of fungal microorganisms by MALDI-TOF mass spectrometry. Biotechnol Adv. 2014;32(1):230–241. doi: 10.1016/j.biotechadv.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Barros Bandeira I, Nunes AG, Vandesmet CS. Malassezia sp.: ma revisão de literatura sobre os aspectos gerais [Malassezia sp.: a literature review on general aspects]. Most Científica em Biomed. 2016;1(1). Accessed April 7, 2023. http://publicacoesacademicas.unicatolicaquixada.edu.br/index.php/mostrabiomedicina/article/viewFile/831/747
- 30.Furlaneto-Maia L, Spcian AFL, Trörn DSW, de Oliveira MT, Furlaneto MC. Estudo da incidência de amostras clínicas do gênero Candida Estudo da incidência de amostras clínicas do gênero Candida isoladas de diversos sítios anatômicos [Study of the incidence of clinical samples of the genus Candida study of the incidence of clini. Acta Sci Heal Sci. 2007;29(1):33–37. doi: 10.4025/actascihealthsci.v29i1.104. [DOI] [Google Scholar]
- 31.Naves PLF, Pinto Santana D, Leão Ribeiro E, Severo Menezes AC. Novas abordagens sobre os fatores de virulência de Candida albicans [New approaches on Candida albicans virulence factors] Rev Ciências Médicas e Biológicas. 2013;12(2):229. doi: 10.9771/cmbio.v12i2.6953. [DOI] [Google Scholar]
- 32.Henrique Reis da Silva M, Henrique Barreto Gotardi A, Ap Silva de Barros A, et al (2014) Isolamento e identificação de microrganismos presentes em superfícies de teclados e mouses de uma Universidade de Três Lagoas, MS. [Isolation and identification of microorganisms present on surfaces of keyboards and mice at a University of Três Lagoas, MS. Colloq Vitae 6(3):83-90. 10.5747/cv.2014.v06.n3.v115
- 33.Eliza A, Senger V, Bizani D. Pesquisa de Staphylococcus aureus em queijo Minas frescal, porduzido de forma artesanal e industrial, comercializado na cidade de Canoas/RS, BRASIL. [Research of Staphylococcus aureus in Minas fresh cheese, produced in an artisanal and industrial way, com. Rev Ciências Ambient. 2011;5(2):25–42. Accessed April 7, 2023. https://revistas.unilasalle.edu.br/index.php/Rbca/article/view/259
- 34.Ranque S, Normand AC, Cassagne C, et al. MALDI-TOF mass spectrometry identification of filamentous fungi in the clinical laboratory. Mycoses. 2014;57(3):135–140. doi: 10.1111/myc.12115. [DOI] [PubMed] [Google Scholar]
- 35.Gouveia BG, Rijo P, Gonçalo TS, Reis CP. Good manufacturing practices for medicinal products for human use. J Pharm Bioallied Sci. 2015;7(2):87–96. doi: 10.4103/0975-7406.154424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Not applicable.