Abstract
Escherichia coli harboring a transmissible locus of stress tolerance (tLST) and the ability to form biofilms represent a serious risk in dairy production. Thus, we aimed to evaluate the microbiological quality of pasteurized milk from two dairy producers in Mato Grosso, Brazil, with a focus on determining the possible presence of E. coli with heat resistance (60 °C/6 min), biofilm-forming potential phenotypes and genotypes, and antimicrobial susceptibility. For this, fifty pasteurized milk samples from producers named A and B were obtained for 5 weeks to investigate the presence of Enterobacteriaceae members, coliforms, and E. coli. For heat resistance, E. coli isolates were exposed to a water bath at 60 °C for 0 and 6 min. In antibiogram analysis, eight antibiotics belonging to six antimicrobial classes were analyzed. The potential to form biofilms was quantified at 570 nm, and curli expression by Congo Red was analyzed. To determine the genotypic profile, we performed PCR for the tLST and rpoS genes, and pulsed-field gel electrophoresis (PFGE) was used to investigate the clonal profile of the isolates. Thus, producer A presented unsatisfactory microbiological conditions regarding Enterobacteriaceae and coliforms for weeks 4 and 5, while all samples analyzed for producer B were contaminated at above-the-limit levels established by national and international legislation. These unsatisfactory conditions enabled us to isolate 31 E. coli from both producers (7 isolates from producer A and 24 isolates from producer B). In this way, 6 E. coli isolates (5 from producer A and 1 from producer B) were highly heat resistant. However, although only 6 E. coli showed a highly heat-resistant profile, 97% (30/31) of all E. coli were tLST-positive. In contrast, all isolates were sensitive to all antimicrobials tested. In addition, moderate or weak biofilm potential was verified in 51.6% (16/31), and the expression of curli and presence of rpoS was not always related to this biofilm potential. Therefore, the results emphasize the spreading of heat-resistant E. coli with tLST in both producers and indicate the biofilm as a possible source of contamination during milk pasteurization. However, the possibility of E. coli producing biofilm and surviving pasteurization temperatures cannot be ruled out, and this should be investigated.
Keywords: Antimicrobial susceptibly, Heat treatment, Locus of heat resistance, Rpos, Transmissible locus of stress tolerance
Introduction
Milk is an important source of vital nutrients, especially proteins and micronutrients, for humans [1]. Milk production has increased by 67% in the last three decades worldwide [1]. In Brazil, the 2020 production reached approximately 25.53 billion liters, with the state of Mato Grosso ranking second in production volume in the Midwest region of the country [2]. Because of this consumption potential, microbiological controls are paramount to ensure food quality and safety, and the pasteurization process is required by both national and international standards for microbiological milk product safety [3–5].
Pasteurization can be categorized as low temperature and long time (LTLT), applying temperatures between 62 and 65 °C for 30 min, or high temperature and short time (HTST), applying temperatures between 72 and 75 °C for 15–20 s, followed by cooling to 4 °C [6,7]. However, temperature abuse during the handling or transport of this milk and a lack of hygiene practices can result in microbial contamination or even an outbreak involving foodborne pathogens [8–10].
On the other hand, some studies using subpasteurization temperatures (between 57 and 68 °C for 15 s or more), commonly used as raw milk thermalization, have demonstrated the survivability of some pathogenic Escherichia coli (E. coli) [11, 12]. In this context, some E. coli pathotypes can cause foodborne diseases, including several forms of diarrhea and even hemolytic uremic syndrome [13, 14]. Furthermore, E. coli is a member of the Enterobacteriaceae family and is the only bacterium in the coliform group that indicates possible fecal contamination [15, 16]. Therefore, the control of Enterobacteriaceae species and coliforms has been described in several legislations and must meet the acceptance limits for commercialized pasteurized milk and dairy products [5, 16–18].
E. coli has been historically classified as heat-sensitive [19]. However, the emergence of heat-resistant E. coli strains isolated from animal-based foods has been reported. Dlusskaya et al. [20] reported that E. coli AW1.7 with a survivability of approximately 5 log CFU/ml in ground beef heated to an internal temperature of 71 °C. Similarly, E. coli AW 1.1 presented a bacterial reduction below 1 log CFU/ml after 5 min at 60 °C [21]. In another study, E. coli FAM23288 showed survivability of approximately 4.9 log CFU/ml after 30 min at 55 °C [22], and E. coli XHR presented growth capacity in MacConkey agar after exposure to 80 °C for 15 min [23]. Strains displaying heat resistance have been associated with gene markers inserted in a mobile island named the locus of heat resistance (LHR), more recently termed the transmissible locus of stress tolerance (tLST) [24]. These genes are responsible for protecting microorganisms against stress factors, including heat shock stress, envelope stress, and oxidative stress [25]. Other studies have associated the presence of pathogenic and nonpathogenic E. coli with tLST in beef produced in Canada, the USA [21, 23, 26–28], and in Brazil by our group [29] and cheese prepared with raw milk [22].
In addition to heat resistance, E. coli has been found to have the capacity for biofilm formation, e.g., isolates from dairy products [30]. Additionally, a considerable portion of tLST-positive E. coli has also been identified to harbor antimicrobial resistance genes [31]. Xu et al. [32] reported that biofilm-embedded tLST-positive E. coli strains are more resistant to chemical sanitizers than tLST-negative E. coli strains. According to a study by Boll et al. [22], the presence of orfE in a plasmid with tLST can encode putative di-guanyl cyclase and cyclic di-GMP (c-di-GMP) enzymes, showing an effect on biofilm formation in E. coli. Biofilms comprise the formation of bacterial multilayers on both biotic and abiotic surfaces, which may promote the persistence of E. coli in the food chain [33]. This can bring challenges to the pasteurized milk chain if the binomial time and temperature are not carefully monitored.
In this context, this work aimed to evaluate the quality of pasteurized milk, investigate the presence of E. coli isolates in this milk, and understand the factors related to persistence or postpasteurization contamination. The study consists of four steps: (i) evaluate the milk quality from two producers (A and B) taking into account the Enterobacteriaceae and coliform counts; (ii) isolate E. coli strains from pasteurized milk; (iii) perform a phenotypic analysis for heat resistance, antimicrobial susceptibility, biofilm potential, and curli expression; and (iv) perform a genotypic analysis searching for genetic similarity profiles of E. coli isolates and verifying the presence of gene markers for the tLST and rpoS genes (involved in stress conditions and curli/cellulose control, respectively).
Materials and methods
Milk sampling
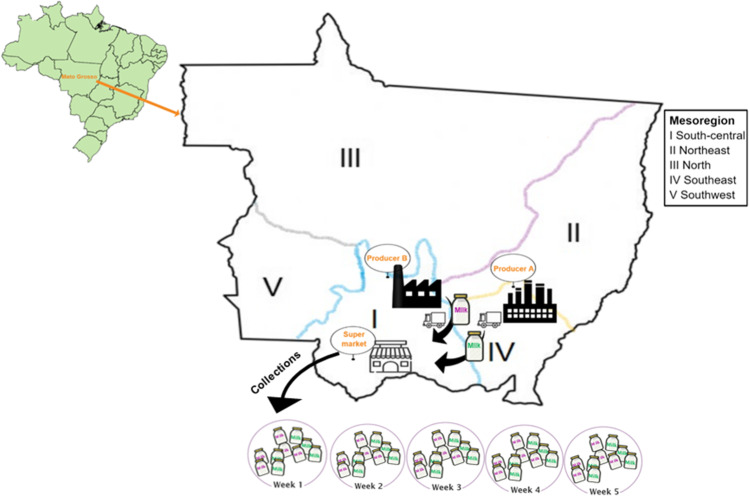
Fifty pasteurized milk samples were obtained from two producers, named A and B (Fig. 1). These producers distribute pasteurized milk in supermarkets and grocery stores throughout the mesoregion of the state of Mato Grosso, Brazil. Sampling was performed over 5 weeks, and five individual samples from each producer were collected weekly. It is important to note that Producer A is monitored by the federal inspection system (SIF), while producer B is regulated by a local Mato Grosso state inspection system (SISE).
Fig. 1.
Collection of pasteurized milk over 5 weeks in supermarkets supplied by two milk producers located in Mato Grosso, Brazil. The green map is Brazil, and the mesoregions of the state of Mato Grosso correspond to the territorial demarcations where the cities and districts are located
Physical–chemical analysis
Alkaline phosphatase and peroxidase tests were performed to verify whether the milk samples were pasteurized correctly by testing with reactive enzyme strips (Cap-Lab, Brazil). Both tests are regulated by Normative Instruction Nr. 76 (Brazilian legislation) [4].
Microbiological analyses
Quantification of Enterobacteriaceae members, total coliforms (35 °C) (TC) and thermotolerant coliforms (45 °C) (TTC), was performed according to the American Public Health Association (APHA) with modifications [34]. Positive E. coli broth (EC broth) (Kasvi, Brazil) was evaluated for the presence of typical E. coli colonies. The mean and standard deviation of the mentioned groups of microorganisms in relation to each collection week were obtained from five experimental replicates. Furthermore, 0.1 ml of EC broth was resuspended through the spread plate technique on plates containing agar Eosin Methylene Blue (EMB) (Kasvi, Spain) with subsequent incubation at 35 °C for 18–24 h. Colonies presenting a metallic green edge color with or without a black center were considered positive (suggestive) for E. coli. All typical colonies were submitted to biochemical tests, such as indole, methyl red, Vogues-Proskauer, and citrate (IMViC). For subsequent analysis, a single typical colony was transferred to 10 ml of lysogenic broth (LB broth) (Kasvi, Brazil) at 35 °C with constant agitation for 18–24 h.
Concerning E. coli identification, each isolate was named the letter of the producer + sample number with two digits + letter and number representing the isolate. For example, B (producer) + 20 (sample) + C3 (isolate). Each week, five samples were collected from each producer: samples 1–5 = week 1, samples 6–10 = week 2, samples 11–15 = week 3, samples 16–20 = week 4, and samples 21–25 = week 5.
Evaluation of heat resistance
E. coli isolates from producers A and B were evaluated through heat treatment at 60 °C in a water bath (model N1040, Centauro, Brazil). The heat treatment was performed in microtubes (2 ml) containing 1.5 ml of bacterial culture in LB broth [35]. The bacterial culture was heated two times: “time to reach T0” or come-up time (time required to reach the target temperature) and 6 min (T6). Treatments T0 and T6 were performed with three independent replicates, each containing duplicate samples (3 × 2). After each thermal exposure, the temperature was rapidly decreased through an ice bath for approximately 30 min. Temperature control was applied using microtubes containing 1.5 ml of LB broth without bacterial growth and a thermometer with an external probe (Equitherm, Brazil). The probe was inserted in the center of the microtube and sealed with adhesive tape. Initial cell concentrations were estimated by optical density (OD) at 600 nm using a spectrophotometer (Model Q8980PT, Quimis, Brazil) and confirmed by plating in plate count agar (PC agar) (Kasvi, Brazil). A high heat resistance wild-type E. coli C31, identified in a previous study by our group, displaying the ability to survive at high temperatures [36], was used as a positive control.
Following the heat treatment, serial dilutions (from 10−1 to 10−9) were prepared using peptone saline solution, and 1 ml of the appropriate dilutions was added through the pour plate technique on plates containing PC agar with subsequent incubation at 35 °C for 18–24 h. After that, colonies were enumerated by plate counting between 30 and 300 colony-forming units per milliliter (CFU/ml). The logarithmic reduction was calculated considering the difference in CFU/ml counts between the unheated strains in relation to those heated to 60 °C for 0 min. Concerning the 6 min exposure, logarithmic reduction was calculated by the difference between CFU/ml counts at T0 and T6. Strains were classified as sensitive (reduction > 5 log), moderate resistance (reduction between 1 and 5 log), or high resistance (reduction < 1 log) under heat treatment [21]. Furthermore, D60°C values were calculated by dividing the incubation time (t) by the log reduction, i.e., D60°C = t/log(T0–T6) [28]. This value measures the time required at 60 °C to kill 90% or 1 log of a microbial population [20].
Antimicrobial susceptibility assays
Antimicrobial susceptibility assays for E. coli were conducted according to the Clinical and Laboratory Standards Institute (CLSI) through the disk-diffusion technique [37]. Thus, eight antibiotics were used: ampicillin (30 μg), cefepime (30 μg), ciprofloxacin (5 μg), enrofloxacin (5 μg), florfenicol (30 μg), gentamicin (10 μg), nitrofurantoin (100 μg), and sulfametoxazole-trimetoprim (25 μg). These antibiotics were chosen since they belong to some of the principal classes of antibiotics (betalactams, fluoroquinolones, phenicals, aminoglycosides, nitrofurantoin, and folate pathway antagonists) used in human and animal therapy [37]. In the test, a typical colony of EMB agar was inoculated onto Mueller–Hinton broth (MH broth) (Sigma‒Aldrich, India) and incubated at 37 °C between 2 and 4 h until achieving growth of approximately 0.5 on the MacFarland scale. Subsequently, 0.1 ml of MH broth growths were streaked for the spread plate technique on MH agar (Kasvi, Italy), and disks containing the respective antibiotic were included, followed by incubation of plates at 37 °C for 18 h. Inhibition zones were then measured in millimeters using a halo-size ruler, and the results were correlated with CLSI standards.
Biofilm potential formation
Biofilm formation assays were performed according to the method described by Bang et al. [38] with modifications. For this, 240 µl of bacterial culture (approximately 8 log CFU/ml) was added in duplicate into 24-well cell culture plates (Corning Incorporated, USA) followed by 2760 µl of sterile LB broth and incubated at 35 °C for 48 h. Sterile LB broth was used as a negative control. After 48 h, the broth was removed using a pipette, and the well plates were rinsed three times with 3 ml sterile phosphate-buffered saline (PBS, pH 7.4) to remove the residual attached cells. Next, the potential biofilms were fixed using 3 ml of 95% ethyl alcohol for 15 min (Química Moderna, Brazil). After that, the plates were dried overnight. Crystal violet dye (Vetec, Brazil) (0.1%; 3 ml) was used to stain the potential biofilm for 15 min. Subsequently, the crystal violet solution was removed by washing with distilled water until all free crystal violet present was completely removed. The plates were air-dried overnight, and the next day, the bound crystal violet dye was removed using 3 ml of 95% ethyl alcohol for 30 min. The OD of the solution containing the crystal violet dye was measured at 570 nm (A570nm) using a spectrophotometer (model Q8980PT, Quimis, Brazil). The results were calculated following parameters established in previous studies [39, 40]. Thus, the results are shown as the mean ± standard deviation (SD) of two independent replicates, with each plate containing duplicate samples (n = 4). For biofilm classification, the absorbance (A570nm) of three times the standard deviation of the negative control was used as the cutoff value (ODc), which was 0.11 in this study. Based on the ODc calculation, the classification was established as follows: nonbiofilm/absence (ODstrain < ODc), weak biofilm (ODc < ODstrain < 2xODc), moderate biofilm (2 × ODc < ODstrain < 4 × ODc), and strong (4 × ODc < ODstrain < 8 × ODc).
Curli expression
E. coli isolates were inoculated on LB agar modified and supplemented with 0.004 g/L of Congo Red dye (Merck, Germany), according to Hassan et al. [41] and Bahri et al. [42]. Isolates were incubated at 25 °C for 4 days and were then checked for different morphotypes, as follows: saw (white and smooth colonies; curli-negative), rdar (red, dry and rough colonies; curli-positive), and intermediate (red and dry or rough colonies; medium curli expression or potentially present) [42].
Pulsed-field gel electrophoresis (PFGE)
PFGE was performed according to the CDC PulseNet protocol [43]. Briefly, the bacterial growth from Columbia agar plates (Merck, USA) (37 °C/18 h) was encased in agarose plugs and lysed with proteinase K (Promega, USA) in a shaking water bath at 55 °C/2 h. The plugs were digested using 10 U of the Xbal I restriction enzyme (Thermo Scientific, USA). DNA fragments were separated using CHEF-DR III equipment (Bio-Rad, USA) with 1% SeaKem® Gold Agarose (Lonza, Switzerland) and 0.5 × TBE (Tris-boric-acid/EDTA) buffer (Bio-Rad, USA). The PFGE conditions were as follows: 6 V/cm for 18 h with pulse times ranging from 6.76 to 35.38 s. Salmonella enterica serovar Branderup H9812 was used as a DNA size marker. Gel images were exported and analyzed using Photo Documenter software (Vilber Lourmat, EU). The dendrogram was constructed using GelJ version 2.0 software by applying the unweighted pair-group with averages (UPGMA) method and the Dice coefficient, with a tolerance index of 2% [44]. Isolates displaying a similarity index above 90% were considered related [45].
DNA extraction and PCR for tLST and rpoS genes
DNA extraction was performed according to Ma and Chui [46], with modifications through thermal lysis. Thus, 0.1 ml of bacterial suspension in LB broth was transferred to PC agar by the spread plate technique and incubated at 35 °C/18–24 h. Then, a single colony grown on the PC agar into 200 μL of ultrapure water was boiled at 100 °C for 10 min and centrifuged at 13,000 × g for 15 min. The obtained supernatants were then collected, and DNA was quantified through a fluorescence technique (QUBIT 2.0 system, Invitrogen, USA).
PCR simplex was conducted for the amplification of orf3-, orf8-, and orf11-targeted primer sequences of tLST described by Ma and Chui [46] and rpoS gene described by Uhlich et al. [47] (Table 1). The tLST primers designed by Ma and Chui [46] provide good coverage across the whole tLST. The cycling conditions for genes of tLST were set as follows: initial denaturation at 95 °C for 5 min, 40 denaturation cycles at 95 °C for 30 s, annealing at 60 °C for 30 s, an extension at 72 °C for 30 s, and a final extension at 72 °C for 10 min. The tLST-positive E. coli AW1.7, provided by Alberta Agriculture and Forestry, was used as a positive control. For the rpoS gene, the cycling conditions comprised initial denaturation at 95 °C for 5 min, 30 denaturation cycles at 95 °C for 60 s, annealing at 59 °C for 30 s, an extension at 72 °C for 60 s and a final extension at 72 °C for 5 min. E. coli ATCC 11229 was used as a positive control, and ultrapure water was used as a negative control.
Table 1.
Primers used in this study
| Target/gene | Sequence | Reference |
|---|---|---|
| orf3 |
F: 5′—CCATTCTTATGTCGGTCCAGAG—3′ R: 5′—CCACCTTGCTGACCTGTT—3′ |
[46] |
| orf8 |
F: 5′—TCGGTAAAGAAAGCGGTCAAG – 3′ R: 5′ – CATCGGAAGGTTGTCGGTTT – 3′ |
|
| orf11 |
F: 5′ – GAAGCGATTGTCCGAGCTAAG – 3′ R: 5′ – TGCTTGCCACTTCGTTATCC – 3′ |
|
| rpoS |
F: 5′—TATCGCCTGGATTACTGGCAAC -3 R: 5′—TAGGACGCTGACGTGTCTTATC -3 |
[47] |
F forward, R reverse
Data analysis
A one-way ANOVA at 5% significance with means separated by Tukey’s post hoc test was used to determine the significant differences between the Enterobacteriaceae results from producer B. A nonparametric analysis (Kruskal‒Wallis test) was performed for TC and TTC to compare significant differences between the sampling weeks for both groups. Statistical differences were verified through pairwise comparisons using SPSS software (version 22).
Results
Physical–chemical tests
The enzymatic tests verified that all samples from producers A and B were efficiently pasteurized. The absence of alkaline phosphatase in all samples indicated adequate pasteurization time and temperature, and the presence of peroxidase indicated adequate temperature during the pasteurization process [4].
Enterobacteriaceae, coliform counts, and presence of E. coli
The results of Enterobacteriaceae and coliform counts indicate that producer A only partially complied with the established legislation [5, 16–18]. Samples from weeks 1 and 2 contained Enterobacteriaceae, TC and TTC below the APHA method’s limit of detection (< 10 CFU/ml for Enterobacteriaceae and < 3.6 MPN/ml for TC and TTC) [34] and below the minimum limit of legislation recommend (< 1 log CFU/ml for Enterobacteriaceae and < 1 log CFU/ml or 7.4 MPN/ml for TC and TTC) [5, 16–18] (Table 2). On the other hand, all milk samples from producer B contained Enterobacteriaceae, TC and TTC above the minimum limit of legislation recommended, with week 3 displaying the greatest Enterobacteriaceae contamination, which was significantly different between weeks 1, 2, and 5 (p < 0.05). The Kruskal‒Wallis test indicated that weeks 2 and 3 displayed the highest counts in producer B samples regarding TC. Concerning TTC, there was no difference between the weeks (Table 2). None of the samples obtained during the 5 weeks of sampling from producer B complied with the limits set by both national and international legislations [5, 16–18] and were, therefore, inadequate for consumption. Thus, the results for both producers could not be statistically compared.
Table 2.
Enterobacteriaceae, TC, and TTC values in pasteurized milk samples obtained from producers A and B during five sampling weeks
| AProducer A | BProducer B | |||||||
|---|---|---|---|---|---|---|---|---|
| Week | Enterobacteriaceae (Log CFU/ml) | TC (MPN/ml) | TTC (MPN/ml) | *Total E. coli isolates |
CEnterobacteriaceae (Log CFU/ml) |
DTC (MPN/ml) |
ETTC (MPN/ml) |
*Total E. coli isolates |
| 1 | < 1 | < 3.6 | < 3.6 | 0 | 4.65 ± 0.94b | 1.10 × 103 b | 1.10 × 103 a | 5 |
| 2 | < 1 | < 3.6 | < 3.6 | 0 | 5.48 ± 0.50b | 1.10 × 104 a | 7.71 × 103 a | 4 |
| 3 | < 1 | 23 | < 3.6 | 0 | 6.54 ± 0.16a | 1.10 × 104 a | 5.32 × 103 a | 5 |
| 4 | 3.1 ± 0.63 | 5.56 × 102 | 1.44 | 3 | 5.67 ± 0.25ab | 1.10 × 103 b | 1.53 × 103 a | 5 |
| 5 | < 1 | 2.04 × 10 | 9.36 | 4 | 5.09 ± 0.48b | 1.10 × 103 b | 1.14 × 103 a | 5 |
ASamples from producer A comprise only the averages obtained each week, without undergoing any statistical analyses. BSamples from producer B were statistically evaluated concerning potential differences between sampling weeks; a,bMeans ± standard deviations in the same row differ significantly (p < 0.05) and were obtained from five experimental replicates. CTukey’s test; DKruskal‒Wallis test, chi-square = 24.000 and p = 0.000; EKruskal‒Wallis test, chi-square = 7.334 and p = 0.119. TC total coliforms, TTC thermotolerant coliforms, MPN/ml most likely number per milliliter, CFU/ml colony forming unit per milliliter. *Total E. coli isolates refer to the number of E. coli per sample collected, and these isolates are identified in Table 3. Minimum limits established for pasteurized milk: < 1 log CFU/ml for Enterobacteriaceae [17, 18], < 1 log CFU/ml or 7.4 MPN/ml for TC and TTC [5, 16]
Furthermore, 31 strains of E. coli were isolated from the positive EC broth followed by inoculation in EMB agar and confirmed by IMViC tests. Of these, 7 strains were from producer A, isolated from the only weeks with verified TTC counts (Table 2). The other 24 strains were isolated from samples obtained from producer B. The weeks with the highest numbers of E. coli isolates were not associated with the highest TTC loads, indicating that low TTC counts did not decrease the risk for the presence of E. coli.
Heat resistance and antimicrobial susceptibility
Regarding producer A, 5/7 isolates presented high heat resistance after 6 min of exposure, and the remaining two (A20C3 and A20C6) showed moderate and sensitive profiles to resistance to heat treatment (Table 3). Concerning producer B, only one isolate (B04C1) exhibited high heat resistance, whereas all 23 were classified as displaying moderate heat resistance (Table 3).
Table 3.
Phenotypic and genotypic characteristics of heat resistance in E. coli isolated from pasteurized milk samples obtained over 5 weeks from two producers from Mato Grosso, Brazil
| Week | Isolate ID | *Heat resistance classification | D60 °C (min) | tLST genes |
|---|---|---|---|---|
| 4 | AA19C6 | High | 13.75 | + |
| AA20C3 | Sensitive | 1.17 | + | |
| AA20C6 | Moderate | 5.95 | + | |
| 5 | AA21C1 | High | 17.62 | + |
| AA22C1 | High | 16.34 | + | |
| AA24C1 | High | 14.43 | + | |
| AA25C2 | High | 17.11 | + | |
| 1 | BB01C3 | Moderate | 5.17 | + |
| BB02C1 | Moderate | 5.07 | + | |
| BB03C4 | Moderate | 4.13 | + | |
| BB04C1 | High | 8.28 | + | |
| BB05C2 | Moderate | 4.11 | + | |
| 2 | BB06C5 | Moderate | 6.91 | + |
| BB07C2 | Moderate | 5.73 | + | |
| BB08C3 | Moderate | 7.69 | + | |
| BB10C2 | Moderate | 5.75 | + | |
| 3 | BB11C1 | Moderate | 6.88 | + |
| BB12C1 | Moderate | 5.21 | + | |
| BB13C1 | Moderate | 5.37 | + | |
| BB14C1 | Moderate | 4.23 | + | |
| BB15C1 | Moderate | 6.40 | + | |
| 4 | BB16C2 | Moderate | 5.77 | + |
| BB17C1 | Moderate | 5.07 | + | |
| BB18C2 | Moderate | 6.61 | + | |
| BB19C1 | Moderate | 7.00 | + | |
| BB20C3 | Moderate | 2.15 | - | |
| 5 | BB21C1 | Moderate | 6.88 | + |
| BB22C1 | Moderate | 11.00 | + | |
| BB23C2 | Moderate | 3.95 | + | |
| BB24C2 | Moderate | 6.00 | + | |
| BB25C1 | Moderate | 4.10 | + |
AE. coli isolate from producer A; BE. coli isolate from producer B; presence or absence of transmissible locus of stress tolerance (tLST) genes. The heat resistance classification and D60°C values were obtained with three independent replicates, each containing duplicate samples (3 × 2)
The highest D60°C values in isolates displaying the high resistance phenotype were observed for E. coli from producer A, ranging from 13.75 to 17.62 min, while D60°C values ranged from 2.15 to 11 min in isolates obtained from producer B.
In relation to the antimicrobial test, the 31 E. coli isolates were susceptible to the eight evaluated antibiotics.
Biofilm formation, curli expression and PFGE
The results showed that 51.6% (16/31) of the isolates showed the potential for biofilm formation (Table 4). Of these, 5 isolates of producer A showed weak biofilm potential in 4 isolates and moderate biofilm potential in one (A24C1). For producer B, 9 isolates were classified as having weak biofilm potential, and 2 isolates (B04C1 and B06C5) showed moderate biofilm potential. However, curli expression, namely as rdar colonies, was identified in 77.4% (24/31) of the isolates (Table 4). Although curli is a major component of biofilms, its absence did not prevent the formation of biofilm in some isolates.
Table 4.
Biofilm formation potential, curli expression phenotype, and identification of rpoS gene in E. coli isolated from pasteurized milk obtained from two producers from Mato Grosso, Brazil
| Week | Isolate ID | Biofilm formation (A570nm) |
*Biofilm classification | **Curli expression | rpoS gene |
|---|---|---|---|---|---|
| 4 | AA19C6 | 0.09 ± 0.01 | Absent | Rdar | + |
| AA20C3 | 0.17 ± 0.02 | Weak | Rdar | + | |
| AA20C6 | 0.21 ± 0.05 | Weak | Rdar | + | |
| 5 | AA21C1 | 0.12 ± 0.01 | Weak | Rdar | + |
| AA22C1 | 0.14 ± 0.04 | Weak | Rdar | + | |
| AA24C1 | 0.29 ± 0.06 | Moderate | Rdar | - | |
| AA25C2 | 0.08 ± 0.001 | Absent | Rdar | + | |
| 1 | BB01C3 | 0.09 ± 0.01 | Absent | Rdar | + |
| BB02C1 | 0.13 ± 0.02 | Weak | Rdar | - | |
| BB03C4 | 0.09 ± 0.01 | Absent | Intermediate | - | |
| BB04C1 | 0.30 ± 0.02 | Moderate | Saw | - | |
| BB05C2 | 0.09 ± 0.004 | Absent | Rdar | - | |
| 2 | BB06C5 | 0.22 ± 0.09 | Moderate | Rdar | + |
| BB07C2 | 0.10 ± 0.01 | Absent | Rdar | + | |
| BB08C3 | 0.12 ± 0.02 | Weak | Rdar | + | |
| BB10C2 | 0.10 ± 0.01 | Absent | Rdar | + | |
| 3 | BB11C1 | 0.14 ± 0.04 | Weak | Intermediate | + |
| BB12C1 | 0.11 ± 0.01 | Absent | Rdar | - | |
| BB13C1 | 0.11 ± 0.02 | Absent | Rdar | + | |
| BB14C1 | 0.10 ± 0.01 | Absent | Rdar | - | |
| BB15C1 | 0.09 ± 0.01 | Absent | Saw | + | |
| 4 | BB16C2 | 0.11 ± 0.02 | Absent | Rdar | + |
| BB17C1 | 0.17 ± 0.03 | Weak | Rdar | + | |
| BB18C2 | 0.09 ± 0.01 | Absent | Intermediate | + | |
| BB19C1 | 0.09 ± 0.005 | Absent | Saw | + | |
| BB20C3 | 0.20 ± 0.06 | Weak | Rdar | + | |
| 5 | BB21C1 | 0.11 ± 0.01 | Absent | Rdar | - |
| BB22C1 | 0.13 ± 0.02 | Weak | Intermediate | + | |
| BB23C2 | 0.22 ± 0.03 | Weak | Rdar | - | |
| BB24C2 | 0.12 ± 0.01 | Weak | Rdar | - | |
| BB25C1 | 0.16 ± 0.002 | Weak | Rdar | - |
*The biofilm classification was obtained based on the cutoff optical density (ODc; 0.11) of two independent replicates, with each plate containing duplicate samples: nonbiofilm (ODstrain < ODc), weak biofilm (ODc < ODstrain < 2xODc), moderate biofilm (2 × ODc < ODstrain < 4 × ODc), and strong biofilm (4 × ODc < ODstrain < 8 × ODc). **Colony characteristics on Congo red agar after 4 days of incubation, comprising rdar (curli-positive colonies), intermediate (medium characteristics), or observed (negative colonies). Presence or absence of rpoS gene (involved in curli/cellulose control in stress conditions) [48]
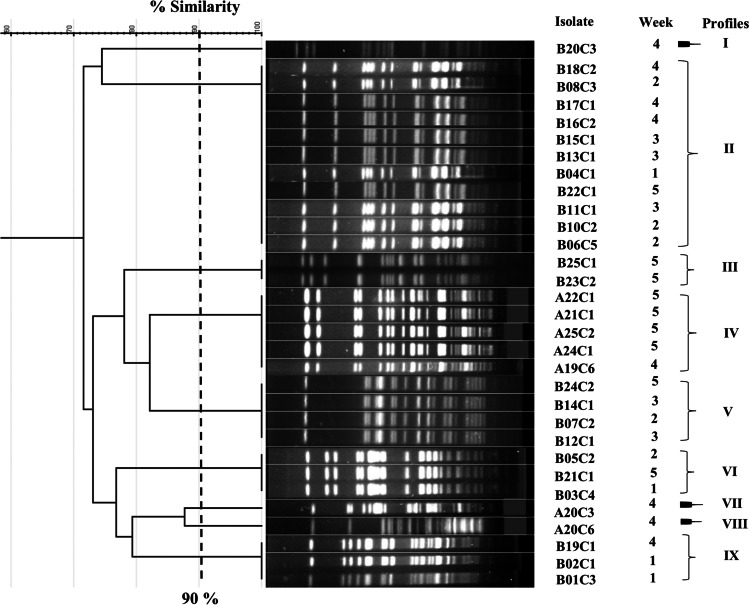
In relation to PFGE, the 31 isolates generated nine profiles with decipherable fragments (Fig. 2). Each profile was composed of clonal isolates with 100% similarity. Three profiles were associated with the isolates from producer A, with clonal profile IV and two orphan profiles obtained from samples from different weeks (different lots). Regarding producer B, five clonal profiles and one orphan profile were identified. Profile II contained the highest number of isolates, and these were obtained from samples of different lots.
Fig. 2.
Dendrogram based on nine PFGE profiles (90% similarity) of E. coli isolated from pasteurized milk samples obtained over 5 weeks from two producers from Mato Grosso, Brazil. Isolates with the initial A were obtained from producer A samples, and isolates with the initial B were obtained from producer B samples
tLST and rpoS genes by PCR
The tLST genes were present in 97% (30/31) of all isolates. The only exception was the B20C3 isolate (Table 3). For the rpoS gene, the findings indicated its presence in 64.5% (20/31) of E. coli isolates.
Discussion
Foodborne pathogens in milk and dairy products have been reported, and among the many causes, lack of hygiene and deficiencies in good manufacturing practices are identified as the main reasons for contamination [14, 49–51]. In this regard, Enterobacteriaceae and coliforms represent important quality indicators to measure a possible failure during postpasteurization contamination [52]. The failure in the postpasteurization process may indicate the presence of pathogenic E. coli serogroups [53]. In this respect, small-scale milk producers may have a tendency to produce lower quality milk than large-scale producers due to several factors, such as low productivity, low capital reserve, and little access to higher qualified professionals, among others [54, 55]. Therefore, in the present study, producer B presented more contaminated milk than producer A; this reason could be because producer B has only a SISE, while producer A has SIF. In this way, we speculate that the lower production demand combined with lower investment capacity can explain the higher contamination verified in producer B.
Furthermore, the implementation and constant monitoring of good hygiene practices (GHP), good manufacturing practices (GMP), and hazard analysis and critical control points (HACCP) are important factors during food processing [56]. However, the inefficient use of these tools may impact food quality and safety [51, 56]. We highlight that the samples from the two evaluated producers did not undergo incorrect pasteurization temperatures (evaluated by phosphatase negative and peroxidase positive in all samples). Even so, high Enterobacteriaceae and coliform counts were above the established standards [5, 16–18] and could be caused by postpasteurization contamination. Corroborating this hypothesis, E. coli isolates from different weeks were noted in a single PFGE profile (Fig. 2). In this regard, notable isolates B04C1 and B06C5 with moderate potential to form biofilms were identified in cluster II, and isolate A24C1, the only isolate from producer A with moderate potential to form biofilm, was identified in cluster IV. This may indicate recurrent contamination during the entire analysis period for producer B samples and during weeks 4 and 5 for producer A. However, isolate B04C1, was the only isolate to present a high heat resistance compared to the other isolates from producer B (Table 3). One hypothesis is that selective pressure may generate different phenotypic responses even in clonal isolates [57].
Milk pasteurization is the only mechanism employed to destroy microorganisms in dairy and dairy products in Brazil [4]. However, subpasteurization temperatures, commonly used in the manufacture of some cheeses, can promote an increase in thermotolerance of E. coli, as has been observed in some strains isolated from raw milk [10]. Although inherent aspects of the food matrix may influence the heat resistance of microorganisms [58], the acquisition of tLST genes by E. coli can provide resistance to times and temperatures beyond those of pasteurization, as was observed in E. coli isolated from a beef processing plant [23]. Our findings reveal E. coli isolates displaying moderate to high heat resistance and a D60°C value exceeding 17 min (isolate A25C2; Table 3). These isolates pose a risk in milk production that applies subpasteurization as a unique microbiological method to control microbial contamination. Furthermore, the adaptive response mediated by selective pressure in E. coli has been suggested for the emergence of tLST genes [24, 59]. In our results, 97% of the isolates presented tLST, which may have contributed to the survival of the isolates at 60 °C/6 min. In addition, even nonclonal isolates harbored tLST (A20C3 and A20C6; Fig. 2), which suggests possible gene dissemination in the milk producers analyzed in the present study. On the other hand, it is necessary to investigate whether these strains are also capable of withstanding HTST pasteurization temperatures (72–75 °C/15 at 20 s).
Another important point in our study is the number of isolates associated with potential to form biofilms (51.6%; Table 4). Biofilm formation in food processing plants can be a vector for pathogen transmission and, in some cases, can be formed by pathogenic microbial communities, such as E. coli O157:H7 [60–62]. Furthermore, microbial communities in biofilms produce extracellular polymeric substances (EPS) that protect them against antimicrobial treatments [41]. The EPS of E. coli is formed especially by polysaccharides such as cellulose, proteins such as curli, and other matrix components [63]. The presence of these components ensures biofilm formation in the early stages, allowing for greater environmental persistence and protecting the bacteria from sanitization [42]. In our results, 77.4% of the isolates expressed curli (Table 4). However, the absence of curli did not prevent the potential to form biofilm (isolate B04C1) under the investigated conditions.
Many other studies have reported that curli is not always crucial for biofilm formation in E. coli since medium conditions, temperature, time, and others need to be considered [32, 40, 61, 64, 65]. Moreover, several genes are related to the expression of biofilm proteins in E. coli [48, 66, 67], and under stress conditions (low nutrient, chlorine, high temperature, and others), the RNA polymerase sigma factor S (product of rpoS gene) can contribute to the expression of the EPS components [48, 66, 68]. Therefore, our initial hypothesis was that rpoS might be involved in the expression of curli as the strains were challenged with stress conditions (60 °C/6 min). Although most isolates showed curli expression characteristics, there was no correlation between curli and rpoS since many isolates that expressed curli did not show the gene (Table 4). In our PCR assays, rpoS was associated with 64.5% of E. coli isolates, which is curious because it is conserved gene in E. coli. In this regard, Stanford et al. [40] identified only 38.8% rpoS in the E. coli isolates evaluated. According to these authors, mutations in rpoS may interfere with PCR detection. A second hypothesis is that storage conditions of E. coli in the laboratory can easily inactivate rpoS, which rarely occurs in E. coli in its natural habitat [69]. However, further studies need to be performed to further investigate the non-detection of this gene in E. coli by PCR.
Overall, the results of this study indicated that even clonal isolates showed distinct phenotypes for heat resistance. Nevertheless, the phenotype for moderate potential to form biofilms and high heat resistance was noted in the same strain (A24C1, B04C1), contributing to the high risk of survival of these strains in the final product. In contrast, all the antimicrobials tested were efficient in inactivating E. coli isolates. This fact indicates that the use of antimicrobials may be occurring correctly. However, the appropriate use of antibiotics in animal production should be considered with larger numbers of samples that can represent the range of producers in the state.
In conclusion, both dairy producers presented unsatisfactory milk contamination levels during the weeks evaluated. This unsatisfactory contamination allowed us to isolate 31 E. coli isolates, and surprisingly, 97% (30/31) of the isolates presented tLST genes and high heat resistance (60 °C/6 min). In addition, through PFGE analyses, a high genetic similarity was identified between the isolates in different weeks, which suggest a recurrent source of contamination present in both dairy producers. Therefore, our study determined that 51.6% of the isolates had biofilm formation potential. Thus, although postpasteurization contamination may have occurred, the heat resistance phenotype was different even for clone isolates. In this sense, the present study is the first to determine the presence of E. coli strains with the potential for heat resistance in milk produced from Brazil. Therefore, further studies can be carried out to determine whether these isolates are able to survive the pasteurization process or were included in the milk in a postpasteurization contamination, or both, as evidenced in isolates A24C1 and B04C1.
Author contribution
Conceptualization: Maxsueli Aparecida Moura Machado, Eduardo Eustáquio de Souza Figueiredo, and Carlos Adam Conte-Junior; methodology: Maxsueli Aparecida Moura Machado, Vinicius Silva Castro, Adelino Cunha-Neto, Deyse Christina Vallim, Rodrigo de Castro Lisbôa Pereira, Jaqueline Oliveira dos Reis, and Patricia Veiga de Almeida; formal analysis and investigation: Maxsueli Aparecida Moura Machado, Vinicius Silva Castro, Diego Galvan, and Adelino da Cunha-Neto; writing—original draft preparation: Maxsueli Aparecida Moura Machado; writing—review and editing: Maxsueli Aparecida Moura Machado, Vinicius Silva Castro, Adelino Cunha-Neto, Deyse Christina Vallim, Rodrigo de Castro Lisbôa Pereira, Jaqueline Oliveira dos Reis, Patricia Veiga de Almeida, Diego Galvan, Eduardo Eustáquio de Souza Figueiredo, and Carlos Adam Conte-Junior; funding acquisition: Maxsueli Aparecida Moura Machado and Eduardo Eustáquio de Souza Figueiredo; resources: Patricia Veiga de Almeida, Deyse Christina Vallim and Carlos Adam Conte-Junior; supervision: Eduardo Eustáquio de Souza Figueiredo, and Carlos Adam Conte-Junior.
Funding
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq (Grant numbers 444465/2020–0, 313119/2020–1, 310181/2021–6, and 200472/2022–4). Authors Maxsueli A. M. Machado, Carlos A. Conte-Junior, and Eduardo E. S. Figueiredo have received research support from CNPq.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Food and Agriculture Organization of the United Nations (FAO) (2021) Milk Facts. http://www.fao.org/3/I9966EN/i9966en.pdf. Accessed 22 July 2021.
- 2.Instituto Brasileiro de Geografia e Estatística (IBGE) (2021) Indicadores IBGE - Estatística da Produção Pecuária out-dez 2020. IBGE, Brasil. Access: https://biblioteca.ibge.gov.br/visualizacao/periodicos/2380/epp_2020_4tri.pdf
- 3.Food and Agriculture Organization of the United Nations (FAO) (2004) Code of hygienic practice for milk and milk products - CAC/RCP 57-2004. Food Agric Organ, United States. Access: https://www.fao.org/fileadmin/user_upload/livestockgov/documents/CXP_057e.pdf
- 4.Brasil (2018) Instrução normativa N° 76, de 26 de novembro de 2018. Ministério da Agric Pecuária e Abast, Brasil. Access: https://wp.ufpel.edu.br/inspleite/files/2019/04/INSTRU%C3%87%C3%83O-NORMATIVA-N%C2%BA-76-DE-26-DE-NOVEMBRO-DE-2018-Di%C3%A1rio-Oficial-da-Uni%C3%A3o-Imprensa-Nacional.pdf
- 5.Food and Drug Administration (FDA) (2017) Grade “A” Pasteurized Milk Ordinance. U S Dep Heal and Hum Serv, FDA. Access: https://www.fda.gov/media/140394/download
- 6.Boor KJ, Wiedmann M, Murphy S, Alcaine S. A 100-Year Review: microbiology and safety of milk handling. J Dairy Sci. 2017;100:9933–9951. doi: 10.3168/jds.2017-12969. [DOI] [PubMed] [Google Scholar]
- 7.Wang G, Zhao T, Doyle MP. Survival and growth of Escherichia coli 0157:H7 in unpasteurized and pasteurized milk. J Food Prot. 1997;60:610–613. doi: 10.4315/0362-028X-60.6.610. [DOI] [PubMed] [Google Scholar]
- 8.Schrijver DK, Buvens G, Possié B et al (2008) Outbreak of verocytotoxin-producing E. coli O145 and O26 infections associated with the consumption of ice cream produced at a farm, Belgium, 2007. Eurosurveillance 10.2807/ese.13.07.08041-en [DOI] [PubMed]
- 9.Germinario C, Caprioli A, Giordano M, et al. Community-wide outbreak of haemolytic uraemic syndrome associated with Shiga toxin 2-producing Escherichia coli O26: H11 in southern Italy, summer 2013. Eurosurveillance. 2016;21:30343. doi: 10.2807/1560-7917.ES.2016.21.38.30343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng S, Hummerjohann J, Stephan R, Hammer P. Short communication: heat resistance of Escherichia coli strains in raw milk at different subpasteurization conditions. J Dairy Sci. 2013;96:3543–3546. doi: 10.3168/jds.2012-6174. [DOI] [PubMed] [Google Scholar]
- 11.Marti R, Muniesa M, Schmid M, Ahrens CH, Naskova J, Hummerjohann J. Short communication: heat-resistant Escherichia coli as potential persistent reservoir of extended-spectrum β-lactamases and Shiga toxin-encoding phages in dairy. J Dairy Sci. 2016;99:8622–8632. doi: 10.3168/jds.2016-11076. [DOI] [PubMed] [Google Scholar]
- 12.Chart H. Clinical significance of Verocytotoxin-producing Escherichia coli O157. World J Microbiol Biotechnol. 2000;16:719–724. doi: 10.1023/A:1008928822352. [DOI] [Google Scholar]
- 13.Denamur E, Clermont O, Bonacorsi S, Gordon D. The population genetics of pathogenic Escherichia coli. Nat Rev Microbiol. 2021;19:37–54. doi: 10.1038/s41579-020-0416-x. [DOI] [PubMed] [Google Scholar]
- 14.Ntuli V, Njage PMK, Buys EM. Characterization of Escherichia coli and other Enterobacteriaceae in producer-distributor bulk milk. J Dairy Sci. 2016;99:9534–9549. doi: 10.3168/jds.2016-11403. [DOI] [PubMed] [Google Scholar]
- 15.Machado MAM, Müller B, Carvalho RCT, de Figueiredo EES. Hygienic sanitary conditions of vacuum packed beef produced by slaughterhouses qualified for export in the Mato Grosso state, Brazil. Cienc Rural. 2018;48:7–10. doi: 10.1590/0103-8478cr20170526. [DOI] [Google Scholar]
- 16.Brasil (2001) Resolução de diretoria colegiada - RDC No 12, de 02 de janeiro de 2001. Ministério da Saúde - MS Agência Nacional de Vigilância Sanitária – ANVISA, Brasil. Access: https://www.gov.br/agricultura/pt-br/assuntos/inspecao/produtos-vegetal/legislacao-1/biblioteca-de-normas-vinhos-e-bebidas/resolucao-rdc-no-12-de-2-de-janeiro-de-2001.pdf/view
- 17.Brasil (2019) Instrução normativa N° 60, de 23 de dezembro de 2019 - Imprensa Nacional. Agência Nac Vigilância Sanitária - ANVISA, Brasil. Access: https://www.in.gov.br/en/web/dou/-/instrucao-normativa-n-60-de-23-de-dezembro-de-2019-235332356
- 18.Union European (EU) (2005) Commission regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off J Eur Union. Access: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02005R2073-20140601&from=EN
- 19.Stringer SC, George SM, Peck MW. Thermal inactivation of Escherichia coli O157:H7. J Appl Microbiol Symp Suppl. 2000;88:79s–89s. doi: 10.1111/j.1365-2672.2000.tb05335.x. [DOI] [PubMed] [Google Scholar]
- 20.Dlusskaya EA, McMullen LM, Gänzle MG. Characterization of an extremely heat-resistant Escherichia coli obtained from a beef processing facility. J Appl Microbiol. 2011;110:840–849. doi: 10.1111/j.1365-2672.2011.04943.x. [DOI] [PubMed] [Google Scholar]
- 21.Mercer RG, Zheng J, Garcia-Hernandez R, Ruan L, Gänzle MG, McMullen LM. Genetic determinants of heat resistance in Escherichia coli. Front Microbiol. 2015;6:1–13. doi: 10.3389/fmicb.2015.00932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boll EJ, Marti R, Hasman H, et al. Turn up the heat-food and clinical Escherichia coli isolates feature two transferrable loci of heat resistance. Front Microbiol. 2017 doi: 10.3389/fmicb.2017.00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guragain M, Smith GE, King DA, Bosilevac JM. Prevalence of extreme heat resistant gram negative bacteria carried by US cattle at harvest. J Food Prot. 2020;83:1438–1443. doi: 10.4315/JFP-20-103. [DOI] [PubMed] [Google Scholar]
- 24.Kamal SM, Simpson DJ, Wang Z, Gänzle M, Römling U. Horizontal transmission of stress resistance genes shape the ecology of beta- and gamma-proteobacteria. Front Microbiol. 2021;12:696522. doi: 10.3389/fmicb.2021.696522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mercer R, Nguyen O, Ou Q, McMullen L, Gänzle MG. Functional analysis of genes comprising the locus of heat resistance in Escherichia coli. Appl Environ Microbiol. 2017;83:1–13. doi: 10.1128/AEM.01400-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guragain M, Brichta-Harhay DM, Bono JL, Bosilevac JM. Locus of heat resistance (LHR) in meat-borne Escherichia coli: screening and genetic characterization. Appl Environ Microbiol. 2021;87:1–13. doi: 10.1128/AEM.02343-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X, Tran F, Klassen MD (2020) Heat resistance in Escherichia coli and its implications on ground beef cooking recommendations in Canada. J Food Saf 1–9 10.1111/jfs.12769
- 28.Zhang P, Tran F, Stanford K, Yang X. Are antimicrobial interventions associated with heat resistant Escherichia coli on meat? Appl Environ Microbiol. 2020 doi: 10.1128/aem.00512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Machado MAM, Castro VS, Carvalho RCT, Figueiredo EES, Conte-Junior CA. Whole-genome sequencing analyses of heat-resistant Escherichia coli isolated from Brazilian beef. Microbiol Resour Announc. 2022;11:e0037122. doi: 10.1128/mra.00371-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marti R, Schmid M, Kulli S, et al. Biofilm formation potential of heat-resistant Escherichia coli dairy isolates and the complete genome of multidrug-resistant, heat-resistant strain FAM21845. Appl Environ Microbiol. 2017 doi: 10.1128/AEM.00628-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang P, Yang X (2022) Genetic characteristics of the transmissible locus of stress tolerance (tLST) and tLST harboring Escherichia coli as revealed by large-scale genomic analysis. Appl Environ Microbiol 88. 10.1128/aem.02185-21 [DOI] [PMC free article] [PubMed]
- 32.Xu ZS, Yang X, Gänzle MG. Resistance of biofilm- and pellicle-embedded strains of Escherichia coli encoding the transmissible locus of stress tolerance (tLST) to oxidative sanitation chemicals. Int J Food Microbiol. 2021;359:109425. doi: 10.1016/j.ijfoodmicro.2021.109425. [DOI] [PubMed] [Google Scholar]
- 33.Dourou D, Beauchamp CS, Yoon Y, et al. Attachment and biofilm formation by Escherichia coli O157:H7 at different temperatures, on various food-contact surfaces encountered in beef processing. Int J Food Microbiol. 2011;149:262–268. doi: 10.1016/j.ijfoodmicro.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Kornacki JL, Johson JL (2015) Enterobacteriaceae, coliforms and Escherichia coli as quality and safety indicators, 101–117. In: Salfinger Y, Tortorello ML (eds) Compendium of Methods for the Microbiological Examination of Foods, 5th ed. American Public Health Association (APHA), Washington, D.C. 10.2105/MBEF.0222
- 35.Figueiredo EES, Yang X, Zhang P, Reuter T, Stanford K. Comparison of heating block and water bath methods to determine heat resistance in Shiga-toxin producing Escherichia coli with and without the locus of heat resistance. J Microbiol Methods. 2019;164:105679. doi: 10.1016/j.mimet.2019.105679. [DOI] [PubMed] [Google Scholar]
- 36.Castro VS, Rosario DKA, Mutz YS, Paletta ACC, Figueiredo EES, Conte-Junior CA. Modelling inactivation of wild-type and clinical Escherichia coli O26 strains using UV-C and thermal treatment and subsequent persistence in simulated gastric fluid. J Appl Microbiol. 2019;127:1564–1575. doi: 10.1111/jam.14397. [DOI] [PubMed] [Google Scholar]
- 37.Clinical and Laboratory Standards Institute (CLSI) (2021) M100 performance standards for antimicrobial susceptibility testing 8:27–39. Access: https://clsi.org/ [DOI] [PMC free article] [PubMed]
- 38.Bang HJ, Park SY, Kim SE, Md Furkanur Rahaman M, Ha S-D. Synergistic effects of combined ultrasound and peroxyacetic acid treatments against Cronobacter sakazakii biofilms on fresh cucumber. LWT. 2017;84:91–98. doi: 10.1016/j.lwt.2017.05.037. [DOI] [Google Scholar]
- 39.Fang Y, Visvalingam J, Zhang P, Yang X. Biofilm formation by Non-O157 Shiga toxin-producing Escherichia coli in monocultures and co-cultures with meat processing surface bacteria. Food Microbiol. 2021;102:103902. doi: 10.1016/j.fm.2021.103902. [DOI] [PubMed] [Google Scholar]
- 40.Stanford K, Tran F, Zhang P, Yang X (2021) Biofilm-forming capacity of Escherichia coli isolated from cattle and beef packing plants: relation to virulence attributes, stage of processing, antimicrobial interventions, and heat tolerance. Appl Environ Microbiol 87. 10.1128/AEM.01126-21 [DOI] [PMC free article] [PubMed]
- 41.Hassan A, Usman J, Kaleem F, Omair M, Khalid A, Iqbal M. Evaluation of different detection methods of biofilm formation in the clinical isolates. Brazilian J Infect Dis. 2011;15:305–311. doi: 10.1016/S1413-8670(11)70197-0. [DOI] [PubMed] [Google Scholar]
- 42.Bahri AA, Wan Abdullah WZ, Lani MN, Salleh W, Alias R. Genotypic and phenotypic characteristics associated with biofilm formation in Escherichia coli and Salmonella spp. Isolated from ulam in Terengganu. Food Res. 2020;4:91–101. doi: 10.1128/AEM.01660-17. [DOI] [Google Scholar]
- 43.Ribot EM, Fair MA, Gautom R, Cameron DN, Hunter SB, Swaminathan B, Barrett TJ. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog Dis. 2006;3:59–67. doi: 10.1089/fpd.2006.3.59. [DOI] [PubMed] [Google Scholar]
- 44.Heras J, Domínguez C, Mata E, Pascual V, Lozano C, Torres C, Zarazaga M. GelJ - a tool for analyzing DNA fingerprint gel images. BMC Bioinformatics. 2015;16:1–8. doi: 10.1186/s12859-015-0703-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong HJ, Lee S, Kim W, An JU, Kim J, Kim D, Cho S. Prevalence, virulence potential, and pulsed-field gel electrophoresis profiling of Shiga toxin-producing Escherichia coli strains from cattle. Gut Pathog. 2017;9:1–16. doi: 10.1186/s13099-017-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma A, Chui L. Identification of heat resistant Escherichia coli by qPCR for the locus of heat resistance. J Microbiol Methods. 2017;133:87–89. doi: 10.1016/j.mimet.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 47.Uhlich GA, Chen C, Cottrell BJ, Hofmann CS, Dudley EG, Strobaugh TP (2013) Phage insertion in mlrA and variations in rpoS limit curli expression and biofilm formation in Escherichia coli serotype O157 : H7. Microbiology 1586–1596. 10.1099/mic.0.066118-0 [DOI] [PubMed]
- 48.Mika F, Hengge R. Small RNAs in the control of RpoS, CsgD, and biofilm architecture of Escherichia coli. RNA Biol. 2014;11(5):494–507. doi: 10.4161/rna.28867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ribeiro Júnior JC, Silva FF, Lima JBA, et al. Short communication: molecular characterization and antimicrobial resistance of pathogenic Escherichia coli isolated from raw milk and Minas Frescal cheeses in Brazil. J Dairy Sci. 2019;102:10850–10854. doi: 10.3168/jds.2019-16732. [DOI] [PubMed] [Google Scholar]
- 50.Berhe G, Wasihun AG, Kassaye E, Gebreselasie K. Milk-borne bacterial health hazards in milk produced for commercial purpose in Tigray, northern Ethiopia. BMC Public Health. 2020;20:894. doi: 10.1186/s12889-020-09016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosario AILS, Castro VS, Santos LF, et al. Shiga toxin–producing Escherichia coli isolated from pasteurized dairy products from Bahia, Brazil. J Dairy Sci. 2021;104:6535–6547. doi: 10.3168/jds.2020-19511. [DOI] [PubMed] [Google Scholar]
- 52.Tortorello ML. Indicator organisms for safety and quality-uses and methods for detection: minireview. J AOAC Int. 2003;86:1208–1217. doi: 10.1093/jaoac/86.6.1208. [DOI] [PubMed] [Google Scholar]
- 53.Fusco V, Chieffi D, Fanelli F, Logrieco AF, Cho GS, Kabisch J, Böhnlein C, Franz CMAP. Microbial quality and safety of milk and milk products in the 21st century. Compr Rev Food Sci Food Saf. 2020;19:2013–2049. doi: 10.1111/1541-4337.12568. [DOI] [PubMed] [Google Scholar]
- 54.Food and Agriculture Organization (FAO) (2010) Status and prospects for smallholder milk production a global perspective, 160. In: by T. Hemme and J Otte Rome. Access: https://www.fao.org/3/i1522e/i1522e00.pdf
- 55.Ledo J, Hettinga KA, Luning PA. A customized assessment tool to differentiate safety and hygiene control practices in emerging dairy chains. Food Control. 2020;111:107072. doi: 10.1016/j.foodcont.2019.107072. [DOI] [Google Scholar]
- 56.Costa M, Brusa V, Padola NL et al (2021) Analysis of scenarios to reduce the probability of acquiring hemolytic uremic syndrome associated with beef consumption. Food Sci Technol Int 1–9. 10.1177/10820132211046124 [DOI] [PubMed]
- 57.Teixeira LAC, Carvalho FT, Vallim DC, et al. Listeria monocytogenes in Export-approved Beef from Mato Grosso, Brazil: prevalence, molecular characterization and resistance to antibiotics and disinfectants. Microorg. 2019;8:18. doi: 10.3390/microorganisms8010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lindsay D, Robertson R, Fraser R, Engstrom S, Jordan K. Heat induced inactivation of microorganisms in milk and dairy products. Int Dairy J. 2021;121:105096. doi: 10.1016/j.idairyj.2021.105096. [DOI] [Google Scholar]
- 59.Wang Z, Hu H, Zhu T, Zheng J, Gänzle MG, Simpson DJ. Ecology and function of the transmissible locus of stress tolerance in Escherichia coli and plant-associated Enterobacteriaceae. mSystems. 2021;6:378–399. doi: 10.1128/mSystems.00378-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chmielewski RAN, Frank JF. Biofilm Formation and control in food processing facilities. Compr Rev Food Sci Food Saf. 2003;2:22–32. doi: 10.1111/j.1541-4337.2003.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 61.Wang R, Kalchayanand N, King DA, Luedtke BE, Bosilevac JM, Arthur TM. Biofilm formation and sanitizer resistance of Escherichia coli O157:H7 strains isolated from “‘High Event Period’” meat contamination 3. J Food Prot. 2014;77:1982–1987. doi: 10.4315/0362-028X.JFP-14-253. [DOI] [PubMed] [Google Scholar]
- 62.Dass SC, Bosilevac JM, Weinroth M, Elowsky CG, Zhou Y, Anandappa A. Wang R (2020) Impact of mixed biofilm formation with environmental microorganisms on E. coli O157:H7 survival against sanitization. npj Sci Food. 2020;41(4):1–9. doi: 10.1038/s41538-020-00076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Galié S, García-Gutiérrez C, Miguélez EM, Villar CJ, Lombó F (2018) Biofilms in the food industry: health aspects and control methods. Front Microbiol 0:898. 10.3389/fmicb.2018.00898 [DOI] [PMC free article] [PubMed]
- 64.Schiebel J, Böhm A, Nitschke J, et al. Genotypic and phenotypic characteristics associated with biofilm formation by human clinical Escherichia coli isolates of different pathotypes. Appl Environ Microbiol. 2017;83:24. doi: 10.1128/AEM.01660-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma A, Neumann N, Chui L. Phenotypic and genetic determination of biofilm formation in heat resistant Escherichia coli possessing the locus of heat resistance. Microorganisms. 2021;9:1–13. doi: 10.3390/microor-ganisms9020403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharma G, Sharma S, Sharma P, Chandola D, Dang S, Gupta S, Gabrani R. Escherichia coli biofilm: development and therapeutic strategies. J Appl Microbiol. 2016;121:309–319. doi: 10.1111/jam.13078. [DOI] [PubMed] [Google Scholar]
- 67.Castro VS, Polo RO, Figueiredo EES, et al. Inconsistent PCR detection of Shiga toxin-producing Escherichia coli: insights from whole genome sequence analyses. PLoS ONE. 2021;16(9):e0257168. doi: 10.1371/journal.pone.0257168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Corona-Izquierdo FP, Membrillo-Hernández J (2002) A mutation in rpoS enhances biofilm formation in Escherichia coli during exponential phase of growth. FEMS Microbiol Lett 211:105–110. 10.1111/j.1574-6968.2002.tb11210.x [DOI] [PubMed]
- 69.Bleibtreu A, Clermont O, Darlu P, Glodt J, Branger C, Picard B, Denamur E. The rpoS gene is predominantly inactivated during laboratory storage and undergoes source-sink evolution in Escherichia coli species. J Bacteriol. 2014;196:4276–4284. doi: 10.1128/JB.01972-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.