Abstract
Glanders is a contagious disease of equids caused by the Gram-negative bacterium Burkholderia mallei. In Brazil, the disease is considered to be reemerging and has been expanding, with records of equids with positive serology in most of the federative units. However, there are few reports describing the genotypic detection of the agent. This study demonstrated the detection of B. mallei by species-specific PCR directly from tissues or from bacterial cultures, followed by amplicon sequencing in equids (equines, mules, and asinines) with positive serology for glanders in all five geographic regions of Brazil. The molecular evidence of B. mallei infection in serologically positive equids in this study expands the possibility of strain isolation and the conduction of epidemiological characterizations based on molecular information. The microbiological detection of B. mallei in cultures from nasal and palate swabs, even in equids without clinical manifestations, raises the possibility of environmental elimination of the agent.
Keywords: Glanders, PCR, DNA sequencing, Necropsy, Zoonosis
Introduction
Glanders is caused by a non-fermenting non-motile Gram-negative bacterium Burkholderia mallei, which mainly affects horses, but also infects mules and donkeys [1]. Burkholderia mallei is considered a zoonotic agent [2] and a potential agent of bioterrorism [3]. Nevertheless, its transmission to humans seems to be uncommon, even in cases of frequent and close contact with infected animals [4].
In Brazil, B. mallei was first described in 1811 [5], and the country was officially considered glanders free in 1960. The disease re-emerged in the country in the 2000s, with the occurrence of cases in the states of Alagoas and Pernambuco [6]. Glanders represent an important socioeconomic problem since the disease control and eradication program provides for mandatory euthanasia of seropositive equids [7, 8] without compensation for owners [9]. Every year, glanders occur in several areas of the country [10], causing serious economic losses and commercial restrictions. The highest frequency of affected farms occurs in the northeast region [11]. From 1999 to 2021, 3164 cases of glanders were reported in Brazil, according to the Animal Health Information System of the Ministry of Agriculture, Livestock and Food Supply (MAPA) (https://indicadores.agricultura.gov.br/saudeanimal).
Despite the evidence of a wide distribution of glanders in Brazil, defined by serology, reports of B. mallei isolation or molecular detection are not frequent, especially outside the northeast region [6, 12–18].
The World Organization for Animal Health (WOAH) considers microbiological culture and PCR as gold standards for glanders confirmation of clinical cases [19]. However, B. mallei has particular culture characteristics, including the requirement for a glycerol-dependent culture medium and slow growth (up to 72 h of incubation) [12]. The sensitivity of the PCR assays for clinical samples is unknown. A negative result, therefore, is no proof of the absence of B. mallei in the sample. Due to these difficulties related to microbiological culture and PCR, these techniques are not recommended to define population freedom from infection, individual animal freedom before movement, eradication policies, or prevalence of infection surveillance, for which serological methods are more suitable [19].
The differentiation of B. mallei strains on a molecular basis, the characterization of genetic diversity, and the definition of transmission events and, therefore, the tracing of infection sources is important knowledge for the definition of public policies [20–22]. Furthermore, genotyping is also important to follow the natural evolution of the genome of B. mallei [23, 24], as the remarkable genome plasticity in this species, mainly caused by insertion element-driven large-scale genetic re-arrangements [20], may impact detection by PCR [25]. Another important aspect of B. mallei genotyping is the possibility of differentiating from Burkholderia pseudomallei infection, which determines similar clinical manifestations [13].
Different genotyping methodologies can be used, such as multi-locus sequence typing, variable numbers of tandem repeat analysis, polymerase chain reaction–high-resolution melting, whole-genome sequencing, and core genome-based multi-locus sequence typing analysis, with varying degrees of resolution [14, 20–23]. However, all these tools are dependent on the isolation of B. mallei in microbiological culture.
This work describes data on the genotypic detection of B. mallei in equids serologically positive for glanders from all geographic regions of Brazil.
Material and methods
Samples
The glanders cases included in this study were defined by serological screening test by ELISA, and confirmatory test by Western blot (Biovetech), according to the Ministry of Agriculture, Livestock and Food Supply (MAPA) Normative Instruction [8], except for one horse from the state of Bahia, which was positive in the ELISA and negative in the Western blot, but was euthanized by the owner’s decision. The animals came from the states of Rio Grande do Sul (n = 3), Santa Catarina (n = 2), São Paulo (n = 1), Mato Grosso (n = 2), Bahia (n = 5), Piauí (n = 1), Maranhão (n = 1), Sergipe (n = 1), Tocantins (n = 1), and Amazonas (n = 1). According to the normative, all animals were euthanized, following recommendations from the National Council for the Control of Animal Experiments. Clinical examination and necropsy of the animals were performed by the Official Veterinary Service in the different units of the Federation. Fragments of organs, with or without lesions suggestive of glanders, as well as nasal swabs from five animals, tracheal swabs from three animals, and palate swabs from one animal, were collected during the necropsy of the animals and sent under refrigeration or frozen, to the Biosafety Level 3 (BSL-3) from Embrapa Beef Cattle, Campo Grande, MS, Brazil. The tissues of the animal from São Paulo were sent to the Instituto Biológico, São Paulo, for B. mallei detection.
Microbiological culture
For processing the samples, the tissues received were previously disinfected with 70% alcohol for five minutes, and then fragments were excised under sterile conditions, mainly delimiting lesions when present. The tissue fragments were placed in microtubes containing 500 µl of Brain Heart Infusion (BHI) broth and were macerated in a TissueLyser equipment (Qiagen, Germany) with a sterile metal bead.
The macerates were plated on blood agar (5% defibrinated sheep blood in the base for blood agar) and 2% glycerin. The same macerates were cultivated in BHI broth 2% glycerin with 100 U/ml penicillin and in BHI broth glycerin without antibiotics. All cultures were carried out at 37 °C. Bacterial growths were followed at 24 h, 48 h, and 72 h. When the presence of growth in the BHI broth was detected, the plating was performed on glycerin blood agar. For the plates that showed bacterial growth, the colonies were subcultured, and this new culture was followed in the same time intervals mentioned above. Those that showed the growth of colonies suggestive of B. mallei were used in a screening process to characterize the morphology and metabolism of these microorganisms in biochemical tests. The screening media were MacConkey agar and 2% glycerin blood agar. In addition to the morphological evaluation of bacterial growth in the culture media, preliminary biochemical tests were performed with triple sugar iron (TSI), oxidase, catalase, sulfide-indole-motility (SIM), oxidation, or fermentation of glucose (OF), in addition to Gram staining. The specific motility test was used to differentiate between B. mallei (non-motile) and B. pseudomallei (motile).
To eliminate contaminating bacteria co-cultured with B. mallei, semi-selective media containing antibiotics and antifungals were used. The colonies were subcultured onto the following media: 2% glycerin blood agar with penicillin and polymyxin B and BM agar containing crystal violet, cycloheximide, ticarcillin disodium, fosfomycin sodium, and polymyxin B [26 – adapted].
DNA extraction and PCR
DNA was extracted from bacterial isolates with a morphological and biochemical profile compatible with B. mallei, following an adapted protocol [27]. Escherichia coli strain TOP10 (Invitrogen) was used as a negative control of DNA extraction. DNA purification from tissues was performed using the DNEasy Blood & Tissue kit (Qiagen, Germany). Then, a polymerase chain reaction (PCR) was performed, targeting IS407-fliP, as recommended by the WOAH [20], using primers described by Abreu and collaborators [12], with an amplicon size of 528 base pairs.
Amplicon sequencing
PCR products were purified according to Werle et al. [28], using the enzymes exonuclease I and shrimp alkaline phosphatase. Sequencing reactions were performed, in duplicate, by the chain termination method using fluorochrome-labeled dideoxynucleotides [29]. Applied Biosystem’s BigDye®Terminator v3.1 kit was used, following the conditions specified by the manufacturer. Reactions were further purified before being sequenced using EDTA and ethanol. Sequence electrophoresis was performed in an ABI 3130XL equipment (Applied Biosystem, USA). The sequences generated by capillary electrophoresis were exported in ABI format and analyzed using the SeqScape® Software v2.1 (Applied Biosystems, USA) program, in which the electropherograms were aligned to a GenBank reference sequence (CP010348.1) and edited. The consensus sequences were submitted to the search for homology using the BLASTn program (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Results
The characterization of the animals included in the study is shown in Table 1. Biological materials (organ fragments and swabs from animals submitted to euthanasia) from 10 Brazilian Federative Units were analyzed in this study, including a sample of B. mallei from equine tissue from the state of São Paulo, which was previously isolated at the Instituto Biológico. Thus, as indicated in Table 1, the five geographic regions of the country were considered. Part of the information regarding the animals is also presented.
Table 1.
Description of the region of origin, sex, age, and clinical alterations of equids with positive serology for glanders in Brazil, which were euthanized, necropsied for detection of Burkholderia mallei
| Region | State | Species | Sex | Age (years) | Clinical manifestations |
|---|---|---|---|---|---|
| South | Rio Grande do Sul | Equine | Female | 8 | None |
| Rio Grande do Sul | Equine | Male | 7 | None | |
| Rio Grande do Sul | Equine | Male | 12 | None | |
| Santa Catarina | Equine | Male | 20 | Respiratory insufficiency and edematous hind legs | |
| Santa Catarina | Equine | Female | 5 | None | |
| Santa Catarina | Equine | Male | 11 | None | |
| Southeast | São Paulo | Equine | Female | 3 | None |
| Northeast | Bahia | Equine | Male | 12 | Nasal secretion |
| Bahia | Equine | Male | 7 | Nasal secretion | |
| Bahia | Equine | Female | 6 | None | |
| Bahia | Equine | Female | 7 | None | |
| Bahia | Equine | Female | 10 | Nasal secretion and lymph node enlargement | |
| Piauí | Equine | Female | 9 | None | |
| Sergipe | Mule | Female | 8 | Discreet cough | |
| Maranhão | Equine | Female | 5 | Declining body score | |
| North | Tocantins | Mule | Female | 16 | None |
| Amazonas | Equine | Female | 5 | None | |
| Midwest | Mato Grosso | Asinine | Female | 2 | None |
| Mato Grosso | Equine | Female | 9 | Declining body score |
After the identification of bacterial colonies with suggestive morphology of B. mallei, those with a compatible tinctorial, biochemical, and cultural profile for this species were selected: Gram-negative coccobacilli, non-motile, do not grow at 42 °C, non-fermenters of sugars, non-producers of H2S, indole negative, metabolize glucose through the oxidative pathway, oxidase variable and positive catalase and on screening culture media, it shows no or little pink colony growth on MacConkey [19].
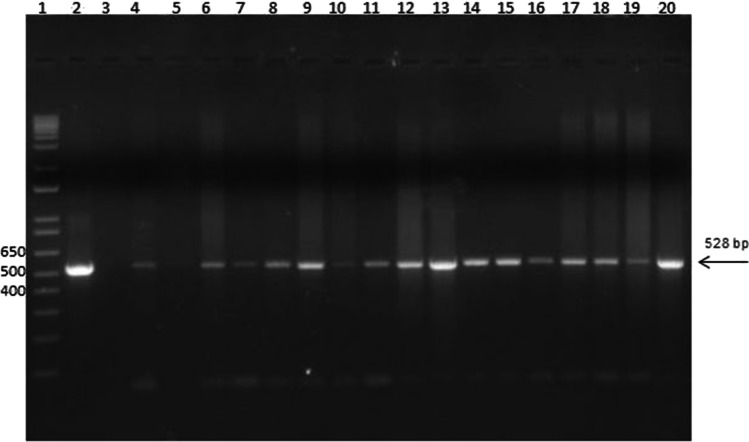
Table 2 shows the results of the PCR analysis. Of the 19 equids included in the study, 18 (94.7%) were positive by PCR directly on the tissue or from the microbiological culture. The only negative horse showed nodules in the lung, spleen, and liver. From the 18 PCR-positive animals, the detection of B. mallei directly in tissues by PCR was possible in 4 (22.28%) equids from the ethmoidal concha, trachea, lung, liver, kidney, renal, and mesenteric lymph nodes. From the PCR of microbiological cultures, it was possible to identify B. mallei in 18/18 (100%) equids, with more frequent sites in the lung and spleen. In three horses (Rio Grande do Sul, Piauí, and Bahia), without clinical manifestations, in which samples of nasal swabs were collected, there was genotypic detection of B. mallei from the microbiological culture, and in one horse, B. mallei was detected from the bacterial culture of a palate swab. Colony PCR detected B. mallei in one horse from the state of Bahia, which was positive in the ELISA, but negative in the Western blot. The results of the amplification of IS407-fliP are shown in Fig. 1. The size of the amplicons was consistent with that expected for B. mallei (528 bp).
Table 2.
Results of PCR directly on tissues and colony PCR for Burkholderia mallei in equids from different geographical regions of Brazil
| State | Species | Sample | Macroscopic lesions | Colony PCR | Tissue PCR |
|---|---|---|---|---|---|
| Rio Grande do Sul | Equine | Nasal swab | Not applicable | Positive | Not applicable |
| Lung | Nodules | Negative | Negative | ||
| Spleen | Nodules | Negative | Negative | ||
| Liver | Nodules | Negative | Negative | ||
| Mesenteric lymph node | No | Negative | Negative | ||
| Retropharyngeal lymph node | No | Negative | Negative | ||
| Deep cervical lymph node | Lymph node enlargement | Negative | Negative | ||
| Pulmonary accessory lobe | No | Negative | Negative | ||
| Mandibular lymph node | No | Negative | Negative | ||
| Mediastinal lymph node | Lymph node enlargement | Negative | Negative | ||
| Pulmonary lymph node | No | Negative | Negative | ||
| Tracheobronchial lymph node | No | Negative | Negative | ||
| Mandibular lymph node | No | Positive | Negative | ||
| Spleen | Nodules | Positive | Negative | ||
| Liver | Nodules | Positive | Negative | ||
| Lung | Nodules | Negative | Negative | ||
| Deep cervical lymph node | No | Negative | Negative | ||
| Mediastinal lymph node | No | Negative | Negative | ||
| Parotid lymph node | No | Negative | Negative | ||
| Retropharyngeal lymph node | No | Negative | Negative | ||
| Mesenteric lymph node | No | Negative | Negative | ||
| Pulmonary accessory lobe | No | Negative | Negative | ||
| Parotid | No | Negative | Negative | ||
| Nasal swab | Not applicable | Negative | Not applicable | ||
| Ethmoidal concha | Pyogranuloma or abscess | Positive | Positive | ||
| Santa Catarina | Equine | Liver | Pyogranuloma or abscess | Positive | Negative |
| Spleen | Pyogranuloma or abscess | Positive | Negative | ||
| Lung | Pyogranuloma or | Negative | Negative | ||
| abscess | |||||
| Pulmonary lymph node | No | Negative | Negative | ||
| Lung | Small pneumonic foci | Positive | Negative | ||
| Liver | Mineralized surface lesion | Negative | Negative | ||
| Kidney | No | Negative | Negative | ||
| Lung | Nodules | Negative | Negative | ||
| Pulmonary lymph node | No | Negative | Negative | ||
| Spleen | Nodules | Negative | Negative | ||
| Liver | Nodules | Negative | Negative | ||
| São Paulo | Equine | Trachea | Purulent tracheitis | Negative | Positive |
| Tracheal swab | Not applicable | Positive | Not applicable | ||
| Deep cervical lymph node | No | Negative | Negative | ||
| Mesenteric lymph node | No | Negative | Negative | ||
| Lung | Pyogranuloma or abscess | Negative | Positive | ||
| Spleen lymph node | No | Negative | Negative | ||
| Spleen | Pyogranuloma or abscess | Negative | Negative | ||
| Heart | No | Negative | Negative | ||
| Liver | Pyogranuloma or abscess | Negative | Positive | ||
| Renal lymph node | No | Negative | Positive | ||
| Kidney | No | Negative | Negative | ||
| Bahia | Equine | Spleen | Pyogranuloma or abscess | Positive | Negative |
| Lung | Pyogranuloma or abscess | Positive | Negative | ||
| Liver | Pyogranuloma or abscess | Negative | Negative | ||
| Liver | Pyogranuloma or abscess | Positive | Negative | ||
| Spleen | Pyogranuloma or abscess | Positive | Negative | ||
| Lung | Pyogranuloma or abscess | Negative | Negative | ||
| Tracheobronchial lymph node | Pyogranuloma or abscess | Positive | Negative | ||
| Lung | Pyogranuloma or abscess | Negative | Negative | ||
| Liver | Pyogranuloma or abscess | Negative | Negative | ||
| Liver | Pyogranuloma or abscess | Positive | Negative | ||
| Spleen | Pyogranuloma or abscess | Negative | Negative | ||
| Lung | Multiple hemorrhagic foci | Negative | Negative | ||
| Nasal swab | Not applicable | Positive | Not applicable | ||
| Palate swab | Not applicable | Positive | Not applicable | ||
| Mandibular lymph node | Pyogranuloma or abscess | Positive | Negative | ||
| Lung | Focal pneumonia | Positive | Negative | ||
| Pulmonary lymph node | Pyogranuloma or abscess | Negative | Negative | ||
| Spleen | Petechiae | Negative | Negative | ||
| Liver | Pyogranuloma or abscess | Negative | Negative | ||
| Piauí | Equine | Pool of pulmonary and mediastinal lymph nodes | No | Positive | Negative |
| Nasal swab | Not applicable | Positive | Not applicable | ||
| Tracheal swab | Not applicable | Negative | Not applicable | ||
| Sergipe | Mule | Liver | Pyogranuloma or abscess | Positive | Negative |
| Lung | Pyogranuloma or abscess | Negative | Negative | ||
| Liver fluid | Not applicable | Negative | Negative | ||
| Mesenteric lymph node | Lymph node enlargement | Negative | Positive | ||
| Lung fluid | Not applicable | Negative | Negative | ||
| Maranhão | Equine | Pulmonary lymph node | Nodules and pyogranuloma or abscess | Positive | Negative |
| Duodenal pancreatic lymph node | No | Negative | Negative | ||
| Lymph node | No | Negative | Negative | ||
| Liver | Nodules and pyogranuloma or abscess | Negative | Negative | ||
| Tracheal swab | Not applicable | Negative | Not applicable | ||
| Nasal swab | Not applicable | Negative | Not applicable | ||
| Tocantins | Mule | Liver | Pyogranuloma or abscess | Positive | Negative |
| Lung | No | Negative | Negative | ||
| Amazonas | Equine | Lung | Nodules | Positive | Negative |
| Prescapular lymph node | No | Negative | Negative | ||
| Liver | Nodules | Negative | Negative | ||
| Mato Grosso | Asinine | Parotid lymph node | Nodules | Positive | Negative |
| Palate | No | Positive | Negative | ||
| Kidney | Pyogranuloma or abscess | Positive | Positive | ||
| Lung | No | Positive | Negative | ||
| Mesenteric lymph node | No | Negative | Negative | ||
| Sublingual lymph node | No | Negative | Negative | ||
| Spleen | Pyogranuloma or abscess | Negative | Negative | ||
| Adrenal | No | Negative | Negative | ||
| Prescapular lymph node | Nodules | Negative | Negative | ||
| Mediastinal lymph node | Nodules | Negative | Negative | ||
| Subiliac lymph node | No | Negative | Negative | ||
| Brain | No | Negative | Negative | ||
| Liver | No | Negative | Negative | ||
| Nasal sinus | No | Negative | Negative | ||
| Equine | Lung | Pyogranuloma or abscess | Positive | Negative | |
| Bladder | No | Positive | Negative | ||
| Liver | Pyogranuloma or abscess | Negative | Negative | ||
| Mediastinal lymph node | No | Negative | Negative | ||
| Kidney | No | Negative | Negative | ||
| Heart | No | Negative | Negative | ||
| Superficial cervical lymph node | No | Negative | Negative | ||
| Spleen | No | Negative | Negative |
Fig. 1.
PCR amplification targeting IS407-fliP for Burkholderia mallei isolates from Brazil. All reactions shown in the figure are PCRs from microbiological cultures. Only one positive sample per animal was included. Arrow: 528 bp. Lane 1: 1 kb plus (Thermo Fisher, USA); lane 2: positive control: DNA from B. mallei (São Paulo) strain 86/19; lane 3: negative control; lane 4: Santa Catarina, equine, male, spleen; lane 5: Santa Catarina, equine, female, lung; lane 6: Bahia, equine, male, spleen; lane 7: Bahia, equine, male, lung; lane 8: Bahia, equine, female, tracheobronchial lymph node; lane 9: Bahia, equine, female, liver; lane 10: Bahia, equine, female, palate swab; lane 11: Rio Grande do Sul, equine, female, nasal swab; lane 12: Rio Grande do Sul, equine, male, mandibular lymph node; lane 13: Rio Grande do Sul, equine, male, ethmoidal concha abscess/piogranuloma; lane 14: Tocantins, mule, female, liver; lane 15: Mato Grosso, asinine, female, kidney; lane 16: Mato Grosso, equine, female; bladder; lane 17: Amazonas, equine, female, lung; lane 18: Piauí, equine, female, lymph node pool; lane 19: Sergipe, mule, female, liver; lane 20: Maranhão, equine, female, pulmonary lymph node
After analyzing the sequenced amplicons, the consensus sequences were obtained. After a homology search, comparing the sequences obtained with the database of the NCBI using the BLASTn program, the best hits of each analysis were obtained, as well as the analysis quality parameters, which are shown in Table 3.
Table 3.
Results of homology searches in BLASTn (NCBI) for sequencing of IS407-fliP PCR amplicons from Burkholderia mallei from tissue cultures and/or swabs of equids from different geographical regions of Brazil
| Isolate | Best hit | Id NCBI | Score | E-value | Identity | Gaps |
|---|---|---|---|---|---|---|
| Bahia, equine, male, spleen | Burkholderia mallei strain Turkey 10 chromosome 1, complete sequence | CP010348.1 | 952 | 0.0 | 515/515 (100%) | 0/515 (0%) |
| Bahia, equine, male, lung | Burkholderia mallei strain Turkey 10 chromosome 1, complete sequence | CP010348.1 | 952 | 0.0 | 518/519 (99%) | 1/519 (0%) |
| Bahia, equine, male, liver | Burkholderia mallei strain Turkey 10 chromosome 1, complete sequence | CP010348.1 | 970 | 0.0 | 524/524 (100%) | 0/524 (0%) |
| Bahia, equine, male, spleen | Burkholderia mallei strain Turkey 10 chromosome 1, complete sequence | CP010348.1 | 968 | 0.0 | 524/524 (100%) | 0/524 (0%) |
| Bahia, equine, female, tracheobronchial lymph node | Burkholderia mallei strain 2002734306 chromosome II, complete sequence | CP009708.1 | 518 | 4e-142 | 383/433 (88%) | 6/433 (1%) |
| Bahia, equine, female, liver | Burkholderia mallei strain Turkey 10 chromosome 1, complete sequence | CP010348.1 | 966 | 0.0 | 523/523 (100%) | 0/523 (0%) |
| Bahia, equine, female, nasal swab | Burkholderia mallei fliP pseudogene, partial sequence; and IS407A transposase (tnpB) gene, partial cds | MK440295.1 | 913 | 0.0 | 494/494 (100%) | 0/494 (0%) |
| Bahia, equine, female palate swab | Burkholderia mallei strain Turkey 10 chromosome 1, complete sequence | CP010348.1 | 963 | 0.0 | 521/521 (100%) | 0/521 (0%) |
| Segipe, mule, female, liver | Burkholderia mallei strain Turkey 10 chromosome 1, complete sequence | CP010348.1 | 970 | 0.0 | 525/525 (100%) | 0/525 (0%) |
| Piauí, equine, female, nasal swab | Burkholderia mallei strain Turkey 10 chromosome 1, complete sequence | CP010348.1 | 946 | 0.0 | 512/512 (100%) | 0/512 (0%) |
| Piauí, equine, female, pulmonary and mediastinal lymph nodes (pool) | Burkholderia mallei strain Turkey 10 clone 1-6.6 fliP mobile element, partial sequence | MK947141.1 | 785 | 0.0 | 425/425 (100%) | 0/425 (0%) |
| Maranhão, equine, female, pulmonary lymph node | Burkholderia mallei strain Turkey 10 chromosome 1, complete sequence | CP010348.1 | 966 | 0.0 | 523/523 (100%) | 0/523 (0%) |
| Tocantins, mule, female, liver | Burkholderia mallei strain Turkey 10 chromosome 1, complete sequence | CP010348.1 | 968 | 0.0 | 524/524 (100%) | 0/524 (0%) |
| Rio Grande do Sul, equine, female, nasal swab | Burkholderia mallei fliP pseudogene, partial sequence; and IS407A transposase (tnpB) gene, partial cds | MK440295.1 | 928 | 0.0 | 502/502 (100%) | 0/502 (0%) |
| Rio Grande do Sul, equine, male, mandibular lymph node | Burkholderia mallei strain Turkey 10 chromosome 1, complete sequence | CP010348.1 | 955 | 0.0 | 517/517 (100%) | 0/517 (0%) |
| Rio Grande do Sul, equine, male, spleen | Burkholderia mallei fliP pseudogene, partial sequence; and IS407A transposase (tnpB) gene, partial cds | MK440295.1 | 719 | 0.0 | 390/391 (99%) | 0/391 (0%) |
| Rio Grande do Sul, equine, male, liver | Burkholderia mallei fliP pseudogene, partial sequence; and IS407A transposase (tnpB) gene, partial cds | MK440295.1 | 928 | 0.0 | 502/502 (100%) | 0/502 (0%) |
| Rio Grande do Sul, equine, male, ethmoidal concha abscess | Burkholderia mallei strain Turkey 10 chromosome 1, complete sequence | CP010348.1 | 968 | 0.0 | 524/524 (100%) | 0/524 (0%) |
| Santa Catarina, equine, male, liver | Burkholderia mallei fliP pseudogene, partial sequence; and IS407A transposase (tnpB) gene, partial cds | MK440295.1 | 939 | 0.0 | 508/508/ (100%) | 0/508 (0%) |
| Santa Catarina, equine, male, spleen | Burkholderia mallei strain Turkey 10 clone 1-6.6 fliP mobile element, partial sequence | MK947141.1 | 678 | 0.0 | 376/380 (99%) | 1/380 (0%) |
| Santa Catarina, equine, female, lung | Burkholderia mallei fliP pseudogene, partial sequence; and IS407A transposase (tnpB) gene, partial cds | MK440295.1 | 920 | 0.0 | 498/498 (100%) | 0/498 (0%) |
| Mato Grosso, donkey, female, kidney | Burkholderia mallei strain Turkey 10 clone 1-6.6 fliP mobile element, partial sequence | MK947140.1 | 911 | 0.0 | 460/470 (98%) | 3/470 (0%) |
| Mato Grosso, donkey, female, palate | Burkholderia mallei fliP pseudogene, partial sequence; and IS407A transposase (tnpB) gene, partial cds | MK440295.1 | 922 | 0.0 | 499/499 (100%) | 0/499 (0%) |
| Mato Grosso, donkey, female, lung | Burkholderia mallei fliP pseudogene, partial sequence; and IS407A transposase (tnpB) gene, partial cds | MK440295.1 | 928 | 0.0 | 502/502 (100%) | 0/502 (0%) |
| Mato Grosso, asinine, female parotid lymph node | Burkholderia mallei fliP pseudogene, partial sequence; and IS407A transposase (tnpB) gene, partial cds | MK440295.1 | 929 | 0.0 | 503/503 (100%) | 0/503 (0%) |
| Mato Grosso, equine, female, bladder | Burkholderia mallei strain Turkey10 chromosome 1, complete sequence | C P010348.1 | 966 | 0.0 | 523/523 (100%) | 0/523 (0%) |
| Mato Grosso, equine, female, Left cranial, lobe lung | Burkholderia mallei strain Turkey10 chromosome 1, complete sequence | C P010348.1 | 957 | 0.0 | 518/518 (100%) | 0/518 (0%) |
| Amazonas, equine, female, lung | Burkholderia mallei strain Turkey10 chromosome 1, complete sequence | C P010348.1 | 970 | 0.0 | 525/525 (100%) | 0/525 (0%) |
| São Paulo, equine, female, tracheal secretion | Burkholderia mallei strain Turkey10 chromosome 1, complete sequence | C P010348.1 | 871 | 0.0 | 490/494 (99%) | 2/494(0%) |
Discussion
Glanders is an important infectious disease that causes serious damage to the equine production chain in countries where it occurs endemic. The control of this disease requires knowledge of epidemiological aspects, among them, the determination of its genetic diversity and its implications in the transmission process. This information fundamentally depends on the isolation of its etiological agent in the tissues of infected horses. This work demonstrated the presence of B. mallei in equids with positive serology for glanders, through PCR directly from tissues or from microbiological culture, in all five regions of Brazil.
Culturing of tissue macerates often results in the accelerated growth of contaminating microorganisms, even in the presence of B. mallei. Thus, preferentially, only isolated colonies, round, punctiform, grayish, with a translucent and shiny halo, and non-hemolytic, were picked [19].
A second selection stage was implemented based on tinctorial, cultural, and biochemical characteristics. In Brazil, previous studies have shown slight variations in the profile of the fermentation of some carbohydrates, such as galactose, glucose, sucrose, maltose, and mannitol, in B. mallei strains from the Northeast region of Brazil, but these variations did not interfere with bacterial identification [30].
The molecular detection of B. mallei in most of the animals included in the study would indicate good specificity of the serological tests used in the official program to control glanders in Brazil. The only animal negative in molecular detection had nodular lesions suggestive of glanders in the lung, spleen, and liver, suggesting a probable low bacterial load in the lesions, making direct detection by PCR and microbiological culture difficult.
In this study, there was greater success in detecting B. mallei from microbiological culture than in PCR directly in tissue. This fact is probably due to the low relative concentration of the pathogen’s DNA in relation to the host’s genetic material, in addition to possible PCR inhibitors present in the tissues.
In three horses (Rio Grande do Sul, Piauí, and Bahia), without clinical manifestations, in which samples of nasal swabs were collected, there was genotypic detection from the microbiological culture, suggesting the respiratory elimination of B. mallei. In one horse, B. mallei was detected from the bacterial culture of a palate swab, which may imply the environmental elimination of the bacteria.
Burkholderia mallei was detected by PCR in two horses from the same farm in the state of Bahia. One of the animals, of high zootechnical value, was positive in ELISA and Western blot. The other was a working animal and was only positive in the screening test (ELISA). By the decision of the animal owner, both were euthanized. The positive horse only in the ELISA presented lesions in the liver, spleen, and lung, with the detection of B. mallei by PCR in lung and spleen cultures. Thus, this horse was considered serologically false-negative, and the pertinent epidemiological implications must be considered [31].
The B. mallei genome is smaller (5.8 Mb) than that of B. pseudomallei (7.2 Mb) or B. thailandensis (6.7 Mb). Whereas these latter species are environmental soil inhabitants, previous studies have shown that B. mallei, an obligate mammalian parasite, does not survive well in the environment [32]. The prediction of the pathways specific to the metabolic capabilities of the B. pseudomallei relative to B. mallei suggests that metabolic abilities essential for environmental survival may have been lost in the genome reduction process in B. mallei [32]. Nevertheless, under favorable conditions, B. mallei can probably survive a few months. Burkholderia mallei can remain viable in tap water for at least one month [19].
The detection of B. mallei in anatomical sites that allow its elimination into the environment suggests that chronically infected equids can potentially spread the infection, especially if there are sufficient humidity conditions for the survival of this bacterium.
The isolation of B. mallei from clinical specimens presents a challenge due to the high occurrence of other bacteria [26]. In addition, due to the low number of bacteria in infected tissues, culture in solid or liquid media is usually negative, especially if the samples come from subclinical or chronic cases [12]. In naturally infected horses from Brazil, kept in quarantine, with chronic infections and showing no clinical signs of glanders, 4 out of 160 clinical samples (1.8%) were positive for B. mallei [12]. However, in the acute phase of infection, the detection of B. mallei in clinical specimens is more frequent. In a study with equids from the states of Pernambuco and Alagoas, Northeastern Brazil, the microbiological isolation and molecular detection of B. mallei was achieved from samples of closed cutaneous nodules from eight different animals with an acute clinical presentation of glanders and serologically positive to the complement fixation test. The animals were used to pull sugar cane carts or transport construction material [30].
The isolates resulting from this study will be characterized by mass spectrometry, and later, genomic sequencing will be carried out. Such strategies are relevant for understanding the genetic diversity of B. mallei in different regions of Brazil. They will also allow the performance of studies aimed at determining the virulence of these isolates, as well as the analysis of the cellular and humoral immune response in experimental inoculation models.
In summary, it was possible to genotypically detect B. mallei in horses from all Brazilian regions with positive serology for the agent, even without clinical disease. Burkholderia mallei was also detected from nasal swabs in three horses and from the palate of an asinine, suggesting environmental elimination of the agent.
Acknowledgements
To the Superintendencies of the Ministry of Agriculture and the Inspectors and Auditors of the Official Veterinary Services of the in the States of Rio Grande do Sul (DSA), Santa Catarina (CIDASC), Mato Grosso (INDEA), Bahia (ADAB), Piauí (ADAPI), Sergipe (Emdagro), Maranhão (AGED), Tocantins (ADAPEC), and Amazonas (ADAF). The excellent work carried out in the clinical evaluations and necropsies of the equids allowed the execution of this article.
To the coordination of the National Equine Health Program/MAPA.
To the Brazilian research agency, CNPq.
Author contribution
Flábio R. Araújo, Lenita R. Santos, and Emanuelle B. Gaspar designed the experiments.
Paula A. Pereira Suniga, Cynthia Mantovani, Maria G. Santos, and Juliana S. Gomes Rieger carried out the experiments – B. mallei cultures from tissues and swabs of euthanized horses seropositive for glanders, DNA extraction from colonies and tissues, PCR, and sequencing.
Paula A. Pereira Suniga, Cynthia Mantovani, Andréa A. Egito, and Flábio R. Araújo analyzed the genomic data.
Paula A. Pereira Suniga, Cynthia Mantovani, Maria G. Santos, Juliana S. Gomes Rieger, Andréa A. Egito, Flábio R. Araújo, Lenita R. Santos, and Emanuelle B. Gaspar wrote the manuscript.
Fernando L. dos Santos performed the analysis of necropsy findings.
Rinaldo A. Mota and Karla P. Chaves – supervision and training in microbiological culture for B. mallei.
José Carlos de Oliveira Filho and Alessandra F. Castro Nassar – necropsy of seropositive horses.
All authors read and approved the manuscript.
Funding
This study was supported by the Department of Agricultural Defense (SDA)/Ministry of Agriculture, Livestock and Food Supply (MAPA) and Brazilian Agriculture Research Corporation (Embrapa) (grant 20.21.10.006.00.00),
CNPq (grants 315857/2021–8 and 403651/2020–4).
CNPq (code 403651/2020-5, UFMS-EMBRAPA cooperation 25/2022).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Responsible Editor: Mariana X Byndloss
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Acharya KP, Marahatta S, Wilson RT. First outbreak of glanders in Nepal and possible implications for the animal sector. Transbound Emerg Dis. 2021;68:3015–3017. doi: 10.1111/tbed.14338. [DOI] [PubMed] [Google Scholar]
- 2.He G, Zeng Y, He Q, Liu T, Li N, Lin H, Zeng M, Li Y, Peng M, Cheng J, Liu W, Yao W. A case report of Burkholderia mallei infection leading to pneumonia. Comb Chem High Throughput Screen. 2023;26(1):241–245. doi: 10.2174/1386207325666220509152221. [DOI] [PubMed] [Google Scholar]
- 3.Bossi P, Tegnell A, Baka A, van Loock F, Hendriks J, Werner A, Maidhof H, Gouvras G. Bichat guidelines for the clinical management of glanders and melioidosis and bioterrorism-related glanders and melioidosis. Euro Surveill. 2004;9(12):35–36. doi: 10.2807/esm.09.12.00507-en. [DOI] [PubMed] [Google Scholar]
- 4.Van Zandt KE, Greer MT, Gelhaus HC. Glanders: an overview of infection in humans. Orphanet J Rare Dis. 2013;8:1–7. doi: 10.1186/1750-1172-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pimentel W. História e organização do serviço veterinário do exército. Revista Militar de Medicina Veterinária. 1938;1:283–322. [Google Scholar]
- 6.Mota RA, Brito MF, Castro FJC, Massa M. Glanders in horses and mules of the states of Pernambuco and Alagoas, Brazil. Pesquisa Veterinária Brasileira. 2000;20:155–159. doi: 10.1590/S0100-736X2000000400005. [DOI] [Google Scholar]
- 7.MAPA (1934) Decreto nº 24.548 de 3 de julho de 1934. http://www.planalto.gov.br/ccivil_03/decreto/1930-1949/d24548.htm Accessed 22 Nov 2022
- 8.MAPA (2018) Portaria SDA n° 35 de 17 de abril de 2018. https://www.gov.br/agricultura/pt-br/assuntos/lfda/legislacao-metodos-da-rede-lfda/copy_of_diagnostico-animal%20arquivos/copy_of_Portaria35de17.04.2018Testeslaboratparamormo.pdf/view. Accessed 22 Nov 2022
- 9.OIE (2018) Chapter 12.10. Infection with Burkholderia mallei (Glanders). OIE Terrestrial Animal Health. https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online%20access/?id=169&L=1&htmfile=chapitre_glanders.html. Accessed 22 Nov 2022
- 10.Resende CF, Dos Santos AM, Filho PMS, De Souza PG, De Azevedo Issa M, De Carvalho Filho MB, et al. Glanders and brucellosis in equids from the Amazon region, Brazil. Acta Tropica. 2022;231:106429. doi: 10.1016/j.actatropica.2022.106429. [DOI] [PubMed] [Google Scholar]
- 11.Fonseca-Rodríguez O, Pinheiro Júnior JW, Mota RA. Spatiotemporal analysis of glanders in Brazil. J Equine Vet. 2019;78:14–19. doi: 10.1016/j.jevs.2019.03.216. [DOI] [PubMed] [Google Scholar]
- 12.Abreu DC, Gomes AS, Tessler DK, Chiebao DP, DelFava C, Romaldini AHCN, et al. Systematic monitoring of glanders-infected horses by complement fixation test, bacterial isolation, and PCR. Vet Anim Sci. 2020;10:100–147. doi: 10.1016/j.vas.2020.100147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falcão MVD, Da Silveira PPM, De Assis Santana VL, Da Rocha LO, Chaves KP, Mota RA. First record of Burkholderia mallei Turkey 10 strain originating from glanderous horses from Brazil. Braz J Microbiol. 2019;50:1125–1127. doi: 10.1007/s42770-019-00113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falcão MVD, Laroucau K, Vorimore F, Deshayes T, De Assis Santana VL, Silva KPC, et al. Molecular characterization of Burkholderia mallei strains isolated from horses in Brazil (2014-2017) Infect Genet Evol. 2022;99:105250. doi: 10.1016/j.meegid.2022.105250. [DOI] [PubMed] [Google Scholar]
- 15.Girault G, Woudstra C, Martin B, Vorimore F, De Assis Santana VL, Fach P, et al. First draft genome for a Burkholderia mallei isolate originating from a glanderous mule from Brazil. Genome Announc. 2017;5:e00579–e617. doi: 10.1128/genomeA.00579-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laroucau K, De Assis Santana VL, Girault G, Martin B, Da Silveira PPM, Machado MB, et al. First molecular characterisation of a Brazilian Burkholderia mallei strain isolated from a mule in 2016. Infect Genet Evol. 2018;57:117–120. doi: 10.1016/j.meegid.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Mota RA, Da Fonseca Oliveira AA, Pinheiro Junior JW, Da Silva LBG, De Farias BM, Rabelo SSA. Glanders in donkeys (Equus asinus) in the state of Pernambuco, Brazil: a case report. Braz J Microbiol. 2010;41:146–149. doi: 10.1590/S1517-83822010000100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rocha LO, De Lima LAR, De Albuquerque RMS, Lages SLS, Nunes ACBT, De Castro RS, et al. Monitoring the outbreak of equine glanders in Alagoas, Brazil: clinical, immunological, molecular, and anatomopathological findings. Ciência Rural [online] 2021;51:e20200834. doi: 10.1590/0103-8478cr20200834. [DOI] [Google Scholar]
- 19.OIE (2018) Chapter 3.6.11 Glanders and mielioidosis. Manual of diagnostic tests and vaccines. https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.06.11_GLANDERS.pdf. Accessed 22 Nov 2022
- 20.Appelt S, Rohleder AM, Jacob D, von Buttlar H, Georgi E, Mueller K, et al. Genetic diversity and spatial distribution of Burkholderia mallei by core genome-based multilocus sequence typing analysis. PLoS One. 2022;17(7):e0270499. doi: 10.1371/journal.pone.0270499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brangsch H, Saqib M, Sial AUR, Melzer F, Linde J, Elschner MC. Sequencing-based genotyping of Pakistani Burkholderia mallei strains: a useful way for investigating glanders outbreaks. Pathogens. 2022;11:614. doi: 10.3390/pathogens11060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scholz HC, Pearson T, Hornstra H, Projahn M, Terzioglu R, Wernery R, et al. Genotyping of Burkholderia mallei from an outbreak of glanders in Bahrain suggests multiple introduction events. PLoS Negl Trop Dis. 2014;8:e3195. doi: 10.1371/journal.pntd.0003195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Losada L, Ronning CM, DeShazer D, Woods D, Fedorova N, Stanley Kim H, et al. Continuing evolution of Burkholderia mallei through genome reduction and large-scale rearrangements. Genome Biol Evol. 2010;2:102–116. doi: 10.1093/gbe/evq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song H, Hwang J, Yi H, Ulrich RL, Yu Y, Nierman WC, Kim HS. The early stage of bacterial genome-reductive evolution in the host. PLoS Pathogens. 2010;6(5):e1000922. doi: 10.1371/journal.ppat.1000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laroucau K, Aaziz R, Vorimore F, Varghese K, Deshayes T, Bertin C, et al. A genetic variant of Burkholderia mallei detected in Kuwait: consequences for the PCR diagnosis of glanders. Transbound Emerg Dis. 2021;68(2):960–963. doi: 10.1111/tbed.13777. [DOI] [PubMed] [Google Scholar]
- 26.Kinoshita Y, Cloutier AK, Rozak DA, Khan MS, Niwa H, Uchida-Fujii E, et al. A novel selective medium for the isolation of Burkholderia mallei from equine specimens. BMC Vet Res. 2019;15(1):1–7. doi: 10.1186/s12917-019-1874-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Embden JD, Cave MD, Crawford JT, Dale JW, Eisenach KD, Gicquel B, et al. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Werle E, Schneider C, Renner M, Völker M, Fiehn W. Convenient single-step, one tube purification of PCR products for direct sequencing. Nucleic Acids Res. 1994;22(20):4354. doi: 10.1093/nar/22.20.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanger F. Sequences, sequences, and sequences. Annu Rev Biochem. 1988;57:1–29. doi: 10.1146/annurev.bi.57.070188.000245. [DOI] [PubMed] [Google Scholar]
- 30.Silva KP, Mota RA, Cunha AP, Silva LB, Leal NC, Cavalcante YV, et al. Caracterização fenotípica e molecular de amostras de Burkholderia mallei isoladas na Região Nordeste do Brasil. Pesquisa Veterinária Brasileira. 2009;29:439–444. doi: 10.1590/S0100-736X2009000500015. [DOI] [Google Scholar]
- 31.Khan I, Wieler LH, Melzer F, Elschner MC, Muhammad G, Ali S, et al. Glanders in animals: a review on epidemiology, clinical presentation, diagnosis and countermeasures. Transbound Emerg Dis. 2013;60(3):204–221. doi: 10.1111/j.1865-1682.2012.01342.x. [DOI] [PubMed] [Google Scholar]
- 32.Nierman WC, DeShazer D, Kim HS, Tettelin H, Nelson KE, Feldblyum T, et al. Structural flexibility in the Burkholderia mallei genome. Proc Natl Acad Sci. 2004;101(39):14246–14251. doi: 10.1073/pnas.0403306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.