Abstract
Adenoid hypertrophy (AH) plays a role as a reservoir for bacterial growth and decreases mucociliary clearance which might contribute to the development of an infection. To compare the presence of AH in the pediatric population presenting with orbital complications as a result of ABRS and the control group radiologically. Patients who were diagnosed with OC as a result of ABRS labeled as case group, and the patients who had undergone computed tomography (CT) for indications other than sinonasal diseases were assigned as control group. Both groups were retrospectively reviewed to measure the adenoid, nasopharynx, and adenoid/nasopharynx ratio (ANR) in the axial and mid-sagittal planes. We compared 52 patients from case group to 57 control group. In the CT axial plane, adenoid length was greater in the OC group compared to the control group, with a significant difference (p-value = 0.02) of 14.2 ± 3.5 mm compared to 11.2 ± 7 mm, respectively. The ANRs were 2.9 in the OC group and 2.8 in the control group, with a p-value of 0.089. In the mid-sagittal plane, only the anteroposterior length was significantly greater in the OC group, with a mean of 19.9 ± 5.3 mm compared to 15.2 ± 8.8 mm in the control group (p-value = 0.007). The process of inflammation increased the anteroposterior length of the adenoids. However, the ANR was similar between the two groups, indicating that adenoid hypertrophy is not directly related as a risk factor for OC in pediatric patients with ARBS.
Keywords: Pediatric acute bacterial rhinosinusitis, Orbital infection, Adenoid hypertrophy
Introduction
Adenoids are lymphoid tissue located in the nasopharynx. They play a role as part of Waldeyer’s Ring in the immune system by providing resistance against upper respiratory tract infection [1]. Hypertrophy of adenoids may contribute to several pathological conditions, such as apnea and chronic suppurative otitis media [2]. Additionally, it might contribute to the development of an infection, as it decreases mucociliary clearance and causes adenoids to act as bacterial reservoirs [3, 4].
Acute bacterial rhinosinusitis (ABRS) is a very common condition in the pediatric population [5]. The pathophysiology of ABRS starts as an inflammatory process of the nasal mucosa that leads to swelling and edema, which result in impaired sinus drainage [6]. Subsequently, the secretion can spread to adjacent compartments, such as the orbit and intracranial fossa [7]. Although the pathophysiology of ABRS is well understood, we still lack evidence for risk factors associated with the development of orbital complications (OC) [5]. The objective of this study is to determine the relationship between adenoid hypertrophy (AH) and OR as a result of ABRS.
Materials and Methods
This study was designed to be a retrospective case series analysis. A screening for all computed tomography (CT) records was done in the tertiary center, King Saud University Medical City, Riyadh, Saudi Arabia, for a 12-year period (2008–2020). An electronic search into the radiological question and reports for keywords included “Acute rhinosinusitis,” “preseptal cellulitis,” “postseptal cellulitis,” “orbital cellulitis,” “subperiosteal abscess,” “orbital abscess,” and “cavernous sinus thrombosis.” Furthermore, all patients were screened for inclusion and exclusion criteria. We included in the OC group patients who were less than 18 years old and presented with OC caused by ABRS confirmed by CT. On the other hand, any image with low quality, patients who were more than 18 years old, patients with head and neck tumors, craniofacial anomalies, or immunodeficiency, hyperactivity of the airway, OC caused by etiology other than ABRS (dacryocystitis, trauma, tumors, or unknown causes) were excluded from the study.
Patients and Grouping
We enrolled patients who met the inclusion criteria as case group. A search of the records was done to reveal their symptoms, diagnosis, and the management measures they underwent. The control group was chosen as patients who had CT scans for reasons other than sinonasal or orbital pathologies.
Radiological Evaluation of Adenoids
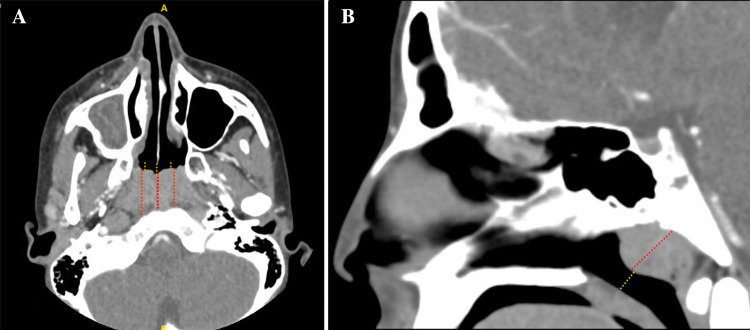
We assessed the adenoid size in the CT scans using two different planes. For the axial plane, at the level of the maximum adenoid dimension (shorter distance between posterior septum and adenoid), the anteroposterior diameter of the adenoid pad was measured with three vertical lines to obtain the average and overcome the curved nature of the adenoid surface. Then, three lines drawn from the anterior border of the adenoid to the beginning of the choanae, representing the length of the nasopharynx, were measured (Fig. 1). After that, the adenoids’ average length was divided by the average nasopharynx length to calculate the adenoid-nasopharynx ratio (ANR-Axial).
Fig. 1.
Demonstration of the lines used to measure the adenoid and nasopharynx in the CT scans. The red dotted lines represent the adenoid length, and dotted yellow lines represent the nasopharynx in the axial plane (A) and mid-sagittal plane (B)
For the sagittal plane, measurements were taken at the midline, which corresponds to the nasal septum seen in the axial cut. The first line was drawn perpendicularly from the maximum adenoid bulging to the basopharyngeal fascia posteriorly. The second line was then measured from the anterior surface of the adenoid to the soft palate to represent the nasopharynx. Finally, the adenoid length was divided along the nasopharynx to calculate the ANR-sagittal (Fig. 1 B). All measurements were recorded in millimeters.
Evaluation of Other Radiological Findings
The Lund–Mackay scoring system was used, as it is a validated tool for assessing every sinus involvement (frontal, anterior ethmoid, posterior ethmoid, maxillary, sphenoid) and the osteomeatal complex with a score of either 0, 1, or 2 for no abnormality, partial opacification, or complete opacification, respectively, for each side independently [8]. Other radiological findings that may contribute to or be involved in the development of OC in ABRS, such as dehiscence of the lamina papyracea (DLP), nasolacrimal duct (NLD) infection, and inferior turbinate hypertrophy (HIT), were examined. In addition, we assessed the presence of other sinonasal radiological findings, such as a deviated nasal septum (DNS), concha bullosa (CB), infraorbital ethmoidal cells, retromaxillary cells, Onodi cells, and optic/carotid dehiscence.
Statistical Analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS-23). Continuous values are presented as either mean ± standard deviation (SD) or as the median based on the normality of distribution. Nonparametric tests included the chi-squared tests for categorical data (Gender, CB, DNS, infraorbital ethmoidal cells, retromaxillary cells, and Onodi cells) and Mann–Whitney U tests for the quantitative data (age, Lund–Mackay score, and ANR mean). P < 0.05 was considered significant.
Ethical Considerations
This retrospective study followed the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of King Saud University, College of Medicine.
Results
Out of the 109 patients included in this study, 64 were male and 45 female, with a p-value of 0.82. The mean age for the OC group was not statistically different from the control group, with p-values of 0.664, 7.8 ± 4.8 years, and 7.7 ± 4.4 years, respectively. For the radiological findings for CB, DNS, infraorbital ethmoidal cells, retromaxillary cells, Onodi cells, and optic or carotid dehiscence, there were no statistical differences between the two groups (Table 1). Summary for the presentation and management for OC patients were mentioned in (Table 2).
Table 1.
Demographic data for the patients involved in the study
| OC of ABRS (n = 52) | Control (n = 57) | Total (n = 109) | p-Value | |
|---|---|---|---|---|
| Male | 35 | 29 | 64 | 0.82 |
| Female | 17 | 28 | 45 | |
| Mean age (SD) | 7.8 (4.8) | 7.7 (4.4) | 8 (4.8) | 0.664 |
| DNS☨ | 2 (3.9%) | 6 (10.5%) | 8 (7.3%) | 0.182 |
| Unilateral CB | 7 (13.5%) | 8 (14%) | 15 (13.8%) | 0.839 |
| Bilateral CB | 6 (11.5%) | 10 (17.5%) | 16 (14.7%) | |
| Infraorbital ethmoidal cells | 2 (3.8%) | 0 (0%) | 2 (1.8%) | 0.135 |
| Retromaxillary cells | 1 (1.9%) | 1 (1.8%) | 2 (1.8%) | 0.948 |
| Onodi cells | 2 (3.9%) | 4 (7%) | 6 (5.5%) | 0.468 |
| Optic/Carotid Dehiscence | 0 (0%) | 0 (0%) | 0 (0%) | / |
SD Standard deviation, CB Concha bullosa, ☨; DNS Deviated nasal septum touching turbinate
Table 2.
Descriptive data for the clinical evaluation for patients presenting with orbital complications as a result of acute sinusitis
| Chandler classification | Class 1 (Preseptal cellulitis) | 11 (21.2%) |
| Class 2 (Orbital cellulitis) | 15 (28.8%) | |
| Class 3 (Subperiosteal Abscess) | 25 (45.1%) | |
| Treatment site | Outpatient | 2 (3.8%) |
| Admission (hospital stay mean) | 50 (96.2%) | |
| Management | Medical management only | 32 (61.5%) |
| FESS with medical management | 2 (3.8%) | |
| External incision and drainage with medical management | 8 (14.8%) | |
| FESS and EIAD with medical management | 8 (14.8%) | |
| Presentation | Proptosis | 23 (44.2%) |
| Chemosis | 17 (32.7%) | |
| Pain | 21 (40.4%) | |
| Restricted eye movement | 29 (42.3%) | |
| Decrease vision | 11 (21.2%) |
FESS Functional endoscopic sinus surgery, EIAD External incision and drainage
Significant differences were observed in the Lund–Mackay score (p-value = 0.0001) and the radiological findings of DLP (p-value = 0.022), NLD (p-value = 0.017), and HIT (p-value = 0.024), as they presented more frequently in the OC group (Table 3).
Table 3.
Radiological findings
| OC of ABRS (n = 52) | Control (n = 57) | Total (n = 109) | p-Value | |
|---|---|---|---|---|
| Mean lund–mackay score (SD) | 10.48 (5.3) | 3.35 (4.5) | 6.75 (6) | 0.0001 |
| Dehiscence LP | 5 (9.6%) | 0 (0%) | 5 (4.6%) | 0.022 |
| NLD infection | 5 (9.6%) | 0 (0%) | 5 (4.6%) | 0.017 |
| Unilateral HIT | 8 (15.4%) | 16 (28.1%) | 24 (22%) | 0.024 |
| Bilateral HIT | 13 (25%) | 19 (33.3%) | 32 (29.4%) |
LP Lamina papyracea, NLD Nasolacrimal duct, HIT Hypertrophied inferior turbinate
For adenoid size, in the adenoid axial length, the OC group showed larger measurements compared to the control group, with a significant difference (p-value = 0.02) of 14.2 ± 3.5 mm compared to 11.2 ± 7 mm. However, the nasopharynx diameter and ANR-axial were statistically insignificantly different between the two groups. For the mid-sagittal plane, only the anteroposterior length was statistically significantly different between the OC and control, with a p-value of 0.007 and means of 19.9 ± 5.3–15.2 ± 8.8 mm, respectively. The superior–inferior axis of the adenoid, anteroposterior nasopharynges, superior-inferior lengths, and ANR-sagittal were all statistically insignificant (Table 4).
Table 4.
Radiological evaluations of the adenoid size between OC and control groups
| OC of ABRS | Control | p-value | |
|---|---|---|---|
| Axial plane | |||
| Adenoid mid-axis | 14.2 ± 3.5 | 11.2 ± 7 | 0.020* |
| Adenoid paramedian | 13 ± 3.8 | 10.2 ± 6.1 | 0.012* |
| Nasopharynx diameter | 6.5 ± 3.7 | 8.2 ± 5.2 | 0.186 |
| ANR | 2.9 ± 2.5 | 2.8 ± 3.2 | 0.089 |
| Mid-sagittal plane | |||
| Adenoid (anteroposterior axis) = length | 19.9 ± 5.3 | 15.2 ± 8.8 | 0.007* |
| adenoid superoinferior axis = width | 9.7 ± 4 | 8.9 ± 5.3 | 0.796 |
| Nasopharynx (anteroposterior axis) = length | 20.9 ± 6 | 18.9 ± 4.6 | 0.077 |
| nasopharynx superoinferior axis = width | 7.4 ± 3.1 | 7.9 ± 4.3 | 0.846 |
| ANR for length | 1.1 ± 0.9 | 0.9 ± 0.6 | 0.156 |
| ANR for width | 1.7 ± 1.1 | 1.8 ± 1.7 | 0.994 |
| ANR for length and width | 1.4 ± 0.8 | 1.4 ± 1 | 0.822 |
*p-value < 0.05 considered statistically significant
ANR Adenoid/nasopharynx ratio
The four groups classified by age (< 4 years, 4–8 years, 9–12 years, and > 12 years) did not show a statistical difference between the OC and control in the mean ANR with p-values of 0.786, 0.403, 0.224, and 0.106, respectively (Table 5).
Table 5.
Measurement of adenoid size for the population subclassified based on age group
| OC of ABRS | Control | p-value | ||
|---|---|---|---|---|
| < 4 years | Number | 13 | 15 | 0.786 |
| Mean ANR | 4.5 (2.3) | 4.6 (2.8) | ||
| 4–8 years | Number | 14 | 13 | 0.403 |
| Mean ANR | 4.4 (1.6) | 4.3 (1.6) | ||
| 9–12 years | Number | 16 | 14 | 0.224 |
| Mean ANR | 4.1 (1.1) | 3.6 (3) | ||
| > 12 years | Number | 16 | 15 | 0.106 |
| Mean ANR | 3.6 (1.3) | 3.2 (6.7) |
Discussion
Orbital infection is the most common complication of ABRS. It ranges from mild swelling of the periorbital soft tissue to orbital abscess collocation and cavernous sinus thrombosis [9, 10].
In our study, AH, which is defined as obstructing the nasopharynx, did not show a direct association with the development of OC in ABRS. The significant difference we observed in the OC-ABRS group in terms of adenoid size in the axial plane and adenoid length in the mid-sagittal plane may have been a part of inflammatory changes resulting from adenitis as part of ABRS. However, ANR values, which are more representative of AH, did not show statistical differences between the two groups in different planes. Furthermore, as adenoid size and nasopharyngeal diameter changes between childhood and adolescence [11], we subdivided the patients based on age into four groups. Between those four groups, there was no statistical difference in ANR values.
Similarly, Wang et al. conducted a cohort study to assess the adenoid diameter in pediatric patients presenting with purulent rhinosinusitis. They found only 15 out of 89 (16.9%) had AH. They concluded that AH is not correlated with ABRS [12]. Additionally, other authors believed that AH is not directly associated with ARBS, like it is related to chronic rhinosinusitis in the pediatric age group [13].
HIT was more common in the OC group, and the scores of the Lund–Mackay staging system were higher in the OC group, as reported in the literature for radiological findings of ARBS [14]. Additionally, DLP and NLD were presented only in the OC group, as they might be explained by the pathway for the spread of the infection beyond the sinuses to the orbit [15].
Grischkan et al. found that adenoid hypertrophy (61%), inferior turbinate hypertrophy (80%), and septal deviation (47%) were more common in patients with OC from ABRS. However, there was no statistically significant difference in their data between the case and control groups. They relied on subjective tools in the form of already written reports from radiologists and screened them for these parameters [16].
AH is diagnosed by clinical history and endoscopic examination of the nasopharynx. Radiological evaluations for adenoids were first introduced in 1979 by Fujioka et al. using lateral radiography and by measuring ANR [17]. Since then, imaging has been reported to be an excellent method for assessing AH. One study showed adenoid size in MRI correlated with the apnea/hypopnea index in children with obstructive sleep apnea [18]. Additionally, cone-beam CT scans showed good accuracy in evaluating adenoid size, with an average sensitivity and specificity of 88–92% and 93–97%, respectively [19, 20]. In our study, we adapted axial and mid-sagittal planes, as they provide the most diagnostically useful information by measuring adenoid dimensions and ANR, as suggested by different authors in the literature [19, 21].
The limitations of our study were that our sample was not a community-based population and included patients who were presented in a tertiary care university hospital. Additionally, the study design employed a retrospective method. Although we evaluated the patients who had CT scans only, we were missing information for the cases who did not require a CT in their course of the disease’s treatment. Furthermore, the gold standard tool for the diagnosis of adenoids is by history taking and nasal endoscope rather than imaging.
Conclusion
AH was not shown to be a risk factor for the spread of acute bacterial rhinosinusitis to the orbit. However, during acute infection, adenoid size may be enlarged but does not cause significant nasopharyngeal narrowing. DLP and NLDI showed radiological presence and was found to be a possible pathway for the spread of the infection to the orbit.
Funding
No funding was received for this study.
Declarations
Conflict of interest
Authors have no conflict of interests.
Ethical Standard
The study was approved by the Institutional Review Board of King Saud University, College of Medicine.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brodsky L. Modern assessment of tonsils and adenoids. Pediatr Clin North Am. 1989;36(6):1551–1569. doi: 10.1016/S0031-3955(16)36806-7. [DOI] [PubMed] [Google Scholar]
- 2.Evcimik MF, Dogru M, Cirik AA, Nepesov MI. Adenoid hypertrophy in children with allergic disease and influential factors. Int J Pediatr Otorhinolaryngol. 2015;79(5):694–697. doi: 10.1016/j.ijporl.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 3.Arnaoutakis D, Collins WO. Correlation of mucociliary clearance and symptomatology before and after adenoidectomy in children. Int J Pediatr Otorhinolaryngol. 2011;75(10):1318–1321. doi: 10.1016/j.ijporl.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Lee D, Rosenfeld RM. Adenoid bacteriology and sinonasal symptoms in children: first place-resident clinical science award 1996. Otolaryngol Neck Surg. 1997;116(3):301–307. doi: 10.1016/S0194-59989770264-X. [DOI] [PubMed] [Google Scholar]
- 5.Holzmann D, Willi U, Nadal D. Allergic rhinitis as a risk factor for orbital complication of acute rhinosinusitis in children. Am J Rhinol. 2001;15(6):387–390. doi: 10.1177/194589240101500606. [DOI] [PubMed] [Google Scholar]
- 6.Wagenmann M, Naclerio RM. Complications of sinusitis. J Allergy Clin Immunol. 1992;90(3):552–554. doi: 10.1016/0091-6749(92)90184-4. [DOI] [PubMed] [Google Scholar]
- 7.García CE, Cunningham MJ, Clary RA, Joseph MP. The etiologic role of frontal sinusitis in pediatric orbital abscesses. Am J Otolaryngol. 1993;14(6):449–452. doi: 10.1016/0196-0709(93)90122-N. [DOI] [PubMed] [Google Scholar]
- 8.Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology. 1993;31(4):183–184. [PubMed] [Google Scholar]
- 9.Baring DEC, Hilmi OJ. An evidence based review of periorbital cellulitis. Clin Otolaryngol. 2011;36(1):57. doi: 10.1111/j.1749-4486.2011.02258.x. [DOI] [PubMed] [Google Scholar]
- 10.Bedwell J, Bauman NM. Management of pediatric orbital cellulitis and abscess. Curr Opin Otolaryngol Head Neck Surg. 2011;19(6):467–473. doi: 10.1097/MOO.0b013e32834cd54a. [DOI] [PubMed] [Google Scholar]
- 11.Gangadhara S, Rajeshwari A, Jain M. Significance of adenoid nasopharyngeal ratio in the assessment of adenoid hypertrophy in children. Res Otolaryngol. 2012;1(1):1–5. [Google Scholar]
- 12.Wang D, Bernheim N, Kaufman L, Clement P. Assessment of adenoid size in children by fibreoptic examination. Clin Otolaryngol Allied Sci. 1997;22(2):172–177. doi: 10.1046/j.1365-2273.1997.00002.x. [DOI] [PubMed] [Google Scholar]
- 13.Bulfamante AM, Saibene AM, Felisati G, Rosso C, Pipolo C. Adenoidal disease and chronic rhinosinusitis in Children:Is there a link? J Clin Med. 2019;8(10):1528. doi: 10.3390/jcm8101528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaikh N, Hoberman A, Kearney DH, Colborn DK, Kurs-Lasky M, Jeong JH, et al. Signs and symptoms that differentiate acute sinusitis from viral upper respiratory tract infection. Pediatr Infect Dis J. 2013;32(10):1061. doi: 10.1097/INF.0b013e31829bb2c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wald ER. Periorbital and orbital infections. Pediatr Rev. 2004;25(9):312–320. doi: 10.1542/pir.25-9-312. [DOI] [PubMed] [Google Scholar]
- 16.Grischkan JM, Elmaraghy CA, Garrett MR, Karanfilov B, Jatana KR. Radiographic findings and clinical correlates in pediatric periorbital infections. Int J Otorhinolaryngol. 2015 doi: 10.13188/2380-0569.1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lionel M. Adenoidal-nasopharyngeal BWYRG, An T, An A, States U. Radiographic Evaluation of adenoidal size in children: ratio
- 18.Arens R, McDonough JM, Costarino AT, Mahboubi S, Tayag-Kier CE, Maislin G, et al. Magnetic resonance imaging of the upper airway structure of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2001;164(4):698–703. doi: 10.1164/ajrccm.164.4.2101127. [DOI] [PubMed] [Google Scholar]
- 19.Major MP, Witmans M, El-Hakim H, Major PW, Flores-Mir C. Agreement between cone-beam computed tomography and nasoendoscopy evaluations of adenoid hypertrophy. Am J Orthod Dentofac Orthop. 2014;146(4):451–459. doi: 10.1016/j.ajodo.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Major MP, Saltaji H, El-Hakim H, Witmans M, Major P, Flores-Mir C. The accuracy of diagnostic tests for adenoid hypertrophy: a systematic review. J Am Dent Assoc. 2014;145(3):247–254. doi: 10.14219/jada.2013.31. [DOI] [PubMed] [Google Scholar]
- 21.Georgalas C, Thomas K, Owens C, Abramovich S, Lack G. Medical treatment for rhinosinusitis associated with adenoidal hypertrophy in children: an evaluation of clinical response and changes on magnetic resonance imaging. Ann Otol Rhinol Laryngol. 2005;114(8):638–644. doi: 10.1177/000348940511400810. [DOI] [PubMed] [Google Scholar]