Abstract
This study aimed to explore the roles of SAP2 and GCN4 in itraconazole (ITR) resistance of C. albicans under different conditions, and their correlations. A total of 20 clinical strains of C. albicans, including 10 ITR resistant strains and 10 sensitive strains, were used. Then, SAP2 sequencing and GCN4 sequencing were performed, and the biofilm formation ability of different C. albicans strains was determined. Finally, real-time quantitative PCR was used to measure the expression of SAP2 and GCN4 in C. albicans under planktonic and biofilm conditions, as well as their correlation was also analyzed. No missense mutations and three synonymous mutation sites, including T276A, G543A, and A675C, were found in SAP2 sequencing. GCN4 sequencing showed one missense mutation site (A106T (T36S)) and six synonymous mutation sites (A147C, C426T, T513C, T576A, G624A and C732T). The biofilm formation ability of drug-resistant C. albicans strains was significantly higher than that of sensitive strains (P < 0.05). Additionally, SAP2 and GCN4 were up-regulated in the ITR-resistant strains, and were both significantly higher in C. albicans under biofilm condition. The mRNA expression levels of SAP2 and GCN4 had significantly positive correlation. The higher expression levels of SAP2 and GCN4 were observed in the ITR-resistant strains of C. albicans under planktonic and biofilm conditions, as well as there was a positive correlation between SAP2 and GCN4 mRNA expression.
Keywords: Invasive candidiasis; C. albicans, SAP2, GCN4, Itraconazole resistance
Introduction
Invasive candidiasis (IC) is a dangerous disease with rapid progress, and the incidence of IC has been increasing year by year. However, due to the limited efficacy of clinical therapeutic drugs and the emergence of severe drug resistance, treatment failure often occurs in patients, and the mortality rate of systemic infection caused by IC has reached up to 45–75% [1, 2]. Candida albicans is the most important opportunistic pathogenic fungus and is the main pathogen causing IC in humans [3, 4]. At present, there are mainly three kinds of antifungal drugs for the treatment of candidiasis, including triazole, polyene, and echinocandin [5]. Among them, azole drugs, such as fluconazole (FCA), itraconazole (ITR), and voriconazole (VRC), have become the most widely used in clinical, and the primary mechanism of their antifungal activity is that through the inhibition of lanosterol 14-α-demethylase, they can block the synthesis of ergosterol, a key component of fungal cell membrane [5, 6]. Due to the long-term irrational drug use, drug-resistant C. albicans strains have been developed, and combined with the limited clinical efficacy of drugs, the number of clinical treatment failure case is increasing [7, 8]. Therefore, it is urgent to deeply unearth the pathogenesis and drug resistance mechanisms of C. albicans, and discover novel antifungal targets so as to develop a new generation of safe and efficient antifungal agents.
The pathogenic mechanism of C. albicans infection is related to a variety of virulence factors, including phenotypic conversion (yeast-mycelium phase conversion), adhesins, and invasive enzymes [9]. Secreted aspartyl proteinase 2 (Sap2), belonging to invasive enzymes, is the most important extracellular proteolytic enzyme in the Saps family, and is the main cause of damage and virulence, which has been considered to be the key virulence pathogenic factor of C. albicans [9, 10]. In clinical azole-resistant strains of C. albicans, the higher expression of SAP2 is involved in nutrient metabolism, cell adhesion, invasion, and destruction of host tissue barrier of C. albicans, thereby increasing its pathogenicity [8, 11, 12]. In addition, another major virulence factor of C. albicans is the ability of biofilm formation [13]. A previous study reported that the drug resistance of fungus in biofilm state was one thousand times higher than that in planktonic state [14]. Other studies have shown that C. albicans in biofilm state was more aggressive, and more resistant to traditional antifungal drugs, which could increase the minimum inhibitory concentration of antifungal drugs by 20,000 times, thus leading to a significant increase in mortality of patients with candidiasis [15–17]. SAP2 can promote the growth of C. albicans biofilm in vivo, and reduce the adhesion of biofilm formation at the initial stage [18], which suggested an important correlation between the important virulence pathogenic factors of C. albicans.
General Control Nonderepressible 4 (GCN4), an amino acid hunger response factor, has been reported to participate in regulating the expression of genes associated with amino acid biosynthetases [19], and play important roles in drug resistance of human fungal pathogens [20]. Under the condition of adequate nutrition, GCN4 can reduce protein synthesis, thus prolonging the replication life of yeast, and increasing the virulence and drug resistance of C. albicans [21–23]. However, under the condition of nutrient deficiency, the expression of GCN4 is upregulated to activate amino acid biosynthesis and promote the phenotype transformation of C. albicans, contributing to the mycelial differentiation and biofilm formation, and then increasing its virulence and drug resistance [24]. In addition, GCN4 was found to be an auxiliary transcriptional regulator of biofilm, and may be associated with the regulation of filamentation in the process of biofilm formation [25–27]. However, the mechanism of GCN4 in ITR-resistant strains of C. albicans in different states, and the relationship between SAP2 and GCN4 remain unclear.
The resistance of C. albicans is increasing year by year, and there is a lack of research on the resistance of anti-fungal drug ITR at home and abroad. Research on ITR-resistant strains will help to discover new resistance mechanisms and further develop new anti-fungal drugs. Therefore, in this study, the mutations of SAP2 and GCN4 in ITR-resistant/sensitive C. albicans strains were measured, and then the expression levels of SAP2 and GCN4 as well as their correlation were analyzed in C. albicans under biofilm and planktonic conditions. Our research will provide a theoretical basis for further clarifying the pathogenesis and azole drug resistance of C. albicans.
Materials and methods
Experimental strains
In this study, 10 clinical C. albicans strains all sensitive to fluconazole (FCA), itraconazole (ITR), and voriconazole (VRC), as well as 10 clinical C. albicans strains only resistant to ITR, were used. These strains were isolated, identified, and stored in the Pathogenic Microorganism and Immunology Laboratory of the Department of Dermatology and Venereology, the Second Hospital of Shanxi Medical University. Besides, these strains were all derived from the sputum of the clinical hospitalized patients. Furthermore, standard strain C. albicans ATCC1106 was obtained from the Fungus and Mycosi Research Center, Peking University Medical Science (Beijing, China). This study was approved by the Ethics Committee of the Second Hospital of Shanxi Medical University (approval no. (2019)YX no. 073).
DNA extraction, and SAP2 and GCN4 sequencing
All the strains were cultured in yeast extract peptone dextrose (YPD) liquid medium (AOBOX Biotechnology, Beijing, China) at 37 °C with cyclotron oscillation rate of 200 rpm for 24 h, and the fungal suspension was used for DNA extraction using a commercial Yeast DNA Extraction kit (Sangon Biotech (Shanghai) Co., Ltd., Shanghai, China) based on the manufacturer’s instructions. The purity and concentration of the isolated DNA were determined using a microplate reader (OD260/280).
According to the SAP2 and GCN4 sequences already shown in GeneBank, the specific primers were designed and synthesized by Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China), and sequences of the primers were displayed in Table 1. The total volume of the PCR was 20 μL, including 0.8 μL forward primer, 0.8 μL reverse primer, 2 μL DNA template, 10 μL Master Mix, and 6.4 μL ddH2O. The PCR reaction was initiated at 94 °C for 5 min, and a total of 35 cycles at 94 °C for 30 s, followed by 56 °C (GCN4)/59 °C (SAP2) for 30 s and 72 °C for 60 s, and 72 °C for 10 min. The PCR products were stored at 4 °C.
Table 1.
Sequences of all primers for PCR and real-time quantitative PCR (RT-qPCR)
| Reaction | Primer | Sequence (5′–3′) |
|---|---|---|
| PCR | SAP2 | F: ATGTTTTTAAAGAATATTTTCATTGCTCTTGCTATTG |
| R: TTAGGTCAAGGCAGAAATACTGGAAG | ||
| GCN4 | F: ATGCCTGCTACTACTCCTATTATT | |
| R: TTAAAATTGAATACCATTAACTCTTAACAATTCT | ||
| RT-PCR | ACT1 | F: TAGGTTTGGAAGCTGCTGGT |
| R: ACGTTCAGCAATACCTGGCA | ||
| SAP2 | F: ACCAATGAAGCCGGTGGTAG | |
| R: TTATTTGTCCCGTGGCAGCA | ||
| GCN4 | F: TGGAGTCGGTTTTCAGCACC | |
| R: ATTGACTTTGGCTCCGTCCA |
The PCR products were detected and analyzed by 1% agarose gel electrophoresis (120 V for 30 min). After that, the successful SAP2 and GCN4 amplification products were sent to Sangon Biotech (Shanghai) Co., Ltd. for bidirectional sequencing. Finally, SnapGene 4.1.8 software was employed to compare the sequencing results of SAP2 and GCN4, and the sequences of SAP2 and GCN4 provided in GeneBank, as well as to observe the sequencing peak maps to find the mutation sites.
Determination of biofilm formation ability
The experimental strains were coated on the YPD agar medium (AOBOX Biotechnology), and after incubated at 37 °C for 72 h, a fresh single C. albicans colony was selected to inoculate to the YPD liquid medium. After incubated at 30 °C with cyclotron oscillation rate of 200 rpm for 24 h, the fungal suspension was centrifuged at 5000 rpm for 2 min, and the sediments were resuspended with YPD liquid medium (0.5 Mc Farlan, about 5 × 106 CFU/mL).
The above fugal suspension (100 μL) was seeded into a 96-well plate, and incubated in a constant temperature incubator at 37 °C for 2 h. After washing with PBS softly, 100 μL YPD liquid medium was added to each well, and incubated for 1 h, 12 h, 24 h, and 48 h. At these different time points, the medium was removed, and the fungi were fixed with 4% paraformaldehyde for 20 min. Then, the fungi were stained with 1% crystal violet dye, and incubated at 37 °C for 30 min. After washing and airing, an inverted microscope (Eppendorf, Germany) was used to observe spore growth and mycelium formation at different time points at 200 × magnification, thus evaluating the biofilm growth and stability of C. albicans.
In addition, the biofilm formation ability of different C. albicans strains was determined. Briefly, the aforementioned fungal suspension (100 μL) was added to a 96-well plate (three duplicate holes for each strain), and then incubated at 37 °C for 48 h. Afterwards, the fungi were fixed with 4% paraformaldehyde for 20 min, and stained with 1% crystal violet dye. After 30 min of incubation at 37 °C, the fungi were washed with distilled water softly, and 100 μL 33% glacial acetic acid was added. After 2 min of incubation, the optical density (OD) at 490 nm was measured using a microplate reader.
Preparation of C. albicans under biofilm and planktonic conditions
The aforementioned fungal suspension was also used to prepare C. albicans under biofilm condition. Briefly, 2 mL fungal suspension was added to a 6-well plate, and incubated at 37 °C for 2 h. After washing with PBS softly, 2 mL fresh YPD liquid medium was added and then the fungi were incubated at 37 °C for 48 h to form mature biofilms. After that, the mature biofilms were scraped with a cell spatula, and added into an Eppendorf (EP) tube containing 1 mL sterile saline, which was C. albicans suspension under biofilm condition.
Furthermore, C. albicans suspension under planktonic condition was prepared. The experimental C. albicans suspension were inoculated in YPD agar medium, and incubated at 37 °C for 48 h. Then, a single colony was chosen to inoculate in YPD liquid, and incubated at 30 °C with cyclotron oscillation rate of 220 rpm for 24 h, which was C. albicans suspension under planktonic condition.
Total RNA isolation and real-time quantitative PCR (RT-qPCR)
Based on the manufacturer’s protocols, total RNA was isolated from different C. albicans strains under biofilm and planktonic conditions using Column Yeast Total RNA Extraction and Purification Kit (Sangon Biotech (Shanghai) Co., Ltd.). The purity and concentration of the isolated total RNA were assessed by a microplate reader. After that, the total RNA was reverse transcribed into cDNA using Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostics Products Co., Ltd., USA) following the manufacturer’s recommendations. The sequences of all primers were shown in Table 1, and ACT1 served as a housekeeping gene. The RT-qPCR reaction was started at 95 °C for 30 s, followed by a total of 40 cycles at 95 °C for 10 s and 60 °C for 30 s. ATCC11006 was used as a control in this research. The relative mRNA expression of SAP2 and GCN4 in each C. albicans strain were calculated by the 2−ΔΔCT method.
Statistical analysis
Three replicates were set up for each experiment (n = 3). SPSS 26.0 software (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. Data were reported as mean ± standard deviation (SD) or median (M) ± quartile range (QR). Student’s t-test or rank sum test was used to analyze the differences between the two groups. A value of P < 0.05 was considered as statistically significant. Additionally, Spearman’s correlation analysis was then employed to analyze the relationship between the SAP2 expression and the GCN4 expression.
Results
Identification of SAP2 mutations
In our study, the SAP2 gene was successfully sequenced in 20 strains of C. albicans, and the sequencing results were shown in Table 2. It was found that there were no missense mutations in these 20 strains, and there were three synonymous mutation sites, including T276A, G543A, and A675C (Table 2). The standard strain ATCC11006 had no mutation.
Table 2.
Base mutation sites and amino acid replacement for SAP2 in Candida albicans strains
| No. of strains | ITR sensitivity | Base mutation sites | Amino acid replacement |
|---|---|---|---|
| 17 | R | G543A | No |
| 40 | R | G543A | No |
| 54 | R | T276A/G543A | No/No |
| 58 | R | T276A/A675C | No/No |
| 63 | R | G543A | No |
| 67 | R | G543A | No |
| 96 | R | G543A | No |
| 105 | R | G543A | No |
| 115 | R | G543A | No |
| 188 | R | T276A/G543A | No/No |
| 53 | S | G543A | No |
| 73 | S | G543A | No |
| 76 | S | A675C | No |
| 80 | S | G543A | No |
| 87 | S | T276A/A675C | No/No |
| 94 | S | T276A/G543A/A675C | No/No/No |
| 95 | S | G543A | No |
| 117 | S | G543A | No |
R, only resistant to ITR; S, susceptible to all ITR, FCA and VRC
Identification of GCN4 mutations
The GCN4 gene was also sequenced in the 20 strains of C. albicans, and 19 of 20 strains were successfully sequenced. Among them, 12 strains of C. albicans had base mutation (Table 3), including one missense mutation site and six synonymous mutation sites (A147C, C426T, T513C, T576A, G624A, and C732T). The missense mutation was A106T (T36S), which meant that after missense mutation, threonine changed to serine at the 36th position. Additionally, we also observed that 4 of 19 strains showed missense mutation (T36S), all of which were T36S, and all appeared in the drug-resistant strains, while no missense mutations were found in the sensitive strains.
Table 3.
Base mutation sites and amino acid replacement for GCN4 in Candida albicans strains
| No. of strains | ITR sensitivity | Base mutation sites | Amino acid replacement |
|---|---|---|---|
| 17 | R | A106Ta/T513C/C732T | T36S/No/No |
| 54 | R | A147C/C732T | No/No |
| 58 | R | G624A | No |
| 67 | R | A106Ta/C732T | T36S/No |
| 96 | R | T513C/C732T | No/No |
| 105 | R | T513C/C732T | No/No |
| 115 | R | A106Ta/T513C/C732T | T36S/No/No |
| 188 | R | A106Ta/C426T/T576A/G624A | T36S/No/No/No |
| 73 | S | C732T | No |
| 80 | S | T513C/C732T | No/No |
| 94 | S | G624A | No |
| 117 | S | T513C/C732T | No/No |
R, only resistant to ITR; S, susceptible to all ITR, FCA, VRC; aMissense mutation sites; T, threonine; S, serine
Biofilm growth and biofilm formation ability
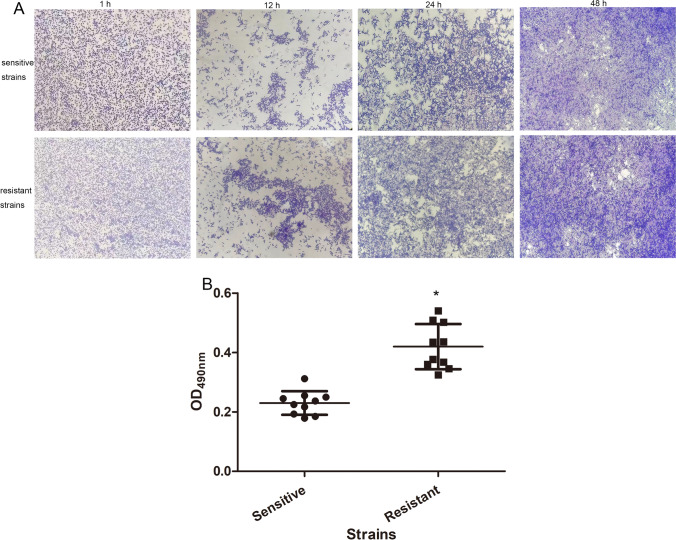
The biofilm growth of C. albicans was observed under an inverted microscope. After 1 h of incubation, a large number of C. albicans were observed, but no obvious budding and mycelial morphology were found. After cultured for 12 h, some C. albicans cells spout and formed mycelia, and there was mutual aggregation between C. albicans cells, as well as some of the mycelia were observed to fuse and form a dense network structure. After 24 h of culture, C. albicans cells were significantly aggregated, and gradually formed clump-like colonies with mycelia. After 48 h of culture, the dense colony composed of cells and mycelia interlaced with each other, forming a membrane network structure covering the whole field of vision (Fig. 1A). Mature biofilms were formed in both drug-resistant and sensitive strains after 48 h culture (Fig. 1A).
Fig. 1.
The biofilm growth of C. albicans under different time points and the biofilm formation ability of different strains. A An inverted microscope was used to observe the biofilm growth of C. albicans at 1 h, 12 h, 24 h, and 48 h. B OD490nm value was measured to assess the biofilm formation ability of sensitive and resistant strains
After that, the biofilm formation ability of different strains was assessed by measuring OD490nm. These data were in accordance with normal distribution, and were expressed mean ± SD. The values of OD490nm in the sensitive strains and resistant strains were respectively 0.23 ± 0.04 and 0.42 ± 0.076, which showed that the value in the resistant strains was significantly higher than that in the sensitive strains (Fig. 1B). The results indicated that the biofilm formation ability of drug-resistant C. albicans strains was higher than that of sensitive strains.
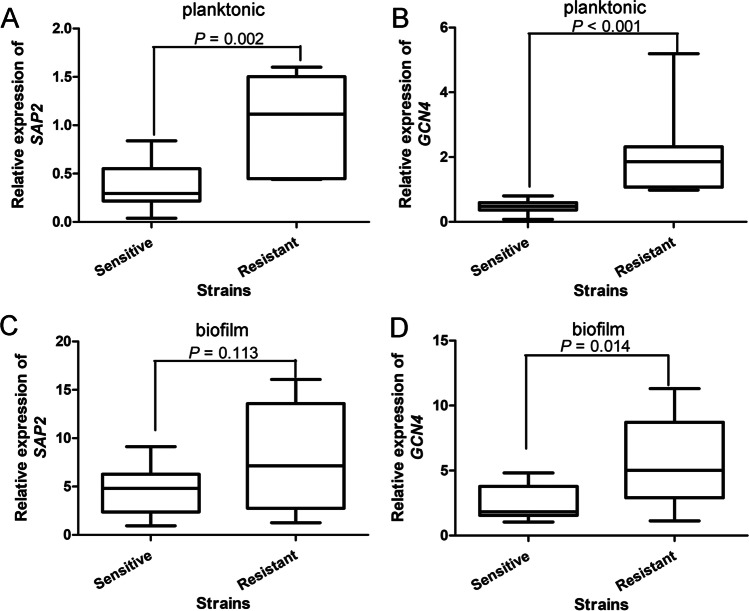
Expression of SAP2 and GCN4 in C. albicans under planktonic and biofilm conditions
The expression of SAP2 and GCN4 in strains of C. albicans under planktonic condition was determined by RT-qPCR. These data did not follow normal distribution, and were reported as M ± QR. It was found that the SAP2 expression in the ITR-resistant strains was significantly higher than that in the sensitive strains (P = 0.002, Fig. 2A). The GCN4 expression in the sensitive and resistant strains of C. albicans was 0.49 ± 0.23 and 1.86 ± 1.2, respectively, which showed that compared with the sensitive strains, the GCN4 expression was significantly increased in the resistant strains (P < 0.001, Fig. 2B).
Fig. 2.
The expression of SAP2 and GCN4 in different strains of C. albicans under planktonic and biofilm conditions. The expression of SAP2 (A) and GCN4 (B) in C. albicans under planktonic condition. The expression of SAP2 (C) and GCN4 (D) in C. albicans under biofilm condition
After that, we also measured the expression of SAP2 and GCN4 in different strains of C. albicans under biofilm condition. As shown in Fig. 2C, the data of SAP2 expression under biofilm condition followed normal distribution, and were shown as mean ± SD. The SAP2 expression was slightly higher in the resistant strains, but had no significant difference between the sensitive and resistant strains (P = 0.113). However, for GCN4, the data were not in accordance with normal distribution, and were expressed as M ± QR. It was found that GCN4 expression was significantly up-regulated in the ITR-resistant strains compared with the sensitive strains of C. albicans (P = 0.014, Fig. 2D). These results indicated that under planktonic and biofilm conditions, SAP2 and GCN4 were both up-regulated in the ITR-resistant strains of C. albicans.
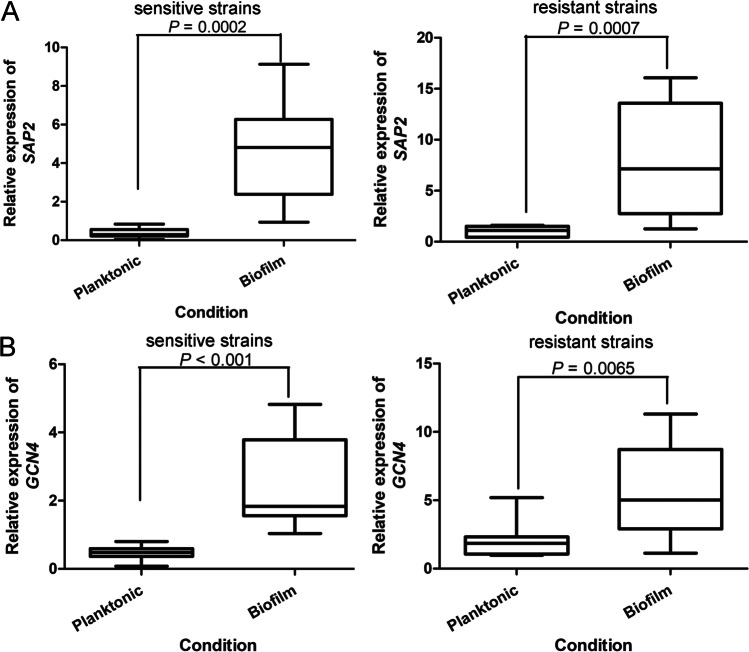
Comparison of SAP2 and GCN4 in C. albicans under different conditions
The expression of SAP2 and GCN4 in sensitive and resistant strains under planktonic and biofilm conditions were further compared. In both sensitive and resistant strains of C. albicans, the SAP2 and GCN4 expression under biofilm condition was both evidently higher than those under planktonic condition (P < 0.001, Fig. 3A, B). These results indicated that SAP2 and GCN4 were both significantly up-regulated in C. albicans strains under biofilm condition.
Fig. 3.
Comparison of SAP2 and GCN4 in sensitive and resistant C. albicans under planktonic and biofilm conditions. A The expression of SAP2 under planktonic and biofilm conditions. B The expression of GCN4 under planktonic and biofilm conditions
Correlation of SAP2 and GCN4 under different conditions
The correlation of SAP2 and GCN4 in C. albicans under planktonic and biofilm conditions was further analyzed using Spearman’s correlation analysis. Under planktonic and biofilm conditions, the correlation between SAP2 and GCN4 was 0.693 and 0.622, which indicated that the mRNA expression levels of SAP2 and GCN4 had significantly positive correlation (P < 0.05).
Discussion
C. albicans is a common opportunistic pathogenic fungus in human, and IC has become one of the primary diseases that threaten life and health in clinical, which brings heavy mental pressure and economic burden to patients’ families and society [28]. Sap2 is considered as the most important member of the Saps family, and can degrade the macromolecular proteins on the mucosal surface through hydrolysis, thus providing nutrition for the growth of C. albicans and increasing the ability of C. albicans to adhere and invade to the host [9]. GCN4 plays an essential role in homeostasis signal transduction of amino acids and transcriptional regulation of MFS-MDR transporter encoding protein and multidrug resistance transporter Tpo1 via participating in the oxidative stress response of C. albicans [29, 30]. In this study, we performed SAP2 sequencing on 20 strains of C. albicans, and found no strains had SAP2 missense mutation, which was in accordance with our previous study [31]. The possible reasons are that the SAP2 gene sequence is relatively conserved and the mutation is rare, so the mutation of the SAP2 gene might not be related to the drug resistance of C. albicans [32]. However, in GCN4 sequencing, it was found that four strains, belonged to ITR resistant strains, appeared missense mutation (T36S), and 12 strains, including 8 resistant strains and 4 sensitive strains, had synonymous mutation. T36S mutation has not been reported, and because the mutation only occurred in the ITR resistant strains, we hypothesized that T36S mutation might be associated with ITR resistance. However, the relationship between T36S mutation and ITR resistance remains to be further investigated, and whether the GCN4 mutation (T36S) has an effect on the GCN4 activity also warrants to be tested from other levels.
The formation of C. albicans biofilms mainly includes cell adhesion, cell proliferation, mycelium and extracellular matrix formation, and cell dispersion [33], which is regulated by a core transcription network [34]. Our study showed that the biofilm formation ability of drug-resistant C. albicans strains was higher than that of sensitive strains. The Saps family has been proved to provide C. albicans with nutrition by degrading proteins, help cells escape immune responses, and contribute to cell adhesion, initiative penetration, and invasion of host cells [35, 36]. Meanwhile, the Saps family plays vital roles in the formation and maintenance of C. albicans mycelia, which is essential for the formation of biofilms. SAP2 is a proteolytic enzyme of C. albicans that aids in adhesion, tissue invasion, and mucosal and systemic infections. A previous study showed that exposure to nicotine could enhance the formation of C. albicans biofilm, accompanied with the higher expression of SAP2 in C. albicans [37]. Another study demonstrated that magnolol could reduce the adhesion, inhibit the formation of mycelium, weaken the vitality and spatial structure of biofilm, as well as decreased cell wall integrity and β-glucan content of C. albicans through down-regulating the expression of various virulence genes, including SAP2, CEK1, MKC1, RHO1, and CDC42. GCN4 expression was significantly up-regulated after the stimulation of various stress conditions, and the life span of yeast was prolonged through inhibiting the synthesis of proteins at translation initiation and transcription stage [38–40]. These reports demonstrated the importance of SAP2 and GCN4 in the pathogenicity and drug resistance of C. albicans.
In the current study, the expression of SAP2 and GCN4 was up-regulated in the ITR-resistant strains under planktonic/biofilm conditions compared with the sensitive strains, and SAP2 and GCN4 were highly expressed in C. albicans in biofilm state relative to the strains in planktonic state. A previous study of Sundaram et al. [24] established GCN4 mutation strains to study the effects of GCN4 on biofilm formation, and found that under the condition of amino acid starvation, GCN4 activity was enhanced, which promoted mycelia differentiation and biofilm formation of C. albicans. This research also illustrated from another perspective that high expression of GCN4 could inhibit the biosynthesis of amino acids, thus promoting the mycelia differentiation and biofilm formation of C. albicans. Our previous study has confirmed that the activity/expression of SAP2 was higher in the resistant strains compared to the sensitive strains, and through the in vivo mouse bacteremia model, the survival rate of the mice treated with resistant strains was lower than that administrated with sensitive strains [41]. These reports, together with our result, it can be inferred that high expression of SAP2 and GCN4 is found in the resistance of C. albicans to ITR under planktonic and biofilm states.
In addition, a previous study used Pearson’s correlation coefficient to observe that there were positive correlations between SAP2 and MDR1, and between CDR1 and CDR2 [41]. Another study also used Pearson’s correlation coefficient to show that there was a positive liner correlation between SAP2 and ERG11 mRNA expression (r = 0.6655, P < 0.001) [31]. In our study, we employed Spearman’s correlation analysis to analyze the correlation between SAP2 and GCN4 in C. albicans under planktonic and biofilm conditions. It was found that SAP2 and GCN4 mRNA expression had significantly positive correlation (P < 0.01) under planktonic (r = 0.693) and biofilm conditions (r = 0.622); which indicated that there may be positive correlation between SAP2 and GCN4 expression in C. albicans under different conditions. However, the specific relationship between SAP2 and GCN4 in ITR-resistant strains also should be further investigated.
However, there are some limitations in this study. Firstly, the relationship between T36S mutation and ITR resistance in C. albicans remains to be further investigated, and whether the GCN4 mutation (T36S) has an effect on the GCN4 activity also warrants to be tested from the protein level. Additionally, our conclusions need to be verified in a larger sample size, and confirmed by other techniques, such as gene knockout, chromatin immunoprecipitation, and fluorescent reporter gene detection.
Conclusion
In conclusion, no missense mutation of SAP2 gene was found in clinical C. albicans, while T36S mutation of GCN4 gene appeared in ITR-resistant strains. ITR-resistant C. albicans strains had stronger ability of biofilm formation. In addition, higher expression levels of SAP2 and GCN4 were observed in the ITR-resistant strains of C. albicans under planktonic and biofilm conditions, as well as there was a positive correlation between SAP2 and GCN4 mRNA expression. Our study preliminarily elaborated the relationships between SAP2 and GCN4, and the biofilm formation and ITR resistance of C. albicans, as well as laid a foundation for the development of new antifungal drugs to treat IC.
Author contribution
Wenli Feng and Jing Yang designed the research. Wenli Feng, Jing Yang, Yan Ma, Luwen Zhang, and Rong Yin did the experiment and obtained the data. Zusha Qiao, Ying Ji, and Yong’an Zhou analyzed and explained the data. Wenli Feng and Jing Yang drafted and revised the manuscript. All authors have read and approved the final version.
Funding
This study was supported by the General Project of the National Natural Science Foundation of China (Project number: 82072262), and Research and Development Key Projects of Shanxi Province (Project number: 201903D321123), Scientific and Technological Activities funding program for Overseas Students of Shanxi Province (Project number:20210030), and Research Project Supported by Shanxi Scholarship Council of China (Project number: 2020–190).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
This study was approved by the Ethics Committee of the Second Hospital of Shanxi Medical University (approval no. (2019)YX no. 073).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Responsible Editor: Rosana Puccia
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wenli Feng, Email: fengwenli@sxmu.edu.cn, Email: wenlifeng2010@163.com.
Jing Yang, Email: yangjing7962@126.com.
References
- 1.Li D, Li T, Bai C, et al. A predictive nomogram for mortality of cancer patients with invasive candidiasis: a 10-year study in a cancer center of North China. BMC Infect Dis. 2021;21(1):021–05780. doi: 10.1186/s12879-021-05780-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srivastava V, Singla RK, Dubey AK. Emerging Virulence, Drug resistance and future anti-fungal drugs for candida pathogens. Curr Top Med Chem. 2018;18(9):759–778. doi: 10.2174/1568026618666180528121707. [DOI] [PubMed] [Google Scholar]
- 3.Pinto-Magalhães S, Martins A, Lacerda S, et al. Candidemia in a Portuguese tertiary care hospital: analysis of a 2-year period. J Mycol Med. 2019;29(4):320–324. doi: 10.1016/j.mycmed.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Priyadarshini E, Rawat K, Prasad T, et al. Antifungal efficacy of Au@ carbon dots nanoconjugates against opportunistic fungal pathogen, Candida albicans. Colloids Surf B Biointerfaces. 2018;163:355–361. doi: 10.1016/j.colsurfb.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Pristov KE, Ghannoum MA. Resistance of Candida to azoles and echinocandins worldwide. Clin Microbiol Infect. 2019;25(7):792–798. doi: 10.1016/j.cmi.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 6.Peyton LR, Gallagher S, Hashemzadeh M. Triazole antifungals: a review. Drugs Today. 2015;51(12):705–718. doi: 10.1358/dot.2015.51.12.2421058. [DOI] [PubMed] [Google Scholar]
- 7.Lee Y, Puumala E, Robbins N, et al. Antifungal drug resistance: molecular mechanisms in Candida albicans and beyond. Chem Rev. 2021;121(6):3390–3411. doi: 10.1021/acs.chemrev.0c00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vila T, Romo JA, Pierce CG, et al. Targeting Candida albicans filamentation for antifungal drug development. Virulence. 2017;8(2):150–158. doi: 10.1080/21505594.2016.1197444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong S, Shi H, Cao D, et al. Novel nanoscale bacteriophage-based single-domain antibodies for the therapy of systemic infection caused by Candida albicans. Sci Rep. 2016;6:32256. doi: 10.1038/srep32256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aoki W, Kitahara N, Miura N, et al. Candida albicans possesses Sap7 as a pepstatin A-insensitive secreted aspartic protease. PLoS One. 2012;7(2):27. doi: 10.1371/journal.pone.0032513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong G, Liu Y, Wu Y, et al. Novel non-peptidic small molecule inhibitors of secreted aspartic protease 2 (SAP2) for the treatment of resistant fungal infections. Chem Commun. 2018;54(96):13535–13538. doi: 10.1039/C8CC07810F. [DOI] [PubMed] [Google Scholar]
- 12.Lohse MB, Gulati M, Johnson AD, et al. Development and regulation of single- and multi-species Candida albicans biofilms. Nat Rev Microbiol. 2018;16(1):19–31. doi: 10.1038/nrmicro.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponde NO, Lortal L, Ramage G, et al. Candida albicans biofilms and polymicrobial interactions. Crit Rev Microbiol. 2021;47(1):91–111. doi: 10.1080/1040841X.2020.1843400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desai JV, Mitchell AP, Andes DR (2014) Fungal biofilms, drug resistance, and recurrent infection. Cold Spring Harb Perspect Med 4(10):a019729 [DOI] [PMC free article] [PubMed]
- 15.Barros PP, Ribeiro FC, Rossoni RD, et al. Influence of Candida krusei and Candida glabrata on Candida albicans gene expression in in vitro biofilms. Arch Oral Biol. 2016;64:92–101. doi: 10.1016/j.archoralbio.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Tsui C, Kong EF, Jabra-Rizk MA (2016) Pathogenesis of Candida albicans biofilm. Pathog Dis 74(4):ftw018 [DOI] [PMC free article] [PubMed]
- 17.Vitális E, Nagy F, Tóth Z, et al. Candida biofilm production is associated with higher mortality in patients with candidaemia. Mycoses. 2020;63(4):352–360. doi: 10.1111/myc.13049. [DOI] [PubMed] [Google Scholar]
- 18.Monika S, Małgorzata B, Zbigniew O. Contribution of aspartic proteases in Candida virulence. Protease inhibitors against Candida infections. Curr Protein Pept Sci. 2017;18(10):1050–62. doi: 10.2174/1389203717666160809155749. [DOI] [PubMed] [Google Scholar]
- 19.Hinnebusch AG. Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol. 2005;59:407–450. doi: 10.1146/annurev.micro.59.031805.133833. [DOI] [PubMed] [Google Scholar]
- 20.Rana A, Gupta N, Thakur A. Post-transcriptional and translational control of the morphology and virulence in human fungal pathogens. Mol Aspects Med. 2021;81(101017):5. doi: 10.1016/j.mam.2021.101017. [DOI] [PubMed] [Google Scholar]
- 21.Bharadwaj PR, Martins RN. Autophagy modulates Aβ accumulation and formation of aggregates in yeast. Mol Cell Neurosci. 2020;104(103466):18. doi: 10.1016/j.mcn.2020.103466. [DOI] [PubMed] [Google Scholar]
- 22.Lee YT, Fang YY, Sun YW, et al. THR1 mediates GCN4 and CDC4 to link morphogenesis with nutrient sensing and the stress response in Candida albicans. Int J Mol Med. 2018;42(6):3193–3208. doi: 10.3892/ijmm.2018.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sundaram A, Grant CM. Oxidant-specific regulation of protein synthesis in Candida albicans. Fungal Genet Biol. 2014;67:15–23. doi: 10.1016/j.fgb.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Sundaram A, Grant CM. A single inhibitory upstream open reading frame (uORF) is sufficient to regulate Candida albicans GCN4 translation in response to amino acid starvation conditions. RNA. 2014;20(4):559–567. doi: 10.1261/rna.042267.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.García-Sánchez S, Aubert S, Iraqui I, et al. Candida albicans biofilms: a developmental state associated with specific and stable gene expression patterns. Eukaryot Cell. 2004;3(2):536–545. doi: 10.1128/EC.3.2.536-545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamthan M, Mukhopadhyay G, Chakraborty N, et al. Quantitative proteomics and metabolomics approaches to demonstrate N-acetyl-D-glucosamine inducible amino acid deprivation response as morphological switch in Candida albicans. Fungal Genet Biol. 2012;49(5):369–378. doi: 10.1016/j.fgb.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez DL, Quail MM, Hernday AD, et al. Transcriptional circuits regulating developmental processes in Candida albicans. Front Cell Infect Microbiol. 2020;10:605711. doi: 10.3389/fcimb.2020.605711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Candel FJ, Pazos Pacheco C, Ruiz-Camps I, et al. Update on management of invasive candidiasis. Rev Esp Quimioter. 2017;30(6):397–406. [PubMed] [Google Scholar]
- 29.Boija A, Klein IA, Sabari BR, et al. Transcription factors activate genes through the phase-separation capacity of their activation domains. Cell. 2018;175(7):1842–1855. doi: 10.1016/j.cell.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandra J, Kuhn DM, Mukherjee PK, et al. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001;183(18):5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng W, Yang J, Ma Y, et al. The effects of secreted aspartyl proteinase inhibitor ritonavir on azoles-resistant strains of Candida albicans as well as regulatory role of SAP2 and ERG11. Immun Inflamm Dis. 2021;9(3):667–680. doi: 10.1002/iid3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu N, Zhang N, Zhang S, et al. Phloretin inhibited the pathogenicity and virulence factors against Candida albicans. Bioengineered. 2021;12(1):2420–2431. doi: 10.1080/21655979.2021.1933824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gulati M, Lohse MB, Ennis CL, et al. In vitro culturing and screening of Candida albicans biofilms. Curr Protoc Microbiol. 2018;50(1):11. doi: 10.1002/cpmc.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pereira R, Dos Santos Fontenelle RO, de Brito EHS, et al. Biofilm of Candida albicans: formation, regulation and resistance. J Appl Microbiol. 2021;131(1):11–22. doi: 10.1111/jam.14949. [DOI] [PubMed] [Google Scholar]
- 35.Chin VK, Lee TY, Rusliza B et al (2016) Dissecting Candida albicans infection from the perspective of C. albicans virulence and omics approaches on host-pathogen interaction: a review. Int J Mol Sci 17(10):1643 [DOI] [PMC free article] [PubMed]
- 36.Höfs S, Mogavero S, Hube B. Interaction of Candida albicans with host cells: virulence factors, host defense, escape strategies, and the microbiota. J Microbiol. 2016;54(3):149–169. doi: 10.1007/s12275-016-5514-0. [DOI] [PubMed] [Google Scholar]
- 37.Haghighi F, Andriasian L, Tran NC et al (2022) Effect of Cigarette and e-cigarette smoke condensates on Candida albicans biofilm formation and gene expression. Int J Environ Res Public Health 19(8):4626. 10.3390/ijerph19084626 [DOI] [PMC free article] [PubMed]
- 38.Hu Z, Xia B, Postnikoff SD, et al. Ssd1 and Gcn2 suppress global translation efficiency in replicatively aged yeast while their activation extends lifespan. Elife. 2018;17(7):35551. doi: 10.7554/eLife.35551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mittal N, Guimaraes JC, Gross T, et al. The Gcn4 transcription factor reduces protein synthesis capacity and extends yeast lifespan. Nat Commun. 2017;8(1):017–00539. doi: 10.1038/s41467-017-00539-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen ZJ, Postnikoff S, Tyler JK. Is Gcn4-induced autophagy the ultimate downstream mechanism by which hormesis extends yeast replicative lifespan? Curr Genet. 2019;65(3):717–720. doi: 10.1007/s00294-019-00936-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng W, Yang J, Pan Y, et al. The correlation of virulence, pathogenicity, and itraconazole resistance with SAP activity in Candida albicans strains. Can J Microbiol. 2016;62(2):173–178. doi: 10.1139/cjm-2015-0457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.