Abstract
Objective
To perform a systematic review and meta-analysis comparing two pre-operative transfusion regimens (conservative versus aggressive) in children with sickle cell disease(SCD) undergoing adenotonsillectomy in terms of post-operative complications, complications related to SCD and transfusion related complications.
Data Sources and Review Methods
A literature review was performed through PubMed, EMBASE, Cochrane, and Ovid databases using the following phrases: (Adenotonsillectomy OR Tonsillectomy) AND (Sickle Cell Disease OR Sickle Cell Trait). Using predetermined inclusion and exclusion criteria, seven articles were selected for systemic review and two control trials were included in meta-analysis.
Results
Out of a total of 3,146 results, seven articles were selected for review and two controlled trials were included in the meta-analysis. There was no statistically significant difference in the rate of primary and secondary hemorrhage between the aggressive and conservative transfusion regimens (RR = 3.1, CI = 0.84–11.4, p-value = 0.089). The rate of sickle cell disease related complications including vaso-occlusive crisis and acute chest syndrome was also not statistically significant between the two transfusion groups (RR = 1.4, CI = 0.7–2.8, p-value = 0.339). Even though, the transfusion related complications did not reach statistical significance, there was a higher complication rate in the group receiving aggressive blood transfusion.
Conclusion
In SCD children undergoing adenotonsillectomy, an aggressive transfusion regimen that focuses on reducing the Hemoglobin S ratio to below 30% has not been shown to be more effective in reducing post-operative complications when compared to a conservative transfusion regimen. Therefore, it is reasonable to utilize a conservative transfusion regimen, thereby reducing the transfusion-associated risks.
Keywords: Sickle cell disease, Tonsillectomy, Adenoidectomy, Adenotonsillectomy, Transfusion, Children, Pediatric, Bleeding, Tonsil, Systematic review
Introduction
Sickle cell disease (SCD) is an autosomal recessive hematologic disorder that occurs due to a single missense point mutation in the DNA of the β-hemoglobin chain, resulting in the substitution of glutamic acid for valine at position 6 of the β-chain. As a result, the hemoglobin molecule transforms from its normal hydrophilic state to a more hydrophobic one. Consequently, the mutated hemoglobin molecules are prone to form large insoluble, occlusive aggregates.[1]. Clinically, these aggregates can cause various clinical presentations, such as chronic hemolytic anemia, vaso-occlusive crises, and gross organ infarction – particularly in the setting of hypoxia, dehydration, and acidosis. Thus, patients with SCD who undergo elective surgery have been shown to be at an increased risk for post-operative complications.[2].
The pre-operative management of SCD patients has been widely debated although no standardized guidelines currently exist. Pre-operative blood transfusions can decrease flow viscosity, increase the total oxygen-carrying capacity, and suppress the production of Hemoglobin S (HbS), thereby minimizing post-operative complications by limiting the amount of peri-operative sickling.[3] Despite the general benefits attributed to pre-operatively transfusing SCD patients, a clear consensus regarding the indications for pre-operative blood transfusions in patients undergoing tonsillectomy has not been established. An overwhelming number of clinical studies have based the validity of pre-operative transfusion on the in vitro data that showed that the flexibility of blood is almost normal when the red blood cell (RBC) mixture contains less than 40% HbS and that clinically vaso-occlusive crisis occurs only when the level of HbS exceeds 50%.[4] Early studies advocated an aggressive transfusion regimen to a HbS ratio below 30% in addition to increasing the hemoglobin (Hgb) level to more than 10 g/dL.[3, 5, 6] However, with improvement in monitoring strategies and better post-operative care, the need for an aggressive protocol has been questioned. [4, 7, 8] A more conservative protocol involving solely increasing the Hgb level to more than 10 g/dL has been shown to be as effective as an aggressive transfusion regimen.[4] The vast majority of clinical studies which compare surgery with and without pre-operative transfusion are retrospective, predisposing them to randomization bias. Another limitation of these studies is the inclusion of various surgical procedures that are not limited to adenotonsillectomy or pediatric populations, causing difficulty in drawing conclusions. A recent Cochrane review regarding the pre-operative transfusion in SCD patients undergoing elective surgical procedures focused on all elective surgeries and included data from adult populations.[4] The purpose of the present study is to do a systematic review and metanalysis regarding the role of preoperative blood transfusion, specifically in children with sickle cell disease undergoing adenotonsillectomy.
Methods
The objective of this study was to determine, based on the current literature, whether an aggressive transfusion regimen is equivalent or superior to a conservative regimen with regards to reducing the complication rates in children with SCD undergoing adenotonsillectomy. The specific parameters compared included: overall mortality, post-operative complications (primary and secondary hemorrhage), complications related to SCD (acute chest syndrome and vaso-occlusive crisis), and transfusion related complications. An aggressive transfusion regimen was defined as a regimen with the objective of getting HbS level less than 30% in addition to increasing the hemoglobin (Hgb) level to more than 10 g/dL. A conservative regimen was defined as a regimen with the objective of having only the Hgb level greater than 10 g/dL, regardless of the HbS percentage.
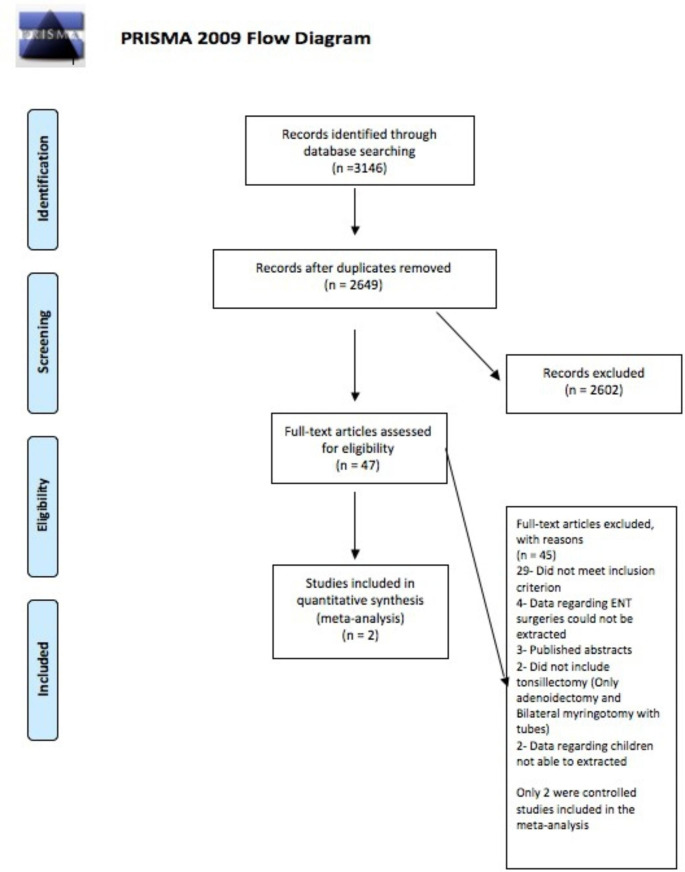
Institutional review board approval was sought for the present study, and the study was found to be exempt. Two independent authors performed a comprehensive literature search in PubMed, EMBASE, Cochrane and Ovid databases using the following phrases: (Adenotonsillectomy OR Tonsillectomy) AND (Sickle Cell Disease OR Sickle Cell Trait) from inception to June 2021. The title and the abstract were screened to determine eligibility based on predefined inclusion criteria, which are outlined in detail below. Clearly irrelevant studies were excluded at this stage. The references of the articles were examined for additional studies that could be included in this analysis. The literature search followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA)[9] (Fig. 1).The inclusion criteria for this study were: 1. A primary research study (a controlled trial or observation study, including case series and case reports). 2.The study included data on pediatric population. 3.Patients diagnosed with SCD.4. The study included data on adenotonsillectomy.5. The literature was composed in the English language. 6. The study was not a duplicate study or a study from the same data set. The exclusion criteria for this study were: (1) Any duplicate studies. (2) Studies in which adenotonsillectomy specific data could not be extracted. 3.Any studies in which pediatric specific data could not be extracted.
Fig. 1.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) showing pathway of inclusion of the studies
Data was extracted from these studies and confirmed by a second author for accuracy. The review authors were not blinded to the names of the authors, institutions, journals or trial outcome. Any disagreement was resolved by consensus. In studies which included other surgeries, the data regarding tonsillectomy/adenoidectomy was extracted. In studies with mixed adult and pediatric populations, data specific to pediatric population was extracted.
Assessment of Quality, Level of Evidence and Risk Bias
The level of evidence was assessed using the Oxford Center for Evidence-Based Medicine Levels.[10] The risk of selection, performance, detection, attrition, and reporting bias in case series were assessed by using a scoring system, which has previously been used for systematic reviews and includes overall scores from 0 (low risk) to 5 (high risk). The following parameters were evaluated using this specific scoring system: sample selection (consecutive or not: 1 indicated no or not stated and 0 indicated consecutive); diagnostic criteria stated (1 indicated not stated and 0 indicated stated); outcomes measured consistently for all patients (1 indicated not consistent and 0 indicated consistent); outcomes reported consistently for all patients (1 indicated not consistent and 0 indicated consistent); and follow-up period of 1 year or more (1 indicated < 1 year and 0 indicated 1 year).
Statistical Analysis
All analyses were conducted using SAS 9.4 software (Cary, NC). To test for differences in complication rates between the 2 transfusion regimens we used generalized linear mixed modeling framework with a logit link. A random effect model was included to account for study clustering. Values were considered significant at the level of p < 0.05.
Results
Study Characteristics
Using our search strategy, a total of 3,146 results were obtained. After excluding duplicates and studies irrelevant to our research question, 47 articles were short listed. Out of these, 8 articles were selected for the systematic review. The 7 studies included in the systematic review have been described in Table 1. Most of the studies on the role of transfusion in adenotonsillectomy are retrospective cohorts with high risk of bias. Only two controlled trials have been done comparing aggressive vs. conservative transfusion regimen and were included in the meta-analysis. Prominent trials which were excluded from the evaluation entirely were the TAPS trial from Howard et al.6, which was not included in the analysis because the ENT surgeries mentioned included adenoidectomy alone; the Vichinsky study4, which included adults and multiple surgical procedures, making it difficult to extract data relevant to adenotosillectomy in children ; and the Al-Jaouni trial[11], which also included data from adults and other surgical procedures making it difficult to extract data specific to our question. The Wali study8, even though non-randomized, was included in the meta-analysis as it specifically pertained to adenotonsillectomy.
Table 1.
Characteristics of the 7 studies included in the systematic review. The studies included in the metanalysis have been highlighted. N/A: Not available; ACS: Acute Chest Syndrome
| Study | Total | Mean Age(Y) | Surgical Method | Type of Study | Risk of Bias | Level of evidence |
Pre-op Hgb (g/dL) | Pre-op HbS | Complications | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | ACS | Pain Crisis | Bleeding | Fever | Atelectasis | Hypoxia | Other | ||||||||||
|
Coker5 (1982) |
9 | 14.4 | Dissection 100% | Retrospective Cohort | 4 | 4 | > 10 | < 45% | 11% | 11% | N/A | N/A | N/A | N/A | N/A | ||
|
Derkay 12 (1991) |
10 | 9 | Dissection or Bovie | Retrospective Cohort | 4 | 4 | > 10 | < 30% | 0% | N/A | N/A | N/A | N/A | N/A | N/A | ||
|
Griffin 2 (1993) |
Total: 74 T& A: 9 |
13 | N/A | Retrospective Cohort | 4 | 4 | > 10 | 56% | 11% | 0% | 0% | 33% | 11% | N/A | |||
| Halvorson (1997) 3 | 75 | 8.7 |
Snare: 5.3% Cold knife: 2.7% KTP: 2.7% Bovie: 89.3% |
Retrospective Cohort | 4 | 4 | > 10 | < 40% | N/A | 9.4% | 4% | 2.7% | 8% | 10.7% | 20% | ||
|
Waldron (1999) 7 |
107 | 9.23 | N/A | Randomized Controlled Trial | 1 | 1b |
Group 1: >10 Group 2: >10 |
Group 1: <30% Group 2: n/a |
22% | 13% | 1.8% | 2.8% | 4.7% | N/A | N/A | ||
|
Wali (2003) 8 |
39 | 9.56 | Dissection-LigaSure | Non-Randomized Controlled Trial | 2 | 2b |
Group 1: >10 Group 2: >10 |
Group 1: <30% Group 2: n/a |
30% | 5% | 18% | 2.5% | 50% | N/A | N/A |
5% (Infection) |
|
|
Duke (2006) 1 |
41 | 8.6 | N/A | Retrospective Outcome measures | 4 | 4 | > 10 | 22% | 8% | 0% | 0% | 10% | N/A | 20% |
2.4% (Airway fire) |
||
Metanalysis
Patient and Surgery Characteristics
Waldron et al.7 conducted a multi-institutional, randomized, controlled trial involving a total of 107 patients undergoing tonsillectomy/adenotonsillectomy with a mean age of 9.23 years. The most common indication was obstructive sleep apnea. The surgical method of tonsillectomy was not mentioned in the study. The patients in this study were randomized into one of two groups. Group one with 48 patients, underwent an aggressive transfusion regimen. Group two, with 59 patients underwent a conservative transfusion regimen. Wali et al.8 studied 39 patients with a mean age of 9.56 years who underwent an adenotonsillectomy by dissection method. Indications for surgery included recurrent tonsillitis, obstructive sleep apnea, or adenoid hypertrophy with an upper airway obstruction. The patients were randomized into one of two groups like the Waldron’s study. Group one, with 14 patients, underwent an aggressive transfusion regimen, while group two, with 25 patients, underwent a conservative transfusion regimen.
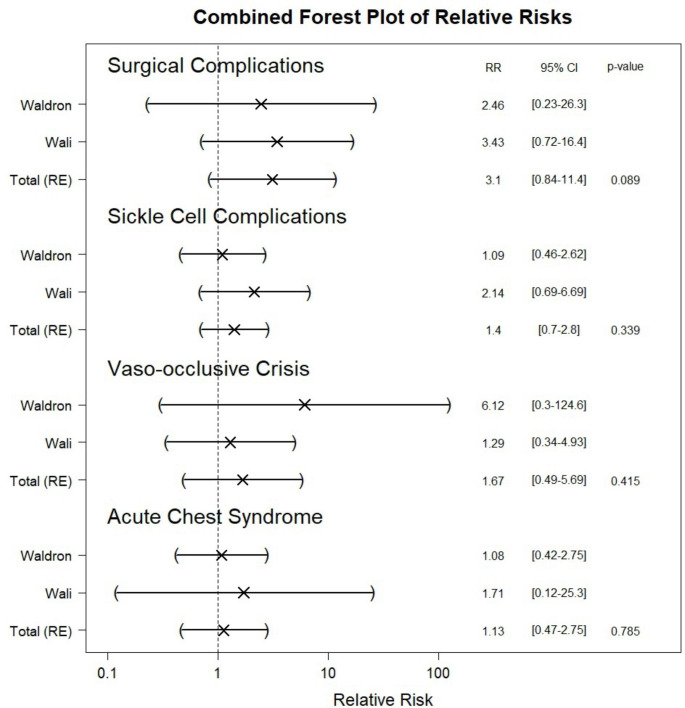
Outcome Analysis: (Fig. 2)
Fig. 2.
Combined forest plot of relative risks of the surgical complications, sickle cell disease related complications in aggressive versus conservative transfusion regimens. (RR = relative risk, CI = Confidence interval)
Primary Outcomes:
Perioperative mortality: No case of death was reported in children undergoing adenotonsillectomy in any of the studies.
Post-operative complications: Post-operative complications included primary and secondary hemorrhage. There was no statistically significant difference between the aggressive and conservative transfusion regimens. (RR = 3.09, CI = 0.84–11.43, p-value = 0.089)
-
Complications related to sickle cell disease: SCD complications included acute chest syndrome and vaso-occlusive crisis. There was no significant difference in the overall rate of complications between the two transfusion regimens. (RR = 1.402, CI = 0.702–2.803, p-value = 0.339)
- Acute Chest Syndrome: Looking specifically at the relative risk of developing post-operative acute chest syndrome between aggressive versus conservative regimen, there was again no statistically significant difference. (Relative Risk = 1.13, Confidence Interval = 0.46–2.74, p-value = 0.785).
- Vaso-occlusive crisis: The relative risk of developing post-operative vaso-occlusive crisis was also not statistically different. (Relative Risk = 1.66, Confidence Interval = 0.488–5.685, p-value = 0.415).
Transfusion related complications: Transfusion-related complications were only reported in the Waldron study. Rates were higher in the aggressive transfusion group. At total of 6 out of 48 patients (12.5%) had transfusion-related complications in the aggressive group while only 2 out of 59 patients (3.3%) in the conservative group suffered from transfusion related complications. Even though the aggressive group had a higher complication rate, it did not reach significance. (Relative Risk = 3.69, p-value = 0.09).
Discussion
Tonsillectomy has been shown to be a safe and effective option to treat obstructive sleep apnea in children with SCD, associated with a reduction in apnea hypopnea index and postoperative emergency room visits. [13] The peri-operative management of this subgroup of patients is unique due to the risk of precipitating a sickle cell crisis. Generally, pre-operative transfusion is recommended in the form of either multiple simple red blood cell transfusions or exchange transfusions. In one of the largest studies on SCD children undergoing tonsillectomy, using a national pedaitric inpatient database, Atwood et al. noticed a significant positive linear trend for the proportion of patients receiving blood transfusion over time from 1997 to 2012. [14] Even though, it is recommended that children with sickle cell anemia receive RBCs transfusion to bring the hemoglobin level to 10 g/dL prior to undergoing surgery, the transfusion protocol remains controversial. [14] Two approaches have been reported based on the absolute hemoglobin level and percentage of HbS. A conservative transfusion is one which corrects anemia by increasing the Hgb levels to more than 10 g/dL without regard for the level of HbS. A more aggressive transfusion approach is where the goal is to correct anemia and reduce the percentage of HbS to less than 30%. The rationale for transfusing SCD patients until the percentage of HbS is less than 30% is to increase the proportion of normal HbA-containing RBCs to a level that will inhibit sickling and the consequent blockage of the microcirculation. Our goal was to review the existing data to see which transfusion regimen will be safe and effective, specifically in children undergoing adenotonsillectomy. A total of 7 studies [1–3, 5, 7, 8, 12] were found to be relevant to the subject and were systematically analyzed. The published literature consists predominantly of single institution, uncontrolled studies using a wide variety of transfusion management strategies. No prospective randomized controlled trial has evaluated the role of routine pre-operative transfusions for these patients.
The role of transfusion in SCD patients undergoing elective surgery has been established by numerous studies. Griffin et al. retrospectively reviewed 74 SCD patients who underwent a variety of major and minor surgical procedures, without preoperative blood transfusion.[2] The rate of complications in children undergoing adenotonsillectomy was close to 56% and included fever, atelectasis and one incident of acute chest syndrome. They identified adenotonsillectomy as one of three procedures that was associated with a higher rate of post-operative complications and recommended routine preoperative transfusion. However, in a large retrospective review of a national inpatient database, Atwood et al. did not find any difference in the rate of complications between the transfused and the non-transfused group. [14]However, the study was limited by its inability to conclude if blood transfusion was given prior to, during, or after tonsillectomy. A 2014 Expert Panel Report based on existing literature recommended that adults and children with sickle cell anemia receive RBCs transfusion to bring the hemoglobin level to 10 g/dL prior to undergoing surgery with general anesthesia.[15] However, the transfusion protocol remains controversial.
Coker et al.[5 ]in a retrospective review of 9 patients undergoing tonsillectomy by dissection method who received an aggressive transfusion regimen to reduce the HbS level to less than 45% and to achieve a Hgb level of 10 g/dL, reported only one patient with a vasocclusive crisis. Derkay et al.[12] reported no complications following an aggressive transfusion regimen (Hgb,10 g/dl, HbS level < 30%) in a retrospective review of 10 patients who underwent adenotonsillectomy or tonsillectomy with the use of either a Bovie or a dissection technique. Similar to earlier studies, Halvorson et al.[3] followed an aggressive transfusion regimen (Hgb > 10 g.dl, HbS ratio < 40%) in 75 children undergoing tonsillectomies with or without adenoidectomies and reported complications including hypoxia (20%), fever (8%), atelectasis (10.7%), pneumonia (6.7%), pain crisis (4%) and acute chest syndrome (2.7%). This group identified two statistically significant risk factors associated with an increase in post-operative complications: pre-operative HbS levels greater than 40% and patients less than 4 years of age and supported pre-operatively transfusing all SCD patients undergoing tonsillectomy to an HbS ratio of less than 40%. While most of the studies were concluding the reduction in complications using an aggressive regime, Vinchinsky et al., in a multicentric study, challenged the idea that an aggressive transfusion regimen, was necessary to reduce post-operative complications. They compared the aggressive regimen to a conservative approach in patients undergoing a variety of elective procedures and concluded that a conservative transfusion regimen is as effective as an aggressive regimen in preventing perioperative complications; furthermore, a conservative approach reduces the risk of transfusion-associated complications by 50%.[4].
The two controlled trials which formed the basis of the current metanalysis are the Waldron and the Wali trial. Waldron et al.[7]conducted a multi-institutional, randomized, controlled trial involving a total of 107 patients undergoing a tonsillectomy/adenotonsillectomy. The surgical method of tonsillectomy was not mentioned in the study. The patients in this study were randomized into two groups with one group of 48 patients undergoing an aggressive transfusion regimen to obtain a pre-operative Hgb level of 10 g/dL and a HbS < 30%. while another group of 59 patients undergoing a conservative transfusion regimen to obtain a Hgb level of 10 g/dL regardless of the HbS percentage. Complications were seen in approximately 21% of patients in both groups. There were no significant differences between the randomized groups in frequencies of complications. Wali et al.[8 ]studied 39 patients with a mean age of 9.56 years who underwent an adenotonsillectomy by dissection method. Like Waldron’s study, the patients were randomized into one of two groups with 14 patients, undergoing an aggressive transfusion regimen, and 25 patients, a conservative transfusion regimen. Complications were seen in approximately 30% of patients in both groups. No statistically significant difference in the incidence or severity of complications was identified between the two groups. In the meta-analysis of the only two controlled studies in the current literature, we found no significant difference between an aggressive transfusion regimen and a conservative regimen when looking at post-operative complications, complications related to SCD and transfusion-related complications. Although there were higher rates of transfusion-related complications in the aggressive transfusion group, this correlation did not reach statistical significance.
Most of the studies dealing with the indication of preoperative transfusion in children with sickle cell disease undergoing adenotonsillectomy are single institutional studies with a high risk on bias. Only one randomized control trial comparing both the transfusion regimens has been conducted. However, the major limitation of the trial is the lack of documentation concerning the technique of tonsillectomy which can be an important factor in the post-operative complication rate. Wali et al. used a uniformed technique of dissection but did not randomize the samples receiving conservative and aggressive therapy.8 Even though earlier studies point to the reduction in complications using an aggressive regimen, the randomized trial and the current metanalysis fail to justify the need of aggressively transfusing children with SCD undergoing adenotonsillectomy. Unnecessary aggressive regimen can cause transfusion related complications and increase the risk of cardiovascular overload, but also escalate the percentage of HbA to at least 55%, which may cause increased blood viscosity inducing a vaso-occlusive crisis.[13].
The current metanalysis identifies a gap in knowledge regarding transfusion regimens for SCD children undergoing adenotonsillectomy. Most of the studies are single institutional with a high risk of bias. Even though multi-institutional randomized studies have been undertaken, they combine data from other elective procedures. There are a variety of confounding factors which make children undergoing adenotonsillectomy different from children undergoing other elective surgeries. Inadequate pain management can result in dehydration and precipitation of a crisis while the surgical technique can influence postoperative bleeding rates.
The current systematic review is not without limitations. Apart from heterogeneity of studies, inherent bias of retrospective review and selection bias regarding data extraction exits. None of the randomized trials specifically focus on adenotonsillectomy. Selection bias in excluding studies in which we could not extract data is also a limitation of the current metanalysis. However, the current study adds important information regarding our understanding of the transfusion regimen for SCD children undergoing adenotonsillectomy. Based on the current evidence, a conservative transfusion regimen appears to be as effective in avoiding sickle cell disease related complications while reducing transfusion related complications, compared to an aggressive regimen.
Conclusion
With respect to current evidence, pre-operative transfusions seem to be indicated for SCD patients undergoing adenotonsillectomy whose Hgb level is less than 10 g/dL. An aggressive transfusion regimen, which focuses on reducing the HbS ratio to below 30%, has not been shown to be more effective in reducing post-operative complications when compared to a conservative regimen. While recent data supports less aggressive transfusion regimens, it remains controversial. There are limited randomized controlled studies, most of which carry a high level of bias and poor quality of evidence. Additional randomized controlled studies are needed to better determine the role of pre-operative transfusions in SCD children undergoing adenotonsillectomy.
Financial Disclosure
No external funding was received in association with this study, and no financial interests are held by any of the authors.
Conflict of Interest
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Duke RL, Scott JP, Panepinto JA, Flanary VA. Perioperative management of sickle cell disease children undergoing adenotonsillectomy. Otolaryngol Head Neck Surg. 2006;134:370–373. doi: 10.1016/j.otohns.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Griffin TC, Buchanan GR. Elective Surgery in Children with Sickle Cell Disease Without Preoperative Blood Transfusion(1993).J Pediatr. 28:681–685 [DOI] [PubMed]
- 3.Halvorson DJ, McKie V, McKie K, et al. Sickle Cell Disease and Tonsillectomy. Arch Otolaryngol Head Neck Surg. 1997;123:689–692. doi: 10.1001/archotol.1997.01900070033005. [DOI] [PubMed] [Google Scholar]
- 4.Vichinsky EP, Haberkern CM, Neumayr L, et al. A Comparison of Conservative and Aggressive Transfusion Regimens in the Perioperative Management of Sickle Cell Disease. N Engl J Med. 1995;333:206–213. doi: 10.1056/NEJM199507273330402. [DOI] [PubMed] [Google Scholar]
- 5.Coker N, Milner P. Elective Surgery in Patients with Sickle Cell Anemia. Arch Otolaryngol. 1982;108:574–576. doi: 10.1001/archotol.1982.00790570040010. [DOI] [PubMed] [Google Scholar]
- 6.Howard J, Malfroy M, Llewelyn C, et al. The Transfusion Alternatives Preoperatively in Sickle Cell Disease (TAPS) study: a randomized,controlled, multicenter clinical trial. Lancet. 2013;381:930–938. doi: 10.1016/S0140-6736(12)61726-7. [DOI] [PubMed] [Google Scholar]
- 7.Waldron P, Pegelow C, Neumayr L, et al. Tonsillectomy, adenoidectomy, and myringotomy in sickle cell disease: perioperative morbidity. Preoperative Transfusion is Sickle Cell Disease Study Group. J Pediatr Hematol Oncol. 1999;21:129–135. doi: 10.1097/00043426-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Wali YA, al Okbi H, Abri R. A comparison of two transfusion regimens in the perioperative management of children with sickle cell disease undergoing adenotonsillectomy. Pediatr Hematol Oncol. 2003;20:7–13. doi: 10.1080/0880010390158487. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Shamseer L, Clarke M, etal Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst reviews. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.OCEBM Levels of Evidence Working Group (2016) The Oxford Levels of Evidence 2. Oxford Centre for Evidence Based Medicine. https://www.cebm.net/index.aspx?o=5653 (accessed
- 11.Al-Jaouni SK, Al-Muhayawi SM, Qari MH, et al. Randomized clinical trial to evaluate the safety of avoiding pre-operative transfusion in sickle cell anemia. Bahrain Med Bull. 2006;28:164–167. [Google Scholar]
- 12.Derkay C, Bray G, Milmoe G, et al. Adenotonsillectomy in Children with Sickle Cell Disease. South Med J. 1991;84:205–218. doi: 10.1097/00007611-199102000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Farrell AN, Goudy SL, Yee ME, Leu RM, Landry AM. Adenotonsillectomy in children with sickle cell disease and obstructive sleep apnea. Int J Pediatr Otorhinolaryngol. 2018;111:158–161. doi: 10.1016/j.ijporl.2018.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atwood CM, Gnagi SH, Teufel IIRJ, Nguyen SA, White DR. Blood transfusion in children with sickle cell disease undergoing tonsillectomy. Int J Pediatr Otorhinolaryngol. 2017;103:117–120. doi: 10.1016/j.ijporl.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Evidence-Based Management of Sickle Cell Disease (2014) :Expert Panel Report, US Department of Health and Human Services